ABSTRACT
Background
Trimethylamine-N-oxide (TMAO), a diet-derived and gut microbiota–related metabolite, is associated with cardiovascular disease (CVD). However, major dietary determinants and specific gut bacterial taxa related to TMAO remain to be identified in humans.
Objectives
We aimed to identify dietary and gut microbial factors associated with circulating TMAO.
Methods
This cross-sectional study included 3972 participants (57.3% women) aged 18–74 y from the Hispanic Community Health Study/Study of Latinos in the United States. Dietary information was collected by 24-h dietary recalls at baseline interview (2008–2011), and baseline serum TMAO and its precursors were measured by an untargeted approach. Gut microbiome was profiled by shotgun metagenomic sequencing in a subset of participants (n = 626) during a follow-up visit (2016–2018). Logistic and linear regression were used to examine associations of inverse-normalized metabolites with prevalent CVD, dietary intake, and bacterial species, respectively, after adjustment for sociodemographic, behavioral, and clinical factors.
Results
TMAO was positively associated with prevalent CVD (case number = 279; OR = 1.34; 95% CI: 1.17, 1.54, per 1-SD). Fish (P = 1.26 × 10−17), red meat (P = 3.33 × 10−16), and egg (P = 3.89 × 10−5) intakes were top dietary factors positively associated with TMAO. We identified 9 gut bacterial species significantly associated with TMAO (false discovery rate <0.05). All 4 species positively associated with TMAO belong to the order Clostridiales, of which 3 might have homologous genes encoding carnitine monooxygenase, an enzyme converting carnitine to trimethylamine (TMA). The red meat–TMAO association was more pronounced in participants with higher abundances of these 4 species compared with those with lower abundance (Pinteraction = 0.013), but such microbial modification was not observed for fish–TMAO or egg–TMAO associations.
Conclusion
In US Hispanics/Latinos, fish, red meat, and egg intakes are major dietary factors associated with serum TMAO. The identified potential TMA-producing gut microbiota and microbial modification on the red meat–TMAO association support microbial TMA production from dietary carnitine, whereas the fish–TMAO association is independent of gut microbiota.
Keywords: cardiovascular disease, diet, gut microbiota, trimethylamine-N-oxide, Hispanic Americans
See corresponding editorial on page 1400.
Introduction
Trimethylamine-N-oxide (TMAO), a potential diet-derived and gut microbiota (GMB)–related metabolite, has been associated with an increased risk of cardiovascular disease (CVD) independent of traditional risk factors in numerous clinic-based studies (1–3). Environmental and host factors, such as GMB, dietary intake, and host genetics, are thought to independently and jointly influence circulating concentrations of TMAO (4), but data from human population-based studies are still limited. Identifying potential drivers for alterations in circulating TMAO could have preventive and therapeutic implications for CVD.
Dietary trimethylamine (TMA)-containing nutrients, including choline, phosphatidylcholine, carnitine, and betaine, abundant in animal foods such as red meat (e.g., beef and pork) and eggs, can be converted to TMAO in humans through a series of physiological processes (1, 5). Specifically, TMA is liberated from TMA-containing nutrients by intestinal bacteria (4), passively absorbed into the circulation system, and then oxidized to TMAO by flavin monooxygenases in the liver (1). In addition to TMAO produced from dietary precursors, preformed TMAO can be absorbed in a manner not involving gut microbes in humans (6) and animals (7). A human feeding study has shown that fish (cod fillet) contains a high concentration of TMAO, and that circulating TMAO was elevated within 15 min of fish consumption (6). However, human population studies assessing the relation between dietary factors and TMAO have been inconclusive (8–11), potentially due to differences in dietary habits, GMB, or host genetic background across populations. Major dietary determinants of circulating TMAO remain open for investigation in human populations.
As to the role of GMB in modulating circulating TMAO, it has been found that antibiotics can suppress blood concentrations of TMAO in mice (1) and humans (2). Two recent studies using 16S rRNA gene amplicon sequencing (16S) data identified several GMB features (e.g., genera from the orders Clostridiales, Bifidobacteriales, and Bacteroidales) associated with circulating TMAO (12, 13). In addition, previous studies have identified that choline TMA-lyase (choline utilization, CutC) and its activator CutD (14), and a 2-component Rieske-type oxygenase/reductase (carnitine monooxygenase, CntA/B) (15), can synthesize TMA from choline and carnitine, respectively. It has been suggested that CutC might be carried by Clostridium XIVa strains and Eubacterium sp. strain AB3007, and CntA might be carried by Escherichia coli (16). However, the specific gut bacterial taxa that can metabolize the TMA-containing nutrients into TMA and thus elevate host TMAO concentrations remain to be identified in human populations. Moreover, the potential interaction between GMB and dietary factors (e.g., red meat intake) on circulating TMAO concentrations also remains unexplored in human populations.
In this study of US Hispanics/Latinos, we aimed to: 1) examine associations of serum TMAO and precursor metabolites (choline, carnitine, and betaine), with prevalent CVD; 2) examine associations of dietary factors with serum concentrations of TMAO and precursor metabolites; and 3) identify GMB features associated with serum TMAO.
Methods
Study design and population
The Hispanic Community Health Study/Study of Latinos (HCHS/SOL) is a prospective community-based cohort study of 16,415 Hispanic/Latino adults aged 18–74 y at recruitment who were living in 4 US urban areas (Bronx, NY; Chicago, IL; Miami, FL; and San Diego, CA). A 2-stage area probability sample design was used to recruit participants, which has been previously described (17, 18). A comprehensive set of interviews and a clinical examination with fasting blood draw were conducted by trained and certified staff at in-person clinic visits from 2008 to 2011. The HCHS/SOL Gut Origins of Latino Diabetes (GOLD) ancillary study was conducted during 2016–2018 to investigate the role of GMB composition in the risk of diabetes and other health outcomes, with a total of 3057 participants enrolled from the HCHS/SOL approximately concurrent with the second in-person visit period from 2014 to 2017 (19). The study was approved by the institutional review boards of corresponding site institutions. Written informed consent was obtained from all participants.
Ascertainment of cardiometabolic diseases
Cardiometabolic diseases were ascertained using information collected at the 2008–2011 examination. Diabetes was defined as either current use of antidiabetic medications, or fasting glucose ≥126 mg/dL, 2-h oral-glucose-tolerance test plasma glucose ≥200 mg/dL, or glycated hemoglobin ≥6.5% (20). Hypertension was defined as systolic/diastolic blood pressure ≥140/90 mmHg or currently taking antihypertensive medications. Dyslipidemia was defined as serum LDL cholesterol ≥160 mg/dL, HDL cholesterol <40 mg/dL, or triglycerides ≥200 mg/dL or currently taking antihyperlipidemic medications (21). CVD was ascertained according to self-reported physician diagnosis of coronary heart disease (CHD) (including a history of a cardiac event or procedure, or electrocardiographic evidence of myocardial infarction observed) or cerebrovascular disease or carotid revascularization (CDCR) (history of stroke, mini-stroke, or transient ischemic attack, or balloon angioplasty or surgery to the arteries in the neck) (21).
Assessment of diet and other covariates
Dietary information was collected by two 24-h dietary recalls as described previously (22, 23). The first recall was performed by in-person interviews during the 2008–2011 examination, and the second recall was conducted via telephone ∼30 d after the first interview. Participants estimated portion sizes with the use of food models (for the in-person interviews) or a food-amount booklet (for the telephone interviews). Foods and nutrients were analyzed using the multiple-pass methods of the Nutrition Data System for Research software (version 11) from the Nutrition Coordinating Center at the University of Minnesota (24). The present analyses mainly focused on 15 major food groups, and 36 major macronutrients and micronutrients were also examined in secondary analyses. Participants who had ≥1 set of 24-h dietary recall data (97% had the first 24-h dietary recall) were included in the current study. Because circulating TMAO concentrations increased over a short time in response to animal source foods (6), we used the first 24-h dietary recall data, which were concurrent with fasting blood draw in terms of the collection time in the main analyses. For those who had no data from the first 24-h dietary recall, data from the second 24-h dietary recall were used.
Sociodemographic and behavioral characteristics of participants, including Hispanic/Latino background, education attainment, annual household income, smoking, and alcohol consumption, in addition to medical and family histories, were collected using structured questionnaires (17, 18). Physical activity was measured using the Global Physical Activity Questionnaire and the metabolic equivalent-hours/day was derived (25). BMI was computed as measured weight (in kilograms) divided by measured height (in meters) squared.
