Abstract
Background
Adequate transplacental passage of maternal thyroid hormone is important for normal fetal growth and development. Maternal overt hypothyroidism and hyperthyroidism are associated with low birth weight but there are still important knowledge gaps regarding the impact of subclinical thyroid function test abnormalities on birth weight, in general or during the late second and third trimester of pregnancy, remains unknown. The aim of this study was to examine associations of maternal thyroid function with birth weight.
Methods
For this individual-participant data meta-analysis we searched Medline (Ovid), Embase.com, Web-of-Science, Cochrane CENTRAL and Google Scholar from inception to March 18th 2018, and published open invitations to join the Consortium on Thyroid and Pregnancy, to identify prospective cohort studies with data on maternal thyroid function during pregnancy and birth weight. We excluded participants with multiple pregnancies, in vitro fertilization, pre-existing thyroid disease or thyroid medication usage, miscarriages and stillbirth. Main outcomes were small for gestational age (SGA), large for gestational age (LGA) (defined by the lowest or highest 10th population-specific percentile of birth weight standardized to gestational age and sex, respectively) and birth weight. We analysed individual participant data using mixed-effects regression models adjusting for maternal age, body mass index, ethnicity, smoking, parity, gestational age at blood sampling, fetal sex and gestational age at birth (the latter two only in case of birth weight continuously). The study protocol was pre-registered at the International Prospective Register of Systematic Reviews (PROSPERO), number CRD42016043496.
Results
From 2,526 published reports, 36 cohorts met the inclusion criteria and were invited to participate of which 15 agreed and after addition of 5 unpublished datasets, a total of 20 cohorts were included. After exclusions, the study population comprised 48,145 mother-child pairs of whom 1,275 (3·1%) had subclinical hypothyroidism (increased TSH with normal FT4) and 929 (2·2%) had isolated hypothyroxinaemia (decreased FT4 with normal TSH). Maternal subclinical hypothyroidism was associated with a higher risk of SGA compared to euthyroidism (11·8% vs. 10·0% respectively, adjusted risk difference 2·4% [95% CI, 0·4 to 4·8]; odds ratio (OR) 1·24 [95% CI 1·04 to 1.48], P=0·015) and lower mean birth weight (adjusted risk difference −38g [95% CI −61 to −15], P=0·001) with a higher effect estimate for measurement in the 3rd trimester compared with the 1st or 2nd trimester. Isolated hypothyroxinaemia was associated with a lower risk of SGA compared to euthyroidism (7·3% vs. 10·0%, adjusted risk difference −2·9 [95% CI, −4·5 to −0·9]; OR, 0·70 [95% CI 0·55 to 0·91], P=0·007) and higher mean birth weight (difference, 45g [95% CI 18 to 73], P=0·001). Each 1-SD higher maternal TSH concentration was associated with lower birth weight (−6g [−10 to −2], per SD, P=0·003), with higher effect estimates in TPOAb-positive than TPO-negative women (P for interaction=0·10). Each 1-SD higher FT4 concentration was associated with lower birth weight (−21g [95% CI −25 to −17] per SD, P<0·0001), with a higher effect estimate for measurement in the 3rd trimester compared with the 1st or 2nd trimester.
Interpretation
Maternal subclinical hypothyroidism in pregnancy is associated with a higher risk of SGA and lower birth weight, whereas isolated hypothyroxinaemia is associated with lower risk of SGA and higher birth weight. There was an inverse, dose-response association of maternal TSH and FT4 (even within the normal range) with birth weight. These results advance our understanding of the complex relationships between maternal thyroid function and fetal outcomes, and should prompt careful consideration of potential risks as well as benefits of levothyroxine therapy during pregnancy.
Introduction
Birth weight is an important marker of fetal growth, development, nutrition and other in utero exposures. Low birth weight or being born small for gestational age (SGA) are major risk factors for neonatal mortality and morbidity, and are associated with a higher risk of non-communicable diseases in later life.1–4 In contrast, being large for gestational age (LGA) is a risk factor for caesarean section, postpartum haemorrhage, new-born hypoglycaemia and obesity in later life.5–7 Thyroid hormone regulates fetal growth and development throughout gestation. Fetal thyroid hormone availability largely depends on the placental transfer of maternal thyroid hormone, particularly during the first 18–20 weeks of pregnancy.8 Overt maternal thyroid disease such as hypothyroidism or (pre-existing) Graves’ hyperthyroidism are well known risk factors for SGA and occur in 0·2% to 1% of pregnancies.8 Milder thyroid function test abnormalities such as subclinical hypothyroidism, hypothyroxinaemia and subclinical hyperthyroidism are up to ten times more frequent. Because some studies showed that mild thyroid function test abnormalities are associated with SGA or LGA9–12 but others did not13,14, it remains to be elucidated whether, or to what extent these are risk factors for SGA or LGA.
Levothyroxine is commonly prescribed during pregnancy in all parts of the world for treatment of overt thyroid dysfunction.15–19 To date, it remains common practice to titrate levothyroxine therapy to high-normal free thyroxine (FT4) concentrations and/or low-normal TSH concentrations as the potential benefits are believed to outweigh potential harms, although the evidence for this remains poor. However, some observational studies suggest that already high-normal FT4 concentrations are associated with impaired fetal growth and lower birth weight, suggesting that levothyroxine treatment comes with the potential risk of overtreatment.20–25 The guidelines of the American Thyroid Association indicate that treatment can be considered for mild thyroid function test abnormalities such as subclinical hypothyroidism or for thyroid peroxidase antibody (TPOAb) positive women with a TSH above 2·5 mU/L, and is recommended for TPOAb positive women when the TSH is >4 mU/L and for all women when the TSH is >10 mU/L.26 Overall, previous observational studies on the association of mild thyroid function test abnormalities with birth weight show conflicting results10,26–32 and most randomized trials of levothyroxine treatment do not report any differences.33–37 Thus far, the interpretation of individual observational studies and randomized trials has been limited by the relatively small sample sizes and use of widely varying definitions of an abnormal thyroid function test. The latter, in combination with different definition of birth weight outcomes, different analysis approaches and scarcity of reporting results for isolated hypothyroxinaemia or continuous association of thyroid function with birth weight, limits the capabilities of an aggregate data meta-analysis. Furthermore, most studies have focused on early pregnancy, and both the clinical relevance of mild thyroid function test abnormalities as well as treatment aims for the second half of pregnancy remain to be elucidated.
The aim of this study was to investigate the associations of maternal thyroid function tests with SGA, LGA and birth weight.
Methods
The Consortium on Thyroid and Pregnancy is a collaboration of prospective birth cohorts that aims to study the association of maternal thyroid function and autoimmunity with adverse pregnancy and child outcomes.38 For the current study, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Individual Patient Data guidelines and pre-registered our study protocol (PROSPERO ID CRD42016043496, appendix p3–6). To identify studies for inclusion, we conducted a systematic search of literature for the publications on the association of thyroid function or autoimmunity with birth weight, published from inception to March 18th 2018, with no language restrictions using several databases (Medline (Ovid), Embase.com, Web-of-Science, Cochrane CENTRAL and Google Scholar, detailed search terms and strategy are in the appendix p7–8). We included cohort studies in which data was collected prospectively and that consecutively included participants from the general population and/or without active selection based on health status and had either TSH or FT4 measurements and data on birth weight available. We excluded studies in which participants received treatment based on (abnormal) thyroid function tests (predominantly hospital-based cohorts) or studies that only included women with (overt) thyroid disease. Possible studies for inclusion were independently assessed for suitability by two authors (TIMK and PNT) and any disagreement was resolved by discussion with a third author (RPP). Investigators from each eligible study were invited to join the consortium using the contact details on the identified reports; if unsuccessful we used contact details of other published studies, contacted their co-authors or department. Upon participation, we collected individual-participant data using a standardized codebook. Quality of the studies and risk of bias was assessed using the Newcastle-Ottawa scale. All cohorts were approved by a local review board and acquired participant informed consent or had been granted exemption from it by the local Ethics Committee. All participants with a measurement of TSH, FT4 or TPOAb (first available) and birth weight were included; any data on thyroglobulin antibodies (TgAb) was collected upon availability. We excluded participants with a miscarriage/stillbirth, pre-existing thyroid disease or thyroid-interfering medication usage, IVF treatment or twin pregnancies. See appendix p8 for more details on methods.
