Abstract
Background
Few studies have examined the impact of treatment-related morbidity on long-term, cause-specific mortality in Hodgkin lymphoma (HL) patients.
Methods
This multicenter cohort included 4919 HL patients, treated before age 51 years between 1965 and 2000, with a median follow-up of 20.2 years. Standardized mortality ratios, absolute excess mortality (AEM) per 10 000 person-years, and cause-specific cumulative mortality by stage and primary treatment, accounting for competing risks, were calculated.
Results
HL patients experienced a 5.1-fold (AEM = 123 excess deaths per 10 000 person-years) higher risk of death due to causes other than HL. This risk remained increased in 40-year survivors (standardized mortality ratio = 5.2, 95% confidence interval [CI] = 4.2 to 6.5, AEM = 619). At age 54 years, HL survivors experienced similar cumulative mortality (20.0%) from causes other than HL to 71-year-old individuals from the general population. Whereas HL mortality statistically significantly decreased over the calendar period (P < .001), solid tumor mortality did not change in the most recent treatment era. Patients treated in 1989-2000 had lower 25-year cardiovascular disease mortality than patients treated in 1965-1976 (4.3% vs 5.7%; subdistribution hazard ratio = 0.65, 95% CI = 0.46 to 0.93). Infectious disease mortality was not only increased after splenectomy but also after spleen irradiation (hazard ratio = 2.81, 95% CI = 1.55 to 5.07). For stage I-II, primary treatment with chemotherapy (CT) alone was associated with statistically significantly higher HL mortality (P < .001 for CT vs radiotherapy [RT]; P = .04 for CT vs RT+CT) but lower 30-year mortality from causes other than HL (15.8%, 95% CI = 9.7% to 23.3%) compared with RT alone (36.9%, 95% CI = 34.0% to 39.8%, P = .001) and RT and CT combined (29.8%, 95% CI = 26.8% to 32.9%, P = .02).
Conclusions
Compared with the general population, HL survivors have a substantially reduced life expectancy. Optimal selection of patients for primary CT is crucial, weighing risks of HL relapse and long-term toxicity.
Over the past decades treatment advances have greatly improved survival of Hodgkin lymphoma (HL), currently resulting in cure rates of more than 80% (1). However, improved prognosis has been accompanied by increased risks of second malignancies (SMN), cardiovascular diseases (CVD), and serious infections (2-9). Whereas many studies have focused on long-term morbidity from treatment-related complications, only a few have examined to what extent such morbidity translates into long-term excess mortality in HL patients (10-12). Knowledge about cause-specific excess mortality provides insight into the ultimate burden from treatment-related late effects.
HL treatment has changed considerably over time towards more effective systemic treatment, with lower doses of alkylating chemotherapy (CT) and radiotherapy (RT) to smaller volumes at lower doses. It is unclear whether, as a result, excess mortality has decreased among more recently treated patients compared with patients treated in earlier decades. Furthermore, because most reports on late treatment effects in HL patients were restricted to 5-year survivors, few studies could directly evaluate treatment-specific HL mortality in relation to long-term mortality from adverse events. This study examines long-term, cause-specific excess mortality in a large, multicenter Dutch cohort of HL patients treated between 1965 and 2000 with up to 45 years of follow-up since HL treatment. We also assessed stage-specific mortality from HL and all other causes according to primary treatment to study the long-term consequences of initial treatment choice.
Methods
Data Collection Procedures
Our multicenter cohort included 4919 HL patients treated before the age of 51 years between 1965 and 2000 in 7 university hospitals or cancer centers or in the affiliated hospitals of 3 regional population-based cancer registries. Patient selection and data collection methods were described previously (2,6,10,13). Data were collected on date of birth and HL diagnosis, treatment, including treatment for relapse (date of start, radiation fields, CT regimens, splenectomy), vital status, and date of most recent medical information. Exact dates of death were obtained by linkage with the Dutch Central Office of Genealogy. Information on cause of death was retrieved from hospital medical records and general practitioners and through linkage with the nationwide cause-of-death registry at Statistics Netherlands for patients with missing causes of death (41%). Causes of death were (re)coded according to the International Classification of Diseases, 10th edition. This study was exempt from institutional review board approval according to Dutch law because existing data from medical files were used.
Statistical Analysis
We compared the observed mortality in our cohort with mortality rates in the general population considering person-years of observation by age, sex, and calendar period. Mortality data for the Dutch general population from Statistics Netherlands for the period 1965-2016 were used as reference rates. Time at risk began at start of treatment and ended at date of death, date of emigration, or January 1, 2018, whichever occurred first. We calculated standardized mortality ratios (SMRs), absolute excess mortality (AEM, expressed per 10 000 person-years), and the corresponding 95% confidence intervals (CI) (14). Tests for heterogeneity and trends of SMRs by follow-up time and other characteristics were performed within collapsed person-time in Poisson regression models. Tests for heterogeneity and trends in AEM were performed in additive Poisson regression models (15).
Cause-specific cumulative mortality treating other causes of death as competing risk was estimated using a nonparametric estimator. The expected cumulative mortality was estimated using the conditional (Ederer II) method (16). To directly compare cumulative HL mortality and (long-term) mortality from all causes other than HL, we performed analyses by stage and primary treatment, ignoring relapse treatment. Differences in stage-, treatment-, and period-specific mortality were evaluated using competing risk models, adjusted for sex, age, and stage (17). Cox regression analysis was used to assess the influence of total treatment on cause-specific mortality, using treatment as the time-varying covariate to account for relapse treatment and using attained age as the primary time-scale. Model fit and model assumptions were assessed using graphic and residual-based methods. P values less than .05 were considered statistically significant. All statistical tests were 2-sided. All analyses were performed using Stata statistical software, version 15 (StataCorp, College Station, TX).
Results
Patient Characteristics
The cohort included 2853 male and 2066 female patients (Table 1). Treatment (including relapse treatment) consisted of RT alone in 23.9%, CT alone in 14.0%, and RT+CT in 62.1% of patients. Of patients treated with RT, 75.4% received supradiaphragmatic RT. Twenty percent underwent splenectomy and 22.1% received spleen irradiation. Patterns of treatment changed over time (Supplementary Methods and Supplementary Table 1, available online). Median age at start of treatment was 27.8 years; median follow-up time was 20.2 years (interquartile range = 12.1-28.6).
Table 1.
