Abstract
Background
Coronavirus disease 2019 (COVID-19) can cause cardiac injury resulting in abnormal right or left ventricular function (RV/LV) with worse outcomes. We hypothesized that two-dimensional (2D) speckle-tracking assessment of LV global longitudinal strain (GLS) and RV free wall strain (FWS) by transthoracic echocardiography can assist as markers for subclinical cardiac injury predicting increased mortality.
Methods
We performed 2D strain analysis via proprietary software in 48 patients hospitalized with COVID-19. Clinical information, demographics, comorbidities, and lab values were collected via retrospective chart review. The primary outcome was in-hospital mortality based on an optimized abnormal LV GLS value via ROC analysis and RV FWS.
Results
The optimal LV GLS cutoff to predict death was −13.8%, with a sensitivity of 85% (95% CI 55–98%) and specificity of 54% (95% CI 36–71%). Abnormal LV GLS >-13.8% was associated with a higher risk of death [unadjusted hazard ratio 5.15 (95% CI 1.13–23.45), p = 0.034], which persisted after adjustment for clinical variables. Among patients with LV ejection fraction (LVEF) >50%, those with LV GLS > −13.8% had higher mortality compared to those with LV GLS <-13.8% (41% vs. 10%, p = 0.030). RV FWS value was higher in patients with LV GLS >-13.8% (−13.7 ± 5.9 vs. −19.6 ± 6.7, p = 0.003), but not associated with decreased survival.
Conclusion
Abnormal LV strain with a cutoff of >−13.8% in patients with COVID-19 is associated with significantly higher risk of death. Despite normal LVEF, abnormal LV GLS predicted worse outcomes in patients hospitalized with COVID-19. There was no mortality difference based on RV strain.
Keywords: COVID-19, Speckle-tracking echocardiography, Left ventricular strain
1. Introduction
Coronavirus disease 2019 (COVID-19) has spread globally since December 2019 and has resulted in more than 1.9 million deaths worldwide (https://coronavirus.jhu.edu/ - last accessed: 01/09/2020). Collective understanding of the pathogenesis of COVID-19, disease course, and management has been increasing but remains limited. It is now known that cardiac injury associated with COVID-19 infection is associated with increased mortality [1]. Use of cardiac biomarkers such as troponin, NT-pro-brain natriuretic peptide (NT-proBNP), creatine phosphokinase, and presence of new right or left ventricular (RV/LV) dysfunction on echocardiogram allows identification of injury that has already occurred. However, our understanding of the impact of subclinical myocardial dysfunction on outcomes in patients with COVID-19 remains limited.
Two-dimensional (2D) speckle-tracking assessment of strain by transthoracic echocardiography (TTE) has been used as a subclinical marker of impaired myocardial function which predicts cardiovascular outcomes [[2], [3], [4]]. Assessment of LV strain has been used to monitor cardiotoxic effects of chemotherapy, as well to predict morbidity and mortality in heart failure [4]. RV strain imaging has been used in patients with pulmonary emboli and pulmonary hypertension [5,6]. Respiratory viral infections including influenza and acute respiratory distress syndrome (ARDS) have been implicated in acute myocardial injury, however, there is profound lack of data elucidating their respective roles on myocardial strain [7,8].
In the most recent study by Li et al. (2020), abnormal right ventricular (RV) strain was associated with worse outcomes in COVID-19 patients and was found to be a predictor of mortality in these patients [9]. We hypothesize that bi-ventricular strain assessment including LV and RV by transthoracic echocardiogram (TTE) can serve as a supplementary marker of outcomes in patients with COVID-19 infection. This study is the first to investigate the relationships between left ventricular global longitudinal strain (LV GLS) and right ventricular free wall strain (RV FWS) on inpatient mortality outcomes in patients hospitalized with COVID-19.
2. Material and methods
2.1. Study population and triage
At our institution, a triage process was implemented for all echocardiogram requests for patients who either were diagnosed with COVID-19 or were considered as patients under investigation (PUI) to determine the appropriateness and necessity of a TTE. This triage system was implemented to prevent disease transmission from the patient to the sonographers and to conserve personal protective equipment. The factors that played an important part in the decision-making included presence of shock with unknown left ventricular ejection fraction (LVEF), profound hypoxemia not explained by current pulmonary disease, concern for ACS, aortic dissection or cardiac tamponade; suspected prosthetic or native valve dysfunction. The patients were triaged to either (a) perform the study; (b) defer the study until the patient either has a negative COVID-19 test result or until point-of-care ultrasound (POCUS) is attempted; or (c) cancel the study at this time as it would not change current management. Our triage strategy has been previously published [10].
From March 2020 to May 2020, a total of 154 TTEs were performed in patients admitted with or undergoing rule out of COVID-19. A total of 109 patients with TTE tested positive for COVID-19 at the time of triage of which 48 had appropriate image quality and ECG tracing for strain assessment. Patients with suboptimal visualization of the left or right ventricular wall throughout the cardiac cycle were excluded. Retrospective chart review was then performed for the included patients.
We performed a retrospective chart review of the electronic medical record (EMR) to obtain data on: patient demographics, medical history (including but not limited to comorbidities and pre-existing cardiovascular conditions), and detailed clinical information at the time of the triage. The primary outcome was the mortality during the same hospital stay. Secondary outcomes included need for the intensive-care unit (ICU), total length of stay, and days requiring mechanical ventilation. Clinical covariates including past medical history, cardiovascular risk factors, pre-existing cardiovascular comorbidities, pre-existing lung disease as well as laboratory values that were collected any time during the hospitalization (troponin T, NT-proBNP, serum creatinine) were included. In-hospital outcomes were assessed and adjudicated by two physicians from March 19 through the time of final data review on Aug 19, 2020.
