ABSTRACT
Background
There is an emerging viewpoint that change in body weight is not sufficiently sensitive to promptly identify clinically meaningful change in body composition, such as skeletal muscle depletion.
Objectives
We aimed to determine whether body weight stability is associated with skeletal muscle depletion and whether skeletal muscle depletion is prognostic of death independently of change in body weight.
Methods
This retrospective cohort included 1921 patients with stage I–III colorectal cancer. Computed tomography (CT)-based skeletal muscle characteristics and body weight were measured at diagnosis and after a mean 15.0-mo follow-up. Body weight stability was defined as weight change less than ±5% during follow-up. Sarcopenia and myosteatosis were defined using established thresholds for patients with cancer. Multivariable-adjusted logistic and flexible parametric proportional hazards survival models were used to quantify statistical associations.
Results
At follow-up, 1026 (53.3%) patients were weight stable. Among patients with weight stability, incident sarcopenia and myosteatosis occurred in 8.5% (95% CI: 6.3%, 10.6%) and 13.5% (95% CI: 11.1%, 15.9%), respectively. Men were more likely to be weight stable than were women (56.7% compared with 49.9%; P = 0.04). Weight-stable men were less likely to develop incident sarcopenia (5.4% compared with 15.4%; P = 0.003) and myosteatosis (9.3% compared with 20.8%; P = 0.001) than weight-stable women. Among all patients, the development of incident sarcopenia (HR: 1.40; 95% CI: 1.02, 1.91) and of myosteatosis (HR: 1.41; 95% CI: 1.05, 1.90) were associated with a higher risk of death, independently of change in body weight. Patient sex did not modify the relation between skeletal muscle depletion and death.
Conclusions
Body weight stability masks clinically meaningful skeletal muscle depletion. Body composition quantified using clinically acquired CT images may provide a vital sign to identify patients at increased risk of death. These data may inform the design of future cachexia trials.
Keywords: cachexia, colorectal neoplasms, obesity, sarcopenia, metabolism, myosteatosis
Introduction
Body weight is routinely measured in patients with cancer (1). Involuntary weight loss of ≥5% is diagnostic of cachexia and associated with poor overall survival (2–4). There is an emerging viewpoint that change in body weight is not sufficiently sensitive to promptly identify clinically meaningful change in body composition, such as skeletal muscle depletion (5–7). This is relevant to oncology because many patients with cancer are older adults, and aging is associated with decreases in skeletal muscle mass without concomitant changes in body weight (8).
The depletion of skeletal muscle, including sarcopenia and myosteatosis, is prognostic in patients with cancer (9–11). Despite the development of automated high-throughput methods to phenotype skeletal muscle using clinically acquired computed tomography (CT) images (12), these methods have not been integrated into oncology practice (13). It is unknown if the measurement of skeletal muscle can be used as a vital sign to identify patients at risk of poor outcome who may benefit from anabolic interventions before the onset of significant and often irreversible weight loss (14, 15).
In a cohort of patients with stage I–III colorectal cancer (CRC), we determined whether body weight stability was associated with skeletal muscle depletion and whether skeletal muscle depletion was prognostic of death independently of change in body weight. We hypothesized that skeletal muscle depletion would occur in patients with weight stability, and that skeletal muscle depletion would be associated with a higher risk of death independently of body weight.
Methods
Study cohort
The C-SCANS (Colorectal-Sarcopenia and Near term Survival) cohort was derived from the Kaiser Permanente Northern California (KPNC) cancer registry, with ascertainment of all patients diagnosed with stage I–III invasive CRC between the years of 2006 and 2011, aged ≥18 y, who underwent surgical resection (n = 4465) (16). Inclusion criteria for this analysis required that patients have body weight and CT imaging measures completed within 3 mo of CRC diagnosis and before surgical resection (n = 3262) and at follow-up after diagnosis (Supplemental Figure 1) (n = 1921). Compared with patients with body weight and CT imaging measures only at baseline, patients with these measures at baseline and follow-up were statistically significantly younger, more likely to have a rectal primary cancer, and less likely to have stage I disease. No other measured variables were associated with patient inclusion. With exceptions at the socioeconomic extremes, KPNC patients are representative of the underlying California population (17). A waiver of written informed consent was obtained by the study investigators, and this study was approved by the KPNC and University of Alberta institutional review boards.
Body weight measures
Body weight was measured and entered into the electronic medical record by a medical assistant following standardized clinical procedures of KPNC (e.g., confirming the scale is set to 0; asking the patient to remove heavy outdoor clothing and, if possible, remove shoes; and the patient being measured while standing without movement on the scale) (18). Longitudinal measures of body weight obtained in clinical practice are valid and reliable compared with measures obtained for research purposes (19). Body weight measures that were closest to the date of CT image acquisition were included in the analysis. Stability of body weight was defined as a change from −4.99% to +4.99%, from baseline to follow-up, which is consistent with our prior analysis (18) and with the observation that weight loss ≥5% is diagnostic of cachexia and associated with poor overall survival (2, 3). In sensitivity analysis, we defined stability of body weight as a change from −2.0% to +2.0%, from baseline to follow-up.
