Abstract
Objective
Hydroxychloroquine has been proposed as a primary prophylactic agent against coronavirus disease 2019 (COVID-19). This study aimed to investigate if patients treated with hydroxychloroquine for a non-COVID-19 indication had a lower risk of verified infection with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) compared with matched controls.
Methods
A cohort comprising all persons in Denmark collecting hydroxychloroquine prescriptions in 2020 and 2019 (i.e., both during and before SARS-CoV-2 was confirmed in Denmark), matched by age and sex with controls, was studied. Data were collected using the Danish national registries, which contain complete information on patient health data, prescriptions and microbiological test results. The main outcome was microbiologically verified SARS-CoV-2 infection.
Results
In total, 5488 hydroxychloroquine users were matched with 54,486 non-users. At baseline, the groups differed in terms of diagnoses of pulmonary disease, cardiovascular disease, renal disease, gastrointestinal/metabolic disease and dementia, as well as treatment with antirheumatic drugs. The final model was adjusted for these potential confounders. Use of hydroxychloroquine for non-COVID-19 indications was not associated with any change in confirmed SARS-CoV-2 (hazard ratio 0.90, 95% confidence interval 0.76–1.07). This result was robust in the propensity-score-matched sensitivity analysis.
Conclusion
This study, which is the largest to date to investigate the primary prophylactic effect of hydroxychloroquine against SARS-CoV-2, does not support any prophylactic benefit of hydroxychloroquine in the prevention of infection with SARS-CoV-2.
Keywords: Hydroxychloroquine, SARS-CoV-2, COVID-19, Prophylaxis, Epidemiology
Introduction
Chloroquine and hydroxychloroquine have been shown to be in-vitro inhibitors of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in infected Vero cells (Liu et al., 2020, Wang et al., 2020, Yao et al., 2020). This contributed to the hypothesis that such drugs could be used as prophylaxis for SARS-CoV-2 infection and for treatment of patients with coronavirus disease 2019 (COVID-19). Hydroxychloroquine is used for long-term treatment of several rheumatic diseases; it has a favourable safety profile (Ruiz-Irastorza et al., 2010, Ponticelli and Moroni, 2017) and a low cost (Ponticelli and Moroni, 2017), which is a key point when facing a pandemic.
Five randomized clinical trials have demonstrated a neutral effect of treatment with hydroxychloroquine in hospitalized patients with COVID-19 (Abd-Elsalam et al., 2020, Group et al., 2020, Self et al., 2020, Tang et al., 2020, WHO Solidarity Trial Consortium et al., 2020). Additionally, three randomized clinical trials reported no benefit of hydroxychloroquine as a postexposure prophylactic agent; two of these trials were against COVID-19 (Boulware et al., 2020, Mitjà et al., 2020) and the latter was against polymerase chain reaction (PCR)-verified SARS-CoV-2 infection (Barnabas et al., 2020).
Hydroxychloroquine has likewise been explored as a primary prophylactic agent against COVID-19 by two randomized trials (Abella et al., 2020, Rajasingham et al., 2020). However, both were stopped too early to reach a firm conclusion.
Currently, four epidemiological studies investigating the primary prophylactic effect of hydroxychloroquine for SARS-CoV-2 infection have been identified (Gendelman et al., 2020, Jung et al., 2020, Bae et al., 2021, Ferreira et al., 2021). Three of these studies reported no effect (Gendelman et al., 2020, Jung et al., 2020, Bae et al., 2021), and the largest study reported a reduced risk of SARS-CoV-2 for persons with chronic hydroxychloroquine use (Ferreira et al., 2021). A recent meta-analysis concluded that there is a need for further evidence on the use of hydroxychloroquine for prophylaxis of COVID-19 (Singh et al., 2021).
This large population-based cohort study, with substantially more events than previously published studies, investigated whether persons treated with hydroxychloroquine prior to the pandemic had a lower risk of PCR-confirmed SARS-CoV-2 infection compared with age- and sex-matched controls.
Methods
Study data
A cohort study was conducted based on nationwide Danish registry data. Data were collected from four registries: (i) the Danish National Patient Registry, which contains information on all admissions to Danish hospitals and hospital outpatient specialist clinic visits (Schmidt et al., 2015); (ii) the National Prescription Registry, which contains information on all prescriptions dispensed in Danish pharmacies [coded according to the Anatomical Therapeutic Chemical (ATC) classification system] (Pottegard et al., 2017); (iii) the Danish Central Personal Registry, which contains information on citizens of Denmark (e.g., age, sex and vital status) (Schmidt et al., 2014); and (iv) the Danish Microbiology Database, where the Danish Departments of Clinical Microbiology (Voldstedlund et al., 2014) and Statens Serum Institut performed laboratory analysis, registration and release of the national SARS-CoV-2 surveillance data for the present study.
