ABSTRACT
Background
Choline deficiency has numerous negative health consequences; although the preponderance of the US population consumes less than the recommended Adequate Intake (AI), clinical assessment of choline status is difficult. Further, several pathways involved in primary metabolism of choline are estrogen-sensitive and the AI for premenopausal women is lower than that for men.
Objectives
We sought to determine whether in vivo magnetic resonance spectroscopy (MRS) of liver and/or isotope-dilution MS of plasma could identify biomarkers reflective of choline intake (preregistered primary outcomes 1 and 2, secondary outcome 1). Determination of whether biomarker concentrations showed sex dependence was a post hoc outcome. This substudy is a component of a larger project to identify a clinically useful biomarker panel for assessment of choline status.
Methods
In a double-blind, randomized, crossover trial, people consumed 3 diets, representative of ∼100%, ∼50%, and ∼25% of the choline AI, for 2-wk periods. We measured the concentrations of choline and several metabolites using 1H single-voxel MRS of liver in vivo and using 2H-labeled isotope dilution MS of several choline metabolites in extracted plasma.
Results
Plasma concentrations of 2H9-choline, unlabeled betaine, and 2H9-betaine, and the isotopic enrichment ratio (IER) of betaine showed highly significant between-diet effects (q < 0.0001), with unlabeled betaine concentration decreasing 32% from highest to lowest choline intake. Phosphatidylcholine IER was marginally significant (q = 0.03). Unlabeled phosphatidylcholine plasma concentrations did not show between-diet effects (q = 0.34). 2H9 (trimethyl)-phosphatidylcholine plasma concentrations (q = 0.07) and MRS-measured total soluble choline species liver concentrations (q = 0.07) showed evidence of between-diet effects but this was not statistically significant.
Conclusions
Although MRS is a more direct measure of choline status, variable spectral quality limited interpretation. MS analysis of plasma showed clear correlation of plasma betaine concentration, but not plasma phosphatidylcholine concentration, with dietary choline intake. Plasma betaine concentrations also correlate with sex status (premenopausal women, postmenopausal women, men).
This trial was registered at clinicaltrials.gov as NCT03726671.
Keywords: nutritional status biomarkers, choline, betaine, sex differences, magnetic resonance spectroscopy, mass spectrometry
Introduction
Choline is an essential nutrient that is not synthesized in amounts in the human body sufficient to meet its needs (1). It is a precursor to acetylcholine, betaine, and the phospholipid backbone glycerophosphocholine. Via its metabolite phosphatidylcholine (PtdCho), choline is essential for the export of lipids from the liver (2, 3) because it is needed for the membranes used in exporting triglycerides as VLDLs (3–6). Diets low in choline result in fatty liver in people (7–9) as well as alterations in other aspects of fatty acid metabolism (10, 11), muscle damage (8, 12), and altered fetal brain development (13). As a precursor to betaine, choline also contributes to cellular methylation potential and the S-adenosylmethionine (SAM):S-adenosylhomocysteine ratio which, in turn, is a metabolic control point that regulates DNA and histone methylation/epigenetic state (14–17) and flux through the sulfur amino acid pathway.
Although the US Institute of Medicine (now the National Academy of Medicine) established a recommended Adequate Intake (AI) in 1998 (18), dietary intake of choline varies widely. Data from the 2009–2012 NHANES suggest that >90% of adult Americans eat less than the AI, with roughly one-quarter of the US adult population consuming <50% of the AI (19). Clinical determination of choline deficiency, however, is difficult. Dietary choline is carried through the portal vein to the liver, where it is metabolized; circulating plasma concentrations are dependent on numerous processes and regulatory steps, and thus plasma choline rarely drops below ∼5 μM, even in severely depleted individuals (7, 20). We are developing a clinically useful panel of biomarkers that can identify choline status before the onset of explicit liver (or other) disease. As a component of this effort, we are examining the correlation of several proxies of choline status. This article presents the results of a planned interim analysis to test several secondary outcomes. Specifically, we compare the efficacy of measurement of 1) isotope dilution MS of several choline metabolites in plasma with 2) 1H single-voxel magnetic resonance spectroscopy (MRS) of the liver as methods to assess changes in the metabolically available choline pool size in humans consuming diets differing in total daily choline intake.
Methods
Study design
The study design was a longitudinal, double-blind, randomized, crossover dietary intervention (NCT03726671). Choline content was based on the National Institute of Medicine–defined AI for adult men (550 mg choline/d). Subjects were provided with all foods and drink for each of three 2-wk controlled feeding arms. These diets were identical except for choline content, which was adjusted by changing the choline (as choline chloride) added to the 3 bread rolls consumed per day. In random order these diet arms delivered 5.1 mmol choline/d (531 mg, 97% AI), 2.5 mmol choline/d (47% AI), or 1.3 mmol choline/d (25% AI) and they are hereafter referred to as the high-choline diet (HC), medium-choline diet (MC), and low-choline diet (LC), respectively. All diets contained a mean of 147 mg betaine/d. The amount of choline in each diet was not adjusted for subject sex or BMI; for premenopausal women, the AI is 425 mg/d. There was a minimum of 2 wk of washout between arms during which the participants returned to their usual diet. On day 12 of each dietary arm, in addition to the food supplied, subjects consumed a single bolus of 2.2 mmol trimethyl-2H9-(methyl) labeled (d9)-choline (as 98% D, methyl-perdeuterated choline chloride; Cambridge Isotope Laboratories). This dose is based on that used by Pynn et al. (21), with an additional ∼25% to account for differential absorption between intravenous and oral delivery. We confirmed the total choline and betaine content of diets using duplicate food portions analyzed by liquid chromatography-stable isotope dilution-multiple reaction monitoring mass spectrometry (LC-SID-MRM/MS) as previously described (22) (see Supplemental Tables 1 and 2 for a nutrient breakdown of the diets, including choline and betaine, and ingredients of the bread rolls). Choline content and stability during storage of choline in the bread rolls were separately determined for each batch produced. Riboflavin (30 mg/d) was added to the bread rolls and was used as a compliance biomarker via 24-h urine collection measures (23–25). Noncompliance for a given dietary arm was defined as day-15 riboflavin 24-h excretion <20% of day-5 riboflavin concentration (all participants exhibited elevated riboflavin at day 5 of each arm). This cutoff resulted in the removal of 6 of the 120 arms from the data. Blood was collected on day 1, and at 6 h, 24 h, 48 h, and 72 h after the day 12 d9-choline bolus. MRS data were collected 72 h postbolus (day 15), after an overnight fast. The protocol was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill and informed consent was obtained.
