ABSTRACT
Background
Research suggests short interpregnancy intervals increase risks for adverse perinatal outcomes, including some birth defects. A hypothesized cause is nutritional depletion, including folic acid (FA).
Objectives
We evaluated associations between short interpregnancy intervals, alone and in combination with FA intake, and the occurrence of select malformations.
Methods
Data were from the National Birth Defects Prevention Study (US case–control, 1997–2011). Participants included multiparous women whose prior pregnancy resulted in live birth. Cases included 8 noncardiac and 6 cardiac defect groups (n = 3219); controls were nonmalformed live-borns (n = 2508). We categorized interpregnancy interval (<6, 6–11, 12–17, and 18–23 mo) and periconceptional FA intake [no FA supplement use and dietary folate equivalents (DFE) <400 µg/d, no FA supplement use and DFE ≥400 µg/d, or any FA supplement use]. We controlled for age, race/ethnicity, income, pregnancy intention, and study center. ORs <0.8 or >1.2 were considered to represent potentially meaningful associations.
Results
ORs for <6 compared with 18–23 mo were >1.2 for 4/8 noncardiac and 3/6 cardiac malformations. Among participants with any FA supplement use, ORs comparing <6 with 6–23 mo were <1.2 for most defects. Conversely, most ORs were >1.2 for <6 mo + no FA supplement use and DFE <400 µg/d compared with 6–23 mo + any FA supplement use. Magnitude and precision varied by defect.
Conclusions
Short interpregnancy intervals were associated with a trend of higher risks for several defects, notably in the absence of FA supplement use. To our knowledge, our study is the first to provide preliminary empirical support that these etiologies may be related to shorter interpregnancy intervals and possible nutritional deficiencies. Because FA intake is highly correlated with other nutrients, and because our estimates were generally imprecise, more research with larger sample sizes is needed to better understand the role of FA compared with other nutrients in each defect-specific etiology.
Keywords: birth defects, birth spacing, folic acid, interpregnancy interval, nutritional deficiency, pregnancy
Introduction
Interpregnancy interval is the time between the end of 1 pregnancy and the start of the next. Short interpregnancy intervals, typically defined as <6 mo, have been associated with complications and adverse outcomes of the subsequent pregnancy (1, 2), including some congenital malformations. The strongest associations have been observed with neural tube defects (NTDs) and gastroschisis, followed by cardiac and cleft defects (adjusted ORs: 2.1–1.4) (3–5). Short intervals have been associated with increased risks for NTDs and cardiac defects in more than 1 study (4, 6, 7).
Women with poorer nutrition and/or low folate may be particularly susceptible to adverse outcomes following short interpregnancy intervals (8, 9). Without the support of supplementation, the demands of pregnancy and lactation can result in reduced folate concentrations through 6 mo postpartum (10). Research supports that when in a state of depletion, nutrients preferentially partition to the mother at the expense of the fetus (11). Furthermore, van Eijsden et al. (12) found that associations between short interpregnancy intervals and low birth weight were strongest among women who did not use folic acid (FA)–containing supplements, weaker among late pregnancy initiators, and absent among early initiators. FA is critical to fetal development (13, 14), protects against the occurrence of NTDs (15, 16), and has been associated with other birth defects (17–19) and other adverse pregnancy outcomes (20). To our knowledge, empirical evidence has yet to be provided to support the nutritional and/or folate depletion hypothesis in the relation between short interpregnancy intervals and increased risks for certain birth defects.
We sought to examine this hypothesis using data from the National Birth Defects Prevention Study (NBDPS). We evaluated associations with short intervals and FA intake separately as well as jointly (e.g., women exposed to both compared to neither). In an attempt to distinguish between the effects of FA and other nutrients, we considered FA intake from diet separately from vitamin supplementation. If short intervals increase defect risks in the presence of no supplement use, as observed in the study by van Eijsden et al. (12), these data could suggest that nutritional depletion may be a part of the biologic mechanism. Furthermore, stronger associations observed among women who also have low dietary folate intake could suggest that FA in particular may play an important role for certain etiologies.
Methods
The NBDPS was a US population-based case–control study that involved surveillance systems in Arkansas, California, Georgia, Iowa, Massachusetts, North Carolina, New Jersey, New York, Texas, and Utah to identify pregnancies affected by major, nonchromosomal birth defects (21). Cases included terminations, fetal losses, and live births. Clinical geneticists confirmed diagnoses; single-gene disorders and chromosomal abnormalities were excluded. Cases were classified according to the presence of various structural malformations. Controls were live-born infants with no major malformations who were randomly selected from birth certificates [Arkansas, Georgia (2001–2009), Iowa, Massachusetts, North Carolina, New Jersey, and Utah] and delivery records from the same hospitals as cases [California, Georgia (1998–2001), New York, and Texas]. Each center obtained study approval from its local institutional review board. To be eligible for the NBDPS, the estimated due date needed to be between October 1, 1997, and December 31, 2011. Among those eligible, consent rates were 67.4% and 64.8% for cases and controls, respectively. Within 2 y of delivery, participants completed a standardized telephone interview, which included questions on reproductive history, demographic characteristics, lifestyle and behaviors, and pregnancy. We conducted our analysis according to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines for case–control studies (22).
We defined interpregnancy interval as the time between the preceding delivery and the start of the study pregnancy. The estimated conception date was calculated as the estimated due date minus 266 d or, for participants missing an estimated due date, as the date of the last menstrual period plus 14 d. When the day was missing for the previous delivery date, we assigned the 15th of the month to compute interpregnancy interval (<0.3% of cases and controls); otherwise, we excluded women with missing dating information (6.8% cases and 5.6% controls). We converted the interval from days to months by dividing by 30.42 and categorized as <6, 6–11, 12–17, or 18–23 mo to be comparable to prior studies (3, 4, 6, 23); we excluded longer intervals because adverse outcomes may be due to a different etiologic mechanism.
In the interview, participants reported product, frequency, and dose of supplements (including multivitamins, prenatal vitamins, and those containing FA only) for the 3 mo before through the duration of pregnancy. These data were classified according to whether the reported supplements contained FA and the specific timing during pregnancy. The time period of interest for our analysis was the 28 d before through the first 56 d of the study pregnancy (herein referred to as “periconception”) because most defects occur early in gestation. Most FA-containing supplements include other vitamins and minerals, although specific contents vary by product (24, 25). In our study, data were not available on other specific components. The NBDPS participants also completed a modified Willett FFQ (26), which included additional questions on cereal intake to improve FA quantification given fortification. The assessment summarized typical diet for the year before the study pregnancy, which we assumed would be similar to that of early pregnancy before recognition. Nutrient matrices were used to estimate average daily intake of macro- and micronutrients, including natural and synthetic folate (27). Due to the greater bioavailability of synthetic folate, we calculated dietary folate equivalents (DFE) as naturally occurring food folate + (1.7 × synthetic folate) (28). FA is found in a variety of foods (29, 30), and some correlation with other nutrients is expected (31); however, dietary patterns do not fully explain variance in FA intake (32). Therefore, examination of dietary FA intake among nonsupplementers can serve as a proxy for the independent effect of FA. Accordingly, we categorized participants into 1 of 3 exclusive groups: any FA-containing supplement use during periconception, no FA-containing supplement use but met the US recommendations for women of childbearing potential (28) based on estimated dietary intake (i.e., DFE ≥400 µg/d), and no FA-containing supplement use and low estimated dietary intake (i.e., DFE <400 µg/d).
We considered an array of potential confounders based on a priori knowledge of associations with interpregnancy interval and at least 1 malformation under study. These factors included maternal and paternal sociodemographic characteristics (study center, age at the prior delivery, race/ethnicity, US born, years of education, and annual household income), reproductive and pregnancy history (gravidity, use of fertility treatment, pregnancy intention, and use of birth control from 3 mo before pregnancy), and health-related behaviors (smoking or alcohol use from 1 mo before through 3 mo into pregnancy). We used a change-in-estimate approach to determine which potential confounding factors we would include in the multivariable regression models (33). Specifically, the set of covariates were those that when added to the model resulted in ≥10% change in the OR estimate for ≥1 defect under study. These factors were study center, maternal age, maternal race/ethnicity, pregnancy intention, and annual household income.
