Graphical abstract
Abbreviations: AIFS, acute invasive fungal rhino-orbital-cerebral sinusitis; COVID-19, coronavirus disease 2019; DM, diabetes mellitus; DCL, disturbed conscious level; PL, perception of light; RT-PCR, reverse transcriptase-polymerase chain reaction
Keywords: Acute invasive fungal rhino-orbital-cerebral sinusitis, Mucormycosis, COVID-19
Abstract
Background
The incidence of devastating opportunistic coinfections in patients with COVID-19 infection, their imaging features and their morbidity and mortality consequences need to be unraveled.
Methods
This is a case series presenting the radiologic features and clinical presentation of acute invasive fungal rhino-orbital-cerebral sinusitis (AIFS) in eight hospitalized patients with confirmed COVID-19 infection.
Results
Our patient cohort presented with symptoms of the invasive fungal disease within 12–35 days from their initial presentation with COVID-19 infection. The cross-sectional imaging features of AIFS associated with COVID-19 infection do not differ from those reported in the literature for AIFS associated with other risk factors, yet our patients had features of aggressive late-stage forms with high morbidity and mortality rate.
Conclusion
AIFS is a possible encounter in patients with COVID-19 patients and radiologists should be familiar with its imaging features.
Introduction
In early March 2021, more than 116 million cases of confirmed corona virus-19 disease (COVID-19) were reported to the World Health Organization with more than 2.5 million deaths.1 Previous studies reported various fungal coinfections in COVID-19 patients.2, 3, 4 COVID-19 patients seem to be more susceptible to fungal coinfections owing to the over-expression of inflammatory cytokines and impairment of the cell-mediated immune response with reduced CD4 and CD8-T lymphocytes. This susceptibility increases with immunocompromised status,5 trauma, diabetes mellitus (DM), and neutropenia. All were reported as predisposing factors.6
Our tertiary referral hospital faced an increased rate of rhino-orbital-cerebral acute invasive fungal sinusitis during the first wave of the COVID-19 pandemic.7 Here, we report the radiologic features of eight patients with confirmed COVID-19 infection admitted to our hospital from May 2020 to February 2021 and presented with AIFS. The available imaging scans were all reviewed for the eight patients. CE-CT and MRI for brain and sinuses were done to seven patients, and non-CE CT scans were done for one patient owing to elevated renal function tests.
Case 1
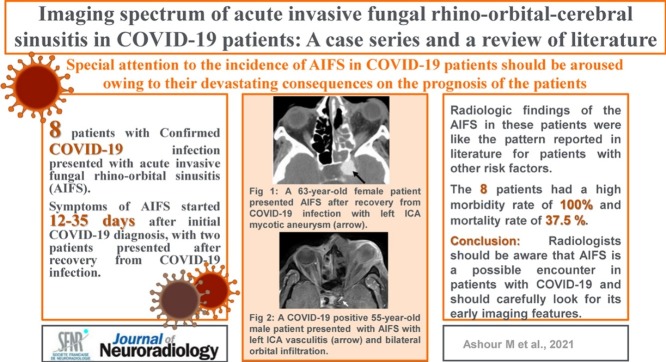
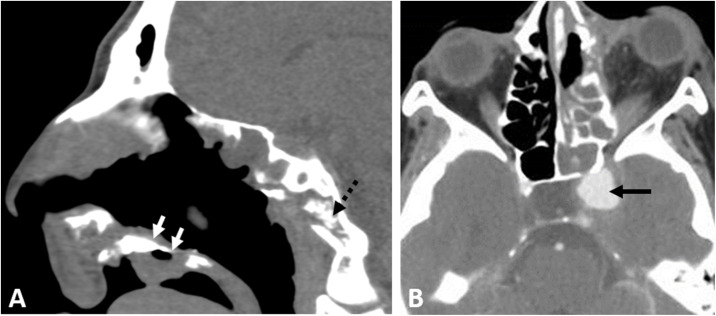
A 47-year-old man with uncontrolled DM type 2 presented to the hospital with disturbed conscious level (DCL), respiratory distress, and right upper eyelid edema with a preceding history of respiratory illness that started 10 days earlier. He was admitted to the ICU. Clinical examination revealed right total ophthalmoplegia, no perception of light (PL), and black nasal crusts. CE-CT scan revealed invasive sinonasal disease with right orbital panophthalmitis. The patient was positive for COVID-19 by reverse transcriptase-polymerase chain reaction (RT-PCR) test. Endoscopic surgical debridement and right orbital exenteration were done. The patient received systemic antifungal (amphotericin B) and ambisome. Histopathology and culture revealed invasive fungi of Aspergillus species. Follow-up by CE-MRI for the sinuses revealed residual cavernous sinus fungal infiltration with perineural spread along the trigeminal nerve and mild non-specific sinus disease (Fig. 1 ). The patient received postoperative amphotericin B and was discharged after controlling his medical condition.