Subject recruitment
Participants were screened and required to be of good health status as determined by self-report, assessment by the study physician, and standard clinical laboratory tests including a comprehensive metabolic panel. Inclusion criteria were age within the range of 17–70 y and BMI (in kg/m2) within the range of 20–30. Exclusion criteria included use of drugs or medications known to be damaging to liver or muscle or that have the potential to alter choline metabolism; a history of hepatic, renal, or other chronic systemic disease; being a substance abuser, current smoker, or consuming >2 alcoholic beverages per day (14/wk); using choline-containing dietary supplements; regularly consuming a diet that might interfere with the study; or allergies to soy proteins or any foods in the required diet. Women who were breastfeeding, pregnant, or planning to become pregnant were excluded owing to the potential risk to the fetus/infant of a low-choline diet. Subjects who performed intense exercise of >1 h every day or other intense muscle-building exercise (such as weightlifting beyond low-weight maintenance repetitions) were also excluded. Ultimately, 40 healthy people (21 premenopausal women, 8 postmenopausal women, 1 perimenopausal woman, 10 men) were included in this planned interim analysis (Table 1). A Consolidated Standards of Reporting Trials diagram is included as Supplemental Figure 1. Power calculations ([F test, between-factors/repeated-measures ANOVA, calculated with G*Power version 3.1.9.7 (26)] showed that a sample size of 30 would be sufficient to yield a power of 0.8 given 3 groups, ρ = 0.5, and an effect size of 0.5. This effect size is readily observed in our data.
TABLE 1.
Participant data (beginning of the study)1
| Premenopausal women | Postmenopausal women | Perimenopausal women | Men | |
|---|---|---|---|---|
| n | 21 | 8 | 1 | 10 |
| Age, y | 34 ± 7 | 54 ± 6 | 49 | 36 ± 10 |
| Height, cm | 165 ± 6 | 166 ± 7 | 161 | 175 ± 4 |
| Weight, kg | 70.8 ± 7.8 | 71.4 ± 7.0 | 74.1 | 71.9 ± 6.5 |
| BMI, kg/m2 | 26.0 ± 3.3 | 26.1 ± 2.5 | 28.6 | 23.5 ± 2.6 |
| Ethnicity, white/black/Hispanic | 14/4/3 | 5/3/0 | 1/0/0 | 9/0/1 |
| FSH, mIU/mL | 4.7–21.5 | 25.8–134.8 | 21.5–25.8 |
Values are mean ± SD or ranges unless otherwise indicated. FSH concentrations as measured by LabCorp were used to classify menopausal status. FSH, follicle-stimulating hormone.
Sample collection
Whole blood was collected in serum-separating tubes (SSTs) for clinical laboratory measurements and in lithium heparin–coated tubes for plasma metabolite analysis. Tubes were gently inverted to mix. Lithium heparin–coated tubes were immediately put on wet ice and centrifuged at 2200 × g at 4°C for 10 min, then returned to the ice bath. Plasma samples were pooled, divided into aliquots, and frozen at −80°C within 1 h of collection time. SSTs were allowed to clot at room temperature then centrifuged at 2200 × g at 4°C for 10 min. SSTs were sent for a comprehensive metabolic panel and creatine kinase testing to the clinical laboratories at Lab Corporation of America, Burlington, NC, which is both Clinical Laboratory Improvement Act (ID 34D0655059) and College of American Pathologists (no. 1396901) accredited. At enrollment, follicle-stimulating hormone and estrogen were also measured in female participants. Urine was collected by participants into a commode specimen collector and immediately poured into a 3-L plastic jug that contained 3 mL acetic acid. The plastic jug was kept cool with ice packs in an insulated cooler during the 24-h collection. On completion, the total volume and collection times were noted. Aliquots of 1 mL were created with and without formic acid (10 µL) and stored at −80°C.
MS
Plasma (choline metabolites) and urine (riboflavin) samples were spiked with stable isotope–labeled internal standards and extracted using a modified Bligh and Dyer extraction as previously described (27). Briefly, samples were extracted with 4 volumes of methanol:chloroform (2:1, vol:vol), mixed on a vortex, held at −20°C for 2–24 h, and mixed on a vortex. Supernatants were collected and pellets re-extracted with methanol:chloroform:water (2:1:0.8, by vol.). Combined supernatants were treated with water and chloroform to induce phase separation. The aqueous phase (choline and betaine) was transferred to HPLC vials for analysis. The organic phase (phosphatidylcholine and sphingomyelin) was diluted with acetonitrile and transferred to HPLC vials for analysis. A series of standards of known concentrations and containing the corresponding internal standards were prepared and treated identically to samples.
Analytes were quantified using LC-SID-MRM/MS (aqueous) or liquid chromatography-stable isotope dilution-mass spectrometry (organic) as previously described (22, 27). Chromatographic separations were performed on an Acquity HILIC 1.7 µm 2.1 × 50 mm column (Waters Corp) using a Waters ACQUITY UPLC system (0.37 mL/min at 40°C). Mobile phases for aqueous analytes were A: 100% water with 0.125% formic acid, and B: 90% acetonitrile/10% water with 10 mM ammonium formate and 0.125% formic acid. Mobile phase A for organic analytes was 10% acetonitrile/90% water and 0.125% formic acid and B was the same as for aqueous metabolites. As reported elsewhere (27), phosphatidylcholine undergoes in-source fragmentation and is detected as phosphocholine. The analytes and their corresponding isotopes were monitored on a Waters TQ detector using characteristic precursor–product ion transitions (Supplemental Table 3). Concentrations of each analyte in the samples were determined using the peak area ratio of the analyte to its isotope (response) and read off a standard curve of response values against standard concentrations for each analyte. The isotopic enrichment ratio (IER) was calculated as the fractional amount of labeled metabolite compared with the total (labeled + unlabeled) metabolite. Given a known quantity of isotopically labeled compound, the IER provides insight into the metabolically available precursor pool (28).
MRS
Magnetic resonance images and spectra were acquired at the Wake Forest School of Medicine Clinical Imaging Core using a 3.0 T scanner (Magnetom Skyra, Siemens Healthcare) with an 18-channel torso coil. Torso images were acquired (T2-weighted 2D half-Fourier acquisition single-shot turbo spin echo imaging) and used for localization of single-voxel MRS. Regions for metabolite measurement were localized to the middle of the right hepatic lobe and adjusted to avoid large vessels and fatty tissue. Single-voxel (3 × 3 × 3 cm3) spectra were collected using point resolved spin-echo spectroscopy with a 1200-Hz spectral width, a recycle time (TR) = 2 s, a spin echo time (TE) = 33 ms, and number of scans = 80. Data collection used 2D Prospective Acquisition Correction navigated acquisition (29) to synchronize acquisition with breathing motions.