Study population
All patients residing in Denmark who collected a prescription for hydroxychloroquine (ATC P01BA02) in both 2020 and 2019 (i.e., initiated treatment before the pandemic emerged in Denmark) were included in the study. This approach was used to eliminate any impact of hydroxychloroquine use in attempts to treat COVID-19. Each recipient was matched randomly by birth year and sex with up to 10 non-treated controls by the Danish Health Data Authority. Information about comorbidities was obtained from the Danish National Patient Registry. Comorbidities were collected as classified in the Charlson comorbidity index (Quan et al., 2005). Grouping of comorbidities was performed as follows. Cardiovascular disease included heart failure, ischaemic heart disease, cerebrovascular disease and peripheral vascular disease. Gastrointestinal and metabolic disease included diabetes with and without complications; mild-, moderate- and severe liver disease; and peptic ulcer disease. Cancer included any malignancy (except malignant neoplasm of skin) and solid metastatic tumours. Pulmonary disease was defined in accordance with the Charlson comorbidity index, with interstitial pulmonary diseases also included. Comorbidities included in this study and their grouping are illustrated in Table S1 (see online Supplementary material). Only comorbidities or groups of comorbidities with prevalence among the study population ≥5% were included in this study.
Diagnoses of ventricular tachycardia, ventricular fibrillation and Torsades de Pointes tachycardia were collected to assess safety.
All subjects were linked to the National Prescription Registry to obtain information on the pharmaceuticals used for outpatient treatment of rheumatoid arthritis and systemic lupus erythematosus in Denmark (ATC code): hydroxychloroquine (P01BA02), methotrexate (L04AX03), sulfasalazine (A07EC01), systemic corticosteroids (H02AB), ciclosporin (L04AD01) and azathioprine (L04AX01).
All subjects were linked to the Danish Microbiology Database in order to access their positive SARS-CoV-2 test results.
The observation time commenced at the time of the first confirmed case of SARS-CoV-2 in Denmark (27 February 2020), and lasted until either (i) time of laboratory-confirmed SARS-CoV-2 infection; (ii) death; or (iii) end of follow-up (30 April 2021), whichever came first.
Intervention
Hydroxychloroquine was the intervention investigated. To avoid bias, users of the drug in both 2019 and 2020 were analysed. Additionally, controls who started hydroxychloroquine in 2020 were excluded (n = 11).
Outcomes
The primary outcome measure was a PCR- or antigenic-verified SARS-CoV-2 infection during the study period (27 February–30 April 2021). The secondary outcome was hospitalization for >12 h within 14 days of a positive SARS-CoV-2-test.
Statistical analysis
Statistical analyses were performed using Statistical Analysis Software 9.4 (SAS Institute, Cary, NC, USA). Baseline comparisons of categorical data, as well as a crude comparison on SARS-CoV-2 in the two groups, were performed using Chi-squared test, where the expected observations were five or more. When expected observations were less than five, Fisher’s exact test was performed. In the case of continuous data, baseline comparisons were performed using Student’s t-test or a non-parametric test, depending on the distributions.
The incidence of a positive SARS-CoV-2 test was compared between cases and controls using an adjusted Cox-proportional hazard regression analysis. Except for rheumatological disorders (a common indication for hydroxychloroquine), adjustments were made for age, sex, admission in the year prior to the study, and possible confounding diagnoses and pharmaceutical treatments with prevalence among the study population of ≥5% (pulmonary disease, cardiovascular disease, gastrointestinal/metabolic disease, cancer, methotrexate, systemic corticosteroids, sulfasalazine), as presented in Table 1 .
Table 1.
