Abstract
Objectives
To describe the clinical and epidemiological characteristics of children with coronavirus disease 2019 (COVID-19) in the state of Negeri Sembilan, Malaysia in the setting of mandatory hospital isolation and quarantine for all confirmed cases.
Methods
A multi-centre, retrospective observational study was performed among children aged ≤12 years with laboratory-proven COVID-19 between 1 February and 31 December 2020.
Results
In total, 261 children (48.7% males, 51.3% females) were included in this study. The median age was 6 years [interquartile range (IQR) 3–10 years]. One hundred and fifty-one children (57.9%) were asymptomatic on presentation. Among the symptomatic cases, fever was the most common presenting symptom. Two hundred and forty-one (92.3%) cases were close contacts of infected household or extended family members. Twenty-one (8.4%) cases had abnormal radiological findings. All cases were discharged alive without requiring supplemental oxygen therapy or any specific treatment during hospitalization. The median duration of hospitalization was 7 days (IQR 6–10 days). One (2.1%) of the uninfected guardians accompanying a child in quarantine tested positive for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) upon discharge.
Conclusions
COVID-19 in children was associated with mild symptoms and a good prognosis. Familial clustering was an important epidemiologic feature in the outbreak in Negeri Sembilan. The risk of transmission of SARS-CoV-2 from children to guardians in hospital isolation was minimal despite close proximity.
Keywords: Clinical features, Epidemiology, Paediatric COVID-19, SARS-CoV-2
Introduction
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), remains an ongoing challenge to countries worldwide. The disease emerged in Wuhan, China in December 2019 and was declared a pandemic by the World Health Organization (WHO) on 11 March 2020 (World Health Organization, 2000). The COVID-19 outbreak in Malaysia occurred in three waves. The first wave started with three cases imported from China via Singapore on 25 January 2020 (Rahman, 2020). This was followed by a quiescent period before a second wave broke out at the beginning of March 2020, culminating in the implementation of a Movement Control Order on 18 March 2020 (Rahman, 2020). The Malaysian health authorities adopted a ‘find, test, trace, isolate’ strategy to curb the spread of COVID-19. As part of the containment strategy, hospital admission and isolation were mandatory for all cases of COVID-19, regardless of symptoms or disease severity (Institute for Health Systems Research Malaysia, 2020).
The state of Negeri Sembilan lies on the west coast of peninsular Malaysia. The state (area 6645 km2) has a total population of approximately 1,100,000 people, including 215,000 children aged ≤12 years. This study describes the epidemiology, clinical characteristics and outcomes of 261 children with COVID-19 in the state of Negeri Sembilan.
Patients and methods
Design
A multi-centre, retrospective observational study was undertaken that included all children aged ≤12 years with laboratory-proven COVID-19 between 1 February and 31 December 2020. All children with confirmed COVID-19 were placed in isolation in three designated hospitals across the state. Children were tested for COVID-19 as part of contact tracing efforts, arrival at a point of entry (airport), or if they had symptoms suggesting COVID-19 (Institute for Health Systems Research Malaysia, 2020). The patients’ hospital medical records were analysed retrospectively. Data were extracted into a manual case report form. Information retrieved included demographic characteristics, symptoms and signs, comorbidities, exposure history, laboratory and radiologic findings, complications, treatment received and outcomes.
Case definitions
Confirmed cases were defined as children who had a positive real-time reverse transcription polymerase chain reaction (RT-PCR) result from a combined nasopharyngeal and oropharyngeal swab. For all patients, nucleic acid detection for SARS-CoV-2 was performed at the National Public Health Laboratory or the state’s tertiary hospital according to the WHO protocol (World Health Organization, 2020a).
The date of diagnosis was defined as the date of onset of symptoms or when the first confirmatory test was conducted, whichever was earlier. Fever was defined as body temperature ≥37.5 °C. The index case was defined as the most likely source based on history; if multiple family members were affected, the first family member who became symptomatic was regarded as the index case.
