Abstract
The ability to learn to use new words is thought to depend on the integrity of the left dorsal temporo-frontal speech processing pathway. We tested this assumption in a chronic aphasic individual (AA) with an extensive left temporal lesion using a new-word learning paradigm. She exhibited severe phonological problems and Magnetic Resonance Imaging (MRI) suggested a complete disconnection of this left-sided white-matter pathway comprising the arcuate fasciculus (AF). Diffusion imaging tractography confirmed the disconnection of the direct segment and the posterior indirect segment of her left AF, essential components of the left dorsal speech processing pathway. Despite her left-hemispheric damage and moderate aphasia, AA learned to name and maintain the novel words in her active vocabulary on par with healthy controls up to 6 months after learning. This exceeds previous demonstrations of word learning ability in aphasia. Interestingly, AA’s preserved word learning ability was modality-specific as it was observed exclusively for written words. Functional magnetic resonance imaging (fMRI) revealed that in contrast to normals, AA showed a significantly right-lateralized activation pattern in the temporal and parietal regions when engaged in reading. Moreover, learning of visually presented novel word–picture pairs also activated the right temporal lobe in AA. Both AA and the controls showed increased activation during learning of novel versus familiar word–picture pairs in the hippocampus, an area critical for associative learning. AA’s structural and functional imaging results suggest that in a literate person, a right-hemispheric network can provide an effective alternative route for learning of novel active vocabulary. Importantly, AA’s previously undetected word learning ability translated directly into therapy, as she could use written input also to successfully re-learn and maintain familiar words that she had lost due to her left hemisphere lesion.
Keywords: Aphasia, Learning, Vocabulary, Anomia, Aphasia treatment
1. Introduction
Previous studies on the neural substrates of learning have delineated a general framework of complementary hippocampal and cortical systems in binding and consolidating memories for novel contents (McClelland, McNaughton, & O’Reilly, 1995) such as new word–referent pairs (Davis & Gaskell, 2009), respectively. Moreover, functional neuroimaging evidence indicates that the left dorsal temporo-frontal pathway involved in word production is also crucial for learning new active vocabulary (Hickok & Poeppel, 2007; Rodríguez-Fornells, Cunillera, Mestres-Missé, & de Diego-Balaguer, 2009). The available evidence from aphasia is in line with these views. Meinzer et al. (2010) showed that the extent of damage to the left hippocampus and surrounding tissue predicts language therapy outcomes in aphasia. Moreover, the few studies on acquisition of new active vocabulary in aphasia indicate that chronic lesions in the left cortical language areas, even in mild aphasia, can severely hamper acquisition and maintenance of novel words (Grossman & Carey, 1987; Gupta, Martin, Abbs, Schwartz, & Lipinski, 2006; McGrane, 2006; Tuomiranta et al., 2011; Tuomiranta, Rautakoski, Rinne, Martin, & Laine, 2012).
In the present paper, we extend this current knowledge on the neurocognition of word learning by presenting behavioral and structural–functional neuroimaging data from a case of aphasia (AA) with a disconnected left dorsal temporo-frontal pathway who nevertheless learned and maintained new active vocabulary on par with healthy controls. The imaging data were expected to shed light on the alternative neural pathways that our patient is using to enable her remarkable learning and maintenance of novel words. We employed a well-studied new-word learning paradigm that involves learning of the names of ancient farming equipment (Laine & Salmelin, 2010). Learning was evaluated with spontaneous naming of the novel objects, a measure that is particularly demanding for individuals with aphasia who almost always suffer from anomia (Laine & Martin, 2006). We employed novel word learning rather than the more traditional approach of re-teaching premorbidly mastered words that have become inaccessible in aphasia, because we wanted to specifically target the word learning mechanisms that encode and store new word–referent associations. Re-teaching of familiar but inaccessible words for an aphasic individual is clinically of utmost importance but, in terms of basic research, makes it difficult to separate the involvement of word learning mechanisms from memory retrieval where access to lost words is re-gained through phonological or semantic cues given by the therapist.