Single-voxel spectra were processed using jMRUI version 6.0b (30) with the Amares plugin (31) and manually inspected to adjust phase if necessary and to ensure there were no artifactual integrations. Choline concentration in the selected voxel was determined by comparing the integral of the peak at 3.2 ppm from the water-suppressed spectrum [total soluble choline species (tCho): choline, phosphocholine, glycerophosphocholine] with the water peak from the unsuppressed spectrum using the relation: tCho = (choline_signal/water_signal) × scale. The scale term accounts for differential choline and water T1 (water, 990 ms; tCho, 842 ms) and T2 (water, 30 ms; tCho, 50 ms) relaxation times (32), TR, TE, the number of protons giving rise to the signals, and the fractional water content of liver (72% of 55.5 M) (33).
Statistics
Repeated-measures mixed-effect analyses and 1-sample t tests were calculated using GraphPad Prism 9.0 and adjusted for multiple comparisons using the 2-stage linear step-up method of Benjamini, Krieger, and Yekutieli (34) with a false discovery rate (FDR) of 5%. The mixed-effect model was chosen to take advantage of the randomized crossover experimental design while being more tolerant of missing values than a repeated-measures ANOVA. One- and 2-factor ANOVAs were calculated using GraphPad Prism 9.0 and adjusted for multiple comparisons using Tukey's test. Data met normality criteria as judged by D'Agostino and Pearson tests and Shapiro–Wilk tests in GraphPad Prism 9.0.
Results
Dietary choline is absorbed from the gut via the portal vein into the liver, where it is phosphorylated to form phosphocholine or oxidized to form betaine. Although circulating plasma concentrations of choline do reflect dietary choline, they are subject to regulation and, as noted, generally do not dip below ∼5 μM, even at very low dietary intake amounts (7, 20). Hence, we sought to identify other biomarkers of choline status.
Metabolite concentrations as a function of choline intake
Liver tCho concentration measured by MRS 72 h after the d9-choline bolus, plasma concentrations of choline [1H9-(methyl) labeled (d0), d9, IER] measured by MS 6 h postbolus, and betaine (d0, d9, IER) and PtdCho (d0, d9, IER) measured by MS 24 h postbolus, as a function of diet, are given in Table 2. Mixed-effect analysis found highly significant [(FDR-adjusted P) q < 0.0001] between-diet effects for d0-choline, d9-choline, d0-betaine, d9-betaine, and IER-betaine, whereas IER-PtdCho was marginally significant (q = 0.03). IER-choline (q = 0.25), d0-PtdCho (q = 0.34), and d9-PtdCho (q = 0.08) were not significant, nor was MRS-measured tCho (q = 0.08). Of the time points sampled, d9-PtdCho was maximal at 24 h. Although plasma d9-betaine concentrations were higher at 6 h postbolus than at 12 h, we have observed in this study (Supplemental Table 4) and pilot studies (data not shown) that buildup rates of plasma concentrations of d9-betaine show substantial interindividual variability and that more consistent data (i.e., smaller relative SD) are obtained at 24 h postbolus. Supplemental Table 4 gives metabolite concentrations measured by MS at 6, 24, 48, and 72 h after the d9-choline bolus.
TABLE 2.
Concentrations of choline metabolites measured by MRS and MS1
| Concentration | |||||
|---|---|---|---|---|---|
| Metabolite | Diet | All2 | Premenopausal female | Postmenopausal female | Male |
| MRS-tCho, mM | LC | 7.80 ± 4.20 | 8.83 ± 4.46 | 8.10 ± 3.80 | 5.36 ± 3.25 |
| MC | 7.99 ± 5.02 | 8.78 ± 5.30 | 7.81 ± 4.78 | 6.66 ± 4.97 | |
| HC | 9.83 ± 4.61 | 10.42 ± 2.92 | 12.15 ± 6.45 | 6.38 ± 3.17 | |
| d0-choline at 6 h, μM | LC | 8.12 ± 2.07 | 7.40 ± 1.61 | 8.77 ± 2.53 | 9.09 ± 2.33 |
| MC | 9.26 ± 2.69 | 8.21 ± 2.29 | 10.70 ± 3.09 | 10.21 ± 2.42 | |
| HC | 10.41 ± 2.84 | 9.30 ± 1.79 | 11.85 ± 3.22 | 11.26 ± 3.70 | |
| d9-choline at 6 h, μM | LC | 0.08 ± 0.13 | 0.06 ± 0.13 | 0.11 ± 0.16 | 0.08 ± 0.09 |
| MC | 0.09 ± 0.13 | 0.08 ± 0.13 | 0.12 ± 0.15 | 0.09 ± 0.10 | |
| HC | 0.12 ± 0.14 | 0.10 ± 0.14 | 0.14 ± 0.17 | 0.11 ± 0.11 | |
| IER-choline at 6 h | LC | 0.0497 ± 0.0175 | 0.0520 ± 0.0202 | 0.0454 ± 0.0133 | 0.0472 ± 0.0152 |
| MC | 0.0513 ± 0.0168 | 0.0536 ± 0.0195 | 0.0487 ± 0.0127 | 0.0488 ± 0.0144 | |
| HC | 0.0509 ± 0.0152 | 0.0545 ± 0.0175 | 0.0464 ± 0.0127 | 0.0476 ± 0.0121 | |
| d0-betaine at 24 h, μM | LC | 38.14 ± 11.77 | 32.83 ± 11.23 | 39.29 ± 6.85 | 49.30 ± 7.81 |
| MC | 43.90 ± 13.69 | 37.08 ± 12.70 | 45.49 ± 6.53 | 56.25 ± 11.05 | |
| HC | 56.89 ± 18.75 | 50.59 ± 19.67 | 57.44 ± 13.13 | 71.25 ± 14.51 | |
| d9-betaine at 24 h, μM | LC | 1.34 ± 0.45 | 1.29 ± 0.51 | 1.27 ± 0.37 | 1.52 ± 0.35 |
| MC | 1.44 ± 0.43 | 1.35 ± 0.49 | 1.44 ± 0.30 | 1.60 ± 0.35 | |
| HC | 1.71 ± 0.55 | 1.72 ± 0.60 | 1.57 ± 0.45 | 1.87 ± 0.50 | |
| IER-betaine at 24 h | LC | 0.0343 ± 0.0075 | 0.0375 ± 0.0078 | 0.0311 ± 0.0068 | 0.0299 ± 0.0041 |
| MC | 0.0324 ± 0.0069 | 0.0354 ± 0.0069 | 0.0310 ± 0.0069 | 0.0277 ± 0.0033 | |
| HC | 0.0299 ± 0.0065 | 0.0337 ± 0.0058 | 0.0264 ± 0.0048 | 0.0256 ± 0.0045 | |
| d0-PtdCho at 24 h, μM | LC | 2175 ± 354 | 2138 ± 347 | 2390 ± 329 | 2099 ± 378 |
| MC | 2228 ± 471 | 2082 ± 356 | 2615 ± 529 | 2209 ± 497 | |
| HC | 2228 ± 493 | 2104 ± 419 | 2612 ± 532 | 2152 ± 510 | |