Serum metabolite measurement
Metabolomic profiling was performed on serum specimens collected during the 2008–2011 examination from 3972 participants randomly selected from the entire study population, using an untargeted LC-MS approach based on the discoveryHD4 platform at Metabolon Inc. Details of sample extraction, separation, and MS analysis have been described elsewhere (26). Briefly, this approach utilized a Waters ACQUITY UPLC and a Thermo Scientific Q-Exactive high-resolution/accurate mass spectrometer interfaced with a heated electrospray ionization (HESI-II) source and Orbitrap mass analyzer operated at 35,000 mass resolution. Metabolite peaks were identified and confirmed using authentic reference standards. Metabolites were measured using AUC of the peaks. TMAO and 3 precursor metabolites (choline, carnitine, and betaine) were captured by this method and were included in the present analysis. The undetectable rates of these 4 metabolites were all <0.1%. Missing values of the 4 metabolites were imputed with half of the detectable minimum values. A rank-based inverse normal transformation was applied to the metabolite data before analyses (27).
Gut microbiome shotgun metagenomics sequencing
In the GOLD ancillary study, enrolled participants were provided with a stool collection kit. For each participant, a single fecal specimen was self-collected using a disposable paper inverted hat (Protocult collection device, ABC Medical Enterprises, Inc.). Detailed sample processing and DNA extraction procedures have been described previously (19). A shallow shotgun metagenomics sequencing, which has been recommended to obtain species-level taxonomic data as an alternative to 16S sequencing at around the same cost for large human microbiome studies where deep whole-metagenome shotgun sequencing can be cost-prohibitive (28), was performed on the Illumina NovaSeq platforms (29). Sequences were boosted using inference from reference genomes of likely genomic content. The adapters and barcode indices were processed following the iTru adapter protocol (30). Shallow shotgun data were left trimmed to remove low-quality bases that had a Phred quality score of ≤25 using prinseq-lite 0.20.4 (31). The quality controlled paired-end data were then concatenated and aligned against the National Center for Biotechnology Information (NCBI) RefSeq representative prokaryotic genome collection (release 82) (32) using default SHOGUN (28) settings. Samples with a coverage depth <100,000 reads per sample were excluded. Finally, the average reads per sample was 436K (range 100K–4122K). Of 3035 samples with sequencing data, 2764 samples passed all quality control metrics and were included in the present analyses. Bowtie2 (33) was used to build the SHOGUN assemblies. The reads that mapped to a single reference genome were labeled with the NCBI taxonomic annotation at the species level. Those reads that mapped to multiple reference genomes were labeled as the last common ancestor of each label according to the NCBI taxonomy (28). Cumulative sum scaling normalization was conducted for the species-level abundance of taxonomic units (34) before analyses.
Statistical analysis
We examined cross-sectional associations of TMAO and its precursor metabolites with prevalence of CVD and its constituent components, CHD and CDCR, in 3827 participants without cancer. For the analysis on dietary factors and serum concentrations of TMAO and its precursor metabolites, we further excluded participants with prevalent CVD (n = 279), with missing dietary information (n = 28), or with extreme dietary energy intake (>6000/<600 kcal/d in men, or >4000/<400 kcal/d in women) (n = 54), leaving 3466 participants (Supplemental Figure 1). Next, in a subgroup of participants (n = 626) who had both metabolite and GMB data, we examined the associations of gut microbial α-diversity and species abundance with TMAO concentrations (Supplemental Figure 1).
Characteristics of the study participants were presented according to quartile of TMAO as means ± SDs or percentages where appropriate. Participant characteristics were compared across the quartile of TMAO by using 1-factor ANOVA for continuous variables and χ2 test for categorical variables. Associations of TMAO and its precursor metabolites with the prevalence of CVD, CHD, and CDCR were assessed using multivariable logistic regression models with adjustment for age, sex, BMI, study field center, Hispanic/Latino background, education, yearly household income, smoking, alcohol consumption, physical activity, and total energy intake. Associations of dietary factors (including food groups and nutrients) with TMAO and its precursor metabolites were assessed using linear regression models, with controlling for the aforementioned covariates plus diabetes, hypertension, and dyslipidemia. To reduce potential influences of extreme values, quintiles were created for all dietary intake variables. For the food groups with a percentage of nonconsumers >20%, nonconsumers were coded as 0, and the other consumers were coded as 1–4 by quartiles. Finally, the continuous quintile ranks (ranged 0–4) were used in the analyses (Supplemental Table 1).
The gut microbial α-diversity and species abundance were examined for associations with serum TMAO using linear regression, after controlling for the aforementioned covariates in addition to use of antibiotics and probiotics. Next, we explored potential effect modification on diet–metabolite associations by gut microbial species. We examined associations between food groups (i.e., fish, red meat, eggs) and TMAO concentrations stratified by abundance of microbial species (i.e., above compared with below median) that were significantly and positively associated with TMAO in our study, because these bacteria might have the potential to produce TMAO and thus might modify the associations of red meat and egg intake with TMAO. In addition, we also calculated a GMB score (range 0–4) based on the abundance of the 4 microbial species positively associated with TMAO (less than median value = 0; and equal to or greater than median value = 1) to represent an overall GMB feature positively associated with TMAO. We then examined associations of food groups with TMAO across 3 levels of the GMB score (0, low GMB score; 1–2, medium GMB score; and 3–4, high GMB score). The interactions between food groups and GMB score on serum TMAO concentrations were tested by including the respective interaction terms in the models (e.g., red meat intake × GMB score). To examine the joint effect of red meat intake and the GMB score on serum TMAO, red meat intake was further categorized as tertiles instead of quintiles to ensure adequate sample sizes in each of 9 subgroups (red meat intake tertiles × 3 GMB score categories). The Benjamini–Hochberg false discovery rate (FDR) method was used for multiple testing correction. All analyses were performed using R version 3.6.0 (https://www.r-project.org/).
Results
Participant characteristics
Characteristics of participants according to quartile of serum TMAO are presented in Table 1. Participants with a higher concentration of TMAO were older and less likely to be women, were more likely to be current smokers, had higher BMI and higher dietary energy intake on average, and were more likely to have diabetes, hypertension, and dyslipidemia, compared with those with a lower concentration of TMAO.
TABLE 1.
Characteristics of participants according to quartiles of serum TMAO concentration1
| Quartiles of TMAO | |||||
|---|---|---|---|---|---|
| Q1 (n = 957) | Q2 (n = 957) | Q3 (n = 957) | Q4 (n = 956) | P | |
| Age, y | 41.3 ± 13.9 | 45.0 ± 13.2 | 47.2 ± 13.6 | 48.4 ± 13.5 | <0.001 |
| Female | 592 (61.9) | 557 (58.2) | 539 (56.3) | 476 (49.8) | <0.001 |
| Education, y | |||||
| 1–10 | 319 (33.4) | 335 (35.1) | 348 (36.4) | 363 (38.0) | 0.56 |
| 11–12 | 256 (26.8) | 253 (26.5) | 249 (26.0) | 241 (25.2) | |
| >13 | 380 (39.8) | 367 (38.4) | 359 (37.6) | 351 (36.8) | |
| Household yearly income, US$ | |||||
| ≤20,000 | 577 (60.3) | 607 (63.4) | 619 (64.7) | 610 (63.8) | 0.37 |
| 20,001–50,000 | 323 (33.8) | 292 (30.5) | 278 (29.0) | 297 (31.1) | |
| >50,000 | 57 (6.0) | 58 (6.1) | 60 (6.3) | 49 (5.1) | |
| Background | |||||
| Dominican | 122 (12.8) | 96 (10.1) | 98 (10.3) | 63 (6.6) | 0.004 |
| Central American | 84 (8.8) | 91 (9.5) | 104 (10.9) | 109 (11.4) | |
| Cuban | 147 (15.4) | 159 (16.7) | 178 (18.6) | 156 (16.3) | |
| Mexican | 359 (37.7) | 332 (34.8) | 329 (34.4) | 365 (38.2) | |
| Puerto Rican | 148 (15.5) | 190 (19.9) | 157 (16.4) | 180 (18.8) | |
| South American | 61 (6.4) | 52 (5.5) | 58 (6.1) | 57 (6.0) | |
| Other/more than one | 32 (3.4) | 34 (3.6) | 32 (3.3) | 25 (2.6) | |
| Smoking | |||||
| Never | 615 (64.3) | 584 (61.0) | 534 (55.8) | 507 (53.1) | <0.001 |
| Former | 161 (16.8) | 182 (19.0) | 196 (20.5) | 213 (22.3) | |
| Current | 180 (18.8) | 191 (20.0) | 227 (23.7) | 234 (24.5) | |
| Alcohol consumption, g/d | 3.3 ± 16.0 | 2.7 ± 12.1 | 3.7 ± 15.7 | 4.2 ± 16.0 | 0.09 |
| BMI, kg/m2 | 29.0 ± 6.1 | 29.7 ± 6.1 | 30.2 ± 6.1 | 30.1 ± 5.8 | <0.001 |
| Physical activity, MET-min/d | 638.9 ± 935.2 | 625.9 ± 974.2 | 625.4 ± 954.6 | 659.8 ± 1059.2 | 0.66 |
| Total energy intake, kcal/d | 1978.5 ± 993.3 | 1977.0 ± 966.5 | 2023.2 ± 1030.1 | 2069.2 ± 1119.1 | 0.03 |
| Diabetes | 138 (14.4) | 141 (14.7) | 201 (21.0) | 267 (27.9) | <0.001 |
| Hypertension | 201 (21.0) | 239 (25.0) | 301 (31.5) | 333 (34.8) | <0.001 |
| Dyslipidemia | 343 (35.8) | 365 (38.2) | 377 (39.4) | 418 (43.8) | 0.004 |
Data are mean ± SD, or n (%). P values were calculated from the 1-factor ANOVA for continuous variables and χ2 test for categorical variables. MET, metabolic equivalent task; Q, quartile; TMAO, trimethylamine-N-oxide.
