Abstract
Background
The purpose of this study was to determine the association between race and long-term cancer outcomes (recurrence and overall survival) within a population of US patients with locoregional colorectal cancer (CRC).
Methods
A cohort study of primary patient data merged with the National Cancer Database as part of a Commission on Cancer Special Study was performed. The study population was a random sample of patients undergoing surgery for stage I to III CRC between years 2006 and 2007 with 5 years of follow-up. Propensity-weighted multivariable Cox regression was performed with pooled results to yield statistical inferences. Prespecified sensitivity analysis was performed only for patients who received guideline concordant care (GCC) of primary CRC. All statistical tests were 2-sided.
Results
The study population included 8176 patients, 9.9% (n = 811) Black and 90.1% (n = 7365) White. Black patients were more likely to be uninsured or underinsured, have lower household income, and lower educational status (all P < .001). Rates of GCC were higher among Black vs White patients with colon cancer (76.9% vs 72.6%, P = .02), and Black and White patients with rectal cancer were treated with radiation at similar rates (69.1% vs 66.6%, P = .64). Black race was independently associated with increased risk of recurrence (hazard ratio [HR] = 1.48, 95% confidence interval [CI] = 1.26 to 1.73) and mortality (HR = 1.37, 95% CI = 1.18 to 1.59). In sensitivity analysis of only patients who received GCC, observed effects for recurrence (HR = 1.51, 95% CI = 1.27 to 1.79) and overall survival (HR = 1.40, 95% CI = 1.18 to 1.66) persisted.
Conclusions
Despite higher rates of GCC for CRC, Black patients experience a higher risk of recurrence and mortality compared with White patients.
Although incidence and mortality rates of colorectal cancer (CRC) are declining in the United States, racial disparities persist. From 2012 to 2016, the incidence of CRC in Black patients was approximately 20% higher than in non-Hispanic White patients (45.7 vs 38.6 per 100 000 population), and the mortality rate was 40% higher (19.0 vs 13.8 per 100 000 population) (1). Black patients present more often with advanced disease but have worse cancer-specific survival for every stage category compared with White patients (1). However, very little is known about racial differences in CRC recurrence rates following definitive treatment.
Recurrence data are not currently available for analysis within national cancer registries; therefore, recurrence rates among CRC patients have been reported predominately in either single-institution studies or within the context of clinical trials. In 1 compilation of 18 randomized trials of stage III patients treated with adjuvant chemotherapy, the majority (80%) of recurrences occurred within the first 3 years (2). To date, only 1 study has reported recurrence data using national cancer registry data, which was collected as part of a Commission on Cancer (CoC) special study. In that study, the median time to detection of recurrence was approximately 15 months, and 5-year cumulative recurrence rates were 20% in colon cancer patients and 24% in rectal cancer patients (3). In the absence of population-based data or multi-institutional studies, however, contemporary data on CRC recurrence outside the context of a clinical trial are sparse, and even less is known about racial differences in recurrence rates within routine clinical practice.
The purpose of this study was to determine the association between race and long-term cancer outcomes (recurrence and overall survival [OS]) within a large population of US patients presenting with locoregional CRC. The hypothesis was that Black patients would experience higher rates of recurrence and worse survival compared with White patients.
Methods
Study Population
Patients older than 18 years with American Joint Committee on Cancer (AJCC) stage I, II, or III adenocarcinoma of the colon or rectum treated with definitive surgical resection in 2006-2007 were identified from the National Cancer Database (NCDB). A random sample of up to 10 patients with CRC from each Commission on Cancer (CoC) facility was selected for detailed primary data collection regarding surveillance testing and recurrence as part of the commission’s special study as previously described (3). For facilities with fewer than 10 eligible patients, data for all eligible patients were abstracted. These data were then merged with corresponding NCDB records. The study period (2006-2007) was selected to allow for a minimum of 5 years of follow-up.
Patient race was determined from predefined NCDB data based on assignment by a CoC registrar according to fixed categories (4). Only patients of White or Black race were included for the primary study analysis. Patients with unknown tumor location, income, education, distance traveled, or race were excluded. Patients who did not undergo any surveillance testing following treatment of the primary cancer were also excluded because they could not be assessed for the primary outcome of recurrence.
The study analysis was considered exempt by The University of Texas MD Anderson Cancer Center Institutional Review Board.
Covariates
CRC clinical and pathologic stage was defined based on the AJCC 6th edition staging manual (5). High-risk stage II colon cancer was defined by the presence of 1 or more of the following features: T4 tumor (based on AJCC T-stage), fewer than 12 lymph nodes (LN) examined, positive margin, high tumor grade (grades 3-4), perineural invasion, or lymphovascular invasion.
