ABSTRACT
Background
Leucine-enriched protein (LEU-PRO) and long-chain (LC) n–3 (ω–3) PUFAs have each been proposed to improve muscle mass and function in older adults, whereas their combination may be more effective than either alone.
Objective
The impact of LEU-PRO supplementation alone and combined with LC n–3 PUFAs on appendicular lean mass, strength, physical performance and myofibrillar protein synthesis (MyoPS) was investigated in older adults at risk of sarcopenia.
Methods
This 24-wk, 3-arm parallel, randomized, double-blind, placebo-controlled trial was conducted in 107 men and women aged ≥65 y with low muscle mass and/or strength. Twice daily, participants consumed a supplement containing either LEU-PRO (3 g leucine, 10 g protein; n = 38), LEU-PRO plus LC n–3 PUFAs (0.8 g EPA, 1.1 g DHA; LEU-PRO+n–3; n = 38), or an isoenergetic control (CON; n = 31). Appendicular lean mass, handgrip strength, leg strength, physical performance, and circulating metabolic and renal function markers were measured pre-, mid-, and postintervention. Integrated rates of MyoPS were assessed in a subcohort (n = 28).
Results
Neither LEU-PRO nor LEU-PRO+n–3 supplementation affected appendicular lean mass, handgrip strength, knee extension strength, physical performance or MyoPS. However, isometric knee flexion peak torque (treatment effect: −7.1 Nm; 95% CI: −12.5, −1.8 Nm; P < 0.01) was lower postsupplementation in LEU-PRO+n–3 compared with CON. Serum triacylglycerol and total adiponectin concentrations were lower, and HOMA-IR was higher, in LEU-PRO+n–3 compared with CON postsupplementation (all P < 0.05). Estimated glomerular filtration rate was higher and cystatin c was lower in LEU-PRO and LEU-PRO+n–3 postsupplementation compared with CON (all P < 0.05).
Conclusions
Contrary to our hypothesis, we did not observe a beneficial effect of LEU-PRO supplementation alone or combined with LC n–3 PUFA supplementation on appendicular lean mass, strength, physical performance or MyoPS in older adults at risk of sarcopenia. This trial was registered at clinicaltrials.gov as NCT03429491.
Keywords: aging, LC n–3 PUFA, leucine, protein, muscle mass, strength, sarcopenia
Introduction
Preserving physical function, mobility and ultimately independence is a priority for older adults (1). Diminished skeletal muscle strength and mass, termed sarcopenia (2), contributes substantially to physical frailty (3), disability (4), falls risk (5), physical dependence (6), reduced quality of life (7) and mortality (8) among older people. As such, solutions to attenuate sarcopenic declines are imperative. Older adults require ∼0.4 g protein/kg per meal to maximally stimulate myofibrillar protein synthesis (MyoPS), ∼67% more protein than is required by younger individuals (0.24 g·kg−1·meal−1) (9). Typically, older adults consume suboptimal protein intakes at breakfast and lunch (∼0.1–0.3 g·kg−1·meal−1) (10, 11), which likely contributes to the chronic state of negative net muscle protein balance that precipitates muscle loss (12).
Numerous studies in older adults have reported that the MyoPS and mixed muscle protein synthesis (MPS) response to a suboptimal protein dose is enhanced by the addition of the amino acid leucine, a potent activator of the mammalian target of rapamycin pathway and “trigger” for MPS (13–16). Furthermore, supplementation of meals with leucine augments integrated rates of MyoPS measured over several days (17). In longer-term (6–13 wk) studies, leucine-enriched protein (LEU-PRO) supplementation resulted in increased skeletal muscle mass and/or increased lower extremity function in healthy (18) and sarcopenic (19) older adults, although this is not a universal finding (20). Interestingly, the improvements were observed in the absence of concomitant exercise training (18, 19). This suggests, as a proof of principle, that nutritional supplementation alone may augment muscle mass and function in individuals who are unable/unwilling to exercise, which represents a significant cohort of older persons (21, 22).
Another emerging nutrient of interest in the sarcopenia field are long-chain (LC) n–3 PUFAs derived from fish oil. A double-blind randomized controlled trial (RCT) demonstrated that LC n–3 PUFA supplementation for 6 mo improved muscle mass (treatment effect: 3.6%; 95% CI: 0.2%, 7.0%), handgrip strength (2.3 kg: 0.8 kg, 3.7 kg) and average 1-repetition-maximum lower- and upper-body strength (4.0%; 0.8%, 7.3%) in sedentary older men and women (23). LC n–3 PUFA supplementation also augmented the mixed MPS response during a hyperaminoacidemic-hyperinsulinemic clamp in older adults (24), suggesting that LC n–3 PUFAs may enhance the sensitivity of older muscle to amino acids.
Therefore, we tested the hypotheses that 1) appendicular lean mass (ALM), strength, and physical performance and MyoPS would increase in older adults supplemented with LEU-PRO and 2) that the addition of LC n–3 PUFA supplementation would further enhance the positive effects of LEU-PRO supplementation. To the best of our knowledge, no studies have investigated whether an additive effect may exist when LC n–3 PUFA supplementation is combined with LEU-PRO supplementation in older adults in the context of a nutrition-only intervention. We also determined the impact of LEU-PRO and LEU-PRO plus LC n–3 PUFA supplementation on markers of metabolic health, given the well-established impact of LC n–3 PUFAs on lipid metabolism (25), as well as indices of renal function.
Methods
Study design
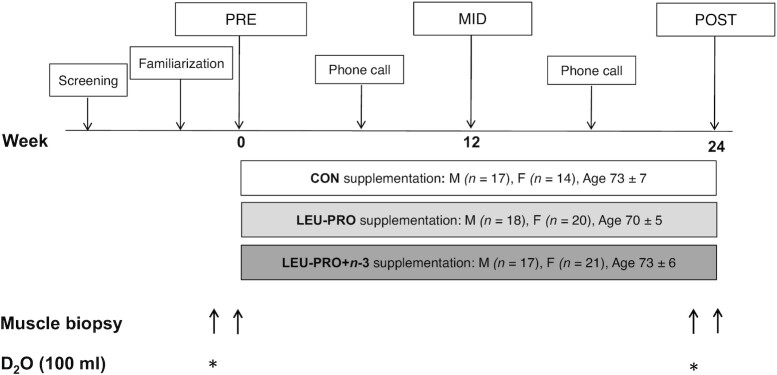
This randomized, double-blind, parallel-group, placebo-controlled trial, approved by the University College Dublin (UCD) Human Research Ethics Committee (permit: LS-16–41-Murphy-Roche), was conducted in 107 older men and women in UCD, Ireland. An overview of the study design is shown in Figure 1. Participants were randomly assigned using the MINIM randomization program, stratified by age and sex, by an independent researcher to 1 of 3 groups: control (CON; n = 31), LEU-PRO (n = 38), or LEU-PRO plus LC n–3 PUFAs (LEU-PRO+n–3; n = 38). Figure 2 shows a CONSORT (Consolidated Standards of Reporting Trials) diagram of the progress from recruitment through to completion of the study. All investigators, study staff and participants were blinded to group allocations, and the randomization code was not broken until statistical analysis of the primary and secondary outcomes was complete. The primary outcome was ALM measured by DXA. Secondary outcomes were as follows: handgrip and lower extremity muscle strength, physical performance, body mass, BMI, fat mass, step count, dietary intake, erythrocyte phospholipid fatty acid composition, biochemical markers of metabolic health and renal function, and in a subcohort of participants, the integrated rate of MyoPS. At the screening visit, participants were informed of all study procedures and were given the option to be included or excluded from the subcohort involving the MyoPS measurements.
FIGURE 1.
Schematic overview of study design. Pre-, mid- and postintervention testing visits involved fasted blood sampling, anthropometry, DXA scanning and the assessment of handgrip and lower extremity strength, physical performance and dietary intake in all participants. A subcohort of participants also took part in the muscle biopsies and D2O loading. CON, control; D2O, deuterated water; LEU-PRO, leucine-enriched protein, LEU-PRO+n–3, leucine-enriched protein plus long-chain n–3 PUFAs; MID, mid-intervention; POST, postintervention, PRE, preintervention. * indicates consumption of 100-mL of D2O.
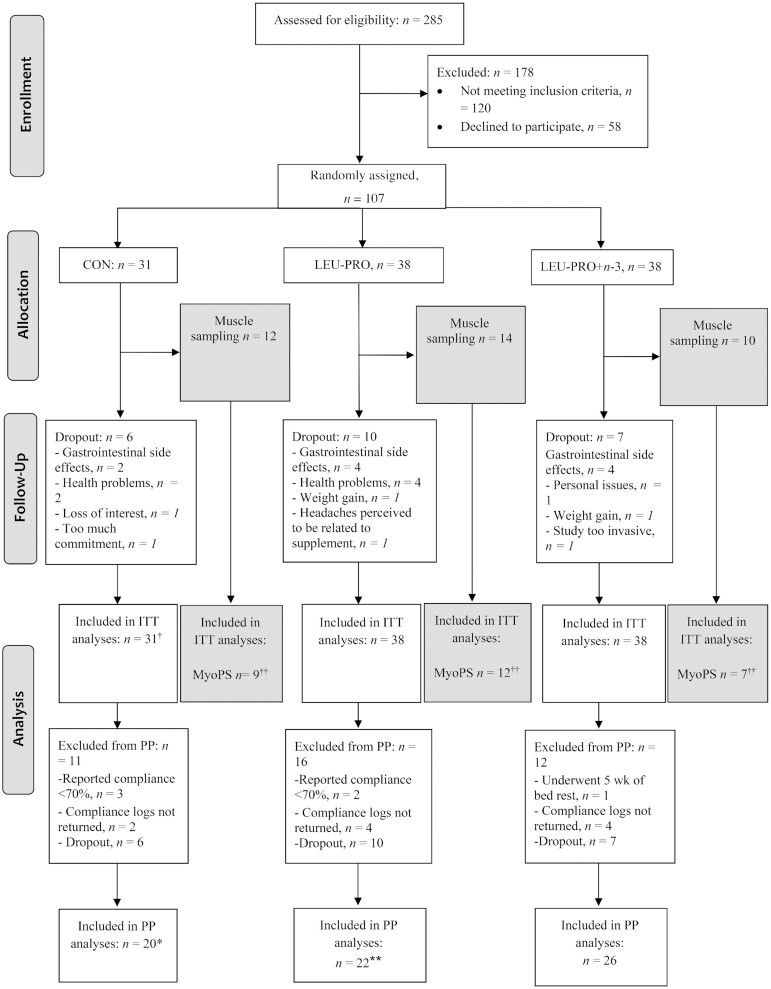
FIGURE 2.
Recruitment, selection, randomization and follow-up of participants. †In biochemical measurements, n = 1 was excluded due to the development of type 2 diabetes during the course of the study. ††Not enough skeletal muscle tissue obtained to perform the MyoPS analyses in n = 2 in CON, n = 2 in LEU-PRO and n = 3 in LEU-PRO+n–3. In a further n = 2 in CON, n = 1 in LEU-PRO, and n = 2 in LEU-PRO+n–3, MyoPS could not be determined either pre- or postintervention due to inadequate muscle tissue. n = 1 in CON withdrew from the optional MyoPS measurement after the initial biopsy but continued with the other study measurements, n = 2 in LEU-PRO and n = 1 in LEU-PRO+n–3 dropped out of the study entirely. *In the physical performance measurements (TUG, FTSTS, SPPB, gait speed, single-leg standing balance) an additional n = 1 was excluded. **In the physical performance measurements an additional n = 2 were excluded. CON, control; FTSTS, 5 times sit-to-stand; ITT, intention-to-treat; LEU-PRO, leucine-enriched protein; LEU-PRO+n–3, leucine-enriched protein plus long-chain n–3 PUFAs; MyoPS, myofibrillar protein synthesis; PP, per protocol; SPPB, short physical performance battery; TUG, Timed Up-and-Go.
Study endpoints were assessed pre- and postsupplementation. In addition, mid-intervention testing was conducted after 12 wk for all variables except for MyoPS. In order to reduce the “practice” effect and enhance reliability, participants were familiarized with all muscle strength and physical performance assessments ∼1 wk prior to preintervention testing (Figure 1). All endpoint testing visits took place in the morning to minimize diurnal variation. In order to control for hydration status, participants were asked to drink a fixed quantity of water (200 mL) 2 h before attending UCD for measurements. Blood sampling, body composition and anthropometry were assessed first at each testing visit, while participants were in the fasted state. Participants were then provided with a standardized snack (cereal bar and juice) prior to muscle strength and physical performance assessments. Recruitment and intervention were conducted between 3 February 2017 and 20 December 2018.