Fig. 1.
A COVID-19 positive 47-year-old male patient presented with acute invasive fungal rhinosinusitis submitted to surgical debridement and right orbital enucleation. Perineural spread of the fungal disease along the trigeminal nerve is demonstrated in (A) axial, and (B) coronal T2WI; the right Meckel’s cave is infiltrated (dashed arrow), with extension along the main trunk of the trigeminal nerve (white arrows).
Case 2
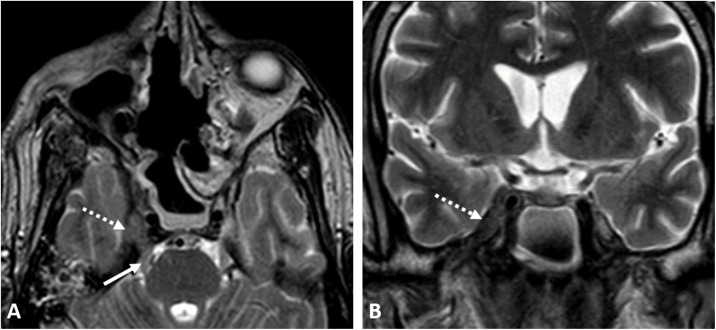
A 65-year-old diabetic woman, with uncontrolled DM type 2, was admitted to the hospital for COVID-19 infection. In the second week after admission, she started to develop rapidly advancing right upper eyelid edema, conjunctival chemosis, and a drop of vision that progressed eventually to right total ophthalmoplegia and no PL, associated with black nasal crusts. CE-MRI revealed right ethmoidal and maxillary sinusitis, with right orbital infiltration involving the optic nerve (Fig. 2 ), right cavernous sinus partial thrombosis with secondary vasculitis of the cavernous segment of the internal carotid artery (ICA), and right posterior watershed acute infarctions (Fig. 2). Surgical debridement was done, histopathology and culture results confirmed the presence of mucormycosis. The patient received postoperative systemic antifungal (amphotericin B) for two weeks and then itraconazole. She was discharged after controlling her medical condition.
Fig. 2.
A COVID-19 positive 65-year-old woman with acute invasive fungal rhinosinusitis; (A) diffusion-weighted image (DWI), showing significant diffusion restriction of the right optic nerve (white arrow) of the ischemic/inflamed right optic nerve. (B) DWI of the brain shows right posterior watershed infarctions (black arrows).
Case 3
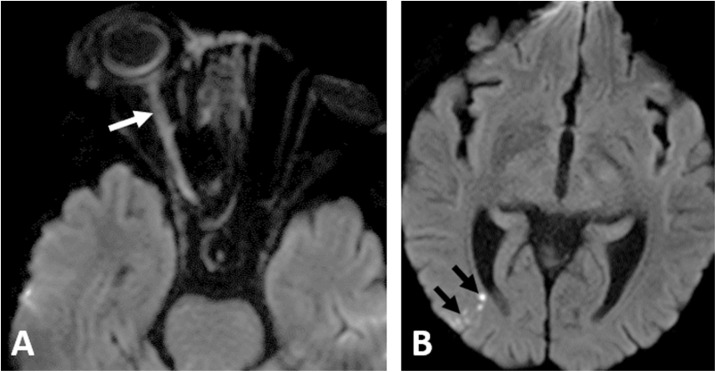
A 67-year-old man with chronic kidney disease. He presented to the hospital with DCL after two weeks of home isolation and medical treatment for COVID-19 infection. He was diagnosed with diabetic ketoacidosis. After 14 days of hospital admission, he complained of a rapidly deteriorating right visual acuity together with conjunctival chemosis. Clinical examination revealed mild orbital proptosis, nasal crusts, and respiratory distress. The patient was admitted to the ICU. Histopathology from nasal biopsy confirmed the presence of mucormycosis. The patient received amphotericin B and surgical debridement was planned upon clinical status improvement. Further deterioration occurred that progressed to right total ophthalmoplegia, and no PL. CE-MRI showed right orbital panophthalmitis, proptosis and posterior contour tenting, focal necrosis of the ethmoidal air cells (Fig. 3 ), and hard palate, and bilateral cavernous sinus invasion. Debridement could not be done because of the deteriorating clinical condition. The patient died in the ICU on day 28.