Exposures
Exposures included subclinical thyroid function test abnormalities, continuous thyroid function test measurements (TSH and FT4), TPOAb and/or TgAb positivity. Overt hyperthyroidism was considered as a subclinical disease entity, considering the transient nature and lack of indication for treatment with anti-thyroid drugs of this biochemically defined entity. We did not have data on TSH receptor antibodies or undiagnosed Graves’ disease, however, we considered this unlikely to affect our results given the prevalence of approximately 0.05%. We did not study the association of overt hypothyroidism with birth weight because treatment for this disease entity is non-controversial, and because the very low prevalence in combination with a relatively large number of women excluded because of pre-existing thyroid disease indicates that women with true overt hypothyroidism are only selectively represented in the included studies. Thyroid function test abnormalities were defined according to cohort-specific 2·5th and 97·5th percentiles for TSH and FT4, in cohorts with TPOAb data, after exclusion of TPOAb positive women. Subclinical hypothyroidism was defined as TSH above the 97·5th percentile and a FT4 within the normal range (2·5th–97·5th percentile). Overt hyperthyroidism was defined as TSH below the 2·5th percentile and a FT4 above the 97·5th percentile. Subclinical hyperthyroidism was defined as a TSH below the 2·5th percentile and a FT4 within the normal range. Isolated hypothyroxinaemia was defined as a FT4 below the 2·5th percentile and a TSH within the normal range. TPOAb and TgAb positivity were based on cohort-specific cut-offs. For continuous TSH and FT4 concentrations as exposure variables, concentrations for all cohorts were log-transformed and then converted to population-specific standard deviation (SD) scores after removal of outliers (+/− 4 SD from the mean) to enable comparison between different cohorts and assays.
Outcomes
The primary outcomes were SGA, LGA and birth weight (as a continuous variable). To define SGA and LGA, birth weight was standardized according to gestational age at birth and fetal sex per cohort. SGA was defined as a standardized birth weight below the 10th cohort-specific percentile, and LGA as a standardized birth weight above the 90th cohort-specific percentile, according to the definition of the World Health Organization.39 Secondary outcomes were low birth weight (LBW; birth weight below 2,500 grams) and macrosomia (birth weight above 4,000 grams).
Statistical analyses
Datasets were merged and all analyses were performed according to the protocol, unless stated otherwise (appendix p8–9). We used linear mixed effect regression models with a random intercept for each cohort to study the association of thyroid function test abnormalities (compared with euthyroidism), TSH, FT4 concentrations or TPOAb, TgAb positivity with birth weight. We used generalized logistic mixed regression models with a random intercept for each cohort to study the association of thyroid function test abnormalities (compared with euthyroidism), TSH, FT4 concentrations or TPOAb, TgAb positivity with SGA, LGA, LBW and macrosomia. All analyses of primary outcomes were also performed using a two-step approach with random-effect models according to DerSimonian and Laird to pool estimates of the cohorts and assess heterogeneity across studies using the I2 statistic and 95% confidence interval. We evaluated for potential publication bias using funnel plots and Egger tests. All models were adjusted for maternal age, BMI, ethnicity, smoking, parity, gestational age at blood sampling, fetal sex and gestational age at birth (the latter two in case of birth weight, LBW and macrosomia outcomes only). To assess the effects of potential confounding, we ran crude models for the primary analysis. Risk differences and corresponding 95% CIs were calculated according to Newcombe and Bender, taking into account baseline risk imprecision calculated using the Wilson score method, and were adjusted for covariates and could thus deviate from non-adjusted percentages.40 We used multilevel multiple imputation for missing data on covariates creating five imputed datasets for pooled analyses.41 See appendix p8–9 for more details on statistical analysis and list of sensitivity analyses.
All statistical analyses were performed using R statistical software version 3.5.1 (R Development Core Team (2018), Vienna, Austria; packages lme4, mice, micemd, metafor, sjPlot).
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
We identified 2,526 reports of which 133 were eligible for inclusion based on title and abstract screening (Figure 1). After reading full texts, and addition of five cohorts identified via personal contacts and open invitations, a total of 36 cohorts were invited to participate. Subsequently, 20 cohorts from Europe, USA, Chile, Pakistan, Japan and Australia responded to our invitation and were able to participate. After exclusions, the final study population included 48,145 participants (Figure 1) with a mean birth weight of 3,400 (SD 536) grams and median gestational age at birth of 39·9 (95% range: 35·5 to 42·0) weeks; 4,771 new-borns were born SGA (9·8%) and 4,736 (9·7%) were born LGA (Table). Subclinical hypothyroidism occurred in 1,275 women (3·1%), isolated hypothyroxinaemia occurred in 929 women (2·2%) out of 41,564 from 17 cohorts with available data on TPOAbs. Cohort-specific characteristics are provided in the appendix p10–17. Compared with participants included in the study, women who were not included because of missing data on birth weight had similar TSH and FT4 concentrations, but a higher rate of TPOAb positivity (12·6% vs. 7·5 %, P=0·0005; appendix p18).
Figure 1.
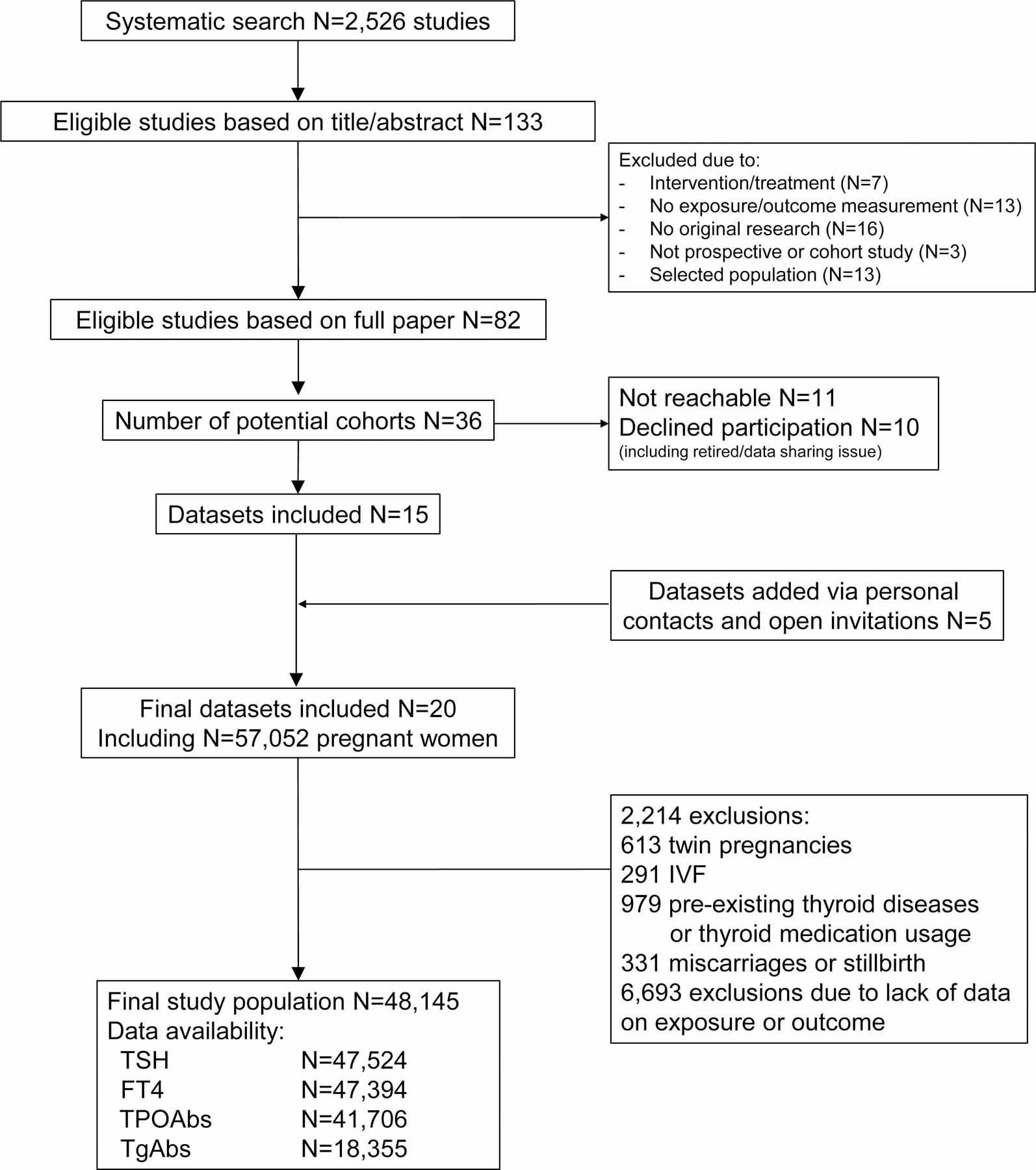
Flowchart of the study and participant selections
Table.