Statistical analysis
Continuous variables are presented as median and interquartile range (IQR), and categorical variables are presented as frequency and percentage (%). Non-parametric two-tailed Mann–Whitney U-tests were used to compare continuous variables, all of which were non-normally distributed. The normality of data distribution was assessed using the Shapiro–Wilk test. Chi-squared tests or Fisher’s exact tests were used to compare categorical variables, as appropriate. All probabilities were two-tailed, and P < 0.05 was considered to indicate statistical significance. Data analysis was performed using SPSS Version 26.0 (IBM Corp., Armonk, NY, USA).
Ethical considerations
This study was registered with the National Medical Research Register (NMRR-20-2924-57631) and approved by the Medical Research and Ethics Committee, Ministry of Health, Malaysia [KKM/NIHSEC/P21-72(4)].
Results
Baseline characteristics
In total, 261 cases were admitted during the 11-month study period. The median age of cases was 6 years (IQR 3–10 years; range 72 days–12 years). The male-to-female ratio was 0.95. Comorbidities were reported for eight cases (3%), with bronchial asthma being the most commonly reported condition (Table 1 ). The median interval between diagnosis and hospital admission was 2 days (IQR 2–4 days).
Table 1.
Demographics, clinical and epidemiological features, laboratory and radiological results, and outcomes of children with coronavirus disease 2019.
| Baseline characteristics of patients | |
|---|---|
| Age, years (median, IQR) | 6 (3–10) |
| <1 | 20 (7.7%) |
| 1–5 | 104 (39.8%) |
| 6–12 | 137 (52.5%) |
| Sex | |
| Male | 127 (48.7%) |
| Female | 134 (51.3%) |
| Ethnicity | |
| Malays | 235 (90%) |
| Indians | 19 (7.3%) |
| Chinese | 6 (2.3%) |
| Other | 1 (0.4%) |
| Symptoms at presentationa | |
| Asymptomatic | 151 (57.9%) |
| Fever | 76 (29.1%) |
| Cough | 37 (14.2%) |
| Rhinorrhoea | 21 (8.0%) |
| Sore throat | 10 (3.8%) |
| Diarrhoea | 11 (4.2%) |
| Vomiting | 4 (1.5%) |
| Pre-existing conditions | |
| None | 253 (96.9%) |
| Asthma | 3 (1.1%) |
| Ex-premature infant (if age <2 years old) | 2 (0.8%) |
| Cerebral palsy | 1 (0.4%) |
| Congenital heart disease | 1 (0.4%) |
| Haemophilia | 1 (0.4%) |
| Index case | |
| Parent | 176 (67.4%) |
| Other household member | 33 (12.6%) |
| Extended family member | 32 (12.3%) |
| School contact | 7 (2.7%) |
| Babysitter | 7 (2.7%) |
| Otherb | 6 (2.3%) |
| Laboratory results (n = 22) | |
| Total white cell count (×109/L) | 8.2 (6.6–9.4) |
| Absolute lymphocyte count (×109/L) | 3.4 (2.8–4.1) |
| Platelet count (×109/L) | 356 (271–402) |
| Chest radiograph (n = 250) | |
| Normal | 229 (91.6%) |
| Interstitial opacities | 15 (6.0%) |
| Unilateral patchy opacities | 4 (1.6%) |
| Bilateral patchy opacities | 2 (0.8%) |
| Length of stay, days (median, IQR) | 7 (6–10) |
| Outcome | |
| Admission to paediatric intensive care unit | 0 (0%) |
| Death | 0 (0%) |
| Discharged alive | 261 (100%) |
IQR, interquartile range.
Some patients presented with more than one symptom.
Close contact with another confirmed case without a social relationship.