Fig. 3.
MRI of both orbits in a COVID-19 positive 67-year-male presented with right orbital proptosis, and ophthalmoplegia. (A) Axial T2WI and (B) axial CE-T1WI demonstrate right panophthalmitis with intra-ocular altered signal, proptosis, and posterior tenting of the right eye globe (dashed arrows) keeping with orbital compartment syndrome. Focal necrosis of the right ethmoidal are cells mucosa is noted (solid white arrow) in (B).
Case 4
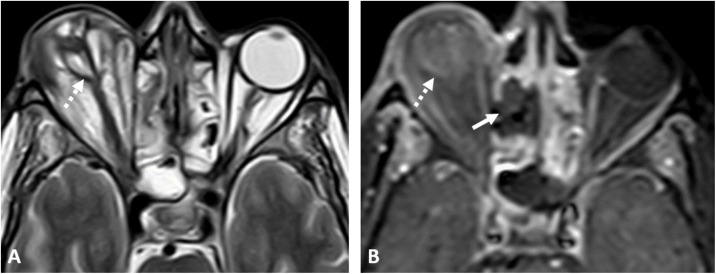
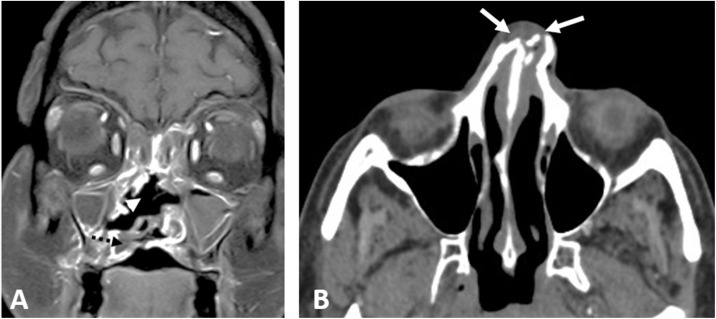
A 42-year-old male patient, with uncontrolled type II DM and a history of recent cerebral infarction 2 months before his presentation to our hospital with fever and nasal regurge after dental extraction. The patient was positive for COVID-19 by RT-PCR. Clinical examination showed right paralytic esotropia, right nasal black crusts, and bilateral oroantral fistulae. CT scan showed features of invasive fungal disease with nasal septum defect, osteonecrosis of the hard palate (Fig. 4A ), the sphenoid bone, and the petrous apex on both sides. The patient received systemic antifungal (amphotericin B). Endoscopic surgical debridement was done and revealed thrombosed sphenopalatine and greater palatine arteries. Histopathology confirmed the presence of mucormycosis. The patient improved and was discharged on supportive treatment and readmitted four months later for palatal reconstruction.
Fig. 4.
Two different patients with acute invasive sinonasal disease; 42-year-male with positive RT-PCR for COVID-19 infection (A) non-CE axial CT of the PNS of; showing destructive soft tissue density mass at the level of the hard palate (white arrow) with osteonecrosis, air locules and the clivus (dashed arrow). A 63-year-old female patient presented after recovery from COVID-19 infection (B) axial CE-CT scan; demonstrates mycotic aneurysm of the cavernous segment of the left ICA (black arrows).