2.2. Echocardiographic analysis
Bedside transthoracic imaging was done using EPIQ 7C and iE33 ultrasound systems (Philips Medical Systems, Andover, Massachusetts). In order to decrease sonographer exposure to COVID while imaging patients, all studies were performed as focused evaluations catered to provide pertinent information needed from the echocardiogram. To decrease time at bedside, offline strain analysis was performed.
Left ventricular ejection fraction was measured by biplane Simpson method, 3-D method, and visual estimation. Right ventricular size and function were assessed using 4-chamber views. Assessment was performed using guidelines from the American Society of Echocardiography.
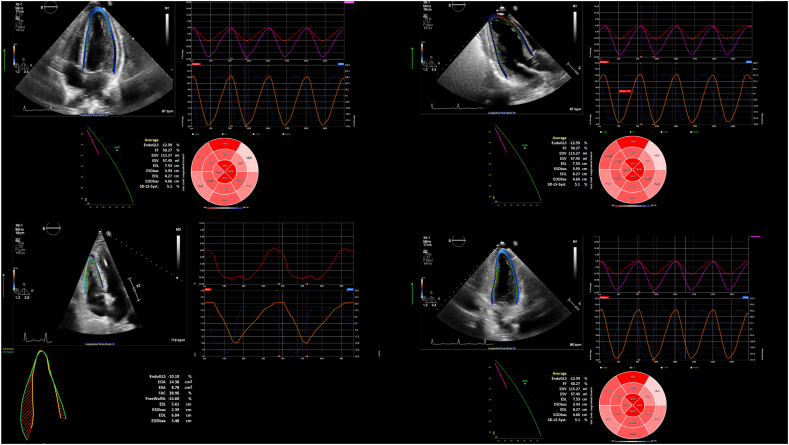
Speckle-tracking analysis of all images was done using off-line image analysis program 2D STRAIN (2D Cardiac Performance Analysis v1.2, Tomtec Imaging Systems GmbH, Unterschleissheim, Germany) which is a validated vendor neutral software for assessment of myocardial deformation and associated speckle tracking [[11], [12], [13]] (Fig. 1, Supplemental Figure). The analysis was performed by one experienced reader after careful selection of appropriate images and cardiac cycles. For all patients, efforts were made to collect the images of the ASE recommended views for cardiac chamber quantification (4-chamber, 2-chamber, and 3-chamber views for the left ventricle, as well as a right ventricular focused view). The images were optimized to acquire the maximum size of the left ventricle, left atrium and the right ventricle during acquisition. From each sequence a complete R-R cycle – end-diastole (ED) start to ED end was selected. The basal and apical anchor points were defined. After automated tracking of the myocardium, manual adjustments of the contours were performed if necessary, and longitudinal strain was computed by the software. An 18-segment bull's eye plot was used for the LV analysis which gives comprehensive information of the strain noted in each segment of the left ventricle (Fig. 1, Supplemental Figure). Strain information on RV fractional area change (FAC), RV global longitudinal strain (RV GLS), RV free wall strain (RV FWS), as well as LV global longitudinal strain (LV GLS), and LV global circumferential strain (LV GCS) were collected. In order to account for inter-observer variability, strain analysis was performed on 15 randomly selected imaging studies by a second experienced reader. Inter-observer variability was assessed using the intraclass correlation coefficient. LV GLS was analyzed using the apical 4- chamber, 3- chamber and 2- chamber views. LV GLS was calculated as the average of the peak longitudinal strain measured in the 12 regions in the apical views. RV FWS was analyzed using the apical 4-chamber views and peak average of regional segment strain (basal, mid and apical) was obtained [14].
Fig. 1.
Echocardiographic measurements of LV GLS.
Offline speckle-tracking analysis of the left ventricle using the analysis software 2D STRAIN (2D Cardiac Performance Analysis v1.2, Tomtec Imaging Systems GmbH, Unterschleissheim, Germany) in an apical 4-chamber view. The myocardial contours are automatically tracked and manually adjusted if needed (left side). An 18-segment bull's eye plot offers information of the strain noted in each segment of the left ventricle (right side). Blue segments (not seen here) reach their longitudinal strain peak before the end systolic period, while red segments reach minimal strain after end-systole. Full resolution images with additional views required for measurement (including right ventricle) are found in the Supplemental Figure. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.3. Statistical analysis
The statistical analyses were performed using Stata 13.1 (StataCorp LP, College Station, Texas). Continuous data were presented as mean ± standard deviation. Categorical variables were presented as absolute and relative frequencies. Group comparisons were performed using unpaired Student's t-test for continuous variables, as well as Fisher's exact test for categorical variables. An optimal cut-off value for LV GLS was calculated using receiver operating characteristic (ROC) analysis based on the maximum of sensitivity and specificity to predict mortality. The resulting binary LV GLS variable was used for univariable and multivariable stepwise (inclusion threshold p < 0.15) Cox proportional-hazards regression if the proportional hazards assumption was not violated. For the Cox regression, the primary outcome (mortality) was used as the dependent variable; abnormal LV GLS, abnormal LV ejection fraction (LVEF ≤50%), abnormal RV FWS, biomarkers (troponin T ≥ 0.01 ng/mL at triage, peak NT-proBNP, peak creatinine), sex, presence of coronary artery disease, atrial fibrillation and congestive heart failure, as well as pre-existing lung disease and smoking status were used as independent variables. Mortality was further assessed using Kaplan-Meier survival estimator with log-rank and Wilcoxon-Breslow test. Inter-observer variability was reported using the intraclass correlation coefficient (ICC). For all analyses, statistical significance was defined as a two-sided p-value <0.05.