Assessment of receipt of treatment of primary colon cancer was based on LN retrieval and chemotherapy. Guideline concordant care (GCC) was defined as 1) removal of at least 12 LN and all nodes negative (AJCC stage I or low-risk stage II), 2) removal of fewer than 12 LN but receipt of chemotherapy (high-risk stage II), or 3) removal of at least 12 LN and 1 or more nodes positive and receipt of chemotherapy (stage III). Failure to receive GCC was defined as 1) removal of fewer than 12 LN and no receipt of chemotherapy (high-risk stage II), or 2) removal of any number of LN with at least 1 or more nodes positive and no receipt of chemotherapy (stage III). Assessment of GCC among patients with rectal cancer was defined as receipt of neoadjuvant or adjuvant radiation in patients with either clinical stage II or III or pathologic stage II or III disease.
Surveillance testing included computed tomography, magnetic resonance imaging, positron emission tomography, or carcinoembryonic antigen tests as previously described (3).
Outcome Measures
The primary outcomes of interest were disease recurrence (locoregional or distant) and OS. Local recurrence was defined as recurrence at the site of primary tumor, anastomosis, or adjacent structure. Regional recurrence was considered recurrence at the regional LN associated with the primary tumor site, and distant recurrence was defined as recurrence outside the local or regional sites. Recurrence could be confirmed either clinically or pathologically. If synchronous locoregional and distant recurrence were diagnosed, both were recorded.
A prespecified sensitivity analysis was performed to assess recurrence rates and OS only among patients who received GCC, defined above.
Statistical Analysis
Baseline clinical and demographic characteristics were compared across racial groups using the χ2 test for categorical variables, t test for means, and Kruskal-Wallis test for medians of continuous variables. Patients were censored at the time of death, loss to follow-up, or end of the surveillance study period (5 years). Cumulative recurrence rates were determined using the Kaplan-Meier method and tested using log-rank test. Multivariable Cox proportional hazards regression model was performed to yield statistical inferences. The proportional hazards assumption was verified graphically using the log-log plot, where the -ln{−ln(survival)} curves of the covariate vs ln(analysis time) were checked. Inverse probability of treatment weighting using propensity score was performed to confirm the findings. Covariates adjusted in Cox and logistic regression for propensity score estimation were based on the previously published recurrence risk model and included age at diagnosis, sex, histology, pathologic tumor stage, index of metastasis (number of positive LN divided by total number of LN examined), lymphovascular invasion, perineural invasion, surgical margin status, chemotherapy, and tumor site, as well as Charlson-Deyo comorbidity index (6). Two variables had greater than 5% missing values: lymphovascular invasion and perineural invasion. Multiple imputation by chained equations was used to substitute predicted values for missing values with 20 imputed values.
All tests were 2-sided, with an alpha of .05. All analyses were performed using SAS software (version 9.1.3; SAS Institute Inc) for data processing and Stata MP (version 13.1; StataCorp) for statistical analyses. Study findings are reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology statement guidelines (7).