Case 5
A 63-year-old female patient with uncontrolled DM and confirmed COVID-19 infection had been discharged from the hospital after improvement and repeated negative RT-PCR. Two days later, she started to develop left upper eyelid edema with reduced visual acuity and presented back to the hospital. Clinical assessment revealed left ophthalmoplegia, no PL, and blackish nasal crusts. CE-CT showed invasive sinonasal disease extending to the left cavernous sinus, left orbit, and left PPF, aneurysm of the cavernous segment of ICA was also noted (Fig. 4B). CE-MRI revealed the underlying left ICA vasculitis and a retrograde extension along the trigeminal nerve. Endoscopic surgical debridement was done, biopsy and culture confirmed the presence of mucormycosis, and postoperative systemic antifungal was given. The patient improved and was discharged after the improvement of the clinical condition. Further management for the ICA aneurysm was planned.
Case 6
A 41-year-old diabetic female patient was admitted to the hospital for a COVID-19 infection. On day 14 of hospital admission, she started to develop progressive facial edema. CE-CT revealed invasive sinonasal disease with erosions of the nasal septum, hard palate, maxillary sinus wall (Fig. 5A ), pterygoid plates, and clivus, with effacement of the skull base fat planes. Surgical debridement was done, histopathology confirmed the presence of mucormycosis. The patient received amphotericin. On day 24, the patient developed left orbital ophthalmoplegia, for which CE-MRI was done and showed bilateral cavernous sinuses infiltration, left temporal meningeal enhancement, and necrotic tissue involving the palate and the infratemporal fossa bilaterally. The patient was kept on medical treatment and the clinical condition improved. Three weeks later, the patient developed acute right-sided weakness and global aphasia, CT head revealed left frontoparietal acute infarctions. The patient improved with medical management and was discharged.
Fig. 5.
Two patients with an acute invasive fungal sinonasal disease and COVID-19 infection; (A) Coronal CE-T1 WI at the level of the bony nasal septum showing a large nasal septum defect (arrowhead), focal non-enhancing necrotic areas at the hard palate (dashed arrow) in a 41-year-old female patient. (B) The initial non-CE-CT at the level of the nasal bones of a 45-year-man; showed soft tissue density obliterating the anterior nasal cavities with fragmented nasal bones (white arrows).
Case 7
A 54-year-old male, with a medical history of DM, hypertension, end-stage renal disease on renal dialysis, and ischemic heart disease. He presented with DCL and respiratory distress and was admitted to the ICU, intubated, and ventilated. RT-PCR test for COVID-19 was positive. On day 16, he started to develop left upper eyelid edema, which progressed rapidly to diffuse facial edema, associated with skin discoloration. The initial CT head showed fragmented nasal bones with soft tissue density with no other significant abnormalities (Fig. 5B) that progressed on the following CT scans to total opacification of the ethmoidal and maxillary sinuses bilaterally. CT chest showed features of acute respiratory distress syndrome (ARDS). Histopathology confirmed the presence of invasive fungal disease. Systemic antifungal treatment was given, and surgical debridement was planned upon clinical improvement. The condition progressed dramatically to bilateral panophthalmitis, associated with a necrosed nasal septum and hard palate. The patient clinical status deteriorated and died on day 31.
Case 8
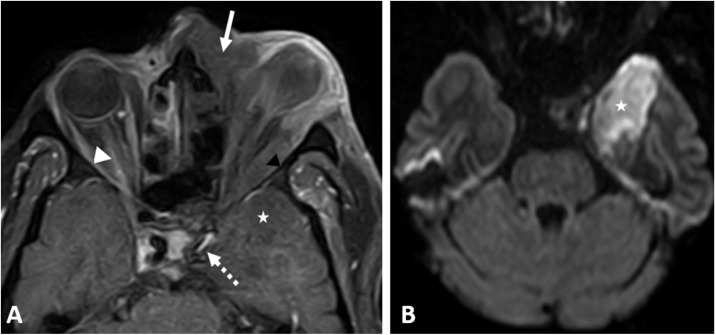
A 50-year-old diabetic male on medical management for a confirmed COVID-19 infection. He presented to our hospital two weeks after the initial respiratory symptoms with left painful orbital proptosis and a drop of vision. Clinical examination showed left panophthalmitis, total ophthalmoplegia and no PL, blackish nasal crusts, and septal ulceration. Combined CT and CE-MRI scans showed pansinusitis, septal perforation, and extension of the inflammatory process to the nasopharynx, and both orbital cavities. Other findings included thrombosis of the left cavernous sinus, temporal lobe meningeal enhancement, left ICA vasculitis, and left frontotemporal infarction (Fig. 6 ). Surgical endoscopic debridement and left orbital enucleation were done. Histopathology confirmed the presence of mucormycosis. On day 5; the clinical condition deteriorated despite the surgical drainage and improvement of the respiratory condition, with a bloody discharge oozing from the left orbital surgical bed. On day 14 the patient developed right-sided paraplegia for which CT head was done and showed significant progression of the left frontal-temporoparietal infarctions. The patient died on day 15.