3. Results
3.1. Study population
The overall mean age of the study population was 58 ± 16 years and 33% (n = 16) of subjects were female. There were no statistically significant age and sex differences across the groups of survivors and deceased. There were 17 who did not survive hospitalization. Overall, cardiovascular risk factors including hypertension and diabetes had a total prevalence of 60% (n = 29) and 38% (n = 18) respectively, whereas manifest coronary artery disease (n = 5) and congestive heart failure (n = 3) were seen at low rates (Table 1). The prevalence of atrial fibrillation, pre-existing lung disease and smoking was higher in the deceased cohort, however, differences were not statistically significant. When comparing baseline characteristics of those included in the study with the excluded subjects based on the imaging quality, there were no statistically significant differences (Supplemental Table 1).
Table 1.
Baseline demographics.
| Demographics | Survived (N = 35) | Died (N = 13) | p-Value |
|---|---|---|---|
| Age (mean ± SD) | 57.63 ± 15.87 | 60.61 ± 16.11 | 0.567 |
| Female sex (n, %) | 13 (37%) | 3 (23%) | 0.288 |
| BMI (mean ± SD) | 29.58 ± 8.64 | 30.77 ± 9.08 | 0.680 |
| Hypertension (n, %) | 20 (57%) | 9 (69%) | 0.522 |
| Diabetes mellitus type 2 (n, %) | 13 (37%) | 5 (38%) | 0.594 |
| Coronary artery disease (n, %) | 3 (9%) | 2 (15%) | 0.413 |
| Chronic kidney disease (n, %) | 6 (17%) | 3 (23%) | 0.463 |
| HFrEF (n, %) | 1 (3%) | 1 (8%) | 0.473 |
| HFpEF (n, %) | 1 (3%) | 0 (0%) | 0.729 |
| Atrial fibrillation (n, %) | 2 (6%) | 3 (23%) | 0.115 |
| Ventricular tachycardia (n, %) | 1 (3%) | 0 (0%) | 0.729 |
| Ischemic stroke (n, %) | 0 (0%) | 1 (8%) | 0.271 |
| Pre-existing lung disease (n, %) | 1 (3%) | 3 (23%) | 0.055 |
| Smoking (n, %) | 2 (6%) | 3 (23%) | 0.115 |
| Biomarkers (mean ± SD) | Survived (N = 35) | Died (N = 13) | p-Value | |
|---|---|---|---|---|
| Triage value | Troponin T | 0.22 ± 0.86 | 0.14 ± 0.17 | 0.731 |
| NT-proBNP | 5462 ± 14,190 | 5454 ± 5216 | 0.998 | |
| Creatinine | 1.67 ± 2.31 | 2.21 ± 1.69 | 0.445 | |
| Peak value | Troponin T | 0.33 ± 0.90 | 0.17 ± 0.20 | 0.549 |
| BNP | 6011 ± 14,097 | 13,783 ± 18,487 | 0.129 | |
| Creatinine | 2.09 ± 2.55 | 3.83 ± 2.74 | 0.045 | |
BMI = body mass index, HFrEF = heart failure with reduced ejection fraction, HFpEF = heart failure with preserved ejection fraction, NT-proBNP = N-terminal pro brain natriuretic peptide.
3.2. Inter-observer variability
Inter-observer variability of strain measurements by ICC showed good to near perfect correlation for all RV and LV strain measurements (Supplemental Table 2). ICC for RV FWS was 0.839 and for LV GLS was 0.871 (both single ICC).
3.3. RV and LV strain values by survival status
RV fractional area change was significantly lower in patients with abnormal RV free wall strain (RV FWS) > −15% [40.84 ± 8.94 vs. 29.18 ± 11.21, p < 0.001]. Similarly, RV FAC was lower when RV global longitudinal strain (RV GLS) was abnormal (> −15%). However, when comparing absolute RV FWS values between survivors and non-survivors, there was no statistically significant difference. Overall, there were no significant differences in mean LV ejection fraction, LV global longitudinal strain, and global circumferential strain (Table 2).
Table 2.
Strain data by survival status.
| Survived (N = 33) | Died (N = 12) | p value | ||
|---|---|---|---|---|
| RV (mean ± SD) | GLS | −14.48 ± 5.63 | −14.77 ± 5.88 | 0.881 |
| FAC | 35.85 ± 11.33 | 35.13 ± 12.42 | 0.855 | |
| FWS | −16.26 ± 1.14 | −16.45 ± 7.90 | 0.938 | |
| Survived (N = 35) | Died (N = 13) | p value | ||
|---|---|---|---|---|
| LV (mean ± SD) | GLS | −13.87 ± 3.57 | −12.62 ± 4.46 | 0.322 |
| GCS | −27.49 ± 6.36 | −25.79 ± 8.68 | 0.460 | |
| EF | 56.50 ± 13.28 | 56.12 ± 18.30 | 0.936 | |
RV = right ventricle, LV = left ventricle, GLS = global longitudinal strain, FAC = fractional area change, FWS = free wall strain, GCS = global circumferential strain, EF = ejection fraction.
3.4. Sensitivity analysis for LV GLS and clinical associations
When using a cut-off value of LV GLS > −15% to predict death, there was no significant association between LV GLS and inpatient mortality. To determine the optimal cut-off value to maximize the sensitivity and specificity of LV GLS to predict death, ROC analysis was performed. A “new abnormal” threshold of −13.8 was calculated, with a sensitivity of 85% (95% CI 55–98%) and a specificity of 54% (95% CI 36–71%). The area under curve was 0.70 (95% CI 0.56–0.83). For RV FWS, the cut off was −25.1, with an AUC of 0.44.
