Abstract
Objective
Plasma Epstein-Barr virus (EBV) DNA is considered a biomarker for nasopharyngeal carcinoma (NPC). However, its long-term role in NPC development is unclear.
Materials and methods
A total of 1363 participants seropositive for EBV VCA-IgA and EBNA1-IgA in a community-based NPC screening program in southern China were tested for plasma EBV DNA levels by real-time qPCR between 2008 and 2015. New NPC cases were confirmed by active follow-up approach and linkage to local cancer registry through the end of 2016. Cox proportional hazards regression analysis was performed to calculate the hazard ratios (HRs) for NPC risk with plasma EBV DNA.
Results
Thirty patients were newly diagnosed during a median 7.5 years follow-up. NPC incidence increased with the plasma EBV DNA load ranging from 281.46 to 10,074.47 per 100,000 person-years in participants with undetectable and ≥ 1000 copies/ml levels; the corresponding cumulative incidence rates were 1.73 and 50%. Furthermore, plasma EBV DNA loads conferred an independent risk for NPC development after adjustment for other risk factors, with HRs of 7.63 for > 3–999 copies/ml and 39.79 for ≥1000 copies/ml. However, the HRs decreased gradually after excluding NPC cases detected in the first 2 to 3 years and became statistically nonsignificant by excluding cases detected during the first 4 years.
Conclusion
Elevated plasma EBV DNA can predict NPC risk over 3 years. Monitoring plasma EBV DNA can be used as a complementary approach to EBV serological antibody-based screening for NPC.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-021-08408-0.
Keywords: Nasopharyngeal carcinoma, Plasma EBV DNA, Risk prediction, Population screening
Introduction
Although nasopharyngeal carcinoma (NPC) is rare in most parts of the world, it is fairly frequent in Southeast Asia [1]. In essence, Epstein-Barr virus (EBV) infection is a necessary cause for approximately 95% of all NPC [2]. Primary EBV infection usually occurs in childhood and the virus preferentially infects within memory B cells in latency in healthy individuals [3]. Rarely, the virus is activated to an active lytic phase by endogenous and environmental stress. This transformation is regarded as the key step for NPC initiation and development, characterized by elevated viral DNA load and antibodies against multiple EBV antigens in circulation [4, 5]. According to the natural history studies for EBV, primary EBV infection occurs earlier in life in developing countries and is typically subclinical. EBV preferentially infects within memory B cells in latency in healthy individuals. The virus can be occasionally reactivated by endogenous and environmental stress, with increased aberrant virus reactivation and antibody responses against multiple EBV antigens, such as VCA-IgA, and EBNA1-IgA. Several long-term prospective studies have reported that elevated levels of antibodies against EBV antigens in serum precede several years of NPC development [6]. The proliferated B lymphocytes could release EBV participles and efficiently mediate cell-to-cell contact for EBV infection into the nasopharyngeal epithelial cells. Under constant attack from the virus, genetic instability increases and subsequently induces tumorigenesis in the susceptible individual. And then, latent EBV infection is established in the malignant nasopharyngeal epithelial cells [7].
Several prospective studies have convinced that elevated EBV-specific immunoglobulin A (IgA) antibodies are correlated with 4–21 fold risks for NPC and precede tumor detection by several years [8, 9]. Testing for serological EBV antibodies has therefore been established as the basis of NPC screening test in the endemic regions [10]. With the conveniences of cheap and easy to measure, they are usually be used as the primary screening markers to stratify NPC risk in NPC screening [11–13]. Among many EBV antibody testing, IgA antibodies against viral capsid antigen (VCA-IgA) and nuclear antigen 1 (EBNA1-IgA) have shown high sensitivity (> 90%) [14]. A community-randomized screening trial has been initiated in southern China to evaluate the efficacy of these two antibodies testing for early detection of NPC [11, 15, 16]. The current data have demonstrated a favorable effect with increased early detection rate and reduced NPC mortality in the screening participants [16]. However, the relatively low specificity (< 90%) of EBV serological antibody resulted in a reduced predictive value (PPV, ~ 5%), which lead to unnecessary psychological/financial burden for individuals without NPC. Therefore, the identification of new prediction factor as a complementary approach in EBV serological positive individuals is of interest.