Epidemiological characteristics
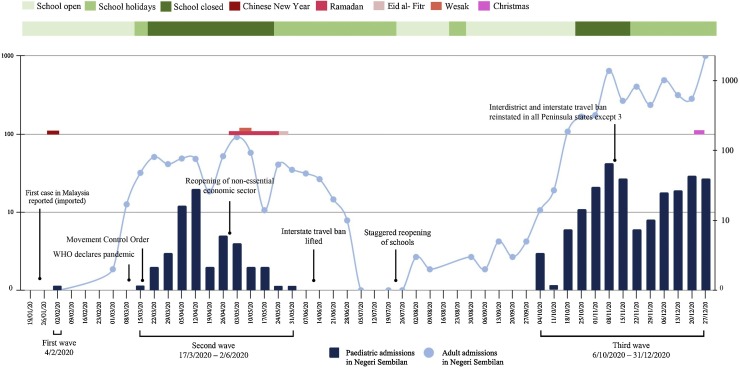
The admissions occurred in three well-defined waves, as shown in Figure 1 , coinciding with the national trend. The first paediatric admission in the state was an imported case from Wuhan, China on 4 February 2020. A 9-year-old Malaysian boy who returned from Wuhan through a humanitarian evacuation mission tested positive along with his father and was hospitalized. He was asymptomatic and had an uneventful 14-day stay in the hospital (See et al., 2020). The subsequent patient was admitted 6 weeks later and was part of the country’s then-largest cluster linked to a mass religious gathering. Fifty-five cases (21%) were admitted during the second wave between mid-March and early June 2020. The third wave of admissions occurred in early October 2020, and accounted for 78% of the paediatric admissions for COVID-19 in 2020. All cases during the study period were detected from public health contact tracing efforts. The majority (92.3%) of cases were close contacts of infected household or extended family members, with either parent being the index case in 67.4% of cases.
Figure 1.
Distribution of hospital admissions for coronavirus disease 2019 during the study period in relation to major public health decision measures, timing of school opening and major religious festivals in the country. WHO, World Health Organization.
Clinical features
One hundred and fifty-one (57.9%) cases were asymptomatic. The median age of asymptomatic cases was higher than that of symptomatic cases [6.9 years (IQR 3.7–9.9) vs 5.3 years (IQR 2.3–9.3); P = 0.012], but no difference was observed between genders. Fever was the most commonly reported presenting symptom, occurring in 76 (29.1%) cases. Among those with fever, 39 (51.3%) did not have any other symptoms. Respiratory symptoms such as cough, rhinorrhoea and sore throat were seen in 56 (21.5%) cases, and diarrhoea was documented in 11 (4.2%) cases. Fever was present in five (45%) cases with diarrhoea. Tachypnoea was recorded in one (0.4%) case. Anosmia or ageusia was reported in <3% of cases. Headache was only reported in the minority (1.5%) of cases. Three (1.1%) cases had co-infection, namely methicillin-susceptible Staphylococcus aureus septicaemia, non-typhoidal salmonella dysentery and scabies.
Of the 250 chest radiographs performed on admission, 21 (8.4%) revealed abnormal results. The most common abnormalities on chest radiograph were interstitial opacities. Lymphopenia was not seen in any of the 22 cases that had complete blood counts.
Table 2 shows a comparison between the clinical features of the cases admitted in the first two waves and the third wave. The median age in the third wave was younger compared with the first two waves. Cases admitted in the third wave had a shorter median duration of hospitalization. Fever was more commonly observed among cases admitted in the third wave, whereas diarrhoea was more commonly observed among cases admitted in the first two waves. The proportion of asymptomatic cases did not differ between the two groups.
Table 2.
Demographic and clinical features of cases admitted in the first two waves vs the third wave.
| Characteristics | First two waves (n = 56) | Third wave (n = 205) | P-value |
|---|---|---|---|
| Age, years (median, IQR) | 8.4 (4.9–10.8) | 6.0 (2.6–9.3) | 0.006 |
| Male gender | 28 (50%) | 99 (48.3%) | 0.821 |
| Length of stay (days) | 12 (9-15) | 7 (6-8) | <0.001 |
| Parental source | 47 (83.9%) | 129 (62.9%) | 0.003 |
| Asymptomatic infection | 34 (60.7%) | 117 (57.1%) | 0.625 |
| Fever | 10 (17.9%) | 66 (32.2%) | 0.036 |
| Cough | 12 (21.4%) | 25 (12.2%) | 0.079 |
| Sore throat | 4 (7.1%) | 6 (2.9%) | 0.229 |
| Rhinorrhoea | 3 (5.4%) | 18 (8.8%) | 0.581 |
| Diarrhoea | 6 (10.7%) | 5 (2.4%) | 0.014 |
| Vomiting | 1 (1.8%) | 3 (1.5%) | 1.000 |
Outcome of hospitalization
All children included in the study recovered and were discharged alive after a median stay of 7 days (IQR 6–10 days). None of the cases required supplemental oxygen therapy or specific treatment with antivirals or steroids. Antibiotics were prescribed for the two patients who had co-infection with Staphylococcus aureus septicaemia and non-typhoidal salmonella dysentery. The child who had scabies co-infection was treated with topical Permethrin. No cases required admission to the paediatric intensive care unit (PICU). None of the paediatric COVID-19 cases in this study subsequently developed multi-system inflammatory syndrome in children.