Baseline characteristics and characteristics after propensity matching.
| Baseline characteristics | ||||
|---|---|---|---|---|
| Entire cohort (n = 60,334) |
Propensity-matched cohort (n = 14,984) |
|||
| Hydroxychloroquine | Non-hydroxychloroquine | Hydroxychloroquine | Non-hydroxychloroquine | |
| n = 5488 | n = 54,846 | n = 4162 | n = 10,822 | |
| Sex | ||||
| Female | 4369 (79.6%) | 43,662 (79.6%) | 3411 (82.0%) | 8982 (83.0%) |
| Age | ||||
| Mean ± SD | 57.3 ± 15.9 | 57.4 ± 15.9 | 56.2 ± 16.6 | 57.8 ± 15.7 |
| Range | 9.0–95.0 | 9.0–95.0 | 9.0–93.0 | 11.0–95.0 |
| Median (IQR) | 59.0 (47.0–70.0) | 59.0 (47.0–70.0) | 57.0 (45.0–69.0) | 59.0 (48.0–70.0) |
| Hospitalization in previous 12 months | ||||
| Yes | 1086 (19.8%) | 6797 (12.4%) | 900 (21.6%) | 2070 (19.1%) |
| Previous diagnosis | ||||
| Pulmonary disease | 675 (12.3%) | 2938 (5.4%) | 540 (13.0%) | 1459 (13.5%) |
| Cardiovascular disease | 657 (12.0%) | 4006 (7.3%) | 537 (12.9%) | 1309 (12.1%) |
| Gastrointestinal/metabolic disease | 443 (8.1%) | 2992 (5.5%) | 362 (8.7%) | 874 (8.1%) |
| Renal disease | 167 (3.0%) | 538 (1.0%) | 155 (3.7%) | 161 (1.5%) |
| Cancer | 353 (6.4%) | 3479 (6.3%) | 296 (7.1%) | 808 (7.5%) |
| Dementia | 35 (0.6%) | 520 (0.9%) | 26 (0.6%) | 106 (1.0%) |
| Concommitant medicine use | ||||
| Methotrexate | 1393 (25.4%) | 451 (0.8%) | 490 (11.8%) | 451 (4.2%) |
| Systemic corticosteroids | 1769 (32.2%) | 3509 (6.4%) | 1294 (30.4%) | 3374 (31.2%) |
| Sulfasalazine | 1168 (21.3%) | 142 (0.3%) | 255 (6.1%) | 142 (1.3%) |
| Azathioprine | 241 (4.4%) | 113 (0.2%) | 229 (5.5%) | 62 (0.6%) |
| Leflunomide | 2 (<0.1%) | 1 (<0.1%) | 1 (<0.1%) | 0 (0%) |
n, number; SD, standard deviation; IQR, interquartile range.
For sensitivity analysis, a Greedy matched propensity-score matching with a case/control ratio of 1:3 was performed via SAS 9.4 ‘PROC PSMATCH’. The same variables used for adjustment in the Cox-proportional hazards regression analysis were used for matching. Matching was based on the logit of the propensity score and with a caliper of 0.25, and an extended common support region was used as per default setting in the SAS procedure. Subsequently, an unadjusted Cox regression was performed on the Greedy-matched population.
To investigate the secondary outcome of hospital admissions, logistic regression analysis was applied, controlling for the previously mentioned parameters.
To account for differences in healthcare seeking behaviour among patients, subgroup analysis was performed on the primary population, using only persons who had a SARS-CoV-2 test performed in the study period.
Furthermore, an adjusted Cox proportional hazards analysis was performed on the group of all recipients of hydroxychloroquine in 2019, with adjustment for the previously mentioned parameters. Model control investigating the proportional hazards assumption and test for linearity was performed to validate the Cox proportional hazards regression. If linearity was not fulfilled, the model was run with the variable converted to ordered categorial variables based on the median and interquartile range to ensure the lack of linearity had no effect on the result.
Specific interaction analysis was performed for hydroxychloroquine and systemic corticosteroids, methotrexate and sulfasalazine, respectively.
Ethical statement
This study was approved by the Danish Data Protection Agency (File No. P-2020-537). In Denmark, retrospective use of register data does not require ethical approval or patient consent. Data were only available for analyses on closed servers via the Danish Health Data Authority.
Reporting was carried out in accordance with the STROBE guidelines (von Elm et al., 2008).