Growing evidence in initial cross-sectional studies have revealed that plasma EBV DNA is a promising NPC indicator. The virus DNA load is also correlated with tumor burden, remission, and recurrence. However, a long-term relationship between plasma EBV DNA load and NPC risk is still lacking. In this study, we examined EBV DNA load in plasma from 1363 EBV-seropositive individuals in a community-based NPC screening program and prospectively evaluate its prediction value for NPC development over 5 years.
Materials and methods
Study population in the parent cohort
This study was based on a large community-based NPC screening program conducted in Sihui County, Guangdong province, southern China from 2008 [15]. The enrolled criteria include (1) residents aged 30–69 years; (2) without a history of NPC; and (3) with an Eastern Cooperative Oncology Group (ECOG) score of 0–2. For each participant, 6 mL of blood was obtained for testing the two screening markers of VCA-IgA (EUROIMMUN AG, Lübeck, Germany) and EBNA1-IgA (Zhongshan Bio-Tech Company, Zhongshan, China) by ELISA. The NPC risk scores were calculated by a risk prediction algorithm (LogitP = − 3.934 + 2.203*VCA-IgA + 4.797*EBNA-IgA) [11, 15], and three risk groups were stratified based on the predefined P scores (low-risk: < 0.65; medium-risk: ≥0.65- < 0.98; and high-risk: ≥0.98). Seropositive individuals were defined as those falling within either the serological high-risk or medium-risk groups.
At recruitment, all participants were asked to complete a structured questionnaire through face-to-face interviews by the trained researchers. The contents in the questionnaire included demographic information, such as sex, age, cigarette smoking, salted food intake, family history of NPC, and education level. Smokers were defined as having smoked at least one cigarette every 1–3 days during a 6-month period. The frequency of consumption of salted food at the time of the study interview was categorized into 2 groups: monthly or less, and weekly and more.
At the end of 2015, a total of 10,209 residents were tested for the screening markers and completed the questionnaire survey at baseline [17] . Written informed consent was obtained from each participant, and this study was approved by the Ethics Review Committee of the Sun Yat-sen University Cancer Center (NCT00941538, Clinical Trials.gov). This study was performed in accordance with the Declaration of Helsinki.
Study population in the current cohort
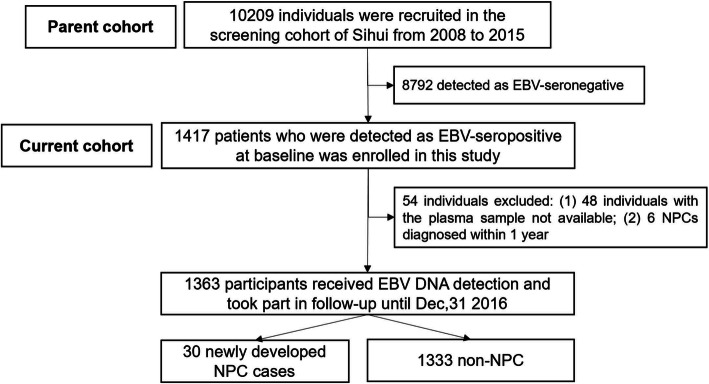
To evaluate the association between plasma EBV DNA load and NPC occurrence in EBV seropositive population, all seropositive participants with EBV antibody scores ≥0.65 (n = 1417) in the parent cohort were selected to test for plasma EBV DNA by real-time quantitative polymerase chain reaction (Fig. 1). Among them, 54 ineligible individuals were excluded, including 48 individuals not available for serum samples, 6 diagnosed with NPC within one year. Therefore, 1363 EBV seropositive subjects were recruited in the analysis, with 397 in the high-risk group and 966 in the medium-risk group.
Fig. 1.
Flow chart of selecting screening participants
Real-time quantitative polymerase chain reaction (qRT-PCR)
Plasma DNA was extracted using the QIAamp Blood Kit (Qiagen, Hilden, Germany), and EBV DNA was detected within the BamHI-W region of the EBV genome by qRT-PCR [18]. The sequences of the primers used were as follows: 5′-GCCAG AGGTA AGTGG ACTTT-3′ (forward) and 5′-TACCA CCTCC TCTTC TTGCT-3′ (reverse). The dual fluorescently labeled oligomer probe sequence was 5′-(FAM) CACAC CCAGG CACAC ACTAC ACAT (TAMRA)-3′. This detection was performed with an Applied Biosystems 7500 Real-Time PCR System (Foster City, CA, USA). Cycle threshold (CT) values were defined as the number of cycles required for exceeding background level for the fluorescent signal. Samples were defined as undetectable if the CT values excess 38 cycles (the lowest limit of reliable quantification [LLOQ]), equal to 0–3 copies/ml of EBV DNA in plasma. Duplicate samples and multiple negative blanks were analyzed, and the mean quantity of each duplicate was used for further concentration calculations. The standard concentration ladders were set at 106, 105, 104, 103, and 102 copies/ ml . The following equation was used to calculate the concentration of plasma EBV DNA: (C: the target concentration in plasma, copies/ml; Q: the target quantity (copies) determined by a sequence detector in a PCR; VDNA: the total volume of DNA obtained after extraction; VPCR: the volume of DNA solution used for PCR; and VEXT: the volume of plasma/serum extracted).