Skeletal muscle measures
Skeletal muscle characteristics were quantified using CT images originally collected for clinical purposes (e.g., initial staging of primary CRC and surveillance of recurrent CRC). A single-slice transverse CT image at the third lumbar vertebra was used for analysis because this anatomical location is correlated with whole-body muscle volume (R2 = 0.86) (20, 21). Tissues were demarcated using Hounsfield Unit (HU) thresholds (22, 23). Cross-sectional muscle area (cm2) was calculated by summing tissue pixels and multiplying by the pixel surface area. Muscle density quantifies the extent of lipid contained within muscle and is a radiologic measure of muscle quality (HU) (24). Fifty CT images were randomly selected to be analyzed by 2 investigators to quantify the reproducibility of the body composition measures, and the remaining CT images were analyzed by a single investigator. The interrater CVs for muscle area and muscle density were 1.2% and 0.7%, respectively (25).
Sarcopenia and myosteatosis were defined using established sex-specific thresholds that are associated with overall survival in patients with cancer (26). Sarcopenia was defined as a skeletal muscle index <43 cm2/m2 for men with a BMI (in kg/m2) <25.0; <53 cm2/m2 for men with a BMI ≥25.0; and <41 cm2/m2 for women irrespective of BMI. Myosteatosis was defined as a muscle density <41 HU for men and women with a BMI <25.0 and <33 HU for men and women with a BMI ≥25.0 (26). Prevalent sarcopenia (or myosteatosis) was defined as meeting the aforementioned thresholds at baseline. The resolution of sarcopenia (or myosteatosis) was defined as the absence of sarcopenia (or myosteatosis) at follow-up among patients with prevalent sarcopenia (or myosteatosis) at baseline. The development of incident sarcopenia (or myosteatosis) was defined as the presence of sarcopenia (or myosteatosis) at follow-up among patients without sarcopenia (or myosteatosis) at baseline.
Survival measures
All-cause death was defined as the time from follow-up CT image acquisition to death from any cause. CRC death was defined as the time from follow-up CT image acquisition to death attributable to CRC. Deaths were identified from the California State Department of Vital Statistics, US Social Security Administration, and KPNC membership and utilization files through 31 December, 2016. Deaths were classified as cancer-specific if CRC was documented as an underlying or contributing cause of death on the death certificate.
Covariates
The KPNC electronic medical record was used to obtain information on age, sex, race and ethnicity, smoking history, and comorbid health conditions using the Charlson comorbidity index (27). The KPNC Cancer Registry was used to obtain information on the anatomical site of cancer, cancer stage (American Joint Committee on Cancer, 7th Edition) (28), and use of chemotherapy and radiation treatment approaches.
Statistical analysis
Baseline characteristics are presented as means ± SDs or counts and percentages. To test for differences in baseline characteristics according to patient sex, the χ2 test was used for categorical variables and the t test for continuous variables. Linear regression models were used to quantify the association between body weight and skeletal muscle characteristics. Results from linear regression models are presented as least-squares means with 95% CIs. The coefficient of partial determination (partial R2) from the regression model was used to quantify the proportion of variance in body weight explained by change in skeletal muscle characteristics, independently of other covariates (29). Logistic regression models were used to estimate the association between skeletal muscle depletion and change in body weight. Results from logistic regression models are presented as ORs or predicted probabilities with 95% CIs. To complement our prior analyses (9, 18), flexible parametric proportional hazards survival models were used to estimate the association between skeletal muscle depletion and overall survival independently of change in body weight (30). Parametric survival models allow for smooth predictions and the estimation of relative and absolute effects, while permitting flexibility in the shape of the baseline hazard function (31). Results from survival models are presented as HRs or predicted probabilities with 95% CIs. In all regression models, nonlinearity of effects was estimated with the use of restricted cubic spline terms, and effect modification by patient sex was evaluated using the likelihood ratio test. In all regression models, model fit was examined using graphical and statistical techniques. All regression models were adjusted for age, race and ethnicity, cancer site, cancer stage, chemotherapy, radiation, smoking history, and the Charlson comorbidity index. The analyses of incident sarcopenia (or myosteatosis) at follow-up were restricted to patients without sarcopenia (or myosteatosis) at baseline. Conversely, the analyses of resolution of prevalent sarcopenia (or myosteatosis) at follow-up were restricted to patients with sarcopenia (or myosteatosis) at baseline. Sensitivity analyses were conducted to determine how robust the association between skeletal muscle depletion and overall survival was to unmeasured or uncontrolled confounding (32). All statistical tests were 2-sided. Stata/MP 15.1 (StataCorp, LLC) was used for all statistical analyses.
Results
The cohort included 1921 patients. The mean ± SD age was 61 ± 11 y (Table 1, Supplemental Table 1). Baseline (at diagnosis) CT imaging was obtained a mean of 6.7 ± 18.7 d after biopsy-confirmed diagnosis of CRC. Body weight was obtained a mean of 3.7 ± 4.1 d before CT image acquisition. At baseline, the mean body weight was 81.2 ± 20.1 kg, BMI 28.2 ± 5.9, muscle area 141.8 ± 37.7 cm2, and muscle density 39.9 ± 9.9 HU. The baseline prevalence of sarcopenia and myosteatosis was 36.5% and 30.7%, respectively.
TABLE 1.