Our analysis included a subset of NBDPS participants with ≥1 previous pregnancy, whose interpregnancy interval was <24 mo, and whose preceding pregnancy resulted in a singleton live birth. We required that the prior pregnancy resulted in live birth because the pregnancy must have lasted long enough for depletion to take effect (34), and live births generally occur after 20 weeks of gestation. Fetal loss after 20 weeks of gestation is speculated to be a confounder (35) because it tends to be associated with shorter interpregnancy intervals (36) and may share underlying etiology with some defects (37). We included isolated cases (affected by only 1 malformation) for defect groups with ≥100 in total and ≥10 in each interpregnancy interval category. We included any defect group meeting these criteria, not just those known to be related to FA, because 1) we aimed to assess short interval associations with birth defects in general, as well as jointly with FA; 2) it is not well established which defects are FA dependent; and 3) non-FA-dependent defects may be affected by other nutrients, such as those co-occurring in multivitamins. For cardiac defects, we included only simple cases (discrete anatomically). We did not consider cardiac conditions that generally are explained by premature delivery or are misclassified (e.g., atrial septal defects) (38), although given the rigorous classification procedures, it is unlikely that such defects would be included in the NBDPS (39). Furthermore, we excluded participants with extreme estimated daily caloric intake (<500 or >3800) or missing information on outcome of the preceding pregnancy, diet, supplement use, or maternal characteristics. We did allow for missing data on income (e.g., refusals to disclose), paternal characteristics, and pregnancy intention using unknown/refused categories.
We used unconditional logistic regression models with Firth's penalized likelihood (40) to estimate ORs and profile likelihood CIs, adjusted for the aforementioned covariates. Firth's penalized likelihood is an alternative estimation approach for rare outcomes (40). We estimated malformation-specific associations with interpregnancy interval (<6, 6–11, and 12–17 mo compared with 18–23 mo) and with FA intake (DFE <400 and ≥400 µg/d among non-FA supplementers compared with any FA supplement use). Following, we evaluated joint effects by estimating ORs for exclusive joint categorizations of these 2 exposures: <6-mo intervals + no FA supplement use and DFE <400 µg/d, <6-mo intervals + no FA supplement use and DFE ≥400 µg/d, <6-mo intervals + any FA supplement use, 6- to 23-mo intervals + no FA supplement use and DFE <400 µg/d, 6- to 23-mo intervals + no FA supplement use and DFE ≥400 µg/d, and 6- to 23-mo intervals + any FA supplement use (reference). We dichotomized at 6 mo because the starkest associations have been observed for <6-mo intervals in other studies (3, 4, 6, 23).
We adjusted for multiple comparisons using the Bonferroni and Dunn method (41, 42) by dividing the original α by the number of comparisons. In our analysis of joint effects of interval length and FA, there are 5 comparisons, so we reported 99% CIs. Although we made this adjustment, we did not accept or reject our hypotheses based on a specific statistical significance threshold, as now advised by the American Statistical Association (43). Rather, we focused on the strength of the association, where we considered ≥20% relative increase (OR > 1.2) or decrease (OR < 0.8) in odds to represent a potentially meaningful association, as well as consistency with prior research and plausibility (44). We also considered precision; however, we did not consider a result to provide evidence only if the 99% confidence bounds excluded 1 because this would be analogous to only accepting results meeting a statistical significance threshold (45). We were cognizant that by evaluating each defect in isolation, the number of exposed cases would be small, leading to imprecise estimates. However, we believed it was important to conduct each analysis by defect group. Had we instead combined all defects, the result might have been a mixture of heterogeneous estimates that could mask effects that only occur among certain defects. We did not report estimates if there was only 1 exposed case or if the model did not converge. We conducted analyses using SAS/STAT software version 9.4 for Windows (SAS Institute) (46).
Results
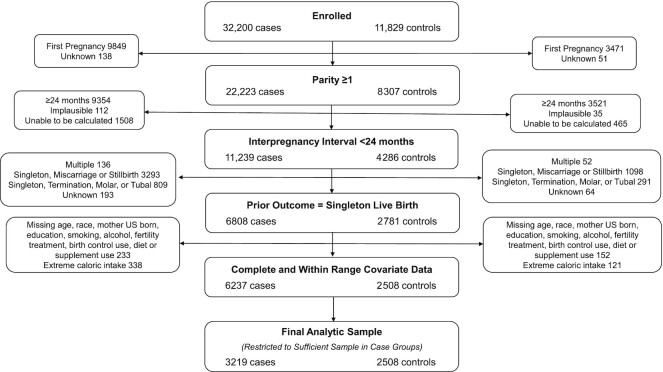
Of the 32,187 cases and 11,814 controls enrolled in the NBDPS, 3219 cases and 2508 controls met the eligibility criteria for our analyses. The primary exclusion reasons were no prior pregnancies, prior interpregnancy interval ≥24 mo, and prior pregnancy ending in miscarriage (Figure 1). The isolated noncardiac defects included anencephaly and craniorachischisis (n = 137), spina bifida (n = 268), cleft palate (n = 261), cleft lip (with or without cleft palate) (n = 599), diaphragmatic hernia (n = 138), hypospadias (second/third degree) (males only, n = 339), craniosynostosis (n = 349), and gastroschisis (n = 154). The simple cardiac defects included tetralogy of Fallot (173), dextro-rotated transposition of the great arteries (d-TGA) (n = 127), coarctation of the aorta (n = 115), hypoplastic left heart syndrome (HLHS) (n = 134), pulmonary valve stenosis (PVS) (n = 251), and ventricular septal defect (VSD, perimembranous) (n = 174).
FIGURE 1.
Eligibility flowchart.
A slightly higher proportion of cases were non-Hispanic white, primiparous (prior to the study pregnancy), unintended pregnancies, and smokers (Table 1). There were slight variations in age and income. Cases and controls were similar with respect to the other characteristics. Differences were more notable among the controls by interpregnancy interval. Mothers with intervals of <6 mo tended to be <25 years old at the prior delivery, whereas women with interpregnancy intervals of ≥6 mo were older. A higher proportion of women with shorter interpregnancy intervals self-identified as black or Hispanic, had lower educational attainment and lower income, had ≥2 prior pregnancies, reported the study pregnancy as unintended, and were smokers. Women with short intervals were also less likely to be born in the United States, use fertility treatment, and be drinkers. Similar patterns were exhibited among the controls who reported no FA supplement use (see Table 1). For all groupings, there were also variations by study center.
TABLE 1.