Characteristics of the total study population (N=48,145*)
| Maternal characteristics | |
| Age, years | 29·0 (5·1) [N=47,661] |
| Gestational age at the time of sampling, weeks | 12·8 (7·0 to 39·7) [N= 47,979] |
| BMI, kg/m2 | 24·0 (4·4) [N=32,586†] |
| Parity, N (%) | |
| 0 | 24,589 (51·1) |
| 1 | 13,805 (28·7) |
| 2 | 4,400 (9·1) |
| ≥3 | 2,486 (5·2) |
| Missing† | 2,865 (6.0) |
| Smoking status, N (%) | |
| Non/past smoker | 39,788 (82·6) |
| Current smoker | 5,038 (10·5) |
| Missing† | 3,319 (6.9) |
| Education, N (%) | |
| Low | 10,085 (20·9) |
| Medium | 11,122 (23.1) |
| High | 11,744 (24·4) |
| Missing† | 15,194 (31.6) |
| Maternal test results | |
| TSH (mU/L) | 1·32 (0·13–4·50) [N=47,524] |
| FT4 (pmol/L) | 13·0 (7·3–21·9) [N=47,394] |
| TPOAb positivity, N (%) | 3,128 (7·5) [N=41,706] |
| TgAb positivity, N (%) | 1,063 (5·8) [N=18,355] |
| Child characteristics | |
| Birth weight, grams | 3,400 (536) |
| Small for gestational age, N (%) | 4,771 (9·9) |
| Large for gestational age, N (%) | 4,736 (9·8) |
| Gestational age at birth, weeks | 39·9 (35·5–42·0) |
| Sex, N (%) | [N=37,181] |
| Female | 19,644 (40.8) |
| Male | 19,054 (39.6) |
| Missing† | 9,447 (19.6) |
Table shows descriptive statistics of the characteristics of all included women as the mean (SD), median (95% range) or count (percentage), as appropriate. Cohort-specific descriptive characteristics are shown in appendix p10–13.
Number of participants with available data on either of thyroid function tests, unless otherwise indicated.
Missing mostly due to lack of data from one or some cohorts.
For detailed description of missing data of covariates per cohort see appendix p14.
Compared with euthyroidism, maternal subclinical hypothyroidism was associated with a higher risk of SGA (11·8% vs 10·0%, adjusted risk difference 2·4 % [95% CI, 0·43 to 4·8]; odds ratio [95% CI]: 1·24 [1·04 to 1·48, P=0·015]; Figure 2A) and lower mean birth weight (estimated mean difference −38g [95% CI −61 to −1]; Figure 2C). Isolated hypothyroxinaemia was associated with a lower risk of SGA (7·3% vs 10·0%, adjusted risk difference −2·9 % [95% CI, −4·5 to −0·9]; odds ratio [95% CI]: 0·70 [0·55 to 0·91, P=0·007]; Figure 2A) and higher mean birth weight (estimated mean difference 45g [95% CI 18 to 73]; Figure 2C). Subclinical hyperthyroidism and overt hyperthyroidism were not associated with SGA or birth weight (Figure 2A), and there was no association of thyroid function test abnormalities with LGA (Figure 2B).
Figure 2.
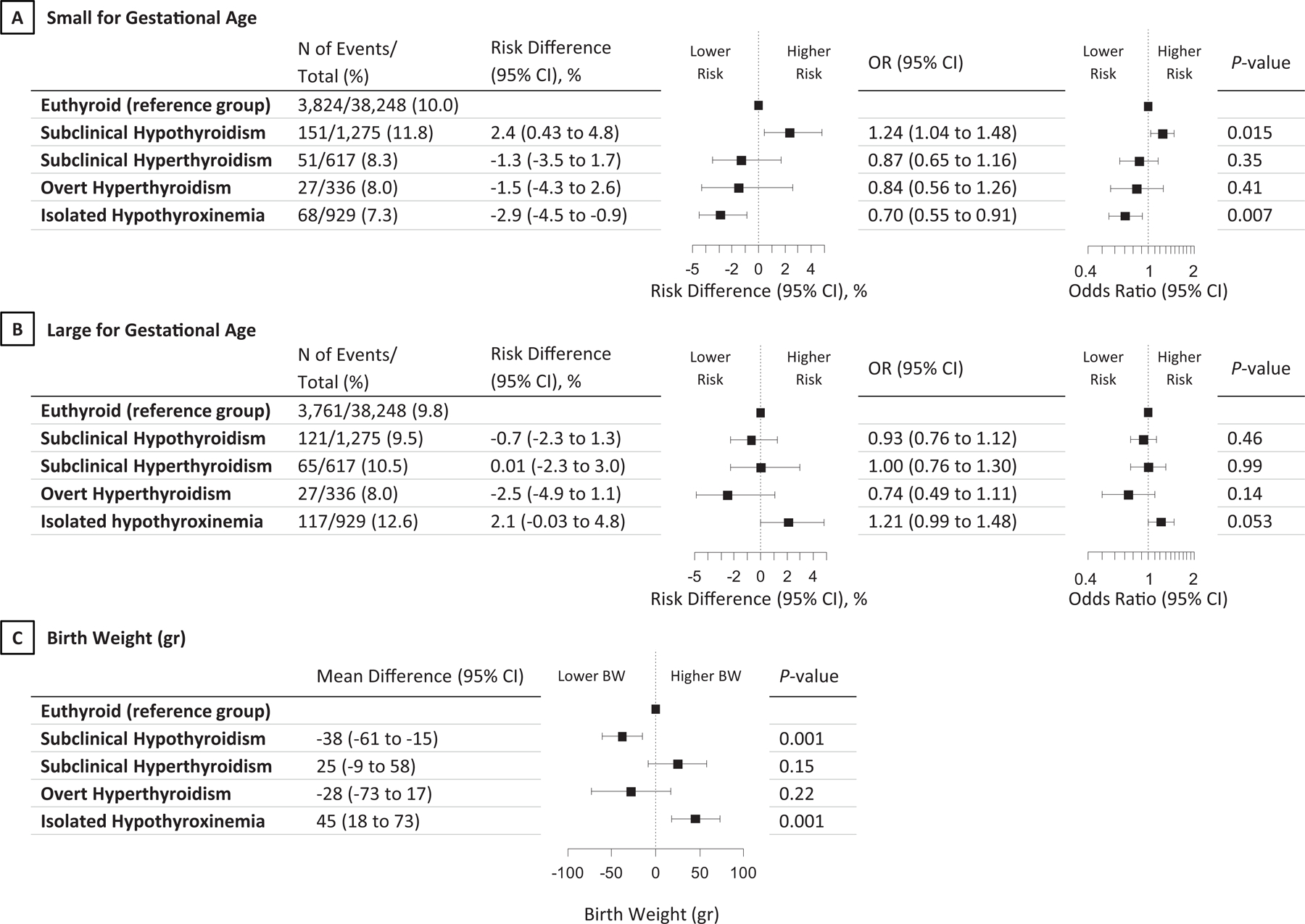
Association of thyroid function test abnormalities with small or large for gestational age and birth weight.