Results
Study Population
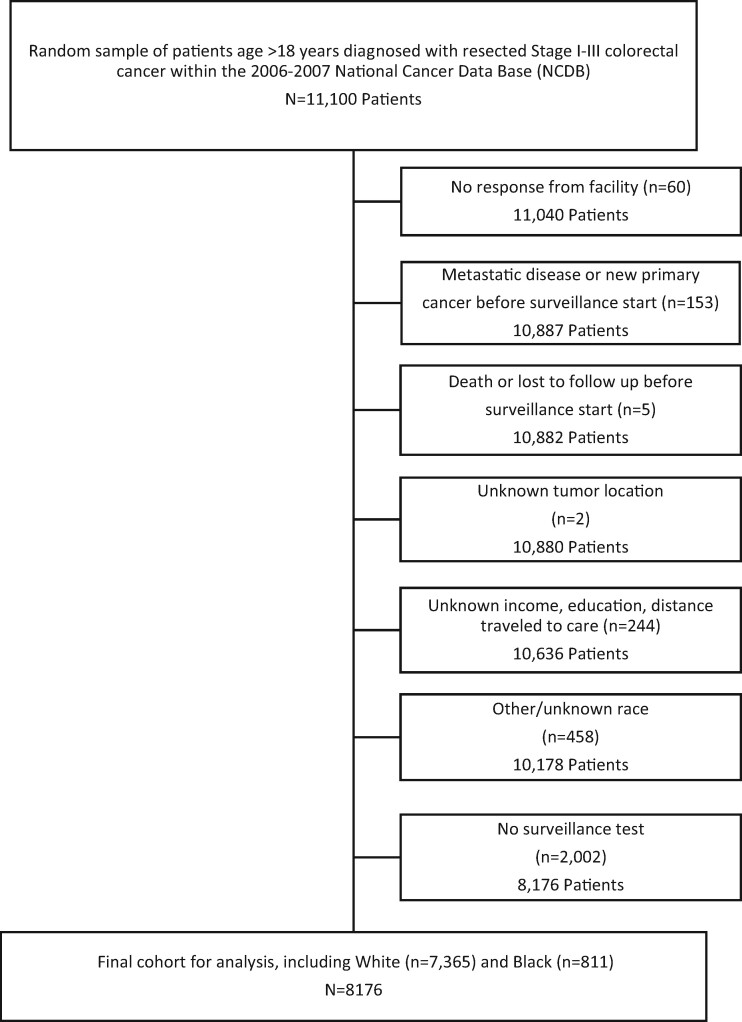
A total of 8176 patients were identified for study inclusion, of whom 9.9% (n = 811) were Black and 90.1% (n = 7365) were White (Figure 1). A larger proportion of Black patients were female (56.6% vs 50.5%) and younger (median 62 years vs 68 years old) compared with White patients (P < .05) (Table 1). Additionally, Black patients were more likely to have Medicaid insurance (8.4% vs 2.9%) or to be uninsured (7.5% vs 2.3%, P < .001). Median household income and zip code–based educational status were also markedly lower among Black patients (P < .001). Within the study cohort, a larger proportion of Black patients was diagnosed with pathologic stage III CRC (44.4% vs 38.1%, P = .005). No difference in tumor sidedness between White and Black patients was observed (P = .11). Black patients were more likely to be treated at an academic facility (31.2% vs 16.0%) and to travel a shorter distance for care (median 47 vs 63 miles, P < .001). Clinical and tumor characteristics were similar among Black and White patients (Supplementary Table 1, available online).
Figure 1.
Cohort selection for surveillance cohort. CRC = colorectal cancer; NCDB = National Cancer Data Base.
Table 1.
Baseline demographics and clinical characteristics of study cohort (N = 8176)
| Characteristics | White (n = 7365) No. (%) | Black (n = 811) No. (%) | P |
|---|---|---|---|
| Median age (interquartile range) | 68 (58-77) | 62 (52-71) | <.001 |
| Sexa | .004 | ||
| Male | 3646 (49.5) | 352 (43.4) | |
| Female | 3717 (50.5) | 459 (56.6) | |
| Comorbidity score | .78 | ||
| 0 | 5195 (70.5) | 564 (69.5) | |
| 1 | 1598 (21.7) | 179 (22.1) | |
| 2 | 572 (7.8) | 68 (8.4) | |
| Insurance status | <.001 | ||
| Private | 2839 (38.5) | 310 (38.2) | |
| Uninsured | 172 (2.3) | 61 (7.5) | |
| Medicaid | 214 (2.9) | 69 (8.5) | |
| Medicare | 3977 (54) | 348 (42.9) | |
| Managed care | 54 (0.7) | 8 (1) | |
| Unknown | 109 (1.5) | 15 (1.8) | |
| Median income | <.001 | ||
| <$30 000 | 1269 (17.2) | 385 (47.5) | |
| $30 000-$35 000 | 1971 (26.8) | 180 (22.2) | |
| $35 000-$45 999 | 2026 (27.5) | 143 (17.6) | |
| $46 000+ | 2099 (28.5) | 103 (12.7) | |
| Proportion without high school degree by zip code | <.001 | ||
| 29% + | 1289 (17.5) | 357 (44.0) | |
| 20%-28.9% | 2043 (27.7) | 268 (33.0) | |
| 14%-19.9% | 2466 (33.5) | 128 (15.8) | |
| <14% | 1567 (21.3) | 58 (7.2) | |
| Population density | <.001 | ||
| Metro area | 5533 (75.1) | 710 (87.5) | |
| Urban area | 1451 (19.7) | 82 (10.1) | |
| Rural area | 186 (2.5) | 7 (0.9) | |
| Unknown | 195 (2.6) | 12 (1.5) | |
| Pathologic stage | .005 | ||
| 0/1 | 1955 (26.5) | 192 (23.7) | |
| 2 | 2577 (35) | 258 (31.8) | |
| 3 | 2808 (38.1) | 360 (44.4) | |
| Unknown | 25 (0.3) | 1 (0.1) | |
| Facility type | <.001 | ||
| Community | 2098 (28.5) | 189 (23.3) | |
| Comprehensive | 4055 (55.1) | 369 (45.5) | |
| Other or unknown | 1179 (16) | 253 (31.2) | |
| Others or unknown | 33 (0.4) | 0 (0) | |
| Distance traveled for care, miles | 63 (24-146) | 47 (19-95) | <.001 |
| Tumor site | .11 | ||
| Right colon, including transverse | 3597 (48.8) | 420 (51.8) | |
| Left colon, rectosigmoid and rectum | 3768 (51.2) | 391 (48.2) |
Unknown sex (n = 2).