Compared with the “new normal group”, those with LV GLS higher than −13.8 (“new abnormal group”) had a significantly higher rate of death (41% vs. 10%, p = 0.022). Length of stay and ventilator days were numerically longer in the survival group, but not statistically significant. Subjects with abnormal LV GLS strain > −13.8 had significantly higher rates of Troponin T > 0.01 ng/mL at the time of TTE (89% vs. 62%, p = 0.04), as well as higher peak creatinine values during admission. NT-proBNP was not statistically significant among groups. Furthermore, abnormal LV GLS was associated with higher values for LV GCS and RV GLS, and RV FWS strain values. Similarly, LVEF and RV FAC were significantly lower in the abnormal LV GLS group (Table 3).
Table 3.
Survival and strain data by abnormal LV strain.
| LV GLS ≤ −13.8 (“New normal”) N = 21 |
LV GLS > −13.8 (“New abnormal”) N = 27 |
P value | ||
|---|---|---|---|---|
| Survival (n, %) | 0.022a | |||
| Died | 2 (10%) | 11 (41%) | ||
| Survived | 19 (90%) | 16 (59%) | ||
| Length of stay | 27.19 ± 21.25 | 24.37 ± 25.68 | 0.686 | |
| ICU days | 15.76 ± 19.83 | 12.81 ± 23.72 | 0.649 | |
| Ventilator days | 14.43 ± 20.16 | 11.22 ± 17.90 | 0.616 | |
| Use of vasopressors | 6 (35%) | 13 (42%) | 0.762a | |
| Vital signs at time of TTE (mean ± SD) | ||||
| Systolic blood pressure | 121 ± 5 | 126 ± 4 | 0.469 | |
| Diastolic blood pressure | 71 ± 3 | 73 ± 3 | 0.635 | |
| Heart rate | 83 ± 3 | 83 ± 3 | 0.953 | |
| Troponinemia ≥ 0.01 at time of TTE (n, %) | 0.040a | |||
| Yes | 13 (62%) | 24 (89%) | ||
| No | 8 (38%) | 3 (11%) | ||
| Biomarkers (mean ± SD) | ||||
| Peak Troponin T | 0.07 ± 0.22 | 0.46 ± 0.99 | 0.086 | |
| Peak NT-proBNP | 5287 ± 11,416 | 10,289 ± 18,042 | 0.282 | |
| Peak Creatinine | 1.53 ± 1.18 | 3.37 ± 3.23 | 0.017 | |
| Echocardiographic data | ||||
| LV (mean ± SD) | EF | 63 ± 5 | 51 ± 17 | 0.003 |
| GCS | −30.48 ± 5.03 | −24.34 ± 7.22 | 0.002 | |
| RV (mean ± SD) | GLS | −17.76 ± 5.32 | −12.00 ± 4.50 | <0.001 |
| FWS | −19.58 ± 6.65 | −13.70 ± 5.90 | 0.003 | |
| FAC | 39.36 ± 10.64 | 32.69 ± 11.48 | 0.052 | |
Fisher's exact test, TTE = transthoracic echocardiogram, NT-proBNP = N-terminal pro brain natriuretic peptide, LV = left ventricle, RV = right ventricle, EF = ejection fraction, GCS = global circumferential strain, GLS = global longitudinal strain, FAC = fractional area change, FWS = free wall strain.
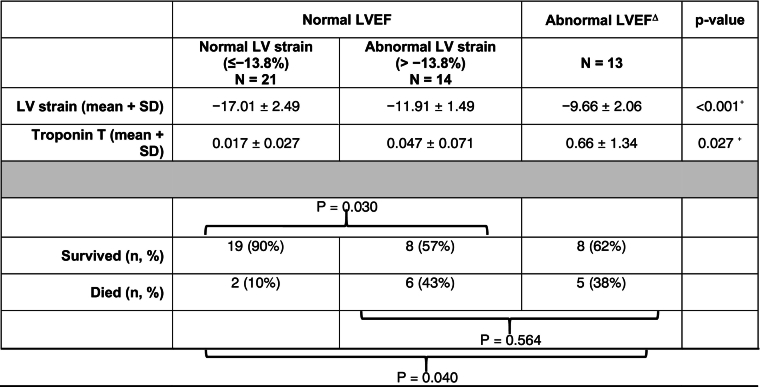
Among patients with normal LV EF ≥50% (n = 35), those with abnormal LV GLS (n = 14) had a significantly higher mortality than those with normal strain (43% vs. 10%, p = 0.030). Furthermore, there rate of in-hospital mortality for patients with normal LVEF but abnormal LV GLS was similar to the mortality rate for patients with abnormal LVEF [43% (6 out of 14) vs. 38% (5 out of 13), p = 0.564] (Table 4).
Table 4.
Survival by LVEF and LV global longitudinal strain.
ΔThere were no patients with abnormal LVEF and normal LV strain ≤−13.8%. +ANOVA, ⁎Fisher's exact test, LV = left ventricle, LVEF = left ventricular ejection fraction.
3.5. Survival analysis
In univariate Cox proportional hazard regression, abnormal LV GLS was significantly associated with death [univariate hazard ratio (HR): 5.15 (95% CI 1.13–23.45), p = 0.034]. After stepwise Cox regression analysis, the association remained significant after adjustment for TTE-derived parameters (abnormal EF, abnormal RV FWS), as well as biomarkers (Troponin T, peak NT-proBNP, peak creatinine) [adjusted HR 4.96 (1.08–22.60), p = 0.039]. When adjusting for clinical covariates, none of the other clinical covariates including sex, coronary artery disease, HFrEF, atrial fibrillation, or pre-existing lung disease met the inclusion criteria of p < 0.15, though smoking status did meet this threshold [adjusted HR 8.56 (1.98–36.90), p = 0.004], (Table 5). The results using different cut-off values for LV GLS to predict death are found in Supplemental Table 3.
