ABSTRACT
Background
The number of APOE-ε4 alleles is a major nonmodifiable risk factor for sporadic Alzheimer disease (AD). There is increasing evidence on the benefits of dietary DHA (22:6n–3) before the onset of AD symptoms, particularly in APOE-ε4 carriers. Brain alterations in the preclinical stage can be detected by structural MRI.
Objectives
We aimed, in middle-aged cognitively unimpaired individuals at increased risk of AD, to cross-sectionally investigate whether dietary DHA intake relates to cognitive performance and to MRI-based markers of cerebral small vessel disease and AD-related neurodegeneration, exploring the effect modification by APOE-ε4 status.
Methods
In 340 participants of the ALFA (ALzheimer and FAmilies) study, which is enriched for APOE-ε4 carriership (n = 122, noncarriers; n = 157, 1 allele; n = 61, 2 alleles), we assessed self-reported DHA intake through an FFQ. We measured cognitive performance by administering episodic memory and executive function tests. We performed high-resolution structural MRI to assess cerebral small vessel disease [white matter hyperintensities (WMHs) and cerebral microbleeds (CMBs)] and AD-related brain atrophy (cortical thickness in an AD signature). We constructed regression models adjusted for potential confounders, exploring the interaction DHA × APOE-ε4.
Results
We observed no significant associations between DHA and cognitive performance or WMH burden. We observed a nonsignificant inverse association between DHA and prevalence of lobar CMBs (OR: 0.446; 95% CI: 0.195, 1.018; P = 0.055). DHA was found to be significantly related to greater cortical thickness in the AD signature in homozygotes but not in nonhomozygotes (P-interaction = 0.045). The association strengthened when analyzing homozygotes and nonhomozygotes matched for risk factors.
Conclusions
In cognitively unimpaired APOE-ε4 homozygotes, dietary DHA intake related to structural patterns that may result in greater resilience to AD pathology. This is consistent with the current hypothesis that those subjects at highest risk would obtain the largest benefits from DHA supplementation in the preclinical stage.
This trial was registered at clinicaltrials.gov as NCT01835717.
Keywords: omega-3 fatty acids, cognition, markers, white matter hyperintensities, cerebral small vessel disease, brain atrophy
See corresponding editorial on page 1396.
Introduction
Alzheimer disease (AD) imposes a huge socioeconomic burden. Given that at the moment there are no approved disease-modifying drugs for AD, preventive strategies are of paramount importance (1). There is a large body of observational evidence on the cognitive benefits of regular consumption of fatty fish (2), which is the main dietary source of DHA (22:6n–3). This fatty acid is critical for brain function and ameliorates AD features (3). Whether dietary intake of DHA relates to cognitive decline and AD is still under debate. A 2015 meta-analysis of 21 epidemiologic studies reported a significantly reduced risk of AD for DHA intake (4). However, randomized controlled trials on DHA supplementation and cognition have yielded mixed results (5). A plausible reason to explain part of this controversy is the influence of the genetic background, in particular the APOE genotype (2, 5, 6), which is the strongest genetic risk factor for sporadic AD (7). In this regard, cognitive benefits of DHA supplementation have been reported in cognitively unimpaired APOE-ε4 carriers, but not in those with AD symptomatology (5, 6). This emphasizes the need for interventions in this population segment before clinical symptoms appear (the so-called preclinical stage).
ApoE has a critical role in lipid transport. APOE-ε4 carriers present a specific isoform of apoE (apoE-ε4) that is less efficient in this function and, in addition, contributes to AD pathogenesis by affecting multiple other pathways, including cerebrovascular derangements and faster brain accumulation of amyloid-β, a pathological hallmark of AD (8). Given that apoe-ε4 operates from the earliest stages of life, it has been suggested that apoE-ε4-related cumulative changes could be observed by MRI in the brains of APOE-ε4 carriers long before the onset of cognitive decline. On one hand, APOE-ε4 carriers, in particular APOE-ɛ4 homozygotes, have an increased burden of MRI markers of cerebral small vessel disease, including white matter hyperintensities (WMHs) (9) and cerebral microbleeds (CMBs) (10), which may confer an increased risk of stroke and AD (11). On the other hand, most studies have reported accelerated atrophy in AD-sensitive regions, related to APOE-ε4 carriership (12).
Given the increasing evidence of APOE-ε4 load as a factor in brain vulnerability in the preclinical stage of AD, we hypothesized that in middle-aged cognitively unimpaired individuals, APOE-ε4 status would modulate the associations of dietary DHA with cognitive performance (direct association) and with MRI-assessed structural brain alterations (inverse association). To ascertain this, we assessed self-reported dietary intake of DHA and searched for associations with performance in neuropsychological testing (episodic memory and executive function), MRI markers of cerebral small vessel disease (WMHs and CMBs), and early AD-related neurodegeneration (cortical thickness in AD-vulnerable regions) in a population enriched with APOE-ε4 carriership (n = 122, noncarriers; n = 157, 1 allele; n = 61, 2 alleles).
Methods
Participants
We conducted this cross-sectional study (NCT01835717) in participants from the ALFA (ALzheimer and FAmilies) study, which is being carried out at the Barcelonaβeta Brain Research Center (BBRC). The protocol of the ALFA study was approved by the Independent Ethics Committee of the “Parc de Salut Mar” (Barcelona, Spain). Detailed information on the study can be found elsewhere (13). In brief, the ALFA parent cohort is comprised of 2743 cognitively unimpaired middle-aged subjects (45–75 y), many of them kindred of AD patients (47.4% of the participants had ≥1 parent diagnosed with AD before the age of 75 y). To ensure unimpaired cognitive status, an initial evaluation of the participants’ neuropsychological status was performed via 4 screening tests. We excluded participants with a Mini-Mental State Examination score < 26, or a Memory Impairment Screen score < 6, or a score on the Time-Orientation subtest of the Barcelona Test II <68, or a Semantic fluency (animals) score < 12. An additional exclusion criterion was a score > 0 on the Clinical Dementia Rating scale, which is derived from a standard clinical impression interview performed with both the participant and a reliable informant to assess cognitive status. At baseline, ALFA participants also provided sociodemographic, anthropometric, clinical, and lifestyle data, along with a blood sample for further genetic analysis, including APOE genotyping. We selected a subgroup of 608 ALFA participants without MRI contraindications, preferentially including APOE-ε4 and APOE-ε2 carriers, to participate in the neuroimaging study, which was also approved by the Ethics Committee of the “Parc de Salut Mar” (Barcelona, Spain). From the 608 participants invited to participate in this study, 595 agreed to undergo MRI and 575 provided valid MRI scans. Because of the protective effects on AD risk of the ε2/ε3 and in particular the ε2/ε2 genotype (14), for this substudy we excluded participants with these genotypes (n = 119, 7 of which were ε2/ε2). All participants accepted the study procedures by signing the study's informed consent form.
Sociodemographic, clinical, and lifestyle data
Data were registered either during the clinical interview or through online self-administered questionnaires. All participants were asked about their family and personal medical history, and medication use was recorded. Participants were considered “hypertensive” if ≥1 of the following conditions was met: 1) self-reported diagnosis; 2) current use of antihypertensive medication; 3) measured systolic blood pressure > 140 mm Hg. “Hypercholesterolemia” was categorized as present if self-reported or if subjects were using cholesterol-lowering medications. Family history of AD was recorded as previously reported (13). In brief, family history was divided into 4 possible groups: “no AD family history,” “maternal,” “paternal,” and “both parents.” This classification was only considered positive if the relative was younger than 75 y at the time of onset of cognitive symptoms. Height, weight, and blood pressure were measured by standard methods.