Baseline characteristics overall and stratified by sex and weight change category1
| Sex stratified | |||||||
|---|---|---|---|---|---|---|---|
| Men (n = 972) | Women (n = 949) | ||||||
| Characteristic | Overall cohort (n = 1921) | Weight stable (n = 548) | Not weight stable (n = 424) | P | Weight stable (n = 478) | Not weight stable (n = 471) | P |
| Age, y | 61 ± 11 | 60 ± 11 | 61 ± 12 | 0.39 | 62 ± 11 | 61 ± 12 | 0.43 |
| Race | 0.017 | 0.19 | |||||
| White | 1212 (63.2) | 330 (60.2) | 293 (69.1) | 309 (64.6) | 280 (59.4) | ||
| Asian | 140 (7.3) | 38 (6.9) | 21 (4.9) | 38 (7.9) | 43 (9.1) | ||
| Hispanic | 319 (16.6) | 72 (13.1) | 54 (12.7) | 46 (9.6) | 62 (13.2) | ||
| Black | 234 (12.2) | 101 (18.4) | 55 (13.0) | 83 (17.4) | 80 (17.0) | ||
| Other or Unknown | 16 (0.7) | 7 (1.3) | 1 (0.2) | 2 (0.4) | 6 (1.3) | ||
| Site | 0.10 | 0.09 | |||||
| Colon | 1302 (67.8) | 355 (64.8) | 253 (59.7) | 361 (75.5) | 333 (70.7) | ||
| Rectum | 619 (32.2) | 193 (35.2) | 171 (40.3) | 117 (24.5) | 138 (29.3) | ||
| Stage | 0.006 | 0.55 | |||||
| I | 361 (18.8) | 126 (23.0) | 63 (14.9) | 93 (19.5) | 79 (16.8) | ||
| II | 594 (30.9) | 177 (32.3) | 147 (34.7) | 135 (28.2) | 135 (28.7) | ||
| III | 966 (50.3) | 245 (44.7) | 214 (50.5) | 250 (52.3) | 257 (54.6) | ||
| Chemotherapy | 1344 (70.0) | 361 (65.9) | 315 (74.3) | 0.005 | 317 (66.3) | 351 (74.5) | 0.006 |
| Radiation | 397 (20.7) | 125 (22.8) | 118 (27.8) | 0.07 | 59 (12.3) | 95 (20.2) | 0.001 |
| Smoking history | 0.04 | 0.003 | |||||
| Never | 921 (48.0) | 242 (44.2) | 158 (37.3) | 288 (60.2) | 233 (49.5) | ||
| Former | 756 (39.4) | 231 (42.1) | 194 (45.8) | 144 (30.1) | 187 (39.7) | ||
| Current | 241 (12.6) | 72 (13.1) | 72 (16.9) | 46 (9.6) | 51 (10.8) | ||
| Unknown | 3 (0.2) | 3 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Charlson Comorbidity Index | 0.15 | 0.10 | |||||
| 0 | 1220 (63.5) | 358 (65.3) | 262 (61.8) | 318 (66.5) | 282 (59.9) | ||
| 1 | 543 (28.3) | 154 (28.1) | 120 (28.3) | 122 (25.5) | 147 (31.2) | ||
| ≥2 | 158 (8.2) | 36 (6.6) | 42 (9.9) | 38 (7.9) | 42 (8.9) | ||
| Height, m | 1.69 ± 0.11 | 1.77 ± 0.07 | 1.76 ± 0.08 | 0.35 | 1.61 ± 0.07 | 1.62 ± 0.07 | 0.16 |
| Body weight, kg | 81.2 ± 20.1 | 89.1 ± 18.3 | 87.2 ± 18.8 | 0.13 | 72.5 ± 18.0 | 75.2 ± 19.8 | 0.03 |
| BMI, kg/m2 | 28.2 ± 5.9 | 28.4 ± 4.9 | 27.9 ± 5.1 | 0.16 | 27.7 ± 6.3 | 28.6 ± 7.2 | 0.06 |
| Muscle area, cm2 | 141.8 ± 37.7 | 171.9 ± 30.4 | 165.4 ± 29.8 | 0.001 | 113.9 ± 19.1 | 113.8 ± 20.4 | 0.97 |
| Sarcopenia | 702 (36.5) | 169 (30.8) | 153 (36.1) | 0.08 | 181 (37.9) | 199 (42.2) | 0.17 |
| Muscle density, HU | 39.9 ± 9.9 | 42.0 ± 9.2 | 40.5 ± 9.5 | 0.01 | 39.5 ± 10.1 | 37.4 ± 10.1 | 0.002 |
| Myosteatosis | 590 (30.7) | 117 (21.3) | 115 (27.1) | 0.04 | 160 (33.5) | 198 (42.0) | 0.006 |
| Time between acquisition of CT images, mo | 15.0 ± 3.8 | 15.0 ± 3.8 | 15.0 ± 3.8 | 0.87 | 15.0 ± 3.8 | 15.1 ± 3.7 | 0.81 |
| Change in body weight between acquisition of CT images, % | −0.4 ± 8.7 | 0.3 ± 2.7 | 0.3 ± 12.0 | 0.97 | −0.1 ± 2.7 | −2.1 ± 12.5 | 0.001 |
1Values are mean ± SD or n (%) unless otherwise indicated. The χ2 test was used to compare distributions of categorical variables and a t test for distributions of continuous variables. All statistical tests were 2-sided. CT, computed tomography; HU, Hounsfield Unit.