Ascertainment of incidence NPC and follow-up
Those in the serological high-risk group were referred to endoscopy examination for clinical evaluation. If suspicious lesions were observed in the endoscopy, nasopharyngeal biopsies were also performed. Pathologically diagnosed patients were immediately given advice for treatment. Those seropositive individuals also entered an accelerated follow-up group with annual screening. Those in the serological low-risk group were recommended for another screening with a 4–5 years interval. All participants were followed annually by linkages with local Cancer, Death and Population Registries. NPC cases were classified according to the World Health Organization (WHO) pathological classification system [19]. Among the 397 participants with EBV high-risk individuals, 247 received the endoscopy examinations and 50 further undertaken nasopharyngeal biopsies. At the end of 2016, 36 NPC cases were identified, with 6 in the first 1 year and 30 diagnosed after 1 year.
Statistical analysis
The person-years of follow-up for each participant were calculated from the date of recruitment to the date of NPC diagnosis, death, emigration, or loss to follow-up or to December 31, 2016, whichever came first. After excluding the NPC cases that occurred in the first year, the annualized NPC incidence rate was calculated by dividing the number of incident NPC cases by the person-years of follow-up. The hazard ratios (HRs) and 95% confidence intervals (CIs) for NPC incidence among the different plasma EBV DNA level groups were calculated by Cox regression analysis adjusted for sex, age, education level, tobacco smoking, family history of NPC, and salted food intake. The cumulative incidences of NPC by plasma EBV DNA level were calculated by Kaplan-Meier plots and compared by the log-rank test. To control the potential bias in our evaluation of NPC risk by EBV DNA load, we analyzed three sub-cohorts by excluding NPC cases detected within the first 2, 3 and 4 years of follow-up. A two-sided test with a P value < 0.05 was considered statistically significant. All analyses were performed by using SAS 9.4 (SAS Institute Inc., Chicago, IL, USA) and R Language 3.5 software (http://www.rproject.org).
Results
Baseline characteristics
A total of 1363 EBV-seropositive participants were recruited to this study, and their baseline characteristics are presented in Table 1. Among them, 649 participants were males (47.62%), with a median age of 49 years (interquartile range [IQR], 43–57); 564 (41.38%) participants had a smoking history (ever and current smokers), and 192 participants (14.09%) had eaten salted food monthly or more. Moreover, 61 participants (4.48%) had a family history of NPC. A total of 875 participants (64.2%) had an education level of more than 6 years.
Table 1.
Baseline characteristics of enrolled participants
| Variables | n(%) | |
|---|---|---|
| Sex, n (%) | male | 649 (47.62%) |
| female | 714 (52.38%) | |
| Age, n (%) | Median age, years (IQR) | 49 (43 ~ 57) |
| 30–39 | 216 (15.85%) | |
| 40–49 | 472 (34.63%) | |
| 50–59 | 461 (33.82%) | |
| 60–69 | 214 (15.70%) | |
| Smoking, n (%)a | never | 799 (58.62%) |
| current or former | 564 (41.38%) | |
| Salted food, n (%) | monthly or less | 1171 (85.91%) |
| weekly and more | 192 (14.09%) | |
| Family history of NPC, n (%) | no | 1302 (95.52%) |
| yes | 61 (4.48%) | |
| Education, n (%) | ≤6 year | 488 (35.8%) |
| > 6 year | 875 (64.2%) | |
| EBV-based risk score, n (%)b | 0.65 - < 0.98 | 966 (70.87%) |
| ≥0.98 | 397 (29.13%) |
aSmoking was defined as ever having smoked at least one cigarette every 1–3 days during a 6-month period; bEBV-based risk score were defined by a predefined logistic regression model
NPC incidence and cumulative incidence by plasma EBV DNA load
Based on the tested plasma EBV DNA levels, only 2.86% (39/1363) of seropositive individuals were detectable for plasma EBV DNA. Through December 31, 2016, with a median of 7.5 years (IQR, 4.2–8.2 years) of follow-up, 30 NPC cases were identified after excluding 6 NPC cases detected in the first year (Table 2). NPC incidence rate in EBV DNA detectable participants (2878.70 per 100,000 person-years) was approximately 10-fold higher than that in EBV DNA undetectable participants (281.46 per 100,000 person-years), (P < 0.05). Furthermore, the incidence rates per 100,000 person-years increased gradually from 2239.01 in the individuals with plasma EBV DNA load > 3–999 copies/ml to 10,074.47 in those with the load ≥1000 copies/ml (P < 0.001).