Participants were asked to provide dietary data by completing a web-based self-administered FFQ. This validated questionnaire has a closed list of 166 items representing typical foods in northeastern Spain (15), including 16 items related to fish and seafood: lean fish; salmon; trout; uncanned sardine; uncanned tuna; mackerel; uncanned bivalves; shrimp, prawn, and crayfish; octopus, baby squid, and squid; tuna canned in oil; tuna canned in brine; sardines canned in oil; sardines canned in brine; anchovy fillets canned in oil; canned clams; and canned cockles. For each food item, participants were asked to indicate their usual consumption from 9 frequency categories, ranging from never or less than once per month to ≥6 times/d. The questionnaire also contained an open section to admit additions to the food list for foods, beverages, and nutritional supplements not included in the closed list of food items. Intakes were converted to mean grams per day using standard reference portion sizes, defined by natural (e.g., 1 orange, 1 slice of bread) or household units (e.g., 1 spoon, 1 cup, 1 glass). We computed intakes of energy and fatty acids including DHA using Spanish food composition tables and the Medisystem 2000 software (Conaycite).
Cognitive testing
During neuropsychological evaluation, participants were administered a cognitive test battery. This battery assessed episodic verbal memory by means of the Memory Binding Test (MBT) (16, 17), as well as executive and reasoning functions using the Wechsler Adult Intelligence Scale (WAIS)-IV including psychomotor speed, visual processing, executive function, and nonverbal and verbal reasoning (Coding, Visual Puzzles, Digit Span, Matrix Reasoning, and Similarities) (18). We computed 2 cognitive composites to assess global episodic memory and executive function by creating z scores for the cognitive measures from the MBT and from the WAIS-IV subtests, respectively. These global measures were calculated by averaging normalized age- and education-regressed scores of all subtests in each domain. As with the individual cognitive tests, higher scores in the different composites represent better cognitive performance, whereas lower scores correspond to worse cognitive performance.
MRI acquisition and processing
MRI scans were acquired on a 3.0-T scanner (GE Discovery MR750 W 3T) using a protocol that included 1 T1-weighted sequence and 3 T2-weighted sequences [fluid-attenuated inversion recovery (FLAIR), fast spin echo (FSE), and gradient echo (GRE)]. The T1-weighted sequence had an isotropic voxel size of 1 mm3 with a matrix size of 256 × 256 × 160, repetition time (TR) = 8.3 ms, echo time (TE) = 3.7 ms, inversion time (TI) = 450 ms, flip angle = 8°. T2 and T2*-weighted sequences, with a voxel size of 1 × 1 × 3 mm, were as follows: FLAIR: TR/TE/TI = 11,000/90/2600 ms, flip angle = 160°; FSE: TR/TE = 5000/85 ms, flip angle = 110°; and GRE: TR/TE = 1300/23 ms, flip angle = 15°. A trained neuroradiologist visually assessed all scans for quality and incidental findings (19).
FLAIR images were assessed for WMHs of presumably vascular origin and automatically segmented using a Bayesian algorithm, as detailed in Sudre et al. (20). Details of the imaging processing can be found in Salvadó et al. (21). Subcortical volumes, cortical thickness, and surface area measures were estimated from 3D T1 MRI using Freesurfer version 5.3.0 (https://surfer.nmr.mgh.harvard.edu) as previously described (22). All segmentations were visually inspected. We computed the AD-signature meta-region of interest consisting of the surface-area weighted average mean cortical thickness in the entorhinal, inferior temporal, middle temporal, and fusiform regions, as described in Jack et al. (23).
CMBs were defined as foci of hypointensity < 10 mm in diameter on the T2* GRE images. The visual assessment of CMBs was performed by consensus of 2 experienced raters blinded to all clinical data and APOE genotype according to the Brain Observer Microbleeds Scale (BOMBS) Criteria (24). Briefly, the BOMBS Criteria are a classification system devised to improve levels of interrater agreement about the presence, number, size, and location of CMBs including 7 anatomical locations, including cortex/gray-white matter junction, subcortical white matter, basal ganglia, internal and external capsule, thalamus, brainstem, and cerebellum. Cortex/gray-white matter junction and subcortical white matter were considered “lobar” locations, whereas basal ganglia, internal and external capsule, thalamus, and cerebellum were considered “deep” locations. The localization of CMBs was marked using ITK-snap (www.itksnap.org) (25).
Statistical analyses
The lack of available literature on the effect of APOE-ε4 genotype on self-reported DHA intake and cognition/neuroimaging in cognitively unimpaired participants (5), coupled with the fact that this study was initially conceived as an exploratory substudy to be conducted in ALFA participants with available data, precluded us from running an a priori power calculation for this substudy (26). However, we conducted sensitivity analyses by using free G-Power 3.1.9.4 software (http://www.gpower.hhu.de) (27) (Supplemental File 1).
We expressed categorical variables as frequencies and percentages, whereas quantitative variables following a normal distribution were expressed as mean (95% CI). The normal distribution of continuous variables was assessed by the Kolmogorov–Smirnov test. Skewed variables, which are reported as medians and IQRs, were rank-transformed for further parametric analyses.
We assessed differences between participants included in the analyses and the whole ALFA population by 1-factor ANOVA, the Kruskal–Wallis test, or the chi-square test, as appropriate.
We next constructed regression models to search for associations between self-reported DHA intake (predictor) and episodic memory and executive function composite scores, WMH burden, prevalence of CMBs, and cortical thickness in the AD signature (outcomes). We did not consider the presence of CMBs in deep brain and lacunar infarcts as outcomes owing to the low number of cases in our population study (n = 14 and n = 12, respectively). For each outcome, we tested the distinct models of APOE-ε4 penetrance, namely the dominant, recessive, and additive effects, as proposed for the analysis of quantitative trait loci (28). Briefly, an additive model predicts an incremental response of the quantitative trait according to the allelic load, whereas a dominant model predicts a common response to 1 copy or 2 copies of the risk allele (i.e., ε4-carriers compared with noncarriers). Finally, a recessive model predicts a common response to 0 copies or 1 copy of the risk allele (i.e., noncarriers and ε4-heterozygotes compared with ε4-homozygotes).
We first searched for associations between DHA and episodic memory and executive function composites using linear regression models, adjusting for gender and total energy intake. Given the documented brain benefits of dietary α-linolenic acid (ALA; 18:3n–3) (the vegetable n–3) (29), we also included self-reported dietary intake of ALA as a confounder. Second, we explored the association between DHA and WMH burden by linear regression models, adjusting for total intracranial volume, gender, age, BMI, hypertension, hypercholesterolemia, total energy intake, and ALA. Third, we evaluated the associations between DHA and the presence of CMBs (in any brain area, and in lobar regions). To this end, we constructed logistic regression models, adjusting for the variables included in the WMH models, except for total intracranial volume. Fourth, we explored the association between DHA and cortical thickness in the AD signature by constructing identical models to those designed for WMHs, but excluding total intracranial volume as a covariate. For each outcome, we further constructed an additional model to assess the interaction DHA × APOE-ε4. In the event of a statistically significant DHA × APOE-ε4 interaction, we stratified the sample by APOE-ε4 status to further search for group-specific associations. To reduce the residual confounding, we further performed a propensity score analysis using a 1:1 matching for selected adjusting covariates, including gender, age (within 2.5 y), BMI (normoweight/overweight/obese), and, when possible, hypertension (yes/no) and hypercholesterolemia (yes/no). We then determined the Pearson correlation coefficients between DHA and standardized residuals outputted from general linear models including the covariates.