TMAO metabolites and CVD
As expected, the 4 metabolites showed a modest-to-moderate correlation with each other except for the lack of correlation between choline and carnitine (Supplemental Figure 2). Serum TMAO was associated with elevated odds of CVD (OR per SD increment: 1.34; 95% CI: 1.17, 1.54), CHD (OR = 1.31; 95% CI: 1.13, 1.53), and CDCR (OR = 1.38; 95% CI: 1.10, 1.74) (model 2 in Table 2). There was no significant heterogeneity in these associations across different Hispanic/Latino subgroups (all P-interaction >0.05; Supplemental Figure 3). In addition, serum betaine, but not choline or carnitine, was also associated with elevated odds of CVD and CDCR (Table 2).
TABLE 2.
Associations of serum TMAO and its precursor metabolites with prevalent cardiovascular disease1
| Q1 | Q2 | Q3 | Q4 | P for trend | Per 1-SD increment | |
|---|---|---|---|---|---|---|
| Cardiovascular disease | ||||||
| TMAO | ||||||
| Cases/participants | 41/957 | 49/957 | 82/957 | 107/956 | 279/3824 | |
| Model 1 | 1.00 (reference) | 1.21 (0.79, 1.85) | 2.09 (1.43, 3.11) | 2.82 (1.96, 4.13) | <0.001 | 1.53 (1.35, 1.73) |
| Model 2 | 1.00 (reference) | 0.89 (0.57, 1.39) | 1.41 (0.94, 2.12) | 1.80 (1.23, 2.69) | <0.001 | 1.34 (1.17, 1.54) |
| Model 3 | 1.00 (reference) | 0.89 (0.57, 1.39) | 1.38 (0.93, 2.10) | 1.78 (1.20, 2.68) | <0.001 | 1.34 (1.17, 1.55) |
| Choline | ||||||
| Cases/participants | 52/957 | 61/957 | 76/957 | 90/956 | 279/3824 | |
| Model 1 | 1.00 (reference) | 1.18 (0.81, 1.74) | 1.50 (1.05, 2.17) | 1.81 (1.27, 2.59) | <0.001 | 1.34 (1.18, 1.51) |
| Model 2 | 1.00 (reference) | 0.94 (0.63, 1.40) | 1.03 (0.70, 1.52) | 0.97 (0.66, 1.44) | 0.97 | 1.07 (0.94, 1.23) |
| Model 3 | 1.00 (reference) | 0.95 (0.63, 1.42) | 1.02 (0.69, 1.51) | 0.95 (0.64, 1.41) | 0.88 | 1.06 (0.92, 1.21) |
| Carnitine | ||||||
| Cases/participants | 66/957 | 64/957 | 66/957 | 83/956 | 279/3824 | |
| Model 1 | 1.00 (reference) | 0.97 (0.68, 1.38) | 1.00 (0.70, 1.43) | 1.28 (0.92, 1.80) | 0.14 | 1.12 (0.99, 1.27) |
| Model 2 | 1.00 (reference) | 0.88 (0.60, 1.29) | 0.88 (0.61, 1.28) | 0.97 (0.67, 1.40) | 0.91 | 1.00 (0.88, 1.14) |
| Model 3 | 1.00 (reference) | 0.88 (0.60, 1.29) | 0.90 (0.61, 1.31) | 0.98 (0.68, 1.42) | 0.98 | 1.01 (0.89, 1.15) |
| Betaine | ||||||
| Cases/participants | 55/957 | 72/957 | 65/957 | 87/956 | 279/3824 | |
| Model 1 | 1.00 (reference) | 1.33 (0.93, 1.92) | 1.20 (0.83, 1.74) | 1.64 (1.16, 2.34) | 0.01 | 1.18 (1.05, 1.34) |
| Model 2 | 1.00 (reference) | 1.39 (0.95, 2.04) | 1.33 (0.90, 1.97) | 1.64 (1.12, 2.41) | 0.02 | 1.16 (1.01, 1.33) |
| Model 3 | 1.00 (reference) | 1.38 (0.94, 2.03) | 1.32 (0.89, 1.97) | 1.59 (1.08, 2.35) | 0.03 | 1.15 (1.00, 1.32) |
| Coronary heart disease | ||||||
| TMAO | ||||||
| Cases/participants | 31/957 | 38/957 | 61/957 | 82/956 | 212/3824 | |
| Model 1 | 1.00 (reference) | 1.24 (0.76, 2.01) | 2.03 (1.32, 3.20) | 2.80 (1.86, 4.34) | <0.001 | 1.52 (1.32, 1.75) |
| Model 2 | 1.00 (reference) | 0.92 (0.56, 1.53) | 1.34 (0.85, 2.15) | 1.76 (1.14, 2.78) | 0.001 | 1.31 (1.13, 1.53) |
| Model 3 | 1.00 (reference) | 0.92 (0.56, 1.53) | 1.35 (0.86, 2.17) | 1.76 (1.13, 2.80) | 0.002 | 1.32 (1.13, 1.55) |
| Choline | ||||||
| Cases/participants | 40/957 | 49/957 | 55/957 | 68/956 | 212/3824 | |
| Model 1 | 1.00 (reference) | 1.24 (0.81, 1.91) | 1.40 (0.92, 2.13) | 1.76 (1.18, 2.64) | 0.005 | 1.32 (1.15, 1.52) |
| Model 2 | 1.00 (reference) | 0.98 (0.63, 1.53) | 0.95 (0.62, 1.49) | 0.92 (0.59, 1.42) | 0.67 | 1.04 (0.90, 1.21) |
| Model 3 | 1.00 (reference) | 0.97 (0.62, 1.52) | 0.94 (0.61, 1.47) | 0.88 (0.57, 1.37) | 0.53 | 1.03 (0.88, 1.20) |
| Carnitine | ||||||
| Cases/participants | 58/957 | 48/957 | 48/957 | 58/956 | 212/3824 | |
| Model 1 | 1.00 (reference) | 0.82 (0.55, 1.21) | 0.82 (0.55, 1.21) | 1.00 (0.69, 1.46) | 1.00 | 1.03 (0.90, 1.18) |
| Model 2 | 1.00 (reference) | 0.71 (0.47, 1.07) | 0.70 (0.46, 1.05) | 0.72 (0.48, 1.07) | 0.13 | 0.91 (0.78, 1.05) |
| Model 3 | 1.00 (reference) | 0.72 (0.47, 1.09) | 0.70 (0.46, 1.07) | 0.73 (0.49, 1.10) | 0.17 | 0.92 (0.79, 1.06) |
| Betaine | ||||||
| Cases/participants | 43/957 | 54/957 | 51/957 | 64/956 | 212/3824 | |
| Model 1 | 1.00 (reference) | 1.27 (0.84, 1.93) | 1.20 (0.79, 1.82) | 1.53 (1.03, 2.28) | 0.06 | 1.15 (1.00, 1.33) |
| Model 2 | 1.00 (reference) | 1.29 (0.84, 1.99) | 1.25 (0.80, 1.94) | 1.44 (0.94, 2.23) | 0.13 | 1.11 (0.95, 1.29) |
| Model 3 | 1.00 (reference) | 1.24 (0.81, 1.92) | 1.23 (0.79, 1.92) | 1.42 (0.92, 2.20) | 0.15 | 1.10 (0.95, 1.29) |
| Cerebrovascular disease or carotid revascularization | ||||||
| TMAO | ||||||
| Cases/participants | 12/957 | 17/957 | 31/957 | 37/956 | 97/3824 | |
| Model 1 | 1.00 (reference) | 1.42 (0.68, 3.07) | 2.64 (1.38, 5.37) | 3.17 (1.69, 6.38) | <0.001 | 1.53 (1.25, 1.88) |
| Model 2 | 1.00 (reference) | 1.09 (0.51, 2.39) | 1.80 (0.92, 3.75) | 2.16 (1.12, 4.46) | 0.007 | 1.38 (1.10, 1.74) |
| Model 3 | 1.00 (reference) | 1.11 (0.52, 2.43) | 1.75 (0.89, 3.67) | 2.16 (1.10, 4.50) | 0.01 | 1.38 (1.09, 1.75) |
| Choline | ||||||
| Cases/participants | 15/957 | 19/957 | 29/957 | 34/956 | 97/3824 | |
| Model 1 | 1.00 (reference) | 1.27 (0.64, 2.56) | 1.96 (1.06, 3.78) | 2.32 (1.28, 4.40) | 0.002 | 1.44 (1.17, 1.76) |
| Model 2 | 1.00 (reference) | 1.04 (0.52, 2.13) | 1.40 (0.73, 2.76) | 1.36 (0.72, 2.68) | 0.25 | 1.20 (0.96, 1.49) |
| Model 3 | 1.00 (reference) | 1.10 (0.54, 2.29) | 1.43 (0.74, 2.89) | 1.37 (0.71, 2.77) | 0.27 | 1.19 (0.95, 1.48) |