Results
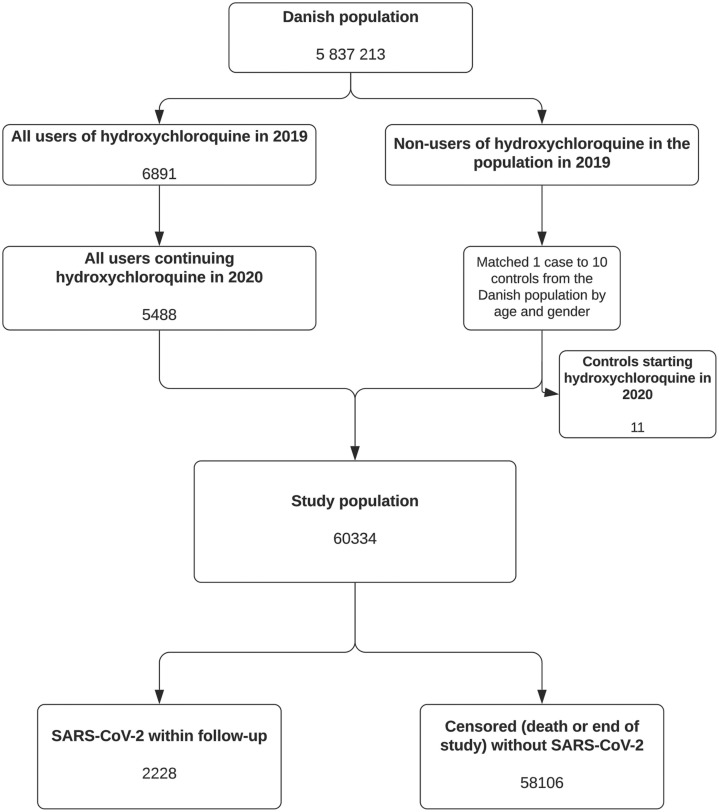
The hydroxychloroquine treatment group consisted of 5488 persons, and these were matched with 54,846 individual non-treated controls as shown in Figure 1 . The baseline characteristics are presented in Table 1. A total of 2228 persons in the study population had a positive SARS-CoV-2 test within the study period. The rate of SARS-CoV-2 test positivity was distributed evenly between the groups: 188/5488 (3,43%) persons in the hydroxychloroquine group and 2040/54,846 (3.72%) persons in the control group (P = 0.27). There was a significant, but minor, skewness in the distribution of persons who had a SARS-CoV-2 test in the study period: 4506/5488 (82.11%) in the hydroxychloroquine group and 43,186/54,846 (78,74%) in the control group (P ≤ 0.01).
Figure 1.
Study flowchart. SARS-CoV-2, severe acute respiratory syndrome coronavirus-2.
The hydroxychloroquine group had significantly more patients with a registered diagnosis of pulmonary disease, cardiovascular disease, gastrointestinal/metabolic disease, renal disease and dementia. This was not the case for the propensity-matched population used for the sensitivity analysis. Furthermore, the hydroxychloroquine group had significantly more persons using antirheumatic drugs.
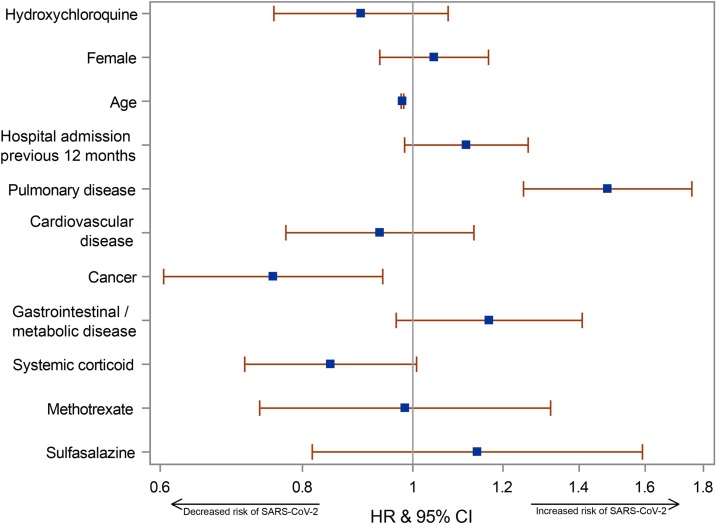
An unadjusted Cox proportional hazards regression model showed no difference in the occurrence of SARS-CoV-2 positivity between the treated and non-treated groups with a hazard ratio (HR) of 0.92 [95% confidence interval (CI) 0.79–1.07]. Adjusting for the previously defined comorbidities and pharmaceutical treatments did not alter the result {HR 0.90, 95% CI 0.76–1.07; see Figure 2 [with Table S2 (see online Supplementary material) for the underlying data]}. There was no difference in ventricular tachycardia (P = 0.37), ventricular fibrillation (P = 0.64) or Torsades de Pointes tachycardia (P = 0.58) between the two groups.
Figure 2.
Forest plot showing the variables in the adjusted Cox proportional hazards regression model, depicting hazard ratio (HR) and confidence interval (CI) for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) positivity on the adjusted variables. Prev., previous.