Outcomes of uninfected guardians hospitalized with infected children
Of the 261 children with COVID-19 hospitalized in the state, 53 (20.3%) were isolated with 47 uninfected guardians (one guardian could be looking after more than one hospitalized child). Of the guardians, 33 were mothers (70.2%), 11 were fathers (23.4%), two were aunts (4.3%) and one was a grandfather (2.1%). The children’s median age was 6 years (IQR 3–9 years) and the median duration of hospitalization was 7 days (IQR 5–9 days). Most (60.4%) of these children were asymptomatic. Upon hospital discharge, 40 (85.1%) of the guardians remained uninfected, with serial combined oronasopharyngeal swabs performed at least twice during hospitalization. Five (10.6%) guardians tested positive on the first oronasopharyngeal swab performed on admission (taken a median of 4 days after the initial swab). One (2.1%) guardian remained uninfected until the second RT-PCR assay was performed before hospital discharge, taken 8 days after the initial test. One (2.1%) guardian had two consecutive negative RT-PCR results during hospitalization, but tested positive for SARS-CoV-2-specific antibodies upon discharge.
Discussion
This article describes the clinical features and epidemiological characteristics of 261 children with COVID-19 in Negeri Sembilan, Malaysia. Paediatric COVID-19 accounts for a small portion of the COVID-19 burden in the state. Children aged ≤12 years comprise 18.8% of the state’s population. However, only 3.2% of the COVID-19 cases in the state were among children aged ≤12 years. This translates into a cumulative rate of infection of 121 cases per 100,000 population aged ≤12 years. The relatively low rate of COVID-19 among children is consistent with the observations reported elsewhere (Livingston and Bucher, 2020, Wu and McGoogan, 2020, Centers for Disease Control and Prevention, 2021, COVID-19 National Incident Room Surveillance Team, 2021).
Remarkably, all paediatric COVID-19 cases in the state were picked up from public health contact tracing efforts. Symptomatic screening of hospitalized children without any epidemiological links to COVID-19 was non-contributory. The criteria for symptomatic surveillance of hospitalized children was dynamic as the pandemic evolved. Nevertheless, 740 hospitalized children were screened in the state’s tertiary hospital (including 90 cases admitted to the PICU), essentially testing 72% of the 1028 admissions for respiratory, gastrointestinal and other febrile illnesses throughout the study period. This suggests that children were primarily infected in familial clusters, which was understandable considering that their sphere of movement has been limited during the pandemic. Similar findings have been observed previously (Lu et al., 2020, CDC COVID-19 Response Team, 2020).
In this study, more than half (57.9%) of the cases were asymptomatic at presentation. Asymptomatic infection in children has been reported in up to one-third of children with COVID-19 (King et al., 2021). The Malaysian public health system structure facilitated expedited laboratory testing, extensive contact tracing and isolation of all positive cases. This presented the opportunity to capture the entire spectrum of clinical presentation of paediatric COVID-19 in the community, and could explain the high rate of asymptomatic infection. Among symptomatic children, fever was the most common presenting symptom, compatible with previously published literature (Castagnoli et al., 2020, CDC COVID-19 Response Team, 2020, King et al., 2021). Symptoms such as anosmia, ageusia and headache were uncommonly reported as young children were less likely to express such symptoms.