| d9-PtdCho at 24 h, μM | LC | 28.25 ± 8.19 | 27.79 ± 8.22 | 30.40 ± 12.69 | 27.82 ± 4.62 |
| MC | 27.42 ± 8.88 | 24.94 ± 8.05 | 32.92 ± 10.95 | 27.98 ± 7.30 | |
| HC | 25.67 ± 5.64 | 25.43 ± 5.28 | 28.94 ± 5.43 | 23.43 ± 6.14 | |
| IER-PtdCho at 24 h, μM | LC | 0.0129 ± 0.0032 | 0.0129 ± 0.0035 | 0.0125 ± 0.0044 | 0.0132 ± 0.0017 |
| MC | 0.0122 ± 0.0030 | 0.0119 ± 0.0030 | 0.0126 ± 0.0040 | 0.0126 ± 0.0023 | |
| HC | 0.0115 ± 0.0021 | 0.0121 ± 0.0022 | 0.0112 ± 0.0020 | 0.0108 ± 0.0018 | |
Values are mean ± SD. Metabolites were measured by single-voxel MRS (liver; soluble choline species at 72 h after the d9-choline bolus) or by MS (plasma; choline at 6 h postbolus, betaine and PtdCho at 24 h postbolus), from study participants on LCs, MCs, or HCs. d0, 1H9-(methyl) labeled; d9, 2H9-(methyl) labeled; HC, high-choline diet; IER, isotopic enrichment ratio; LC, low-choline diet; MC, medium-choline diet; MRS, magnetic resonance spectroscopy; PtdCho, phosphatidylcholine; tCho, total soluble choline species.
For MRS: n (all) = 37 (LC), 34 (MC), 32 (HC); n (premenopausal female) = 21 (LC), 17 (MC), 17 (HC); n (postmenopausal female) = 6 (LC), 8 (MC), 8 (HC); n (male) = 9 (LC), 9 (MC), 6 (HC). For MS: n (all) = 39 (LC), 38 (MC), 37 (HC); n (premenopausal female) = 21 (LC), 20 (MC), 19 (HC); n (postmenopausal female) = 7 (LC), 8 (MC), 8 (HC); n (male) = 10 (LC), 10 (MC), 9 (HC). Data for the single perimenopausal female are included in “All” but not listed separately.
Fold-change analysis of choline metabolites
Although the observed metabolite concentrations at the MC dietary intake amount were consistently in between the concentrations for the HC and LC diets, a given metabolite concentration is not, in general, a linear function of intake of a metabolic precursor. In addition, the MC diet represents a fairly mild metabolic challenge. Consequently, our fold-change (FC) analysis focuses on relative changes in metabolite concentrations between the HC and LC diets.
We calculated FC for each metabolite using (concentration on the LC diet)/(concentration on the HC diet) at the 6 h time point (choline), the 24 h time point (betaine, PtdCho) or the single 72 h measurement point (MRS-tCho). Calculated across all participants, d0-betaine (FC = 0.68, q = 4.2e−15), d9-betaine (FC = 0.77, q = 1.6e−10), d9-choline (FC = 0.78, q = 5.1e−6), and d0-choline (FC = 0.82, q = 3.15e−11) concentrations decreased noticeably going from the HC to the LC diet, consistent with the mixed-effect analysis of raw metabolite concentrations. Figure 1 shows -log2(FC) for each metabolite.
FIGURE 1.

Choline metabolites as a function of dietary choline intake. Box and whiskers plot of -log2(concentration FC) of MRS-derived tCho (n = 30) and MS-derived d0, d9, and IER for choline, betaine, and PtdCho (n = 36). Choline was measured at 6 h after the d9-choline bolus, betaine and PtdCho at 24 h postbolus. MRS scans were performed on day 15 (72 h postbolus). Positive values of -log2(FC) indicate higher concentrations for HCs than for LCs. For each metabolite, the box spans the IQR, the horizontal line is the median, + marks the mean, and whiskers span the full data range. Adjusted P using FDR (5%): *q < 0.0001; †q = 0.006; ‡q = 0.019; no symbol: q > 0.1. d0, 1H9-(methyl) labeled; d9, 2H9-(methyl) labeled; FC, fold-change; HC, high-choline diet; IER, isotopic enrichment ratio; LC, low-choline diet; MRS, magnetic resonance spectroscopy; PtdCho, phosphatidylcholine; tCho, total soluble choline species.
The IER, expressed as d9-metabolite/(d9-metabolite + d0-metabolite), is a measure of newly formed deuterium-labeled metabolite (derived from the isotopic d9-choline bolus on day 12) compared with the pool of unlabeled metabolite present in the tissue. Whereas d0-betaine was sensitive to and positively correlated with dietary choline intake (FC = 0.68, q = 4.2e−15), betaine IER was considerably less responsive to choline intake (FC = 1.14, q = 1.2e−6). Under the dietary conditions used in this study, we expected that the plasma concentration of d0-betaine would be sensitive to d0-choline amounts in the HC/MC/LC, but would not be affected by the single d9-choline bolus. Surprisingly, we observed that concentrations of both d0- and d9-betaine increased after the d9-choline bolus. Consequently, the betaine IER is less sensitive because both numerator and denominator change together in the same direction. The origin of this effect is unclear but may reflect the different kinetic properties (KM, Vmax) of choline dehydrogenase (CHDH) and choline kinase (ChoK): at low concentrations in the liver, choline is preferentially phosphorylated by ChoK; at higher concentrations choline is predominantly oxidized to betaine by CHDH (35). Alternatively, the observed effect could be caused by upregulation of CHDH protein at higher concentrations of choline. However, hepatic CHDH expression is not affected by choline availability (36). There also may be other substrate-inducible processes. Regardless of mechanism, the observed dependence of hepatic choline metabolic fate (i.e., oxidation or phosphorylation) on hepatic choline concentration may be of relevance to studies that use bolus dosing of choline.