Table 2.
Univariate and multivariable Cox analyses for risk factors of NPC
| Variables | Participants(n) | Person-Years | NPC Cases(n) | Incidence Rate Per 100,000 Person-Years |
Crude HR(95%CIs) | P-value | Fully Adjusted HR(95%CIs)b | P-value |
|---|---|---|---|---|---|---|---|---|
| Sex | ||||||||
| male | 649 | 4009.16 | 18 | 448.97 | Reference | Reference | ||
| female | 714 | 4405.70 | 12 | 272.37 | 0.611 (0.294–1.269) | 0.186 | 1.381 (0.413–4.617) | 0.601 |
| Age | ||||||||
| 30–49 | 688 | 4470.52 | 15 | 335.53 | Reference | Reference | ||
| 50–69 | 675 | 3944.34 | 15 | 380.29 | 1.099 (0.537–2.250) | 0.796 | 0.832 (0.389–1.783) | 0.637 |
| Smoking | ||||||||
| never | 799 | 4929.13 | 11 | 223.16 | Reference | Reference | ||
| current or former | 564 | 3485.73 | 19 | 545.08 | 2.415 (1.149–5.076) | 0.020 | 3.566 (1.055–12.060) | 0.041 |
| Salted food | ||||||||
| monthly or less | 1171 | 7073.05 | 25 | 353.45 | Reference | Reference | ||
| weekly and more | 192 | 1341.81 | 5 | 372.63 | 1.050 (0.402–2.745) | 0.921 | 0.867 (0.329–2.288) | 0.774 |
| Family history | ||||||||
| no | 1302 | 8025.25 | 25 | 311.52 | Reference | Reference | ||
| yes | 61 | 389.61 | 5 | 1283.34 | 4.227 (1.618–11.043) | 0.003 | 2.838 (1.029–7.826) | 0.044 |
| Education | ||||||||
| ≤ 6 year | 488 | 2967.21 | 14 | 471.82 | Reference | Reference | ||
| > 6 year | 875 | 5447.65 | 16 | 293.70 | 0.638 (0.312–1.308) | 0.220 | 0.433 (0.191–0.982) | 0.045 |
| EBV DNA (copies/ml) | ||||||||
| ≤ 3(undetectable) | 1324 | 8171.70 | 23 | 281.46 | Reference | Reference | ||
| > 3 (detectable) | 39 | 243.16 | 7 | 2878.70 | 10.476 (4.495–24.414) | < 0.0001 | 10.049 (4.227–23.887) | < 0.0001 |
| > 3–999 | 35 | 223.31 | 5 | 2239.01 | 8.123 (3.088–21.370) | 7.627 (2.83–20.558) | ||
| ≥ 1000 | 4 | 19.85 | 2 | 10,074.47 | 37.763 (8.890–160.414) | < 0.0001a | 39.789 (8.406–188.348) | < 0.0001 |
aP trend for the comparision among EBV DNA undetectable group, DNA levels at > 3–999 copies/ml and DNA levels ≥1000 copies/ml
b Maximum adjustment: all the aforementioned variables were used for adjustment including sex, age, smoking status, NPC family history, salted food,education level, EBV DNA
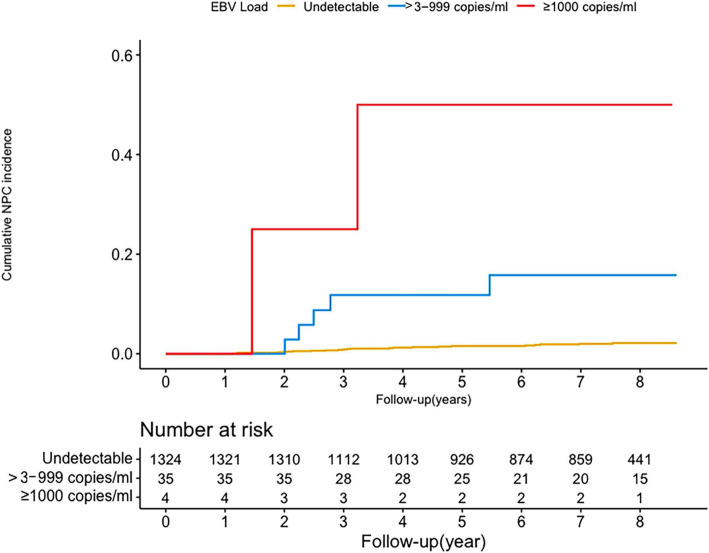