The complete blood count laboratory results from the study cases were unremarkable. This was unsurprising as lymphopenia has only been reported in a minority of paediatric cases of COVID-19 (Lu et al., 2020, Ma et al., 2020). Chest X-ray abnormalities were seen in 21 (8.4%) cases with a chest radiograph on admission. Notably, more than half (52.4%) of the patients with abnormal radiological findings were asymptomatic. The absence of symptoms despite radiologic evidence of pneumonia has been documented previously (Lu et al., 2020). None of the cases required supplemental oxygen or treatment with antivirals, suggesting a benign course of illness even in those with radiological abnormalities. Although extremes of age have been reported as predictors of severe disease in children (Graff et al., 2021), the 20 infants in the present study had an uneventful course of hospitalization. The favourable outcomes were consistent with previous reports, indicating that children with COVID-19 typically have a good prognosis (Castagnoli et al., 2020, Lu et al., 2020, Wu and McGoogan, 2020). Several factors have been proposed to explain the differences in severity between children and adults with COVID-19 (Zimmermann and Curtis, 2021), but none are conclusive. In Latin America, poorer outcomes have been described compared with other studies from China, Europe and North America, with low socio-economic conditions associated with more severe disease (Antúnez-Montes et al., 2021). Outcome disparities between the rich and poor are possibly due to children living in poverty being more prone to food scarcity and malnutrition, as well as delayed presentation to hospital due to the lack of access to health care. In the present study population, 22% of the children belonged to families in the bottom 40% of low-income earners. However, all children in the cohort had good outcomes regardless of their socio-economic background.
This study investigated how the patients’ demographics and clinical features evolved over the year. The median age of cases in the third wave was significantly lower than the median age of cases in the first two waves. The shift toward younger ages has also been reported in other studies, which was more pronounced in the adult population with a broader age variation (Boehmer et al., 2020, Eurosurveillance Editorial Team, 2020, COVID-19 National Incident Room Surveillance Team, 2021). The length of hospitalization during the third wave was significantly shorter compared with the first two waves. The shorter length of hospitalization was due to a change in the hospital discharge protocol, in line with WHO’s recommendations of a time-based discharge criteria instead of the need for negative RT-PCR tests (World Health Organization, 2020b).
This study adds new information to existing reports on the transmission of SARS-CoV-2 in a hospital setting. It highlights the outcomes of uninfected guardians who were quarantined with infected children, a situation which invariably arose with mandatory isolation of all confirmed cases. The guardians were placed in a separate isolation room from the positive COVID-19 patients. Such situations raise concerns about transmission from children to their uninfected guardians. During hospitalization, the guardians were advised to wear three-ply surgical masks, perform regular hand hygiene, and avoid sharing feeding utensils with their infected child. The children were mostly unable to comply with wearing face masks. Apart from the single guardian infected upon hospital discharge, no cases of SARS-CoV-2 transmission from children to guardians were observed in the isolation setting despite close proximity. Five guardians who were thought to be uninfected tested positive on admission, suggesting that they were already infected before hospitalization. The single guardian with two consecutive negative RT-PCR tests but who demonstrated positive SARS-CoV-2-specific antibodies upon discharge had probably been infected much earlier and had recovered by the time of admission.
This study has several important implications. The results reveal a high degree of asymptomatic infection among children with COVID-19. The actual burden of disease is thus grossly underestimated. The presenting symptoms among the symptomatic cases were non-specific and mimicked many other common viral respiratory and gastrointestinal illnesses in childhood. Therefore, a testing strategy focusing solely on children with lower respiratory tract signs or abnormal chest radiographs would fail to identify most cases. Similarly, contact tracing of symptomatic individuals alone would be biased as asymptomatic cases preclude detection. This study revealed that the majority of cases had an epidemiological link to an infected household adult. Given these findings, symptomatic screening for COVID-19 among hospitalized children without any epidemiological links may not be cost-effective or beneficial for disease containment.
The outcomes of this study suggest that a classification of severity that includes a radiological diagnosis of pneumonia may not be necessary for children. Although radiological imaging may improve diagnostic accuracy, the radiation risk needs to be balanced against the benefits. Moreover, the good outcomes and the absence of any specific treatment despite the radiological abnormalities suggest that radiological imaging should not be performed routinely. Based on these findings, a clinical-based classification of disease severity as opined by Buonsenso et al. (2021) may warrant further evaluation. In addition, lung ultrasonography is potentially a useful tool in the diagnosis and monitoring of paediatric COVID-19, with the advantages of absence of radiation exposure and ease of use at point of care (Musolino et al., 2020, Musolino et al., 2021).