Participants
Urban community-dwelling men and women were recruited via newspaper advertisements, recreational clubs and posters. Inclusion criteria were as follows: ≥65 y of age, increased risk of sarcopenia as defined by having low skeletal muscle mass index determined by bioelectrical impedance analysis (BIA) [<6.75 kg/m2 in females and <10.75 kg/m2 in males (26)] and/or low handgrip strength [<20 kg females, <30 kg males (27)], BMI (kg/m2) of 19–35, nonsmokers, no major cognitive impairment [Mini-Mental State Examination (MMSE) score ≥21] and generally healthy according to responses to a standard health screening questionnaire. Exclusion criteria included self-reported 1) malignancy in the past 5 y (except for adequately treated prostate cancer without evidence of metastases, localized bladder cancer, cervical carcinoma in situ, breast cancer in situ or nonmelanoma skin cancer); 2) diabetes mellitus; 3) advanced renal disease; 4) conditions affecting ability to consume, digest and/or absorb the study drink (e.g., cow-milk protein allergy, inflammatory bowel disease); 5) neuromuscular disease; 6) significant body mass changes (>3 kg) in the 1 mo period prior to the study; 7) total walking incapacity; 8) participation in a structured, regular (at least twice per week) and progressive muscle-strengthening program; 9) usual protein intake at breakfast >0.5 g protein/kg (assessed via diet history taken at the screening visit); 10) regular LC n–3 PUFA supplementation and not willing to cease consumption ≥6 wk prior to and for the duration of the 24-wk study; and 11) use of medications interfering with the nutrition intervention (e.g., corticosteroids for systemic use, hormone replacement therapy, insulin, high-dose anti-inflammatories, simvastatin).
Nutrition intervention
The active supplements provided 21.2 g protein/d, which included 6.2 g leucine/d, with or without 4 g LC n–3 PUFAs/d; full compositional details of supplements (CON, LEU-PRO and LEU-PRO+n–3) are shown in Table 1. Participants received individual servings of their assigned, ready-to-drink, juice-based supplement in identical 200-mL cartons. All of the supplements were taste- and energy-matched and were manufactured by Smartfish (Norway). The LEU-PRO and LEU-PRO+n–3 beverages contained a combination of whey protein and a peptide carrier enriched with free leucine (PepForm; Glanbia Nutritionals). The LEU-PRO+n–3 supplements contained fish oil, and the LEU-PRO and CON supplements were formulated with a mixture of high-oleic sunflower and corn oil. Maltodextrin was added to the CON supplement to make it isocaloric with the 2 LEU-PRO–containing supplements. To supplement lower-protein meals, participants were asked to consume 2 of their supplements per day: one directly before breakfast and the other directly before their second light meal of the day (lunch or evening meal depending on the individual's meal pattern).
TABLE 1.
Nutritional composition of the supplements per single (200 mL) serving1
| Supplements | |||
|---|---|---|---|
| CON | LEU-PRO | LEU-PRO+n–3 | |
| Energy, kcal | 200 | 209 | 209 |
| Fat, g | 8.9 | 10.1 | 10.1 |
| SFAs, g | 1.0 | 1.6 | 2.1 |
| MUFAs, g | 2.5 | 2.8 | 4.5 |
| PUFAs, mg | 5.3 | 5.5 | 2.8 |
| n–6 PUFAs, g | 5.3 | 5.5 | 0.4 |
| n–3 PUFAs, g | 0.02 | 0.03 | 2.4 |
| EPA, mg | <0.01 | <0.01 | 0.78 |
| DHA, mg | <0.01 | <0.01 | 1.14 |
| DPA, mg | <0.01 | <0.01 | 0.11 |
| Protein, g | 0.2 | 10.6 | 10.6 |
| Leucine, g | 0.0 | 3.1 | 3.1 |
| Carbohydrate, g | 29.7 | 18.8 | 18.8 |
| Fiber, g | 1.3 | 0.9 | 0.9 |
CON, control; DPA, docosapentaenoic acid; LEU-PRO, leucine-enriched protein; LEU-PRO+n–3, leucine-enriched protein plus long-chain n–3 PUFAs.
Participants were instructed to maintain their habitual diet for the duration of the intervention. In anticipation that participants may be less hungry than usual when consuming the 2 × 200-mL supplements each day, the research dietitians and nutritionists emphasized the importance of maintaining the habitual protein content of the diet and instructed participants to reduce their intake of carbohydrate- or fat-rich, protein-poor foods (i.e., butter, sweets, cakes, desserts, bread, rice, potatoes, etc.) if their appetite was lower at mealtimes. Participants were instructed to avoid taking any new nutritional supplements throughout the intervention and to maintain their habitual physical activity for the duration of the study.
Body composition and anthropometry
ALM (sum of the fat-free soft tissue mass of arms and legs) and fat mass were evaluated via DXA (GE-LUNAR iDXA; Aymes Medical). Body mass was assessed using a calibrated scale (SECA), and height was measured using a stadiometer (Holtain).
Muscle strength
Isometric knee extensor and flexor peak torque and isokinetic knee extensor peak torque were measured on the self-reported dominant leg using a dynamometer (Cybex NORM; Humac). After a submaximal warm-up, participants completed 4 maximal leg extension and flexion contractions at 90° of knee flexion, each separated by 60 s of rest, followed by 3 maximal isokinetic knee extensions at 2 different angular velocities—60°/s and 120°/s—with 10 s of rest separating each contraction and 60 s between velocities. The repetition yielding the highest peak torque value in each test was used for further analysis.
Handgrip strength was measured on the right and left side using a hydraulic hand dynamometer (Saehan SH5001; Glanford Electronics Ltd.). On each side, 2 measurements of grip strength were recorded. If the relative difference between these 2 measurements was >10%, a third measurement was performed. The maximal value of the 2 reproducible measures was retained for analyses.
Physical performance
Physical performance was assessed via the Short Performance Physical Battery (SPPB) (28), the Timed Up-and-Go test (TUG) (29) and the single-leg standing balance test (30) according to standard protocols. As exploratory outcomes, the continuous SPPB components of 5 times sit-to-stand (FTSTS) time (seconds) and gait speed (meters/second) were examined separately from the composite SPPB score. The TUG test and the single-leg standing balance test were repeated twice, with the average of 2 tests used in the analysis.
MyoPS
The integrated rate of MyoPS was measured over two 72-h periods: preintervention (immediately prior to the beginning of supplementation) and postintervention (during the final week of supplementation), as described previously (17). On the first day of the preintervention 72-h MyoPS measurement period, participants reported to the laboratory in the fasted state and provided a saliva sample and a muscle biopsy from the vastus lateralis of their nondominant leg. Biopsies were obtained under local lidocaine anesthesia by an experienced medical doctor using the microbiopsy technique (Biofeather, Medax) (31). The tissue samples were frozen immediately in liquid nitrogen, then stored at −80°C. Participants then consumed a single 100-mL oral bolus of deuterated water (D2O; 70 atom%; Isowater) to label newly synthesized muscle proteins. Following this, participants returned home and were asked to continue their usual diets and daily activities. A second muscle biopsy was obtained from the same leg 72 h later. Total body water 2H enrichment was used as a surrogate for plasma alanine labeling and was determined from saliva swabs collected daily during the 72-h MyoPS measurement period. Saliva swabs were collected in the mornings before eating or drinking and were frozen for further analysis. During the 72-h MyoPS measurement period, participants were asked to keep a detailed, weighed food record and to wear a pedometer (Piezo SC-StepX™; StepsCount) to record daily step count. Participants were asked to refrain from alcohol and strenuous exercise for 2 d before, and for the duration of, the 72-h MyoPS measurement period.
The same procedure was repeated postintervention with 2 exceptions: 1) participants were asked to replicate their daily step count from the preintervention MyoPS measurement period and 2) participants were provided with a copy of their preintervention 72-h weighed food record, which was modified by the research dietitian to include one of their allocated supplements at breakfast and one at their second light meal. In order to match energy intake to the preintervention MyoPS measurement period, some protein-poor high-fat/-carbohydrate foods were removed and/or portion sizes of these foods were reduced (mainly biscuits, cakes, desserts or bread) to account for the additional energy provided by the supplements. Participants were asked to replicate this modified preintervention food record during the postintervention MyoPS measurement period.
Myofibrillar-enriched proteins were isolated from the muscle biopsies (∼30 mg) and the incorporation of deuterium (2H) into protein-bound alanine was determined using a GC-pyrolysis-isotope-ratio mass spectrometer (MAT 253; ThermoFisher Scientific) equipped with a pyrolysis oven using a 60-m DB-17MS column and 5-m precolumn (no. 122–4762; Agilent) and GC-Isolink, as previously described (32). Samples were measured in quadruplicate along with a series of known standards every 12 injections. Saliva samples were analyzed for 2H enrichment in duplicate on an isotope ratio mass spectrometer (Delta V Advantage, fitted with a Gasbench II; ThermoFisher Scientific), as previously described (32). The fractional synthetic rate (FSR) of myofibrillar protein was determined from the incorporation of deuterium-labeled alanine into protein, using the enrichment of body water (corrected for the mean number of deuterium moieties incorporated per alanine, 3.7) as the surrogate for precursor labeling between subsequent biopsies according to the following standard equation:
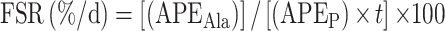 |
(1) |
where, APEAla = deuterium enrichment of protein bound alanine, APEP = mean precursor enrichment over the time period and t is the time between biopsies.
Biochemical markers of renal and metabolic health
Fasted, resting morning venous blood samples were collected from each participant's antecubital vein. Serum, plasma and erythrocyte aliquots were stored at −80°C for the assessment of the various analytes in duplicate at completion of the study. Serum concentrations of triacylglycerol (TG), total cholesterol, HDL cholesterol, creatinine (via enzymatic colorimetric method), urea, cystatin c, glucose and high-sensitivity C-reactive protein (hsCRP) were analyzed using a RxDaytona chemical autoanalyzer (Randox Laboratories). LDL-cholesterol concentration was calculated using the Friedewald equation (33). ELISA kits were used to measure serum insulin (Crystal Chem), serum insulin-like growth factor I (IGF-I; Crystal Chem) and plasma total and high-molecular-weight adiponectin (ELISA Genie) concentrations, as previously described (34). Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) cystatin c equation (35). The CKD-EPI cystatin c equation was chosen instead of creatinine-based estimates of eGFR as the latter have the potential to be influenced by dietary protein intake and muscle mass (36, 37). Total serum 25-hydroxyvitamin D [25(OH)D; sum of 25(OH)D2 + 25(OH)D3] concentrations were quantified using LC–tandem MS (LC-MS/MS; API 4000; AB Sciex).
Erythrocyte membrane phospholipid composition was measured as described previously (38). Briefly, lipids from isolated erythrocytes were extracted in chloroform:methanol (2:1 vol:vol) containing butylated hydroxytoluene (0.01%) as an antioxidant and heptadecanoic acid (17:0) as an internal standard. After lipid extraction and transmethylation with BF3/methanol, the lipid phase containing the fatty acid methyl esters was dissolved in hexane and analyzed using a Hewlett-Packard 5890 Series II gas chromatograph with a Varian CP-SIL capillary column (100 m; internal diameter, 0.25 mm) and flame-ionization detector. Individual fatty acids were detected in accordance with the retention times of standards. Erythrocyte fatty acid data are reported as relative abundance (percentage composition).
Diet and physical activity
Participants wore a pedometer (Piezo SC-StepX™, StepsCount) to measure their daily step count for 3 d immediately before their pre-, mid- and postintervention visits. Dietary intake was assessed via a 24-h recall performed by a research dietitian or nutritionist using the 5-step multiple-pass method (39) at pre-, mid- and postintervention visits.
Medical history and medication use
A lifestyle questionnaire was used to obtain information on history of diseases/illnesses, smoking history, current use of medication, physical activity habits and dietary supplement use. Any alterations to medications or new medications prescribed to participants by their doctor were documented by the researchers at each testing appointment or during phone calls at week 6 and week 18.
Compliance
Participants were provided with daily supplement logs and were asked to record the time of day supplements and meals were consumed. Logs were collected from the participants at the mid- and postintervention visits. Compliance was calculated as the percentage of supplement consumed relative to the total prescribed. Compliance was also verified in LEU-PRO+n–3 via erythrocyte EPA (20:5n–3) and DHA (22:6n–3) phospholipid content.
Sample size
We determined our sample size based on the findings of Bauer et al. (19), which showed that, in sarcopenic older adults, supplementation with leucine-enriched whey protein twice daily for 13 wk resulted in an increase in ALM compared with the placebo group. Based on a group-by-time interaction in a mixed ANOVA, using a partial η2 of 0.02 and a correlation among repeated measures of 0.57, we calculated that a sample size of 104 would be required at an α level of 0.05 and β level of 0.80, with the assumption of an ∼20% dropout rate in all groups (G*Power v3.1).