Maternal characteristics by case–control status, interpregnancy interval, and folic acid intake, National Birth Defect Prevention Study (United States, 1997–2011)1
| Folic acid intake (controls) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Interpregnancy interval (controls) | No FA supplement use (B1–P2) | Any FA supplement use (B1–P2) | ||||||||
| Cases2 | Controls | <6 mo | 6–11 mo | 12–17 mo | 18–23 mo | DFE <400 µg | DFE ≥400 µg | |||
| n | 3219 | 2508 | 249 | 681 | 842 | 736 | 233 | 397 | 1878 | |
| Maternal age at prior delivery, % | ||||||||||
| <20 y | % | 13.6 | 14.2 | 16.9 | 16.0 | 13.3 | 12.5 | 24.0 | 28.2 | 10.0 |
| 20–24 y | % | 28.1 | 26.8 | 37.8 | 27.3 | 25.1 | 24.6 | 35.6 | 32.5 | 24.5 |
| 25–29 y | % | 29.5 | 31.9 | 24.5 | 30.7 | 32.5 | 34.7 | 20.2 | 25.2 | 34.7 |
| ≥30 y | % | 28.9 | 27.2 | 20.9 | 26.0 | 29.1 | 28.3 | 20.2 | 14.1 | 30.8 |
| Paternal age at prior delivery, % | ||||||||||
| <20 y | % | 5.5 | 5.9 | 8.8 | 7.3 | 5.0 | 4.6 | 10.7 | 10.6 | 4.3 |
| 20–24 y | % | 21.4 | 21.4 | 27.3 | 23.4 | 20.4 | 18.6 | 31.8 | 28.5 | 18.6 |
| 25–29 y | % | 29.0 | 28.2 | 25.7 | 26.7 | 29.0 | 29.6 | 22.8 | 24.9 | 29.6 |
| ≥30 y | % | 41.3 | 41.8 | 35.3 | 40.2 | 42.4 | 44.8 | 29.2 | 29.0 | 46.1 |
| Unknown/refused | % | 2.8 | 2.7 | 2.8 | 2.4 | 3.2 | 2.3 | 5.6 | 7.1 | 1.4 |
| Maternal race/ethnicity, % | ||||||||||
| Non-Hispanic white | % | 66.8 | 63.6 | 47.4 | 58.3 | 70.4 | 66.3 | 42.9 | 38.8 | 71.5 |
| Non-Hispanic black | % | 6.2 | 8.6 | 15.3 | 11.0 | 5.3 | 7.9 | 19.3 | 14.4 | 6.1 |
| Hispanic | % | 20.6 | 22.1 | 32.9 | 25.1 | 19.1 | 19.2 | 32.2 | 41.1 | 16.9 |
| Other | % | 6.3 | 5.6 | 4.4 | 5.6 | 5.1 | 6.7 | 5.6 | 5.8 | 5.6 |
| Paternal race/ethnicity, % | ||||||||||
| Non-Hispanic white | % | 63.8 | 61.7 | 45.4 | 54.8 | 68.4 | 65.9 | 40.3 | 36.0 | 69.8 |
| Non-Hispanic black | % | 8.0 | 9.9 | 18.1 | 12.6 | 6.1 | 8.8 | 20.2 | 15.1 | 7.5 |
| Hispanic | % | 21.8 | 22.1 | 31.3 | 25.6 | 19.7 | 18.6 | 32.2 | 41.1 | 16.9 |
| Other | % | 5.4 | 5.3 | 3.2 | 6.0 | 4.4 | 6.3 | 5.6 | 6.6 | 5.0 |
| Unknown/refused | % | 1.1 | 1.1 | 2.0 | 1.0 | 1.4 | 0.4 | 1.7 | 1.3 | 1.0 |
| Mother US born, % | ||||||||||
| Yes | % | 83.6 | 81.8 | 79.5 | 80.3 | 82.8 | 82.9 | 78.1 | 70.0 | 84.8 |
| No | % | 16.4 | 18.2 | 20.5 | 19.7 | 17.2 | 17.1 | 21.9 | 30.0 | 15.2 |
| Father US born, % | ||||||||||
| Yes | % | 81.0 | 79.8 | 74.7 | 78.4 | 81.2 | 81.3 | 75.5 | 61.7 | 84.2 |
| No | % | 18.4 | 19.5 | 24.5 | 21.0 | 17.7 | 18.5 | 22.8 | 36.8 | 15.4 |
| Unknown/refused | % | 0.6 | 0.7 | 0.8 | 0.6 | 1.1 | 0.3 | 1.7 | 1.5 | 0.4 |
| Maternal education, y | ||||||||||
| <12 | % | 17.1 | 16.4 | 26.9 | 17.9 | 14.3 | 13.7 | 29.2 | 35.3 | 10.8 |
| 12 | % | 23.0 | 23.7 | 32.1 | 25.7 | 20.9 | 22.2 | 32.6 | 32.8 | 20.7 |
| >12 | % | 59.9 | 60.0 | 41.0 | 56.4 | 64.9 | 64.1 | 38.2 | 32.0 | 68.6 |
| Paternal education, y | ||||||||||
| <12 | % | 16.2 | 15.4 | 23.7 | 17.6 | 13.7 | 12.6 | 25.3 | 30.7 | 11.0 |
| 12 | % | 27.4 | 26.9 | 35.3 | 28.9 | 23.8 | 25.8 | 35.2 | 31.0 | 25.0 |
| >12 | % | 54.0 | 55.2 | 37.4 | 51.1 | 59.6 | 60.1 | 33.5 | 33.3 | 62.6 |
| Unknown/refused | % | 2.5 | 2.4 | 3.6 | 2.4 | 3.0 | 1.5 | 6.0 | 5.0 | 1.4 |
| Annual household income, % | ||||||||||
| <$10,000 | % | 17.6 | 17.6 | 29.3 | 21.4 | 14.9 | 13.3 | 31.3 | 35.5 | 12.1 |
| $10,000–$50,000 | % | 43.9 | 43.3 | 42.2 | 43.5 | 42.9 | 43.9 | 46.8 | 46.1 | 42.2 |
| >$50,000 | % | 34.7 | 35.5 | 22.1 | 31.0 | 39.2 | 39.8 | 17.2 | 12.9 | 42.5 |
| Unknown/refused | % | 3.9 | 3.7 | 6.4 | 4.1 | 3.1 | 3.0 | 4.7 | 5.5 | 3.1 |
| Gravidity, % | ||||||||||
| 1 | % | 43.0 | 41.6 | 36.6 | 39.7 | 43.5 | 43.1 | 34.3 | 37.0 | 43.5 |
| ≥2 | % | 57.0 | 58.4 | 63.5 | 60.4 | 56.5 | 56.9 | 65.7 | 63.0 | 56.5 |
| Fertility treatment, % | ||||||||||
| Yes | % | 2.3 | 2.1 | 0.0 | 1.8 | 2.6 | 2.5 | 0.9 | 0.8 | 2.5 |
| No | % | 97.7 | 97.9 | 100.0 | 98.2 | 97.4 | 97.6 | 99.1 | 99.2 | 97.5 |
| Pregnancy intention, % | ||||||||||
| Intended | % | 39.3 | 39.7 | 16.9 | 17.8 | 21.3 | 22.0 | 31.8 | 26.5 | 43.5 |
| Unintended/ambivalent | % | 41.5 | 40.2 | 59.4 | 50.7 | 35.6 | 29.2 | 54.1 | 56.2 | 35.1 |
| Unknown/refused | % | 19.2 | 20.1 | 23.7 | 31.6 | 43.1 | 48.8 | 14.2 | 17.4 | 21.4 |
| Any birth control (B3–), % | ||||||||||
| Yes | % | 30.1 | 31.4 | 29.7 | 31.3 | 32.4 | 30.8 | 25.8 | 28.5 | 32.7 |
| No | % | 69.9 | 68.6 | 70.3 | 68.7 | 67.6 | 69.2 | 74.3 | 71.5 | 67.3 |
| Smoking (B1–P3), % | ||||||||||
| Yes | % | 16.1 | 14.9 | 20.5 | 16.7 | 13.7 | 12.8 | 18.9 | 20.2 | 13.3 |
| No | % | 83.9 | 85.1 | 79.5 | 83.3 | 86.3 | 87.2 | 81.1 | 79.9 | 86.7 |
| Alcohol use (B1–P3), % | ||||||||||
| Yes | % | 31.5 | 31.4 | 25.3 | 32.5 | 31.8 | 31.9 | 30.0 | 24.7 | 33.0 |
| No | % | 68.5 | 68.6 | 74.7 | 67.6 | 68.2 | 68.1 | 70.0 | 75.3 | 67.0 |
| Study center location, % | ||||||||||
| Arkansas | % | 12.8 | 11.3 | 16.9 | 11.9 | 10.1 | 10.3 | 9.4 | 11.6 | 11.5 |
| California | % | 12.2 | 10.1 | 10.8 | 10.7 | 10.0 | 9.2 | 12.5 | 14.9 | 8.7 |
| Iowa | % | 10.9 | 12.3 | 8.4 | 12.3 | 13.1 | 12.6 | 9.0 | 9.6 | 13.3 |
| Massachusetts | % | 12.0 | 11.0 | 8.4 | 12.0 | 10.6 | 11.3 | 9.0 | 5.3 | 12.4 |
| New Jersey | % | 4.4 | 4.0 | 4.4 | 5.0 | 3.6 | 3.5 | 3.0 | 4.0 | 4.2 |
| New York | % | 7.8 | 8.7 | 6.8 | 8.2 | 9.1 | 9.4 | 9.0 | 8.1 | 8.8 |
| Texas | % | 8.6 | 10.6 | 16.9 | 11.6 | 9.4 | 9.0 | 13.3 | 18.4 | 8.6 |
| CDC/Atlanta | % | 9.4 | 9.5 | 11.7 | 10.4 | 8.6 | 9.0 | 12.5 | 11.3 | 8.7 |
| North Carolina | % | 7.4 | 7.9 | 10.0 | 7.5 | 7.5 | 7.9 | 9.4 | 7.3 | 7.8 |
| Utah | % | 14.4 | 14.7 | 5.6 | 10.3 | 18.2 | 17.8 | 12.9 | 9.6 | 16.0 |
B1–P2, 1 mo before pregnancy through the first 2 mo of pregnancy; B1–P3, 1 mo before pregnancy through the first 3 mo of pregnancy; B3–, any time since 3 mo prior to pregnancy; FA, folic acid.