Over the last four decades, different aspects of verbal learning in aphasia have been probed in experimental studies. Early studies quantified aphasic individuals’ ability to re-learn to produce familiar words (e.g., Sarno, Silverman, & Sands, 1970) or compared the learning rate and capacity of aphasic versus healthy participants with word list learning tasks (e.g., Tikofsky, 1971). More recently, the effects of short-term memory on verbal learning in aphasia have been a focus of inquiry (e.g., Freedman & Martin, 2001; Martin & Saffran, 1999). Several investigations have also added challenge to the verbal learning tasks through introducing partly novel materials (e.g., Breitenstein, Kamping, Jansen, Schomacher, & Knecht, 2004; Freed, Marshall, & Nippold, 1995; Marshall, Freed, Karow, 2001; Marshall, Neuburger, & Phillips, 1992). Of particular interest for the present paper are studies that have utilized a design where genuinely novel referents have been paired with genuinely novel names (Grossman & Carey, 1987; Gupta et al., 2006; Laganaro, Di Pietro, & Schnider, 2006; McGrane, 2006; Morrow, 2006; Tuomiranta et al., 2011, 2012). Looking at active vocabulary acquisition as measured by spoken naming, of these investigations one showed practically no learning (Gupta et al., 2006), one probed only passive vocabulary (Morrow, 2006), and one did not measure naming accuracy (Laganaro et al., 2006). Three investigations reported statistically significant short-term novel word learning that varied between aphasic participants (McGrane, 2006; Tuomiranta et al., 2011, 2012). Not surprisingly, in the latter two studies that included also healthy controls, the performance levels of the individuals with aphasia were impaired. Only two studies (Grossman & Carey, 1987; Tuomiranta et al., 2012) reported some long-term maintenance of novel referent-word pairs in aphasic participants, and in the latter study that included healthy controls, the long-term maintenance of the aphasic individuals was significantly impaired. In summary, the previous literature indicates that some individuals with aphasia are able to acquire at least some novel active vocabulary, even though their learning outcomes are impaired in relation to normal performance both in the short-term and long-term. Nevertheless, these findings inspired us to look further into the word learning abilities of individuals with aphasia and led to the discovery of the present case which, to our surprise, showed learning and maintenance of novel active vocabulary on par with healthy controls.
Current views on the neural substrates of language differentiate two major left-sided pathways: a dorsal stream (linking perisylvian language areas and inferior frontal regions) for sound-motor connections and a ventral stream (connecting temporal and prefrontal regions via the extreme capsule) for auditory comprehension (Hickok & Poeppel, 2007; Kümmerer et al., 2013; Parker et al., 2005; Saur et al., 2008, 2010; Ueno, Saito, Rogers, & Lambon Ralph, 2011). The lesion in our patient affected especially a major component of the dorsal pathway, namely the arcuate fasciculus (AF), a large left-lateralized (Catani et al., 2007; Nucifora, Verma, Melhem, Gur, & Gur, 2005) white-matter fiber bundle with three segments (Catani, Jones, & ffytche, 2005). In humans, the direct segment of the AF connects the posterior part of the superior and middle temporal regions (Wernicke’s territory) to the posterior frontal regions (Broca’s territory) (Catani et al., 2005; Fernández-Miranda et al., 2008). The AF has been linked to the transmission, manipulation, and articulation of phonological information, verbal working memory, and verbal repetition of speech (Catani & Mesulam, 2008; Hickok & Poeppel, 2007; Marchina et al., 2011; Rilling et al., 2008; Saur et al., 2008). AF lesions (Damasio & Damasio, 1980) as well as damage to the left supramarginal gyrus (SMG) and the temporo-parietal junction (Fridriksson et al., 2010) have been associated with conduction aphasia.
The indirect segment of the AF is divided into the anterior part, linking Broca’s territory and adjacent premotor areas with the inferior parietal cortex (Geschwind’s territory), and the posterior section, connecting the inferior parietal cortex with Wernicke’s territory (Catani et al., 2005; Fernández-Miranda et al., 2008). Crucially to the present investigation, as an interface between phonological input and articulatory representations, the left AF is considered to be necessary for learning to produce new words (Aboitiz, 2012; Hickok & Poeppel, 2007; Rodríguez-Fornells et al., 2009; Schultze, Vargha-Khadem, & Mishkin, 2012). This view predicts that learning of new active vocabulary becomes impaired if the left AF is disconnected. However, to our knowledge, the effects of left AF disconnection on the acquisition and long-term maintenance of novel words have not been examined before. Given the intactness of our patient’s right hemisphere including the right AF, it is of interest to see to what extent right-hemispheric structures could contribute to her word learning. Interestingly, recent functional neuroimaging results indicate considerable variability in normal individuals’ engagement of right-hemispheric regions e.g., in reading (Seghier, Lee, Schofield, Ellis, & Price, 2008).