Patient characteristics
| Characteristic | HL patients, No. (%) (n = 4919) |
|---|---|
| Sex | |
| Male | 2853 (58.0) |
| Female | 2066 (42.0) |
| Age at HL treatment, y | |
| Median (IQR) | 27.8 (21.4-36.4) |
| <25 | 1941 (39.5) |
| 25-34 | 1575 (32.0) |
| 35-50 | 1403 (28.5) |
| Treatment period | |
| 1965-1976 | 1121 (22.8) |
| 1977-1988 | 1588 (32.3) |
| 1989-2000 | 2210 (44.9) |
| HL treatmenta | |
| RT alone | 1175 (23.9) |
| CT alone | 668 (14.0) |
| RT+CT | 3056 (62.1) |
| RT field | |
| No RT | 688 (14.0) |
| Supradiaphragmatic RT | 2006 (40.8) |
| Infradiaphragmatic RT | 254 (5.2) |
| Supra- and infradiaphragmatic RT | 1700 (34.5) |
| RT field unknown | 271 (5.5) |
| Spleen irradiation | |
| No | 3562 (72.4) |
| Yes | 1086 (22.1) |
| RT field unknown | 271 (5.5) |
| Splenectomy | |
| No | 3730 (75.8) |
| Yes | 988 (20.1) |
| Unknown | 201 (4.1) |
| Anthracyclines | |
| No CT | 1175 (23.9) |
| No anthracycline-containing CT | 1440 (29.3) |
| ≤210-g/m2 anthracycline dose | 941 (19.1) |
| >210-g/m2 anthracycline dose | 880 (17.9) |
| Yes, anthracycline dose unknown | 263 (5.4) |
| CT regimen unknown | 220 (4.5) |
| Procarbazine | |
| No CT | 1175 (23.9) |
| No procarbazine-containing CT | 697 (14.2) |
| ≤4.2-g/m2 procarbazine dose | 1044 (21.2) |
| >4.2-g/m2 procarbazine dose | 1410 (28.7) |
| Yes, procarbazine dose unknown | 373 (7.6) |
| CT regimen unknown | 226 (4.6) |
| Vital status at end of follow-up | |
| Alive | 2632 (53.5) |
| Dead | 2180 (44.3) |
| Emigrated or lost to follow-up | 107 (2.2) |
| Follow-up after HL treatment, y | |
| Median (IQR) | 20.2 (12.1-28.6) |
| 0-4 | 566 (11.5) |
| 5-9 | 368 (7.5) |
| 10-19 | 1489 (30.3) |
| 20-29 | 1448 (29.4) |
| 30-39 | 792 (16.1) |
| ≥40 | 256 (5.2) |
| Attained age at end of follow-up, y | |
| Median (IQR) | 50.1 (40.3-59.7) |
| <40 | 1194 (24.3) |
| 40-49 | 1244 (25.3) |
| 50-59 | 1285 (26.1) |
| ≥60 | 1196 (24.3) |
Including relapse treatment. CT = chemotherapy; HL = Hodgkin lymphoma; IQR = interquartile range; RT = radiotherapy.
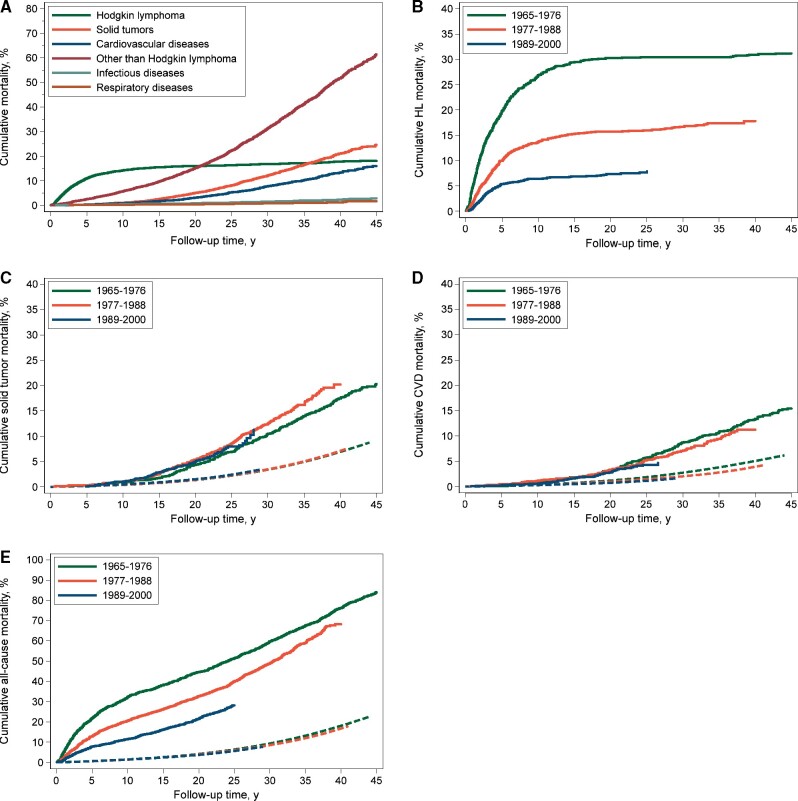
Cumulative Mortality by Cause of Death
Up to January 2018, 2180 patients (44.3%) had died; cause of death was available for 96%. A total 706 patients (32.4%) died of HL, 712 (32.7%) died of SMN, 393 (18.0%) of diseases of the circulatory system, 69 (3.2%) of infectious diseases, and 38 (1.7%) of respiratory diseases (Table 2). Cumulative mortality from HL steeply increased until 5 years of follow-up and leveled off thereafter. At 10 years of follow-up, the cumulative HL mortality was 14.1% (95% CI = 13.1% to 15.2%; Figure 1, A). Cumulative mortality from solid tumors started to increase after 10 years, leading to 5% cumulative mortality (95% CI = 4.4% to 5.7%) at 20 years and 21.0% (95% CI = 19.2% to 22.8%) at 40 years of follow-up. Cumulative CVD mortality also increased after 10 years, with a cumulative mortality of 3.1% (95% CI = 2.6% to 3.6%) at 20 years and 13.2% (95% CI = 11.7% to 14.7%) at 40 years. Cumulative mortality rates of infectious diseases and respiratory diseases were 2.3% (95% CI = 1.7% to 3.0%) and 1.2% (95% CI = 0.8% to 1.8%) at 40 years, respectively.
Table 2.