Fig. 6.
A COVID-19 positive 55-year-old male patient; (A) CE-T1 WI showing extension of the sinonasal inflammatory tissue to the left orbital cavity (white arrow), with left orbital panophthalmitis and proptosis of the left deformed eye globe, non-enhancing swollen muscles are noted (black arrowhead), and right perioptic neuritis (white arrowhead). Left ICA critical attenuation (dashed arrow) with left frontal (not demonstrated here) and left temporal infarction (asterisk) that shows diffusion restriction in diffusion weighted image (B).
Discussion
The eight presented cases illustrate the imaging findings of AIFS associated with COVID-19 infection. We searched Pubmed and Web of Science for similarly reported cases with the terms ‘invasive fungal sinusitis’ or ‘mucormycosis’ and ‘COVID-19’ or ‘SARS-COV-2’ with language restriction (English), and no date restrictions (up to March 9, 2021). We found 18 articles and case reports with a total of 15 cases reported worldwide with confirmed acute invasive fungal orbital-rhinosinusitis associated with COVID-19 infection. The first case was reported by Werthman-Ehrenreich8 for a 33-year-old female patient, who was positive for COVID-19 and developed mucormycosis with orbital compartmental syndrome. Her CT head showed significant mucosal thickening of the maxillary and ethmoidal sinuses, MRI brain showed ischemic infarctions and multi-focal cerebral edema that progressed to bifrontal abscesses on the later scan.8
The second case was reported by Mehta and Pandey9 for a 60-year-old male diabetic patient who developed orbital symptoms on day 10 from the start of COVID-19 symptoms. MRI scan showed significant mucosal thickening in the right frontal, maxillary, and ethmoidal sinuses, right orbital proptosis with a soft tissue swelling in the right preseptal, and retrobulbar tissues. Mekonnen et al.10 reported a case of AIFR associated with COVID-19 ARDS. CT scans showed opacification of the right-sided paranasal sinuses, lamina papyracea dehiscence, right orbital proptosis, and cellulitis. Similar other three cases of COVID-19 related ARDS were reported by Sebastian et al.[1], 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 presented with AIFR with orbital cellulitis 10–15 days after the onset of COVID-19 illness their CT scans showed variable sinuses opacification with internal soft tissue density. Waisel-Haiat et al.12 reported a case of a female patient who presented with ROM in a late stage of COVID-19 illness. CT scan revealed left-sided mucosal thickening of the maxillary, sphenoid, and maxillary sinuses, left orbital proptosis with periorbital and midfacial soft tissue swelling.
Sen et al.13 presented a case series of 6 patients with ROM, five of them were presented after recovery from COVID-19, and only one patient presented with both infections concurrently. The radiological findings were not reported in detail, yet five patients had intracranial extension to the pachymeninges (n = 1), brain parenchyma (n = 2), cavernous sinus (n = 4), and ICA thrombosis (n = 1).13 Ahmadikia et al.14 also reported a case presented with mucormycosis 20 days after recovery. CT was done after sinus surgery showed maxillary sinus mild mucosal thickening.
Our eight patients were symptomatic for COVID-19 infection and required hospital admission at different time points throughout their illness. Like the few previously reported cases of AIFS in patients with COVID-19,8, 9, 10, 11, 12, 13, 14 most of our patients presented predominantly within a late stage of COVID-19 infection; the symptoms of AIFS started 12–35 days after the initial COVID-19 diagnosis, with two of those patients presented with AIFS after recovery from COVID-19 infection.