The transmission of SARS-CoV-2 from children to uninfected guardians in an isolation setting was minimal in this study. This could be explained by the various infection control measures applied during isolation. Alternatively, this finding suggests that children are less efficient spreaders of SARS-CoV-2. The low transmission potential from children to household members has been described previously in epidemiological studies in South Korea (Park et al., 2020, Kim et al., 2021).
These results are subject to several limitations. The patients’ symptoms were self-reported, which carries the risk of under-reporting, and younger children may have been less able to describe their symptoms fully. The authors acknowledge recall and documentation biases on the clinical history involving presenting symptoms, which might explain the significant diarrhoea among admissions during the first two waves and fever among admissions during the third wave. The documented symptoms were baseline symptoms from patients upon hospital admission, and this study did not capture longitudinal data regarding the development of new symptoms. Secondly, laboratory and radiological investigations were not performed routinely in every individual. Chest radiographs were performed in the vast majority of patients as the pandemic evolved and reports on radiological abnormalities in asymptomatic cases emerged, whereas blood investigations were only performed as clinically indicated. Finally, the outcomes described were short-term outcomes upon hospital discharge. More extensive series with long-term follow-up are needed to identify long-term sequelae of COVID-19 in children.
Conclusion
In conclusion, this study showed that COVID-19 in children was associated with mild symptoms and good prognosis. Given the high proportion of asymptomatic patients, identification of children who are likely to be infected is challenging. Familial clustering was an important epidemiologic feature in the outbreak in Negeri Sembilan, and close household contact was the primary mode of SARS-CoV-2 infection in children. The risk of transmission of SARS-CoV-2 from infected children to their accompanying guardians in a hospital isolation setting was minimal.
Conflict of interest statement
None declared.
Funding
None.
Ethical approval
This study was approved by the Medical Research and Ethics Committee, Ministry of Health, Malaysia [KKM/NIHSEC/P21-72(4)].
Author contributions
DNCE, TKK, MMA and EJK made substantial contributions to the conception and design of the work. CL, LML, FMM, MFAR and HAR made substantial contributions to data collection and data analysis. DNCE, TKK, CL, MMA, LML, FMM, MFAR, HAR and EJK made substantial contributions to the interpretation of data for the manuscript. DNCE, CL and MMA drafted the initial manuscript. DNCE, TKK and EJK supported the literature review. All authors reviewed and revised the manuscript for important intellectual content, giving approval of the version to be published and gave their agreement to be accountable for all aspects of the work.
Acknowledgements
The authors wish to thank all healthcare workers involved in the treatment of paediatric cases of COVID-19 in the state. In addition, the authors thank the Director-General of Health Malaysia for his permission to publish this article.
References
- Antúnez-Montes O.Y., Escamilla M.I., Figueroa-Uribe A.F., Arteaga-Menchaca E., Lavariega-Saráchaga M., Salcedo-Lozada P. COVID-19 and multisystem inflammatory syndrome in Latin American children: a multinational study. Pediatr Infect Dis J. 2021;40:e1–e6. doi: 10.1097/INF.0000000000002949. [DOI] [PubMed] [Google Scholar]
- Boehmer T.K., DeVies J., Caruso E., van Santen K.L., Tang S., Black C.L. Changing age distribution of the COVID-19 pandemic – United States, May–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1404–1409. doi: 10.15585/mmwr.mm6939e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonsenso D., Parri N., De Rose C., Valentini P. Toward a clinically based classification of disease severity for paediatric COVID-19. Lancet Infect Dis. 2021;21:22. doi: 10.1016/S1473-3099(20)30396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagnoli R., Votto M., Licari A., Brambilla I., Bruno R., Perlini S. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020;174:882–889. doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