| Carnitine | ||||||
| Cases/participants | 18/957 | 27/957 | 21/957 | 31/956 | 97/3824 | |
| Model 1 | 1.00 (reference) | 1.51 (0.83, 2.81) | 1.17 (0.62, 2.23) | 1.75 (0.98, 3.21) | 0.13 | 1.19 (0.97, 1.46) |
| Model 2 | 1.00 (reference) | 1.7 (0.91, 3.26) | 1.23 (0.63, 2.43) | 1.69 (0.91, 3.23) | 0.24 | 1.14 (0.92, 1.41) |
| Model 3 | 1.00 (reference) | 1.67 (0.89, 3.23) | 1.26 (0.64, 2.48) | 1.66 (0.89, 3.20) | 0.25 | 1.14 (0.92, 1.41) |
| Betaine | ||||||
| Cases/participants | 18/957 | 26/957 | 21/957 | 32/956 | 97/3824 | |
| Model 1 | 1.00 (reference) | 1.46 (0.80, 2.72) | 1.17 (0.62, 2.23) | 1.81 (1.02, 3.30) | 0.09 | 1.21 (0.99, 1.48) |
| Model 2 | 1.00 (reference) | 1.62 (0.87, 3.10) | 1.54 (0.79, 3.03) | 2.16 (1.16, 4.15) | 0.03 | 1.27 (1.01, 1.58) |
| Model 3 | 1.00 (reference) | 1.72 (0.91, 3.34) | 1.58 (0.81, 3.16) | 2.04 (1.07, 4.00) | 0.05 | 1.23 (0.99, 1.55) |
Data are ORs (95% CIs) from logistic regression models. Model 1 was the crude model; model 2 was adjusted for age, sex, BMI, study field center, Hispanic/Latino background, physical activity, alcohol consumption, smoking, education, yearly household income, and total energy intake; and model 3 was further adjusted for intakes of fish, red meat, and eggs. Q, quartile; TMAO, trimethylamine-N-oxide.
Diet and TMAO metabolites
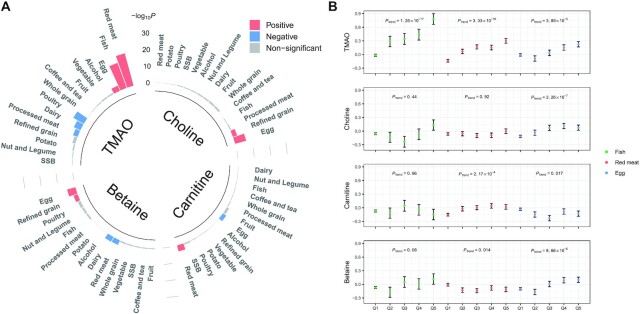
We next assessed associations of dietary factors with serum concentrations of TMAO and its precursor metabolites. After multivariable adjustment for sociodemographic and behavioral factors plus medical history, higher intakes of red meat (P = 6.74 × 10−21), fish (P = 9.24 × 10−17), and eggs (P = 5.11 × 10−4) were strongly associated with a higher concentration of TMAO, whereas intakes of poultry (P = 5.78 × 10−8), dairy (P = 5.10 × 10−5), and processed meat (P = 0.001) were inversely associated with serum TMAO (Figure 1A; Supplemental Table 2). In addition, egg and refined grain intakes were positively associated with serum choline (P = 1.37 × 10−7 and 0.002, respectively) and betaine (P = 6.59 × 10−6 and 0.003, respectively). Red meat intake was positively associated with serum carnitine (P = 1.85 × 10−4). The associations of red meat, fish, and egg intakes with serum TMAO were generally consistent and no significant heterogeneity was observed across Hispanic/Latino subgroups (all P-interaction >0.05, Supplemental Table 3). In sensitivity analyses, we also examined associations of red meat, fish, and egg intakes with serum metabolite concentrations using dietary data combined from the two 24-h dietary recalls (average of 2 recalls) or from the second 24-h dietary recall alone. As expected, the associations were relatively weaker using data based on combined dietary recalls or the second 24-h dietary recall compared with those using the first dietary recall (Supplemental Table 4). In addition, we also examined associations of TMAO and related metabolites with prevalent CVD after further adjustment for intakes of food groups positively associated with TMAO (i.e., red meat, fish, and eggs), and the associations were only slightly attenuated (model 3 in Table 2).
FIGURE 1.
Associations of food groups with serum TMAO and its precursor metabolites. (A) Associations (−log10P) of 15 food groups with serum TMAO and its precursor metabolites in 3466 individuals. P values were estimated from linear regression after adjustment for age, sex, BMI, study field center, Hispanic/Latino background, education, yearly household income, smoking, alcohol consumption, total energy intake, physical activity, diabetes, hypertension, and dyslipidemia. Red bars indicate positive associations (FDR-adjusted P < 0.05), blue bars indicate inverse associations (FDR-adjusted P < 0.05), and gray bars indicate nonsignificance (FDR-adjusted P ≥ 0.05). (B) Circulating concentrations of TMAO and its precursor metabolites (inverse normal transformation) across quintiles of fish, red meat, and egg intakes in 3466 individuals. The dots and error bars are the means and SEs estimated from linear regression models, which included all 15 food groups in the same model after multivariable adjustment for age, sex, BMI, study field center, Hispanic/Latino background, education, yearly household income, smoking, alcohol consumption, total energy intake, physical activity, diabetes, hypertension, and dyslipidemia. Numbers of individuals were 3191, 71, 64, 67, and 73 across the quintiles of fish intake; 1708, 434, 474, 401, and 449 across the quintiles of red meat intake; and 2339, 288, 270, 282, and 287 across the quintiles of egg intake. FDR, false discovery rate; SSB, sugar-sweetened beverage; TMAO, trimethylamine-N-oxide.
We then included all 15 food groups in 1 model (mutual adjustment) to assess independent relations of food groups with TMAO and its precursor metabolites. Red meat, fish, and egg intakes remained strongly and positively associated with serum TMAO concentrations. As shown in Figure 1B, the ascending trends of TMAO were significant as intakes of fish (P = 1.26 × 10−17), red meat (P = 3.33 × 10−16), and eggs (P = 3.89 × 10−5) increased. In addition, red meat intake was positively associated with serum carnitine (P = 2.17 × 10−4), and egg intake was positively associated with serum choline (P = 2.26 × 10−7) and betaine (P = 8.66 × 10−6), whereas fish intake was not associated with any of these TMAO precursors (all P > 0.05).