Figure 2 shows the cumulative incidences of NPC by plasma EBV DNA level during the follow-up period. There was a biological gradient trend of the cumulative NPC incidence with plasma EBV DNA levels increasing (P < 0.05). Approximately 50% of individuals with plasma EBV DNA loads ≥1000 copies/ml developed NPC during the follow-up period, compared with 14.29% of those with > 3–999 copies/ml and 1.74% with undetectable plasma EBV DNA loads (both P < 0.001). When we chose 500 copies/ml and 1500 copies/ml as the highest cutoff values, the same trends were found in the three grade groups with EBV DNA levels (both P < 0.001) (Supplementary Table 1).
Fig. 2.
Cumulative incidence of nasopharyngeal carcinoma by plasma EBV DNA levels. The cumulative incidence of NPC in the 1363 participants. The log-rank test shows that higher levels of plasma EBV DNA present higher risks of NPC development. a EBV DNA positive vs. undetectable (P < 0.001). b Comparison between EBV DNA undetectable, > 3 ~ 999 copies/ml, and ≥ 1000 copies/ml (P < 0.001)
NPC risk by plasma EBV DNA load
The NPC risks associated with different plasma EBV DNA levels are shown in Table 2. Compared with participants with undetectable EBV DNA levels, those with EBV DNA loads of > 3–999 copies/ml and ≥ 1000 copies/ml had crude HRs of 8.123 (95% CI: 3.088–21.370; P < 0.0001) and 37.763 (95% CI: 8.890–160.414; P < 0.0001), respectively. After full adjustment for potential risk factors, including age, sex, smoking, family history of NPC, education, and salted food intake, the same trend was found, with P < 0.0001. Meanwhile, we found that smoking, family history of NPC, and education level were also significantly associated with NPC development, and the adjusted HRs were of similar magnitude, as seen in multiple regression analyses (Table 2).
NPC risk by plasma EBV DNA level considering different follow-up durations
To our knowledge, plasma EBV DNA in NPC patients may originate from tumor cells. To minimize the potential reverse causality, we further evaluated the association of NPC risk with plasma EBV DNA levels after excluding all new cases diagnosed in the first 2, 3 or 4 years. Compared to the HR of NPC risk in participants with undetectable EBV DNA, a gradual decrease in HRs with detectable plasma EBV DNA was observed after excluding cases diagnosed in the first 2 (10.878, 95% CI: 4.215–28.072; P < 0.0001) or 3 years (5.139, 95% CI: 1.136–23.252; P = 0.0336). Moreover, the HR of NPC risk was further decreased to 4.054 (95% CI: 0.500–32.845; P = 0.1898) after excluding cases diagnosed in the first 4 years and became statistically nonsignificant (Table 3).
Table 3.