2. Methods
2.1. Participants
Participant AA was a 60-year-old monolingual Finnish-speaking female with chronic aphasia caused by a subarachnoid hemorrhage and subsequent infarction 2 years and 9 months before this experiment started. AA had surgery for a large, ruptured aneurysm located in the first bifurcation of the left middle cerebral artery, and the aneurysm was ligated [a surgical clip remained in her brain, causing some local distortion in the Magnetic Resonance Imaging (MRI) signal from her left inferior frontal regions]. AA’s extensive left temporal lobe lesion encompassed the left superior and middle temporal gyri, but spared the left inferior and medial temporal areas including the hippocampus and the parahippocampal gyrus (see Fig. 1(A) for a structural MRI image from the chronic stage). AA was strongly right-handed, and all her close relatives had also been right-handed. AA had 15 years of formal education and had worked as a nurse.
Fig. 1 –

Depiction of the left-hemispheric structural lesion and intact right hemisphere connectivity in AA. (A) Sagittal (top row), coronal (middle row) and axial (bottom) views in native space of AA’s T1 structural image showing her extensive lesion in the left temporal lobe. (B) AA’s left-hemisphere parieto-frontal tracts as reconstructed using probabilistic tractography. (C) AA’s intact right-sided arcuate fasciculus neural pathway as reconstructed using deterministic tractography (Red color – Arcuate Fasciculus direct segment, Yellow – Arcuate Fasciculus posterior segment, Green – Arcuate Fasciculus anterior segment).
The language deficits in AA were in line with her lesion locus. She had been globally aphasic at least up to 3 months post-onset. AA received regular (one to four sessions/week) speech and language therapy from about 1 week to 3 months post-onset. After that, therapy sessions became more irregular and ended at 18 months post-onset. The present testing took place 33–50 months post-onset, at which point AA suffered from moderate aphasia. She exhibited fluent speech, severe anomia, very poor word repetition with some semantic errors, and an inability to repeat pseudowords (see below for the details of the neuropsychological evaluation). These symptoms met the criteria for deep dysphasia. In contrast, she could read single words and pseudowords aloud quite well, attesting to functional orthography-to-phonology conversion that has been related to medial-inferior occipital and ventral occipito-temporal brain regions (Price & Devlin, 2011).
For behavioral Experiment 1 on word learning, we recruited also five healthy, right-handed, monolingual Finnish-speaking controls (59–64 years old, 1 male) with an educational background comparable to that of AA. For brain imaging, we recruited seven healthy, right-handed, monolingual Finnish-speaking female controls (56–64 years old) with different educational backgrounds. The exclusion criteria for both control groups were a history of neurological or psychiatric illness, developmental learning and language impairments, including dyslexia. AA and both control groups were assessed by the same researcher. All participants received information about the study in written and spoken form and gave their written consent. The study was approved by the Ethics Committee of the Hospital District of Southwest Finland.
2.2. Neuropsychological examination
For AA, the background language tests included the Finnish versions of the Boston Diagnostic Aphasia Examination (BDAE) (Laine, Niemi, Koivuselkä-Sallinen, & Tuomainen, 1997), the Boston Naming Test (BNT) (Laine, Koivuselkä-Sallinen, Hänninen, & Niemi, 1997), the Temple Assessment of Language and Short-term Memory in Aphasia (TALSA) (Tuomiranta, Laine, & Martin, 2009), and the 36-item Token Test (De Renzi & Faglioni, 1978). In addition, several experimental tasks (Karttunen & Renvall, 2005; Laine, Kujala, Niemi, & Uusipaikka, 1992, 1998; Renvall, Laine, Laakso, & Martin, 2003; Renvall & Tuomiranta, 2010) were administered. Lexical-semantic processing was measured with odd-one-out tasks using pictures and words (Laine et al., 1992), picture sorting (Laine et al., 1992), word–picture matching (Laine et al., 1992), and category-specific word–picture matching (Laine et al., 1998). Furthermore, possible word length and frequency effects on repetition and oral reading of words and pseudowords were probed (Renvall et al., 2003), as well as the effects of imageability and length on word repetition (Renvall & Tuomiranta, 2010). Lexical decision of words varied for imageability and frequency (Karttunen & Renvall, 2005) was measured both auditorily and orthographically. The Edinburgh Inventory (Oldfield, 1971) was administered to specify handedness, and the Corsi Block Tapping Task (De Renzi & Nichelli, 1975) to measure visuospatial span.