Model control
The proportional hazards assumption for the Cox regression was met. Linearity was not confirmed for age, so this variable was tested as a categorical variable in four categories, which did not change the results (Table S3, see online Supplementary material). No interaction was found between hydroxychloroquine and either systemic corticosteroids, methotrexate or sulfasalazine.
Sensitivity analysis
The sensitivity analysis was a propensity-matched cohort in which the main finding was confirmed: HR 1.01 (95% CI 0.92–1.31). The baseline characteristics of the propensity-matched cohort are displayed in Table 1.
Secondary outcome
The secondary outcome of hospital admission within 14 days of SARS-CoV-2 positivity (Tables S4 and S5 and Figure S1, see online Supplementary material) was an infrequent event, both in the hydroxychloroquine group and among controls (n = 175). The result was neutral, but was limited by a lack of power: odds ratio 1.44 (95% CI 0.78–2.65).
Subgroup analyses
The subgroup consisting of all persons tested for SARS-CoV-2 in the study period also confirmed the primary analysis (HR 0.88, 95% CI 0.74–1.05) (Table S6 and Figure S2, see online Supplementary material).
Repeating the primary analysis on the group of recipients of hydroxychloroquine in 2019 and their controls yielded similar results with an HR of 0.93 (95% CI 0.80–1.09) (Table S7 and Figure S3, see online Supplementary material). Due to the nature of the Danish registries, the study had 100% follow-up on both the primary and secondary outcomes.
Discussion
The present analysis, based on nationwide data and with complete follow-up for confirmed SARS-CoV-2 infection, did not find a beneficial effect of hydroxychloroquine for primary prophylaxis against infection with SARS-CoV-2.
The result was robust and the signal remained unchanged in a propensity-matched cohort sensitivity analysis. This was also the case in the subgroups of all SARS-CoV-2-tested persons and hydroxychloroquine recipients in 2019. In addition, no prophylactic effect of the drug regarding hospital admission following confirmed SARS-CoV-2 was found, but the latter analysis was limited by a lack of power.
A lower incidence of SARS-CoV-2 positivity was observed with increasing age. This is likely to be a result of the social behaviour of these groups, especially considering that a higher risk of admission amongst positive patients was also found to increase with age, as reported previously (Ioannou et al., 2020, Reilev et al., 2020).
In agreement with these results, three epidemiological studies did not find any difference in the risk of SARS-CoV-2 between persons using hydroxychloroquine and others (Gendelman et al., 2020, Jung et al., 2020, Bae et al., 2021). These studies were, however, limited by few events in the hydroxychloroquine groups (n = 3–16) and thus most likely insufficient power. A larger observational study from Portugal reported decreased risk of SARS-CoV-2 for people receiving chronic hydroxychloroquine treatment (Ferreira et al., 2021) by crosslinking their national databases on drug prescription, obligatory notifiable diseases and a database containing all SARS-CoV-2 tests performed. Although interesting, the study was limited by a lack of important information on the subjects (e.g., comorbidities), which, in turn, could affect social behaviour.
To date, two randomized controlled trials investigating hydroxychloroquine as primary prophylaxis for COVID-19 have been performed (Abella et al., 2020, Rajasingham et al., 2020). Both trials were performed using healthcare workers as subjects. Abella et al. (2020) investigated a dose of 600 mg daily, which was higher than the recommended daily dose for rheumatological disorders, whereas Rajasingham et al. (2020) used two different dosing regimens with either 400 mg once or twice weekly. Both trials were terminated prematurely with very few events and are consequently inconclusive, but do not conflict with the present results.
Postexposure prophylactic properties of hydroxychloroquine were investigated by Boulware et al. (2020) and Mitjà et al. (2020) against COVID-19. Neither of these studies demonstrated any effect on the occurrence of the disease. The study by Boulware et al. was limited by a lack of microbiological verification on all subjects and by using a short-term but high dosing regimen (Boulware et al., 2020). This was not a limitation in the other study which had PCR-verified SARS-CoV-2 as an outcome; however, their inclusion period after contact of up to 7 days may have limited the study (Mitjà et al., 2020). A recent study investigated the postexposure prophylactic abilities of hydroxychloroquine against PCR-verified SARS-CoV-2 infection without the aforementioned limitations, and reached the same conclusion (Barnabas et al., 2020).