Causes of death in HL patients (n = 4919)
| Cause of death | ICD-10 | Observed (%) | SMR (95% CI) | AEM |
|---|---|---|---|---|
| HLa | C81 | 706 (32.4) | — | 73.3 |
| Causes of death other than HL | A00-Y98 (C81 excluded) | 1474 (67.6) | 5.1 (4.8 to 5.3) | 122.9 |
| Infections (including pneumonia and influenza)b | A00-B99, J10-J18 | 69 (3.2) | 8.3 (6.5 to 10.5) | 6.3 |
| Malignant neoplasms other than HL | C00-C97 (C81 excluded) | 712 (32.7) | 5.9 (5.5 to 6.4) | 61.5 |
| Digestive organs | C15, C26, C48 | 165 (7.6) | 5.1 (4.4 to 6.0) | 13.8 |
| Esophagus | C15 | 37 (1.7) | 7.8 (5.5 to 10.7) | 3.4 |
| Stomach | C16 | 31 (1.4) | 6.8 (4.6 to 9.6) | 2.7 |
| Colon | C18 | 25 (1.1) | 2.8 (1.8 to 4.1) | 1.7 |
| Rectum, sigmoid, anus | C19-C21 | 20 (0.9) | 6.7 (4.1 to 10.4) | 1.8 |
| Pancreas | C25 | 31 (1.4) | 5.0 (3.4 to 7.1) | 2.6 |
| Respiratory organs | C30-C38, C45 | 230 (10.6) | 6.7 (5.9 to 7.6) | 20.3 |
| Lung, bronchus, trachea | C33-C34 | 204 (9.4) | 6.4 (5.6 to 7.4) | 17.9 |
| Mesothelioma | C45 | 21 (1.0) | 15.5 (9.6 to 23.8) | 2.0 |
| Female breast | C50 | 55 (2.5) | 4.4 (3.3 to 5.7) | 4.4 |
| Urogenital tract | C51-C68 | 39 (1.8) | 2.0 (1.4 to 2.7) | 2.0 |
| Other, ill-defined, and unspecified sites | C76, C80 | 36 (1.7) | 6.9 (4.9 to 9.6) | 3.2 |
| Non-HL | C82-C85 | 88 (4.0) | 25.7 (20.6 to 31.7) | 8.8 |
| Leukemiac | C91-C96 | 53 (2.4) | 15.6 (11.7 to 20.4) | 5.2 |
| Diseases of the blood and blood-forming organs | D50-D89 | 8 (0.4) | 12.1 (5.2 to 23.9) | 0.8 |
| Endocrine, nutritional, and metabolic diseases | E00-E90 | 12 (0.6) | 1.7 (0.9 to 2.9) | 0.5 |
| Mental and behavioral disorders | F00-F99 | 5 (0.2) | 1.1 (0.4 to 2.6) | 0.1 |
| Diseases of nervous system and sense organs | G00-H95 | 10 (0.5) | 1.3 (0.6 to 2.4) | 0.2 |
| Diseases of circulatory system | I00-I99 | 393 (18.0) | 5.5 (5.0 to 6.1) | 33.4 |
| Myocardial infarction | I21-I22 | 92 (4.2) | 3.7 (3.0 to 4.5) | 7.0 |
| Other ischemic heart diseases | I20, I23-I25 | 31 (1.4) | 4.7 (3.2 to 6.7) | 2.5 |
| Cerebrovascular diseases | I60-I69 | 30 (1.4) | 2.9 (1.5 to 3.3) | 1.8 |
| Other diseases of heart and circulatory systemd | I00-I05, I26-I28, I30-I52, I70-I99 | 235 (10.8) | 8.9 (7.8 to 10.1) | 21.7 |
| Respiratory diseases (excluding pneumonia and influenza) | J00-J99 (J10-J18 excluded) | 38 (1.7) | 3.6 (2.6 to 5.0) | 2.9 |
| Diseases of digestive system | K00-K93 | 32 (1.5) | 2.9 (2.0 to 4.1) | 2.2 |
| Diseases of genitourinary system | N00-N99 | 4 (0.2) | 1.6 (0.4 to 4.2) | 0.2 |
| Symptoms, signs, ill-defined, and unknown causes of mortalitye | R00-R99 | 106 (4.9) | 7.2 (5.9 to 8.7) | 9.5 |
| External causes of mortality | V01-Y98 | 46 (2.1) | 1.7 (1.2 to 2.2) | 1.9 |
SMR for HL is not provided. Because all patients in our cohort were diagnosed with HL, the SMR would reflect an overestimation of observed HL deaths compared with those expected in the general population. AEM = absolute excess mortality per 10 000 person-years; CI = confidence interval; HL = Hodgkin lymphoma; ICD-10 = International Classification of Diseases version 10; SMR = standardized mortality ratio.
Including 31 deaths from septicemia and 26 deaths from pneumonia.
An additional 26 patients died from myelodysplastic syndrome, but mortality rates in the general population are not specified for the entire observation period.
Including 75 deaths from heart failure, 15 deaths from cardiomyopathy, 48 deaths from valvular heart disease, 11 deaths from diseases of the pericardium, and 12 deaths from cardiac dysrhythmias. Historical mortality rates of these cardiovascular disease subtypes are not available.
Including 96 deaths from unknown and unspecified causes.
Figure 1.
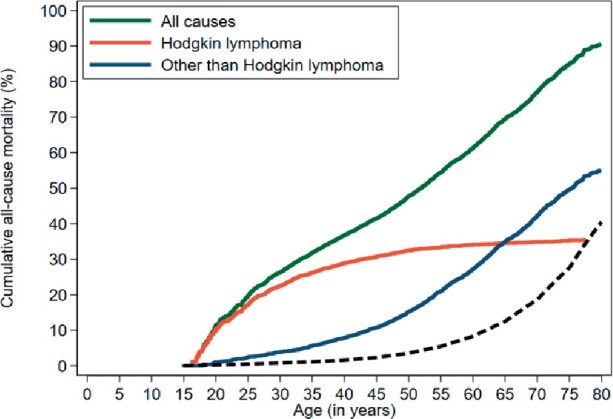
Cumulative mortality from all causes, Hodgkin lymphoma (HL), and other than HL by attained age. Solid lines represent the observed cumulative mortality, and dashed line represents the expected other-cause mortality based on general population rates.