Betaine and PtdCho are the major choline metabolites released by the liver into the circulation. Relative to betaine, plasma PtdCho concentration is considerably less sensitive to diet. Over the choline diet range used in this study, d0-PtdCho was invariant (FC = 1.01, q = 0.27), whereas d9-PtdCho (FC = 1.09, q = 0.025) and PtdCho IER (FC = 1.08, q = 0.019) were slightly negatively correlated with dietary choline content. Lower concentrations of d9-PtdCho (and smaller IERs) at higher amounts of dietary (d0) choline are expected. Under HC conditions, the hepatic choline pool is higher in d0-choline concentration, and also enriched in d0-choline relative to d9-choline, compared with under LC conditions. The utility of the IER is to identify relative concentrations of newly formed metabolites; given the large (∼2 mM) plasma pool of extant unlabeled PtdCho (Table 2), this approach is limited in its sensitivity.
Single-voxel MRS measurement, although a direct measure of liver total choline, was not found to be a reliable indicator of dietary choline intake across the diet variation studied. Although the observed mean FC is similar to that of plasma betaine and choline concentrations (0.87), there was substantial variability among subjects (see the Discussion), and the FC was not statistically significant (q = 0.1) (Figure 1). Of the 30 MRS comparisons, 17 showed decreased tCho and 13 increased tCho as choline intake decreased from the HC to the LC diet. In contrast, 36 of 36 subjects exhibited decreased plasma betaine concentrations, and 35 of 36 subjects exhibited decreased plasma choline concentrations, under the same dietary conditions (Supplemental Table 5).
Plasma concentrations of betaine were stratified by sex
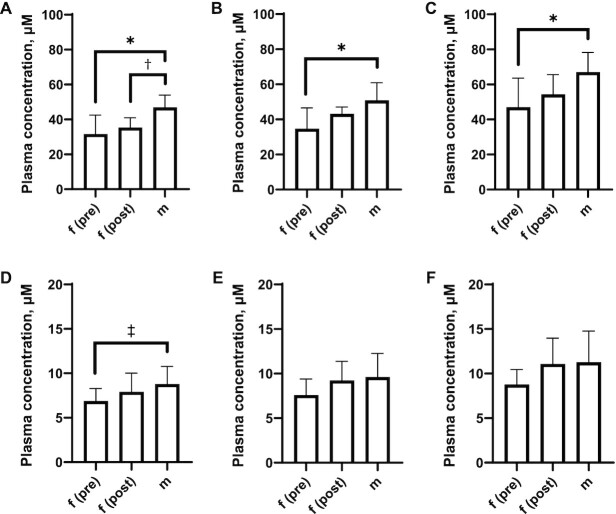
We observed sex-dependent differences in plasma concentrations of betaine and choline as a function of dietary choline intake. At a given dietary intake amount, males had significantly higher betaine concentrations than did premenopausal females (Figure 2A–C), consistent with previous reports (37). Postmenopausal females had plasma betaine concentrations intermediate to those of males and premenopausal females. Males also had higher plasma choline concentrations than did premenopausal females across all diets, but statistical significance was only observed at the lower choline intake amounts (Figure 2D–F). Repeated-measures 2-factor ANOVA of plasma choline:betaine ratios showed significant between-diet (LC compared with HC, MC compared with HC) but not between-sex effects (Supplemental Figure 2).
FIGURE 2.
Plasma choline and betaine concentrations as a function of sex status and dietary choline intake amount. Between-sex comparisons of plasma (d0-)betaine concentration at the end of each arm for (A) LC, (B) MC, and (C) HC and plasma (d0-)choline concentration at the end of each arm for (D) LC, (E) MC, and (F) HC. Error bars show SDs. Adjusted P using Tukey's test: *P(adjusted) < 0.005; †P(adjusted) < 0.05; ‡P(adjusted) < 0.02. d0, 1H9-(methyl) labeled; f(post), postmenopausal female; f(pre), premenopausal female; HC, high-choline diet; LC, low-choline diet; m, male; MC, medium-choline diet.
Daily choline intake was not adjusted for sex (intake is relative to the AI for adult males) or plasma volume (we note that the daily choline recommended AI is also not adjusted for BMI). However, because of the repeated-measures nature of this study, and the fact that there were no substantial changes in weight throughout the entirety of this trial, the conclusions were not affected by use of plasma concentration as opposed to total plasma amount.
Discussion
Of the metabolites investigated, and over the dietary choline range used in this study, plasma betaine concentrations were the most responsive biomarker of recent dietary choline intake, with plasma choline concentrations also somewhat responsive. This is consistent with previous findings (38), which also found that plasma choline, but not PtdCho, reflected dietary choline intake.
Bolus compared with steady-state administration of d9-choline
Previous studies (38, 39) used extended d9-choline intake (3 wk, 15% total dietary intake of choline as d9). Such dosing can establish an isotopic steady state which is necessary to allow interpretations of d3- and d6-PtdCho concentrations in addition to d9-metabolite concentrations. This is not practical in a clinical setting and it requires a considerable amount of d9-choline: 15% of daily AI is 1.58 mmol choline, equivalent to 221 mg d9-choline chloride. Over 3 wk, this amounts to 4.64 g d9-choline chloride per person per experimental arm. Administering d9-choline chloride as a single bolus, as in this study, decreases the required d9-choline chloride to 307 mg (2.2 mmol) per person, but we lose the ability to trace secondary metabolic products. In addition, although we retain the ability to calculate IER, we cannot relate IER as easily to pool size: for PtdCho, extant d0-PtdCho is too high; for betaine, biosynthesis and secretion are fast and would require sampling at additional time points in the first 24 h; for choline, the plasma concentrations of d9-choline are too low for a reliable calculation of IER.