All analyses were adjusted for maternal age, BMI, ethnicity, smoking, parity, gestational age at blood sampling, fetal sex and gestational age at birth (the latter two for birth weight only). Risk differences and 95% CIs were back-calculated from the results of multivariable models and adjusted for baseline risk imprecision.
When analysed as a continuous variable, each 1-SD higher maternal TSH concentration was associated with a higher risk of SGA (OR 1·05 [95% CI 1·01 to 1·08] per SD, Figure 3A) and lower mean birth weight (−6g [95% CI −10 to −2] per SD, Figure 3B). Each 1-SD higher FT4 concentration was associated with a higher risk of SGA (OR 1·07 [95% CI 1·0 to 1·11] per SD, Figure 3A), a lower risk of LGA (OR 0·91 [95% CI 0·88 to 0·94] per SD, Figure 3A) and lower mean birth weight (−21g [95%CI −25 to −17] per SD, Figure 3B). When considered across the full FT4 range, the approximated difference in birth weight was ~200 grams (Figure 3B). Effect estimates remained similar when analyses were confined to TSH or FT4 concentrations within the normal range (Figure 3B). TPOAb and TgAb positivity were not associated with SGA, LGA or birth weight (appendix p19).
Figure 3.
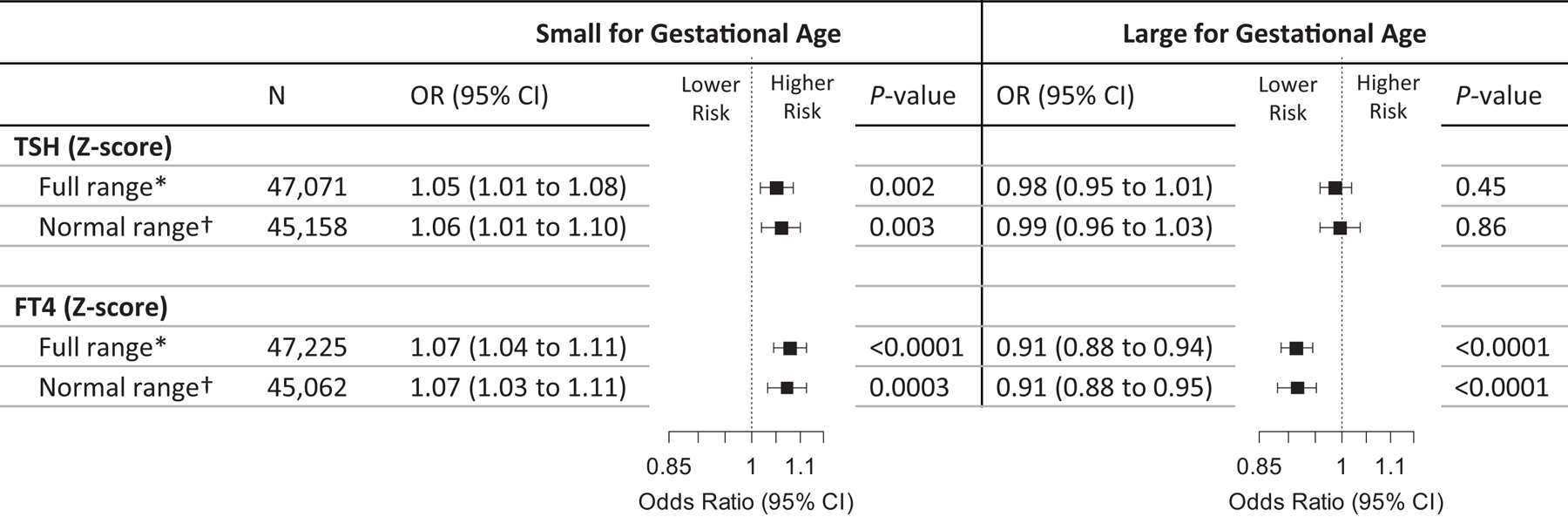
Association of TSH and FT4 concentrations with small or large for gestational age and birth weight.
Figures show the association of maternal TSH and FT4 in full range or within the normal range (2.5th–97.5th percentiles) with small or large for gestational age (panel A) and birth weight in grams (panel B). All analyses were adjusted for maternal age, BMI, ethnicity, smoking, parity, gestational age at blood sampling, fetal sex and gestational age at birth (the latter two for birth weight only).
* After exclusion of outliers of TSH (n=453) or FT4 (n=169).
† Normal range (2.5th–97.5th percentiles) is defined based on cohort-specific absolute measurements of TSH or FT4, which in the standardized data corresponds to TSH Z-score range of −4.2 to 1.8 and FT4 Z-score range of −2.2 to 2.5.
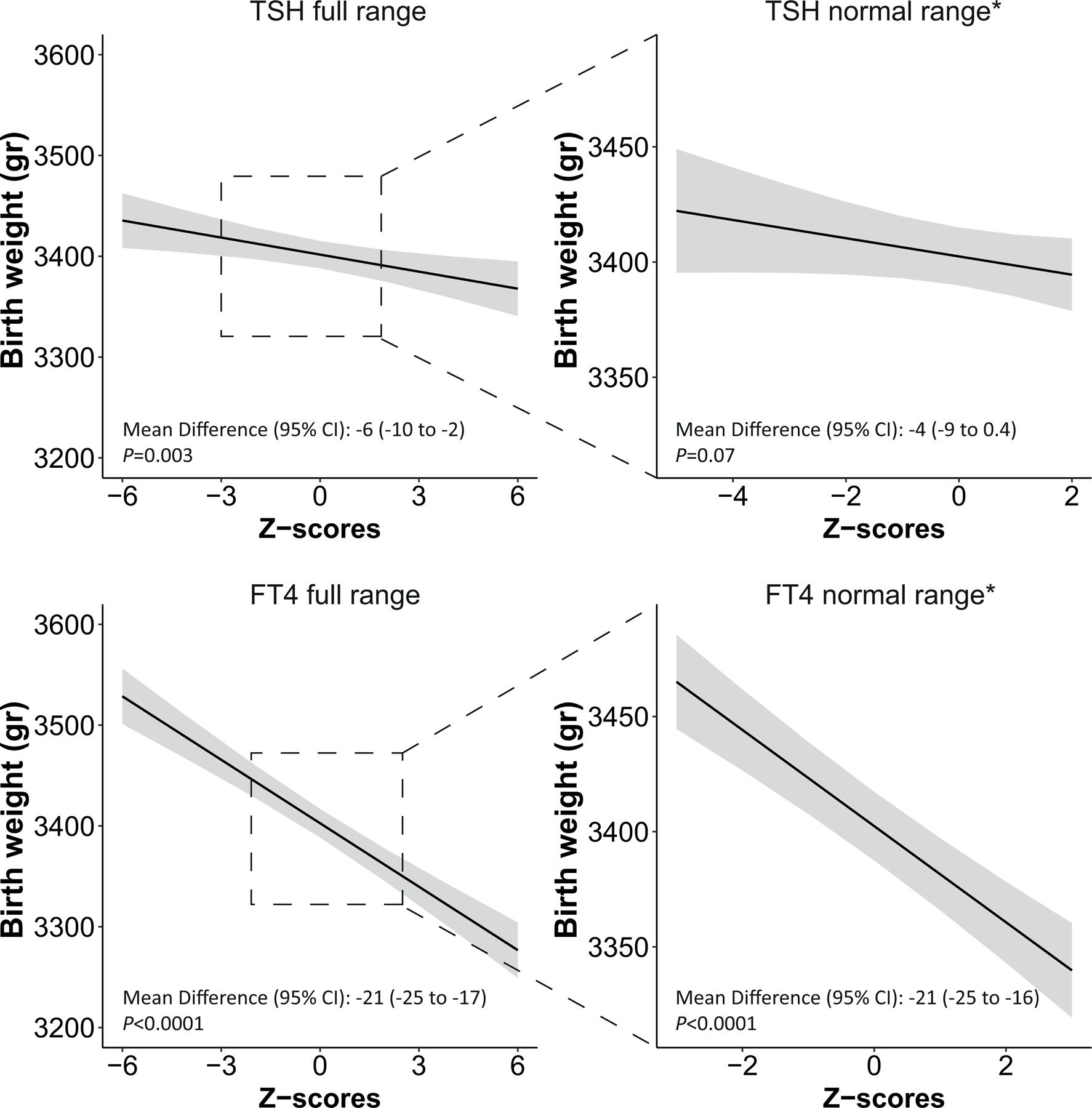
The association of FT4 with birth weight differed according to the gestational age at blood sampling (P for interaction=0·0002). Subsequent stratified analyses showed that the effect estimates of the association of FT4 with birth weight were 2 and 3-times larger when the FT4 concentration was measured during the 2nd or 3rd trimesters compared to the 1st trimester (β [95% CI] for birth weight: −13, −22 and −36 in 1st, 2nd and 3rd trimesters, respectively; Figure 4, appendix p21). Also, estimates of the association of subclinical hypothyroidism with birth weight measured in 2nd and 3rd trimesters were 2 and 5 times larger compared to 1st trimester (β [95% CI] for birth weight: −20, −33 and −75 in 1st, 2nd and 3rd trimesters, respectively; appendix p21).