Predictive value of EBV DNA for NPC development by different follow-up duration
| Variables | > 2 years | > 3 years | > 4 years | |||
|---|---|---|---|---|---|---|
| Adjusted HR(95%CIs) | P-value | Adjusted HR(95%CIs) | P-value | Adjusted HR(95%CIs) | P-value | |
| ≤3(undetectable) | Reference | Reference | Reference | |||
| > 3 (detectable) | 10.878 (4.215–28.072) | < 0.0001a | 5.139 (1.136–23.252) | 0.0336a | 4.054 (0.500–32.845) | 0.1898a |
| > 3–999 | 9.637 (3.491–26.607) | < 0.0001b | 2.785 (0.359–21.619) | 0.3273b | 4.472 (0.552–36.257) | 0.3739b |
| ≥1000 | 30.111 (3.662–247.587) | 33.560 (3.996–281.872) | – |
aHRs are adjusted by sex, age, smoking status, NPC family history, salted food,education level; b P trend for the comparision among EBV DNA undetectable group, DNA levels at > 3–999 copies/ml and DNA levels ≥1000 copies/ml
Discussion
As plasma EBV DNA has been shown to be a promising indicator of NPC tumor presence and is widely used to predict NPC prognosis in clinical practice [15], we further evaluate its risk prediction value in the EBV seropositive individuals in an NPC screening cohort. Our prospective cohort study provides compelling evidence that plasma EBV DNA load is strongly associated with an increased risk of NPC over 3 years. The association is independent of several potential confounders, including age, sex, education level, family history of cancer, cigarette smoking, salted food intake. Moreover, the higher virus DNA load revealed a gradual increasing risk for NPC, with the cumulative NPC incidence increased gradually from 1.74% in individuals with plasma EBV DNA undetectable to 14.29% in those with > 3–999 copies/ml and 50% with ≥1000 copies/ml.
To the best of our knowledge, this study is the largest and longest prospective follow-up study on the association between plasma EBV DNA loads and NPC outcomes. All the evidence in this study suggest plasma EBV DNA can be used as a complementary approach to EBV serological antibody-based screening for NPC. Closer clinical monitoring and even more sensitive diagnostic examination should be recommended for these extremely high-risk individuals with both circulating EBV DNA and antibodies positive, especially in the first several years. Since magnetic resonance imaging (MRI) examination of nasopharynx has shown higher sensitivity to capture minor, early-stage NPC difficult to visualize by endoscopy and to maximize NPC detection within EBV-based NPC screening programs, using plasma EBV DNA as an auxiliary approach can minimize false seropositive screening test results and reduce cost burden associated with unnecessary referrals [20].
However, plasma EBV-DNA testing is not ideal as a primary screening tool. Even we utilized a cutoff at the LLOQ, only 21.8% NPC cases with EBV seropositive are detectable by plasma EBV DNA in this cohort, that is nearly 80% NPC cases would be missed. Similar to our result, several previous population-based cross-sectional screening studies have also reported the sensitivity of plasma EBV DNA (56.4–73.0%) is inferior to EBV antibody testing alone (> 90%) in NPC screening [21].
Our study has strengths and limitations. This study was based on a large population-based prospective screening cohort with a long-term follow-up and designed specifically to evaluate EBV-based serological screening for the early detection of NPC. In addition, the EBV seropositive population received active follow-up and comprehensive clinical examinations, thus largely reducing diagnostic bias and errors in this cohort. Nevertheless, several limitations to this study should also be mentioned. First, although this study was based on a longitudinal cohort, we did not retest and monitor the fluctuation pattern for EBV DNA during the follow-up period. We don’t know how many percentages of the participants had transient or chronic EBV infection at the time of testing. Monitoring the fluctuation pattern of plasma EBV DNA and NPC occurrence in a large cohort with longer follow-up is still needed in the future. Moreover, the association between plasma EBV DNA load and NPC onset was only investigated in a high-risk area, hence, our results might not be applicable to other areas. More prospective studies are warranted to confirm our findings in other regions.
In summary, we evaluate plasma EBV DNA loads and the risk of NPC development in a large-scale NPC screening program in an endemic area. This study indicates that plasma EBV DNA can predict an increased risk of NPC over 3 years and be used as a complementary approach to EBV serological antibody-based screening to improve NPC screening efficiency.
Supplementary Information
Acknowledgments
None.
Abbreviations
- NPC
Nasopharyngeal Carcinoma
- EBV
Epstein-Barr Virus
- IgA
Immunoglobulin A
- HR
Hazard Ratios
- CIs
Confidence Intervals
- IQR
Interquartile Range
Authors’ contributions
Sumei Cao designed the study and revised the manuscript. Wenjie Chen and Wenna Xu took part in designing and conducting the experiment. Wenjie Chen contributed to writing the manuscript. Haiyun Wang conceived and designed the experiment. Shanghang Xie and Dongfeng Lin did the main part of the experiment. Xiaoxia Chen and Xueqi Li analyzed the data and conducted the results. The authors read and approved the final manuscript.