For all regression analyses, standard diagnostic checks on the residuals from the fitted models showed no evidence of any failure of the assumptions of normality and homogeneity of the residual variance.
Statistical significance was set at the P < 0.05 level in all cases. Analyses were performed using SPSS software, release 20.0 (IBM Corp.). Figures were built using R software (R Foundation for Statistical Computing; http://www.r-project.org/).
Results
Of 456 participants that were not APOE-ε2/ε2 or APOE-ε2/ε3 with MRI scans, 12 were removed from the study owing to the presence of incidental findings, motion artifacts, or segmentation problems. After further excluding participants with incomplete dietary data (n = 64) and those who reported total energy intake outside predefined limits [>4000 or <800 kcal/d in men and >3500 or <500 kcal/d in women (30), n = 40], 340 participants remained in the present analyses. None of the participants reported consumption of fish oil or foods supplemented with DHA. Supplemental Figure 1 depicts the flowchart of participants throughout the study. Compared with the whole cohort, participants included in the analyses more often had a prior history of hypercholesterolemia and prevalence of APOE-ε4 carriership (P < 0.001, both). Table 1 shows the characteristics of the study's population by number of APOE-ε4 alleles. Supplemental Table 1 displays information regarding the 40 participants excluded for reporting total energy intake outside the predefined limits.
TABLE 1.
Demographic, clinical, genetic, lifestyle, and neuroimaging data of the study population by number of APOE-ε4 alleles1
| APOE-ε4 alleles, n | |||
|---|---|---|---|
| Variable | None (n = 122) | One2 (n = 157) | Two (n = 61) |
| Women | 80 (65.6) | 81 (51.6) | 40 (65.6) |
| Age, y | 58.7 (57.2, 60.2) | 58.0 (56.8, 59.1) | 54.2 (52.6, 55.8) |
| Parental history of AD before 75 y | |||
| No AD family history | 57 (46.7) | 69 (43.9) | 26 (42.6) |
| Paternal | 17 (13.9) | 30 (19.1) | 8 (13.1) |
| Maternal | 48 (39.3) | 52 (33.1) | 21 (34.4) |
| Both parents | 0 (0.0) | 6 (3.8) | 6 (9.8) |
| Hypertension | 32 (26.2) | 32 (20.4) | 12 (19.7) |
| Hypercholesterolemia | 47 (38.5) | 56 (35.7) | 24 (39.3) |
| Years of education | 13.5 (12.9, 14.2) | 14.0 (13.4, 14.5) | 13.5 (12.6, 14.4) |
| Smoking | |||
| Never smoker | 17 (13.9) | 28 (17.8) | 5 (8.3) |
| Current smoker | 31 (25.4) | 34 (21.7) | 19 (31.7) |
| Former smoker | 74 (60.7) | 95 (60.5) | 36 (60.0) |
| Weight, kg | 73.1 (70.8, 75.5) | 74.7 (72.4, 77.0) | 72.9 (69.3, 76.4) |
| BMI, kg/m2 | 26.7 (26.0, 27.3) | 26.8 (26.1, 27.4) | 27.0 (25.9, 28.1) |
| Dietary data | |||
| Energy, kcal/d | 2467 (2369, 2565) | 2430 (2332, 2527) | 2353 (2217, 2489) |
| Seafood, g/d | 107 (97, 117) | 106 (94, 119) | 114 (93, 134) |
| Fatty fish, g/d | 60 (53, 67) | 55 (50, 60) | 60 (46, 74) |
| DHA, g/d | 0.82 (0.74, 0.90) | 0.76 (0.70, 0.82) | 0.77 (0.66, 0.88) |
| ALA, g/d | 1.06 (1.01, 1.12) | 1.04 (0.98, 1.09) | 1.06 (0.98, 1.14) |
| Neuroimaging data | |||
| WMH burden, cm3 | 2.19 [1.99–3.98] | 2.02 [0.99–3.69] | 1.91 [1.11–4.37] |
| Prevalence of microbleeds, any brain area | 21 (17.2) | 29 (18.7) | 12 (19.7) |
| Prevalence of microbleeds, lobar brain | 19 (15.6) | 22 (14.2) | 9 (14.8) |
| Prevalence of microbleeds, deep brain | 3 (2.5) | 7 (4.5) | 4 (6.6) |
| Lacunar infarcts | 5 (4.1) | 7 (4.5) | 0 (0.0) |
| Cortical thickness in the AD signature, mm | 2.85 [2.79–2.91] | 2.86 [2.80–2.92] | 2.85 [2.76–2.93] |
Values are n (%) or mean (95% CI), except for WMH burden and cortical thickness in the AD signature, which are median [IQR]. AD, Alzheimer disease; ALA, α-linolenic acid; WMH, white matter hyperintensity.
Includes ε2/ε4 (n = 29) and ε3/ε4 (n = 128) genotypes.
Results of the association between self-reported dietary DHA intake and cognitive performance are presented in Table 2 (episodic memory) and Table 3 (executive function). We did not find statistically significant associations between DHA intake and episodic memory or executive function. Table 4 displays multivariate associations for WMH burden. As observed, no statistically significant associations were observed for DHA or the interaction DHA × APOE-ε4 in any model. Table 5 presents multiple logistic regression models for the presence of CMBs. DHA showed a trend toward a lower prevalence of lobar CMBs (P ≤ 0.055, whichever was the model). The interaction DHA × APOE-ε4 was found to be statistically nonsignificant in any model. We next searched for associations between dietary DHA and cortical thickness in the AD signature (Table 6). No statistically significant associations were observed for DHA. However, the interaction DHA × APOE-ε4 in the recessive model (P = 0.045) prompted us to search for associations after separating by APOE-ε4 homozygosis. With this approach, we observed a statistically significant direct association between DHA and cortical thickness in the AD signature in APOE-ε4 homozygotes but not in nonhomozygotes (Supplemental Table 2). To reduce the residual confounding, we next performed a propensity score analysis by matching each homozygote to a nonhomozygote of the cohort. Given that 2 homozygotes could not be matched because of BMI extreme values, 59 pairs (n = 118) were considered for this subanalysis. Supplemental Table 3 details the characteristics of the 2 groups. No between-group significant differences were observed concerning the variables used for matching, predictor, or outcome of interest. With this approach, the strength of the DHA × APOE-ε4 interaction increased (Supplemental Table 4). Like the model including the total population, a statistically significant direct association between DHA and cortical thickness in the AD signature was observed in APOE-ε4 homozygotes but not in matched nonhomozygotes (Figure 1, Supplemental Table 5).
TABLE 2.