Table 5.
Cox proportional hazards regression: Survival analysis by strain.
| Dependent variable: death Independent variables (below) |
Univariate Cox regression | aMultivariate Cox regression (TTE variables) | aMultivariate Cox regression (Biomarkers) | aMultivariate Cox regression (Clinical comorbidities) | ||||
|---|---|---|---|---|---|---|---|---|
| LV GLS > −13.8 % | 5.15 (1.13–23.45) | 0.034 | 5.15 (1.13–23.45) | 0.034 | 4.96 (1.08–22.60) | 0.039 | 7.13 (1.41–35.48) | 0.016 |
| Abnormal EF < 50% | 2.29 (0.74–7.10) | 0.151 | KO | N/A | ||||
| RV FWS > −15 % | 1.10 (0.37–3.28) | 0.859 | KO | N/A | ||||
| Triage Troponin T ≥ 0.01 ng/mL | 3.91 (0.86–17.71) | 0.076 | KO | N/A | ||||
| Peak NT-proBNP | 1.00 (1.00–1.00) | 0.364 | KO | N/A | ||||
| Peak Creatinine | 1.12 (0.95–1.32) | 0.162 | KO | N/A | ||||
| Female sex | 0.63 (0.18–2.32) | 0.497 | KO | N/A | ||||
| Coronary artery disease | 2.06 (0.45–9.52) | 0.354 | KO | N/A | ||||
| HFrEF | 1.23 (0.16–9.76) | 0.547 | KO | N/A | ||||
| Atrial fibrillation | 4.82 (1.23–18.90) | 0.024 | KO | N/A | ||||
| Pre-existing lung disease | 5.73 (1.52–21.67) | 0.010 | KO | N/A | ||||
| Smoking | 5.14 (1.37–19.29) | 0.01 | 8.56 (1.98–36.90) | 0.004 | ||||
Stepwise regression, KO (knock out) = not included in model, TTE = transthoracic echocardiogram, LV GLS = left ventricular global longitudinal strain, EF = ejection fraction, RV FWS = right ventricular free wall strain, NT-proBNP = N-terminal pro brain natriuretic peptide, HFrEF = heart failure with reduced ejection fraction.
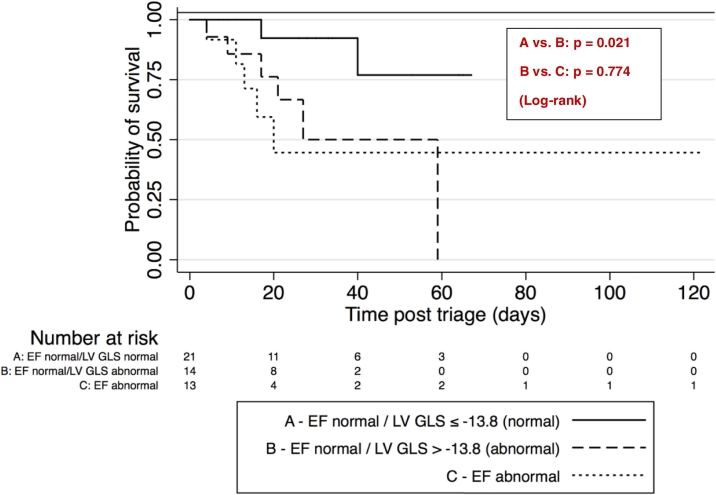
The Kaplan-Meier estimate function is shown in Fig. 2 and demonstrates a higher mortality in those patients with normal LVEF, but abnormal LV GLS > −13.8% compared to those with normal LVEF and LV GLS ≤ -13.8% (p = 0.021). When comparing the former group to those with abnormal LVEF, there was no statistically significant difference in mortality (p = 0.774).
Fig. 2.
Survival curves by LV EF and LV GLS status.
4. Discussion
In this paper, we investigated the effects of abnormal RV and LV strain on mortality of patients hospitalized with COVID-19 and found that abnormal LV strain is associated with a significantly higher risk of in-hospital death, particularly in patients with a normal LV EF. This association persisted after adjusting for echocardiographic markers, clinical risk factors and potential confounders including elevated troponin, underlying heart and lung disease, and smoking status. Conversely, we did not find a statistically significant association between RV strain abnormalities and survival status.
4.1. Right-ventricular strain in COVID-19
TTE during the COVID-19 pandemic is used for rapid assessment of cardiac function. However, granular data on echocardiographic profiles on patients with COVID-19 are limited. Szekely et al. observed that more patients showed RV pathology including dilation and dysfunction than LV pathology (39% vs. 26%, respectively) [15]. The largest series to date by Li et al. with 120 patients demonstrated that subclinical RV dysfunction assessed by TAPSE, RV FAC, and RV FWS was a significant predictor of survival status independent of ARDS [9] with a total mortality of 32.5% (n = 13) in patients with abnormal RV strain. This is comparable to our study with 39% in patients with any LV or RV strain abnormalities. However, in our population, death rates seemed to be mostly driven by abnormal LV function, not by RV function. Moreover, in our study, we detected no deaths in patients with abnormal RV FWS alone when they had normal LV GLS.