Cumulative 10-year HL mortality decreased from 26.7% in 1965-1976 to 6.4% in 1989-2000 (subdistribution hazard ratio [sHR] = 0.30, 95% CI = 0.24 to 0.36, adjusted for age and sex and restricted to the first 25 years of follow-up; Figure 2, B). From the age of 50 years, HL patients reached a given cumulative mortality approximately 15-20 years earlier than individuals from the general population. For example, when HL survivors reached the age of 54 years, they already experienced similar cumulative mortality (20.0%) from causes other than HL to 71-year-old individuals from the general population (Figure 1). Cumulative mortality from solid tumors did not change over the calendar period (Figure 2, C). The 25-year cumulative mortality from solid tumors was 6.8% (95% CI = 5.4% to 8.4%) in 1965-1976 and 7.9% (95% CI = 6.4% to 9.6%) in 1989-2000. However, cumulative mortality from CVD was statistically significantly lower for patients treated in 1989-2000 than for patients treated from 1965 to 1976 (4.3% vs 5.7%, sHR = 0.65, 95% CI = 0.46 to 0.93; Figure 2, D).
Figure 2.
Cumulative mortality from major disease categories in the entire cohort (A) and cumulative mortality by treatment period from Hodgkin lymphoma (HL) (B), solid tumors (C), cardiovascular disease (CVD) (D), and all causes (E). Solid lines represent the observed cumulative mortality, and dashed lines represent the expected mortality based on general population rates.
Comparison With the General Population
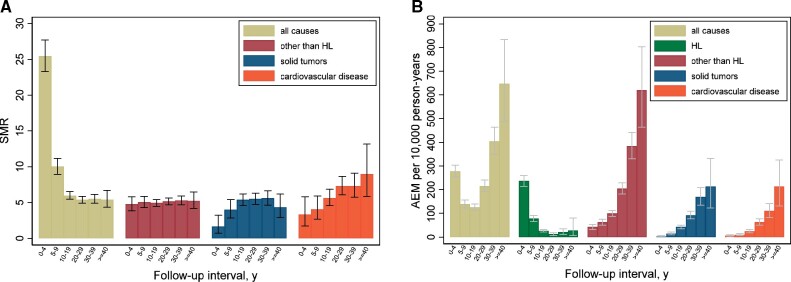
Compared with the general population, mortality due to causes other than HL increased 5.1-fold (95% CI = 4.8-fold to 5.3-fold), corresponding to 122.9 excess deaths per 10 000 person-years (Table 2). AEM from HL amounted to 73.3 per 10 000 person-years. Solid tumors and CVD contributed most (65.7%) to the excess mortality from causes other than HL, with AEMs of 47.4 and 33.4 per 10 000 person-years, respectively. For SMN (overall SMR = 5.9), greater than 15-fold increased SMRs were observed for mesothelioma, leukemia, and non-HL. Of all SMN, lung cancer contributed most to AEM from cancer, accounting for 29.1% of excess cancer deaths, followed by digestive tract malignancies (22.4% of excess cancer deaths). Excess mortality due to female breast cancer was low, with 4.4 excess deaths per 10 000 person-years, as was excess mortality for sites with high SMRs but low background rates in the population (eg, mesothelioma).
For diseases of the circulatory system (SMR = 5.5, 95% CI = 5.0 to 6.1), myocardial infarction contributed most to excess mortality (21.0% of excess circulatory deaths), followed by nonischemic diseases of the circulatory system and cerebrovascular disease (65.0% of excess circulatory deaths). HL patients also experienced more than eightfold increased mortality from infections (AEM = 6.3) and threefold increased mortality from respiratory and digestive diseases (AEMs = 2.9 and 2.2, respectively).
The SMR for causes of death other than HL remained increased for at least 40 years after HL treatment (SMR for ≥40 years = 5.2, 95% CI = 4.2 to 6.5). The AEM increased rapidly during follow-up (Figure 3), resulting in 619 excess deaths per 10 000 person-years after 40 or more years (Ptrend < .001). At 25 years after HL, AEM from causes other than HL (AEM = 86.5) exceeded that of HL mortality (AEM = 82.3). The SMR for CVD was already elevated in the first 5 years of follow-up and increased thereafter (Ptrend < .001), whereas a statistically significantly elevated SMR for solid tumors was observed from 5 years of follow-up onwards (P < .001). For infectious disease, the SMR decreased during follow-up (SMRs for <15 years and ≥30 years follow-up: 12.0, 95% CI = 8.1 to 17.3; and 6.2, 95% CI = 3.5 to 10.3, respectively, Ptrend = .03; Supplementary Figure 1, available online).
Figure 3.
Standardized mortality ratios (SMR) (A) and absolute excess mortality (AEM) (B) from major disease categories by follow-up interval.
For both solid tumors and CVD, SMRs decreased with increasing age at HL treatment (Ptrend < .001; Supplementary Table 2, available online). However, AEM from these diseases strongly increased with older age at treatment and with attained age; the increasing trend of AEM with attained age was also seen within age at treatment categories (Ptrend < .001). In patients who were 25 years old or younger at HL treatment and reached age 50 years or older, we observed 141.4 excess deaths from solid tumors and 108.9 excess deaths per 10 000 person-years from CVD. In patients treated at 35-50 years old with an attained age of 50 years and older, we observed 69.7 excess deaths from solid tumors and 32.3 excess deaths per 10 000 person-years from CVD.
Associations Between Treatment and Cause-Specific Mortality
In multivariable analysis (Table 3), patients with supradiaphragmatic RT had higher solid tumor mortality risk than patients not receiving such treatment (HR = 2.02, 95% CI = 1.54 to 2.65). Mortality after supradiaphragmatic RT was only statistically significantly increased from 10 years after initial treatment (10-19 years of follow-up: HR = 2.27, 95% CI = 1.4 to 3.7; and ≥20 years of follow-up: HR = 2.0, 95% CI = 1.4 to 2.8). Notably, receiving infradiaphragmatic RT or more than 4.2 g/m2 procarbazine-containing CT dose was associated with 1.5-fold (HR = 1.53, 95% CI = 1.26 to 1.85) increased mortality risk from solid cancers. For CVD mortality, patients who received supradiaphragmatic RT had a 4.3-fold (HR = 4.36, 95% CI = 2.74 to 6.94) increased risk to die from CVD, whereas anthracycline-containing CT was not associated with CVD mortality. However, in a subanalysis, mortality from heart failure and cardiomyopathy was increased after both greater than 210 g/m2 anthracycline-containing CT dose (HR = 2.10, 95% CI = 1.00 to 4.38) and supradiaphragmatic RT (HR = 7.27, 95% CI = 2.21 to 23.92). Mortality from infectious diseases was statistically significantly increased after splenectomy (HR = 2.52, 95% CI = 1.40 to 4.53) and after splenic irradiation (HR = 2.81, 95% CI = 1.55 to 5.07). Infectious disease mortality was also increased after receipt of both low- and high-dose anthracycline-containing CT (HR = 2.67, 95% CI = 1.19 to 5.98; and HR = 2.35, 95% CI = 1.12 to 4.94, respectively). Furthermore, supradiaphragmatic RT was associated with increased mortality from respiratory diseases (HR = 7.91, 95% CI = 1.85 to 33.88) and infradiaphragmatic RT with increased mortality from digestive tract diseases (HR = 2.41, 95% CI = 1.15 to 5.06; Supplementary Table 3, available online).