Cases include isolated noncardiac defects: anencephaly and craniorachischisis (n = 137), spina bifida (n = 268), cleft palate (n = 261), cleft lip (with or without cleft palate) (n = 599), diaphragmatic hernia (n = 138), hypospadias (second/third degree) (males only, n = 339), craniosynostosis (n = 349), and gastroschisis (n = 154); and simple cardiac defects: tetralogy of Fallot (173), dextro-rotated transposition of the great arteries (n = 127), coarctation of the aorta (n = 115), hypoplastic left heart syndrome (n = 134), pulmonary valve stenosis (n = 251), and ventricular septal defect (perimembranous) (n = 174).
A higher proportion of controls with shorter intervals reported no periconceptional FA supplement use compared with controls with longer intervals (29.7%, 29.5%, 22.8%, and 22.2% for <6, 6–11, 12–17, and 18–23 mo, respectively). As seen in other studies (47), these differences appeared to be explained by other socioeconomic factors because the association between interpregnancy interval and supplement use was null upon adjustment for confounders. Among non-FA supplementers, there were no appreciable differences between interval and dietary FA intake (data not shown).
A higher proportion of cases compared with controls had interpregnancy intervals of <6 mo (11.8% compared with 9.9% respectively). We observed positive associations with intervals of <6 mo, compared with 18–23 mo, for 4/8 noncardiac and 3/6 cardiac malformations studied (Table 2). The most notable increases were observed for gastroschisis (OR: 2.3; 99% CI: 1.1, 4.7), PVS (OR: 1.7; 99% CI: 0.92, 3.2), d-TGA (OR: 1.7; 99% CI: 0.69, 4.0), and craniosynostosis (OR: 1.5; 99% CI: 0.86, 2.6). Positive associations were also observed with 6–11 mo for gastroschisis and PVS and with 12–17 mo for PVS and d-TGA. An inverse association was observed with anencephaly with 6- to 11-mo intervals.
TABLE 2.
Associations between interpregnancy interval categories and isolated birth defects, National Birth Defect Prevention Study (United States, 1997–2011)1
| Interpregnancy interval | |||||
|---|---|---|---|---|---|
| <6 mo | 6–11 mo | 12–17 mo | 18–23 mo | ||
| Noncardiac defects | |||||
| Anencephaly/craniorachischisis | n | 14 | 32 | 45 | 46 |
| aOR (99% CI) | 0.80 (0.33, 1.8) | 0.71 (0.38, 1.3) | 0.85 (0.48, 1.5) | 1.00 | |
| Spina bifida | n | 26 | 74 | 86 | 82 |
| aOR (99% CI) | 0.84 (0.44, 1.5) | 0.89 (0.57, 1.4) | 0.89 (0.58, 1.4) | 1.00 | |
| Cleft palate | n | 31 | 71 | 90 | 69 |
| aOR (99% CI) | 1.3 (0.69, 2.3) | 1.1 (0.67, 1.7) | 1.1 (0.72, 1.7) | 1.00 | |
| Cleft lip (w/wo cleft palate) | n | 84 | 152 | 188 | 175 |
| aOR (99% CI) | 1.4 (0.91, 2.1) | 0.92 (0.66, 1.3) | 0.91 (0.68, 1.2) | 1.00 | |
| Diaphragmatic hernia | n | 14 | 38 | 50 | 36 |
| aOR (99% CI) | 1.1 (0.43, 2.4) | 1.1 (0.58, 2.0) | 1.2 (0.67, 2.1) | 1.00 | |
| Hypospadias second/third degree | n | 29 | 81 | 127 | 102 |
| aOR (99% CI) | 1.0 (0.55, 1.8) | 0.92 (0.60, 1.4) | 1.1 (0.74, 1.6) | 1.00 | |
| Craniosynostosis | n | 39 | 81 | 127 | 102 |
| aOR (99% CI) | 1.5 (0.86, 2.6) | 0.97 (0.63, 1.5) | 1.1 (0.73, 1.5) | 1.00 | |
| Gastroschisis | n | 29 | 56 | 38 | 31 |
| aOR (99% CI) | 2.3 (1.1, 4.7) | 1.8 (0.98, 3.4) | 1.0 (0.55, 2.0) | 1.00 | |
| Cardiac defects | |||||
| Tetralogy of Fallot | n | 22 | 51 | 50 | 50 |
| aOR (99% CI) | 1.3 (0.64, 2.7) | 1.1 (0.65, 1.9) | 0.88 (0.52, 1.5) | 1.00 | |
| d-Transposition of the great arteries | n | 15 | 30 | 55 | 27 |
| aOR (99% CI) | 1.7 (0.69, 4.0) | 1.2 (0.58, 2.4) | 1.7 (0.95, 3.3) | 1.00 | |
| Coarctation of the aorta | n | 12 | 28 | 42 | 33 |
| aOR (99% CI) | 1.1 (0.41, 2.6) | 0.89 (0.44, 1.8) | 1.1 (0.58, 2.0) | 1.00 | |
| Hypoplastic left heart syndrome | n | 10 | 33 | 53 | 38 |
| aOR (99% CI) | 1.1 (0.38, 2.6) | 1.1 (0.59, 2.1) | 1.2 (0.69, 2.1) | 1.00 | |
| Pulmonary valve stenosis | n | 34 | 78 | 85 | 54 |
| aOR (99% CI) | 1.7 (0.92, 3.2) | 1.5 (0.95, 2.5) | 1.4 (0.86, 2.2) | 1.00 | |
| VSD perimembranous | n | 20 | 57 | 51 | 46 |
| aOR (99% CI) | 1.1 (0.52, 2.3) | 1.2 (0.72, 2.1) | 0.97 (0.56, 1.7) | 1.00 | |
Adjusted OR estimated from multivariable logistic regression with Firth's penalized likelihood with 99% profile likelihood CIs, controlling for maternal age at the prior delivery, maternal race/ethnicity, annual household income, pregnancy intention, and study center. aOR, adjusted OR; VSD, ventricular septal defect; w/wo, with and without.
A higher proportion of cases compared with controls did not supplement with FA and had estimated DFE <400 µg/d (11.6% compared with 9.3%, respectively). We observed positive associations with no FA supplement use and DFE <400 µg/d, compared with any FA supplement use, for 5/8 noncardiac and 4/6 cardiac defects (Table 3). The strongest associations were observed for d-TGA (OR: 1.7; 99% CI: 0.77, 3.5), cleft palate (OR: 1.6; 99% CI: 0.93, 2.6), PVS (OR: 1.6; 99% CI: 0.90, 2.6), and spina bifida (OR: 1.5; 99% CI: 0.91, 2.5). Although estimates were imprecise, adjusted ORs for no FA supplement use and DFE ≥400 µg/d, compared with any FA supplement use, were >1.2 for 1/8 noncardiac and 3/6 cardiac defects; conversely, inverse associations were observed for 1/8 noncardiac and 1/6 cardiac defects.
TABLE 3.
Associations between FA intake and isolated birth defects, National Birth Defect Prevention Study (United States, 1997–2011)1
| FA Intake | ||||
|---|---|---|---|---|
| DFE <400 µg and no FA supplement use | DFE ≥400 µg and no FA supplement use | Any FA supplement use (B1–P2) | ||
| Noncardiac defects | ||||
| Anencephaly/craniorachischisis | n | 16 | 16 | 105 |
| aOR (99% CI) | 1.2 (0.53, 2.4) | 0.64 (0.29, 1.3) | 1.00 | |
| Spina bifida | n | 40 | 39 | 189 |
| aOR (99% CI) | 1.5 (0.91, 2.5) | 0.82 (0.49, 1.3) | 1.00 | |
| Cleft palate | n | 36 | 35 | 190 |
| aOR (99% CI) | 1.6 (0.93, 2.6) | 0.92 (0.53, 1.5) | 1.00 | |
| Cleft lip (w/wo cleft palate) | n | 70 | 101 | 428 |
| aOR (99% CI) | 1.3 (0.90, 2.0) | 1.1 (0.74, 1.5) | 1.00 | |
| Diaphragmatic hernia | n | 12 | 29 | 97 |
| aOR (99% CI) | 1.1 (0.43, 2.3) | 1.4 (0.77, 2.6) | 1.00 | |
| Hypospadias second/third degree | n | 28 | 35 | 276 |
| aOR (99% CI) | 1.1 (0.61, 1.9) | 0.89 (0.52, 1.5) | 1.00 | |
| Craniosynostosis | n | 32 | 39 | 278 |
| aOR (99% CI) | 1.3 (0.73, 2.1) | 0.97 (0.59, 1.6) | 1.00 | |
| Gastroschisis | n | 26 | 36 | 92 |
| aOR (99% CI) | 1.4 (0.74, 2.7) | 1.1 (0.62, 1.9) | 1.00 | |
| Cardiac defects | ||||
| Tetralogy of Fallot | n | 21 | 29 | 123 |
| aOR (99% CI) | 1.5 (0.73, 2.7) | 1.3 (0.69, 2.2) | 1.00 | |
| d-Transposition of the great arteries | n | 16 | 21 | 90 |
| aOR (99% CI) | 1.7 (0.77, 3.5) | 1.4 (0.66, 2.6) | 1.00 | |
| Coarctation of the aorta | n | 11 | 19 | 85 |
| aOR (99% CI) | 1.2 (0.47, 2.7) | 1.3 (0.63, 2.6) | 1.00 | |
| Hypoplastic left heart syndrome | n | 13 | 14 | 107 |
| aOR (99% CI) | 1.1 (0.48, 2.4) | 0.78 (0.34, 1.6) | 1.00 | |
| Pulmonary valve stenosis | n | 35 | 34 | 182 |
| aOR (99% CI) | 1.6 (0.90, 2.6) | 0.92 (0.53, 1.5) | 1.00 | |
| VSD perimembranous | n | 18 | 31 | 125 |
| aOR (99% CI) | 1.1 (0.54, 2.2) | 1.1 (0.62, 1.9) | 1.00 | |
Adjusted OR estimated from multivariable logistic regression with Firth's penalized likelihood with 99% profile likelihood CIs, controlling for maternal age at the prior delivery, maternal race/ethnicity, annual household income, pregnancy intention, and study center. aOR, adjusted OR; B1–P2, 1 mo before pregnancy through the first 2 mo of pregnancy; FA, folic acid; VSD, ventricular septal defect; w/wo, with and without.