Abnormal RV strain is a known predictor of longer-term adverse events [16] (including all-cause mortality [[17], [18], [19]] in HFrEF and stress cardiomyopathy), whereas research on short-term outcomes (e.g. in myocardial infarction [20] and pulmonary embolism [21]) has been rather limited. The lack of impact of RV strain on acute mortality might be explained by histopathological findings suggesting that abnormal RV strain is the functional correlate for myocardial fibrosis [22] which is a gradual process not easily detected during an acute hospitalization. We also acknowledge important limitations in our study including selection bias introduced by the strict triage process at our institution that was specifically directed towards the cardiac assessment of patients whose clinical picture and symptoms appeared to be out of proportion to COVID-19-related lung disease. Hence, TTEs were preferentially performed on sicker individuals in which decreased LV function from primary myocardial injury was already hypothesized. Furthermore, patients with extensive lung disease tended to have worse image quality not always adequate for strain analysis and strain analysis might not necessarily comparable knowing that values are often vendor-dependent to a certain degree.
4.2. Left-ventricular strain in COVID-19
Hypotheses to explain LV dysfunction in COVID-19 including direct myocardial damage and inflammatory cytokine storm are under active investigation [23]. The theory of direct myocardial damage finds support in a recent report Churchill et al. who found that almost half of patients with high-sensitivity troponin T (hs-TNT) levels ≥50 ng/mL had evidence of LV dysfunction [24]. While Li et al. and Szekely et al. documented that mean LVEF was generally ≥50% and did not show statistically significant differences after comparison by survival status, myocardial strain status, or clinical severity of COVID-19, our group has observed in a prior analysis of our COVID-19 population that patients with LVEF <50% had significantly higher mortality than those with LVEF ≥50% [25].
Our most notable finding is that mortality is increased even when changes in left ventricular function in patients with COVID-19 are considered seemingly “subclinical”. In our patient population with COVID-19, LVEF in both survivor and non-survivor groups was ≥50%, limiting predictive information based on LVEF alone. However, when LV GLS was also assessed, those individuals with normal LVEF but abnormal LV GLS had a mortality risk closer to those with abnormal LVEF. Our survival analysis further incorporated adjustments for other clinical parameters. While volume status has been shown by Burns et al. to acutely influence LV systolic strain [26], the association of abnormal LV strain with mortality was independent of NT-proBNP levels at the time of TTE which was used as a surrogate for volume status and was not significantly different across our comparison groups.
LV strain traditionally has been used to predict long-term outcomes in patients with asymptomatic type 2 diabetes [27], patients treated with anthracyclines [28], as well as in patients with aortic stenosis with preserved ejection fraction [29]. Only little data exists on the role of abnormal LV strain in acute settings. While our population was limited to patients hospitalized with COVID-19, this study to our knowledge is the first showing that abnormal LV GLS was significantly and independently from LVEF associated with increased acute, inpatient mortality. This might signal that the scope of abnormal LV GLS might possibly useful in other conditions in which myocardial injury might not be detected in the form of overtly reduced LVEF.
While COVID-19-related myocardial changes are found in both RV and LV, our work should incite further discussion about their potentially differential relationships with inpatient and long-term outcomes. Our work suggests that LV strain analysis might serve as an incremental predictive factor for mortality in patients with COVID-19, especially where (normal) LVEF alone would fail to unveil pertinent information on mortality.
4.3. Comparison of LV and RV strain
We found that LV GLS with an optimal cutoff threshold of −13.8% was able to predict survival status in patients hospitalized with COVID-19. Due to the nature of ROC analysis, this is expectedly less negative than the pooled normal value from a meta-analysis using healthy individuals which is reported to be −19.5% (95% CI −20.4 – −18.9%) [30], and less negative than the 5th percentile in a healthy general population (−18.4%) [31]. Our threshold ranges between the mean LV GLS of patients with acute pulmonary embolism (−16%) [32] and those with stress cardiomyopathy (−11.6%) [33], or acute decompensated heart failure (−9.8%) [34]. Based on our review of literature, this is the first report to provide a LV GLS cutoff value for presumed acute myocardial injury in the setting of COVID-19.
Patients with this “new abnormal” cut-off for LV GLS showed a mean RV FWS of −13.7% which is comparable to a value of −13.1% found in patients with acute decompensated heart failure associated with adverse events in 36-month follow-up [34]. This was also less negative than −23.3%, a lower limit of normal of 3-segment RV FWS originating from a reference group of 276 healthy volunteers [35].
In Li et al.'s COVID-19 population, the mean 3-segment RV FWS was −18.9% in non-survivors [9], while our corresponding group showed a comparable mean of −16.5%. However, neither segmentation into quartiles nor sensitivity analysis for RV FWS was able to predict survival outcomes in our study.
To our knowledge, only one other study to this date has compared the effects of both RV and LV strain on clinical outcomes. Cameli et al. showed that in patients with HFrEF referred for heart transplant, RV FWS (AUC 0.87) with a cutoff of −15% was a better predictor of adverse outcome events than LV GLS (AUC 0.26) with a cutoff of −8.1% [36]. A key difference to our study is that in our study the majority of patients (35 out of 48) had normal ejection fraction, whereas the same cannot be said of an advanced heart failure population. In the latter population, RV parameters gradually gain importance in the prediction of mortality as LV parameters including strain and EF lose their clinical utility as disease progresses. In contrast, in a study population like ours in which most patients are presumed to have subclinical myocardial damage, we showed that LV strain can uncover these subclinical differences.
Even though we did not detect a survival difference in patients with COVID-19 when considering abnormal RV strain status, RV strain possibly still plays a role: We observed that the mean of all RV strain parameters including GLS, FWS, and FAC diverge when stratifying by abnormal LV GLS status. This suggests that LV GLS likely has an effect modifying influence on RV strain.
Overall, our work is the first to compare RV and LV strain parameters in an acute setting, more specifically when an acute myocardial injury (here due to COVID-19) is suspected, in a population with comparably low prevalence of manifest cardiovascular comorbidities.