Table 3.
Multivariable Cox regression analyses of potential risk factors for solid tumor, cardiovascular disease, and infectious disease mortalitya
| Risk factors | Solid tumors (n = 568) |
Cardiovascular disease (n = 363)b |
Infectious disease (n = 69) |
|||
|---|---|---|---|---|---|---|
| No.c | HR (95% CI) | No.c | HR (95% CI) | No.c | HR (95% CI) | |
| Sex | ||||||
| Male | 323 | 1.00 (Ref.) | 231 | 1.00 (Ref.) | 45 | 1.00 (Ref.) |
| Female | 245 | 0.88 (0.75 to 1.05) | 132 | 0.57 (0.46 to 0.71) | 24 | 0.71 (0.43 to 1.18) |
| Age at HL treatment, y | ||||||
| <25 | 165 | 1.00 (Ref.) | 102 | 1.00 (Ref.) | 17 | 1.00 (Ref.) |
| 25-34 | 197 | 0.69 (0.56 to 0.86) | 107 | 0.73 (0.55 to 0.97) | 25 | 1.47 (0.74 to 2.93) |
| 35-50 | 206 | 0.48 (0.38 to 0.61) | 154 | 0.71 (0.53 to 0.97) | 27 | 1.31 (0.61 to 2.82) |
| Supradiaphragmatic RT | ||||||
| No RT or no supradiaphragmatic RT | 66 | 1.00 (Ref.) | 21 | 1.00 (Ref.) | 8 | 1.00 (Ref.) |
| Yes | 482 | 2.02 (1.54 to 2.65) | 331 | 4.36 (2.74 to 6.94) | 60 | 2.17 (0.99 to 4.79) |
| Infradiaphragmatic RT | ||||||
| No RT or no infradiaphragmatic RT | 254 | 1.00 (Ref.) | 166 | 1.00 (Ref.) | 26 | 1.00 (Ref.) |
| Yes, no spleen | 139 | 1.46 (1.16 to 1.85) | 89 | 1.21 (0.90 to 1.63) | 19 | 1.70 (0.87 to 3.30) |
| Yes, including spleen | 155 | 1.52 (1.23 to 1.87) | 97 | 1.38 (1.06 to 1.80) | 23 | 2.81 (1.55 to 5.07) |
| Splenectomy | ||||||
| No | 367 | 1.00 (Ref.) | 224 | 1.00 (Ref.) | 38 | 1.00 (Ref.) |
| Yes | 178 | 1.02 (0.82 to 1.26) | 126 | 1.04 (0.79 to 1.36) | 29 | 2.52 (1.40 to 4.53) |
| CT | ||||||
| Anthracyclines | ||||||
| No CT or no anthracyclines | 406 | 1.00 (Ref.) | 280 | 1.00 (Ref.) | 37 | 1.00 (Ref.) |
| ≤210-g/m2 anthracycline dose | 52 | 0.87 (0.62 to 1.21) | 22 | 0.68 (0.40 to 1.14) | 11 | 2.67 (1.19 to 5.98) |
| >210-g/m2 anthracycline dose | 85 | 1.19 (0.92 to 1.55) | 45 | 1.29 (0.89 to 1.87) | 12 | 2.35 (1.12 to 4.94) |
| Procarbazine | ||||||
| No CT or no procarbazine | 251 | 1.00 (Ref.) | 205 | 1.00 (Ref.) | 22 | 1.0 (Ref.) |
| ≤4.2-g/m2 procarbazine dose | 81 | 1.09 (0.82 to 1.47) | 47 | 1.00 (0.69 to 1.45) | 11 | 0.91 (0.39 to 2.09) |
| >4.2-g/m2 procarbazine dose | 204 | 1.53 (1.26 to 1.85) | 86 | 0.87 (0.66 to 1.13) | 21 | 1.37 (0.74 to 2.53) |
Attained age was used as the primary x-axis in all models. CI = confidence interval; CT = chemotherapy; HL = Hodgkin lymphoma; HR = hazard ratio; RT = radiotherapy.
Excluding deaths from cerebrovascular disease. Model stratified for treatment period.
For variables with missing values, the number of patients in each category do not add up to the total number of patients. Missing values for each variable were assigned to a “missing” category and analyzed as such in the model. Hazard ratios for this category showed no statistical significance and are not presented in this table.