Results for dietary nutrients in relation to metabolites were generally in agreement with those for food groups (Supplemental Table 2). Dietary intakes of total protein, animal protein, thiamin, vitamin B-12, calcium, zinc, and selenium, which were largely from animal source foods, were strongly associated with a higher concentration of TMAO after the multivariable adjustment (all FDR-adjusted P ≤ 0.011). In addition, dietary EPA and DHA, which were mainly from fish intake, were positively associated with serum TMAO (both FDR-adjusted P ≤ 0.003). As expected, dietary choline was positively correlated with serum concentrations of TMAO (FDR-adjusted P < 0.001) and choline (FDR-adjusted P < 0.001).
GMB and TMAO
We firstly assessed the relation between 3 different measures of microbiome α-diversity (i.e., Chao1, Shannon, and Simpson) and serum concentrations of TMAO, and did not find significant associations (Supplemental Table 5).
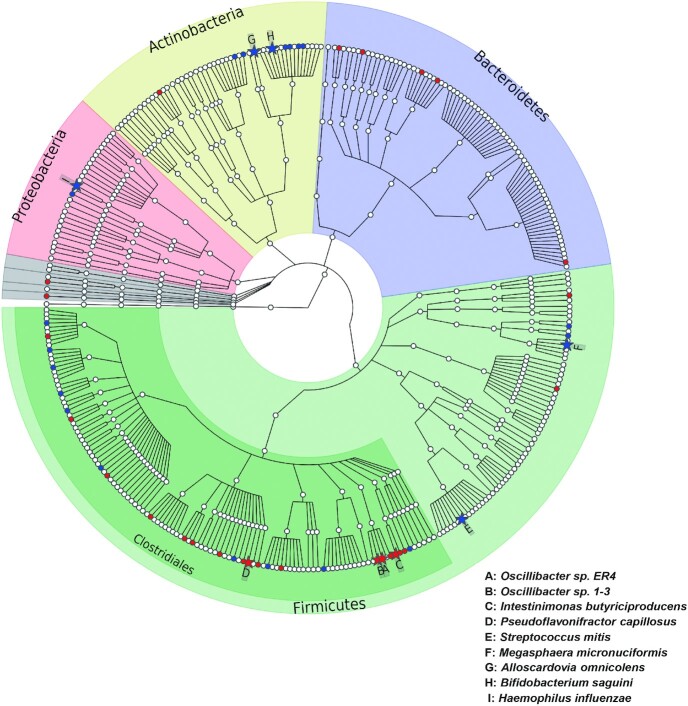
Among the 339 identified gut microbial species (average reads ≥10 and prevalence ≥15%), 9 were significantly associated with serum TMAO concentrations after adjusting for age, sex, BMI, study field center, Hispanic/Latino background, education, family income, smoking, alcohol consumption, total energy intake, physical activity, diabetes, hypertension, dyslipidemia, and antibiotic and probiotic use (FDR-adjusted P < 0.05; Figure 2, Supplemental Table 6). Four of the 9 species, belonging to the order Clostridiales (i.e., Oscillibacter sp. ER4, Oscillibacter sp. 1-3, Intestinimonas butyriciproducens, and Pseudoflavonifractor capillosus), were positively associated with serum TMAO, whereas the other 5 species (i.e., Megasphaera micronuciformis, Streptococcus mitis, Alloscardovia omnicolens, Bifidobacterium saguini, and Haemophilus influenzae) were inversely associated with serum TMAO. We did not find significant associations of these 9 species with fish, red meat, or egg intake.
FIGURE 2.
Phylogenetic tree of taxonomic features in association with serum TMAO concentrations. Species associated with TMAO at FDR-adjusted P < 0.05 were highlighted as solid stars and noted by capital letters, and species with a raw P < 0.05 were highlighted as solid circles. Solid stars or circles with red indicate positive associations whereas blue indicates inverse associations. P values were estimated from linear regression models in 626 individuals after controlling for age, sex, BMI, study field center, Hispanic/Latino background, physical activity, alcohol consumption, smoking, education, yearly household income, total energy intake, diabetes, hypertension, dyslipidemia, and use of antibiotics and probiotics. FDR, false discovery rate; TMAO, trimethylamine-N-oxide.
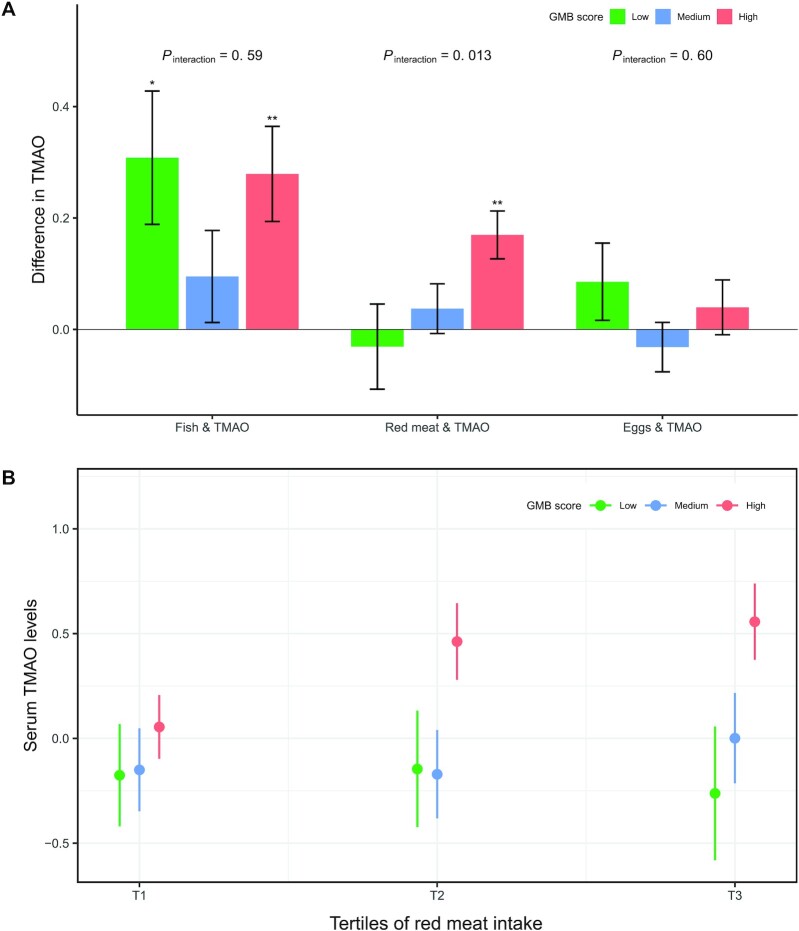
We then focused on 4 species that were positively associated with TMAO and might have the potential to produce TMA, hypothesizing that they might modify the associations of TMAO with red meat and egg intake. As shown in Figure 3A, there was a significant interaction between red meat intake and the GMB score based on these 4 species on serum TMAO (P-interaction = 0.013), with a stronger association between red meat intake and serum TMAO in participants with a higher GMB score compared with those with a lower score. Higher red meat intake was significantly associated with higher serum TMAO only in participants with a high GMB score, but not in those with a low or medium score (Figure 3B). No such microbial modification was observed on the associations of fish or egg intake with TMAO (Figure 3A). Similar results were observed when we analyzed each of these 4 species individually (Supplemental Figure 4). Higher red meat intake was significantly associated with higher serum TMAO only in participants with higher abundance of these species (above median).
FIGURE 3.
Associations of fish, red meat, and egg intake with TMAO according to the GMB score. (A) Data are effect size (β coefficients and SEs) of food intake (per quintile) on serum TMAO levels (inverse-normal transformed) estimated from linear regression after adjustment for age, sex, BMI, study field center, Hispanic/Latino background, physical activity, alcohol consumption, smoking, education, yearly household income, total energy intake, diabetes, hypertension, dyslipidemia, and use of antibiotics and probiotics. The GMB score (range 0–4) was calculated based on the abundance of the 4 microbial species positively associated with TMAO (less than median value = 0; equal to or greater than median value = 1): 0, low GMB score ( n = 137); 1–2, medium GMB score (n = 241); and 3–4, high GMB score (n = 248). * , ** Significant difference in TMAO concentration were estimated from multivariable linear regression models: *P < 0.05 and **P < 0.01 . P-interaction values were calculated by adding a multiplicative factor in the multivariable linear regression models. (B) TMAO levels (inverse-normal transformed) across tertiles (instead of quintiles to ensure adequate sample sizes) of red meat intake according to GMB score. Data are means and SEs estimated from linear regression after adjustment for age, sex, BMI, study field center, Hispanic/Latino background, physical activity, alcohol consumption, smoking, education, yearly household income, total energy intake, diabetes, hypertension, and dyslipidemia. P-trend values across tertiles of red meat intake were 0.83, 0.40, and 6.21 × 10−4 in the low, medium, and high GMB score groups, respectively. Numbers of individuals were 70, 119, and 126 across the low, medium, and high GMB score groups in the first tertile of red meat intake; 38, 64, and 52 across the 3 GMB score groups in the second tertile of red meat intake; and 28, 55, and 69 across the 3 GMB score groups in the third tertile of red meat intake, respectively. GMB, gut microbiota; TMAO, trimethylamine-N-oxide.