Participant AA had moderate aphasia (severity rating 2 in the BDAE Finnish version; Laine, Niemi, et al., 1997). Details of the neuropsychological examination are presented in Table 1. Apart from anomia and word–pseudoword repetition problems, also lexical-semantics (Laine et al., 1992, 1998; Tuomiranta et al., 2009) were mildly impaired. AA’s speech output was fluent and contained phonological and morphosyntactic errors. Predominant naming errors were phonological paraphasias and semantic errors. Auditory comprehension was impaired with both single words and sentences (De Renzi & Faglioni, 1978; Laine, Niemi, et al., 1997; Tuomiranta et al., 2009). Her auditory-verbal spans were minimal (1–2 items) and showed no effect of response mode (oral repetition or pointing) (Tuomiranta et al., 2009) while nonverbal spatial span (De Renzi & Nichelli, 1975) was clearly superior (5 items). AA’s lexical decision ability was somewhat stronger for written than spoken stimuli (Karttunen & Renvall, 2005). Reading comprehension was slightly impaired at the single-word level and clearly compromised when reading short text passages (Laine, Niemi, et al., 1997). Her writing was dysgraphic and dysgrammatic (Laine, Niemi, et al., 1997). AA showed no post-stroke hemiplegia, neglect, or hemianopia.
Table 1.
Language and cognitive test results of AA. mths, months post-onset
| Task | Score | Post-onset |
|---|---|---|
| Naming (Boston Naming Test) | 19 of 60 | 33 mths |
| Word discrimination (BDAE) | 58.5 of 72 (40th percentile) | 33 mths |
| Body-part identification (BDAE) | 12 of 20 (20th percentile) | 33 mths |
| Commands (BDAE) | 9 of 15 (40th percentile) | 33 mths |
| Complex ideational material (BDAE) | 5 of 12 (40th percentile) | 33 mths |
| Word–picture matching (reading/BDAE) | 9 of 10 (50th percentile) | 33 mths |
| Reading comprehension of sentences and paragraphs (BDAE) | 6 of 10 (60th percentile) | 33 mths |
| Written confrontation naming (BDAE) | 6 of 10 (60th percentile) | 33 mths |
| Digit span: response by repetition/pointing | 1/1 | 33 mths |
| Word span: response by repetition/pointing | 2/2 | 33 mths |
| Visual spatial span ISO (Corsi Block Tapping Task) | 5 | 33 mths |
| Semantic odd-one-out (pictures) | 7 of 12 | 38 mths |
| Semantic odd-one-out (words) | 9 of 12 | 38 mths |
| Picture sorting | 50 of 50 | 38 mths |
| Word–picture matching | 20 of 20 | 38 mths |
| Category-specific word–picture matching | 45 of 48 | 38 mths |
| Oral reading of words/pseudowords | 89 of 90/73 of 90 | 39 mths |
| Repetition of words/pseudowords | 47 of 90*/5 of 90 | 39 mths |
| Repetition of words varied for imageability high/low | 21 of 40/13 of 40 | 39 mths |
| The Token Test (auditorily) | 10 of 36 | 41 mths |
| The Token Test (reading) | 25 of 36 | 41 mths |
| Auditory lexical decision | 152 of 160 | 50 mths |
| Orthographic lexical decision | 156 of 160 | 50 mths |
word length effect with longer words more difficult to repeat than shorter ones; Yates’ corrected χ2 = 28.95, df = 2, p<.001; BDAE, Boston Diagnostic Aphasia Examination; ISO, in serial order.
The control participants (five in the behavioral study and seven in the neuroimaging study) completed a set of language and short-term memory tasks: confrontation naming (Laine, Koivuselkä-Sallinen, et al., 1997), narrative production (Laine, Niemi, et al., 1997), semantic fluency (Laine, Niemi, et al., 1997), digit and word spans (Tuomiranta et al., 2009), semantic odd-one-out decision (Laine et al., 1992), as well as a questionnaire to assure that their language performances were within normal limits.
2.3. Behavioral experiments
In the first behavioral experiment tapping Learning and long-term maintenance of novel word–picture pairs, AA and the five controls were trained in their native language Finnish to name 20 novel objects that carried equally unfamiliar names. At the time of the experiment, AA was 33–39 months post-onset. There were four training sessions with altogether 20 exposures per novel word. A pretest assured that the objects and their names were unfamiliar to all participants. Stimuli were presented auditorily and orthographically, and the participants were required to repeat the names (12 sec per item). The participants were requested not to train at home. Learning and maintenance were measured by picture naming accuracy (last response) at 1 day, 1 week, 4 weeks, 8 weeks and 6 months post-training. One-phoneme distortion was accepted as a correct response. The target items were black-and-white line-drawings of pieces of ancient farming equipment (Laine & Salmelin, 2010; for a sample item, see Fig. 2(A)) paired with their real names. The names (4–8 phonemes/graphemes, 2–3 syllables) are unfamiliar to modern-day Finnish-speakers.