On the basis of data from a previous leucine supplementation study (17) in which MyoPS was measured over a 3-d period using D2O in older men, we calculated that a sample size of 27 would be sufficient to detect a difference in integrated MyoPS between groups of 0.11%/d with an SD of 0.10%/d, using an α of 0.05 and a β of 0.80 (G*Power v3.1).
Adverse events
Participants were asked to report any adverse events to the researchers when they occurred; otherwise, this information was collected at each testing appointment (mid, post) and during the 6-wk and 18-wk phone calls.
Statistical analyses
All data were entered using the double data-entry method for quality control. In the primary analyses, data were analyzed using the intention-to-treat approach, wherein all participants who were randomly assigned were included in the analyses in their intended group, regardless of noncompliance, protocol deviations, withdrawal and anything that happened after randomization (40). Linear mixed-model analyses were conducted with group (CON, LEU-PRO, LEU-PRO+n–3), time (mid, post) and the group-by-time interaction as fixed factors; participants as a random factor; and preintervention values as covariates. Contrasts were constructed to estimate the differences between the CON and the 2 intervention groups (LEU-PRO and LEU-PRO+n–3) postintervention, with preintervention values as covariates. In addition, mid-intervention comparisons were conducted via contrasts as a secondary exploratory analysis, with the exception of MyoPS, which was only assessed pre- and postintervention. Linear mixed models are largely robust even to quite severe violations of model assumptions (41). Nevertheless, major violations of homoscedasticity and normality of residuals were ruled out by graphing and visually inspecting the data. One of the participants in the CON group developed type 2 diabetes (T2D) during the study; this 1 participant majorly influenced the overall outcomes for the circulating biochemical measures. As such, specifically for the biochemical data, we present a modified intention-to-treat analysis excluding this participant for clarity. No adjustments were made for multiple testing for secondary outcomes due to the exploratory nature of the study. Missing values in outcome variables were not imputed because mixed models handle missing data well by maximum likelihood estimation at the observation (time-point) level, avoiding listwise deletion (42). To assess the magnitude of between-group differences in skeletal muscle outcomes postintervention, standardized effect sizes were calculated using Cohen's d and interpreted using thresholds of 0.2, 0.5 and 0.8 for small, moderate and large, respectively (43).
In addition to the intention-to-treat analyses, 2 sensitivity analyses were performed to determine the robustness of trial findings (44). If the findings of sensitivity analyses are consistent with those of the primary analysis, this indicates that the findings are robust, thereby increasing confidence in the results (44). First, a per-protocol analysis was conducted in which we excluded participants who withdrew from the intervention, those who were not compliant with the supplementation or those who violated the protocol. Noncompliance was determined using the self-report supplement logs and defined as either consumption of <70% of the allocated supplements over the 24-wk intervention or missing/incomplete supplement logs. In the second sensitivity analysis, participants with outlying observations (± >3 SDs from the mean) were removed. As percentage compliance values were not normally distributed, between-group differences in compliance were assessed via a Kruskal-Wallis H test. All analyses were performed using SPSS (version 24.0; IBM). Results are presented as means ± SDs unless otherwise specified. For the primary outcome, we performed a Bonferroni adjustment on the 2 primary contrasts; therefore, significance was accepted as of P < 0.025. For all secondary and exploratory contrasts, significance was accepted as P < 0.05.
Results
Characteristics of study participants “at risk” of sarcopenia
Participants who participated in this study were deemed to be at risk of sarcopenia, whereby BIA-derived skeletal muscle mass index was low as per the Janssen et al. (26) criteria, in line with values denoting moderate-to-high physical disability risk (Table 2). Handgrip strength and physical performance (SPPB, gait speed, TUG) were normal according to the most recent thresholds set by the European Working Group on Sarcopenia in Older People (2) (Table 2). All participants self-reported as being of White race. The baseline characteristics of the subcohort who underwent the MyoPS measurements (Supplemental Table 1) were generally similar to the overall cohort.
TABLE 2.
Baseline characteristics of participants1
| CON (n = 31) | LEU-PRO (n = 38) | LEU-PRO+n–3 (n = 38) | |
|---|---|---|---|
| Female sex, n | 14 | 20 | 21 |
| Age, y | 73 ± 7 | 70 ± 5 | 73 ± 6 |
| Body mass, kg | 71.9 ± 10.0 | 69.0 ± 12.8 | 72.4 ± 12.7 |
| BMI, kg/m2 | 25.4 ± 2.8 | 24.8 ± 3.4 | 26.7 ± 3.2 |
| Fat mass, kg | 23.0 ± 6.2 | 21.2 ± 7.3 | 25.0 ± 5.9 |
| MMSE | 29 ± 2 | 29 ± 2 | 29 ± 1 |
| Number of medical conditions, n (%) | |||
| 0 | 10 (32.3) | 17 (44.7) | 15 (39.5) |
| 1 | 8 (25.8) | 6 (15.8) | 9 (23.7) |
| 2 | 6 (19.3) | 10 (26.3) | 9 (23.7) |
| ≥3 | 7 (22.6) | 5 (13.2) | 5 (13.1) |
| Number of medications | 2 ± 3 | 1 ± 2 | 2 ± 2 |
| SMMI (BIA), kg/m2 | |||
| Female | 5.73 ± 0.45 | 5.66 ± 0.37 | 5.84 ± 0.59 |
| Male | 8.32 ± 0.70 | 8.64 ± 0.81 | 8.79 ± 0.87 |
| ALMI (DXA), kg/m2 | |||
| Female | 6.38 ± 0.51 | 6.10 ± 0.46 | 6.29 ± 0.60 |
| Male | 7.70 ± 0.60 | 7.99 ± 0.92 | 8.10 ± 0.69 |
| Handgrip strength, kg | |||
| Female | 22.9 ± 4.9 | 22.7 ± 6.0 | 19.4 ± 4.3 |
| Male | 39.0 ± 7.5 | 38.8 ± 6.5 | 37.3 ± 6.1 |
| SPPB | 11 ± 2 | 11 ± 1 | 11 ± 1 |
| Gait speed, m/s | 0.76 ± 0.17 | 0.72 ± 0.12 | 0.77 ± 0.17 |
| FTSTS, s | 12.2 ± 2.3 | 11.6 ± 2.6 | 12.2 ± 3.1 |
| TUG, s | 7.0 ± 1.3 | 6.7 ± 1.0 | 7.0 ± 1.4 |
| Single-leg standing balance, s | 26.3 ± 23.6 | 26.1 ± 19.2 | 28.0 ± 21.5 |
| Isometric knee extension MVC, Nm | |||
| Female | 104 ± 25 | 95 ± 29 | 85 ± 27 |
| Male | 159 ± 42 | 164 ± 41 | 176 ± 36 |
| Isometric knee flexion MVC, Nm | |||
| Female | 35 ± 7 | 37 ± 11 | 32 ± 10 |
| Male | 57 ± 17 | 60 ± 16 | 63 ± 20 |
| TG, mmol/L | 1.1 ± 0.4 | 1.1 ± 0.4 | 1.2 ± 0.6 |
| HDL cholesterol, mmol/L | 1.6 ± 0.5 | 1.7 ± 0.4 | 1.8 ± 0.5 |
| LDL cholesterol, mmol/L | 3.3 ± 0.9 | 3.6 ± 0.8 | 3.5 ± 0.9 |
| Glucose, mmol/L | 5.8 ± 0.8 | 5.6 ± 0.6 | 5.7 ± 0.5 |
| Insulin, mU/L | 4.8 ± 3.7 | 4.6 ± 3.5 | 6.2 ± 3.1 |
| HOMA-IR | 1.4 ± 1.3 | 1.2 ± 1.0 | 1.6 ± 0.9 |
| hsCRP, mg/L | 1.5 ± 1.3 | 1.2 ± 0.9 | 1.9 ± 1.4 |
| Cystatin C, mg/L | 0.91 ± 0.18 | 0.79 ± 0.11 | 0.92 ± 0.20 |
| Physical activity, steps/d | 8192 ± 5142 | 8354 ± 4125 | 8257 ± 3906 |
| Oily fish consumption, portions/wk | 1.5 ± 2.3 | 1.1 ± 1.0 | 1.4 ± 1.7 |
Values are means ± SDs unless otherwise specified. ALMI, appendicular lean mass index; BIA, bioelectrical impedance analysis; CON, control; FTSTS, 5 times sit-to-stand; hsCRP, high-sensitivity C-reactive protein; LEU-PRO, leucine-enriched protein; LEU-PRO+n–3, leucine-enriched protein plus long-chain n–3 PUFAs; MMSE, Mini-Mental State Examination; MVC, maximal voluntary contraction; SMMI, skeletal muscle mass index; SPPB, Short Physical Performance Battery; TG, triacylglycerol; TUG, Timed Up-and-Go.
ALM was not affected by LEU-PRO supplementation alone or in combination with LC n–3 PUFA supplementation
In both the intention-to-treat and the sensitivity analyses (outlying values removed, per protocol) there were no differences between CON and either LEU-PRO or LEU-PRO+n–3 in adjusted ALM mid- or postintervention (P > 0.05; Table 3, Supplemental Figure 1). In both the intention-to-treat and the sensitivity analyses there were no differences between CON and either of the 2 intervention groups in fat mass, body mass or BMI at mid- or postintervention (P > 0.05; Table 3). Across all groups there was an increase in body mass over the 24-wk intervention (Table 3).
TABLE 3.
Anthropometric and DXA-derived body composition1
| Estimated between-group difference vs. CON at POST, mean (95% CI) | P | ||||
|---|---|---|---|---|---|
| Change from PRE | |||||
| PRE | MID2 | POST2 | |||
| Adjusted ALM,3 kg | |||||
| CON4 | 12.9 ± 2.8 | 0.12 ± 0.46 | 0.11 ± 0.46 | ||
| LEU-PRO5 | 12.6 ± 3.3 | 0.13 ± 0.36 | 0.11 ± 0.42 | 0.01 (−0.20, 0.22) | 0.93 |
| LEU-PRO+n–36 | 12.4 ± 3.1 | 0.07 ± 0.32 | 0.02 ± 0.31 | −0.08 (−0.29, 0.13) | 0.44 |
| Body mass (kg) | |||||
| CON4 | 71.9 ± 10.0 | 0.9 ± 1.7 | 1.3 ± 2.1 | ||
| LEU-PRO7 | 69.0 ± 12.8 | 0.9 ± 1.4 | 1.9 ± 1.6 | 0.7 (−0.2, 1.6) | 0.12 |
| LEU-PRO+n–38 | 72.4 ± 12.7 | 0.6 ± 1.6 | 1.1 ± 1.9 | −0.1 (−1.0, 0.8) | 0.84 |
| BMI, kg/m2 | |||||
| CON4 | 25.4 ± 2.8 | 0.34 ± 0.58 | 0.57 ± 0.95 | ||
| LEU-PRO7 | 24.8 ± 3.4 | 0.33 ± 0.50 | 0.69 ± 0.57 | 0.27 (−0.05, 0.61) | 0.10 |
| LEU-PRO+n–38 | 26.7 ± 3.2 | 0.22 ± 0.60 | 0.40 ± 0.67 | 0.05 (−0.28, 0.37) | 0.77 |
| Fat mass (kg) | |||||
| CON4 | 23.0 ± 6.2 | 0.5 ± 1.1 | 0.9 ± 1.4 | ||
| LEU-PRO5 | 21.2 ± 7.3 | 0.6 ± 0.9 | 1.1 ± 1.3 | 0.2 (−0.5, 0.8) | 0.65 |
| LEU-PRO+n–36 | 25.0 ± 5.9 | 0.8 ± 1.0 | 1.4 ± 1.7 | 0.5 (−0.2, 1.2) | 0.13 |
PRE value, change from PRE-to-MID and change from PRE-to-POST values are means ± SDs. Data represent the total cohort and were analyzed by an intention-to-treat approach using a linear mixed model with group (CON, LEU-PRO, LEU-PRO+n–3), time (MID, POST) and the group-by-time interaction as fixed factors; participants as a random factor; and PRE value as a covariate. Estimated between-group differences represent LEU-PRO − CON and LEU-PRO+n–3 − CON at POST and are derived from the linear mixed model and adjusted for PRE value. ALM, appendicular lean mass; CON, control; LEU-PRO, leucine-enriched protein; LEU-PRO+n–3, leucine-enriched protein plus long-chain n–3 PUFAs; MID, mid-intervention; POST, postintervention, PRE, preintervention.
PRE-to-MID changes represent participants with both PRE and MID data, PRE-to-POST changes represent participants with both PRE and POST data.