Guideline Concordance
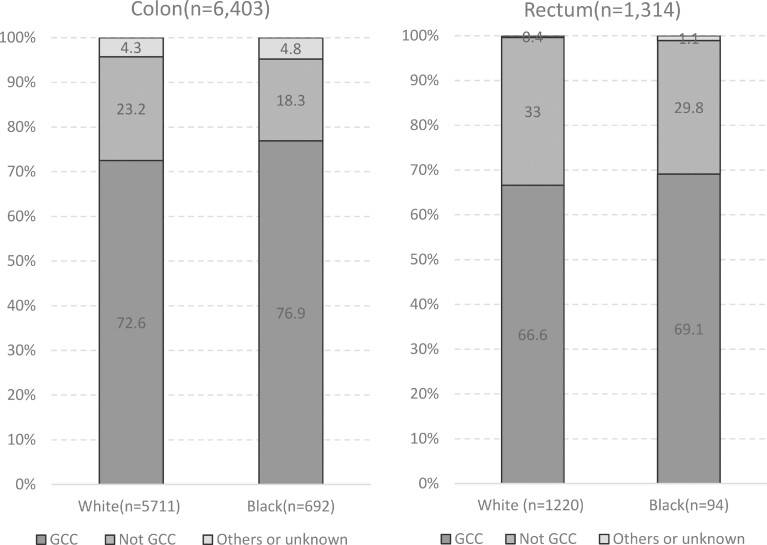
Among patients with colon cancer, rates of adequate lymphadenectomy (removal of at least 12 LN) did not differ by race (Black, 72.2% vs White, 69.6%, P = .06). Among patients with high-risk stage II colon cancer, no difference was observed in rates of adjuvant chemotherapy use (Black, 37.5% vs White, 30.5%, P = .45). However, a greater proportion of Black patients with stage III colon cancer were treated with adjuvant chemotherapy compared with their White counterparts (83.1% vs 76.3%, P = .005). Overall rates of guideline concordant colon cancer care were higher among Black patients compared with White patients (76.9% vs 72.6%, P = .02). Black and White patients with clinical stage II or III or pathologic stage II or III rectal cancer were treated with radiation at similar rates (69.1% vs 66.6%, P = .64) (Figure 2).
Figure 2.
Treatment of primary colon and rectal cancer by race. Colon cancer guideline concordant care (GCC) was defined as removal of at least 12 lymph nodes (LN) and all LN negative (American Joint Committee on Cancer stage I or low-risk stage II); or removal of fewer than 12 LN but the patient received adjuvant chemotherapy (high-risk stage II); or removal of at least 12 LN and 1 or more LN positive and the patient received adjuvant chemotherapy (stage III). Rectal cancer GCC was defined as receipt of neoadjuvant or adjuvant radiation in patients with either clinical stage II or III or pathologic stage II or III disease.
Primary Outcome: Recurrence and OS
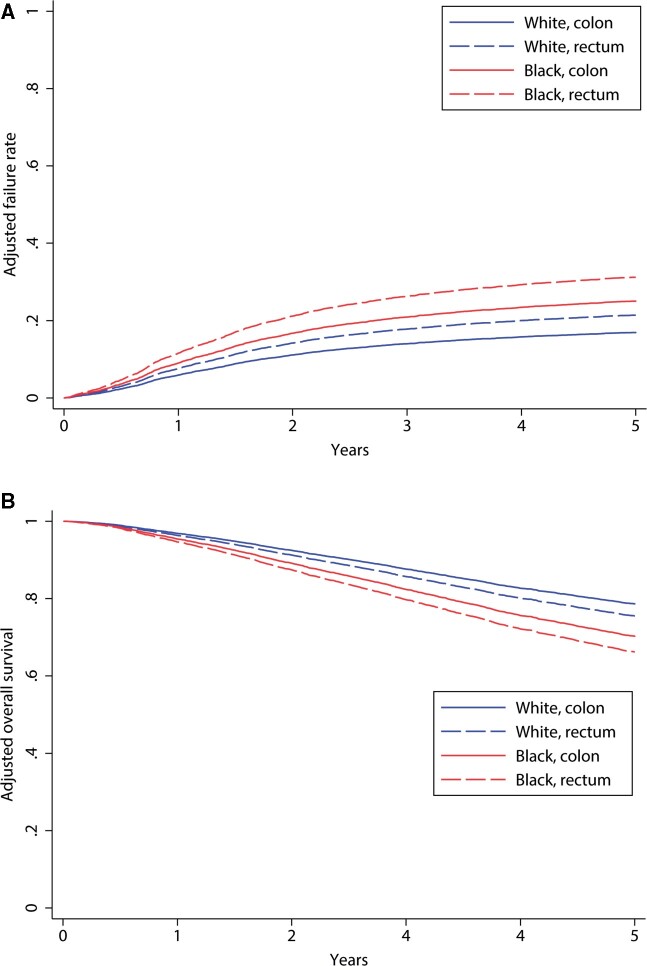
Black race was independently associated with increased risk of CRC recurrence and worse OS by adjusted analysis (Figure 3, A and B). By regression analysis adjusting for clinical factors previously demonstrated to be predictive of recurrence, risk of recurrence was also higher in Black patients (hazard ratio [HR] = 1.53, 95% confidence interval [CI] = 1.32 to 1.78). When socioeconomic factors, specifically insurance status, income, and population density of residence, were added to the model, Black race remained associated with risk of recurrence (HR = 1.48, 95% CI = 1.26 to 1.73) (Table 2). Similar findings were observed for OS. Black race was associated with increased risk of death (HR = 1.44, 95% CI = 1.26 to 1.66) compared with White race. This finding also persisted after adjusting for measured socioeconomic factors (HR = 1.37, 95% CI = 1.18 to 1.59) (Table 2). These findings were also confirmed by propensity weighted analysis (data not shown).