Results from both our study and a previous study suggest that regulation of betaine metabolism may be more complex than commonly appreciated. Our study differed from Yan et al. (39) in 1) using a bolus as opposed to long-term administration of d9-choline and 2) using a fixed amount of dietary d9-choline (2.2 mmol) and a variable amount of d0-choline (1.319, 2.637, or 5.275 mmol/d) as opposed to a fixed ratio of d9- to d0-choline with a variable total amount (0.79 mmol d9/d and 4.48 mmol d0/d or 1.72 mmol d9/d and 8.97 mmol d0/d). Comparing these 2 approaches, we expected to see a substantially decreasing IER with increasing dietary choline intake, whereas Yan et al. expected to see no change in IER with increasing dietary choline intake. Instead, we observed a muted decrease in betaine IER corresponding to a dietary choline increase which can be traced to the increase in d0-betaine after ingestion of the d9-choline bolus at each dietary choline amount. Yan et al., similarly, saw an increase in IER (choline, betaine, PtdCho) as dietary (d0-) choline increased (men only, n = 23). Yan et al. concluded that their:
observed increase in isotope enrichment from precursor d9-choline to several products in blood and urine indicates that pools are in flux and that more than one precursor pool exists for derivation of subsequent products. The high enrichment of betaine, relative to that of choline, may also reflect an increased release of more enriched products as a result of gut-level oxidation of choline to methylamines.
We note that the HC, MC, and LC used in this study contained the same amount of betaine, so differences in plasma d0-betaine concentration were not caused by differences in ingestion of betaine.
Single-voxel 1H MRS as a monitor of systemic choline availability
Analysis of localized choline concentration by 1H MRS has been reported as a promising measure of hepatocellular carcinoma (40), although whether choline is elevated or depressed in/near tumors is unclear (33). In this study, we found that although the mean FC [and mean -log2(FC)] were suggestive of responsiveness to diet, the overall variability drove significance >0.1. In part this discrepancy reflects the functional difficulty in data collection (magnetic resonance hardware, technician skill, subject compliance, voxel location). Spectra for comparative analysis of tumor and normal tissue are typically acquired in the same imaging session for a given patient. In our case we compared spectra acquired ≥4 wk apart for each subject (each diet condition), and across 40 persons over a period of multiple years. Spectral quality (line widths, water suppression) varied within and especially between subjects. This leads to another confounder of data analysis (accurate measurement of signal in the face of widely variable data quality). Similar variability across and within subjects has been reported (41). In addition, the MRS signal at 3.2 ppm may include contributions from phosphoethanolamine and other phosphomonoesters (40). We also have not accounted for differences in liver fat, because we assumed a uniform 72% water content to standardize the unsuppressed MRS spectra (see the Methods). However, the effects of differential liver fat are minimized by our use of a crossover design, because only within-subject/between-diet changes will affect FC. Overall, therefore, our results suggest that MRS may reflect hepatic choline concentrations, because the mean -log2(FC) was >0. However, the variability in our particular data set makes its utility marginal.
Implications
Plasma PtdCho concentrations were not significantly decreased after 2 wk of LC compared with HC. Although Zeisel et al. (8) observed decreased plasma PtdCho in response to 3 wk consuming a choline-deficient diet, that study used 0.125 mmol choline/d (∼2.5% AI) for the low-choline arm, whereas the lowest amount used here was 1.3 mmol/d. Plasma choline concentrations do reflect dietary intake and decrease as choline decreases from 5.1 to 1.3 mmol/d, but they do not drop below ∼5 μM even at very low amounts (0.5 mmol/d) of choline intake (7, 20). Rather, the primary effect observed is in circulating betaine concentrations, which decreased ∼30% as dietary choline decreased from 5.1 to 1.3 mmol/d.
Premenopausal females have lower circulating betaine concentrations than do males or postmenopausal females. One possible contributor to this lower circulating betaine is greater utilization of hepatic betaine as a methyl-donor to form SAM, which is used by phosphatidylethanolamine methyltransferase (PEMT) to methylate phosphatidylethanolamine and produce PtdCho. PEMT activity is induced by estrogen, and this pathway is one of the major consumers of hepatic SAM (17). Whether females with functional polymorphisms in the PEMT gene have higher plasma concentrations of betaine is currently unknown. Betaine, unlike choline, is stored in cells, and plasma concentration is not necessarily representative of tissue concentration (42). That said, higher plasma betaine concentrations are correlated with increased fibroblast growth factor 21 concentrations and correlate closely with insulin sensitivity (43). In addition, plasma betaine concentration is inversely correlated with plasma total homocysteine (44, 45), which is implicated in certain cardiovascular diseases (46).
Conclusions
In summary, we report measurements of several choline metabolites as a function of dietary choline intake. Across the intake range studied (1.3–5.1 mmol choline/d, ∼25%–100% adult male AI), plasma choline and betaine concentrations were strongly correlated with choline intake amounts, whereas liver tCho measured by single-voxel MRS was weakly correlated. Plasma PtdCho concentrations were not correlated. In light of a study that found plasma PtdCho decreased in response to very low (0.5 mmol/d) choline intake (8), decreased PtdCho biosynthesis may become of clinical concern as choline intake drops to <25% of the AI. We also observed stratification by sex status of plasma betaine concentration (premenopausal women < postmenopausal women < men) across the choline intake range studied. Consequently, the health consequences of a choline-deficient diet may depend, in part, on sex status.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—SHZ: designed the research and had primary responsibility for the final content; JMS, SH, WBF, and DRK: conducted the research; DAH: analyzed the data and wrote the manuscript; JMS, SH, and SHZ: helped edit the manuscript; and all authors: read and approved the final manuscript. SHZ has an equity interest in SNP Therapeutics, a company that identifies people with certain genetic polymorphisms that are associated with health problems and develops medical foods to treat these problems. He has had grant funding from Balchem Company, a company that makes choline for diet supplements and animal feed, and is on advisory boards for ProTara, ByHeart, and Ingenuity Foods, all companies with an interest in choline relative to their products. All the other authors report no conflicts of interest.
Notes
Supported by NIH/National Institute of Diabetes and Digestive and Kidney Diseases grants R01 DK115380 (to SHZ) and P30 DK056350 (to SHZ).
Supplemental Figures 1 and 2 and Supplemental Tables 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Present address for SH: Research Resources Center, University of Illinois at Chicago, Chicago, USA.
Abbreviations used: AI, Adequate Intake; CHDH, choline dehydrogenase; ChoK, choline kinase; d0, 1H9-(methyl) labeled; d9, 2H9-(methyl) labeled; FC, fold-change; FDR, false discovery rate; HC, high-choline diet; IER, isotopic enrichment ratio; LC, low-choline diet; LC-SID-MRM/MS, liquid chromatography-stable isotope dilution-multiple reaction monitoring mass spectrometry; MC, medium-choline diet; MRS, magnetic resonance spectroscopy; PEMT, phosphatidylethanolamine methyltransferase; PtdCho, phosphatidylcholine; SAM, S-adenosylmethionine; SST, serum-separating tube; tCho, total soluble choline species; TE, spin echo time; TR, recycle time.