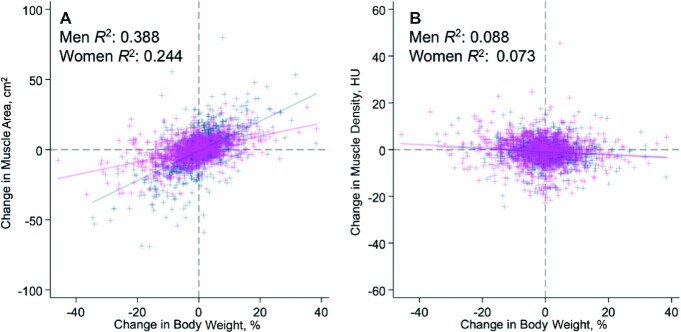
Follow-up body weight and CT imaging were obtained at a mean of 15.0 ± 3.8 mo after baseline. At follow-up, body weight, BMI, and muscle density decreased when compared with baseline, and the magnitude of the difference varied by patient sex (Supplemental Table 2). The percentage of variance of change in body weight explained by muscle area and muscle density was 29.6% (P < 0.001) and 7.2% (P < 0.001), respectively; patient sex modified the proportion of variance explained by muscle area (Figure 1A) (P < 0.001) but not muscle density (Figure 1B) (P = 0.69).
FIGURE 1.
Association of change in body weight with change in muscle area (A) and muscle density (B) among 972 men (navy blue) and 949 women (magenta). The percentage of variance of change in body weight explained by muscle area and muscle density was 29.6% (P < 0.001) and 7.2% (P < 0.001), respectively; patient sex modified the proportion of variance explained by muscle area (P < 0.001) but not muscle density (P = 0.69). R2 values represent the coefficient of partial determination for change in body weight in a multivariable-adjusted regression model. Note the differential y-axis scaling and units of measure. HU, Hounsfield Unit.
At follow-up, 1026 (53.3%) patients were classified as weight stable. In a multivariable-adjusted logistic regression model, patients classified as weight stable were more likely to be older (OR per 5-y increase: 1.07; 95% CI: 1.02, 1.13), less likely to receive chemotherapy (OR: 0.69; 95% CI: 0.55, 0.86), less likely to be a former (OR: 0.74; 95% CI: 0.60, 0.91) or current (OR: 0.72; 95% CI: 0.54, 0.98) smoker, and more likely to have a higher baseline muscle area (OR per 10 cm2 increase: 1.10; 95% CI: 1.04, 1.16) and muscle radiodensity (OR per 10-HU increase: 1.23; 95% CI: 1.08, 1.41).
Among weight-stable patients, incident sarcopenia and myosteatosis occurred in 8.5% (95% CI: 6.3%, 10.6%) and 13.5% (95% CI: 11.1%, 15.9%), respectively. Among weight-stable patients, the resolution of prevalent sarcopenia and myosteatosis occurred in 20.0% (95% CI: 15.9%, 24.2%) and 17.1% (95% CI: 12.7%, 21.4%), respectively. In sensitivity analyses, results were similar when body weight stability was defined as a change from −2.0% to +2.0%, from baseline to follow-up.
Men were significantly more likely to be weight stable than were women (56.7% compared with 49.9%; P = 0.04). In a multivariable-adjusted logistic regression model, weight-stable men were more likely to be of Hispanic ethnicity (OR: 2.00; 95% CI: 1.31, 3.06), less likely to receive chemotherapy (OR: 0.65; 95% CI: 0.48, 0.90), less likely to have ≥3 comorbid health conditions (OR: 0.59; 95% CI: 0.35, 0.98), and more likely to have a higher baseline muscle area (OR per 10-cm2 increase: 1.11; 95% CI: 1.04, 1.19). In a multivariable-adjusted logistic regression model, weight-stable women were more likely to be older (OR per 5-y increase: 1.09; 95% CI: 1.01, 1.18), less likely to receive chemotherapy (OR: 0.72; 95% CI: 0.53, 0.99) or radiation (OR: 0.54; 95% CI: 0.32, 0.91), to be a former smoker (OR: 0.61; 95% CI: 0.45, 0.81), and more likely to have a higher baseline muscle radiodensity (OR per 10-HU increase: 1.35; 95% CI: 1.12, 1.62).
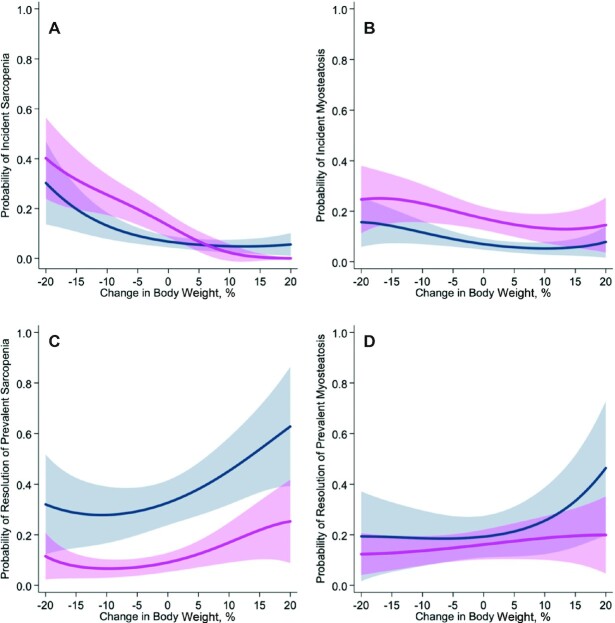
Weight-stable men were less likely to develop incident sarcopenia (5.4% compared with 15.4%; P = 0.003) (Figure 2A) and myosteatosis (9.3% compared with 20.8%; P = 0.001) (Figure 2B) than were weight-stable women. Weight-stable men were more likely to experience resolution of prevalent sarcopenia (34.6% compared with 11.7%; P < 0.001) (Figure 2C) but not resolution of prevalent myosteatosis (17.7% compared with 15.7%; P = 0.72) (Figure 2D) than were weight-stable women.
FIGURE 2.