- CDC COVID-19 Response Team Coronavirus disease 2019 in children – United States, February 12–April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:422–426. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . CDC; Atlanta, GA: 2021. Demographic trends of COVID-19 cases and deaths in the US reported to CDC. Available at: https://stacks.cdc.gov/view/cdc/99332. [Accessed 19 March 2021] [Google Scholar]
- COVID-19 National Incident Room Surveillance Team COVID-19 Australia: Epidemiology Report 32: Four-week reporting period ending 3 January 2021. Commun Dis Intell (2018) 2021;45:6. doi: 10.33321/cdi.2021.45.1. [DOI] [PubMed] [Google Scholar]
- Eurosurveillance Editorial Team Updated rapid risk assessment from ECDC on coronavirus disease (COVID-19) pandemic in the EU/EEA and the UK: resurgence of cases. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.32.2008131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff K., Smith C., Silveira L., Jung S., Curran-Hays S., Jarjour J. Risk factors for severe COVID-19 in children. Pediatr Infect Dis J. 2021;40:e137–45. doi: 10.1097/INF.0000000000003043. [DOI] [PubMed] [Google Scholar]
- Institute for Health Systems Research Malaysia . Institute for Health Systems Research Malaysia; Shah Alam: 2020. Universal health coverage and COVID-19 preparedness & response. Available at: https://www.ihsr.moh.gov.my/images/publication_material/techreport/wpro-covid_final.pdf. [Accessed 19 March 2021] [Google Scholar]
- Kim J., Choe Y.J., Lee J., Park Y.J., Park O., Han M.S. Role of children in household transmission of COVID-19. Arch Dis Child. 2021 doi: 10.1136/archdischild-2020-319910. Published Online First: 7 August 2020. In press. [DOI] [PubMed] [Google Scholar]
- King J.A., Whitten T.A., Bakal J.A., McAlister F.A. Symptoms associated with a positive result for a swab for SARS-CoV-2 infection among children in Alberta. CMAJ. 2021;193:E1–9. doi: 10.1503/cmaj.202065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston E., Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA. 2020;323:1335. doi: 10.1001/jama.2020.4344. [DOI] [PubMed] [Google Scholar]
- Lu X., Zhang L., Du H., Zhang J., Li Y.Y., Qu J. SARS-CoV-2 infection in children. N Engl J Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H., Hu J., Tian J., Zhou X., Li H., Laws M.T. A single-center, retrospective study of COVID-19 features in children: a descriptive investigation. BMC Med. 2020;18:123. doi: 10.1186/s12916-020-01596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musolino A.M., Supino M.C., Buonsenso D., Ferro V., Valentini P., Magistrelli A. Lung ultrasound in children with COVID-19: preliminary findings. Ultrasound Med Biol. 2020;46:2094–2098. doi: 10.1016/j.ultrasmedbio.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musolino A.M., Supino M.C., Buonsenso D., Papa R.E., Chiurchiù S., Magistrelli A. Lung ultrasound in the diagnosis and monitoring of 30 children with coronavirus disease 2019. Pediatr Pulmonol. 2021;56:1045–1052. doi: 10.1002/ppul.25255. [DOI] [PubMed] [Google Scholar]
- Park Y.J., Choe Y.J., Park O., Park S.Y., Kim Y.M., Kim J. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020;26:2465–2468. doi: 10.3201/eid2610.201315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman F. 2020. The Malaysian response to COVID-19: building preparedness for ‘surge capacity’, testing efficiency and containment. Available at: https://kpkesihatan.com/2020/06/16/the-malaysian-response-to-covid-19-building-preparedness-for-surge-capacity-testing-efficiency-and-containment/. [Accessed 19 March 2021] [Google Scholar]
- See K.C., Liew S.M., Ng D.C.E., Chew E.L., Khoo E.M., Sam C.H. COVID-19: four paediatric cases in Malaysia. Int J Infect Dis. 2020;94:125–127. doi: 10.1016/j.ijid.2020.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO; Geneva: 2000. WHO Director-General’s opening remarks at the media briefing on COVID-19. Available at: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. [Accessed 31 March 2021] [Google Scholar]
- World Health Organization . WHO; Geneva: 2020. Laboratory testing for coronavirus disease (COVID-19) in suspected human cases: interim guidance. Available at: https://apps.who.int/iris/handle/10665/331329. [Accessed 19 March 2021] [Google Scholar]
- World Health Organization . WHO; Geneva: 2020. Clinical Management of COVID-19: interim guidance. Available at: https://apps.who.int/iris/handle/10665/332196. [Accessed 19 March 2021] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Zimmermann P., Curtis N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch Dis Child. 2021;106:429–439. doi: 10.1136/archdischild-2020-320338. [DOI] [PubMed] [Google Scholar]