Contributor Information
David A Horita, Nutrition Research Institute, University of North Carolina at Chapel Hill, Kannapolis, NC, USA.
Sunil Hwang, Nutrition Research Institute, University of North Carolina at Chapel Hill, Kannapolis, NC, USA.
Julie M Stegall, Nutrition Research Institute, University of North Carolina at Chapel Hill, Kannapolis, NC, USA.
Walter B Friday, Nutrition Research Institute, University of North Carolina at Chapel Hill, Kannapolis, NC, USA.
David R Kirchner, Nutrition Research Institute, University of North Carolina at Chapel Hill, Kannapolis, NC, USA.
Steven H Zeisel, Nutrition Research Institute, University of North Carolina at Chapel Hill, Kannapolis, NC, USA; Department of Nutrition, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Data Availability
Data described in the article will be made publicly and freely available pending completion of the full study.
References
- 1. Zeisel SH, Klatt KC, Caudill MA. Choline. Adv Nutr. 2018;9(1):58–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Noga AA, Vance DE. A gender-specific role for phosphatidylethanolamine N-methyltransferase-derived phosphatidylcholine in the regulation of plasma high density and very low density lipoproteins in mice. J Biol Chem. 2003;278(24):21851–9. [DOI] [PubMed] [Google Scholar]
- 3. Noga AA, Zhao Y, Vance DE. An unexpected requirement for phosphatidylethanolamine N-methyltransferase in the secretion of very low density lipoproteins. J Biol Chem. 2002;277(44):42358–65. [DOI] [PubMed] [Google Scholar]
- 4. Hörl G, Wagner A, Cole LK, Malli R, Reicher H, Kotzbeck P, Köfeler H, Höfler G, Frank S, Bogner-Strauss JGet al. Sequential synthesis and methylation of phosphatidylethanolamine promote lipid droplet biosynthesis and stability in tissue culture and in vivo. J Biol Chem. 2011;286(19):17338–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vance JE, Vance DE. The role of phosphatidylcholine biosynthesis in the secretion of lipoproteins from hepatocytes. Can J Biochem Cell Biol. 1985;63(8):870–81. [DOI] [PubMed] [Google Scholar]
- 6. Song J, da Costa KA, Fischer LM, Kohlmeier M, Kwock L, Wang S, Zeisel SH. Polymorphism of the PEMT gene and susceptibility to nonalcoholic fatty liver disease (NAFLD). FASEB J. 2005;19(10):1266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fischer LM, daCosta KA, Kwock L, Stewart PW, Lu T-S, Stabler SP, Allen RH, Zeisel SH. Sex and menopausal status influence human dietary requirements for the nutrient choline. Am J Clin Nutr. 2007;85(5):1275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zeisel SH, Da Costa KA, Franklin PD, Alexander EA, Lamont JT, Sheard NF, Beiser A. Choline, an essential nutrient for humans. FASEB J. 1991;5(7):2093–8. [PubMed] [Google Scholar]
- 9. Buchman AL, Dubin MD, Moukarzel AA, Jenden DJ, Roch M, Rice KM, Gornbein J, Ament ME. Choline deficiency: a cause of hepatic steatosis during parenteral nutrition that can be reversed with intravenous choline supplementation. Hepatology. 1995;22(5):1399–403. [PubMed] [Google Scholar]
- 10. Deminice R, de Castro GSF, Francisco LV, da Silva LECM, Cardoso JFR, Frajacomo FTT, Teodoro BG, dos Reis Silveira L, Jordao AA. Creatine supplementation prevents fatty liver in rats fed choline-deficient diet: a burden of one-carbon and fatty acid metabolism. J Nutr Biochem. 2015;26(4):391–7. [DOI] [PubMed] [Google Scholar]
- 11. Mehedint MG, Zeisel SH. Choline's role in maintaining liver function: new evidence for epigenetic mechanisms. Curr Opin Clin Nutr Metab Care. 2013;16(3):339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. da Costa KA, Corbin KD, Niculescu MD, Galanko JA, Zeisel SH. Identification of new genetic polymorphisms that alter the dietary requirement for choline and vary in their distribution across ethnic and racial groups. FASEB J. 2014;28(7):2970–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Trujillo-Gonzalez I, Friday WB, Munson CA, Bachleda A, Weiss ER, Alam NM, Sha W, Zeisel SH, Surzenko N. Low availability of choline in utero disrupts development and function of the retina. FASEB J. 2019;33(8):9194–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haws SA, Yu D, Ye C, Wille CK, Nguyen LC, Krautkramer KA, Tomasiewicz JL, Yang SE, Miller BR, Liu WHet al. Methyl-metabolite depletion elicits adaptive responses to support heterochromatin stability and epigenetic persistence. Mol Cell. 2020;78(2):210–23.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dominguez-Salas P, Moore SE, Baker MS, Bergen AW, Cox SE, Dyer RA, Fulford AJ, Guan Y, Laritsky E, Silver MJet al. Maternal nutrition at conception modulates DNA methylation of human metastable epialleles. Nat Commun. 2014;5:3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ideraabdullah FY, Zeisel SH. Dietary modulation of the epigenome. Physiol Rev. 2018;98(2):667–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ye C, Sutter BM, Wang Y, Kuang Z, Tu BP. A metabolic function for phospholipid and histone methylation. Mol Cell. 2017;66(2):180–93.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Institute of Medicine.. Dietary Reference Intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington (DC): National Academies Press; 1998. [PubMed] [Google Scholar]
- 19. Wallace TC, Fulgoni VL 3rd. Assessment of total choline intakes in the United States. J Am Coll Nutr. 2016;35(2):108–12. [DOI] [PubMed] [Google Scholar]
- 20. Fischer LM, da Costa KA, Kwock L, Galanko J, Zeisel SH. Dietary choline requirements of women: effects of estrogen and genetic variation. Am J Clin Nutr. 2010;92(5):1113–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pynn CJ, Henderson NG, Clark H, Koster G, Bernhard W, Postle AD. Specificity and rate of human and mouse liver and plasma phosphatidylcholine synthesis analyzed in vivo. J Lipid Res. 2011;52(2):399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao X, Zeisel SH, Zhang S. Rapid LC-MRM-MS assay for simultaneous quantification of choline, betaine, trimethylamine, trimethylamine N-oxide, and creatinine in human plasma and urine. Electrophoresis. 2015;36(18):2207–14. [DOI] [PubMed] [Google Scholar]