Multivariable-adjusted association of change in body weight with probability of developing sarcopenia during follow-up among patients without sarcopenia at baseline (A); developing myosteatosis at follow-up among patients without myosteatosis at baseline (B); resolution of sarcopenia at follow-up among patients with sarcopenia at baseline (C); and resolution of myosteatosis at follow-up among patients with myosteatosis at baseline (D), among men (navy blue) and women (magenta). Weight-stable men (n = 548) were less likely to develop incident sarcopenia (5.4% compared with 15.4%; P = 0.003) and myosteatosis (9.3% compared with 20.8%; P = 0.001) than were weight-stable women (n = 478). Weight-stable men (n = 548) were more likely to experience resolution of prevalent sarcopenia (34.6% compared with 11.7%; P < 0.001) but not resolution of prevalent myosteatosis (17.7% compared with 15.7%; P = 0.72) than were weight-stable women (n = 478). Solid lines represent point estimates and shaded areas represent the corresponding 95% CIs estimated from a multivariable-adjusted logistic regression model.
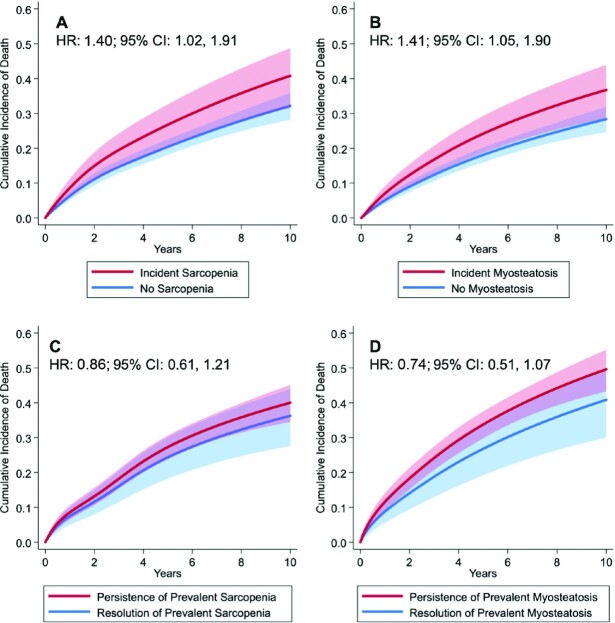
Patients were observed for a mean of 5.4 ± 2.4 y, during which 519 deaths attributable to all causes were observed. Among all patients, the development of incident sarcopenia (HR: 1.40; 95% CI: 1.02, 1.91) (Figure 3A) and of myosteatosis (HR: 1.41; 95% CI: 1.05, 1.90) (Figure 3B) were associated with a higher risk of death independently of change in body weight. The resolution of prevalent sarcopenia (HR: 0.86; 95% CI: 0.61, 1.21) (Figure 3C) and of myosteatosis (HR: 0.74; 95% CI: 0.51, 1.07) (Figure 3D) were not associated with the risk of death independently of change in body weight. Weight change did not modify the relation of incident sarcopenia (P = 0.77) or myosteatosis (P = 0.63) with death, nor the resolution of prevalent sarcopenia (P = 0.23) or myosteatosis (P = 0.75) with death. The joint development of incident sarcopenia and myosteatosis was not multiplicatively associated with death (P = 0.95) nor was the joint resolution of prevalent sarcopenia and myosteatosis (P = 0.64). Patient sex did not modify the relation of incident sarcopenia (P = 0.26) and myosteatosis (P = 0.15), nor resolution of prevalent sarcopenia (P = 0.89) or myosteatosis (P = 0.84), with death.
FIGURE 3.
Multivariable-adjusted 10-y cumulative incidence of all-cause death by developing sarcopenia during follow-up among patients without sarcopenia at baseline (A); developing myosteatosis at follow-up among patients without myosteatosis at baseline (B); resolution of sarcopenia at follow-up among patients with sarcopenia at baseline (C); and resolution of myosteatosis at follow-up among patients with myosteatosis at baseline (D). Among all patients, the development of incident sarcopenia (HR: 1.40; 95% CI: 1.02, 1.91) and of myosteatosis (HR: 1.41; 95% CI: 1.05, 1.90) were associated with a higher risk of death independently of change in body weight. The resolution of prevalent sarcopenia (HR: 0.86; 95% CI: 0.61, 1.21) and of myosteatosis (HR: 0.74; 95% CI: 0.51, 1.07) were not associated with the risk of death independently of change in body weight. Solid lines represent point estimates and shaded areas represent the corresponding 95% CIs estimated from a flexible parametric proportional hazards survival model.
Sensitivity analysis demonstrated that an unmeasured or uncontrolled confounder must have a minimum HR of 1.85 to explain away the observed association of sarcopenia or myosteatosis with death. Results were similar in analyses of CRC death (Supplemental Figure 2).
Discussion
In a population-based sample of 1921 patients with stage I–III CRC who survived a median of 15 mo after diagnosis, body weight stability masked dynamic changes in skeletal muscle. Despite body weight stability, 1 in 8 and 1 in 7 patients developed incident sarcopenia and myosteatosis, respectively. Compared with men, women were particularly vulnerable to experience skeletal muscle depletion; 1 in 5 women with body weight stability developed incident sarcopenia or myosteatosis during the follow-up period. Our findings highlight that the development of incident skeletal muscle depletion was associated with overall survival independently of change in body weight. In all patients, the development of incident sarcopenia and of myosteatosis were independently associated with a 40% higher RR of death; the absolute difference in the risk of death at 10 y was ∼8.5%.