Our assessment of the joint effects of interval length and FA intake was hindered by imprecision and small numbers of jointly exposed cases. Still, we noted some general patterns (Figure 2). Among participants who supplemented with FA, ORs comparing intervals of <6 mo to 6–23 mo were <1.2 for 6/8 cardiac defects [anencephaly (OR: 0.73; 99% CI: 0.25, 1.7), hypospadias (OR: 0.74; 99% CI: 0.34, 1.4), spina bifida (OR: 0.96; 99% CI: 0.46, 1.8), diaphragmatic hernia (OR: 1.1; 99% CI: 0.41, 2.4), craniosynostosis (OR: 1.1; 99% CI: 0.59, 2.0), and cleft palate (OR: 1.1; 99% CI: 0.57, 2.1)] and 3/6 cardiac defects [coarctation of the aorta (OR: 0.95; 99% CI: 0.30, 2.4), tetralogy of Fallot (OR: 1.0; 99% CI: 0.41, 2.3), and VSD (OR: 1.1; 99% CI; 0.48, 2.3)]; estimates were >1.2 for the other 2/8 noncardiac defects [cleft lip (OR: 1.3; 99% CI: 0.84, 2.0) and gastroschisis (OR: 1.3; 99% CI: 0.52, 2.7)] and 3/6 cardiac defects [PVS (OR: 1.4; 99% CI: 0.74, 2.5), d-TGA (OR: 1.4; 99% CI: 0.51, 3.1), and HLHS (OR: 1.3; 99% CI: 0.50, 3.0)]. In contrast, with intervals of <6 mo + no FA supplement use and DFE <400 µg/d, ORs were >1.2 for 6/8 noncardiac defects [gastroschisis (OR: 3.4; 99% CI: 0.93, 10.1), hypospadias (OR: 3.3; 99% CI: 0.93, 9.7), craniosynostosis (OR: 3.1; 99% CI: 0.89, 8.9), anencephaly (OR: 2.4; 99% CI: 0.47, 8.2), cleft palate (OR: 2.0; 99% CI: 0.48, 6.4), and cleft lip (OR: 1.8; 99% CI: 0.66, 4.4)] and 4/6 cardiac defects [coarctation of the aorta (OR: 3.3; 99% CI: 0.51, 13.1), tetralogy of Fallot (OR: 3.3; 99% CI: 0.77, 10.7), PVS (OR: 2.5; 99% CI: 0.65, 7.5), and VSD (OR: 1.3; 99% CI: 0.13, 5.6)]. With intervals <6 mo + no FA supplement use and DFE ≥400 µg/d, OR estimates were comparatively lower but still >1.2 for 6/8 noncardiac defects [craniosynostosis (OR: 2.6; 99% CI: 0.88, 6.5), gastroschisis (OR: 2.2; 99% CI: 0.72, 5.7), cleft lip (OR: 1.8; 99% CI: 0.84, 3.5), diaphragmatic hernia (OR: 1.7; 99% CI: 0.34, 5.6), cleft palate (OR: 1.4; 99% CI: 0.38, 3.9), and spina bifida (OR: 1.3; 99% CI: 0.40, 3.5)] and 3/6 cardiac defects [d-TGA (OR: 2.8; 99% CI: 0.7, 8.7), tetralogy of Fallot (OR: 2.1; 99% CI: 0.57, 6.1), and coarctation of the aorta (OR: 1.3; 99% CI: 0.14, 5.8)].
FIGURE 2.
Forest plot of joint associations between short interpregnancy intervals of <6 mo + no FA supplement use and DFE <400 µg (black triangles), <6 mo + no FA supplement use and DFE ≥400 µg (gray triangles), <6 mo + any FA supplementation (white triangles), 6–23 mo + no FA supplement use and DFE <400 µg (black circles), 6–23 mo + no FA supplement use and DFE ≥400 µg (gray circles), and 6–23 mo + any FA supplement use (white circles) (reference group) with risks for select isolated birth defects. ORs (shapes) and 99% profile likelihood CIs (error bars) were computed from multivariable logistic regression models, with Firth's penalized likelihood, controlling for maternal age at the prior delivery, maternal race/ethnicity, annual household income, pregnancy intention, and study center. Cases include isolated noncardiac defects: anencephaly and craniorachischisis (n = 137), spina bifida (n = 268), cleft palate (n = 261), cleft lip (with or without cleft palate) (n = 599), diaphragmatic hernia (n = 138), hypospadias (second/third degree) (males only, n = 339), craniosynostosis (n = 349), and gastroschisis (n = 154); and simple cardiac defects: tetralogy of Fallot (173), d-TGA (n = 127), coarctation of the aorta (n = 115), HLHS (n = 134), PVS (n = 251), and VSD (perimembranous) (n = 174). Controls were nonmalformed live-borns (n = 2508). d-TGA, dextro-rotated transposition of the great arteries; DFE, dietary folate equivalents; FA, folic acid; HLHS, hypoplastic left heart syndrome; NC, not calculated; PVS, pulmonary valve stenosis; VSD, ventricular septal defect.
Discussion
In this multisite, population-based case–control study, we found a trend of associations between short interpregnancy intervals and several malformations. To our knowledge, our study provides the first empirical evidence to suggest that nutritional depletion may be a part of the underlying biologic mechanism (Figure 3). For most defects, short intervals were not associated with increased risks among FA supplementers. The strongest associations with short intervals, although imprecise, were observed among women who did not supplement and had DFE <400 µg/d. We were not able to explore other specific nutrients and several of the defects have not previously been associated with FA, raising the possibility that the mechanism may not be attributed to FA specifically. However, the fact that short intervals were not associated with increased risks for most defects among women who took supplements, many of which presumably contained multiple nutrients, while nonsupplementers with low dietary folate exhibited increased risks, points to FA being a possible underlying factor for certain defects.
FIGURE 3.
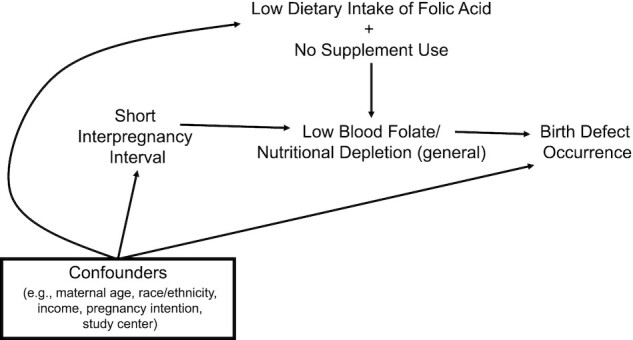
Conceptual model for nutrition/folate depletion as an explanation for the association between short interpregnancy intervals and increased risks for birth defects. Nutrient (folate) concentrations naturally decrease during pregnancy and postpartum. Under this paradigm, short interpregnancy intervals increase the risk for low blood folate (and nutritional depletion in general) at the start of the next pregnancy. This deficit then increases the risk for birth defect occurrence. Social and reproductive factors are related to likelihood of rapid repeat pregnancy and risks for birth defects. Such factors are also associated with dietary intake and supplement use, which modify the short interval–birth defect pathway by directly altering maternal serum concentrations.