Figure 3.
Regression-adjusted recurrence rates and overall survival rates by tumor site and race (N = 8176).
Table 2.
Regression-adjusted risk of recurrence and OS among Black compared with White patients (N = 8176)
| Modela | Total cohort |
Colon cancer |
Rectal cancer |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Recurrence | 1.53 (1.32 to 1.78) | <.001 | 1.55 (1.31 to 1.82) | <.001 | 1.44 (1.00 to 2.07) | .047 |
| OS | 1.44 (1.26 to 1.66) | <.001 | 1.40 (1.22 to 1.63) | <.001 | 1.74 (1.23 to 2.46) | .002 |
| Model + SESb | ||||||
| Recurrence | 1.48 (1.26 to 1.73) | <.001 | 1.53 (1.28 to 1.82) | <.001 | 1.31 (0.89 to 1.93) | .16 |
| OS | 1.37 (1.18 to 1.59) | <.001 | 1.35 (1.15 to 1.59) | <.001 | 1.61 (1.11 to 2.32) | .01 |
Adjusted for age at diagnosis, sex, histology, pathologic tumor stage, index of metastasis, lymphovascular invasion, perineural invasion, surgical margin status, receipt of chemotherapy, tumor site, and Charlson-Deyo comorbidity index. CI = confidence interval; HR = hazard ratio; OS = overall survival; SES = socioeconomic status.
Added adjustment for SES also performed, including median income quartile, education, and population density.
Secondary Outcomes and Sensitivity Analysis
Among those with locoregional or distant recurrent disease, White patients were treated with surgical therapy either alone or in combination with chemotherapy or radiation more often than Black patients (24.0% [n = 288] vs 16.8% [n = 32], P = .04). Accordingly, White patients were treated less often with chemotherapy or chemoradiation alone compared with Black patients (40.9% [n = 490] vs 49.0% [n = 93], P = .04).
Sensitivity analysis was performed to include only the cohort of patients who received GCC for their primary CRC (Supplementary Table 2, available online). This demonstrated a similar increased hazard for recurrence and mortality among Black compared with White patients (HR = 1.51, 95% CI = 1.27 to 1.79; and HR = 1.40, 95% CI = 1.18 to 1.66) (Supplementary Table 3, available online).
Discussion
In this study of a real-world population of US patients with locoregional CRC, statistically significant racial disparities in recurrence and survival were identified. Black patients with colon cancer appeared to receive equivalent or superior quality of care compared with their White counterparts, measured by increased adjuvant chemotherapy use among stage III patients and higher rates of overall GCC. However, despite receiving equivalent or superior quality of care, rates of recurrence were higher among Black compared with White patients, and OS was worse. There were measurable differences in socioeconomic status between Black and White patients, including insurance status and household income; however, even after controlling for these differences, Black patients were still at higher risk for recurrence and mortality. To our knowledge, this is the first report of racial differences in CRC recurrence rates within the routine, clinical practice setting.
These findings are consistent with secondary analyses of patients enrolled in clinical trials in the United States, which have also found shorter time to recurrence, worse disease-free survival, and worse OS in Black patients (8-10). In a secondary analysis of patients with stage III colon cancer enrolled on the Alliance N0147 clinical trial and treated with 5-FU, leucovorin, and oxaliplatin (FOLFOX) chemotherapy, Black patients younger than 50 years had a shorter disease-free survival compared with White patients (HR = 2.84, 95% CI = 1.73 to 4.66), as did those with N1 disease (HR = 1.54, 95% CI = 1.04 to 2.29) (9). A pooled analysis of over 14 000 patients with stage II and III colon cancer enrolled in 12 randomized adjuvant chemotherapy trials also demonstrated worse recurrence-free survival in Black compared with White patients (3-year recurrence-free survival = 64.4% vs 72.1%, HR = 1.14, 95% CI = 1.04 to 1.24) (10).