These results may have implications for clinical care. Body weight remains an important and easily measured vital sign. Involuntary weight loss is associated with poor overall survival in patients with various types and stages of cancer (3, 4). However, emerging data suggest that skeletal muscle depletion is also associated with clinical outcome. In 2 meta-analyses, sarcopenia and myosteatosis at the time of cancer diagnosis were associated with 40% and 75% higher RRs of death, respectively (33, 34). Our study extends these prior findings by demonstrating that the development of incident sarcopenia and myosteatosis after cancer diagnosis is associated with poor overall survival. Moreover, skeletal muscle depletion is associated with treatment-related toxicity, declines in physical functioning, and poorer quality of life (13).
These results may have implications for clinical research. Cancer cachexia is a multifactorial syndrome defined by an ongoing loss of skeletal muscle mass, with or without the loss of fat mass, that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment (2). The current diagnostic criterion for cachexia is body weight loss ≥5% (or body weight loss ≥2% in patients with BMI <20) or sarcopenia (2). Our results demonstrate that in some, but not all cases, weight loss may not be an adequate surrogate measure of skeletal muscle depletion. This is of regulatory importance because randomized clinical trial programs testing therapeutic agents for cancer cachexia have used weight loss as an eligibility criterion in patients with stage III and IV cancer (35). Our observation that body weight stability masks clinically meaningful changes in skeletal muscle may be useful to refine eligibility criteria of future therapeutic trials for cachexia in order to identify patients who may benefit from anabolic intervention before the onset of weight loss (36, 37).
In our cohort, women with stable body weight were more likely to develop sarcopenia and myosteatosis than were weight-stable men. In the study that validated the thresholds that we used to define sarcopenia and myosteatosis, sarcopenia, but not myosteatosis, was more common among women than men (53% compared with 31%; P < 0.001) (26). The reasons for this dimorphism have not been completely elucidated. Men and women have distinct muscle fiber characteristics, hormonal actions, and mitochondrial differences that may collectively explain the increased susceptibility of women to developing sarcopenia and myosteatosis (38). In our cohort, the prognostic association of incident sarcopenia or myosteatosis with death did not differ by patient sex. Although the susceptibility to sarcopenia and myosteatosis may differ between men and women, the clinical consequence of these syndromes (e.g., risk of death) is similar between the sexes.
There are several limitations to this study. This retrospective cohort study used clinically acquired data. We did not have information on patient behaviors that may have influenced body weight and body composition, such as dietary patterns, supplement consumption, or participation in specific physical activities such as weightlifting exercise. We were unable to differentiate intentional and unintentional weight changes. Body composition was measured at the third lumbar vertebra. Although this region is correlated with whole-body muscle volume (20, 21), anatomic differences in the distribution of muscle mass between men and women may influence our findings. The extent to which changes in the third lumbar vertebra reflect change in whole-body skeletal muscle has not been explored. We lacked measures of body weight or body composition before the cancer diagnosis; therefore, we cannot rule out the possibility that some patients experienced tumor-induced changes in body weight and composition. To be included in our analysis, patients were required to have repeat CT imaging, which is indicated for patients with a higher risk of disease recurrence (39). Body weight and composition were measured at 2 time points, thus we could not explore the role of weight cycling trajectories over time.
There are several strengths of this study, including the large population-based sample. Body mass and body composition were measured objectively, and body composition was quantified using gold-standard CT imaging (40). The time interval between baseline and follow-up measures was of sufficient duration to allow stabilization of acute treatment-related alterations in body weight (41).
In conclusion, body weight stability masks clinically meaningful change in skeletal muscle. The measurement of body composition measures using clinically acquired CT images may provide a useful vital sign to identify patients at risk of poor outcome and inform the design of therapeutic trials for cancer cachexia.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—JCB, BJC, CMP, CHK, and JAM: provided substantial contributions to the conception or design of the work; JCB: drafted the work; and all authors: participated in the acquisition, analysis, or interpretation of data for the work; revised the work critically for important intellectual content; agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; and read and approved the final manuscript. JAM reports receiving institutional research funding from Boston Biomedical, has served as an advisor or consultant to Ignyta and COTA Healthcare, and has served on a grant review panel for the National Comprehensive Cancer Network funded by Taiho Pharmaceutical. All other authors report no conflicts of interest.
Notes
Supported by National Cancer Institute of the NIH awards R00-CA218603 (to JCB), K01-CA226155 (to EMCF), R01-CA175011 (to BJC), and R25-CA203650 (to JCB) and National Institute of General Medical Sciences of the NIH award U54-GM104940 (to JCB). JAM is supported by the Douglas Gray Woodruff Chair Fund, the Guo Shu Shi Fund, Anonymous Family Fund for Innovations in Colorectal Cancer, Project P Fund, and the George Stone Family Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funding agency had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Supplemental Tables 1 and 2 and Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: CRC, colorectal cancer; CT, computed tomography; HU, Hounsfield Unit; KPNC, Kaiser Permanente Northern California.
Contributor Information
Justin C Brown, Cancer Metabolism Program, Pennington Biomedical Research Center, Baton Rouge, LA, USA; Stanley S Scott Cancer Center, Louisiana State University Health Sciences Center, New Orleans, LA, USA.
Bette J Caan, Kaiser Permanente Northern California, Oakland, CA, USA.
Elizabeth M Cespedes Feliciano, Kaiser Permanente Northern California, Oakland, CA, USA.
Jingjie Xiao, Covenant Health Palliative Institute, Edmonton, Alberta, Canada.