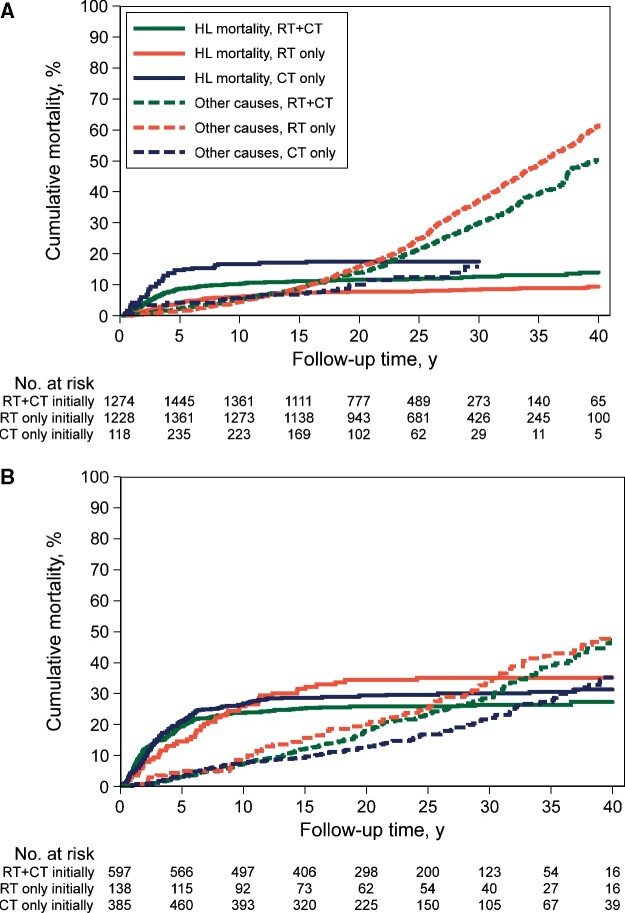
Treatment-Specific Cumulative Mortality From HL and Other Causes
We compared stage-specific HL mortality with mortality from any cause other than HL according to initial HL treatment (Figure 4). Stage I-II patients treated with RT alone experienced lower 30-year HL mortality (8.4%, 95% CI = 7.0% to 10.1%) compared with RT+CT (12.6%, 95% CI = 10.8% to 14.5%) and CT alone (17.5%, 95% CI = 11.63% to 24.5%); sHRs = 0.58, 95% CI = 0.45 to 0.75; and 0.63, 95% CI = 0.41 to 0.97, respectively, adjusted for age, treatment period, and stage (sHR for CT vs RT+CT = 1.58, 95% CI = 1.03 to 2.42). Mortality from causes other than HL varied much more, with lower 30-year mortality after CT alone (15.8%, 95% CI = 9.7% to 23.3%) compared with RT alone (36.9%, 95% CI = 34.0% to 39.8%) and RT+CT combined (29.8%, 95% CI = 26.8% to 32.9%) the subdistribution hazard ratios were 0.49 (95% CI = 0.32 to 0.76) and 0.59 (95% CI = 0.38 to 0.91), respectively. For stage III-IV, HL mortality did not differ between primary treatment groups, whereas mortality from causes other than HL was lower for CT alone compared with RT+CT (sHR = 0.67, 95% CI = 0.51 to 0.87) and RT alone (sHR = 0.49, 95% CI = 0.33 to 0.71). Stratified by treatment period, similar cumulative mortality patterns were observed (Supplementary Figure 2, available online). In the most recent period (1989-2000), 20-year cumulative mortality for stage I-II from causes other than HL varied statistically significantly between RT alone (16.8%, 95% CI = 13.4% to 20.4%) and RT+CT (12.0%, 95% CI = 9.7% to 15.5%; sHR for RT vs RT+CT = 1.51; 95% CI = 1.14 to 2.01), but not with CT alone (13.0%; 95% CI = 6.6% to 21.5%).
Figure 4.
Cumulative mortality from Hodgkin lymphoma (HL) and all causes other than HL by stage and treatment. A) Cumulative mortality in patients with stage I-II disease. B) Cumulative mortality in patients with stage III-IV disease. CT = chemotherapy; RT = radiotherapy.
Mortality analysis by total treatment received also showed that 30-year mortality from causes other than HL was lowest in patients who had received primary treatment with CT alone (21.0%, 95% CI = 16.3% to 26.1%) compared with those who had primary treatment with RT+CT alone (29.4%, 95% CI = 26.6% to 32.2%), RT alone (35.6%, 95% CI = 32.2% to 38.9%), and salvage treatment (31.6%, 95% CI = 28.9% to 34.3%, P values for all comparisons <.001, adjusted for age, treatment period, and stage) (Supplementary Figure 3, available online).
Discussion
In this large, multicenter cohort study including patients treated 1965-2000, we showed that HL patients remain at substantially increased risk of death due to causes other than HL even 40 years after initial treatment. Solid tumors and CVD contributed most (65.7%) to AEM from causes other than HL. At the age of 54 years, HL survivors experienced the same cumulative mortality (20.0%) from causes other than HL as 71-year-old individuals from the general population. Whereas HL mortality substantially decreased in more recent treatment periods, mortality from solid tumors did not decline. However, patients treated in the most recent treatment period (1989-2000) did experience lower CVD mortality than patients treated 1965-1976. Infectious disease mortality was more than twofold increased not only among patients who underwent splenectomy, but also in patients receiving RT to the spleen. According to primary HL treatment, early-stage patients who received CT alone had statistically significantly higher 30-year mortality from HL, but lower mortality from other causes (15.8%), compared with RT alone (36.9%) and RT+CT combined (28.9%).
All-cause mortality decreased in more recently treated HL patients, mainly due to decreasing HL mortality over time, as previously described in early-stage adult HL patients (12) and in childhood HL patients (18,19). CVD mortality was also lower in more recently treated HL patients (1989-2000) compared with patients treated 1965-1976. Although a decrease in CVD mortality was also observed in adolescent and young adult HL survivors (20), we previously found no decrease in CVD incidence among more recently treated patients (8). In the general population, CVD mortality has also declined over the past decades, whereas CVD incidence remained relatively stable, suggesting that improved treatment of ischemic heart disease has reduced CVD mortality (21). Therefore, the decrease in CVD mortality in our study may not reflect lower cardiotoxicity of more recent treatments, but rather better treatment and improved outcome of CVD in general.
Mortality from solid tumors did not decrease among more recently treated HL patients. This was also observed in childhood HL patients (18,19) and early-stage HL patients (12). We previously reported that solid tumor incidence following HL treatment also did not decrease over time, possibly because lower dosed and smaller volume RT regimens were introduced only recently, or because favorable changes in RT were offset by unfavorable changes in CT (5). A decline in solid tumor mortality would actually be expected, because for certain cancer types treatment has improved and, consequently, mortality in the general population has decreased (eg, breast cancer) (22,23).
Other mortality studies in HL patients often report at which point during follow-up cumulative mortality from causes other than HL surpasses HL mortality (11,12). It is, however, not surprising those frequently occurring diseases in the general population, like cancer and CVD, also cause strongly increasing mortality with age in HL survivors. In our opinion, it is more relevant at which point in time AEM from causes other than HL exceeds excess HL mortality. We showed that this occurred at 25 years of follow-up.
To our knowledge, our study is the first to show that solid tumor mortality after HL is increased after not only RT but also after high-dose procarbazine-containing CT. Previous studies showed that procarbazine-containing regimens were associated with increased incidence of lung cancer (24,25) and gastrointestinal cancers (5,26-29). Lung and gastrointestinal cancers contributed much more to excess mortality than breast cancer due to the more favorable prognosis of the latter cancer (22,23). Although contemporary HL treatment regimens lack procarbazine or contain low doses (except BEACOPP), it is important to realize that there is a large population of HL survivors at risk of excess mortality due to high-dose procarbazine treatment in the past.