In this study, the finding that Black patients with colon cancer were more likely to receive GCC than White patients was unexpected. Most prior studies have demonstrated persistent racial disparities in CRC treatment, including lower rates of surgery for stage I and stage IV CRC, adjuvant chemotherapy for stage III colon cancer, radiation for stage II or III rectal cancer, and targeted therapy for stage IV CRC (11-15). The cohort of Black patients treated at CoC sites and included in this study was more often from a metro area (87.5% vs 75.1) and more likely to receive care at an academic or research facility than White patients (31.2% vs 16.0%), which could explain higher rates of GCC. It is certainly possible that this finding is not generalizable to patients treated at non-CoC sites.
Because Black patients experience higher recurrence rates and worse survival even in the context of randomized controlled trials in which patients receive standard therapy, it has been suggested that underlying differences in tumor biology may be responsible for disparate outcomes. Recent evidence suggests that KRAS mutation rates differ by race, with a higher proportion of KRAS codon 13 mutations among Black patients (16,17). Additionally, in a secondary analysis of the CALGB/SWOG 80405 randomized trial in metastatic CRC patients, a higher rate of extended RAS mutations (noncodon 12 or 13) was identified in Black compared with White patients, and these extended mutations were associated with worse OS (17). Correlative studies of patients with metastatic CRC treated in the CALGB/SWOG 80405 randomized trial recently investigated the association of consensus molecular subtypes and tumor mutational burden with OS and progression-free survival (PFS) (18,19). Although consensus molecular subtypes and tumor mutational burden are prognostic for survival, the specific profiles of each did not differ by race ; however, this was not the primary focus of either analysis.
Several studies have recently demonstrated an association between patient race and tumor sidedness. A Surveillance, Epidemiology, and End Results Program analysis of stage IV CRC patients found that right-sided cancers were more common in Black compared with White patients (odds ratio = 1.45, 95% CI = 1.33 to 1.58) and that right-sided cancers were associated with a greater risk of death (HR = 1.27, 95% CI = 1.22 to 1.32) (20). Several smaller state or hospital-based cohort studies also found higher rates of proximal cancers in Black compared with White patients (21-23). In this study, a statistically significant difference in the frequency of proximal tumors between White and Black patients was not seen (48.8% vs 51.8%, respectively, P = .11). However, based on recent data as well as the findings presented in this study, further study of potential differences in tumor biology appears warranted.
In addition to tumor biology, differences in socioeconomic status likely contribute to much of the racial disparity in CRC outcomes. A recent pooled analysis of randomized controlled trials in a variety of cancer types found that patients with Medicaid or without insurance did not receive the same benefit of experimental therapy as did patients with private insurance (24). This would suggest that other unmeasured social determinants of health, such as financial strain or transportation access, for example, influence outcomes, even in the setting of equal access to high-quality treatment (25). Many of the same social determinants of health that correlate with insurance status may also correlate with race and could affect multiple aspects of care delivery, such as treatment adherence or access to survivorship care, for example. Therefore, it is likely that the racial differences in recurrence rates and survival identified here also reflect unmeasured differences in socioeconomic conditions and health-care access. Prioritization of collecting better data specific to social determinants of health will be critical to make meaningful progress in cancer health disparities research.
Several studies have identified racial differences in response to therapy and treatment toxicity in both the locoregional and metastatic setting. In a large randomized controlled trial of patients with high-risk stage II or stage III colon cancer treated with 5-FU chemotherapy, African American patients had lower rates of treatment-related toxicity than Caucasian patients, including lower rates of diarrhea, nausea, vomiting, stomatitis, and overall toxicity (P < .05) (8). Similarly, a secondary analysis of the National Cancer Institute-sponsored (N9741) randomized trial of patients with metastatic CRC treated with standard chemotherapy found lower response rates in Blacks compared with Whites (28% vs 41%, P = .008) and lower rates of grade 3 or higher adverse events among Black patients (34% vs 48%, P = .004) (26). Finally, a prospective observational study of patients with metastatic CRC treated with bevacizumab also found lower response rates in Black compared with White patients (37.5% vs 46.3%; adjusted odds ratio = 0.67, 95% CI = 0.50 to 0.90) (27). Taken together, these findings seem to suggest racial differences in sensitivity to systemic therapy that deserve further investigation.