Skeletal muscle biopsies which were performed in n = 36 participants on the nondominant vastus lateralis 72 h and ∼ 1 h prior to DXA scanning. This resulted in variable degree of local muscle edema which had the potential to impact ALM values. Therefore, “adjusted” ALM was calculated in all participants (including those who underwent biopsies and those who did not) by summing lean soft tissue in the arms plus the dominant leg.
PRE n = 31, MID n = 28, POST n = 25.
PRE n = 38, MID n = 27, POST n = 28.
PRE n = 38, MID n = 31, POST n = 31.
PRE n = 38, MID n = 29, POST n = 28.
PRE n = 38, MID n = 33, POST n = 31.
Leg flexion strength, but not leg extension or handgrip strength, was lower following LEU-PRO+n–3 supplementation compared with CON
Intention-to-treat analysis demonstrated that there were no impacts of either LEU-PRO or LEU-PRO+n–3 supplementation on handgrip strength, isometric and isokinetic knee extension peak torque, or any of the physical performance tests mid- or postintervention (all P > 0.05; Table 4, Supplemental Figure 1). However, isometric knee flexion peak torque was lower in LEU-PRO+n–3 compared with CON postintervention (P < 0.01; Table 4), with a small effect size (d = −0.43, Supplemental Table 2). Isometric knee flexion peak torque did not differ between CON and LEU-PRO+n–3 mid-intervention (−2.3 Nm; 95% CI : −7.4 Nm, 2.8 Nm; P = 0.38), or between CON and LEU-PRO either mid-intervention (−1.5 Nm; −6.9 Nm, 3.8 Nm; P = 0.57) or postintervention (P = 0.32; Table 4, Supplemental Figure 1). The per-protocol analysis confirmed that isometric knee flexion peak torque was lower in LEU-PRO+n–3 compared with CON postintervention (−7.3 Nm; −13.6 Nm, −1.1 Nm; P = 0.02). The sensitivity analyses yielded similar results to the intention-to-treat for all other strength and physical performance measures, except for FTSTS time, which was higher (i.e., performance worsened) in LEU-PRO+n–3 compared with CON postintervention in the per-protocol analysis (between-group effect: 1.7 s; 95% CI: 0.4 s, 3.0 s; P < 0.01) and when outliers were removed (1.1 s; 0.1 s, 2.2 s; P < 0.05).
TABLE 4.
Strength and physical performance1
| Change from PRE | Estimated between-group difference vs. CON at POST, mean (95% CI) | ||||
|---|---|---|---|---|---|
| PRE | MID2 | POST2 | P | ||
| Handgrip strength, kg | |||||
| CON3 | 31.7 ± 10.3 | −0.1 ± 3.2 | 0.5 ± 3.5 | ||
| LEU-PRO4 | 30.3 ± 10.2 | 0.6 ± 2.9 | 1.0 ± 3.1 | 0.5 (−1.4, 2.3) | 0.61 |
| LEU-PRO+n–35 | 27.3 ± 10.3 | −0.2 ± 3.8 | 0.0 ± 3.3 | −0.8 (−2.6, 1.0) | 0.40 |
| Isometric knee extension peak torque, Nm | |||||
| CON3 | 134.1 ± 44.9 | 0.3 ± 17.6 | 3.7 ± 17.9 | ||
| LEU-PRO4 | 126.7 ± 50.0 | 2.2 ± 23.7 | 8.7 ± 23.7 | 4.9 (−5.8, 15.5) | 0.37 |
| LEU-PRO+n–36 | 125.4 ± 55.1 | −2.0 ± 18.4 | −1.1 ± 25.5 | −6.5 (−16.8, 3.9) | 0.22 |
| Isokinetic knee extension peak torque at 60°/s, Nm | |||||
| CON3 | 91.8 ± 33.8 | −1.6 ± 14.6 | −2.6 ± 16.0 | ||
| LEU-PRO7 | 86.4 ± 31.1 | −0.1 ± 13.0 | −0.1 ± 18.2 | 2.4 (−5.3, 10.0) | 0.55 |
| LEU-PRO+n–38 | 84.2 ± 40.3 | 0.1 ± 12.0 | −2.6 ± 13.3 | 0.1 (−7.4, 7.5) | 0.99 |
| Isokinetic knee extension peak torque at 120°/s, Nm | |||||
| CON3 | 67.4 ± 24.8 | −1.3 ± 11.0 | 1.9 ± 11.7 | ||
| LEU-PRO7 | 62.3 ± 25.7 | −0.1 ± 15.1 | −0.6 ± 13.4 | −2.0 (−8.5, 4.6) | 0.56 |
| LEU-PRO+n–38 | 63.8 ± 30.7 | −3.0 ± 9.6 | −2.2 ± 12.6 | −3.2 (−9.6, 3.2) | 0.32 |
| Isometric knee flexion peak torque, Nm | |||||
| CON9 | 47.9 ± 17.2 | 0.8 ± 11.8 | 4.0 ± 9.3 | ||
| LEU-PRO10 | 48.0 ± 17.9 | −0.5 ± 10.0 | 1.2 ± 10.1 | −2.8 (−8.3, 2.8)0.320.32 | 0.32 |
| LEU-PRO+n–311 | 45.8 ± 22.0 | −1.1 ± 8.7 | −2.8 ± 11.6 | −7.1 (−12.5, −1.8) | <0.01 |
| SPPB score | |||||
| CON3 | 10.7 ± 1.6 | 0.3 ± 0.9 | 0.3 ± 0.9 | ||
| LEU-PRO12 | 11.1 ± 1.0 | 0.4 ± 1.2 | −0.1 ± 1.4 | −0.1 (−0.6, 0.4) | 0.75 |
| LEU-PRO+n–36 | 10.7 ± 1.3 | 0.0 ± 1.3 | −0.1 ± 1.1 | −0.3 (−0.8, 0.2) | 0.29 |
| Gait speed, m/s | |||||
| CON13 | 0.76 ± 0.17 | −0.02 ± 0.10 | −0.04 ± 0.08 | ||
| LEU-PRO12 | 0.72 ± 0.12 | 0.00 ± 0.08 | 0.00 ± 0.10 | 0.03 (−0.02, 0.08) | 0.25 |
| LEU-PRO+n–314 | 0.77 ± 0.17 | 0.00 ± 0.01 | 0.02 ± 0.11 | 0.04 (−0.01, 0.09) | 0.08 |
| FTSTS, s | |||||
| CON15 | 12.2 ± 2.3 | −0.6 ± 2.0 | −0.1 ± 1.8 | ||
| LEU-PRO16 | 11.6 ± 2.6 | −0.2 ± 2.4 | 0.7 ± 2.8 | 0.7 (−0.5, 1.9) | 0.25 |
| LEU-PRO+n–317 | 12.2 ± 3.1 | −0.2 ± 2.4 | 1.1 ± 2.5 | 1.0 (−0.2, 2.2) | 0.09 |
| TUG, s | |||||
| CON18 | 7.0 ± 1.3 | 0.0 ± 0.8 | 0.0 ± 0.8 | ||
| LEU-PRO4 | 6.7 ± 1.0 | 0.2 ± 0.8 | 0.3 ± 1.0 | 0.3 (−0.2, 0.7) | 0.27 |
| LEU-PRO+n–319 | 7.0 ± 1.4 | 0.2 ± 1.1 | 0.3 ± 0.9 | 0.2 (−0.3, 0.6) | 0.42 |
| Single-leg standing balance, s | |||||
| CON20 | 26.3 ± 23.6 | 2.0 ± 13.1 | 0.8 ± 13.6 | ||
| LEU-PRO4 | 26.2 ± 19.1 | 7.6 ± 16.8 | 3.1 ± 15.7 | 1.5 (−6.5, 9.5) | 0.71 |
| LEU-PRO+n–321 | 28.0 ± 21.5 | 8.2 ± 17.4 | 6.5 ± 16.1 | 4.4 (−3.5, 12.3) | 0.27 |
PRE value, change from PRE-to-MID and change from PRE-to-POST values are means ± SDs. Data represent the total cohort and were analyzed by an intention-to-treat approach using a linear mixed model with group (CON, LEU-PRO, LEU-PRO+n–3), time (MID, POST) and the group-by-time interaction as fixed factors; participants as a random factor; and PRE value as a covariate. Estimated between-group differences represent LEU-PRO − CON and LEU-PRO+n–3 − CON at POST and are derived from the linear mixed model and adjusted for PRE value. CON, control; FTSTS, 5 times sit-to-stand; LEU-PRO, leucine-enriched protein; LEU-PRO+n–3, leucine-enriched protein plus long-chain n–3 PUFAs; MID, mid-intervention; POST, postintervention; PRE, preintervention; SPPB, Short Physical Performance Battery; TUG, Timed Up-and-Go.
PRE-to-MID changes represent participants with both PRE and MID data, PRE-to-POST changes represent participants with both PRE and POST data.
PRE n = 31, MID n = 28, POST n = 25.
PRE n = 36, MID n = 28, POST n = 28.
PRE n = 36, MID n = 32, POST n = 30.
PRE n = 36, MID n = 33, POST n = 31.
PRE n = 36, MID n = 28, POST n = 27.
PRE n = 36, MID n = 33, POST n = 30.
PRE n = 29, MID n = 27, POST n = 24.
PRE n = 35, MID n = 28, POST n = 28.
PRE n = 36, MID n = 32, POST n = 31.
PRE n = 36, MID n = 29, POST n = 28.
PRE n = 30, MID n = 26, POST n = 24.
PRE n = 36, MID n = 29, POST n = 31.
PRE n = 30, MID n = 26, POST n = 23.
PRE n = 35, MID n = 29, POST n = 28.
PRE n = 35, MID n = 28, POST n = 31.
PRE n = 30, MID n = 27, POST n = 24.
PRE n = 35, MID n = 31, POST n = 31.
PRE n = 30, MID n = 27, POST n = 34.
PRE n = 36, MID n = 31, POST n = 30.
MyoPS was not affected by LEU-PRO with or without LC n–3 PUFA supplementation
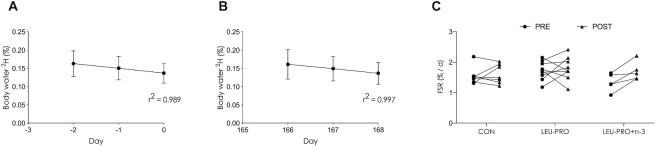
Figure 3 shows the mean body water deuterium enrichment over the 3-d MyoPS measurement period preintervention (Figure 3A) and postintervention (Figure 3B) in the subcohort of participants who took part in the MyoPS assessments. Body water enrichment followed a linear decay pattern (r2 = 0.989 pre, r2 = 0.997 post). Integrated rates of MyoPS preintervention were 1.64% ± 0.35%/d in CON, 1.71% ± 0.31%/d in LEU-PRO and 1.38% ± 0.29%/d in LEU-PRO+n–3, and postintervention were 1.61% ± 0.32%/d in CON, 1.79% ± 0.38%/d in LEU-PRO and 1.74% ± 0.29%/d in LEU-PRO+n–3. In the intention-to-treat analysis, the integrated rate of MyoPS was similar in CON compared with both LEU-PRO (between-group effect: 0.14 %/d; 95% CI: −0.25 %/d, 0.52 %/d; P = 0.46) and LEU-PRO+n–3 (0.26 %/d; 95% CI: −0.26 %/d, 0.78 %/d; P = 0.31) postintervention (Figure 3C). Both sensitivity analyses revealed results similar to the intention-to-treat analysis.
FIGURE 3.
Linear time course of body water deuterium enrichment (%) following oral bolus of 100 mL D2O (70 atoms %) in participants preintervention (A) and postintervention (B) in the subcohort of participants who underwent the MyoPS measurements. Values are means ± SDs; n = 28. The bolus was consumed on day −3 before the intervention began and on day 165 of supplementation. (C) The integrated myofibrillar FSR (%/d) pre- and postintervention. Each line represents 1 participant (n = 7 in CON, n = 9 in LEU-PRO, n = 5 in LEU-PRO+n–3). Data from participants for whom paired measurements were not available (e.g., MyoPS data only available either pre- or postintervention) are not shown. Data were analyzed by an intention-to-treat approach using a linear mixed model with group (CON, LEU-PRO, LEU-PRO+n–3) as a fixed factor, participant as a random factor and preintervention values as covariates. Data from participants with both paired (pre and post) and unpaired (pre or post) MyoPS data were included in the analysis as per the intention-to-treat approach (at preintervention: n = 9 in CON, n = 12 in LEU-PRO and n = 6 in LEU-PRO+n–3; at postintervention: n = 7 in CON, n = 9 in LEU-PRO and n = 6 in LEU-PRO+n–3). There were no differences in MyoPS between groups (P > 0.05). CON, control; D2O, deuterated water; FSR, fractional synthetic rate; LEU-PRO, leucine-enriched protein; LEU-PRO+n–3, leucine-enriched protein plus long-chain n–3 PUFAs; MyoPS, myofibrillar protein synthesis; PRE, preintervention; POST, postintervention.