4.4. Limitations
We acknowledge that one of the biggest limitations of this study is the relatively low number of diagnostic echocardiograms, due to the significant number of studies excluded due to image quality and focused nature of TTEs in this patient population. Differences in imaging quality and clinical characteristics compared to other study populations such as in China were not considered. Precision of our results from adjusted analyses were limited by our cohort size and thus low numbers of events. Furthermore, our study might be subject to selection bias due to the careful selection of patients appropriate for TTE by our triage criteria. The lack of long-term follow-up precludes any conclusions on post-discharge mortality, although all patients included had a final disposition at the time of study. Additionally, strain computation itself might also be vendor-dependent. Our work should be considered hypothesis generating and further research including prospective work should be initiated. Importantly, more widespread use of strain imaging in patients with COVID-19 is feasible since it requires no added scanning time and may enable future research including the incorporation of longitudinal follow-up data of serial echocardiograms, using myocardial strain in treatment monitoring, as well as its use in lung disease.
5. Conclusions
In patients hospitalized with COVID-19, abnormal LV strain is an early marker for increased risk of death independent of LVEF. This association persists after further adjustment for biomarkers, clinical risk factors including underlying heart and lung disease, as well as smoking status. Conversely, such association was not present with echocardiographic RV strain markers in our study.
CRediT authorship contribution statement
Jakob Park: Conceptualization, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Yekaterina Kim: Conceptualization, Investigation, Writing – original draft, Data curation. Jason Pereira: Data curation. Kerrilynn C. Hennessey: Data curation. Kamil F. Faridi: Conceptualization, Supervision, Writing – review & editing. Robert L. McNamara: Conceptualization, Supervision, Writing – review & editing. Eric J. Velazquez: Supervision. David J. Hur: Conceptualization, Supervision, Writing – review & editing. Lissa Sugeng: Conceptualization, Supervision, Writing – review & editing. Vratika Agarwal: Conceptualization, Formal analysis, Investigation, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ahjo.2021.100018.
Contributor Information
Jakob Park, Email: Jakob.Park@yale.edu.
Vratika Agarwal, Email: vratika.agarwal@yale.edu.
Appendix A. Supplementary data
Supplementary Tables and Figures
References
- 1.Shi S., Qin M., Shen B., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kraigher-Krainer E., Shah A.M., Gupta D.K., et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2014 doi: 10.1016/j.jacc.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mizuguchi Y., Oishi Y., Miyoshi H., Iuchi A., Nagase N., Oki T. The functional role of longitudinal, circumferential, and radial myocardial deformation for regulating the early impairment of left ventricular contraction and relaxation in patients with cardiovascular risk factors: a study with two-dimensional strain Im. J. Am. Soc. Echocardiogr. 2008 doi: 10.1016/j.echo.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Saito M., Negishi K., Eskandari M., et al. Association of left ventricular strain with 30-day mortality and readmission in patients with heart failure. J. Am. Soc. Echocardiogr. 2015;28(6):652–666. doi: 10.1016/j.echo.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Hardegree E.L., Sachdev A., Villarraga H.R., et al. Role of serial quantitative assessment of right ventricular function by strain in pulmonary arterial hypertension. Am. J. Cardiol. 2013 doi: 10.1016/j.amjcard.2012.08.061. [DOI] [PubMed] [Google Scholar]
- 6.Fine N.M., Chen L., Bastiansen P.M., et al. Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Circ. Cardiovasc. Imaging. 2013 doi: 10.1161/CIRCIMAGING.113.000640. [DOI] [PubMed] [Google Scholar]
- 7.Ito T.I., Akamatsu K.I., Fujita S., Kanzaki Y., Ukimura A., Hoshiga M. 2019. Transient Depression of Myocardial Function After Influenza Virus Infection: A Study of Echocardiographic Tissue Imaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zochios V., Parhar K., Tunnicliffe W., Roscoe A., Gao F. The right ventricle in ARDS. Chest. 2017;152(1):181–193. doi: 10.1016/j.chest.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Li Y., Li H., Zhu S., et al. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc. Imaging. April 2020 doi: 10.1016/j.jcmg.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hennessey K.C., Shah N., Soufer A., et al. Inpatient transthoracic echocardiography during the coronavirus disease 2019 (COVID-19) pandemic: evaluating a new triage process. J. Am. Soc. Echocardiogr. July 2020 doi: 10.1016/j.echo.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amundsen B.H., Helle-Valle T., Edvardsen T., et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography. J. Am. Coll. Cardiol. 2006 doi: 10.1016/j.jacc.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 12.Korinek J., Wang J., Sengupta P.P., et al. Two-dimensional strain-A Doppler-independent ultrasound method for quantitation of regional deformation: validation in vitro and in vivo. J. Am. Soc. Echocardiogr. 2005 doi: 10.1016/j.echo.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Cheng S., Larson M.G., McCabe E.L., et al. Age- and sex-based reference limits and clinical correlates of myocardial strain and synchrony: the framingham heart study. Circ. Cardiovasc. Imaging. 2013 doi: 10.1161/CIRCIMAGING.112.000627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Il'Giovine Z.J., Mulder H., Chiswell K., et al. Right ventricular longitudinal strain reproducibility using vendor-dependent and vendor-independent software. J. Am. Soc. Echocardiogr. 2018 doi: 10.1016/j.echo.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szekely Y., Lichter Y., Taieb P., et al. The Spectrum of cardiac manifestations in coronavirus disease 2019 (COVID-19) - a systematic echocardiographic study. Circulation. May 2020 doi: 10.1161/circulationaha.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verhaert D., Mullens W., Borowski A., et al. Right ventricular response to intensive medical therapy in advanced decompensated heart failure. Circ. Heart Fail. 2010;3(3):340–346. doi: 10.1161/CIRCHEARTFAILURE.109.900134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iacoviello M., Citarelli G., Antoncecchi V., et al. Right ventricular longitudinal strain measures independently predict chronic heart failure mortality. Echocardiography. 2016;33(7):992–1000. doi: 10.1111/echo.13199. [DOI] [PubMed] [Google Scholar]