Figure 4.
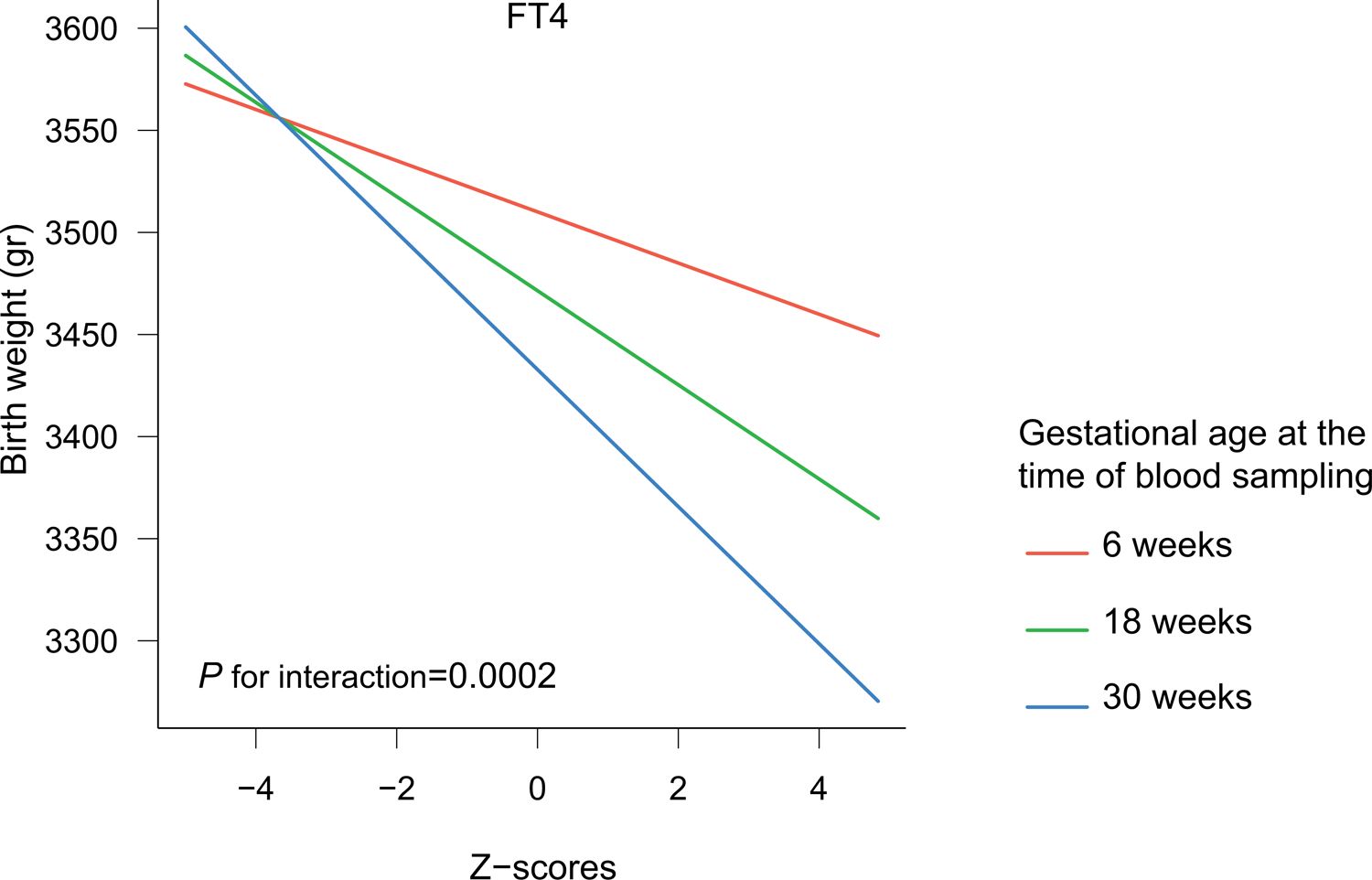
Association of FT4 Z-scores with birth weight according to gestational age at the time of sampling.
Figure shows the association of FT4 Z-scores with birth weight (grams) stratified by gestational age at the time of sampling. The analysis was adjusted for maternal age, BMI, ethnicity, smoking, parity, gestational age at blood sampling and fetal sex and gestational age at birth.
There was evidence that the association of TSH and FT4 with birth weight differed according to TPOAb status (P for interaction=0·10 and 0·09, respectively). In the subsequent stratified analysis, effect estimates of the negative association of TSH with birth weight were 4-times higher for TPOAb positive women than TPOAb negative women (β [95% CI] −17g [−32 to −2·5] per SD vs. −4.7g [−9 to −0·2] per SD, respectively; appendix p24&37). In contrast, for FT4, the negative effect estimate of the association with birth weight in TPOAb positive women was almost half the estimate for TPOAb negative women (β [95% CI] −10 [−25 to 4] per SD vs. −21 [−26 to −17] per SD, respectively; appendix p24&37).
There was evidence that association of FT4 with birth weight differed according to maternal age and BMI (P for interaction 0·078 and 0·003, respectively) but not fetal sex or smoking status (appendix p20). When stratified by maternal age there was not a meaningful difference in the association of FT4 with birth weight between the two groups (β [95% CI] for birth weight: −22 [−27 to −17] and −19 [−25 to −13] for maternal age <30 or ≥30 years, respectively; appendix p22). Stratified analyses showed that the negative effect estimate of the association of FT4 with birth weight was larger in women with a BMI ≥30 kg/m2 compared to those with a BMI of 18–25 kg/m2 (appendix p23). There was also evidence that the association of TSH with birth weight differed according to maternal age (P for interaction=0.11) but not gestational age at the time of sampling, fetal sex, BMI, or smoking (appendix p20). When stratified by maternal age, the negative association of TSH with birth weight was nearly 4-times higher for women aged ≥30 years compared to <30 (appendix p22). Results of analyses on low birth weight or macrosomia yielded results similar to those of SGA or LGA (appendix p25–26). Additional adjustment for gestational diabetes mellitus or preeclampsia did not change the results (appendix p27–32). Results of the crude analyses can be found in appendix p33–34. Using Newcastle-Ottawa Quality Assessment Scales we did not identify any risk of bias in the included cohorts (appendix p35–36). Results of two-step meta-analyses were similar to one-step analyses with I2 statistics ranging between 0 to 51·5% indicative of low to moderate heterogeneity. Moreover, funnel plots did not indicate publication bias or unexpected differences in effect estimates between the included studies (appendix p38–45).