Physical activity levels, as assessed by step count, were similar between preintervention (CON: 9204 ± 6023; LEU-PRO: 8393 ± 2937; LEU-PRO+n–3: 7353 ± 2163 steps/d) and postintervention (CON: 8470 ± 2519; LEU-PRO: 7936 ± 3203; LEU-PRO+n–3: 7502 ± 3866 steps/d, P = 0.86) MyoPS measurement periods, and did not differ between groups (P = 0.62). Protein intakes were higher in the LEU-PRO and LEU-PRO+n–3 groups postintervention, during the MyoPS measurement period, and were higher than in the CON group (all P < 0.05; Supplemental Table 3). There were no differences in energy, fat or carbohydrate intakes between pre- and postintervention measurement periods, or between groups (all P > 0.05).
Indices of renal function were better maintained in the LEU-PRO and LEU-PRO+n–3 groups relative to the control, whereas LEU-PRO+n–3 supplementation had inconsistent effects on metabolic health
The modified intention-to-treat analysis revealed that indices of kidney function were not compromised by either of the LEU-PRO–containing supplements. Indeed, eGFR was higher and cystatin c was lower in both LEU-PRO (P < 0.01) and LEU-PRO+n–3 (P < 0.01) compared with CON postintervention (Table 5). Serum creatinine concentration was lower in both LEU-PRO (P = 0.02) and LEU-PRO+n–3 (P = 0.02; Table 5) compared with CON postintervention, whereas urea concentration was higher in LEU-PRO+n–3 compared with CON (P < 0.01) postintervention. The modified intention-to-treat analysis revealed no impacts of LEU-PRO supplementation on indices of metabolic health. In contrast, in the LEU-PRO+n–3 group, serum TG (P < 0.05) and plasma total adiponectin concentrations were lower (P < 0.01) and HOMA-IR was higher (P = 0.04; Table 5) compared with CON postintervention, indicative of mixed effects of LEU-PRO+n–3 supplementation on metabolic health.
TABLE 5.
Circulating biochemical indices of metabolic health and renal function1
| Estimated between-group difference vs. CON at POST, mean (95% CI) | ||||
|---|---|---|---|---|
| Change from PRE | ||||
| PRE | POST2 | P | ||
| eGFR, mL·min−1·1.73 m−2 | ||||
| CON3 | 83.7 ± 18.7 | −7.3 ± 12.3 | ||
| LEU-PRO4 | 95.5 ± 11.5 | −0.5 ± 8.9 | 8.8 (2.6, 15.1) | 0.006 |
| LEU-PRO+n–35 | 81.7 ± 19.5 | −0.2 ± 8.9 | 6.9 (1.8, 12.1) | 0.009 |
| Cystatin C, mg/L | ||||
| CON3 | 0.91 ± 0.18 | 0.07 ± 0.12 | ||
| LEU-PRO4 | 0.79 ± 0.11 | −0.01 ± 0.08 | −0.11 (−0.18, −0.04) | 0.001 |
| LEU-PRO+n–35 | 0.92 ± 0.20 | 0.01 ± 0.12 | −0.08 (−0.13, −0.02) | 0.011 |
| Creatinine, μmol/L | ||||
| CON3 | 79.1 ± 13.5 | 3.0 ± 6.3 | ||
| LEU-PRO4 | 76.5 ± 16.1 | −1.4 ± 10.1 | −5.4 (−9.9, −1.0) | 0.016 |
| LEU-PRO+n–35 | 81.2 ± 15.6 | −2.1 ± 8.0 | −5.0 (−9.3, −0.7) | 0.023 |
| Urea, mmol/L | ||||
| CON3 | 6.2 ± 1.8 | −0.7 ± 1.5 | ||
| LEU-PRO4 | 5.7 ± 1.4 | 0.0 ± 1.3 | 0.5 (−0.2, 1.1) | 0.150 |
| LEU-PRO+n–35 | 6.0 ± 1.3 | 0.7 ± 1.2 | 1.3 (0.7, 1.9) | <0.001 |
| TG, mmol/L | ||||
| CON3 | 1.07 ± 0.44 | 0.13 ± 0.60 | ||
| LEU-PRO4 | 1.05 ± 0.36 | 0.17 ± 0.46 | 0.00 (−0.24, 0.25) | 0.975 |
| LEU-PRO+n–35 | 1.24 ± 0.57 | −0.12 ± 0.40 | −0.24 (−0.47, −0.01) | 0.045 |
| Total cholesterol, mmol/L | ||||
| CON3 | 5.40 ± 1.08 | 0.02 ± 0.54 | 0.886 | |
| LEU-PRO4 | 5.76 ± 0.95 | −0.06 ± 0.78 | −0.03 (−0.41, 0.35) | 0.886 |
| LEU-PRO+n–35 | 5.83 ± 1.18 | 0.17 ± 0.77 | 0.17 (−0.19, 0.54) | 0.350 |
| LDL cholesterol, mmol/L | ||||
| CON3 | 3.30 ± 0.89 | −0.05 ± 0.52 | ||
| LEU-PRO4 | 3.61 ± 0.78 | −0.09 ± 0.66 | 0.03 (−0.29, 0.35) | 0.866 |
| LEU-PRO+n–35 | 3.51 ± 0.90 | 0.18 ± 0.66 | 0.26 (−0.05, 0.57) | 0.101 |
| HDL cholesterol, mmol/L | ||||
| CON3 | 1.61 ± 0.45 | 0.02 ± 0.15 | ||
| LEU-PRO4 | 1.68 ± 0.36 | −0.04 ± 0.16 | −0.06 (−0.18, 0.06) | 0.327 |
| LEU-PRO+n–35 | 1.75 ± 0.48 | 0.05 ± 0.32 | 0.03 (−0.09, 0.15) | 0.589 |
| Total adiponectin, μg/mL | ||||
| CON6 | 17.2 ± 15.2 | 1.2 ± 6.1 | ||
| LEU-PRO7 | 17.8 ± 14.0 | −0.8 ± 5.9 | −1.9 (−5.3, 1.4) | 0.256 |
| LEU-PRO+n–38 | 15.4 ± 12.02 | −2.9 ± 9.0 | −4.5 (−7.7, −1.2) | 0.008 |
| HMW adiponectin, μg/mL | ||||
| CON6 | 3.02 ± 1.77 | −0.16 ± 0.97 | ||
| LEU-PRO7 | 3.17 ± 1.93 | 0.25 ± 1.17 | 0.47 (−0.16, 1.08) | 0.140 |
| LEU-PRO+n–38 | 3.14 ± 1.70 | −0.11 ± 1.36 | 0.16 (−0.44, 0.75) | 0.602 |
| Insulin, mU/L | ||||
| CON9 | 4.8 ± 3.7 | −0.4 ± 1.9 | ||
| LEU-PRO10 | 4.6 ± 3.5 | 0.3 ± 1.2 | 1.1 (−0.3, 2.5) | 0.094 |
| LEU-PRO+n–35 | 6.2 ± 3.1 | 0.6 ± 2.2 | 1.2 (−0.1, 2.5) | 0.063 |
| Glucose, mmol/L | ||||
| CON3 | 5.74 ± 0.76 | 0.05 ± 0.40 | ||
| LEU-PRO4 | 5.56 ± 0.55 | 0.04 ± 0.40 | −0.08 (−0.34, 0.19) | 0.568 |
| LEU-PRO+n–35 | 5.75 ± 0.51 | 0.17 ± 0.45 | 0.15 (−0.10, 0.40) | 0.248 |
| HOMA-IR | ||||
| CON11 | 1.3 ± 1.3 | −0.1 ± 0.5 | ||
| LEU-PRO10 | 1.2 ± 1.0 | 0.1 ± 0.3 | 0.3 (−0.1, 0.7) | 0.126 |
| LEU-PRO+n–312 | 1.6 ± 0.9 | 0.2 ± 0.6 | 0.4 (0.0, 0.7) | 0.036 |
| hsCRP, mg/L | ||||
| CON3 | 1.51 ± 1.34 | 0.16 ± 0.74 | ||
| LEU-PRO4 | 1.21 ± 0.93 | 0.41 ± 1.23 | 0.20 (−0.48, 0.88) | 0.568 |
| LEU-PRO+n–35 | 1.88 ± 1.44 | 0.32 ± 1.83 | 0.38 (−0.28, 1.04) | 0.255 |
| IGF-I, ng/mL | ||||
| CON13 | 133.5 ± 70.7 | −0.5 ± 39.5 | ||
| LEU-PRO14 | 128.3 ± 44.7 | 6.6 ± 33.8 | 9.4 (−12.1, 31.0) | 0.389 |
| LEU-PRO+n–35 | 152.4 ± 54.7 | 12.0 ± 54.1 | 17.0 (−3.4, 38.3) | 0.100 |
| 25(OH)D, nmol/L | ||||
| CON15 | 67.8 ± 32.2 | −2.0 ± 21.8 | ||
| LEU-PRO16 | 71.5 ± 23.3 | −5.3 ± 18.2 | −2.6 (−11.4, 6.1) | 0.553 |
| LEU-PRO+n–38 | 68.9 ± 25.1 | 0.1 ± 17.7 | 2.5 (−5.9, 11.0) | 0.550 |
PRE value and change from PRE-to-POST value are means ± SDs. Data represent the total cohort and were analyzed by an intention-to-treat approach using a linear mixed model with group (CON, LEU-PRO, LEU-PRO+n–3), time (MID, POST) and the group-by-time interaction as fixed factors; participants as a random factor; and PRE values as covariates. MID-intervention: data not shown. Estimated between-group differences represent LEU-PRO − CON and LEU-PRO+n–3 − CON at POST and are derived from the linear mixed model and adjusted for PRE value. CON, control; eGFR, estimated glomerular filtration rate; HMW, high molecular weight; hsCRP, high-sensitivity C-reactive protein; IGF-I, insulin-like growth factor I; LEU-PRO, leucine-enriched protein; LEU-PRO+n–3, leucine-enriched protein plus long-chain n–3 PUFAs; MID, mid-intervention; POST, postintervention, PRE, preintervention; TG, triacylglycerol; 25(OH)D, 25-hydroxyvitamin D.
PRE-to-POST changes represent participants with both PRE and POST data.
PRE n = 27, POST n = 22.
PRE n = 37, POST n = 27.
PRE n = 37, POST n = 31.
PRE n = 28, POST n = 21.
PRE n = 35, POST n = 26.
PRE n = 38, POST n = 31.
PRE n = 29, POST n = 22.
PRE n = 33, POST n = 26.
PRE n = 27, POST n = 21.
PRE n = 36, POST n = 31.
3PRE n = 29, POST n = 23.
PRE n = 36, POST n = 27.
PRE n = 30, POST n = 24.
PRE n = 36, POST n = 27.
In contrast to the modified intention-to-treat analyses, neither of the sensitivity analyses revealed differences in TG or total adiponectin concentrations between groups postintervention (all P > 0.05). Similarly, in the per-protocol analysis, HOMA-IR was not different between CON and either of the 2 intervention groups postintervention (both P > 0.05). However, in the analysis wherein outlying values were removed, HOMA-IR was lower in CON compared with both LEU-PRO and LEU-PRO+n–3 (both P < 0.02). Both sensitivity analyses revealed results similar to the modified intention-to-treat analyses for all the other biochemical markers.
Supplementation intervention effectively increased protein and leucine intake and LC n–3 PUFA status
As intended, our intervention successfully increased protein and leucine intake at breakfast in both the LEU-PRO (P < 0.01) and LEU-PRO+n–3 (P < 0.01) intervention groups compared with CON, whereas leucine (both P < 0.01) but not protein intake increased at lunch (LEU-PRO: P = 0.30; LEU-PRO+n–3: P = 0.73; Table 6). Daily protein intake (expressed as grams/day and grams/kilogram per day) was higher than CON in LEU-PRO mid-intervention (P = 0.02; data not shown) and postintervention (P = 0.01; Table 6) and in LEU-PRO+n–3 mid-intervention (P < 0.01; data not shown) but not postintervention (P = 0.09; Table 6). In order to energy-match the supplements, the carbohydrate content of the CON supplements was higher; this resulted in a greater carbohydrate intake (grams/day and % of energy) in the CON group diet compared with both LEU-PRO and LEU-PRO+n–3 (P < 0.01). Dietary n–6 PUFA intake was lower and EPA and DHA intakes were higher in LEU-PRO+n–3 compared with CON mid- and postintervention (P < 0.01), but were similar between CON and LEU-PRO (P > 0.05) treatments. Erythrocyte phospholipid fatty acid composition demonstrated that EPA and DHA concentrations were elevated and that n–6 PUFA concentrations were reduced following LEU-PRO+n–3 supplementation, compared with CON, both mid- and postsupplementation (P < 0.01; Supplemental Table 4). There were no differences in erythrocyte phospholipid fatty acid composition between CON and LEU-PRO mid- and postintervention.