The World Health Organization (WHO) has released a living guideline on drugs to prevent COVID-19, which makes a strong recommendation against hydroxychloroquine as prophylaxis against COVID-19 (Lamontagne et al., 2021). On the other hand, Singh et al. recently published a systematic review and meta-analysis on the prevention and treatment of COVID-19 with hydroxychloroquine, concluding that there is a need for further evidence on prevention (Singh et al., 2021). Two pre-print systematic reviews and meta-analyses on randomized clinical trials have investigated hydroxychloroquine as prophylaxis against COVID-19. The first showed a pooled risk ratio of SARS-CoV-2 of 0.86 (95% CI 0.70–1.06) (García-Albéniz et al., 2021). The other concluded, on the basis of low-grade evidence, that hydroxychloroquine may have no effect on prophylaxis of SARS-CoV-2 infection and furthermore no important effect on hospital admission (Bartoszko et al., 2021).
Of the randomized controlled trials investigating hydroxychloroquine as prophylaxis, four reported information on admissions (Barnabas et al., 2020, Boulware et al., 2020, Mitjà et al., 2020, Rajasingham et al., 2020). In total, 51 hospitalizations occurred across all four studies. Two trials, which were both on postexposure prophylaxis, classified admissions based on their relation to COVID-19. Both had an equal distribution of COVID-19-related admissions between the treatment and control arms (Barnabas et al., 2020, Rajasingham et al., 2020).
Thus, despite the strong WHO recommendations, the evidence on the effect of hydroxychloroquine prophylaxis is insufficient. When comparing the above-mentioned results on SARS-CoV-2 with the present results, the neutral results line up. However, due to the sparseness of data, a minor benefit of hydroxychloroquine cannot be ruled out. Particularly regarding the effect on hospitalization, the evidence is sparse and limited by few events. However, the neutral results line up with the present results which, although limited in power, report no difference in admissions following SARS-CoV-2.
This study has various strengths. The Danish nationwide and complete registries were used, which ensured homogenous and complete availability of data in the cohort, and thus there was no loss to follow-up. These data comprised all confirmed positive SARS-CoV-2 PCR- and antigen-verified tests performed through all public hospitals and public test centres in Denmark during the study, accounting for nearly all tests performed in Denmark. All positive PCR and antigen tests performed in Denmark are required by law to be reported to Statens Serum Institut. The data also included all prescriptions collected from pharmacies on the defined pharmaceuticals, and data on diagnosis registered at all inpatient and outpatient visits to hospitals in Denmark. Additionally, the study was conducted in a country with universal healthcare, free testing and without any risk of third-centre referral bias. Availability of these data entailed a large cohort consisting of all recipients of hydroxychloroquine who had started the drug before the pandemic emerged in Denmark, and the numbers of exposed and non-exposed individuals and the number of events, were in fact, higher than in any other published study. The size of the cohort makes it more generalizable amongst other populations. Furthermore, the Danish registries ensured no loss to follow-up as all persons, either admitted or dead, are registered.
Despite the abovementioned strengths, this study also has some limitations. First, the design of the primary analyses does not take into account that differences in behaviour or living conditions of the groups could exist, illustrated by, for example, the decreased risk of SARS-CoV-2 in patients with cancer. Using propensity-score matching for the sensitivity analyses will likely have reduced the effect of the two mentioned biases, although this is far from certain. Second, the availability of testing amongst the population represents a possible bias, especially at the beginning of the pandemic. A second wave hit Denmark in the autumn of 2020, when testing possibilities were abundant, thus reducing this bias; data on the amount of testing performed in Denmark are supplied in Table S8 and Figure S4 (see online Supplementary material). Third, there is a possibility of residual confounding, but currently, no obvious connection exists between a known rheumatological disorder and the risk of contracting SARS-CoV-2. Fourth, data on the so-called ‘biological agents’ (tumour necrosis factor antagonists etc.) are lacking as data on biological treatment are not included in the Danish National Patient Registry, so the effect of these could not be taken into account. Furthermore, data on dosing information were not included, although it seems fair to assume that treatment followed the Danish recommended doses of 200–400 mg hydroxychloroquine per day (Danish Rheumatology Society, 2019). Finally, it seems worth noting that, although there seemed to be some discrete differences in some of the baseline variables in the propensity-matched cohort (Table 1), only the differences in methotrexate and systemic corticosteroids were of note.