- 18.Motoki H., Borowski A.G., Shrestha K., et al. Right ventricular global longitudinal strain provides prognostic value incremental to left ventricular ejection fraction in patients with heart failure. J. Am. Soc. Echocardiogr. 2014;27(7):726–732. doi: 10.1016/j.echo.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Finocchiaro G., Kobayashi Y., Magavern E., et al. Prevalence and prognostic role of right ventricular involvement in stress-induced cardiomyopathy. J. Card. Fail. 2015;21(5):419–425. doi: 10.1016/j.cardfail.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Gorter T.M., Lexis C.P.H., Hummel Y.M., et al. 2016. Right Ventricular Function After Acute Myocardial Infarction Treated With Primary Percutaneous Coronary Intervention (from the Glycometabolic Intervention as Adjunct to Primary Percutaneous Coronary Intervention in ST-Segment Elevation Myocardial Infarction III Trial) [DOI] [PubMed] [Google Scholar]
- 21.Sugiura E., Dohi K., Onishi K., et al. Reversible right ventricular regional non-uniformity quantified by speckle-tracking strain imaging in patients with acute pulmonary thromboembolism. J. Am. Soc. Echocardiogr. 2009;22(12):1353–1359. doi: 10.1016/j.echo.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Lisi M., Cameli M., Righini F.M., et al. RV longitudinal deformation correlates with myocardial fibrosis in patients with end-stage heart failure. JACC Cardiovasc. Imaging. 2015;8(5):514–522. doi: 10.1016/j.jcmg.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 23.Clerkin K.J., Fried J.A., Raikhelkar J., et al. COVID-19 and cardiovascular disease. Circulation. 2020;141(20):1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 24.Churchill T.W., Bertrand P.B., Bernard S., et al. Echocardiographic features of COVID-19 illness and association with cardiac biomarkers. J. Am. Soc. Echocardiogr. 2020;33(8):1053–1054. doi: 10.1016/j.echo.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faridi K.F., Hennessey K.C., Shah N., et al. Left ventricular systolic function and inpatient mortality in patients hospitalized with Coronavirus Disease 2019 (COVID-19) J. Am. Soc. Echocardiogr. 2020;0(0) doi: 10.1016/j.echo.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burns A.T., La Gerche A., D'hooge J., Macisaac A.I., Prior D.L. Left ventricular strain and strain rate: characterization of the effect of load in human subjects. Eur. J. Echocardiogr. 2010;11(3):283–289. doi: 10.1093/ejechocard/jep214. [DOI] [PubMed] [Google Scholar]
- 27.Ng A.C.T., Delgado V., Bertini M., et al. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am. J. Cardiol. 2009;104(10):1398–1401. doi: 10.1016/j.amjcard.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 28.Ali M.T., Yucel E., Bouras S., et al. Myocardial strain is associated with adverse clinical cardiac events in patients treated with anthracyclines. J. Am. Soc. Echocardiogr. 2016;29(6):522–527.e3. doi: 10.1016/j.echo.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 29.Salaun E., Casalta A.C., Donal E., et al. Apical four-chamber longitudinal left ventricular strain in patients with aortic stenosis and preserved left ventricular ejection fraction: analysis related with flow/gradient pattern and association with outcome. Eur. Heart J. Cardiovasc. Imaging. 2018;19(8):868–878. doi: 10.1093/ehjci/jex203. [DOI] [PubMed] [Google Scholar]
- 30.Yingchoncharoen T., Agarwal S., Popović Z.B., Marwick T.H. Normal ranges of left ventricular strain: a meta-analysis. J. Am. Soc. Echocardiogr. 2013;26(2):185–191. doi: 10.1016/j.echo.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Kuznetsova T., Herbots L., Richart T., et al. Left ventricular strain and strain rate in a general population. Eur. Heart J. 2008;29(16):2014–2023. doi: 10.1093/eurheartj/ehn280. [DOI] [PubMed] [Google Scholar]
- 32.Takamura T., Dohi K., Onishi K., et al. Reversible left ventricular regional non-uniformity quantified by speckle-tracking displacement and strain imaging in patients with acute pulmonary embolism. J. Am. Soc. Echocardiogr. 2011;24(7):792–802. doi: 10.1016/j.echo.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Alashi A., Isaza N., Faulx J., et al. Characteristics and outcomes of patients with takotsubo syndrome: incremental prognostic value of baseline left ventricular systolic function. J. Am. Heart Assoc. August 2020 doi: 10.1161/JAHA.120.016537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamada-Harimura Y., Seo Y., Ishizu T., et al. Incremental prognostic value of right ventricular strain in patients with acute decompensated heart failure. Circ. Cardiovasc. Imaging. 2018;11(10) doi: 10.1161/CIRCIMAGING.117.007249. [DOI] [PubMed] [Google Scholar]
- 35.Muraru D., Onciul S., Peluso D., et al. Sex- and method-specific reference values for right ventricular strain by 2-dimensional speckle-tracking echocardiography. Circ. Cardiovasc. Imaging. 2016;9(2) doi: 10.1161/CIRCIMAGING.115.003866. [DOI] [PubMed] [Google Scholar]
- 36.Cameli M., Righini F.M., Lisi M., et al. Comparison of right versus left ventricular strain analysis as a predictor of outcome in patients with systolic heart failure referred for heart transplantation. Am. J. Cardiol. 2013;112(11):1778–1784. doi: 10.1016/j.amjcard.2013.07.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables and Figures