Funding
South China Cohort of Chronic Diseases, Grant/Award Number (2017YFC0907102); Planned Science and Technology Project of Guangdong Province (2017A02021503, 2019B020230002); Heath&Medical Collaborate Innovation Project of Guangzhou City, China (201803040003); the National Key R&D Program of China, Grant/Award Number (2016YFC0902001); Sun Yat-Sen University Clinical Research 5010 Program, Grant/Award Number (2013012); the Natural Science Foundation of China (81872700, 82073625).
Availability of data and materials
Data and materials of this work are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
A written informed consent was obtained from each participant and this study was approved by the Ethics Review Committee of the Sun Yat-sen University Cancer Center (NCT00941538, Clinical Trials.gov).
Consent for publication
All the authors are approved of this submission.
Competing interests
None.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wen-Jie Chen and Wen-Na Xu contributed equally to this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4(10):757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 3.Lo KW, To KF. Huang DP. Focus on nasopharyngeal carcinoma. Cancer Cell. 2004;5(5):423–428. doi: 10.1016/S1535-6108(04)00119-9. [DOI] [PubMed] [Google Scholar]
- 4.Chang Y, Lee HH, Chang SS, Hsu TY, Wang PW, Chang YS, Takada K, Tsai CH. Induction of Epstein-Barr virus latent membrane protein 1 by a lytic transactivator Rta. J Virol. 2004;78(23):13028–13036. doi: 10.1128/JVI.78.23.13028-13036.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sivachandran N, Wang X, Frappier L. Functions of the Epstein-Barr virus EBNA1 protein in viral reactivation and lytic infection. J Virol. 2012;86(11):6146–6158. doi: 10.1128/JVI.00013-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young LS, Yap LF, Murray PG. Epstein-Barr virus: more than 50 years old and still providing surprises. Nat Rev Cancer. 2016;16(12):789–802. doi: 10.1038/nrc.2016.92. [DOI] [PubMed] [Google Scholar]
- 7.McKenzie J, El-Guindy A. Epstein-Barr virus lytic cycle reactivation. Curr Top Microbiol Immunol. 2015;391:237–261. doi: 10.1007/978-3-319-22834-1_8. [DOI] [PubMed] [Google Scholar]
- 8.Cao SM, Liu Z, Jia WH, Huang QH, Liu Q, Guo X, Huang TB, Ye W, Hong MH. Fluctuations of epstein-barr virus serological antibodies and risk for nasopharyngeal carcinoma: a prospective screening study with a 20-year follow-up. PLoS One. 2011;6(4):e19100. doi: 10.1371/journal.pone.0019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chien YC, Chen JY, Liu MY, Yang HI, Hsu MM, Chen CJ, Yang CS. Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N Engl J Med. 2001;345(26):1877–1882. doi: 10.1056/NEJMoa011610. [DOI] [PubMed] [Google Scholar]
- 10.Zhang XM, Zhong JM, Tang MZ, Zhang XG, Liao J, Zheng YM, Deng H, Zeng Y. Comparison of IgA/VCA, IgA/EA, IgG/EA in immunoenzyme methods and ZEBRA ELISA in early diagnosis of nasopharyngeal carcinoma. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2006;20(3):263–265. [PubMed] [Google Scholar]
- 11.Liu Y, Huang Q, Liu W, Liu Q, Jia W, Chang E, Chen F, Liu Z, Guo X, Mo H, Chen J, Rao D, Ye W, Cao S, Hong M. Establishment of VCA and EBNA1 IgA-based combination by enzyme-linked immunosorbent assay as preferred screening method for nasopharyngeal carcinoma: a two-stage design with a preliminary performance study and a mass screening in southern China. Int J Cancer. 2012;131(2):406–416. doi: 10.1002/ijc.26380. [DOI] [PubMed] [Google Scholar]