All our patients had comorbidities; DM (n = 6), hypertension (n = 2), end stage renal disease (n = 2), hyperlipidemia (n = 2), ischemic heart disease (n = 1), previous cerebral stroke (n = 1).
The sinonasal imaging findings we encountered were as follows; opacification of the nasal cavity (n = 8), anterior ethmoidal (n = 8), posterior ethmoidal (n = 8), maxillary (n = 7), sphenoid (n = 7), and frontal sinuses (n = 5), according to the degree of sinonasal mucosal thickening; mild thickening (n = 0), moderate thickening approaching half of the luminal involvement (n = 4), severe thickening up to total sinus opacification (n = 4). Extension to the nasolacrimal duct/sac (n = 6), hard palate (n = 6), pterygopalatine fossa (n = 5), and peri-antral fat (n = 7) were also noticed as well as bone dehiscence (n = 7), septal ulceration (n = 7). Regarding laterality of the disease process; unilateral (n = 3), bilateral (n = 5). For the pattern of the disease process, the eight patients showed an infiltrative pattern of the inflammatory process (n = 8).
Orbital infiltration was unilateral in four patients with the following findings: panophthalmitis (n = 4), orbital compartment syndrome (n = 1), and optic nerve ischemia/inflammation (n = 2). Bilateral orbital panophthalmitis occurred in two patients. Intracranial complications were: perineural spread (n = 6), cavernous sinus involvement (n = 6), meningeal/epidural infiltration (n = 3), ICA vasculitis/thrombosis (n = 4), ICA mycotic aneurysm (n = 1), intracerebral abscess (n = 2), and cerebral infarctions (n = 3).
Radiologic findings of the AIFS in these patients were not peculiar from the pattern previously reported in literature for patients with AIFS with other risk factors.15, 16 Yet our patients had radiologic features of aggressive late-stage forms of the disease process with a consequent long-term morbidity rate of 100% and a high mortality rate of 37.5%.
Invasive fungal rhinosinusitis is categorized into acute and chronic forms, the chronic form is either chronic invasive rhinosinusitis (CIFS) or chronic granulomatous invasive rhinosinusitis (CGFS).17 Our patients presented with a rapidly progressing course that is recognized in the AIFS in contrary to the indolent chronic course that characterizes the chronic invasive forms of fungal rhino-sinusitis that usually arise in immunocompetent hosts.18 The radiologic features that were encountered in the included cohort were of a predominant infiltrative pattern and bone dehiscence; these features differ from those described in patients with the chronic forms of invasive fungal sinusitis. Cho et al.19 reported seven patients with CIFS and four patients with CGFS, five out of those eleven patients presented with an infiltrative pattern while the other six patients had a mass forming pattern. No bone sclerosis was noted in our patient’s cohort while all patients described by Cho SJ et al. presented with bone sclerosis.19
Owing to the limited number of our patients, we cannot establish a theory for the associated incidence of both infections, yet from the predominant preceding presentation of COVID-19 among our patients with co-existing health problems, we suggest that COVID-19 may have added to the existing health debilitating factors that made the patients more susceptible to opportunistic secondary infections. Also, prolonged periods of hospital admission might have increased this incidence. Prior recent studies reported other risk factors as COVID-19 associated dysregulated immune response that can induce the incidence of secondary fungal infections.5 Other risk elements that may increase the odds for fungal infections include corticosteroid therapy,20 and the empirical use of a broad spectrum of antibiotics.21
This case series aims to raise the concerns for early diagnosis of AIFS in patients with COVID-19 infection.
Conclusion
AIFS in COVID-19 patients shows variable imaging features similar to AIFS with other risk factors and radiologists should be familiar with these radiologic features.
Funding
No funding was received for this study.
Disclosure
Part of the data reported here were used in a brief report on mucormycosis incidence in the COVID-19 era provisionally accepted by Frontiers in Medicine. (Front. Med. | doi: https://doi.org/10.3389/fmed.2021.645270).
Presentation
This manuscript has not been presented in any meeting in part or whole.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments.
Informed consent
For this type of study informed consent was not required and waived by the ethical committee.
References
- 1.World Health Organization 2021, Coronavirus disease (COVID19) pandemic, accessed 8 March 2021, <https://www.who.int/emergencies/diseases/novel-coronavirus-2019>.