Prior literature suggests a possible role of FA in the interpregnancy interval mechanism (8, 10) and birth defect etiology (48–51). Randomized controlled trials and observational studies have conclusively shown daily FA supplement use more than halves NTD risk (48). Although less conclusive, meta-analyses suggest modest protective effects for cleft palate (RR: 0.7; 95% CI: 0.1, 10.9), cleft lip (RR: 0.8; 95% CI: 0.1, 4.4), and cardiovascular defects (RR: 0.6; 95% CI: 0.2, 1.3) (48), which are further supported by pre- and post-FA fortification studies (49, 51) and have led some to consider these defects as “folate sensitive” (52). At least 1 study has reported reduced risk for gastroschisis with sustained FA supplement use in the first trimester (OR: 0.3; 95% CI: 0.1, 0.7) (50). Similarly, we observed associations with DFE <400 µg among nonsupplementers, compared with any FA supplement use, for spina bifida, clefts, gastroschisis, and 4/6 of the heart defects; our observed association with craniosynostosis has not been previously reported.
A challenge in observational study of any isolated nutrient is its potential correlation with other nutrients (31). We cannot rule out the possibility that associations may be explained by other nutrients, the specifics of which may vary for a given defect. For instance, research suggests that 1-carbon micronutrients (e.g., B-6, choline, and methionine), alone or in combination with FA, may decrease risks for NTDs (53) and defects not typically associated with FA, including hypospadias (54) and craniosynostosis (55). Higher quality diets (e.g., those containing more fruits and vegetables) have been found to decrease risks for a variety of defects [e.g., anencephaly, tetralogy of Fallot, craniosynostosis, cleft lip (56), hypospadias (57), and gastroschisis (50)]. Future research should explore this complexity to clarify each defect-specific mechanism.
In a retrospective Canadian cohort (1999–2007), Chen et al. (3) found that short intervals were more strongly associated with defects they defined as “folate-independent” (OR: 1.9; 95% CI: 1.1, 3.2) compared with “folate-dependent” defects [i.e., NTDs, clefts, cardiac defects, urinary tract defects, and limb defects (OR: 1.2; 95% CI: 0.9, 1.6)]. Similarly, we also found associations with a couple of defects not typically linked to FA. The largest limitation of Chen et al.’s study was that they did not have data on supplement use or diet (3). Our NTD associations did not manifest until we also accounted for FA intake. A prior NBDPS study found no difference in associations between <12-mo intervals and gastroschisis among women who supplemented with a multivitamin compared with those who did not; however, that former analysis did not consider diet (5). This is an important distinction, particularly in the age of fortification (58, 59).
Our design addresses certain limitations of prior research. The efficiency gained by sampling controls from the population base afforded us resources to capture detailed FA information from supplements and diet and relatively large numbers of specific defects with rigorous case classification. Still, several malformations were too rare to be examined. Although trends appeared fairly consistent across various defects, our estimates were imprecise. Some findings may have been due to chance, which may explain, for instance, the inverse associations observed with anencephaly. We were able to adjust for a number of covariates, although residual confounding cannot be entirely dismissed. We did not control for family history of defects, illicit drug use, or certain medications (e.g., antiepileptic drugs) because they tend to be rare (60–62) and are not known to be strongly related to interpregnancy interval (35). We chose not to adjust for BMI at the start of the study pregnancy, a risk factor for several birth defects (63), because the interpregnancy interval length correlates with changes in BMI between pregnancies (64, 65). BMI and breastfeeding should be examined in future research because it is not known the degree to which these factors may confound or modify the relations under study. We relied on maternal recall up to 2 y after the study pregnancy. Although the Willett FFQ has been validated (26), the added questions on cereal intake have not. For these reasons, errors in DFE estimates may have occurred. However, because many people lack knowledge about specific nutritional content of food (66), we do not believe this misclassification would be differential with respect to case status. Prior research of recall bias in relation to nutrition and breast cancer has supported this speculation (67). There may be reporting errors in FA supplement use. We conjecture that women who were truly supplementers were unlikely to report being non-FA supplementers, corresponding with near-perfect specificity; however, women who did not actually supplement may have reported taking FA supplements given the recommendations (68), which would correspond with imperfect sensitivity and may be differential with respect to case status. Even large errors in recall may have only minor impact on case–control findings when the predominant errors are nondifferential (random) or relate to differential sensitivity (69), as we believe them to be in our study. Future researchers should investigate dose, frequency, and timing and, as previously noted, the distinct role of FA compared with other nutrients [e.g., iron (8)].
Our study adds to the growing literature indicating that closely spaced pregnancies are at increased susceptibility to adverse outcomes. Short intervals were associated with a trend to increased risks for certain malformations, notably in the absence of FA supplement use. These associations do not appear to be explained by social or reproductive factors, including pregnancy intention. As seen in other studies (70, 71), supplement use occurred less often among unintended pregnancies. This finding reinforces the current recommendation that all women of childbearing potential should consume 400 µg of FA daily (68), which can be achieved with regular intake of most multivitamins. Replication of our findings is needed. To provide greater insight into the etiology and generalizability, future research should include larger sample sizes; data to evaluate correlated nutrient intake and absorption, diet quality, breastfeeding, and interpregnancy BMI; and be performed in countries without FA fortification. Such evidence, if found, could suggest that defect risks and/or other adverse outcomes associated with short birth spacing may be lessened by support from multivitamins and/or supplemental FA.
Acknowledgments
This study represents a secondary use of the data, which were collected prior to the conception of the specific research question examined in this study. We thank Natalie Archer for her replication of the case–control counts and exposure and covariate distributions of this analysis, in accordance with the NBDPS/BD-STEPS data replication policy. We also thank the mothers who participated in this study and the clinical coordinators, without whom this research would not be possible.
The authors’ responsibilities were as follows—JMP: analyzed the data and wrote the initial draft of the manuscript; MMY, KDG, MTA, and MMW: provided critical review and edits to the manuscript; and all authors: formulated the initial study question and analytic plan and read and approved the final manuscript. The authors report no conflicts of interest.
Notes
This work was supported through CDC cooperative agreements under PA 96043, PA 02081, FOA DD09-001, FOA DD13-003, and NOFO DD18-001 to the Centers for Birth Defects Research and Prevention participating in the National Birth Defects Prevention Study and/or the Birth Defects Study To Evaluate Pregnancy exposureS. In addition, this work was supported by grant DK56350 from the Nutrition Epidemiology Core of the University of North Carolina Clinical Nutrition Research Center. KDG was supported in part by NIH grant T32HD052458. The study sponsors were not directly involved in the design, analysis, or summary of findings.
Abbreviations: DFE, dietary folate equivalents; d-TGA, dextro-rotated transposition of the great arteries; FA, folic acid; HLHS, hypoplastic left heart syndrome; NBDPS, National Birth Defects Prevention Study; NTD, neural tube defect; PVS, pulmonary valve stenosis; VSD, ventricular septal defect.
Contributor Information
Julie M Petersen, Department of Epidemiology, Boston University School of Public Health, Boston, MA USA.
Mahsa M Yazdy, Center for Birth Defects Research and Prevention, Massachusetts Department of Public Health, Boston, MA USA.
Kelly D Getz, Center for Pediatric Clinical Effectiveness, The Children's Hospital of Philadelphia, Philadelphia, PA USA; Departments of Biostatistics, Epidemiology and Informatics, and Pediatrics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Marlene T Anderka, Center for Birth Defects Research and Prevention, Massachusetts Department of Public Health, Boston, MA USA.
Martha M Werler, Department of Epidemiology, Boston University School of Public Health, Boston, MA USA.
Data Availability
Data described in the manuscript, code book, and analytic code may be made available upon request. The process for accessing the data used in this study is described at https://www.cdc.gov/ncbddd/birthdefects/nbdps-public-access-procedures.html.