In conclusion, this study did not find any protective effect of primary prophylaxis with hydroxychloroquine against SARS-CoV-2 infection among pre-existing users of this drug. Rather, very neutral estimates were seen in both primary and secondary analyses, as well as the sensitivity analyses. These findings are in agreement with small, randomized trials of primary and postexposure prophylaxis, as well as the WHO guideline on drugs to prevent COVID-19. Based on this study and the abundance of neutral data on hydroxychloroquine for COVID-19, this study does not support the use of hydroxychloroquine for prophylaxis against SARS-CoV-2.
Conflict of interest
PS reports personal fees from Boehringer Ingelheim outside the submitted work. CT reports personal fees from TEVA outside the submitted work. KEJH reports personal fees from AstraZeneca, personal fees from TEVA and personal fees from Chiesi outside the submitted work. CSU reports personal fees from AstraZeneca; personal fees and non-financial support from GSK; personal fees from Chiesi; personal fees from TEVA; grants and personal fees from Sanofi Genzyme; personal fees from Orion Pharma; personal fees from Actelion; grants and personal fees from Boehringer-Ingelheim; grants and personal fees from Mundipharma; and grants, personal fees and non-financial support from Novartis outside the submitted work. TBS reports personal fees from Amgen, grants from Sanofi Pasteur, grants from GE Healthcare, personal fees from Sanofi Pasteur, and personal fees from Novartis outside the submitted work.
All other authors declare no competing interests.
Funding
This work was supported by the Novo Nordisk Foundation (Grant No. NNF20SA0062834). The research salary of PS was sponsored by Copenhagen University Hospital-Herlev and Gentofte. The trial was not supported in any way by the pharmaceutical industry. The funding sources have not had and will not have any influence on trial design, data collection, analysis or reporting.
Data sharing
Statistical source code used for generation of the results can be obtained via the corresponding author. Source data collected for this study will not be made available to others due to Danish legislation regarding data sharing on population data. However, Danish citizens who have a legitimate reason can apply for access to the data via the Danish National Health Authority (https://sundhedsdatastyrelsen.dk/da/forskerservice/ansog-om-data). The study protocol is available at http://coptrin.dk/wp-content/uploads/2020/08/hydrchloroq-profylakse-final.pdf.
References
- Abd-Elsalam S., Esmail E.S., Khalaf M., Abdo E.F., Medhat M.A., Abd El Ghafar M.S. Hydroxychloroquine in the treatment of COVID-19: a multicenter randomized controlled study. Am J Trop Med Hyg. 2020;103:1635–1639. doi: 10.4269/ajtmh.20-0873. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Abella B.S., Jolkovsky E.L., Biney B.T., Uspal J.E., Hyman M.C., Frank I. Efficacy and safety of hydroxychloroquine vs placebo for pre-exposure SARS-CoV-2 prophylaxis among health care workers: a randomized clinical trial. JAMA Intern Med. 2020;181(2):195–202. doi: 10.1001/jamainternmed.2020.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae S., Ghang B., Kim Y.J., Lim J.S., Yun S.C., Kim Y.G. Recent hydroxychloroquine use is not significantly associated with positive PCR results for SARS-CoV-2: a nationwide observational study in South Korea. Viruses. 2021;13(2) doi: 10.3390/v13020329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnabas R.V., Brown E.R., Bershteyn A., Stankiewicz Karita H.C., Johnston C., Thorpe L.E. Hydroxychloroquine as postexposure prophylaxis to prevent severe acute respiratory syndrome coronavirus 2 infection: a randomized trial. Ann Intern Med. 2020;174(3):344–352. doi: 10.7326/M20-6519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoszko J.J., Siemieniuk R.A., Kum E., Qasim A., Zeraatkar D., Ge L. Prophylaxis for COVID-19: living systematic review and network meta-analysis. BMJ. 2021;373 doi: 10.1136/bmj.n949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware D.R., Pullen M.F., Bangdiwala A.S., Pastick K.A., Lofgren S.M., Okafor E.C. A randomized trial of hydroxychloroquine as postexposure prophylaxis for COVID-19. N Engl J Med. 2020;383:517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danish Rheumatology Society . 2019. Behandling med hydroxychloroquin [Treatment with hydroxychloroquine] Available at: https://danskreumatologi.dk/laegemidler/hydroxychloroquin/. [Accessed 21 December 2020] [Google Scholar]