TABLE 6.
Dietary intake1
| CON | LEU-PRO | LEU-PRO+n–3 | ||||
|---|---|---|---|---|---|---|
| PRE | POST | PRE | POST | PRE | POST | |
| n | 29 | 25 | 35 | 28 | 37 | 30 |
| Energy, kcal·d−1 | 1902 ± 532 | 2173 ± 564 | 2010 ± 571 | 2057 ± 457 | 1755 ± 420 | 1865 ± 470 |
| Protein,2 g·d−1 | ||||||
| Food | 78 ± 33 | 82 ± 23 | 84 ± 26 | 79 ± 22 | 77 ± 25 | 73 ± 23 |
| Total | 79 ± 34 | 82 ± 23 | 84 ± 26 | 100 ± 23* | 77 ± 25 | 92 ± 25 |
| Protein, g·kg BM−1·d−1 | 1.13 ± 0.48 | 1.16 ± 0.36 | 1.24 ± 0.42 | 1.43 ± 0.32* | 1.08 ± 0.35 | 1.28 ± 0.36 |
| Breakfast | 0.24 ± 0.13 | 0.23 ± 0.10 | 0.28 ± 0.14 | 0.38 ± 0.10* | 0.24 ± 0.12 | 0.33 ± 0.14* |
| Lunch | 0.31 ± 0.18 | 0.37 ± 0.28 | 0.37 ± 0.26 | 0.45 ± 0.21 | 0.34 ± 0.26 | 0.36 ± 0.20 |
| Dinner | 0.49 ± 0.31 | 0.50 ± 0.26 | 0.50 ± 0.23 | 0.56 ± 0.21 | 0.44 ± 0.28 | 0.51 ± 0.21 |
| Snack | 0.08 ± 0.14 | 0.07 ± 0.09 | 0.10 ± 0.13 | 0.05 ± 0.07 | 0.05 ± 0.07 | 0.07 ± 0.11 |
| Leucine, mg·d−1 | 6356 ± 2704 | 6512 ± 1800 | 6995 ± 2815 | 12,332 ± 2351* | 6132 ± 1934 | 11,315 ± 3157* |
| Breakfast | 1339 ± 764 | 1281 ± 597 | 1587 ± 919 | 4253 ± 780* | 1491 ± 863 | 3904 ± 1305* |
| Lunch | 1800 ± 1031 | 2024 ± 1457 | 2034 ± 1435 | 3870 ± 1640* | 1819 ± 1439 | 3538 ± 1584* |
| Dinner | 2730 ± 1808 | 2759 ± 1374 | 2834 ± 1689 | 3773 ± 1547* | 2614 ± 1678 | 3344 ± 1770 |
| Snack | 487 ± 863 | 447 ± 574 | 541 ± 768 | 435 ± 880 | 281 ± 382 | 530 ± 1179 |
| CHO, g·d−1 | 214 ± 62 | 268 ± 68 | 226 ± 78 | 229 ± 60* | 200 ± 66 | 200 ± 57* |
| Fat, g·d−1 | 80 ± 34 | 85 ± 30 | 82 ± 32 | 80 ± 24 | 69 ± 25 | 76 ± 28 |
| SFAs, g·d−1 | 33 ± 14 | 32 ± 13 | 32 ± 15 | 28 ± 12 | 27 ± 13 | 25 ± 12 |
| MUFAs, g·d−1 | 28 ± 17 | 27 ± 13 | 29 ± 12 | 26 ± 9 | 23 ± 10 | 28 ± 11 |
| PUFAs, g·d−1 | 11 ± 6 | 18 ± 7 | 13 ± 9 | 19 ± 5 | 10 ± 5 | 14 ± 5* |
| n–6 PUFAs | 6.5 ± 4.3 | 14.6 ± 5.4 | 7.7 ± 7.7 | 15.2 ± 4.3 | 6.4 ± 4.8 | 6.8 ± 3.5* |
| n–3 PUFAs | 1.6 ± 2.3 | 1.2 ± 1.0 | 1.7 ± 2.3 | 1.5 ± 1.9 | 1.6 ± 1.9 | 5.4 ± 1.9* |
| EPA | 0.07 ± 0.20 | 0.06 ± 0.16 | 0.07 ± 0.21 | 0.19 ± 0.53 | 0.04 ± 0.14 | 1.40 ± 0.54* |
| DHA | 0.16 ± 0.39 | 0.12 ± 0.29 | 0.15 ± 0.43 | 0.26 ± 0.74 | 0.09 ± 0.26 | 2.06 ± 0.74* |
| Fiber (AOAC), g·d−1) | 22 ± 8 | 25 ± 8 | 25 ± 10.1 | 21 ± 7* | 23 ± 10 | 21 ± 7* |
| Protein, % of total energy intake | 16.7 ± 5.3 | 15.2 ± 3.2 | 17.1 ± 3.9 | 19.6 ± 3.3* | 17.6 ± 4.5 | 19.9 ± 4.0* |
| CHO, % of total energy intake | 45.6 ± 7.5 | 49.8 ± 5.6 | 45.0 ± 9.7 | 44.6 ± 6.7* | 45.6 ± 8.4 | 43.5 ± 8.0* |
| Fat, % of total energy intake | 37.4 ± 9.3 | 34.8 ± 5.4 | 36.3 ± 7.7 | 34.8 ± 6.3 | 35.4 ± 8.8 | 36.2 ± 7.8 |
| Alcohol, % of total energy intake | 0.4 ± 1.5 | 0.2 ± 0.9 | 1.6 ± 5.5 | 0.8 ± 2.2 | 1.5 ± 3.9 | 0.3 ± 1.5 |
Values are means ± SDs. Data represent the total cohort and were analyzed by an intention-to-treat approach using a linear mixed model with group (CON, LEU-PRO, LEU-PRO+n–3), time (MID, POST) and the group-by-time interaction as fixed factors; participants as a random factor; and PRE values as covariates. MID-intervention data not shown for clarity. AOAC, Association of Analytical Chemists; BM, body mass; CHO, carbohydrate; CON, control; LEU-PRO, leucine-enriched protein; LEU-PRO+n–3, leucine-enriched protein plus long-chain n–3 PUFAs; MID, mid-intervention; POST, postintervention; PRE, preintervention. *Different from CON at that time point, P < 0.05.
Protein “Food” indicates protein intake from food only. Protein “Total” indicates protein from food plus supplement intake. All other values in the table represent intake from food plus study supplements.
In terms of habitual activity, daily step counts were similar between the CON and both intervention groups at mid-intervention (CON: 7728 ± 4396; LEU-PRO: 9421 ± 4731; LEU-PRO+n–3: 8927 ± 4198 steps/d; P > 0.05) and postintervention (CON: 7524 ± 3111; LEU-PRO: 8094 ± 3932; LEU-PRO+n–3: 8013 ± 3445 steps/d; P > 0.05).
There was high level of compliance with supplementation. Compliance (based on daily self-report logs) with supplement consumption was high [median (IQR): CON = 93% (87–95%); LEU-PRO = 89% (83–94%); LEU-PRO+n–3= 92 (87–97%)] and did not differ between groups (P = 0.36). One serious adverse event possibly related to nutritional supplementation was reported: the onset of T2D in 1 participant within the CON group. Adverse metabolic effects of the CON supplement were not apparent in any other member of the cohort (Table 5). The most commonly reported side effects that may have been related to the supplements were gastrointestinal symptoms (including nausea, bloating, diarrhea, constipation and reflux), feelings of fullness and weight gain. One or more gastrointestinal symptoms were reported in 4 participants in CON (13%), 9 participants in LEU-PRO (24%) and 5 participants in LEU-PRO+n–3 (13%).
Discussion
Contrary to our hypothesis, we report no beneficial impact of 24 wk of supplementation with LEU-PRO alone or in combination with LC n–3 PUFAs on ALM, strength, physical performance or integrated MyoPS in older adults at increased risk of sarcopenia. To our knowledge, this is the first study to assess the putative efficacy of combined LEU-PRO and LC n–3 PUFA supplementation (formulated without other active nutrients; e.g., vitamin D, creatine) on skeletal muscle health in older adults, in the context of a nutrition-only intervention.
We hypothesized that supplementation with LEU-PRO would increase ALM, strength, physical performance and MyoPS in older adults, and that the addition of LC n–3 PUFA supplementation would further enhance the positive effects of LEU-PRO supplementation on these outcomes. Although we observed no impact of supplementation on ALM or the majority of strength and physical performance measures, unexpectedly, we observed that one of the measures of leg strength (knee flexor peak torque) was lower after the intervention in the group supplemented with LEU-PRO+n–3 relative to the control group. Furthermore, the sensitivity analyses indicated that FTSTS performance, a well-established measure of physical performance (2), worsened in the LEU-PRO+n–3 group compared with the CON group. Interestingly, a deterioration was not apparent following supplementation with LEU-PRO alone, suggesting that participants responded adversely to the LC n–3 PUFA component of the supplements. This is a surprising finding as Smith and colleagues (23) demonstrated that supplementation with a similar dose of LC n–3 PUFAs [3.4 g (Smith et al.) vs. 3.8 g (present study) EPA + DHA/d] for the same duration (6 mo) improved whole-body strength and thigh muscle volume in 44 healthy older men and women. Another double-blind RCT showed that low-dose LC n–3 PUFA supplementation (1.2 g EPA + DHA/d) for 6 mo resulted in a small improvement in gait speed in 126 postmenopausal women (45). Nevertheless, the impact of LC n–3 PUFA supplementation on muscle mass and function is inconsistent (46, 47). The longest and largest trial in this area to date reported no effect of 3 y of supplementation (∼1 g EPA + DHA/d) on handgrip strength or FTSTS performance in 1679 older adults (46). Similarly, another large double-blind RCT recently reported no effect of 3 y of supplementation with a similar dose of LC n–3 PUFAs (∼1 g EPA + DHA/d) on SPPB in healthy older adults (48). To our knowledge, however, no studies have reported an adverse effect of LC n–3 PUFA supplementation relative to the control.
In terms of critically evaluating whether we observed a true negative impact of LEU-PRO+n–3 supplementation, it is noteworthy that the lower leg flexion strength we observed postintervention in the LEU-PRO+n–3 group relative to the control group was partially due to an unexpected increase in leg flexion strength within the control group between the mid- and postintervention time points, in addition to a smaller decline in leg flexion strength within the LEU-PRO+n–3 group. Nonetheless, taken together with the finding that FTSTS performance worsened (∼1 s), these data are consistent with a small deterioration in muscle function following LEU-PRO+n–3 supplementation. One possible explanation for this finding is that, despite randomization, the LEU-PRO+n–3 group was more metabolically “challenged” at baseline. This group had a higher baseline BMI, fat mass and HOMA-IR, with slightly elevated inflammation indicated by hsCRP, all indicative of elevated risk of insulin resistance (34, 49), compared with the other groups. It is possible that this underlying metabolic/inflammatory phenotype may have predisposed them to greater deterioration over the 24-wk intervention period. Although this is speculative, CRP is a proposed biomarker of frailty (50) and is prospectively associated with the loss of strength (51) and incident mobility limitation (52) among older adults. Furthermore, greater fat mass has been reported to exacerbate anabolic resistance (53) and declines in muscle quality (54) and physical performance (55) with aging. As such, the LEU-PRO+n–3 group may have been at slightly higher risk of decline at the study outset.
Another important finding from our study is that LEU-PRO supplementation did not impact on ALM, strength or physical performance. This corroborates the findings of several double-blind RCTs that reported no effect of supplementation with leucine (56, 57), essential amino acids (58) and/or protein (20, 59) on muscle mass and strength in nonexercising older adults. In congruence with these previous studies, in the current trial, baseline protein intake was 1.1–1.2 g·kg−1·d−1, which is within the levels recommended to preserve muscle mass in healthy older adults (1.0–1.2 g·kg−1·d−1) (60, 61) and may explain the lack of observed effect of the leucine/protein supplementation (62). Conversely, per-meal leucine intake at baseline was likely suboptimal (∼1.5 g at breakfast, ∼ 2 g at lunch) and increased with supplementation to ∼3.5–4 g leucine/meal, which is in line with the leucine dose estimated to maximally stimulate MyoPS in this cohort [∼3.5 g leucine/meal based on the mean body mass of ∼72 kg and the leucine content of 0.4 g whey protein isolate/kg per meal (9)]. Interestingly, despite habitual protein intakes of 1–1.3 g·kg−1·d−1, 2 double-blind RCTs reported improvements in muscle mass and/or physical performance following supplementation with LEU-PRO (3 g leucine, 20 g protein) plus vitamin D (20 μg) once-daily in healthy (18) and twice-daily in sarcopenic (19) older adults. The reason for the inconsistency between these 2 trials and ours is unclear. Indeed, the leucine content and the timing of the supplementation were similar across all 3 studies, whereas, phenotypically, our participants (who had low muscle mass and/or handgrip strength) fell in-between the healthy and the sarcopenic participants in the 2 aforementioned trials. It is possible that the vitamin D component of the supplements in the 2 previous studies may have enhanced the beneficial effects of the LEU-PRO (63, 64).