References
- 1. Conde-Agudelo A, Rosas-Bermudez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295(15):1809–23. [DOI] [PubMed] [Google Scholar]
- 2. Ahrens KA, Nelson H, Stidd RL, Moskosky S, Hutcheon JA. Short interpregnancy intervals and adverse perinatal outcomes in high-resource settings: an updated systematic review. Paediatr Perinat Epidemiol. 2019;33(1):O25–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen I, Jhangri GS, Chandra S. Relationship between interpregnancy interval and congenital anomalies. Am J Obstet Gynecol. 2014;210(6):564.e1–e8. [DOI] [PubMed] [Google Scholar]
- 4. Ekin A, Gezer C, Taner CE, Ozeren M, Mat E, Solmaz U. Impact of interpregnancy interval on the subsequent risk of adverse perinatal outcomes. J Obstet Gynaecol Res. 2015;41(11):1744–51. [DOI] [PubMed] [Google Scholar]
- 5. Getz KD, Anderka MT, Werler MM, Case AP. Short interpregnancy interval and gastroschisis risk in the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol. 2012;94(9):714–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kwon S, Lazo-Escalante M, Villaran MV, Li CI. Relationship between interpregnancy interval and birth defects in Washington state. J Perinatol. 2012;32(1):45–50. [DOI] [PubMed] [Google Scholar]
- 7. Todoroff K, Shaw GM. Prior spontaneous abortion, prior elective termination, interpregnancy interval, and risk of neural tube defects. Am J Epidemiol. 2000;151(5):505–11. [DOI] [PubMed] [Google Scholar]
- 8. King JC. The risk of maternal nutritional depletion and poor outcomes increases in early or closely spaced pregnancies. J Nutr. 2003;133(5 Suppl 2):1732S–6S. [DOI] [PubMed] [Google Scholar]
- 9. Conde-Agudelo A, Rosas-Bermudez A, Castano F, Norton MH. Effects of birth spacing on maternal, perinatal, infant, and child health: a systematic review of causal mechanisms. Stud Fam Plann. 2012;43(2):93–114. [DOI] [PubMed] [Google Scholar]
- 10. Smits LJ, Essed GG. Short interpregnancy intervals and unfavourable pregnancy outcome: role of folate depletion. Lancet. 2001;358(9298):2074–7. [DOI] [PubMed] [Google Scholar]
- 11. Winkvist A, Habicht JP, Rasmussen KM. Linking maternal and infant benefits of a nutritional supplement during pregnancy and lactation. Am J Clin Nutr. 1998;68(3):656–61. [DOI] [PubMed] [Google Scholar]
- 12. van Eijsden M, Smits LJ, van der Wal MF, Bonsel GJ.. Association between short interpregnancy intervals and term birth weight: the role of folate depletion. Am J Clin Nutr. 2008;88(1):147–53. [DOI] [PubMed] [Google Scholar]
- 13. Fox JT, Stover PJ.. Folate-mediated one-carbon metabolism. Vitam Horm. 2008;79:1–44. [DOI] [PubMed] [Google Scholar]
- 14. James P, Sajjadi S, Tomar AS, Saffari A, Fall CHD, Prentice AM, Shrestha S, Issarapu P, Yadav DK, Kaur Let al. Candidate genes linking maternal nutrient exposure to offspring health via DNA methylation: a review of existing evidence in humans with specific focus on one-carbon metabolism. Int J Epidemiol. 2018;47(6):1910–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet North Am Ed. 1991;338(8760):131–7. [PubMed] [Google Scholar]
- 16. Werler MM, Shapiro S, Mitchell AA. Periconceptional folic acid exposure and risk of occurrent neural tube defects. JAMA. 1993;269(10):1257–61. [PubMed] [Google Scholar]
- 17. Canfield MA, Collins JS, Botto LD, Williams LJ, Mai CT, Kirby RS, Pearson K, Devine O, Mulinare J; National Birth Defects Preventin Network . Changes in the birth prevalence of selected birth defects after grain fortification with folic acid in the United States: findings from a multi-state population-based study. Birth Defect Res A. 2005;73(10):679–89. [DOI] [PubMed] [Google Scholar]
- 18. Meyer RE, Brown AB. Folic acid and birth defects prevention: a public health success story. N C Med J. 2004;65(3):157–8. [PubMed] [Google Scholar]
- 19. Taruscio D, Carbone P, Granata O, Baldi F, Mantovani A. Folic acid and primary prevention of birth defects. Biofactors. 2011;37(4):280–4. [DOI] [PubMed] [Google Scholar]
- 20. Zhang Q, Wang Y, Xin X, Zhang Y, Liu D, Peng Z, He Y, Xu J, Ma X. Effect of folic acid supplementation on preterm delivery and small for gestational age births: a systematic review and meta-analysis. Reprod Toxicol. 2017;67:35–41. [DOI] [PubMed] [Google Scholar]
- 21. Yoon PW, Rasmussen SA, Lynberg MC, Moore CA, Anderka M, Carmichael SL, Druschel C, Hobbs CA, Romitti PA, Langlois PHet al. The National Birth Defects Prevention Study. Public Health Rep. 2001;116(Suppl 1):32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–7. [DOI] [PubMed] [Google Scholar]
- 23. Mburia-Mwalili A, Yang W. Interpregnancy interval and birth defects. Birth Defects Res A Clin Mol Teratol. 2015;103(11):904–12. [DOI] [PubMed] [Google Scholar]
- 24. DeSalvo K, Stamm CA, Borgelt LM. Evaluation of reported contents in prescription and over-the-counter prenatal multivitamins. J Am Pharm Assoc. 2018;58(3):258. [DOI] [PubMed] [Google Scholar]
- 25. Saldanha LG, Dwyer JT, Andrews KW, Brown LL, Costello RB, Ershow AG, Hardy CJ, Pehrsson PR. Is nutrient content and other label information for prescription prenatal supplements different from nonprescription products?. J Acad Nutr Diet. 2017;117(9):1429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Willett WC, Reynolds RD, Cottrell-Hoehner S, Sampson L, Browne ML. Validation of a semi-quantitative food frequency questionnaire: comparison with a 1-year diet record. J Am Diet Assoc. 1987;87(1):43–7. [PubMed] [Google Scholar]
- 27. U.S. Department of Agriculture, Agricultural Research Service , USDA National Nutrient Database for Standard Reference. (release 27, August 2014, revised May 2015). [Internet].Methods and Application of Food Composition Laboratory. Available from: http://www.ars.usda.gov/nea/bhnrc/mafcl. [Google Scholar]
- 28. Institute of Medicine.. Dietary Reference Intakes (DRIs): recommended dietary allowances and adequate intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. [Internet]Washington (DC): Institute of Medicine, National Academies, Food and Nutrition Board. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes; 1998. Available from: https://www.nal.usda.gov/sites/default/files/fnic_uploads/recommended_intakes_individuals.pdf. [Google Scholar]
- 29. U.S. Department of Agriculture . Agricultural Research Service. [Internet]. FoodData Central; 2019. Available from: fdc.nal.usda.gov. [Google Scholar]
- 30. Carmel R. Folic acid. In: Ross AC, Caballero BH, Cousins RJ, Tucker KL, Ziegler, TR, editors. Modern nutrition in health and disease. Baltimore (MD): Lippincott Williams & Wilkins; 2005. 11th. ed., p. 470–81. [Google Scholar]
- 31. Willett WC. Issues in analysis and presentation of dietary data. Nutritional epidemiology. New York: Oxford University Press; 2012. [Google Scholar]
- 32. Moskal A, Pisa PT, Ferrari P, Byrnes G, Freisling H, Boutron-Ruault MC, Cadeau C, Nailler L, Wendt A, Kühn Tet al. Nutrient patterns and their food sources in an International Study Setting: report from the EPIC study. PLoS One. 2014;9(6):e98647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kleinbaum DG, Kupper L, Morgenstern H. Epidemiologic research: principles and quantitative methods. New York: Nostrand Reinhold; 1982. [Google Scholar]
- 34. Kangatharan C, Labram S, Bhattacharya S. Interpregnancy interval following miscarriage and adverse pregnancy outcomes: systematic review and meta-analysis. Hum Reprod Update. 2017;23(2):221–31. [DOI] [PubMed] [Google Scholar]
- 35. Hutcheon JA, Moskosky S, Ananth CV, Basso O, Briss PA, Ferre CD, Frederiksen BN, Harper S, Hernández-Díaz S, Hirai AHet al. Good practices for the design, analysis, and interpretation of observational studies on birth spacing and perinatal health outcomes. Paediatr Perinat Epidemiol. 2019;33(1):O15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Regan AK, Gissler M, Magnus MC, Haberg SE, Ball S, Malacova E, Nassar N, Leonard H, Pereira G. Association between interpregnancy interval and adverse birth outcomes in women with a previous stillbirth: an international cohort study. Lancet. 2019;393(10180):1527–35. [DOI] [PubMed] [Google Scholar]