Our study is the first, to our knowledge, to examine the effect of spleen irradiation on mortality from infectious diseases in adult HL survivors. Infection-related mortality was even slightly more strongly increased after spleen irradiation than after splenectomy; excess mortality was increased throughout follow-up. A recent study from the Childhood Cancer Survivor Study reported that a dose as low as 10-20 Gy to the spleen was associated with infectious disease mortality (30). Although we did not observe an effect of alkylating CT on infectious disease mortality (30), we found an association with anthracycline-containing CT. Because anthracyclines were always given in combination with bleomycin (ABV[D] regimen), this might be due to pulmonary toxicity of bleomycin. In splenectomized patients, vaccinations have been recommended since the late 1980s, whereas vaccination after splenic irradiation was introduced more recently. We examined vaccination status among 48 patients who had received splenectomy or spleen irradiation and first visited a survivorship clinic (31,32). Whereas 77% of splenectomized patients had received vaccinations, only 17% of spleen-irradiated patients had been vaccinated. This suggests that the rather high infectious disease mortality after splenic irradiation compared with splenectomy may be due to a much lower vaccination grade. Dutch surveillance guidelines now recommend similar vaccinations following splenectomy and spleen irradiation (31,32).
Most studies on morbidity and mortality from adverse effects of treatment in (childhood) HL patients only include 5-year survivors (5,8,18-20,33,34). We deliberately included all HL patients to examine the long-term consequences of initial treatment choice on HL mortality and mortality from all other causes. Initial treatment with CT alone was associated with lower long-term mortality from causes other than HL than other treatments, for both stage I-II and III-IV disease, whereas differences in HL mortality between CT alone and combined modality were relatively small. However, our results are not based on random allocation of treatment, and therefore conclusions regarding the effects of primary treatment modality on HL mortality warrant cautious interpretation. Stage migration may also affect HL mortality results, although analyses adjusted for stage and treatment period yielded similar results. When we examined the effect of initial treatment choice on mortality in the most recent treatment period (1989-2000), differences in mortality due to causes other than HL between various treatments were smaller, possibly because follow-up was not yet long enough for major late sequelae to become clinically manifest. It is also possible, however, that more contemporary treatments are associated with smaller differences in mortality from treatment-related complications.
Our study has a few limitations. First, CT doses were missing for part of the patients; however, for both anthracyclines and procarbazine, less than 10% of patients had missing dose data. Furthermore, it is important to realize that cause-specific mortality is determined by the incidence of adverse events as well as prognosis. As a consequence, when studying associations of treatment with cause-specific mortality, trends over calendar time can also be affected by changes in treatment and prognosis of the adverse events under study. Unfortunately, data on treatment of adverse events in our study population were not available. Finally, our cohort included patients treated 1965-2000, and HL treatment changed considerably over the study period (1,35). Although HL treatment also changed after 2000, survival in the more recent treatment periods in our cohort is high and a large proportion of patients are still alive. Therefore, our findings remain relevant in the light of survivorship care. Currently, treatment is more personalized than before, generally leading to lower exposure to alkylating agents, anthracyclines, and bleomycin and less radiation exposure because of smaller volumes, lower doses, and improved RT techniques (35,36). We therefore expect excess mortality to decrease in more recently treated patients.
HL treatment substantially reduces life expectancy of cured HL patients. Increased mortality from adverse events of HL treatment persists for at least 40 years. Optimal selection of patients for primary CT is crucial because salvage treatment is not always successful and is associated with high mortality from causes other than HL. Therefore, weighing risks of HL relapse and long-term toxicity is of paramount importance.
Funding
This work was supported by the Dutch Cancer Society (NKI 2010–4720).
Notes
Role of the funder: The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Disclosures: None of the authors have disclosures or conflict of interest.
Prior presentations: An abstract from preliminary analyses of this study was presented at the American Society for Clinical Oncology Annual Meeting, Chicago, USA, 2014; the International Symposium on Hodgkin lymphoma, Cologne, Germany, 2018; and the North-American Symposium on Late Complications after Childhood Cancer, Atlanta, USA, 2019.
Author contributions: SV, MS, BMPA, and FEvL designed the study; CPMJ, LAD, EJP, RWMvdM, JMZ, MB, MRN, KMSV, LCMK, PJL, ADGK, JMR, WJP, DJvS, GWvI, JPdB, BMPA provided study patients; SV, MS, and AMvE collected and assembled data; SV, MS, BMPA and FEvL analyzed and interpreted data; SV, MS, BMPA, and FEvL drafted the manuscript. All authors commented upon the manuscript draft and approved the final manuscript.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author; however, restrictions apply to the availability of cause-of-death data because these are only accessible through Statistics Netherlands.
Supplementary Material
Authors contributed equally.