Associations between dietary DHA and episodic memory composite scores in the studied population1
| Variable | Model | APOE-ε4 in the model | Estimate (95% CI) | P | R 2 |
|---|---|---|---|---|---|
| DHA | Unadjusted | — | 0.039 (−0.184, 0.261) | 0.733 | <0.001 |
| Adjusted2 | Carrier/noncarrier3 | 0.006 (−0.227, 0.239) | 0.957 | 0.021 | |
| Number of alleles4 | 0.005 (−0.228, 0.238) | 0.964 | 0.021 | ||
| Homozygote/nonhomozygote5 | −0.001 (−0.234, 0.233) | 0.997 | 0.017 | ||
| DHA × APOE-ε4 | Unadjusted | Carrier/noncarrier3 | −0.330 (−0.782, 0.123) | 0.153 | 0.002 |
| Adjusted6 | −0.381 (−0.836, 0.074) | 0.101 | 0.011 | ||
| Unadjusted | Number of alleles4 | −0.156 (−0.454, 0.142) | 0.304 | 0.010 | |
| Adjusted6 | −0.171 (−0.470, 0.127) | 0.260 | 0.025 | ||
| Unadjusted | Homozygote/nonhomozygote5 | −0.035 (−0.599, 0.530) | 0.904 | 0.004 | |
| Adjusted6 | −0.024 (−0.588, 0.540) | 0.933 | 0.017 |
n = 340. Data are presented for 1 g/d of DHA, obtained by multiple linear regression analyses. Episodic memory composite scores were calculated by averaging normalized age- and education-regressed scores of all subtests in the domain. ALA, α-linolenic acid.
Including APOE-ε4, gender, self-reported energy intake, and ALA intake as covariates.
Distributed into n = 218 carriers and n = 122 noncarriers.
Distributed into n = 122 with 0 alleles, n = 157 with 1 allele, and n = 61 with 2 alleles.
Distributed into n = 61 homozygotes and n = 279 nonhomozygotes.
Including gender, self-reported energy intake, and ALA intake as covariates.
TABLE 3.
Associations between dietary DHA and executive function composite scores in the studied population1
| Variable | Model | APOE-ε4 in the model | Estimate (95% CI) | P | R 2 |
|---|---|---|---|---|---|
| DHA | Unadjusted | — | −0.038 (−0.189, 0.112) | 0.617 | 0.001 |
| Adjusted2 | Carrier/noncarrier3 | −0.005 (−0.160, 0.151) | 0.953 | 0.051 | |
| Number of alleles4 | −0.004 (−0.159, 0.151) | 0.959 | 0.051 | ||
| Homozygote/nonhomozygote5 | −0.005 (−0.160, 0.150) | 0.951 | 0.051 | ||
| DHA × APOE-ε4 | Unadjusted | Carrier/noncarrier3 | −0.121 (−0.429, 0.186) | 0.438 | 0.004 |
| Adjusted6 | −0.061 (−0.366, 0.243) | 0.692 | 0.051 | ||
| Unadjusted | Number of alleles4 | −0.019 (−0.222, 0.184) | 0.856 | 0.002 | |
| Adjusted6 | −0.003 (−0.203, 0.196) | 0.975 | 0.051 | ||
| Unadjusted | Homozygote/nonhomozygote5 | 0.123 (−0.260, 0.505) | 0.528 | 0.002 | |
| Adjusted6 | 0.086 (−0.289, 0.462) | 0.651 | 0.052 |
n = 340. Data are presented for 1 g/d of DHA, obtained by multiple linear regression analyses. Executive function composite scores were calculated by averaging normalized age- and education-regressed scores of all subtests in the domain. ALA, α-linolenic acid.
Including APOE-ε4, gender, self-reported energy intake, and ALA intake as covariates.
Distributed into n = 218 carriers and n = 122 noncarriers.
Distributed into n = 122 with 0 alleles, n = 157 with 1 allele, and n = 61 with 2 alleles.
Distributed into n = 61 homozygotes and n = 279 nonhomozygotes.
Including gender, self-reported energy intake, and ALA intake as covariates.
TABLE 4.
Associations between dietary DHA and WMH burden in the studied population1
| Variable | Model | APOE-ε4 in the model | Estimate (95% CI) | P | R 2 |
|---|---|---|---|---|---|
| DHA | Unadjusted | — | −375 (−1232, 483) | 0.391 | 0.002 |
| Adjusted2 | Carrier/noncarrier3 | −250 (−1119, 618) | 0.571 | 0.112 | |
| Number of alleles4 | −234 (−1102, 633) | 0.596 | 0.113 | ||
| Homozygote/nonhomozygote5 | −244 (−1107, 618) | 0.578 | 0.123 | ||
| DHA × APOE-ε4 | Unadjusted | Carrier/noncarrier3 | −1488 (−3235, 259) | 0.095 | 0.012 |
| Adjusted6 | −918 (−2624, 788) | 0.291 | 0.115 | ||
| Unadjusted | Number of alleles4 | −1089 (−2239, 62) | 0.064 | 0.013 | |
| Adjusted6 | −661 (−1781, 459) | 0.247 | 0.117 | ||
| Unadjusted | Homozygote/nonhomozygote5 | −1511 (−3682, 659) | 0.172 | 0.011 | |
| Adjusted6 | −845 (−2944, 1253) | 0.429 | 0.124 |
n = 340. Data are presented for 1 g/d of DHA, obtained by multiple linear regression analyses. WMH burden was rank-transformed. ALA, α-linolenic acid; WMH, white matter hyperintensity.
Including APOE-ε4, total intracranial volume, gender, age, BMI, hypercholesterolemia, hypertension, self-reported energy intake, and ALA intake as covariates.
Distributed into n = 218 carriers and n = 122 noncarriers.
Distributed into n = 122 with 0 alleles, n = 157 with 1 allele, and n = 61 with 2 alleles.
Distributed into n = 61 homozygotes and n = 279 nonhomozygotes.
Including total intracranial volume, gender, age, BMI, hypercholesterolemia, hypertension, self-reported energy intake, and ALA intake as covariates.
TABLE 5.
Associations between dietary DHA and prevalence of CMBs in the studied population1
| Presence of CMBs, any brain area (n = 62 cases) | Presence of CMBs, lobar brain (n = 50 cases) | |||||
|---|---|---|---|---|---|---|
| Variable | Model | APOE-ε4 in the model | OR (95% CI) | P | OR (95% CI) | P |
| DHA | Unadjusted | — | 0.702 (0.357, 1.379) | 0.304 | 0.469 (0.197, 1.113) | 0.064 |
| Adjusted2 | Carrier/noncarrier3 | 0.585 (0.279, 1.228) | 0.156 | 0.446 (0.195, 1.018) | 0.055 | |
| Number of alleles4 | 0.578 (0.276, 1.211) | 0.146 | 0.441 (0.193, 1.004) | 0.051 | ||
| Homozygote/nonhomozygote5 | 0.575 (0.275, 1.204) | 0.142 | 0.445 (0.195, 1.014) | 0.054 | ||
| DHA × APOE-ε4 | Unadjusted | Carrier/noncarrier3 | 1.056 (0.264, 4.228) | 0.939 | 0.566 (0.123, 2.610) | 0.466 |
| Adjusted6 | 1.347 (0.308, 5.894) | 0.693 | 0.682 (0.134, 3.461) | 0.644 | ||
| Unadjusted | Number of alleles4 | 1.109 (0.453, 2.717) | 0.821 | 0.706 (0.253, 1.972) | 0.506 | |
| Adjusted6 | 1.194 (0.462, 3.090) | 0.714 | 0.750 (0.252, 2.230) | 0.605 | ||
| Unadjusted | Homozygote/nonhomozygote5 | 1.324 (0.255, 6.873) | 0.739 | 0.719 (0.102, 5.089) | 0.741 | |
| Adjusted6 | 1.226 (0.224, 6.711) | 0.814 | 0.702 (0.096, 5.151) | 0.728 | ||
n = 338. Values are ORs and 95% CIs for 1 g/d of DHA, obtained by logistic regression models, unless otherwise indicated. ALA, α-linolenic acid; CMB, cerebral microbleed.