- 2.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim D., Quinn J., Pinsky B., et al. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA. 2020;323(20):2085–2086. doi: 10.1001/jama.2020.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song G., Liang G., Liu W. Fungal co-infections associated with global COVID-19 pandemic: a clinical and diagnostic perspective from China. Mycopathologia. 2020;31:18. doi: 10.1007/s11046-020-00462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornely O., Alastruey-Izquierdo A., Arenz D., et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology incooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19(12):e405–21. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fouad Y.A., Abdelaziz T.T., Askoura A., et al. Spike in rhino-orbital-cerebral mucormycosis cases presenting to a tertiary care center during the COVID-19 pandemic. Front Med. 2021;8:716. doi: 10.3389/fmed.2021.645270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am J Emerg. 2021;42:264.e5–264.e8. doi: 10.1016/j.ajem.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta S., Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus. 2020;12(9) doi: 10.7759/cureus.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mekonnen Z., Ashraf D., Jankowski T., et al. Acute invasive rhino-orbital mucormycosis in a patient with COVID-19-associated acute respiratory distress syndrome. Ophthalmic Plast Reconstr Surg. 2021;37(2):e40–e80. doi: 10.1097/IOP.0000000000001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sebastian S., Kumar V., Gupta M., et al. Covid associated invasive fungal sinusitis. Indian J Otolaryngol Head Neck Surg. 2021;25:1–4. doi: 10.1007/s12070-021-02471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waizel-Haiat S., Guerrero-Paz J., Sanchez-Hurtado L., et al. A case of fatal rhino-orbital mucormycosis associated with new onset diabetic ketoacidosis and COVID-19. Cureus. 2021;13(2) doi: 10.7759/cureus.13163. Published online 2021 Feb 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sen M., Lahane S., Lahane T. Mucor in a viral land: a tale of two pathogens. Indian J Ophthalmol. 2021;69(2):244–252. doi: 10.4103/ijo.IJO_3774_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmadikia K., Hashemi S., Khodavaisy S., et al. The double‐edged sword of systemic corticosteroid therapy in viral pneumonia: a case report and comparative review of influenza‐associated mucormycosis versus COVID‐19 associated mucormycosis. Mycoses. 2021:1–11. doi: 10.1111/myc.13256. 00: [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DelGaudio J., Swain R., Kingdom T., et al. Computed tomographic findings in patients with invasive fungal sinusitis. Arch Otolaryngol Head Neck Surg. 2003;129:236–240. doi: 10.1001/archotol.129.2.236. [DOI] [PubMed] [Google Scholar]
- 16.Middlebrooks E., Frost C., De Jesus R., et al. Acute invasive fungal rhinosinusitis: a comprehensive update of CT findings and design of an effective diagnostic imaging model. Am J Neuroradiol. 2015;36(8):1529–1535. doi: 10.3174/ajnr.A4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deutsch P., Whittaker J., Prasad S. Invasive and non-invasive fungal rhinosinusitis a review and update of the evidence. Medicina. 2019;55(7):319. doi: 10.3390/medicina55070319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vakharia K., Durand M., Hamilos D., et al. An atypical case of chronic invasive fungal sinusitis and its management. Otolaryngol Head Neck Surg. 2010;142:150–151. doi: 10.1016/j.otohns.2009.06.743. [DOI] [PubMed] [Google Scholar]
- 19.Cho S.J., Choi Y.J., Cho K., et al. Image findings in patients with chronic invasive fungal infection of paranasal sinuses. J Neuroradiol. 2021 doi: 10.1016/j.neurad.2021.02.005. S0150-9861(21)00046-8. Epub ahead of print. PMID: 33639140. [DOI] [PubMed] [Google Scholar]
- 20.Ni Y.-N., Chen G., Sun J., et al. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care. 2019;23(1):99. doi: 10.1186/s13054-019-2395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du R.-H., Liu L.-M., Yin W., et al. Hospitalization and critical care of 109 decedents with COVID-19 pneumonia in Wuhan, China. Ann Am Thorac Soc. 2020;17(7):839–846. doi: 10.1513/AnnalsATS.202003-225OC. [DOI] [PMC free article] [PubMed] [Google Scholar]