References
- 1. Borchmann P, Eichenauer DA, Engert A. State of the art in the treatment of Hodgkin lymphoma. Nat Rev Clin Oncol. 2012;9(8):450–459. [DOI] [PubMed] [Google Scholar]
- 2. van Leeuwen FE, Klokman WJ, Veer MB, et al. Long-term risk of second malignancy in survivors of Hodgkin's disease treated during adolescence or young adulthood. J Clin Oncol. 2000;18(3):487–497. [DOI] [PubMed] [Google Scholar]
- 3. Ng AK, Bernardo MV, Weller E, et al. Second malignancy after Hodgkin disease treated with radiation therapy with or without chemotherapy: long-term risks and risk factors. Blood. 2002;100(6):1989–1996. [DOI] [PubMed] [Google Scholar]
- 4. Swerdlow AJ, Higgins CD, Smith P, et al. Second cancer risk after chemotherapy for Hodgkin's lymphoma: a collaborative British cohort study. J Clin Oncol. 2011;29(31):4096–4104. [DOI] [PubMed] [Google Scholar]
- 5. Schaapveld M, Aleman BM, van Eggermond AM, et al. Second cancer risk up to 40 years after treatment for Hodgkin's lymphoma. N Engl J Med. 2015;373(26):2499–2511. [DOI] [PubMed] [Google Scholar]
- 6. Aleman BM, van den Belt-Dusebout AW, De Bruin ML, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109(5):1878–1886. [DOI] [PubMed] [Google Scholar]
- 7. Myrehaug S, Pintilie M, Tsang R, et al. Cardiac morbidity following modern treatment for Hodgkin lymphoma: supra-additive cardiotoxicity of doxorubicin and radiation therapy. Leuk Lymphoma. 2008;49(8):1486–1493. [DOI] [PubMed] [Google Scholar]
- 8. van Nimwegen FA, Schaapveld M, Janus CP, et al. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med. 2015;175(6):1007–1017. [DOI] [PubMed] [Google Scholar]
- 9. Andersson A, Enblad G, Gustavsson A, et al. Long term risk of infections in Hodgkin lymphoma long-term survivors. Br J Haematol. 2011;154(5):661–663. [DOI] [PubMed] [Google Scholar]
- 10. Aleman BM, van den Belt-Dusebout AW, Klokman WJ, et al. Long-term cause-specific mortality of patients treated for Hodgkin's disease. J Clin Oncol. 2003;21(18):3431–3439. [DOI] [PubMed] [Google Scholar]
- 11. Ng AK, Bernardo MP, Weller E, et al. Long-term survival and competing causes of death in patients with early-stage Hodgkin's disease treated at age 50 or younger. J Clin Oncol. 2002;20(8):2101–2108. [DOI] [PubMed] [Google Scholar]
- 12. Patel CG, Michaelson E, Chen YH, et al. Reduced mortality risk in the recent era in early-stage Hodgkin lymphoma patients treated with radiation therapy with or without chemotherapy. Int J Radiat Oncol Biol Phys. 2018;100(2):498–506. [DOI] [PubMed] [Google Scholar]
- 13. van Eggermond AM, Schaapveld M, Lugtenburg PJ, et al. Risk of multiple primary malignancies following treatment of Hodgkin lymphoma. Blood. 2014;124(3):319–327, quiz 466. [DOI] [PubMed] [Google Scholar]
- 14. Breslow NE, Day NE. Statistical methods in cancer research. Volume II--The design and analysis of cohort studies. IARC Sci Publ. 1987;(82):1–406. https://www.ncbi.nlm.nih.gov/pubmed/3329634 [PubMed] [Google Scholar]
- 15. Boshuizen HC, Feskens EJ. Fitting additive Poisson models. Epidemiol Perspect Innov. 2010;7(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ederer F, Axtell LM, Cutler SJ. The relative survival rate: a statistical methodology. Natl Cancer Inst Monogr. 1961;6:101–121. [PubMed] [Google Scholar]
- 17. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 18. Armstrong GT, Yasui Y, Robison LL. Reduction in late mortality after childhood cancer. N Engl J Med. 2016;375(3):290–292. [DOI] [PubMed] [Google Scholar]
- 19. Fidler MM, Reulen RC, Winter DL, et al. ; British Childhood Cancer Survivor Study Steering Group. Long term cause specific mortality among 34 489 five year survivors of childhood cancer in Great Britain: population based cohort study. BMJ. 2016;354:i4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Henson KE, Reulen RC, Winter DL, et al. Cardiac mortality among 200 000 five-year survivors of cancer diagnosed at 15 to 39 years of age: the teenage and young adult cancer survivor study. Circulation. 2016;134(20):1519–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koopman C, Vaartjes I, van Dis I, et al. Explaining the decline in coronary heart disease mortality in the Netherlands between 1997 and 2007. PLoS One. 2016;11(12):e0166139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–2106. [DOI] [PubMed] [Google Scholar]
- 23. Davies C, Godwin J, Gray R, et al. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. [DOI] [PubMed] [Google Scholar]
- 24. Travis LB, Gospodarowicz M, Curtis RE, et al. Lung cancer following chemotherapy and radiotherapy for Hodgkin's disease. J Natl Cancer Inst. 2002;94(3):182–192. [DOI] [PubMed] [Google Scholar]
- 25. Swerdlow AJ, Schoemaker MJ, Allerton R, et al. Lung cancer after Hodgkin's disease: a nested case-control study of the relation to treatment. J Clin Oncol. 2001;19(6):1610–1618. [DOI] [PubMed] [Google Scholar]
- 26. van den Belt-Dusebout AW, Aleman BMP, Besseling G, et al. Roles of radiation dose and chemotherapy in the etiology of stomach cancer as a second malignancy. Int J Radiat Oncol Biol Phys. 2009;75(5):1420–1429. [DOI] [PubMed] [Google Scholar]
- 27. Morton LM, Dores GM, Curtis RE, et al. Stomach cancer risk after treatment for Hodgkin lymphoma. J Clin Oncol. 2013;31(27):3369–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Eggermond AM, Schaapveld M, Janus CP, et al. Infradiaphragmatic irradiation and high procarbazine doses increase colorectal cancer risk in Hodgkin lymphoma survivors. Br J Cancer. 2017;117(3):306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dores GM, Curtis RE, van Leeuwen FE, et al. Pancreatic cancer risk after treatment of Hodgkin lymphoma. Ann Oncol. 2014;25(10):2073–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weil BR, Madenci AL, Liu Q, et al. Late infection-related mortality in asplenic survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2018;36(16):1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nijdam A, Dekker N, Aleman BMP, et al. Setting up a National Infrastructure for Survivorship Care after treatment for Hodgkin lymphoma. Br J Haematol. 2019;186(4):e103–e108. [DOI] [PubMed] [Google Scholar]
- 32. Dekker N, van 't Veer MB, Aleman BM, et al. [ The BETER survivorship care initiative for Hodgkin lymphoma; tailored survivorship care for late effects of treatment]. Ned Tijdschr Geneeskd. 2015;159:A9269. [PubMed] [Google Scholar]
- 33. Bhakta N, Liu Q, Yeo F, et al. Cumulative burden of cardiovascular morbidity in paediatric, adolescent, and young adult survivors of Hodgkin's lymphoma: an analysis from the St Jude Lifetime Cohort Study. Lancet Oncol. 2016;17(9):1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Armstrong GT, Liu W, Leisenring W, et al. Occurrence of multiple subsequent neoplasms in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2011;29(22):3056–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Specht L, Yahalom J, Illidge T, et al. Modern radiation therapy for Hodgkin lymphoma: field and dose guidelines from the International Lymphoma Radiation Oncology Group (ILROG). Int J Radiat Oncol Biol Phys. 2014;89(4):854–862. [DOI] [PubMed] [Google Scholar]
- 36. Eichenauer DA, Aleman BMP, Andre M, et al. Hodgkin lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Supplement_4):iv19–iv29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author; however, restrictions apply to the availability of cause-of-death data because these are only accessible through Statistics Netherlands.