Including APOE-ε4, gender, age, BMI, hypercholesterolemia, hypertension, self-reported energy intake, and ALA intake as covariates.
Distributed into n = 216 carriers and n = 122 noncarriers.
Distributed into n = 122 with 0 alleles, n = 155 with 1 allele, and n = 61 with 2 alleles.
Distributed into n = 61 homozygotes and n = 277 nonhomozygotes.
Including gender, age, BMI, hypercholesterolemia, hypertension, self-reported energy intake, and ALA intake as covariates.
TABLE 6.
Associations between dietary DHA and cortical thickness in the AD signature in the studied population1
| Variable | Model | APOE-ε4 in the model | Estimate (95% CI) | P | R 2 |
|---|---|---|---|---|---|
| DHA | Unadjusted | — | 0.003 (−0.026, 0.031) | 0.858 | <0.001 |
| Adjusted2 | Carrier/noncarrier3 | −0.003 (−0.032, 0.026) | 0.859 | 0.096 | |
| Number of alleles4 | −0.003 (−0.032, 0.026) | 0.842 | 0.096 | ||
| Homozygote/nonhomozygote5 | −0.003 (−0.032, 0.026) | 0.848 | 0.099 | ||
| DHA × APOE-ε4 | Unadjusted | Carrier/noncarrier3 | 0.055 (−0.002, 0.113) | 0.060 | 0.013 |
| Adjusted6 | 0.033 (−0.024, 0.089) | 0.257 | 0.100 | ||
| Unadjusted | Number of alleles4 | 0.048 (0.010, 0.086) | 0.014 | 0.019 | |
| Adjusted6 | 0.035 (−0.002, 0.072) | 0.067 | 0.105 | ||
| Unadjusted | Homozygote/nonhomozygote5 | 0.084 (0.012, 0.155) | 0.022 | 0.016 | |
| Adjusted6 | 0.071 (0.002, 0.141) | 0.045 | 0.110 |
n = 339. Data are presented for 1 g/d of DHA, obtained by multiple linear regression analyses. Cortical thickness in the AD signature was rank-transformed. ALA, α-linolenic acid.
Including APOE-ε4, gender, age, BMI, hypercholesterolemia, hypertension, self-reported energy intake, and ALA intake as covariates.
Distributed into n = 217 carriers and n = 122 noncarriers.
Distributed into n = 122 with 0 alleles, n = 156 with 1 allele, and n = 61 with 2 alleles.
Distributed into n = 61 homozygotes and n = 278 nonhomozygotes.
Including gender, age, BMI, hypercholesterolemia, hypertension, self-reported energy intake, and ALA intake as covariates.
FIGURE 1.
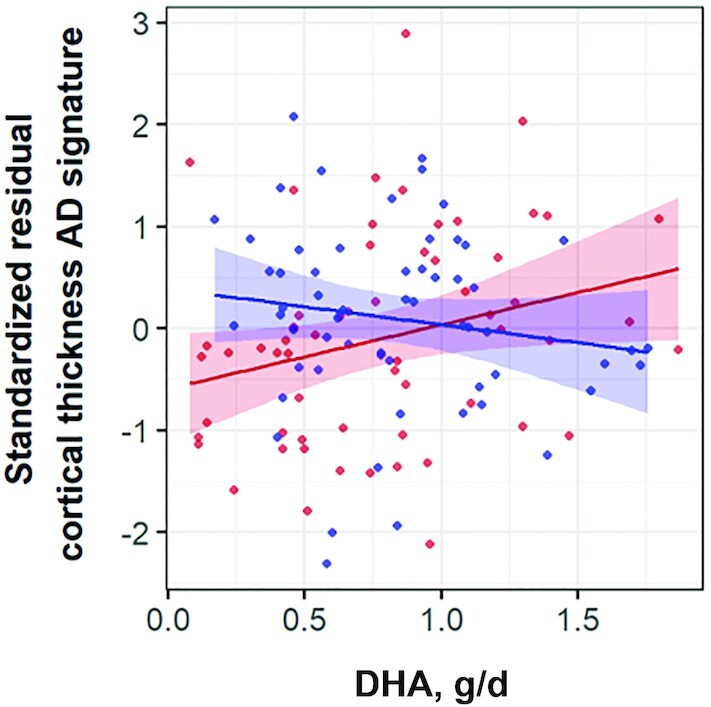
Scatterplot of the association between self-reported dietary intake of DHA and standardized residuals of cortical thickness in the AD signature by Jack et al. (23), outputted from a general linear model including gender, age, BMI, hypercholesterolemia, hypertension, energy intake, and α-linolenic acid intake, in APOE-ε4 homozygotes (n = 59, in red) and nonhomozygotes matched for selected adjusting covariates (n = 59, in blue). P value for the DHA × APOE-ε4 interaction = 0.025. Pearson correlation coefficient = −0.151 (P = 0.253) for APOE-ε4 homozygotes; Pearson correlation coefficient = 0.267 (P = 0.041) for matched nonhomozygotes. AD, Alzheimer disease.
Finally, sensitivity results including the 40 participants excluded for reporting total energy intake outside the predefined limits can be found in Supplemental Tables 6 (episodic memory composite scores), 7 (executive function composite scores), 8 (WMH burden), 9 (CMBs), and 10 (cortical thickness in the AD signature). For the latter, the statistical significance for the DHA × APOE-ε4 interaction in the recessive model weakened (P = 0.098).
Discussion
In this cross-sectional study conducted in middle-aged cognitively unimpaired individuals from a cohort enriched by APOE-ε4 carriership, we observed that in APOE-ε4 homozygotes higher self-reported dietary DHA intake was related to a lower prevalence of CBMs in lobar regions of the brain (i.e., not in the basal ganglia) and to a greater cortical thickness in the so-called AD signature, which includes regions known to undergo atrophy in AD.
Three aspects of our results merit highlighting. First, despite the increasing amount of research on diet (including nutrients, foods, and dietary patterns) and MRI-assessed markers of AD, this is the first study that we know of to examine how APOE-ε4 modulates the association between dietary DHA and structural AD features in the preclinical stage. Second, we described a beneficial association for DHA only in APOE-ε4 homozygotes. The high prevalence of APOE-ε4 homozygotes in our sample provided us with unprecedented statistical power to test separate models of genetic penetrance, which may capture distinct levels of vulnerability to risk alleles conferring a predisposition to AD. Third, our results reinforce the hypothesis that this genetically disadvantaged population might benefit the most from an intervention related to dietary DHA.