Discussion
In this individual participant data meta-analysis, we show that, compared to euthyroidism, maternal subclinical hypothyroidism during pregnancy is a risk factor for SGA and is associated with lower birth weight. By contrast, isolated hypothyroxinaemia was associated with higher birth weight but not LGA. Maternal TSH and FT4 concentrations were both inversely associated with birth weight, with the association of FT4 being most apparent during later pregnancy, whereas the association of TSH with birth weight was most apparent in TPOAb-positive women.
One of the main results of this study is that higher FT4 concentrations are associated with lower birth weight, even within the normal range. For TSH concentrations, the associations with birth weight were less evident and not present within the normal range. These continuous analyses can be interpreted in various ways. First, together with results from other studies, the negative association of maternal FT4 with birth weight in this study can strengthen hypotheses about the effects of thyroid hormone on the developing fetus. Since circulating maternal FT4 crosses the placenta, and maternal FT4 concentrations are correlated with new-born FT4 concentrations,42,43 the negative association of maternal FT4 with birth weight could reflect a direct thyroid hormone effect. The negative dose-dependent association of FT4 with birth weight can also be further extrapolated to fetal growth restriction typically seen in pregnancies complicated by Graves’ hyperthyroidism.44,45 We hypothesize that such an effect is mediated by an increase in new-born lipid and protein catabolism causing a reduction in caloric availability, which could be further complicated by a higher placenta vascular resistance.46,47 Yet, the point estimates for overt hyperthyroidism in this study warrant further studies, although it is possible that the association of FT4 concentrations with birth weight is partly caused by high hCG concentrations. Overt hyperthyroidism in the current study may reflect transient gestational thyrotoxicosis rather than Graves’ hyperthyroidism, and high hCG concentrations have been associated with a higher birth weight.48 An alternative underlying mechanism could be through lower T3, as a recent study showed that lower maternal T3 is associated with lower birth weight.22 Although it remains to be elucidated whether T3 passes the placenta, further studies on the association of maternal T3 concentrations with pregnancy outcomes seem warranted also because T3 concentrations are lower in individuals treated with levothyroxine therapy.26
Second, the continuous analyses for FT4 and TSH support the associations identified for isolated hypothyroxinaemia in this study, since both lower FT4 concentrations as well as isolated hypothyroxinaemia were associated with higher birth weight. Interestingly, the association of subclinical hypothyroidism with birth weight was in the opposite direction of the association of continuous FT4 and isolated hypothyroxinaemia, and TSH in the normal range (as is the case for isolated hypothyroxinemia) was not associated with birth weight. This suggests important differences in the underlying pathophysiological mechanisms. Subclinical hypothyroidism is more common in TPOAb positive women and likely reflects a lower thyroid functional capacity. The latter is reflected by a considerable attenuation of the hCG-mediated increase in FT4 and decrease in TSH concentrations in women with subclinical hypothyroidism as compared to euthyroid women.49 On the other hand, neither TPOAb positivity nor an impaired thyroidal response to hCG seem to play a role in women with isolated hypothyroxinaemia.49 We speculate that isolated hypothyroxinaemia is a thyroid function test abnormality that is specific for pregnancy and may not necessarily represent thyroid gland hypofunction.8 It has also been suggested that minor aberrations of thyroid function during pregnancy may arise from dysfunction of the uteroplacental unit, rather than from thyroid dysfunction.50 Further studies are required to elucidate the underlying physiology of such gestational thyroid function test abnormalities.
Thyroid hormone regulates different metabolic and anabolic processes in both mother and fetus throughout gestation. It controls fetal growth by facilitating placentation and regulation of metabolism, fetal glucose and oxygen consumption as well as other co-factors directly affecting skeletal growth, tissue differentiation and accretion.51–53 One of the sensitivity analyses in this study showed that the negative association of FT4 with birth weight is amplified during the 2nd and 3rd trimester. These differences most likely reflect an amplification of the metabolic effect of thyroid hormone on fetal growth due to an increased fetal nutritional demand and increased fetal growth rate associated with the progression of pregnancy.51,54 Our results indicate that maternal thyroid function also during later pregnancy is a relevant determinant of fetal development. These results highlight the relevance of follow-up thyroid function testing when levothyroxine therapy is started during early pregnancy and warrant further studies preferably utilizing repeated thyroid function tests.
Worldwide, levothyroxine is one of the most often prescribed drugs during pregnancy 15–19, and the dosage is commonly targeted to achieve high-normal FT4 concentrations. The extrapolation of data from population-based studies for defining treatment targets remains valuable, as is reflected by recent data on levothyroxine overtreatment and child neurocognition.8,55 This study in untreated, otherwise healthy women shows that a higher maternal FT4 concentration within the normal range is associated with lower birth weight and a higher risk of SGA. This suggests that levothyroxine therapy comes with a potential risk of overtreatment, especially when targeting high-normal FT4 concentrations. Consistent with the results of this study, a recent randomized trial showed that low-dose levothyroxine treatment of either subclinical hypothyroidism or isolated hypothyroxinaemia was associated with a higher risk of SGA, albeit statistically non-significant (for subclinical hypothyroidism, levothyroxine 10% vs placebo 8%; for isolated hypothyroxinaemia, levothyroxine 9% vs placebo 8%).37 Further studies are needed to investigate whether the changes in TSH or FT4 concentrations that occur during levothyroxine therapy in pregnancy are related to treatment benefits and/or harms and differ per underlying thyroid function test abnormality.
Strengths and limitations
In the current study, we were able to utilize detailed individual-participant data on thyroid function, birth weight and potential confounders from 19 prospective, population-based cohorts, allowing standardization of the definition of thyroid function test abnormalities and analysing potential dose-dependent associations. One of the limitations of the current study is the interpretation of the results on overt hyperthyroidism, since we had limited statistical power for this group and TSH receptor antibody concentrations were not available. Secondly, the interpretation of the results could be affected by pregnancy-related changes in thyroid binding proteins that could interfere with FT4 immunoassays. However, gestational changes in FT4 concentrations as assessed by immunoassays are highly similar to those measured with liquid chromatography-mass spectrometry or equilibrium dialysis.56,57 Thirdly, we could not invite 5 studies that were published while conducting statistical analyses for the current study (appendix p8) and that we could not collect data on previous stillbirths, renal disease or previous SGA. Finally, due to the observational nature of the included studies, we cannot exclude any residual or unmeasured confounding, which limits any conclusions on causality of the identified associations.
Conclusion
This large individual participant data meta-analysis shows that subclinical hypothyroidism is a risk factor for SGA and that isolated hypothyroxinaemia is associated with higher birth weight. Furthermore, we identified a dose-dependent negative association of maternal FT4 with birth weight that was most prominent during late pregnancy. This indicates that there is a potential risk of overtreatment when titrating levothyroxine to high-normal FT4 concentrations and underlines the importance of follow-up thyroid function testing when levothyroxine therapy is started during early pregnancy.
Supplementary Material
Research in context.
Evidence before this study
Studies on hypothyroidism and hyperthyroidism implicate that adequate maternal thyroid hormone availability is required for optimal fetal growth and development. Studies on the association of mild thyroid function test abnormalities with birth weight report heterogeneous results. Some studies indicate that high FT4 concentrations are associated with lower birth weight which could have implications for the treatment target in women already on levothyroxine therapy. We searched Medline (Ovid), Embase.com, Web-of-Science, Cochrane CENTRAL and Google Scholar up to March 18th, 2018 and collected data on serum thyroid function tests and antibody status during pregnancy and birth weight from prospective cohort studies including treatment-naïve pregnant women.
Added value of this study
This individual participant data meta-analysis showed that subclinical hypothyroidism is associated with lower birth weight. We also identified that a higher maternal FT4, even within the normal range, is associated with lower birth weight and that isolated hypothyroxinaemia was associated with higher birth weight. The association of FT4 with birth weight was more apparent in the second and third trimester as compared to the first trimester of pregnancy.
Implications of all the available evidence
There was a continuous negative association of maternal FT4 with birth weight that was most prominent in the second and third trimester of pregnancy. This implicates that there could be a potential risk of overtreatment when titrating levothyroxine to high-normal FT4 concentrations during pregnancy and emphasize the need for treatment monitoring during later pregnancy.