Discussion
In a population-based representative sample of US Hispanic/Latino adults, we found that higher serum concentrations of TMAO, a diet-derived, gut microbial–related metabolite, were associated with a higher prevalence of CVD. Since 2011, it has been reported that elevated circulating TMAO is associated with an increased risk of major adverse cardiovascular events (including myocardial infarction, stroke, or death) (1, 2, 5). The relation between TMAO and CVD was examined in subsequent studies of different populations and confirmed in a meta-analysis of 19 clinical-based cohorts, which mainly included Caucasian and black participants (3). To the best of our knowledge, this is the first study to report a positive association between TMAO and prevalent CVD in US Hispanic/Latino adults of diverse backgrounds, though further studies with prospective data in this population are needed.
Our analyses demonstrated both GMB-dependent (e.g., red meat) and GMB-independent (e.g., fish) dietary sources of circulating TMAO in human populations. GMB-dependent TMAO, meaning the fraction that is produced by gut bacterial metabolism, was mainly from dietary choline and carnitine, both of which are abundant in eggs, liver, and a variety of meat (9, 35, 36). However, TMAO also naturally exists in seafood in a preformed state (37, 38). A randomized controlled trial confirmed that eggs had the highest content of choline, beef had the highest concentration of carnitine, whereas fish had 650 times more TMAO compared with eggs and beef (6). Along with another dietary intervention study, these reports found that circulating TMAO increased over a short time after consumption of fish (6, 39). A few observational studies conducted in general populations have examined the associations of food groups with TMAO and yielded various results (8–11, 40). For example, fish and red meat intakes were positively correlated with circulating TMAO in studies from Germany and Italy (10, 11), whereas another study from Germany found that consumption of dairy, but not meat, eggs, or fish, was positively associated with plasma TMAO (8). In addition, a study from China found that consumption of fish but not red meat was associated with elevated urinary TMAO (9). Our current study found that fish, red meat, and eggs were 3 major dietary determinants of serum TMAO in US Hispanics/Latinos; and that red meat and egg intakes, but not fish intake, were positively associated with serum concentrations of TMAO precursors. Our findings provide strong support for 2 suggested major pathways of TMAO in human circulation (6, 39).
Previous studies in mice and humans indicated that circulating TMAO from choline or carnitine was GMB dependent (1, 5). However, the specific taxa that might be associated with TMAO production have not been fully understood. Recently, several studies have explored the relation between gut bacterial taxa and circulating concentrations of TMAO in humans (5, 12, 13, 41, 42). For example, 3 intervention studies including 20 to 60 participants found that several genera (e.g., Clostridium clusters XIVa) belonging to the order Clostridiales were associated with elevated TMAO (5, 41, 42). Strains from Clostridium XIVa have been suggested to possess a choline TMA-lyase gene (cutC) (16). Another 2 recent studies in general populations also found that genera belonging to the orders Clostridiales, Bacteroidales, or Desulfovibrionales were positively associated with circulating TMAO (12, 13). Partially consistent with previous results, the 4 gut microbial species that were positively associated with serum TMAO in our study all belong to the order Clostridiales. More specifically, 2 microbial species (i.e., Oscillibacter sp. ER4 and Oscillibacter sp. 1-3) identified in our study, belong to Oscillibacter, a genus that was previously reported to be associated with circulating TMAO concentrations (42, 43) and cerebrovascular disease (44). Moreover, our bacterial gene alignment analysis indicated that 3 of these 4 gut microbial species (Oscillibacter sp. 1-3, Pseudoflavonifractor capillosus, and Intestinimonas butyriciproducens) possess homologous genes encoding carnitine monooxygenase (CntA/B), an enzyme that converts carnitine to TMA (Supplemental Table 7). This suggests that these species might have the potential to produce TMA, though further analytical and experimental studies are needed to demonstrate the TMA-producing capability of these species.
The identified gut microbial species and their potential capability to produce TMA from carnitine were further supported by a significant microbial modification on the red meat–TMAO association observed in this study. This finding suggests that the positive association between red meat and circulating TMAO is dependent on these gut bacterial taxa, which might contribute to the processing of carnitine from dietary red meat to TMA (10, 45). As expected, we did not find such microbial modification for the association between fish intake and serum TMAO, further supporting fish as a GMB-independent diet source of circulating TMAO (6, 39). We also did not find such microbial modification for the association between egg intake, a major dietary source of choline (10), and serum TMAO, which is in line with our bacterial gene alignment results, namely that these gut microbial species contain carnitine monooxygenase genes (cntA/B), but not choline TMA-lyase gene (cutC) (Supplemental Table 7). Consistently, a recent dietary intervention study also suggested that higher intake of dietary red meat could increase systemic TMAO concentrations through microbial TMA production from dietary carnitine, but not choline (45). Nevertheless, because the dose–response relation between egg consumption and circulating TMAO has been demonstrated in this and previous studies (2, 46), further studies are needed to clarify the gut microbial pathway linking dietary egg and choline consumption to microbial TMA and TMAO production in humans.
This study also identified a number of gut microbial species inversely associated with serum TMAO. Among them, it is noteworthy that Bifidobacterium saguini (FDR-adjusted P < 0.05) and several others (i.e., B. longum, B. breve, B. gallinarum, and B. bifidum with a raw P < 0.05 but did not pass FDR), belong to Bifidobacterium, a genus that has been widely reported to be inversely associated with circulating TMAO concentrations in humans (12, 47) and mice (48). In support of these findings, some members of Bifidobacterium and other gut microbiota, such as the Streptococcaceae family (Streptococcus mitis, inversely associated with serum TMAO in the current study, belongs to this family), have been found to convert TMAO to TMA in mice and pure culture (49), although the conversion rate was relatively low.
Several limitations of our study need to be acknowledged. A major limitation is that ascertainment of dietary intake and serum metabolites preceded sampling of GMB by a median of 7.2 y, although human GMB has been found to be notably stable over a long period (50). This time lag might bias the observed association between gut microbiota and serum TMAO. However, our results have strong biological plausibility, because several identified TMAO-associated species have the potential to produce TMA from carnitine. Due to the cross-sectional study design and its observational nature, our study is unable to make causal inference. We used an untargeted metabolomic approach that did not allow us to obtain absolute concentrations of serum metabolites, although this would not influence association results. Other limitations include uncontrolled [e.g., kidney function measures (51)] or unknown confounding factors, self-reported dietary recalls with inevitable measurement errors, and shallow shotgun sequencing data (28). Finally, the present study included US Hispanics/Latinos, who have a distinctive dietary pattern and GMB composition (19), and triadmixed genetic backgrounds; and hence, it should take caution to generalize our findings.
In summary, our study found a cross-sectional association between TMAO and CVD, and demonstrated that fish, red meat, and egg intakes were major dietary determinants of circulating TMAO concentrations in US Hispanics/Latinos. We identified several gut microbial species that might have the potential to produce TMA, positively associated with serum TMAO. Moreover, the association between red meat intake and serum TMAO could depend on these specific gut microbial species, supporting the essential role of gut microbiota in TMA and TMAO production from dietary carnitine. Our findings provide evidence from a human population study supporting diet-derived TMAO formation in the human circulation, in both a GMB-independent and GMB-dependent manner. This in turn might have important implications for future efforts to prevent CVD related to this pathway in human populations through diet and GMB modification.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—ZM, G-CC, QQ: conceived the study; ZM, ZW: performed statistical analyses; ZM: drafted the manuscript; G-CC, ZW, QQ: critically revised the manuscript; MLD, RDB, RCK, QQ: collected the data and specimens from the HCHS/SOL participants and obtained funding; ZW, MU, YVB, GH, RSB, RK: did the gut microbial sequencing analysis; MU, RDB: did the processing of the HCHS/SOL fecal samples; BY, EB: did the metabolome profiling analysis; JL, JSW-N, LH, JC, YZ, RK: edited and reviewed the manuscript; QQ: is the guarantor of this work and had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
The Hispanic Community Health Study/Study of Latinos (HCHS/SOL) is a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (HHSN268201300001I/N01-HC-65233), University of Miami (HHSN268201300004I/N01-HC-65234), Albert Einstein College of Medicine (HHSN268201300002I/N01-HC-65235), University of Illinois at Chicago (HHSN268201300003I/N01-HC-65236 Northwestern University), and San Diego State University (HHSN268201300005I/N01-HC-65237). The following Institutes/Centers/Offices have contributed to the HCHS/SOL through a transfer of funds to the NHLBI: National Institute on Minority Health and Health Disparities (NIMHD), National Institute on Deafness and Other Communication Disorders, National Institute of Dental and Craniofacial Research, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Neurological Disorders and Stroke, and NIH Institution-Office of Dietary Supplements.