We did not find any significant association between self-reported dietary DHA intake and cognitive performance. In addition to the limited sample size (which might complicate the detection of weak associations), a plausible reason to explain in part such a finding could be the fact that our participants are highly educated and had high-range scores in most tests, even though we used challenging cognitive tasks to avoid the ceiling effects observed in such a population in regular tests used at memory clinics. Similarly, we did not find any relevant association between DHA and WMH burden, a marker of cerebral small vessel disease which has been found to have an impact on memory and executive functions in the same study population (31). Despite the long-known vascular protection ascribed to dietary intake of omega (ω)-3 fatty acids of marine origin (32), the issue of whether intake of DHA (or consumption of its parent foods) relates to WMH remains controversial. Most studies on the topic to date have been conducted in US populations. Whereas increasing blood concentrations of marine-derived ω-3 fatty acids were found to be associated with a lesser WMH burden in 2 cross-sectional studies (33, 34), self-reported fish consumption was not a significant independent predictor of WMH burden in the Northern Manhattan Study (35). A plausible explanation for our neutral findings on DHA and WMH other than the limited sample size might be the existence of a threshold of protection, largely exceeded by our population (mean DHA intake > 0.7 g/d), above which further benefits would not be observed. This would be similar to the prevention of ischemic heart disease, for which few benefits are observed beyond DHA intake of 0.5 mg/d (36). Further research is needed to elucidate whether cerebrovascular benefits of dietary DHA would be mainly observed in populations from countries in which fish consumption is customarily low, e.g., the United States (37). In contrast, we observed an inverse association between DHA intake and the presence in lobar regions of CMBs, which have been associated with accumulation of amyloid proteins in the walls of blood vessels (38). Unfortunately, the absence of biomarkers of amyloid-β deposition (determination in cerebrospinal fluid or by amyloid-β positron emission tomography) in our population precluded us confirming whether the inverse association between DHA and presence of lobar CMBs is mediated by the pathway relating DHA and amyloid-β, as has been suggested in 2 previous observational studies (39, 40).
Of note, we also uncovered a relevant finding for dietary DHA concerning a specific pattern of cortical areas (entorhinal, inferior temporal, middle temporal, and fusiform regions) whose thinning has been found to be associated with AD risk and progression [the “AD signature” established by Jack et al. (23)]. Interestingly, statistical significance was restricted to individuals carrying 2 copies of the APOE-ε4 allele, which are the strongest genetic risk factor for sporadic AD, and are suggested to have higher DHA requirements (5, 6) due to apoE-ε4-related disturbed lipid metabolism (5). Although causality cannot be inferred in our study given its cross-sectional nature, these 2 findings support the potential benefits of dietary DHA in managing APOE-ε4-related risk before symptomatology appears, as has been hypothesized (5). This notion is in alignment with the current point of view that lifestyle-related interventions would yield more benefits in subpopulations at increased risk (1), which further reinforces the need for identification of at-risk individuals.
Our study is not free of limitations. First, its cross-sectional nature precluded us from exploring whether self-reported intake of DHA related to longitudinal changes in MRI-assessed structural AD features. Second, we do not have data concerning core AD biomarkers. Third, the use of objective biomarkers of DHA intake (DHA determination in adipose tissue or RBCs) reflects long-term exposure more accurately than DHA estimation from self-reported food consumption. Finally, our cohort is enriched by family history of sporadic AD and APOE-ε4 carriership, which may limit the generalizability of the findings to other age groups or populations. In contrast, the strengths of the present study include the precise clinical characterization of the participants, the large number of APOE-ε4 homozygotes, the use of a validated FFQ, and adjustment for a wide array of potential confounders in multivariable analyses.
In conclusion, self-reported dietary DHA was found to be associated with several beneficial brain neuroimaging phenotypes related to cerebrovascular pathology or AD-related neurodegeneration in middle-aged cognitively unimpaired individuals at increased genetic risk of sporadic AD. Further research is needed to explore the effectiveness of interventions involving dietary supplementation with this fatty acid at the preclinical stage of AD, particularly in subjects at high risk of the disease.
Supplementary Material
Acknowledgments
This publication is part of the ALFA (ALzheimer and FAmilies) study. We express our most sincere gratitude to the ALFA project participants from the Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona, Spain, without whom this research would not have been possible. Collaborators of the ALFA study are Annabella Beteta, Anna Brugulat-Serrat, Alba Cañas, Noemí Carranza, Carme Deulofeu, Ruth Dominguez, Maria Emilio, Laura Hernandez, Gema Huesa, Jordi Huguet, Iva Knezevic, Paula Marne, Tania Menchón, Albina Polo, Sandra Pradas, Mahnaz Shekari, Anna Soteras, Marc Vilanova, and Natàlia Vilor-Tejedor.
The authors’ responsibilities were as follows—AS-V, J-DG, and JLM: designed the study; EMA-U, GS-B, MS-C, MM-A, OG-R, JMG-d-E, MC-B, GO, CF, GS, RC, SI, FB, and HS: acquired the data; CM, KF, and J-DG: contributed to data quality assurance and data quality analysis; AS-V, NS, J-DG, and JLM: drafted the manuscript; and all authors: revised the manuscript and read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported by “la Caixa” Foundation (ID 100010434) under agreement LCF/PR/GN17/10300004 (to JLM). In addition, supported by Universities and Research Secretariat, Ministry of Business and Knowledge of the Catalan Government grant no. 2017-SGR-892 (to JLM). AS-V is the recipient of Instituto de Salud Carlos III Miguel Servet fellowship grant CP II 17/00029. EMA-U is the recipient of Alzheimer's Association research grant AARG 2019-AARG-644641 and holds “Ramón y Cajal” fellowship RYC2018-026053-I. MS-C received funding from the European Union's Horizon 2020 Research and Innovation Program under Marie Sklodowska-Curie action grant agreement no. 752310 and is currently supported by Instituto de Salud Carlos III grant PI19/00155 and Spanish Ministry of Science, Innovation, and Universities Juan de la Cierva Programme grant IJC2018-037478-I. FB is supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre. JD-G holds “Ramón y Cajal” fellowship RYC-2013-13054.
Supplemental Figure 1, Supplemental Tables 1–10, and Supplemental File 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
The complete list of collaborators of the ALFA Study can be found in the Acknowledgments.
Abbreviations used: AD, Alzheimer disease; ALA, α-linolenic acid; ALFA, ALzheimer and FAmilies; BOMBS, Brain Observer Microbleeds Scale; CMB, cerebral microbleed; FLAIR, fluid-attenuated inversion recovery; FSE, fast spin echo; GRE, gradient echo; MBT, Memory Binding Test; TE, echo time; TI, inversion time; TR, repetition time; WAIS, Wechsler Adult Intelligence Scale; WMH, white matter hyperintensity.
Contributor Information
Aleix Sala-Vila, Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona, Spain; Hospital del Mar Medical Research Institute (IMIM), Barcelona, Spain; Fatty Acid Research Institute, Sioux Falls, SD, USA.
Eider M Arenaza-Urquijo, Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona, Spain; Hospital del Mar Medical Research Institute (IMIM), Barcelona, Spain; Center for Biomedical Research Network on Frailty and Healthy Aging (CIBERFES), Madrid, Spain.
Gonzalo Sánchez-Benavides, Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona, Spain; Hospital del Mar Medical Research Institute (IMIM), Barcelona, Spain; Center for Biomedical Research Network on Frailty and Healthy Aging (CIBERFES), Madrid, Spain.