Acknowledgements
We gratefully acknowledge the contribution of study participants across all cohorts included in the current study and extend our gratitude to study staff, data managers, general practitioners, hospitals and midwives who have made these separate studies possible. We also gratefully acknowledge the contributions of Shafqat Mukhtar MD (Department of Gynecology and Obstetrics, Shaikh Zayed Medical Complex, Lahore, Pakistan), Professor Andrew Hattersley, Beverley Shields PhD, Rachel Freathy PhD, Robin Beaumont PhD (all University of Exeter) to data collection and management, none of whom received any form of compensation.
Funding
This work was supported by replication studies grant 401.16.020 from the Netherlands Organization for Scientific Research.
Funding
This work was supported by a Replication Studies Grant (2016, number 401.16.020) from the Netherlands Organization for Scientific Research (NWO; grant to RPP, TIMK and EAPS). Cohort-specific grants are described in the appendix. None of the funders have been involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Declaration of interests
RPP has served as a consultant to Berlin-Chemie AG, GoodLife Fertility BV, and Institut Biochimique SA. TIMK has received personal fees from Berlin Chemie, Goodlife Healthcare, and Quidel. BV has received honoraria from Berlin-Chime. EO has received grants from the National Institutes of Health. TV has received grants from the Netherlands Organization for Health Research and Development. CD has received grants from the Chief Scientist Office (Scotland) and the British Heart Foundation. SMN has received grants from the Chief Scientist Office (Scotland) and reports grants and personal fees from Roche Diagnostics, during the conduct of the study. LC reported serving as a consultant to Pfizer. TM reported serving as a consultant to Abbott Diagnostics and Roche. ENP has received grants from the Sociedad Quimica y Minera de Chile and personal fees from the Institut Biochimique SA. The rest of the authors declare no competing interests.
References
- 1.Zimmermann E, Gamborg M, Sorensen TI, Baker JL. Sex Differences in the Association Between Birth Weight and Adult Type 2 Diabetes. Diabetes 2015; 64(12): 4220–5. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Z, Kris-Etherton PM, Hartman TJ. Birth weight and risk factors for cardiovascular disease and type 2 diabetes in US children and adolescents: 10 year results from NHANES. Matern Child Health J 2014; 18(6): 1423–32. [DOI] [PubMed] [Google Scholar]
- 3.Stuart A, Amer-Wahlin I, Persson J, Kallen K. Long-term cardiovascular risk in relation to birth weight and exposure to maternal diabetes mellitus. Int J Cardiol 2013; 168(3): 2653–7. [DOI] [PubMed] [Google Scholar]
- 4.Spracklen CN, Wallace RB, Sealy-Jefferson S, et al. Birth weight and subsequent risk of cancer. Cancer Epidemiol 2014; 38(5): 538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geserick M, Vogel M, Gausche R, et al. Acceleration of BMI in Early Childhood and Risk of Sustained Obesity. New England Journal of Medicine 2018; 379(14): 1303–12. [DOI] [PubMed] [Google Scholar]
- 6.Khambalia AZ, Algert CS, Bowen JR, Collie RJ, Roberts CL. Long-term outcomes for large for gestational age infants born at term. J Paediatr Child Health 2017; 53(9): 876–81. [DOI] [PubMed] [Google Scholar]
- 7.Weissmann-Brenner A, Simchen MJ, Zilberberg E, et al. Maternal and neonatal outcomes of large for gestational age pregnancies. Acta Obstet Gynecol Scand 2012; 91(7): 844–9. [DOI] [PubMed] [Google Scholar]
- 8.Korevaar TIM, Medici M, Visser TJ, Peeters RP. Thyroid disease in pregnancy: new insights in diagnosis and clinical management. Nat Rev Endocrinol 2017; 13(10): 610–22. [DOI] [PubMed] [Google Scholar]
- 9.Monen L, Kuppens SM, Hasaart TH, et al. Maternal thyrotropin is independently related to Small for Gestational Age neonates at term. Clinical endocrinology 2015; 82(2): 254–9. [DOI] [PubMed] [Google Scholar]
- 10.Chen L-M, Du W-J, Dai J, et al. Effects of subclinical hypothyroidism on maternal and perinatal outcomes during pregnancy: a single-center cohort study of a Chinese population. PloS one 2014; 9(10): e109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cleary-Goldman J, Malone FD, Lambert-Messerlian G, et al. Maternal thyroid hypofunction and pregnancy outcome. Obstetrics and gynecology 2008; 112(1): 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Yd, Han Y, Huang K, et al. The impact of isolated maternal hypothyroxinaemia on the incidence of large-for-gestational-age infants: the Ma’anshan Birth Cohort study. BJOG: An International Journal of Obstetrics & Gynaecology 2018; 125(9): 1118–25. [DOI] [PubMed] [Google Scholar]
- 13.Kumru P, Erdogdu E, Arisoy R, et al. Effect of thyroid dysfunction and autoimmunity on pregnancy outcomes in low risk population. Arch Gynecol Obstet 2015; 291(5): 1047–54. [DOI] [PubMed] [Google Scholar]
- 14.Maraka S, Ospina NM, O’Keeffe DT, et al. Subclinical Hypothyroidism in Pregnancy: A Systematic Review and Meta-Analysis. Thyroid 2016; 26(4): 580–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demailly R, Escolano S, Quantin C, Tubert-Bitter P, Ahmed I. Prescription drug use during pregnancy in France: a study from the national health insurance permanent sample. Pharmacoepidemiol Drug Saf 2017; 26(9): 1126–34. [DOI] [PubMed] [Google Scholar]
- 16.Engeland A, Bjorge T, Klungsoyr K, Hjellvik V, Skurtveit S, Furu K. Trends in prescription drug use during pregnancy and postpartum in Norway, 2005 to 2015. Pharmacoepidemiol Drug Saf 2018; 27(9): 995–1004. [DOI] [PubMed] [Google Scholar]
- 17.Smolina K, Hanley GE, Mintzes B, Oberlander TF, Morgan S. Trends and Determinants of Prescription Drug Use during Pregnancy and Postpartum in British Columbia, 2002–2011: A Population-Based Cohort Study. PLoS One 2015; 10(5): e0128312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tinker SC, Broussard CS, Frey MT, Gilboa SM. Prevalence of prescription medication use among non-pregnant women of childbearing age and pregnant women in the United States: NHANES, 1999–2006. Matern Child Health J 2015; 19(5): 1097–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishigori H, Obara T, Nishigori T, et al. Drug Use before and during Pregnancy in Japan: The Japan Environment and Children’s Study. Pharmacy (Basel) 2017; 5(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haddow JE, Craig WY, Neveux LM, et al. Implications of High Free Thyroxine (FT4) concentrations in euthyroid pregnancies: the FaSTER trial. J Clin Endocrinol Metab 2014; 99(6): 2038–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medici M, Timmermans S, Visser W, et al. Maternal thyroid hormone parameters during early pregnancy and birth weight: the Generation R Study. J Clin Endocrinol Metab 2013; 98(1): 59–66. [DOI] [PubMed] [Google Scholar]
- 22.Zhang C, Yang X, Zhang Y, et al. Association Between Maternal Thyroid Hormones and Birth Weight at Early and Late Pregnancy. J Clin Endocrinol Metab 2019. [DOI] [PubMed]
- 23.Johns LE, Ferguson KK, Cantonwine DE, Mukherjee B, Meeker JD, McElrath TF. Subclinical Changes in Maternal Thyroid Function Parameters in Pregnancy and Fetal Growth. J Clin Endocrinol Metab 2018; 103(4): 1349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shields BM, Knight BA, Hill A, Hattersley AT, Vaidya B. Fetal thyroid hormone level at birth is associated with fetal growth. J Clin Endocrinol Metab 2011; 96(6): E934–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leon G, Murcia M, Rebagliato M, et al. Maternal thyroid dysfunction during gestation, preterm delivery, and birthweight. The Infancia y Medio Ambiente Cohort, Spain. Paediatr Perinat Epidemiol 2015; 29(2): 113–22. [DOI] [PubMed] [Google Scholar]