In line with a lack of observed effect of LEU-PRO and LEU-PRO+n–3 on ALM, we report no impact of supplementation on the integrated rate of MyoPS measured over several days. Higher supplemental doses of isolated leucine (5 g/meal × 3 times/d) (17) and LEU-PRO (15 g protein, 4.2 g leucine × 2 times/d) (15) have previously been reported to enhance integrated MyoPS rates in older men and women, respectively. However, using a LEU-PRO dose very similar to the current study (10 g protein, 3 g leucine × 2 times/d), Devries et al. (15) recently reported that supplementation only enhanced MyoPS when combined with resistance exercise and not in rested conditions in older women. As such, a higher dose of leucine/LEU-PRO may be required to augment MyoPS and potentially muscle mass in older adults, at least in situations where resistance exercise is not performed. Nonetheless, the MyoPS data in the current study should be interpreted with caution as we obtained both pre- and postintervention MyoPS rates in only 7, 9 and 5 participants in the CON, LEU-PRO and LEU-PRO+n–3 groups, respectively. As our sample size calculation estimated 9 participants per group, we may have been underpowered to detect an effect of supplementation, particularly with respect to the LEU-PRO+n–3 intervention. Interestingly, mean MyoPS rates increased following supplementation by 0.37%/d in the LEU-PRO+n–3 group compared with 0.06%/d in the CON and 0.07%/d in the LEU-PRO groups. This may be indicative of a positive effect of LEU-PRO+n–3 supplementation on MyoPS and warrants further investigation in fully powered studies.
It is important to highlight that, despite having extensive experience in obtaining muscle biopsies (∼30 mg/sample) in younger and healthy older adults using the microbiopsy technique (65–67), ∼33% of the samples we obtained in the current cohort of older adults with low muscle mass were insufficient for performing the MyoPS analysis. The challenge of obtaining muscle biopsy samples was also demonstrated in a recent study of frail older adults using the Bergstrom technique (68). This is a critical consideration when planning future studies in this population in order to prevent biopsy failures and unnecessary risk among vulnerable older adults.
Limitations of the current study include that, while we present P values from hypothesis tests for secondary/exploratory variables, this study was not statistically powered specifically for these outcomes nor did we correct for multiplicity. Nonetheless, we have endeavored to examine all treatment effects for the magnitude of the difference based on our subject matter expertise, irrespective of the P value. In addition, the small sample size for the MyoPS data may have limited our ability to detect effects of supplementation.
In conclusion, contrary to our hypothesis, we report no beneficial impact of 24 wk of supplementation with LEU-PRO or LEU-PRO+n–3 on ALM, strength, physical performance or integrated MyoPS in older adults at risk of sarcopenia. Further research is warranted to investigate the impact of combined protein and LC n–3 PUFA supplementation and to determine whether different subgroups of older adults may respond differently.
Supplementary Material
Acknowledgments
We thank Brian Reilly, Stacey McDonnell, Aoife Courtney and Alan Maloney for help with participant recruitment, scheduling, testing, and their technical assistance. We thank Marilena Cignarella, Danica Murphy and David Kenny for their help with data entry.
The authors’ responsibilities were as follows—CHM, BE, HMR, GDV, CAC, and SNM: designed the trial; CHM, EMF, GDV, DS, and EdMC: collected the data; KAJM, JMGS, JPBG, LJCvL, AM, and AC: analyzed samples and provided technical support; CHM: performed the statistical analysis; CHM and HMR: wrote the manuscript; BE, GDV, CAC, SNM, EMF, KAJM, EdMC, DS, JMGS, JPBG, AM, AC, RS, and LJCvL: critically revised the manuscript for important intellectual content; HMR: had primary responsibility for the final content; and all authors: read and approved the final manuscript. The authors report no conflicts of interests.
Notes
This work was supported by the Department of Agriculture, Food and the Marine Food Institutional Research Measure grant entitled NUTRIMAL “Novel Nutritional Solutions for the Prevention of Malnutrition” (grant 14F822), the European Union's Horizon 2020 Research and Innovation Program under the Marie Skłodowska-Curie Grant Agreement No. 666010, and a Research Fellowship awarded to CHM by the European Society of Clinical Nutrition and Metabolism (ESPEN). HMR was supported by funding from the Joint Programming Initiative Healthy Diet for a Healthy Life (JPI HDHL) EU Food Biomarkers Alliance “FOODBAll” with Science Foundation Ireland (14/JPHDHL/B3076). Glanbia Plc and Smartfish® supplied the supplement ingredients, and Smartfish® produced the ready-to-drink control and intervention supplements for this study. However, neither Glanbia Plc nor Smartfish® played a role in data collection, data analysis or in the writing of this manuscript.
Supplemental Tables 1–4 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: ALM, appendicular lean mass; BIA, bioelectrical impedance analysis; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CON, control; D2O, deuterated water; eGFR, estimated glomerular filtration rate; FSR, fractional synthetic rate; FTSTS, 5 times sit-to-stand; hsCRP, high sensitivity C-reactive protein; IGF-I, insulin-like growth factor I; LC, long-chain; LEU-PRO, leucine-enriched protein; LEU-PRO+n–3, leucine-enriched protein plus long-chain n–3 PUFAs; MPS, muscle protein synthesis; MVC, maximal voluntary contraction; MyoPS, myofibrillar protein synthesis; RCT, randomized controlled trial; SPPB, Short Physical Performance Battery; TG, triacylglycerol; TUG, Timed Up and Go; T2D, type 2 diabetes; UCD, University College Dublin; 25(OH)D, 25-hydroxyvitamin D.
Contributor Information
Caoileann H Murphy, School of Public Health, Physiotherapy and Sport Science, University College Dublin, Dublin, Ireland.
Ellen M Flanagan, School of Public Health, Physiotherapy and Sport Science, University College Dublin, Dublin, Ireland.
Giuseppe De Vito, School of Public Health, Physiotherapy and Sport Science, University College Dublin, Dublin, Ireland; Department of Biomedical Sciences, University of Padua, Padua, Italy.
Davide Susta, School of Health and Human Performance, Dublin City University, Dublin, Ireland; Department of Normal Physiology, IM Sechenov First Moscow State Medical University, Moscow, Russia.
Kathleen A J Mitchelson, School of Public Health, Physiotherapy and Sport Science, University College Dublin, Dublin, Ireland.
Elena de Marco Castro, School of Public Health, Physiotherapy and Sport Science, University College Dublin, Dublin, Ireland.
Joan M G Senden, Department of Human Movement Sciences, Maastricht University, Maastricht, Netherlands.
Joy P B Goessens, Department of Human Movement Sciences, Maastricht University, Maastricht, Netherlands.
Agnieszka Mikłosz, Department of Physiology, Medical University of Bialystok, Bialystok, Poland.
Adrian Chabowski, Department of Physiology, Medical University of Bialystok, Bialystok, Poland.
Ricardo Segurado, School of Public Health, Physiotherapy and Sport Science, University College Dublin, Dublin, Ireland; UCD Centre for Support and Training in Analysis and Research (CSTAR), University College Dublin, Dublin, Ireland.
Clare A Corish, School of Public Health, Physiotherapy and Sport Science, University College Dublin, Dublin, Ireland.
Sinead N McCarthy, Teagasc Food Research Centre, Ashtown, Dublin, Ireland.
Brendan Egan, School of Health and Human Performance, Dublin City University, Dublin, Ireland.
Luc J C van Loon, Department of Human Movement Sciences, Maastricht University, Maastricht, Netherlands.
Helen M Roche, School of Public Health, Physiotherapy and Sport Science, University College Dublin, Dublin, Ireland; Institute for Global Food Security, Queen's University Belfast, Belfast, United Kingdom.
Data Availability
Data described in the manuscript, code book and analytic code will be made available upon request pending application and approval.
References
- 1. Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, Cesari M, Chumlea WC, Doehner W, Evans Jet al. . Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AAet al. . Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke Get al. . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. [DOI] [PubMed] [Google Scholar]
- 4. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–63. [DOI] [PubMed] [Google Scholar]
- 5. Schaap LA, van Schoor NM, Lips P, Visser M. Associations of sarcopenia definitions, and their components, with the incidence of recurrent falling and fractures: the Longitudinal Aging Study Amsterdam. J Gerontol A Biol Sci Med Sci. 2018;73:1199–204. [DOI] [PubMed] [Google Scholar]
- 6. Akune T, Muraki S, Oka H, Tanaka S, Kawaguchi H, Tokimura F, Yoshida H, Suzuki T, Nakamura K, Yoshimura N. Incidence of certified need of care in the long-term care insurance system and its risk factors in the elderly of Japanese population-based cohorts: the ROAD study. Geriatr Gerontol Int. 2014;14:695–701. [DOI] [PubMed] [Google Scholar]
- 7. Trombetti A, Reid KF, Hars M, Herrmann FR, Pasha E, Phillips EM, Fielding RA. Age-associated declines in muscle mass, strength, power, and physical performance: impact on fear of falling and quality of life. Osteoporos Int. 2016;27:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Landi F, Cruz-Jentoft AJ, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E, Bernabei R, Onder G. Sarcopenia and mortality risk in frail older persons aged 80 years and older: results from ilSIRENTE study. Age Ageing. 2013;42:203–9. [DOI] [PubMed] [Google Scholar]
- 9. Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, Phillips SM. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci. 2015;70:57–62. [DOI] [PubMed] [Google Scholar]
- 10. Tieland M, Borgonjen-Van den Berg KJ, van Loon LJ, de Groot LC. Dietary Protein Intake in Dutch Elderly People: a Focus on Protein Sources. Nutrients. 2015;7:9697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hone M, Nugent AP, Walton J, McNulty BA, Egan B. Habitual protein intake, protein distribution patterns and dietary sources in Irish adults with stratification by sex and age. J Hum Nutr Diet. 2020;33:465–76. [DOI] [PubMed] [Google Scholar]
- 12. Murphy CH, Oikawa SY, Phillips SM. Dietary protein to maintain muscle mass in aging: a case for per-meal protein recommendations. J Frailty Aging. 2016;5:49–58. [DOI] [PubMed] [Google Scholar]
- 13. Wall BT, Hamer HM, de Lange A, Kiskini A, Groen BB, Senden JM, Gijsen AP, Verdijk LB, van Loon LJ. Leucine co-ingestion improves post-prandial muscle protein accretion in elderly men. Clin Nutr. 2013;32:412–9. [DOI] [PubMed] [Google Scholar]
- 14. Devries MC, McGlory C, Bolster DR, Kamil A, Rahn M, Harkness L, Baker SK, P SM. Leucine, not total protein, content of a supplement is the primary determinant of muscle protein anabolic responses in healthy older women. J Nutr. 2018;148:1088–95. [DOI] [PubMed] [Google Scholar]
- 15. Devries MC, McGlory C, Bolster DR, Kamil A, Rahn M, Harkness L, Baker SK, Phillips SM. Protein leucine content is a determinant of shorter- and longer-term muscle protein synthetic responses at rest and following resistance exercise in healthy older women: a randomized, controlled trial. Am J Clin Nutr. 2018;107:217–26. [DOI] [PubMed] [Google Scholar]
- 16. Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291:E381–7. [DOI] [PubMed] [Google Scholar]
- 17. Murphy CH, Saddler NI, Devries MC, McGlory C, Baker SK, Phillips SM. Leucine supplementation enhances integrative myofibrillar protein synthesis in free-living older men consuming lower- and higher-protein diets: a parallel-group crossover study. Am J Clin Nutr. 2016;104:1594–606. [DOI] [PubMed] [Google Scholar]
- 18. Chanet A, Verlaan S, Salles J, Giraudet C, Patrac V, Pidou V, Pouyet C, Hafnaoui N, Blot A, Cano Net al. . Supplementing breakfast with a vitamin d and leucine-enriched whey protein medical nutrition drink enhances postprandial muscle protein synthesis and muscle mass in healthy older men. J Nutr. 2017;147:2262–71. [DOI] [PubMed] [Google Scholar]