Marc Suárez-Calvet, Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona, Spain; Hospital del Mar Medical Research Institute (IMIM), Barcelona, Spain; Center for Biomedical Research Network on Frailty and Healthy Aging (CIBERFES), Madrid, Spain; Neurology Service, Hospital del Mar, Barcelona, Spain.
Marta Milà-Alomà, Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona, Spain; Hospital del Mar Medical Research Institute (IMIM), Barcelona, Spain; Center for Biomedical Research Network on Frailty and Healthy Aging (CIBERFES), Madrid, Spain.
Oriol Grau-Rivera, Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona, Spain; Hospital del Mar Medical Research Institute (IMIM), Barcelona, Spain; Neurology Service, Hospital del Mar, Barcelona, Spain.
José M González-de-Echávarri, Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona, Spain; Hospital del Mar Medical Research Institute (IMIM), Barcelona, Spain; Center for Biomedical Research Network on Frailty and Healthy Aging (CIBERFES), Madrid, Spain.
Marta Crous-Bou, Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona, Spain; Center for Biomedical Research Network on Frailty and Healthy Aging (CIBERFES), Madrid, Spain; Department of Epidemiology, Harvard TH Chan School of Public Health, Boston, MA, USA; Unit of Nutrition and Cancer, Cancer Epidemiology Research Program, Catalan Institute of Oncology (ICO)–Bellvitge Biomedical Research Institute (IDIBELL), L'Hospitalet de Llobregat, Barcelona, Spain.
Carolina Minguillón, Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona, Spain; Hospital del Mar Medical Research Institute (IMIM), Barcelona, Spain.
Karine Fauria, Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona, Spain.
Grégory Operto, Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona, Spain; Hospital del Mar Medical Research Institute (IMIM), Barcelona, Spain; Center for Biomedical Research Network on Frailty and Healthy Aging (CIBERFES), Madrid, Spain.
Carles Falcón, Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona, Spain; Hospital del Mar Medical Research Institute (IMIM), Barcelona, Spain; Center for Biomedical Research Network on Bioengineering, Biomaterials, and Nanomedicine (CIBERBBN), Madrid, Spain; Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain.
Gemma Salvadó, Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona, Spain; Hospital del Mar Medical Research Institute (IMIM), Barcelona, Spain; Center for Biomedical Research Network on Frailty and Healthy Aging (CIBERFES), Madrid, Spain.
Raffaele Cacciaglia, Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona, Spain; Hospital del Mar Medical Research Institute (IMIM), Barcelona, Spain; Center for Biomedical Research Network on Frailty and Healthy Aging (CIBERFES), Madrid, Spain.
Silvia Ingala, Department of Radiology and Nuclear Medicine, Amsterdam Neuroscience, Vrije Universiteit Amsterdam, Amsterdam UMC, Amsterdam, Netherlands.
Frederik Barkhof, Department of Radiology and Nuclear Medicine, Amsterdam Neuroscience, Vrije Universiteit Amsterdam, Amsterdam UMC, Amsterdam, Netherlands; Institute of Neurology, University College London, London, United Kingdom; Institute of Healthcare Engineering, University College London, London, United Kingdom.
Helmut Schröder, Hospital del Mar Medical Research Institute (IMIM), Barcelona, Spain; Center for Biomedical Research Network on Epidemiology and Public Health (CIBERESP), Instituto de Salud Carlos III, Madrid, Spain.
Nikolaos Scarmeas, 1st Department of Neurology, Aiginition Hospital, National and Kapodistrian University of Athens Medical School, Athens, Greece; Department of Neurology, The Gertrude H Sergievsky Center, Taub Institute for Research in Alzheimer's Disease and the Aging Brain, Columbia University, New York, NY, USA.
Juan-Domingo Gispert, Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona, Spain; Hospital del Mar Medical Research Institute (IMIM), Barcelona, Spain; Center for Biomedical Research Network on Bioengineering, Biomaterials, and Nanomedicine (CIBERBBN), Madrid, Spain; Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain.
José L Molinuevo, Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona, Spain; Hospital del Mar Medical Research Institute (IMIM), Barcelona, Spain; Center for Biomedical Research Network on Frailty and Healthy Aging (CIBERFES), Madrid, Spain; Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain.
ALFA study:
Annabella Beteta, Anna Brugulat-Serrat, Alba Cañas, Noemí Carranza, Carme Deulofeu, Ruth Dominguez, Maria Emilio, Laura Hernandez, Gema Huesa, Jordi Huguet, Iva Knezevic, Paula Marne, Tania Menchón, Albina Polo, Sandra Pradas, Mahnaz Shekari, Anna Soteras, Marc Vilanova, and Natàlia Vilor-Tejedor
Data Availability
Data described in the article will be made available upon request pending application and approval.
References
- 1. Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. 2018;14:653–6. [DOI] [PubMed] [Google Scholar]
- 2. Scarmeas N, Anastasiou CA, Yannakoulia M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018;17:1006–15. [DOI] [PubMed] [Google Scholar]
- 3. Dyall SC. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci. 2015;7:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang Y, Chen J, Qiu J, Li Y, Wang J, Jiao J. Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: a dose-response meta-analysis of 21 cohort studies. Am J Clin Nutr. 2015;103:330–40. [DOI] [PubMed] [Google Scholar]
- 5. Yassine HN, Braskie MN, Mack WJ, Castor KJ, Fonteh AN, Schneider LS, Harrington MG, Chui HC. Association of docosahexaenoic acid supplementation with Alzheimer disease stage in apolipoprotein E ε4 carriers: a review. JAMA Neurol. 2017;74:339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pontifex M, Vauzour D, Minihane AM. The effect of APOE genotype on Alzheimer's disease risk is influenced by sex and docosahexaenoic acid status. Neurobiol Aging. 2018;69:209–20. [DOI] [PubMed] [Google Scholar]
- 7. Riedel BC, Thompson PM, Brinton RD. Age, APOE and sex: triad of risk of Alzheimer's disease. J Steroid Biochem Mol Biol. 2016;160:134–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Williams T, Borchelt DR, Chakrabarty P. Therapeutic approaches targeting Apolipoprotein E function in Alzheimer's disease. Mol Neurodegeneration. 2020;15:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rojas S, Brugulat-Serrat A, Bargalló N, Minguillón C, Tucholka A, Falcon C, Carvalho A, Morán S, Esteller M, Gramunt Net al. Higher prevalence of cerebral white matter hyperintensities in homozygous APOE- ɛ 4 allele carriers aged 45–75: results from the ALFA study. J Cereb Blood Flow Metab. 2018;38:250–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ingala S, Mazzai L, Sudre CH, Salvadó G, Brugulat-Serrat A, Wottschel V, Falcon C, Operto G, Tijms B, Gispert JDet al. The relation between APOE genotype and cerebral microbleeds in cognitively unimpaired middle- and old-aged individuals. Neurobiol Aging. 2020;95:104–14. [DOI] [PubMed] [Google Scholar]