- 26.Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017; 27(3): 315–89. [DOI] [PubMed] [Google Scholar]
- 27.Männistö, Vääräsmäki M, Pouta A, et al. Perinatal outcome of children born to mothers with thyroid dysfunction or antibodies: a prospective population-based cohort study. The Journal of Clinical Endocrinology & Metabolism 2009; 94(3): 772–9. [DOI] [PubMed] [Google Scholar]
- 28.Casey BM, Dashe JS, Wells CE, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstetrics & Gynecology 2005; 105(2): 239–45. [DOI] [PubMed] [Google Scholar]
- 29.Vrijkotte TGM, Hrudey EJ, Twickler MB. Early maternal thyroid function during gestation is associated with fetal growth, particularly in male newborns. The Journal of Clinical Endocrinology & Metabolism 2017; 102(3): 1059–66. [DOI] [PubMed] [Google Scholar]
- 30.Karakosta P, Alegakis D, Georgiou V, et al. Thyroid dysfunction and autoantibodies in early pregnancy are associated with increased risk of gestational diabetes and adverse birth outcomes. J Clin Endocrinol Metab 2012; 97(12): 4464–72. [DOI] [PubMed] [Google Scholar]
- 31.Johns LE, Ferguson KK, Cantonwine DE, Mukherjee B, Meeker JD, McElrath TF. Subclinical Changes in Maternal Thyroid Function Parameters in Pregnancy and Fetal Growth. The Journal of Clinical Endocrinology & Metabolism 2017; 103(4): 1349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bliddal S, Boas M, Hilsted L, Friis-Hansen L, Tabor A, Feldt-Rasmussen U. Thyroid function and autoimmunity in Danish pregnant women after an iodine fortification program and associations with obstetric outcomes. Eur J Endocrinol 2015; 173(6): 709–18. [DOI] [PubMed] [Google Scholar]
- 33.Lazarus JH, Bestwick JP, Channon S, et al. Antenatal Thyroid Screening and Childhood Cognitive Function. New England Journal of Medicine 2012; 366(6): 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casey BM, Thom EA, Peaceman AM, et al. Treatment of Subclinical Hypothyroidism or Hypothyroxinemia in Pregnancy. New England Journal of Medicine 2017; 376(9): 815–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nazarpour S, Tehrani FR, Simbar M, Tohidi M, Majd HA, Azizi F. Effects of levothyroxine treatment on pregnancy outcomes in pregnant women with autoimmune thyroid disease. European journal of endocrinology 2017; 176(2): 253–65. [DOI] [PubMed] [Google Scholar]
- 36.Nazarpour S, Ramezani Tehrani F, Simbar M, et al. Effects of Levothyroxine on Pregnant Women With Subclinical Hypothyroidism, Negative for Thyroid Peroxidase Antibodies. The Journal of Clinical Endocrinology & Metabolism 2017; 103(3): 926–35. [DOI] [PubMed] [Google Scholar]
- 37.Dhillon-Smith RK, Middleton LJ, Sunner KK, et al. Levothyroxine in Women with Thyroid Peroxidase Antibodies before Conception. New England Journal of Medicine 2019; 380(14): 1316–25. [DOI] [PubMed] [Google Scholar]
- 38.Consortium on T, Pregnancy-Study Group on Preterm B, Korevaar TIM, et al. Association of Thyroid Function Test Abnormalities and Thyroid Autoimmunity With Preterm Birth: A Systematic Review and Meta-analysis. JAMA 2019; 322(7): 632–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health O. Physical status: The use of and interpretation of anthropometry, Report of a WHO Expert Committee. 1995. [PubMed]
- 40.Newcombe RG, Bender R. Implementing GRADE: calculating the risk difference from the baseline risk and the relative risk. BMJ Evidence-Based Medicine 2014; 19(1): 6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jolani S, Debray TP, Koffijberg H, van Buuren S, Moons KG. Imputation of systematically missing predictors in an individual participant data meta-analysis: a generalized approach using MICE. Stat Med 2015; 34(11): 1841–63. [DOI] [PubMed] [Google Scholar]
- 42.Korevaar TI, Chaker L, Jaddoe VW, Visser TJ, Medici M, Peeters RP. Maternal and Birth Characteristics Are Determinants of Offspring Thyroid Function. J Clin Endocrinol Metab 2016; 101(1): 206–13. [DOI] [PubMed] [Google Scholar]
- 43.Momotani N, Noh J, Oyanagi H, Ishikawa N, Ito K. Antithyroid drug therapy for Graves’ disease during pregnancy. Optimal regimen for fetal thyroid status. N Engl J Med 1986; 315(1): 24–8. [DOI] [PubMed] [Google Scholar]
- 44.Cooper DS, Laurberg P. Hyperthyroidism in pregnancy. Lancet Diabetes Endocrinol 2013; 1(3): 238–49. [DOI] [PubMed] [Google Scholar]
- 45.Aggarawal N, Suri V, Singla R, et al. Pregnancy outcome in hyperthyroidism: a case control study. Gynecol Obstet Invest 2014; 77(2): 94–9. [DOI] [PubMed] [Google Scholar]
- 46.Kim B Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid 2008; 18(2): 141–4. [DOI] [PubMed] [Google Scholar]
- 47.Belkacemi L, Nelson DM, Desai M, Ross MG. Maternal undernutrition influences placental-fetal development. Biology of reproduction 2010; 83(3): 325–31. [DOI] [PubMed] [Google Scholar]
- 48.Barjaktarovic M, Korevaar TI, Jaddoe VW, et al. Human chorionic gonadotropin (hCG) concentrations during the late first trimester are associated with fetal growth in a fetal sex-specific manner. Eur J Epidemiol 2017; 32(2): 135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Korevaar TI, de Rijke YB, Chaker L, et al. Stimulation of Thyroid Function by Human Chorionic Gonadotropin During Pregnancy: A Risk Factor for Thyroid Disease and a Mechanism for Known Risk Factors. Thyroid 2017; 27(3): 440–50. [DOI] [PubMed] [Google Scholar]
- 50.Laurberg P, Andersen SL, Pedersen IB, Andersen S, Carlé A. Screening for overt thyroid disease in early pregnancy may be preferable to searching for small aberrations in thyroid function tests. Clinical endocrinology 2013; 79(3): 297–304. [DOI] [PubMed] [Google Scholar]
- 51.Barjaktarovic M, Korevaar TI, Chaker L, et al. The association of maternal thyroid function with placental hemodynamics. Hum Reprod 2017; 32(3): 653–61. [DOI] [PubMed] [Google Scholar]
- 52.Fowden AL, Forhead AJ. Endocrine mechanisms of intrauterine programming. Reproduction 2004; 127(5): 515–26. [DOI] [PubMed] [Google Scholar]
- 53.Forhead AJ, Fowden AL. Thyroid hormones in fetal growth and prepartum maturation. Journal of Endocrinology 2014; 221(3): R87–R103. [DOI] [PubMed] [Google Scholar]
- 54.Roland MCP, Friis CM, Voldner N, et al. Fetal growth versus birthweight: the role of placenta versus other determinants. PLoS one 2012; 7(6): e39324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hales C, Taylor PN, Channon S, et al. Controlled Antenatal Thyroid Screening II: effect of treating maternal sub-optimal thyroid function on child behaviour. J Clin Endocrinol Metab 2019. [DOI] [PubMed]
- 56.Anckaert E, Poppe K, Van Uytfanghe K, Schiettecatte J, Foulon W, Thienpont LM. FT4 immunoassays may display a pattern during pregnancy similar to the equilibrium dialysis ID-LC/tandem MS candidate reference measurement procedure in spite of susceptibility towards binding protein alterations. Clinica Chimica Acta 2010; 411(17–18): 1348–53. [DOI] [PubMed] [Google Scholar]
- 57.Kahric-Janicic N, Soldin SJ, Soldin OP, West T, Gu J, Jonklaas J. Tandem mass spectrometry improves the accuracy of free thyroxine measurements during pregnancy. Thyroid 2007; 17(4): 303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