This work is supported by the NIDDK R01DK119268 and NHLBI R01HL060712, and other funding sources for this study include UM1 HG008898 from the National Human Genome Research Institute, R01MD011389 from the NIMHD, and R01HL140976 from the NHLBI. ZM was supported by Fudan University Exchange Program Scholarship for Doctoral Students.
Supplemental Tables 1–7 and Supplemental Figures 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: CDCR, cerebrovascular disease or carotid revascularization; CHD, coronary heart disease; Cnt, carnitine monooxygenase; Cut, choline utilization; CVD, cardiovascular disease; FDR, false discovery rate; GMB, gut microbiota; GOLD, Gut Origins of Latino Diabetes; HCHS/SOL, Hispanic Community Health Study/Study of Latinos; NCBI, National Center for Biotechnology Information; TMA, trimethylamine; TMAO, trimethylamine-N-oxide.
Contributor Information
Zhendong Mei, State Key Laboratory of Genetic Engineering, Human Phenome Institute, and School of Life Sciences, Fudan University, Shanghai, China; Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA.
Guo-Chong Chen, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA.
Zheng Wang, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA.
Mykhaylo Usyk, Department of Pediatrics, Albert Einstein College of Medicine, Bronx, NY, USA.
Bing Yu, Department of Epidemiology, Human Genetics, and Environmental Sciences, School of Public Health, The University of Texas Health Science Center at Houston, Houston, TX, USA.
Yoshiki Vazquez Baeza, Department of Pediatrics, University of California, San Diego, La Jolla, CA, USA.
Greg Humphrey, Department of Pediatrics, University of California, San Diego, La Jolla, CA, USA.
Rodolfo Salido Benitez, Department of Pediatrics, University of California, San Diego, La Jolla, CA, USA.
Jun Li, Department of Nutrition and Department of Epidemiology, Harvard TH Chan School of Public Health, Boston, MA, USA.
Jessica S Williams-Nguyen, Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Martha L Daviglus, Institute of Minority Health Research, University of Illinois College of Medicine, Chicago, IL, USA.
Lifang Hou, Institute for Public Health and Medicine, Northwestern University, Chicago, IL, USA.
Jianwen Cai, Department of Biostatistics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Yan Zheng, State Key Laboratory of Genetic Engineering, Human Phenome Institute, and School of Life Sciences, Fudan University, Shanghai, China; Ministry of Education Key Laboratory of Public Health Safety, School of Public Health, Fudan University, Shanghai, China.
Rob Knight, Department of Pediatrics, University of California, San Diego, La Jolla, CA, USA; Department of Computer Science and Engineering, Jacobs School of Engineering, and Center for Microbiome Innovation, University of California San Diego, La Jolla, CA, USA.
Robert D Burk, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA; Department of Pediatrics, Albert Einstein College of Medicine, Bronx, NY, USA; Department of Microbiology and Immunology, and Department of Obstetrics, Gynecology and Women's Health, Albert Einstein College of Medicine, Bronx, NY, USA.
Eric Boerwinkle, Department of Epidemiology, Human Genetics, and Environmental Sciences, School of Public Health, The University of Texas Health Science Center at Houston, Houston, TX, USA.
Robert C Kaplan, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA; Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Qibin Qi, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA; Department of Nutrition and Department of Epidemiology, Harvard TH Chan School of Public Health, Boston, MA, USA.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending application.
References
- 1. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, DuGar B, Feldstein AE, Britt EB, Fu X, Chung Y-Met al. . Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tang WW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta-analysis of prospective studies. J Am Heart Assoc. 2017;6(7):e004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. He Z, Chen Z-Y. The origin of trimethylamine-N-oxide (TMAO) and its role in development of atherosclerosis. J Food Bioactives. 2018;2(2):28–36. [Google Scholar]
- 5. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li Let al. . Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cho CE, Taesuwan S, Malysheva OV, Bender E, Tulchinsky NF, Yan J, Sutter JL, Caudill MA. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: a randomized controlled trial. Mol Nutr Food Res. 2017;61(1):1600324. [DOI] [PubMed] [Google Scholar]
- 7. Bjørndal B, Ramsvik M, Lindquist C, Nordrehaug J, Bruheim I, Svardal A, Nygård O, Berge R. A phospholipid-protein complex from Antarctic krill reduced plasma homocysteine levels and increased plasma trimethylamine-N-oxide (TMAO) and carnitine levels in male Wistar rats. Marine Drugs. 2015;13(9):5706–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rohrmann S, Linseisen J, Allenspach M, von Eckardstein A, Müller D. Plasma concentrations of trimethylamine-N-oxide are directly associated with dairy food consumption and low-grade inflammation in a German adult population. J Nutr. 2016;146(2):283–9. [DOI] [PubMed] [Google Scholar]
- 9. Yu D, Shu XO, Rivera ES, Zhang X, Cai Q, Calcutt MW, Xiang YB, Li H, Gao YT, Wang TJet al. . Urinary levels of trimethylamine-N-oxide and incident coronary heart disease: a prospective investigation among urban Chinese adults. J Am Heart Assoc. 2019;8(1):e010606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krüger R, Merz B, Rist MJ, Ferrario PG, Bub A, Kulling SE, Watzl B. Associations of current diet with plasma and urine TMAO in the KarMeN study: direct and indirect contributions. Mol Nutr Food Res. 2017;61(11):1700363. [DOI] [PubMed] [Google Scholar]
- 11. Barrea L, Annunziata G, Muscogiuri G, Laudisio D, Di Somma C, Maisto M, Tenore GC, Colao A, Savastano S. Trimethylamine N-oxide, Mediterranean diet, and nutrition in healthy, normal-weight adults: also a matter of sex?. Nutrition. 2019;62:7–17. [DOI] [PubMed] [Google Scholar]
- 12. Manor O, Zubair N, Conomos MP, Xu X, Rohwer JE, Krafft CE, Lovejoy JC, Magis AT. A multi-omic association study of trimethylamine N-oxide. Cell Rep. 2018;24(4):935–46. [DOI] [PubMed] [Google Scholar]
- 13. Fu BC, Hullar MA, Randolph TW, Franke AA, Monroe KR, Cheng I, Wilkens LR, Shepherd JA, Madeleine MM, Le Marchand Let al. . Associations of plasma trimethylamine N-oxide, choline, carnitine, and betaine with inflammatory and cardiometabolic risk biomarkers and the fecal microbiome in the Multiethnic Cohort Adiposity Phenotype Study. Am J Clin Nutr. 2020;111:1226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Craciun S, Balskus EP. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc Natl Acad Sci U S A. 2012;109(52):21307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhu Y, Jameson E, Crosatti M, Schäfer H, Rajakumar K, Bugg TD, Chen Y. Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proc Natl Acad Sci U S A. 2014;111(11):4268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rath S, Heidrich B, Pieper DH, Vital M. Uncovering the trimethylamine-producing bacteria of the human gut microbiota. Microbiome. 2017;5(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. LaVange LM, Kalsbeek WD, Sorlie PD, Avilés-Santa LM, Kaplan RC, Barnhart J, Liu K, Giachello A, Lee DJ, Ryan Jet al. . Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20(8):642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sorlie PD, Avilés-Santa LM, Wassertheil-Smoller S, Kaplan RC, Daviglus ML, Giachello AL, Schneiderman N, Raij L, Talavera G, Allison Met al. . Design and implementation of the Hispanic community health study/study of Latinos. Ann Epidemiol. 2010;20(8):629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaplan RC, Wang Z, Usyk M, Sotres-Alvarez D, Daviglus ML, Schneiderman N, Talavera GA, Gellman MD, Thyagarajan B, Moon J-Yet al. . Gut microbiome composition in the Hispanic Community Health Study/Study of Latinos is shaped by geographic relocation, environmental factors, and obesity. Genome Biol. 2019;20(1):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schneiderman N, Llabre M, Cowie CC, Barnhart J, Carnethon M, Gallo LC, Giachello AL, Heiss G, Kaplan RC, LaVange LMet al. . Prevalence of diabetes among Hispanics/Latinos from diverse backgrounds: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Diabetes Care. 2014;37(8):2233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Daviglus ML, Talavera GA, Avilés-Santa ML, Allison M, Cai J, Criqui MH, Gellman M, Giachello AL, Gouskova N, Kaplan RCet al. . Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA. 2012;308(17):1775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Siega-Riz AM, Sotres-Alvarez D, Ayala GX, Ginsberg M, Himes JH, Liu K, Loria CM, Mossavar-Rahmani Y, Rock CL, Rodriguez Bet al. . Food-group and nutrient-density intakes by Hispanic and Latino backgrounds in the Hispanic Community Health Study/Study of Latinos. Am J Clin Nutr. 2014;99(6):1487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang X, Jung M, Mossavar-Rahmani Y, Sotres-Alvarez D, Espinoza Giacinto RA, Pirzada A, Reina SA, Casagrande SS, Wang T, Avilés-Santa MLet al. . Macronutrient intake, diagnosis status, and glycemic control among US Hispanics/Latinos with diabetes. J Clin Endocrinol Metab. 2016;101(4):1856–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schakel SF, Buzzard IM, Gebhardt SE. Procedures for estimating nutrient values for food composition databases. J Food Compos Anal. 1997;10(2):102–14. [Google Scholar]
- 25. Arredondo EM, Sotres-Alvarez D, Stoutenberg M, Davis SM, Crespo NC, Carnethon MR, Castañeda SF, Isasi CR, Espinoza RA, Daviglus MLet al. . Physical activity levels in US Latino/Hispanic adults: results from the Hispanic community health study/study of Latinos. Am J Prev Med. 2016;50(4):500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zheng X, Zhao A, Xie G, Chi Y, Zhao L, Li H, Wang C, Bao Y, Jia W, Luther Met al. . Melamine-induced renal toxicity is mediated by the gut microbiota. Sci Transl Med. 2013;5(172):172ra22. [DOI] [PubMed] [Google Scholar]
- 27. Yang J, Loos RJ, Powell JE, Medland SE, Speliotes EK, Chasman DI, Rose LM, Thorleifsson G, Steinthorsdottir V, Mägi Ret al. . FTO genotype is associated with phenotypic variability of body mass index. Nature. 2012;490(7419):267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hillmann B, Al-Ghalith GA, Shields-Cutler RR, Zhu Q, Gohl DM, Beckman KB, Knight R, Knights D. Evaluating the information content of shallow shotgun metagenomics. Msystems. 2018;3(6):e00069–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Costello M, Fleharty M, Abreu J, Farjoun Y, Ferriera S, Holmes L, Granger B, Green L, Howd T, Mason Tet al. . Characterization and remediation of sample index swaps by non-redundant dual indexing on massively parallel sequencing platforms. BMC Genomics. 2018;19(1):332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Glenn TC, Nilsen RA, Kieran TJ, Sanders JG, Bayona-Vásquez NJ, Finger JW, Pierson TW, Bentley KE, Hoffberg SL, Louha Set al. . Adapterama I: universal stubs and primers for 384 unique dual-indexed or 147,456 combinatorially-indexed Illumina libraries (iTru & iNext). PeerJ. 2019;7:e7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schmieder R, Edwards R. Quality control and preprocessing of metagenomic datasets. Bioinformatics. 2011;27(6):863–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Al-Ghalith G, Knights D. BURST enables optimal exhaustive DNA alignment for big data. Zenodo [Internet]2020. Available from:http://doi.org/10.5281/zenodo.3779009. [Google Scholar]
- 33. Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paulson JN, Stine OC, Bravo HC, Pop M. Differential abundance analysis for microbial marker-gene surveys. Nat Methods. 2013;10(12):1200–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Patterson KY, Bhagwat SA, Williams JR, Howe JC, Holden J, Zeisel S, Dacosta K, Mar M. USDA database for the choline content of common foods, release two (2008) [Internet] [accessed 11 February 2021]. Nutrient Data Laboratory, Beltsville Human Nutrition Research Center, ARS, USDA; 2008. Version current 1 November 2019. Available from: 10.15482/USDA.ADC/1178141. [DOI] [Google Scholar]
- 36. Malinowska AM, Szwengiel A, Chmurzynska A. Dietary, anthropometric, and biochemical factors influencing plasma choline, carnitine, trimethylamine, and trimethylamine-N-oxide concentrations. Int J Food Sci Nutr. 2017;68(4):488–95. [DOI] [PubMed] [Google Scholar]
- 37. Cho CE, Caudill MA. Trimethylamine-N-oxide: friend, foe, or simply caught in the cross-fire?. Trends Endocrinol Metab. 2017;28(2):121–30. [DOI] [PubMed] [Google Scholar]
- 38. Landfald B, Valeur J, Berstad A, Raa J. Microbial trimethylamine-N-oxide as a disease marker: something fishy? Microb Ecol Health Dis. 2017;28(1):1327309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cheung W, Keski-Rahkonen P, Assi N, Ferrari P, Freisling H, Rinaldi S, Slimani N, Zamora-Ros R, Rundle M, Frost Get al. . A metabolomic study of biomarkers of meat and fish intake. Am J Clin Nutr. 2017;105(3):600–8. [DOI] [PubMed] [Google Scholar]
- 40. Genoni A, Christophersen CT, Lo J, Coghlan M, Boyce MC, Bird AR, Lyons-Wall P, Devine A. Long-term Paleolithic diet is associated with lower resistant starch intake, different gut microbiota composition and increased serum TMAO concentrations. Eur J Nutr. 2020;59:1845–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu W-K, Chen C-C, Liu P-Y, Panyod S, Liao B-Y, Chen P-C, Kao H-L, Kuo H-C, Kuo C-H, Chiu THet al. . Identification of TMAO-producer phenotype and host–diet–gut dysbiosis by carnitine challenge test in human and germ-free mice. Gut. 2019;68(8):1439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schmedes M, Brejnrod AD, Aadland EK, Kiilerich P, Kristiansen K, Jacques H, Lavigne C, Graff IE, Eng Ø, Holthe Aet al. . The effect of lean-seafood and non-seafood diets on fecal metabolites and gut microbiome: results from a randomized crossover intervention study. Mol Nutr Food Res. 2019;63(1):1700976. [DOI] [PubMed] [Google Scholar]
- 43. Wang S, Xia G, He Y, Liao S, Yin J, Sheng H, Zhou H. Distribution characteristics of trimethylamine N-oxide and its association with gut microbiota. Nan Fang Yi Ke Da Xue Xue Bao. 2016;36(4):455–60. [PubMed] [Google Scholar]
- 44. Yin J, Liao SX, He Y, Wang S, Xia GH, Liu FT, Zhu JJ, You C, Chen Q, Zhou Let al. . Dysbiosis of gut microbiota with reduced trimethylamine-N-oxide level in patients with large-artery atherosclerotic stroke or transient ischemic attack. J Am Heart Assoc. 2015;4(11):e002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, Koeth RA, Li L, Wu Y, Tang WWet al. . Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J. 2019;40(7):583–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Miller CA, Corbin KD, da Costa K-A, Zhang S, Zhao X, Galanko JA, Blevins T, Bennett BJ, O'Connor A, Zeisel SH. Effect of egg ingestion on trimethylamine-N-oxide production in humans: a randomized, controlled, dose-response study. Am J Clin Nutr. 2014;100(3):778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang C, Yin A, Li H, Wang R, Wu G, Shen J, Zhang M, Wang L, Hou Y, Ouyang Het al. . Dietary modulation of gut microbiota contributes to alleviation of both genetic and simple obesity in children. EBioMedicine. 2015;2(8):968–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen M-I, Yi L, Zhang Y, Zhou X, Ran L, Yang J, Zhu J-D, Zhang Q-Y, Mi M-T. Resveratrol attenuates trimethylamine-N-oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. MBio. 2016;7(2):e02210–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hoyles L, Jiménez-Pranteda ML, Chilloux J, Brial F, Myridakis A, Aranias T, Magnan C, Gibson GR, Sanderson JD, Nicholson JKet al. . Metabolic retroconversion of trimethylamine N-oxide and the gut microbiota. Microbiome. 2018;6(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RLet al. . The long-term stability of the human gut microbiota. Science. 2013;341(6141):1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stubbs JR, House JA, Ocque AJ, Zhang S, Johnson C, Kimber C, Schmidt K, Gupta A, Wetmore JB, Nolin TDet al. . Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J Am Soc Nephrol. 2016;27(1):305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending application.