- 11. Cannistraro RJ, Badi M, Eidelman BH, Dickson DW, Middlebrooks EH, Meschia JF. CNS small vessel disease: a clinical review. Neurology. 2019;92:1146–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fouquet M, Besson FL, Gonneaud J, La Joie R, Chételat G. Imaging brain effects of APOE4 in cognitively normal individuals across the lifespan. Neuropsychol Rev. 2014;24:290–9. [DOI] [PubMed] [Google Scholar]
- 13. Molinuevo JL, Gramunt N, Gispert JD, Fauria K, Esteller M, Minguillon C, Sánchez-Benavides G, Huesa G, Morán S, Dal-Ré Ret al. The ALFA project: a research platform to identify early pathophysiological features of Alzheimer's disease. Alzheimers Dement (N Y). 2016;2:82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reiman EM, Arboleda-Velasquez JF, Quiroz YT, Huentelman MJ, Beach TG, Caselli RJ, Chen Y, Su Y, Myers AJ, Hardy Jet al. Exceptionally low likelihood of Alzheimer's dementia in APOE2 homozygotes from a 5,000-person neuropathological study. Nat Commun. 2020;11:667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schröder H, Covas MI, Marrugat J, Vila J, Pena A, Alcántara M, Masiá R. Use of a three-day estimated food record, a 72-hour recall and a food-frequency questionnaire for dietary assessment in a Mediterranean Spanish population. Clin Nutr. 2001;20:429–37. [DOI] [PubMed] [Google Scholar]
- 16. Buschke H. Rationale of the memory binding test. In: Nilsson L, Ohta N, editors. Dementia and memory. Hove, United Kingdom: Psychology Press; 2014. p. 55–61. [Google Scholar]
- 17. Gramunt N, Buschke H, Sánchez-Benavides G, Lipton RB, Peña-Casanova J, Diéguez-Vide F, Masramon X, Gispert JD, Fauria K, Camí Jet al. Reference data of the Spanish Memory Binding Test in a midlife population from the ALFA Study (Alzheimer's and Family). J Alzheimers Dis. 2015;48:613–25. [DOI] [PubMed] [Google Scholar]
- 18. Wechsler D. Escala de Inteligencia Wechsler Para Adultos IV (Spanish version). Madrid, Spain: Pearson; 2012. [Google Scholar]
- 19. Brugulat-Serrat A, Rojas S, Bargalló N, Conesa G, Minguillón C, Fauria K, Gramunt N, Molinuevo JL, Gispert JD. Incidental findings on brain MRI of cognitively normal first-degree descendants of patients with Alzheimer's disease: a cross-sectional analysis from the ALFA (Alzheimer and Families) project. BMJ Open. 2017;7:e013215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sudre CH, Cardoso MJ, Bouvy WH, Biessels GJ, Barnes J, Ourselin S. Bayesian model selection for pathological neuroimaging data applied to white matter lesion segmentation. IEEE Trans Med Imaging. 2015;34:2079–102. [DOI] [PubMed] [Google Scholar]
- 21. Salvadó G, Brugulat-Serrat A, Sudre CH, Grau-Rivera O, Suárez-Calvet M, Falcon C, Fauria K, Cardoso MJ, Barkhof F, Molinuevo JLet al. Spatial patterns of white matter hyperintensities associated with Alzheimer's disease risk factors in a cognitively healthy middle-aged cohort. Alzheimers Res Ther. 2019;11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fischl B. FreeSurfer. Neuroimage. 2012;62:774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jack CR Jr, Wiste HJ, Weigand SD, Therneau TM, Lowe VJ, Knopman DS, Gunter JL, Senjem ML, Jones DT, Kantarci Ket al. Defining imaging biomarker cut points for brain aging and Alzheimer's disease. Alzheimers Dement. 2017;13:205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cordonnier C, Potter GM, Jackson CA, Doubal F, Keir S, Sudlow CL, Wardlaw JM, Al-Shahi Salman R. Improving interrater agreement about brain microbleeds: development of the Brain Observer MicroBleed Scale (BOMBS). Stroke. 2009;40:94–9. [DOI] [PubMed] [Google Scholar]
- 25. Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–28. [DOI] [PubMed] [Google Scholar]
- 26. Anderson SF. Best (but oft forgotten) practices: sample size planning for powerful studies. Am J Clin Nutr. 2019;110:280–5. [DOI] [PubMed] [Google Scholar]
- 27. Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. [DOI] [PubMed] [Google Scholar]
- 28. Clarke GM, Anderson CA, Pettersson FH, Cardon LR, Morris AP, Zondervan KT. Basic statistical analysis in genetic case-control studies. Nat Protoc. 2011;6:121–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blondeau N, Lipsky RH, Bourourou M, Duncan MW, Gorelick PB, Marini AM. Alpha-linolenic acid: an omega-3 fatty acid with neuroprotective properties—ready for use in the stroke clinic?. Biomed Res Int. 2015:519830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Willett W. Nutritional epidemiology. 3rd ed. New York, NY: Oxford University Press; 2013. [Google Scholar]
- 31. Brugulat-Serrat A, Salvadó G, Sudre CH, Grau-Rivera O, Suárez-Calvet M, Falcon C, Sánchez-Benavides G, Gramunt N, Fauria K, Cardoso MJet al. Patterns of white matter hyperintensities associated with cognition in middle-aged cognitively healthy individuals. Brain Imaging Behav. 2020;14:2012–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rimm EB, Appel LJ, Chiuve SE, Djoussé L, Engler MB, Kris-Etherton PM, Mozaffarian D, Siscovick DS, Lichtenstein AH; American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology . Seafood long-chain n-3 polyunsaturated fatty acids and cardiovascular disease: a Science Advisory from the American Heart Association. Circulation. 2018;138:e35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bowman GL, Silbert LC, Howieson D, Dodge HH, Traber MG, Frei B, Kaye JA, Shannon J, Quinn JF. Nutrient biomarker patterns, cognitive function, and MRI measures of brain aging. Neurology. 2012;78:241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tan ZS, Harris WS, Beiser AS, Au R, Himali JJ, Debette S, Pikula A, Decarli C, Wolf PA, Vasan RSet al. Red blood cell ω-3 fatty acid levels and markers of accelerated brain aging. Neurology. 2012;78:658–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gardener H, Scarmeas N, Gu Y, Boden-Albala B, Elkind MS, Sacco RL, DeCarli C, Wright CB. Mediterranean diet and white matter hyperintensity volume in the Northern Manhattan Study. Arch Neurol. 2012;69:251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–99. [DOI] [PubMed] [Google Scholar]
- 37. Micha R, Khatibzadeh S, Shi P, Andrews KG, Engell RE, Mozaffarian D; Global Burden of Diseases Nutrition and Chronic Diseases Expert Group (NutriCoDE) . Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: a systematic analysis including 266 country-specific nutrition surveys. BMJ. 2014;348:g2272. Erratum in: BMJ 2015;350:h1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Benedictus MR, Goos JD, Binnewijzend MA, Muller M, Barkhof F, Scheltens P, Prins ND, van der Flier WM. Specific risk factors for microbleeds and white matter hyperintensities in Alzheimer's disease. Neurobiol Aging. 2013;34:2488–94. [DOI] [PubMed] [Google Scholar]
- 39. Yassine HN, Feng Q, Azizkhanian I, Rawat V, Castor K, Fonteh AN, Harrington MG, Zheng L, Reed BR, DeCarli Cet al. Association of serum docosahexaenoic acid with cerebral amyloidosis. JAMA Neurol. 2016;73:1208–16. [DOI] [PubMed] [Google Scholar]
- 40. Gu Y, Schupf N, Cosentino SA, Luchsinger JA, Scarmeas N. Nutrient intake and plasma β-amyloid. Neurology. 2012;78:1832–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article will be made available upon request pending application and approval.