- 23. Jay S, DuRant RH, Litt IF, Linder CW, Shoffitt T. Riboflavin, self-report, and serum norethindrone. Comparison of their use as indicators of adolescent compliance with oral contraceptives. Am J Dis Child. 1984;138(1):70–3. [DOI] [PubMed] [Google Scholar]
- 24. Ramanujam VMS, Anderson KE, Grady JJ, Nayeem F, Lu L-JW. Riboflavin as an oral tracer for monitoring compliance in clinical research. Open Biomark J. 2011;4:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang M, Liu H, Huang X, Shao L, Xie X, Wang F, Yang J, Pei P, Zhang Z, Zhai Yet al. A novel LC-MS/MS assay for vitamin B1, B2 and B6 determination in dried blood spots and its application in children. J Chromatogr B. 2019;1112:33–40. [DOI] [PubMed] [Google Scholar]
- 26. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. [DOI] [PubMed] [Google Scholar]
- 27. Koc H, Mar MH, Ranasinghe A, Swenberg JA, Zeisel SH. Quantitation of choline and its metabolites in tissues and foods by liquid chromatography/electrospray ionization-isotope dilution mass spectrometry. Anal Chem. 2002;74(18):4734–40. [DOI] [PubMed] [Google Scholar]
- 28. Sørensen LP, Gormsen LC, Nielsen S. VLDL-TG kinetics: a dual isotope study for quantifying VLDL-TG pool size, production rates, and fractional oxidation in humans. Am J Physiol Endocrinol Metab. 2009;297(6):E1324–30. [DOI] [PubMed] [Google Scholar]
- 29. Salibi N, Xu J, Taouli B. 1H single voxel spectroscopy with 2D PACE motion control. Magnetom Flash. 2006;3:60–2. [Google Scholar]
- 30. Stefan D, Cesare FD, Andrasescu A, Popa E, Lazariev A, Vescovo E, Strbak O, Williams S, Starcuk Z, Cabanas Met al. Quantitation of magnetic resonance spectroscopy signals: the jMRUI software package. Meas Sci Technol. 2009;20(10):104035. [Google Scholar]
- 31. Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997;129(1):35–43. [DOI] [PubMed] [Google Scholar]
- 32. Weis J, Kullberg J, Ahlström H. Multiple breath-hold proton spectroscopy of human liver at 3T: relaxation times and concentrations of glycogen, choline, and lipids. J Magn Reson Imaging. 2018;47(2):410–17. [DOI] [PubMed] [Google Scholar]
- 33. Ter Voert E, Heijmen L, van Asten JJA, Wright AJ, Nagtegaal ID, Punt CJA, de Wilt JHW, van Laarhoven HWM, Heerschap A. Levels of choline-containing compounds in normal liver and liver metastases of colorectal cancer as recorded by 1H MRS. NMR Biomed. 2019;32(1):e4035. [DOI] [PubMed] [Google Scholar]
- 34. Benjamini YK, Abba M, Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika. 2006;93(3):491–507. [Google Scholar]
- 35. Zeisel SH, Story DL, Wurtman RJ, Brunengraber H. Uptake of free choline by isolated perfused rat liver. Proc Natl Acad Sci U S A. 1980;77(8):4417–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Slow S, Garrow TA. Liver choline dehydrogenase and kidney betaine-homocysteine methyltransferase expression are not affected by methionine or choline intake in growing rats. J Nutr. 2006;136(9):2279–83. [DOI] [PubMed] [Google Scholar]
- 37. Lever M, Sizeland PC, Bason LM, Hayman CM, Chambers ST. Glycine betaine and proline betaine in human blood and urine. Biochim Biophys Acta. 1994;1200(3):259–64. [DOI] [PubMed] [Google Scholar]
- 38. Veenema K, Solis C, Li R, Wang W, Maletz CV, Abratte CM, Caudill MA. Adequate Intake levels of choline are sufficient for preventing elevations in serum markers of liver dysfunction in Mexican American men but are not optimal for minimizing plasma total homocysteine increases after a methionine load. Am J Clin Nutr. 2008;88(3):685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yan J, Wang W, Gregory JF 3rd, Malysheva O, Brenna JT, Stabler SP, Allen RH, Caudill MA. MTHFR C677T genotype influences the isotopic enrichment of one-carbon metabolites in folate-compromised men consuming d9-choline. Am J Clin Nutr. 2011;93(2):348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reischauer C, Hock A, Kolokythas O, Binkert CA, Gutzeit A. Fully navigated 3 T proton magnetic resonance spectroscopy of liver metastases with inner-volume saturation. Abdom Radiol. 2017;42(11):2615–22. [DOI] [PubMed] [Google Scholar]
- 41. Fischbach F, Schirmer T, Thormann M, Freund T, Ricke J, Bruhn H. Quantitative proton magnetic resonance spectroscopy of the normal liver and malignant hepatic lesions at 3.0 Tesla. Eur Radiol. 2008;18(11):2549–58. [DOI] [PubMed] [Google Scholar]
- 42. Slow S, Lever M, Chambers ST, George PM. Plasma dependent and independent accumulation of betaine in male and female rat tissues. Physiol Res. 2009;58(3):403–10. [DOI] [PubMed] [Google Scholar]
- 43. Ejaz A, Martinez-Guino L, Goldfine AB, Ribas-Aulinas F, De Nigris V, Ribó S, Gonzalez-Franquesa A, Garcia-Roves PM, Li E, Dreyfuss JMet al. Dietary betaine supplementation increases Fgf21 levels to improve glucose homeostasis and reduce hepatic lipid accumulation in mice. Diabetes. 2016;65(4):902–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Holm PI, Ueland PM, Vollset SE, Midttun Ø, Blom HJ, Keijzer MB, den Heijer M. Betaine and folate status as cooperative determinants of plasma homocysteine in humans. Arterioscler Thromb Vasc Biol. 2005;25(2):379–85. [DOI] [PubMed] [Google Scholar]
- 45. Schwahn BC, Chen Z, Laryea MD, Wendel U, Lussier-Cacan S, Genest J Jr, Mar MH, Zeisel SH, Castro C, Garrow Tet al. Homocysteine-betaine interactions in a murine model of 5,10-methylenetetrahydrofolate reductase deficiency. FASEB J. 2003;17(3):1–25. [DOI] [PubMed] [Google Scholar]
- 46. Lever M, George PM, Elmslie JL, Atkinson W, Slow S, Molyneux SL, Troughton RW, Richards AM, Frampton CM, Chambers ST. Betaine and secondary events in an acute coronary syndrome cohort. PLoS One. 2012;7(5):e37883. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article will be made publicly and freely available pending completion of the full study.