Erin Weltzien, Kaiser Permanente Northern California, Oakland, CA, USA.
Carla M Prado, Faculty of Arts, University of Alberta, Edmonton, Alberta, Canada.
Candyce H Kroenke, Kaiser Permanente Northern California, Oakland, CA, USA.
Adrienne Castillo, Kaiser Permanente Northern California, Oakland, CA, USA.
Marilyn L Kwan, Kaiser Permanente Northern California, Oakland, CA, USA.
Jeffrey A Meyerhardt, Dana-Farber Cancer Institute, Boston, MA, USA.
Data Availability
Data described in the article, code book, and analytic code will not be made available because this was a retrospective cohort study using electronic medical records. The requirement for informed consent was waived by the institutional review board. Consequently, study subjects did not explicitly consent for their data to be shared publicly. Moreover, study subjects were all treated within a single health system for an uncommon disease, and our ability to preserve subject anonymity cannot be guaranteedt.
References
- 1. Ligibel JA, Jones LW, Brewster AM, Clinton SK, Korde LA, Oeffinger KC, Bender CM, Tan W, Merrill JK, Katta Set al. . Oncologists’ attitudes and practice of addressing diet, physical activity, and weight management with patients with cancer: findings of an ASCO survey of the oncology workforce. J Oncol Pract. 2019;15(6):e520–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani Get al. . Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–95. [DOI] [PubMed] [Google Scholar]
- 3. Martin L, Senesse P, Gioulbasanis I, Antoun S, Bozzetti F, Deans C, Strasser F, Thoresen L, Jagoe RT, Chasen Met al. . Diagnostic criteria for the classification of cancer-associated weight loss. J Oncol Pract. 2015;33(1):90–9. [DOI] [PubMed] [Google Scholar]
- 4. Gannavarapu BS, Lau SKM, Carter K, Cannon NA, Gao A, Ahn C, Meyer JJ, Sher DJ, Jatoi A, Infante Ret al. . Prevalence and survival impact of pretreatment cancer-associated weight loss: a tool for guiding early palliative care. J Oncol Pract. 2018;14(4):e238–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bruggeman AR, Kamal AH, LeBlanc TW, Ma JD, Baracos VE, Roeland EJ. Cancer cachexia: beyond weight loss. J Oncol Pract. 2016;12(11):1163–71. [DOI] [PubMed] [Google Scholar]
- 6. Strulov Shachar S, Williams GR. The obesity paradox in cancer—moving beyond BMI. Cancer Epidemiol Biomarkers Prev. 2017;26(1):13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gonzalez MC, Correia M, Heymsfield SB. A requiem for BMI in the clinical setting. Curr Opin Clin Nutr Metab Care. 2017;20(5):314–21. [DOI] [PubMed] [Google Scholar]
- 8. Gallagher D, Ruts E, Visser M, Heshka S, Baumgartner RN, Wang J, Pierson RN, Pi-Sunyer FX, Heymsfield SB. Weight stability masks sarcopenia in elderly men and women. Am J Physiol Endocrinol Metab. 2000;279(2):E366–75. [DOI] [PubMed] [Google Scholar]
- 9. Brown JC, Caan BJ, Meyerhardt JA, Weltzien E, Xiao J, Cespedes Feliciano EM, Kroenke CH, Castillo A, Kwan ML, Prado CM. The deterioration of muscle mass and radiodensity is prognostic of poor survival in stage I–III colorectal cancer: a population-based cohort study (C-SCANS). J Cachexia Sarcopenia Muscle. 2018;9(4):664–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kurk SA, Peeters PHM, Dorresteijn B, de Jong PA, Jourdan M, Creemers GM, Erdkamp FLG, de Jongh FE, Kint PAM, Poppema BJet al. . Loss of skeletal muscle index and survival in patients with metastatic colorectal cancer: secondary analysis of the phase 3 CAIRO3 trial. Cancer Med. 2020;9(3):1033–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blauwhoff-Buskermolen S, Versteeg KS, de van der Schueren MA, Den Braver NR, Berkhof J, Langius JA, Verheul HM. Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J Clin Oncol. 2016;34(12):1339–44. [DOI] [PubMed] [Google Scholar]
- 12. Wang B, Torriani M. Artificial intelligence in the evaluation of body composition. Semin Musculoskelet Radiol. 2020;24(1):30–7. [DOI] [PubMed] [Google Scholar]
- 13. Brown JC, Cespedes Feliciano EM, Caan BJ. The evolution of body composition in oncology—epidemiology, clinical trials, and the future of patient care: facts and numbers. J Cachexia Sarcopenia Muscle. 2018;9(7):1200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prado CM, Sawyer MB, Ghosh S, Lieffers JR, Esfandiari N, Antoun S, Baracos VE. Central tenet of cancer cachexia therapy: do patients with advanced cancer have exploitable anabolic potential?. Am J Clin Nutr. 2013;98(4):1012–19. [DOI] [PubMed] [Google Scholar]
- 15. Aversa Z, Costelli P, Muscaritoli M. Cancer-induced muscle wasting: latest findings in prevention and treatment. Ther Adv Med Oncol. 2017;9(5):369–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Caan BJ, Meyerhardt JA, Kroenke CH, Alexeeff S, Xiao J, Weltzien E, Feliciano EC, Castillo AL, Quesenberry CP, Kwan MLet al. . Explaining the obesity paradox: the association between body composition and colorectal cancer survival (C-SCANS Study). Cancer Epidemiol Biomarkers Prev. 2017;26(7):1008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gordon NP. Similarity of the Kaiser Permanente senior member population in northern California to the non-Kaiser Permanente covered and general population of seniors in northern California: statistics from the 2009 California Health Interview Survey. Oakland, CA: Kaiser Permanente Northern California Division of Research; 2012. [Google Scholar]