- 19. Bauer JM, Verlaan S, Bautmans I, Brandt K, Donini LM, Maggio M, McMurdo ME, Mets T, Seal C, Wijers SLet al. . Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2015;16:740–7. [DOI] [PubMed] [Google Scholar]
- 20. Kirk B, Mooney K, Cousins R, Angell P, Jackson M, Pugh JN, Coyles G, Amirabdollahian F, Khaiyat O. Effects of exercise and whey protein on muscle mass, fat mass, myoelectrical muscle fatigue and health-related quality of life in older adults: a secondary analysis of the Liverpool Hope University-Sarcopenia Ageing Trial (LHU-SAT). Eur J Appl Physiol. 2020;120:493–503. [DOI] [PubMed] [Google Scholar]
- 21. Strain T, Fitzsimons C, Kelly P, Mutrie N. The forgotten guidelines: cross-sectional analysis of participation in muscle strengthening and balance & co-ordination activities by adults and older adults in Scotland. BMC Public Health. 2016;16:1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bennie JA, Pedisic Z, van Uffelen JG, Charity MJ, Harvey JT, Banting LK, Vergeer I, Biddle SJ, Eime RM. Pumping iron in Australia: prevalence, trends and sociodemographic correlates of muscle strengthening activity participation from a national sample of 195,926 adults. PLoS One. 2016;11:e0153225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smith GI, Julliand S, Reeds DN, Sinacore DR, Klein S, Mittendorfer B. Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am J Clin Nutr. 2015;102:115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ, Mittendorfer B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr. 2011;93:402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jans A, van Hees AM, Gjelstad IM, Sparks LM, Tierney AC, Risérus U, Drevon CA, Schrauwen P, Roche HM, Blaak EE. Impact of dietary fat quantity and quality on skeletal muscle fatty acid metabolism in subjects with the metabolic syndrome. Metabolism. 2012;61:1554–65. [DOI] [PubMed] [Google Scholar]
- 26. Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159:413–21. [DOI] [PubMed] [Google Scholar]
- 27. Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, Corsi AM, Rantanen T, Guralnik JM, Ferrucci L. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–60. [DOI] [PubMed] [Google Scholar]
- 28. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. [DOI] [PubMed] [Google Scholar]
- 29. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–8. [DOI] [PubMed] [Google Scholar]
- 30. Giorgetti MM, Harris BA, Jette A. Reliability of clinical balance outcome measures in the elderly. Physiother Res Int. 1998;3:274–83. [DOI] [PubMed] [Google Scholar]
- 31. Hayot M, Michaud A, Koechlin C, Caron MA, Leblanc P, Préfaut C, Maltais F. Skeletal muscle microbiopsy: a validation study of a minimally invasive technique. Eur Respir J. 2005;25:431–40. [DOI] [PubMed] [Google Scholar]
- 32. Holwerda AM, Paulussen KJM, Overkamp M, Smeets JSJ, Gijsen AP, Goessens JPB, Verdijk LB, van Loon LJC. Daily resistance-type exercise stimulates muscle protein synthesis in vivo in young men. J Appl Physiol. 2018;124:66–75. [DOI] [PubMed] [Google Scholar]
- 33. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 34. Tierney AC, McMonagle J, Shaw DI, Gulseth HL, Helal O, Saris WH, Paniagua JA, Gołąbek-Leszczyñska I, Defoort C, Williams CMet al. . Effects of dietary fat modification on insulin sensitivity and on other risk factors of the metabolic syndrome—LIPGENE: a European randomized dietary intervention study. Int J Obes. 2011;35:800–9. [DOI] [PubMed] [Google Scholar]
- 35. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YLet al. . Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tangri N, Stevens LA, Schmid CH, Zhang YL, Beck GJ, Greene T, Coresh J, Levey AS. Changes in dietary protein intake has no effect on serum cystatin C levels independent of the glomerular filtration rate. Kidney Int. 2011;79:471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD 3rd, Zhang YLet al. . Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McGlory C, Gorissen SHM, Kamal M, Bahniwal R, Hector AJ, Baker SK, Chabowski A, Phillips SM. Omega-3 fatty acid supplementation attenuates skeletal muscle disuse atrophy during two weeks of unilateral leg immobilization in healthy young women. FASEB J. 2019;33:4586–97. [DOI] [PubMed] [Google Scholar]
- 39. Conway JM, Ingwersen LA, Moshfegh AJ. Accuracy of dietary recall using the USDA five-step multiple-pass method in men: an observational validation study. J Am Diet Assoc. 2004;104:595–603. [DOI] [PubMed] [Google Scholar]
- 40. Gupta SK. Intention-to-treat concept: a review. Perspect Clin Res. 2011;2:109–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schielzeth H, Dingemanse N, Westneat D, Allegue H, Teplitsky C, Réale D, Dochtermann N, Garamszegi L, Araya Y. Robustness of linear mixed-effects models to violations of distributional assumptions. Methods Ecol Evol. 2020;111:141. [Google Scholar]
- 42. Allison PD. Handling missing data by maximum likelihood: SAS Global Forum. Orlando (FL): Statistical Horizons, 2012. [Google Scholar]
- 43. McLeod LD, Cappelleri JC, Hays RD. Best (but oft-forgotten) practices: expressing and interpreting associations and effect sizes in clinical outcome assessments. Am J Clin Nutr. 2016;103:685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. de Souza RJ, Eisen RB, Perera S, Bantoto B, Bawor M, Dennis BB, Samaan Z, Thabane L. Best (but oft-forgotten) practices: sensitivity analyses in randomized controlled trials. Am J Clin Nutr. 2016;103:5–17. [DOI] [PubMed] [Google Scholar]
- 45. Hutchins-Wiese HL, Kleppinger A, Annis K, Liva E, Lammi-Keefe CJ, Durham HA, Kenny AM. The impact of supplemental n-3 long chain polyunsaturated fatty acids and dietary antioxidants on physical performance in postmenopausal women. J Nutr Health Aging. 2013;17:76–80. [DOI] [PubMed] [Google Scholar]
- 46. Rolland Y, Barreto PS, Maltais M, Guyonnet S, Cantet C, Andrieu S, Vellas B. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain lifestyle intervention on muscle strength in older adults: secondary analysis of the Multidomain Alzheimer Preventive Trial (MAPT). Nutrients. 2019;11:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Krzymińska-Siemaszko R, Czepulis N, Lewandowicz M, Zasadzka E, Suwalska A, Witowski J, Wieczorowska-Tobis K. The effect of a 12-week omega-3 supplementation on body composition, muscle strength and physical performance in elderly individuals with decreased muscle mass. Int J Environ Res Public Health. 2015;12:10558–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bischoff-Ferrari HA, Vellas B, Rizzoli R, Kressig RW, da Silva JAP, Blauth M, Felson DT, McCloskey EV, Watzl B, Hofbauer LCet al. . Effect of vitamin D supplementation, omega-3 fatty acid supplementation, or a strength-training exercise program on clinical outcomes in older adults: the DO-HEALTH randomized clinical trial. JAMA. 2020;324:1855–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yubero-Serrano EM, Delgado-Lista J, Tierney AC, Perez-Martinez P, Garcia-Rios A, Alcala-Diaz JF, Castano JP, Tinahones FJ, Drevon CA, Defoort Cet al. . Insulin resistance determines a differential response to changes in dietary fat modification on metabolic syndrome risk factors: the LIPGENE study. Am J Clin Nutr. 2015;102:1509–17. [DOI] [PubMed] [Google Scholar]
- 50. Rodriguez-Mañas L, Araujo de Carvalho I, Bhasin S, Bischoff-Ferrari HA, Cesari M, Evans W, Hare JM, Pahor M, Parini A, Rolland Yet al. . ICFSR Task Force perspective on biomarkers for sarcopenia and frailty. J Frailty Aging. 2020;9:4–8. [DOI] [PubMed] [Google Scholar]
- 51. Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119, 6:526.e9–17. [DOI] [PubMed] [Google Scholar]
- 52. Penninx BW, Kritchevsky SB, Newman AB, Nicklas BJ, Simonsick EM, Rubin S, Nevitt M, Visser M, Harris T, Pahor M. Inflammatory markers and incident mobility limitation in the elderly. J Am Geriatr Soc. 2004;52:1105–13. [DOI] [PubMed] [Google Scholar]
- 53. Smeuninx B, McKendry J, Wilson D, Martin U, Breen L. Age-related anabolic resistance of myofibrillar protein synthesis is exacerbated in obese inactive individuals. J Clin Endocrinol Metab. 2017;102:3535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fabbri E, Chiles Shaffer N, Gonzalez-Freire M, Shardell MD, Zoli M, Studenski SA, Ferrucci L. Early body composition, but not body mass, is associated with future accelerated decline in muscle quality. J Cachexia Sarcopenia Muscle. 2017;8:490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mikkola TM, von Bonsdorff MB, Salonen MK, Simonen M, Pohjolainen P, Osmond C, Perälä MM, Rantanen T, Kajantie E, Eriksson JG. Body composition as a predictor of physical performance in older age: a ten-year follow-up of the Helsinki Birth Cohort Study. Arch Gerontol Geriatr. 2018;77:163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Verhoeven S, Vanschoonbeek K, Verdijk LB, Koopman R, Wodzig WK, Dendale P, van Loon LJ. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am J Clin Nutr. 2009;89:1468–75. [DOI] [PubMed] [Google Scholar]
- 57. Leenders M, Verdijk LB, van der Hoeven L, van Kranenburg J, Hartgens F, Wodzig WK, Saris WH, van Loon LJ. Prolonged leucine supplementation does not augment muscle mass or affect glycemic control in elderly type 2 diabetic men. J Nutr. 2011;141:1070–6. [DOI] [PubMed] [Google Scholar]
- 58. Kim HK, Suzuki T, Saito K, Yoshida H, Kobayashi H, Kato H, Katayama M. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: a randomized controlled trial. J Am Geriatr Soc. 2012;60:16–23. [DOI] [PubMed] [Google Scholar]
- 59. Zhu K, Kerr DA, Meng X, Devine A, Solah V, Binns CW, Prince RL. Two-year whey protein supplementation did not enhance muscle mass and physical function in well-nourished healthy older postmenopausal women. J Nutr. 2015;145:2520–6. [DOI] [PubMed] [Google Scholar]
- 60. Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, Phillips S, Sieber C, Stehle P, Teta Det al. . Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013;14:542–59. [DOI] [PubMed] [Google Scholar]
- 61. Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, Cederholm T, Cruz-Jentoft A, Krznaric Z, Nair KSet al. . Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33:929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tieland M, Franssen R, Dullemeijer C, van Dronkelaar C, Kyung Kim H, Ispoglou T, Zhu K, Prince RL, van Loon LJC, de Groot L. The impact of dietary protein or amino acid supplementation on muscle mass and strength in elderly people: individual participant data and meta-analysis of RCT's. J Nutr Health Aging. 2017;21:994–1001. [DOI] [PubMed] [Google Scholar]
- 63. Salles J, Chanet A, Giraudet C, Patrac V, Pierre P, Jourdan M, Luiking YC, Verlaan S, Migné C, Boirie Yet al. . 1,25(OH)2-Vitamin D3 enhances the stimulating effect of leucine and insulin on protein synthesis rate through Akt/PKB and mTOR mediated pathways in murine C2C12 skeletal myotubes. Mol Nutr Food Res. 2013;57:2137–46. [DOI] [PubMed] [Google Scholar]
- 64. Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian J, Petermans J, Reginster JY, Bruyère O. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2014;99:4336–45. [DOI] [PubMed] [Google Scholar]
- 65. Krause M, Crognale D, Cogan K, Contarelli S, Egan B, Newsholme P, De Vito G. The effects of a combined bodyweight-based and elastic bands resistance training, with or without protein supplementation, on muscle mass, signaling and heat shock response in healthy older people. Exp Gerontol. 2019;115:104–13. [DOI] [PubMed] [Google Scholar]
- 66. Minnock D, Annibalini G, Le Roux CW, Contarelli S, Krause M, Saltarelli R, Valli G, Stocchi V, Barbieri E, De Vito G. Effects of acute aerobic, resistance and combined exercises on 24-h glucose variability and skeletal muscle signalling responses in type 1 diabetics. Eur J Appl Physiol. 2020;120:2677–91. [DOI] [PubMed] [Google Scholar]
- 67. Cogan KE, Evans M, Iuliano E, Melvin A, Susta D, Neff K, De Vito G, Egan B. Co-ingestion of protein or a protein hydrolysate with carbohydrate enhances anabolic signaling, but not glycogen resynthesis, following recovery from prolonged aerobic exercise in trained cyclists. Eur J Appl Physiol. 2018;118:349–59. [DOI] [PubMed] [Google Scholar]
- 68. Wilson D, Breen L, Lord JM, Sapey E. The challenges of muscle biopsy in a community based geriatric population. BMC Res Notes. 2018;11:830. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book and analytic code will be made available upon request pending application and approval.