There are several limitations to this study. First, the purpose of the study was to measure differences in outcomes between Black and White patients; therefore, patients of other racial and ethnic groups were excluded (other race, n = 362; unknown race, n = 96). Because patients were not prospectively enrolled to include equal numbers of patients in each race category, the cohort size of Black patients (n = 811) was much smaller than the cohort of White patients (n = 7365). This resulted in a small number of Black patients with rectal cancer (n = 94), thereby limiting subgroup analysis. Differences were observed in measured socioeconomic factors between Black and White patients. Although these were included in the multivariable regression model, it is possible that other confounders related to social determinants of health, such as marital status, exist that were not available within the dataset. Because the study cohort is comprised of patients diagnosed in 2006-2007, these findings may not be representative of contemporary clinical outcomes. These dates were selected to allow at least 5 years of follow-up data for detection of recurrence. Because this is a retrospective study, it is possible that recurrence could be underestimated. To account for this, if patients sought treatment or follow-up at another institution, registrars were asked to collect these data for data completeness. Additionally, it would be expected that any variation in recurrence detection would be similar between racial groups and would be unlikely to alter the findings. Selection criteria for the study cohort could introduce potential bias, specifically the exclusion of patients who did not receive surveillance testing. However, the proportions of Black and White patients without any surveillance testing did not differ (21.9% vs 19.4%, P = .05). Additionally, the patient population within the NCDB consists of patients treated at CoC sites. It is possible that the observed racial differences in receipt of GCC are unique to the population of patients treated at CoC sites and may not be generalizable to patients treated in other clinical settings. Despite this limitation, because the NCDB represents 70% of newly diagnosed cancer cases nationwide, it is considered an acceptable representation of the national cancer patient population. Finally, NCDB does not capture data on performance status or response to therapy, which could result in unmeasured confounding. Despite receiving equivalent or superior quality of care, Black patients experience a higher risk of recurrence and worse OS compared with White patients.
Funding
This work was supported by the Patient-Centered Outcomes Research Institute (PCORI) Award (CE13-04–6855, GJC). This work was also supported, in part, by the National Cancer Institute of the National Institutes of Health (P30CA016672; The University of Texas, MD Anderson Cancer Center Support Grant) and U10CA180821 (to the Alliance for Clinical Trials in Oncology).
Notes
Role of the funders: The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript and the decision to submit the manuscript for publication.
Disclosures: The authors have no conflicts of interest to report.
Prior presentations: This study has not been previously presented.
Acknowledgements: The authors would like to acknowledge Amanda Cuddy for her administrative support related to the PCORI Award and the American College of Surgeons Commission on Cancer for their support in conducting the special study.
Disclaimers: All statements in this publication, including, its findings, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee, or the National Institutes of Health.
Author contributions: Rebecca A. Snyder, MD, MPH- conceptualization, investigation, methodology, supervision, validation, writing- original, writing- review & editingChung-Yuan Hu, MPH, PhD- data curation, formal analysis, investigation, methodology, software, validation, visualization, writing – review & editingSyed Nabeel Zafar, MBBS; MPH- investigation, methodology, validation, writing- review & editingAmanda Francescatti, MS- data curation, funding acquisition, project administration, resources, validation, writing- review & editingGeorge J. Chang, MD, MS- conceptualization, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, writing- review & editing.
Data Availability
Data cannot be shared for ethical/privacy reasons.
Supplementary Material
References
- 1. Siegel RL, Miller Kd G, Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020; 70(3):145–164. [DOI] [PubMed] [Google Scholar]
- 2. Sargent DJ, Wieand HS, Haller DG, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23(34):8664–8670. [DOI] [PubMed] [Google Scholar]
- 3. Snyder RA, Hu CY, Cuddy A, et al. ; for the Alliance for Clinical Trials in Oncology Network Cancer Surveillance Optimization Working Group. Association between intensity of posttreatment surveillance testing and detection of recurrence in patients with colorectal cancer. JAMA. 2018;319(20):2104–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American College of Surgeons. Cancer. Facility oncology registry data standards (FORDS): revised for 2004. http://www.facs.org/∼/media/files/quality%20programs/cancer/coc/fords/fordsrevised0605.ashx. 2005. Accessed April 1, 2020.