- 18. Meyerhardt JA, Kroenke CH, Prado CM, Kwan ML, Castillo A, Weltzien E, Cespedes Feliciano EM, Xiao J, Caan BJ. Association of weight change after colorectal cancer diagnosis and outcomes in the Kaiser Permanente Northern California population. Cancer Epidemiol Biomarkers Prev. 2017;26(1):30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xiao L, Lv N, Rosas LG, Au D, Ma J. Validation of clinic weights from electronic health records against standardized weight measurements in weight loss trials. Obesity. 2017;25(2):363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, Heymsfield SB, Heshka S. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004;97(6):2333–8. [DOI] [PubMed] [Google Scholar]
- 21. Schweitzer L, Geisler C, Pourhassan M, Braun W, Glüer CC, Bosy-Westphal A, Müller MJ. What is the best reference site for a single MRI slice to assess whole-body skeletal muscle and adipose tissue volumes in healthy adults?. Am J Clin Nutr. 2015;102(1):58–65. [DOI] [PubMed] [Google Scholar]
- 22. Lieffers JR, Mourtzakis M, Hall KD, McCargar LJ, Prado CM, Baracos VE. A viscerally driven cachexia syndrome in patients with advanced colorectal cancer: contributions of organ and tumor mass to whole-body energy demands. Am J Clin Nutr. 2009;89(4):1173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prado CM, Cushen SJ, Orsso CE, Ryan AM. Sarcopenia and cachexia in the era of obesity: clinical and nutritional impact. Proc Nutr Soc. 2016;75(2):188–98. [DOI] [PubMed] [Google Scholar]
- 24. Aubrey J, Esfandiari N, Baracos VE, Buteau FA, Frenette J, Putman CT, Mazurak VC. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol. 2014;210(3):489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown JC, Caan BJ, Prado CM, Weltzien E, Xiao J, Cespedes Feliciano EM, Kroenke CH, Meyerhardt JA. Body composition and cardiovascular events in patients with colorectal cancer: a population-based retrospective cohort study. JAMA Oncol. 2019;5(7):967–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB, Baracos VE. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31(12):1539–47. [DOI] [PubMed] [Google Scholar]
- 27. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–51. [DOI] [PubMed] [Google Scholar]
- 28. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4. [DOI] [PubMed] [Google Scholar]
- 29. Nagelkerke NJD. A note on a general definition of the coefficient of determination. Biometrika. 1991;78(3):691–2. [Google Scholar]
- 30. Royston P, Parmar MK. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Statist Med. 2002;21(15):2175–97. [DOI] [PubMed] [Google Scholar]
- 31. Rutherford MJ, Crowther MJ, Lambert PC. The use of restricted cubic splines to approximate complex hazard functions in the analysis of time-to-event data: a simulation study. J Statist Comput Simulation. 2015;85(4):777–93. [Google Scholar]
- 32. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268–74. [DOI] [PubMed] [Google Scholar]
- 33. Aleixo GFP, Shachar SS, Nyrop KA, Muss HB, Malpica L, Williams GR. Myosteatosis and prognosis in cancer: systematic review and meta-analysis. Crit Rev Oncol Hematol. 2020;145:102839. [DOI] [PubMed] [Google Scholar]
- 34. Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer. 2016;57:58–67. [DOI] [PubMed] [Google Scholar]
- 35. Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y, Fearon KC. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 2016;17(4):519–31. [DOI] [PubMed] [Google Scholar]
- 36. Fearon K, Argiles JM, Baracos VE, Bernabei R, Coats A, Crawford J, Deutz NE, Doehner W, Evans WJ, Ferrucci Let al. . Request for regulatory guidance for cancer cachexia intervention trials. J Cachexia Sarcopenia Muscle. 2015;6(4):272–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Prado CM, Purcell SA, Laviano A. Nutrition interventions to treat low muscle mass in cancer. J Cachexia Sarcopenia Muscle. 2020;11(2):366–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rosa-Caldwell ME, Greene NP. Muscle metabolism and atrophy: let's talk about sex. Biol Sex Differ. 2019;10(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meyerhardt JA, Mangu PB, Flynn PJ, Korde L, Loprinzi CL, Minsky BD, Petrelli NJ, Ryan K, Schrag DH, Wong SLet al. . Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol. 2013;31(35):4465–70. [DOI] [PubMed] [Google Scholar]
- 40. Heymsfield SB, Adamek M, Gonzalez MC, Jia G, Thomas DM. Assessing skeletal muscle mass: historical overview and state of the art. J Cachexia Sarcopenia Muscle. 2014;5(1):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Winkels RM, Snetselaar T, Adriaans A, van Warmerdam LJ, Vreugdenhil A, Slooter G, Straathof J-W, Kampman E, van Lieshout R, Beijer S. Changes in body weight in patients with colorectal cancer treated with surgery and adjuvant chemotherapy: an observational study. Cancer Treat Res Commun. 2016;9:111–15. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article, code book, and analytic code will not be made available because this was a retrospective cohort study using electronic medical records. The requirement for informed consent was waived by the institutional review board. Consequently, study subjects did not explicitly consent for their data to be shared publicly. Moreover, study subjects were all treated within a single health system for an uncommon disease, and our ability to preserve subject anonymity cannot be guaranteedt.