- 12.Wang WY, Twu CW, Chen HH, Jiang RS, Wu CT, Liang KL, Shih YT, Chen CC, Lin PJ, Liu YC, Lin JC. Long-term survival analysis of nasopharyngeal carcinoma by plasma Epstein-Barr virus DNA levels. Cancer. 2013;119(5):963–970. doi: 10.1002/cncr.27853. [DOI] [PubMed] [Google Scholar]
- 13.Pickard A, Chen CJ, Diehl SR, Liu MY, Cheng YJ, Hsu WL, Sun B, Hsu MM, Chen IH, Chen JY, Yang CS, Mittl BL, Chou SP, Ruggles DD, Goldstein AM, Hildesheim A: Epstein-Barr virus seroreactivity among unaffected individuals within high-risk nasopharyngeal carcinoma families in Taiwan. Int J Cancer 2004, 111(1):117–123, 1, DOI: 10.1002/ijc.20222. [DOI] [PubMed]
- 14.Chen GH, Liu Z, Ji MF, Pfeiffer RM, Huang QH, Lu YQ, et al. Prospective assessment of a nasopharyngeal carcinoma risk score in a population undergoing screening. Int J Cancer. 2021;148(10):2398-406. 10.1002/ijc.33424. [DOI] [PubMed]
- 15.Liu Z, Ji MF, Huang QH, Fang F, Liu Q, Jia WH, Guo X, Xie SH, Chen F, Liu Y, Mo HY, Liu WL, Yu YL, Cheng WM, Yang YY, Wu BH, Wei KR, Ling W, Lin X, Lin EH, Ye W, Hong MH, Zeng YX, Cao SM. Two Epstein-Barr virus-related serologic antibody tests in nasopharyngeal carcinoma screening: results from the initial phase of a cluster randomized controlled trial in southern China. Am J Epidemiol. 2013;177(3):242–250. doi: 10.1093/aje/kws404. [DOI] [PubMed] [Google Scholar]
- 16.Ji MF, Sheng W, Cheng WM, Ng MH, Wu BH, Yu X, Wei KR, Li FG, Lian SF, Wang PP, Quan W, Deng L, Li XH, Liu XD, Xie YL, Huang SJ, Ge SX, Huang SL, Liang XJ, He SM, Huang HW, Xia SL, Ng PS, Chen HL, Xie SH, Liu Q, Hong MH, Ma J, Yuan Y, Xia NS, Zhang J, Cao SM. Incidence and mortality of nasopharyngeal carcinoma: interim analysis of a cluster randomized controlled screening trial (PRO-NPC-001) in southern China. Ann Oncol. 2019;30(10):1630–1637. doi: 10.1093/annonc/mdz231. [DOI] [PubMed] [Google Scholar]
- 17.Hu T, Lin CY, Xie SH, Chen GH, Lu YQ, Ling W, Huang QH, Liu Q, Cao SM. Smoking can increase nasopharyngeal carcinoma risk by repeatedly reactivating Epstein-Barr virus: an analysis of a prospective study in southern China. Cancer Med. 2019;8(5):2561–2571. doi: 10.1002/cam4.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao JY, Li YH, Gao HY, Wu QL, Cui NJ, Zhang L, Cheng G, Hu LF, Ernberg I, Zeng YX. Comparison of plasma Epstein-Barr virus (EBV) DNA levels and serum EBV immunoglobulin a/virus capsid antigen antibody titers in patients with nasopharyngeal carcinoma. Cancer. 2004;100(6):1162–1170. doi: 10.1002/cncr.20099. [DOI] [PubMed] [Google Scholar]
- 19.Shanmugaratnam K, Sobin LH. The World Health Organization histological classification of tumours of the upper respiratory tract and ear. A commentary on the second edition. Cancer. 1993;71(8):2689–2697. doi: 10.1002/1097-0142(19930415)71:8<2689::AID-CNCR2820710843>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 20.King AD, Woo JKS, Ai QY, Chan JSM, Lam WKJ, Tse IOL, Bhatia KS, Zee BCY, Hui EP, Ma BBY, Chiu RWK, van Hasselt AC, Chan ATC, Lo YMD, Chan KCA. Complementary roles of MRI and endoscopic examination in the early detection of nasopharyngeal carcinoma. Ann Oncol. 2019;30(6):977–982. doi: 10.1093/annonc/mdz106. [DOI] [PubMed] [Google Scholar]
- 21.Liu W, Chen G, Gong X, Wang Y, Zheng Y, Liao X, Liao W, Song L, Xu J, Zhang X. The diagnostic value of EBV-DNA and EBV-related antibodies detection for nasopharyngeal carcinoma: a meta-analysis. Cancer Cell Int. 2021;21(1):164. doi: 10.1186/s12935-021-01862-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and materials of this work are available from the corresponding author on reasonable request.