- 5.American Joint Committee on Cancer. American Joint Committee on Cancer AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010. [Google Scholar]
- 6. Zafar SN, Hu CY, Snyder RA, et al. Predicting risk of recurrence after colorectal cancer surgery in the United States: an analysis of a Special Commission on Cancer national study. Ann Surg Oncol. 2020;27(8):2740–2749. [DOI] [PubMed] [Google Scholar]
- 7. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. [DOI] [PubMed] [Google Scholar]
- 8. McCollum AD, Catalano PJ, Haller DG. Outcomes and toxicity in African-American and Caucasian patients in a randomized adjuvant chemotherapy trial for colon cancer. J Natl Cancer Inst. 2002;94(15):1160–1167. [DOI] [PubMed] [Google Scholar]
- 9. Yoon HH, Shi Q, Alberts SR, et al. Racial differences in BRAF/KRAS mutation rates and survival in stage III colon cancer patients. J Natl Cancer Inst. 2015;107(10):djv186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yothers G, Sargent DJ, Wolmark N, et al. ; on behalf of the ACCENT Collaborative Group. Outcomes among black patients with stage II and III colon cancer receiving chemotherapy: an analysis of ACCENT adjuvant trials. J Natl Cancer Inst. 2011;103(20):1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Demissie K, Oluwole OO, Balasubramanian BA, Osinubi OO, August D, Rhoads GG. Racial differences in the treatment of colorectal cancer: a comparison of surgical and radiation therapy between Whites and Blacks. Ann Epidemiol. 2004;14(3):215–221. [DOI] [PubMed] [Google Scholar]
- 12. Dimou A, Syrigos KN, Saif MW. Disparities in colorectal cancer in African-Americans vs Whites: before and after diagnosis. World J Gasteroenterol. 2009;15(30):3734–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gross CP, Smith BD, Wolf E, Andersen M. Racial disparities in cancer therapy: did the gap narrow between 1992 and 2002? Cancer. 2008;112(4):900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lai Y, Wang C, Civan JM, et al. Effects of cancer stage and treatment differences on racial disparities in survival from colon cancer: a United States population-based study. Gastroenterology. 2016;150(5):1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murphy CC, Harlan LC, Warren JL, Geiger AM. Race and insurance differences in the receipt of adjuvant chemotherapy among patients with stage III colon cancer. J Clin Oncol. 2015;33(23):2530–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sylvester BE, Huo D, Khramtsov A, et al. Molecular analysis of colorectal tumors within a diverse patient cohort at a single institution. Clin Cancer Res. 2012;18(2):350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sehdev A, Niedzwiecki D, Venook AP, et al. ; Cancer and Leukemia Group B (Alliance) and SWOG. Association of RAS mutations with race in metastatic colorectal cancer: CALGB/SWOG 80405 (ALLIANCE). J Clin Oncol. 2018;36(4_suppl):638–638. [Google Scholar]
- 18. Innocenti F, Ou FS, Qu X, et al. Mutational analysis of patients with colorectal cancer in CALGB/SWOG 80405 identifies new roles of microsatellite instability and tumor mutational burden for patient outcome. J Clin Oncol. 2019;37(14):1217–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lenz HJ, Ou FS, Venook AP, et al. Impact of consensus molecular subtype on survival in patients with metastatic colorectal cancer: results from CALGB/SWOG 80405 (Alliance). J Clin Oncol. 2019;37(22):1876–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Charlton ME, Kahl AR, Greenbaum AA, et al. KRAS testing, tumor location, and survival in patients with stage IV colorectal cancer: SEER 2010-2013. J Natl Compr Canc Netw. 2017;15(12):1484–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carethers JM, Murali B, Yang B, et al. Influence of race on microsatellite instability and CD8+ T cell infiltration in colon cancer. PLoS One. 2014;9(6):e100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rodriguez EA, Tamariz L, Palacio A, Li H, Sussman DA. Racial disparities in the presentation and treatment of colorectal cancer: a statewide cross-sectional study. J Clin Gastroenterol. 2018;52(9):817–820. [DOI] [PubMed] [Google Scholar]
- 23. Xicola RM, Gagnon M, Clark JR, et al. Excess of proximal microsatellite-stable colorectal cancer in African Americans from a multiethnic study. Clin Cancer Res. 2014;20(18):4962–4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Unger JM, Blanke CD, LeBlanc M, et al. Association of patient demographic characteristics and insurance status with survival in cancer randomized clinical trials with positive findings. JAMA Netw Open. 2020;3(4):e203842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Snyder RA, Chang GJ. Insurance status as a surrogate for social determinants of health in cancer clinical trials. JAMA Netw Open. 2020;3(4):e203890. [DOI] [PubMed] [Google Scholar]
- 26. Sanoff HK, Sargent DJ, Green EM, McLeod HL, Goldberg RM. Racial differences in advanced colorectal cancer outcomes and pharmacogenetics: a subgroup analysis of a large randomized clinical trial. J Clin Oncol. 2009;27(25):4109–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Polite BN, Sing A, Sargent DJ, et al. Exploring racial differences in outcome and treatment for metastatic colorectal cancer: results from a large prospective observational cohort study (BRiTE). Cancer. 2012;118(4):1083–1090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data cannot be shared for ethical/privacy reasons.