- Ferreira A., Oliveira E.S.A., Bettencourt P. Chronic treatment with hydroxychloroquine and SARS-CoV-2 infection. J Med Virol. 2021;93:755–759. doi: 10.1002/jmv.26286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Albéniz X., Amo Jd, Polo R., Morales-Asencio J.M., Hernán M.A. Systematic review and meta-analysis of randomized trials of hydroxychloroquine for the prevention of COVID-19. medRxiv. 2021 doi: 10.1007/s10654-022-00891-4. 2020.09.29.20203869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman O., Amital H., Bragazzi N.L., Watad A., Chodick G. Continuous hydroxychloroquine or colchicine therapy does not prevent infection with SARS-CoV-2: insights from a large healthcare database analysis. Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group R.C., Horby P., Mafham M., Linsell L., Bell J.L., Staplin N. Effect of hydroxychloroquine in hospitalized patients with COVID-19. N Engl J Med. 2020;383:2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou G.N., Locke E., Green P., Berry K., O’Hare A.M., Shah J.A. Risk factors for hospitalization, mechanical ventilation, or death among 10131 US veterans with SARS-CoV-2 infection. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S.Y., Kim M.S., Kim M.C., Choi S.H., Chung J.W., Choi S.T. Effect of hydroxychloroquine pre-exposure on infection with SARS-CoV-2 in rheumatic disease patients: a population-based cohort study. Clin Microbiol Infect. 2020;27(4):611–617. doi: 10.1016/j.cmi.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamontagne F., Agoritsas T., Siemieniuk R., Rochwerg B., Bartoszko J., Askie L. A living WHO guideline on drugs to prevent COVID-19. BMJ. 2021;372:n526. doi: 10.1136/bmj.n526. [DOI] [PubMed] [Google Scholar]
- Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitjà O., Corbacho-Monné M., Ubals M., Alemany A., Suñer C., Tebé C. A cluster-randomized trial of hydroxychloroquine for prevention of COVID-19. N Engl J Med. 2020;384(5):417–427. doi: 10.1056/NEJMoa2021801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponticelli C., Moroni G. Hydroxychloroquine in systemic lupus erythematosus (SLE) Expert Opin Drug Saf. 2017;16:411–419. doi: 10.1080/14740338.2017.1269168. [DOI] [PubMed] [Google Scholar]
- Pottegard A., Schmidt S.A.J., Wallach-Kildemoes H., Sorensen H.T., Hallas J., Schmidt M. Data resource profile: the Danish national prescription registry. Int J Epidemiol. 2017;46 doi: 10.1093/ije/dyw213. 798-f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan H., Sundararajan V., Halfon P., Fong A., Burnand B., Luthi J.C. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- Rajasingham R., Bangdiwala A.S., Nicol M.R., Skipper C.P., Pastick K.A., Axelrod M.L. Hydroxychloroquine as pre-exposure prophylaxis for COVID-19 in healthcare workers: a randomized trial. Clin Infect Dis. 2020;72(11):e835–e842. doi: 10.1093/cid/ciaa1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilev M., Kristensen K.B., Pottegard A., Lund L.C., Hallas J., Ernst M.T. Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort. Int J Epidemiol. 2020;49:1468–1481. doi: 10.1093/ije/dyaa140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Irastorza G., Ramos-Casals M., Brito-Zeron P., Khamashta M.A. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis. 2010;69:20–28. doi: 10.1136/ard.2008.101766. [DOI] [PubMed] [Google Scholar]
- Schmidt M., Pedersen L., Sorensen H.T. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–549. doi: 10.1007/s10654-014-9930-3. [DOI] [PubMed] [Google Scholar]
- Schmidt M., Schmidt S.A., Sandegaard J.L., Ehrenstein V., Pedersen L., Sorensen H.T. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self W.H., Semler M.W., Leither L.M., Casey J.D., Angus D.C., Brower R.G. Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: a randomized clinical trial. JAMA. 2020;324(21):2165–2176. doi: 10.1001/jama.2020.22240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B., Ryan H., Kredo T., Chaplin M., Fletcher T. Chloroquine or hydroxychloroquine for prevention and treatment of COVID-19. Cochrane Database Syst Rev. 2021;2 doi: 10.1002/14651858.CD013587.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W., Cao Z., Han M., Wang Z., Chen J., Sun W. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voldstedlund M., Haarh M., Molbak K. MiBa board of representatives. The Danish microbiology database (MiBa) 2010 to 2013. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.1.20667. [DOI] [PubMed] [Google Scholar]
- von Elm E., Altman D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Solidarity Trial Consortium, Pan H., Peto R., Henao-Restrepo A.M., Preziosi M.P., Sathiyamoorthy V. Repurposed antiviral drugs for COVID-19 – Interim WHO Solidarity Trial Results. N Engl J Med. 2020;384(6):497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71(15):732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]