- 37. Lawn JE, Blencowe H, Waiswa P, Amouzou A, Mathers C, Hogan D, Flenady V, Frøen JF, Qureshi ZU, Calderwood Cet al. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet. 2016;387(10018):587–603. [DOI] [PubMed] [Google Scholar]
- 38. Radzik D, Davignon A, van Doesburg N, Fournier A, Marchand T, Ducharme G. Predictive factors for spontaneous closure of atrial septal defects diagnosed in the first 3 months of life. J Am Coll Cardiol. 1993;22(3):851–3. [DOI] [PubMed] [Google Scholar]
- 39. Botto LD, Lin AE, Riehle-Colarusso T, Malik S, Correa A, National Birth Defects Prevention Study . Seeking causes: classifying and evaluating congenital heart defects in etiologic studies. Birth Defect Res A. 2007;79(10):714–27. [DOI] [PubMed] [Google Scholar]
- 40. Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80(1):27–38. [Google Scholar]
- 41. Bonferroni CE. Teoria statistica delle classi e calcolo delle probabilità. Pubblicazioni del R Istituto Superiore di Scienze Economiche e Commerciali di Firenze; 1936. [Google Scholar]
- 42. Dunn OJ. Multiple comparisons among means. J Am Statist Assoc. 1961;56(293):52–64. [Google Scholar]
- 43. Wasserstein RL, Lazar NA. The ASA statement on p-values: context, process, and purpose. The American Statistician. 2016;70(2):129–33. [Google Scholar]
- 44. Hill AB. The environment and disease: association or causation?. J R Soc Med. 2015;108(1):32–7.. (Original work published 1965) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Greenland S, Senn SJ, Rothman KJ, Carlin JB, Poole C, Goodman SN, Altman DG. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol. 2016;31(4):337–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. SAS Institute , SAS/STAT, version 9.4 for Windows. Cary (NC): SAS Institute. [Google Scholar]
- 47. Nilsen RM, Mastroiacovo P, Gunnes N, Alsaker ER, Bjorke-Monsen AL, Eussen SJ, Haugen M, Johannessen A, Meltzer HM, Stoltenberg C. Folic acid supplementation and interpregnancy interval. Paediatr Perinat Epidemiol. 2014;28(3):270–4. [DOI] [PubMed] [Google Scholar]
- 48. De-Regil LM, Pena-Rosas JP, Fernandez-Gaxiola AC, Rayco-Solon P. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev. 2015;; 2015(12):CD007950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Godwin KA, Sibbald B, Bedard T, Kuzeljevic B, Lowry RB, Arbour L. Changes in frequencies of select congenital anomalies since the onset of folic acid fortification in a Canadian birth defect registry. Can J Public Health. 2008;99(4):271–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Paranjothy S, Broughton H, Evans A, Huddart S, Drayton M, Jefferson R, Rankin J, Draper E, Cameron A, Palmer SR. The role of maternal nutrition in the aetiology of gastroschisis: an incident case–control study. Int J Epidemiol. 2012;41(4):1141–52. [DOI] [PubMed] [Google Scholar]
- 51. Yang W, Carmichael SL, Shaw GM. Folic acid fortification and prevalences of neural tube defects, orofacial clefts, and gastroschisis in California, 1989 to 2010. Birth Defects Res A Clin Mol Teratol. 2016;106(12):1032–41. [DOI] [PubMed] [Google Scholar]
- 52. Wilson RD, Genetics Committee . Pre-conception folic acid and multivitamin supplementation for the primary and secondary prevention of neural tube defects and other folic acid–sensitive congenital anomalies. J Obstet Gynaecol Can. 2015;37(6):534–52. [DOI] [PubMed] [Google Scholar]
- 53. Petersen JM, Parker SE, Crider KS, Tinker SC, Mitchell AA, Werler MM. One-carbon cofactor intake and risk of neural tube defects among women who meet folic acid recommendations: a multicenter case–control study. Am J Epidemiol. 2019;188(6):1136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Carmichael SL, Yang W, Correa A, Olney RS, Shaw GM, National Birth Defects Prevention Study . Hypospadias and intake of nutrients related to one-carbon metabolism. J Urol. 2009;181(1):315–21.; discussion 21. [DOI] [PubMed] [Google Scholar]
- 55. Carmichael SL, Rasmussen SA, Lammer EJ, Ma C, Shaw GM, National Birth Defects Prevention Study . Craniosynostosis and nutrient intake during pregnancy. Birth Defects Res A Clin Mol Teratol. 2010;88(12):1032–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Carmichael SL, Yang W, Gilboa S, Ailes E, Correa A, Botto LD, Feldkamp ML, Shaw GM, National Birth Defects Prevention Study . Elevated body mass index and decreased diet quality among women and risk of birth defects in their offspring. Birth Defects Res A Clin Mol Teratol. 2016;106(3):164–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. de Kort CA, Nieuwenhuijsen MJ, Mendez MA. Relationship between maternal dietary patterns and hypospadias. Paediatr Perinat Epidemiol. 2011;25(3):255–64. [DOI] [PubMed] [Google Scholar]
- 58. Dietrich M, Brown CJ, Block G. The effect of folate fortification of cereal-grain products on blood folate status, dietary folate intake, and dietary folate sources among adult non-supplement users in the United States. J Am Coll Nutr. 2005;24(4):266–74. [DOI] [PubMed] [Google Scholar]
- 59. Quinlivan EP, Gregory JF 3rd. Effect of food fortification on folic acid intake in the United States. Am J Clin Nutr. 2003;77(1):221–5. [DOI] [PubMed] [Google Scholar]
- 60. Green RF, Olney RS, Reefhuis J, Botto LD, Romitti PA, National Birth Defects Prevention Study . Maternal reports of family history from the National Birth Defects Prevention Study, 1997–2001. Genet Med. 2008;10(1):37–45. [DOI] [PubMed] [Google Scholar]
- 61. van Gelder MM, Reefhuis J, Caton AR, Werler MM, Druschel CM, Roeleveld N, National Birth Defects Prevention Study . Maternal periconceptional illicit drug use and the risk of congenital malformations. Epidemiology. 2009;20(1):60–6. [DOI] [PubMed] [Google Scholar]
- 62. Werler MM, Ahrens KA, Bosco JL, Mitchell AA, Anderka MT, Gilboa SM, National Birth Defects Prevention Study . Use of antiepileptic medications in pregnancy in relation to risks of birth defects. Ann Epidemiol. 2011;21(11):842–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009;301(6):636–50. [DOI] [PubMed] [Google Scholar]
- 64. Bender W, Hirshberg A, Levine LD.. Interpregnancy body mass index changes: distribution and impact on adverse pregnancy outcomes in the subsequent pregnancy. Am J Perinatol. 2019;36(5):517–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ziauddeen N, Roderick PJ, Macklon NS, Alwan NA. The duration of the interpregnancy interval in multiparous women and maternal weight gain between pregnancies: findings from a UK population-based cohort. Sci Rep. 2019;9(1):9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Persoskie A, Hennessy E, Nelson WL. US consumers’ understanding of nutrition labels in 2013: the importance of health literacy. Prev Chronic Dis. 2017;14:E86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Friedenreich CM, Howe GR, Miller AB.. Recall bias in the association of micronutrient intake and breast cancer. J Clin Epidemiol. 1993;46(9):1009–17. [DOI] [PubMed] [Google Scholar]
- 68. CDC.. Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR Recomm Rep. 1992;41(RR-14):1–7. [PubMed] [Google Scholar]
- 69. Drews CD, Greeland S. The impact of differential recall on the results of case–control studies. Int J Epidemiol. 1990;19(4):1107–12. [DOI] [PubMed] [Google Scholar]
- 70. Dott M, Rasmussen SA, Hogue CJ, Reefhuis J, National Birth Defects Prevention Study . Association between pregnancy intention and reproductive-health related behaviors before and after pregnancy recognition, National Birth Defects Prevention Study, 1997–2002. Matern Child Health J. 2010;14(3):373–81. [DOI] [PubMed] [Google Scholar]
- 71. Rosenberg KD, Gelow JM, Sandoval AP.. Pregnancy intendedness and the use of periconceptional folic acid. Pediatrics. 2003;111(5 Pt 2):1142–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data described in the manuscript, code book, and analytic code may be made available upon request. The process for accessing the data used in this study is described at https://www.cdc.gov/ncbddd/birthdefects/nbdps-public-access-procedures.html.