Fig. 2 –
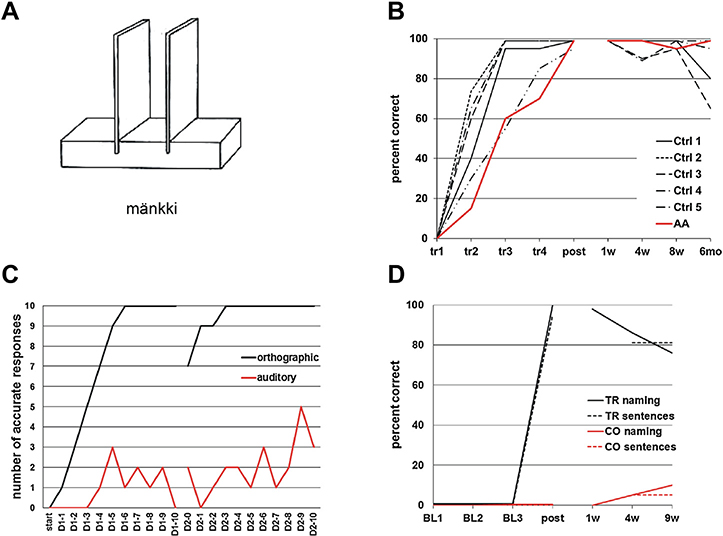
Behavioral results on word learning. (A) Experiment 1: Sample item of a novel word–object pair. Stimulus names were presented auditorily and orthographically, and the participants were required to repeat each name and to associate it with the new object. (B) Experiment 1: Individual word learning and maintenance curves for AA and the five control participants (Ctrl, control participant; tr, training session; post, post-training session; w, week; mo, month). (C) Experiment 2: Novel word learning curves for AA in the orthographic (10 words) and auditory (10 words) condition (D1, day 1; D2, day 2). (D) Experiment 3: AA’s learning curves for familiar words re-acquired through home training and measured by word retrieval during isolated naming and sentence production (TR, trained items; CO, untrained control items; BL, baseline session; post, post-training session; w, week).
In the second experiment that addressed Orthographic versus auditory short-term learning of novel word–picture pairs, we administered to AA two similar learning tasks in an ABBA design, either with auditory (A) or orthographic (B) word presentation. AA was instructed to read aloud (A) or repeat (B) the words paired with images. Each list was trained over two sessions during two consecutive days. The first training session had 10 training and 10 test (picture naming) cycles. The second training session was identical to the first except for a retention test (picture naming) performed at the session start. The measure for learning success was picture naming accuracy (last response, one-phoneme target word deviance allowed). The targets were 20 new items similar to the ones in Experiment 1 but with pseudoword names (4–5 phonemes/graphemes, 2 syllables). The pseudowords comprised of four equally complex subsets of 5 items (average bigram frequency balanced across the conditions), with 2 lists per condition (visual versus auditory). AA was 39 months post-onset during this experiment.
The third word learning task administered to AA 44–47 months post-onset was Re-learning of everyday object names. For this task, we selected 63 names of familiar items that she could not produce accurately in either three baseline picture naming tests or two baseline sentence production tests. AA chose the semantic categories and approved of the items to match her own interests and needs. These items were divided into 42 trained objects and 21 untrained control objects. The stimuli included 63 color photographs of household objects, plants, animals, music instruments and handicraft items. Another set of 63 color photographs of the same objects in action situations was created for sentence production. The target words included 5–14 phonemes/graphemes and 2–6 syllables. Sixty-nine percent of the targets were compounds.
The names were taught through orthography: AA was shown an object picture with its written name, and was prompted to read the name aloud. The computerized training program proceeded automatically and showed each stimulus for 12 sec. Using a laptop, AA performed self-administered home training and kept a training diary. She accomplished 36 training cycles (equal number of exposures per word) during 18 days. At this point, she was confident about being able to name the trained items. Post-training picture naming and sentence production tests were administered during the next two days. Learning maintenance was studied 1 week (picture naming accuracy) as well as 4 and 9 weeks (picture naming accuracy and target word production accuracy in sentence context) post-training.
2.4. Functional magnetic resonance imaging (fMRI) tasks
The fMRI experiments were administered when AA was 50 months post-onset. The first fMRI experiment was a Reading paradigm that probed reading pathways in AA and seven healthy females. The experiment included two baseline conditions: (i) viewing of meaningless false-font strings and (ii) rest with a fixation point. The active task included (iii) 48 familiar Finnish words and (iv) 48 pseudowords following the Finnish orthotactic rules. Words and pseudowords were matched by length (2–3 syllables) and presented in lowercase Courier font. The false-font strings created from the real font stimuli of each block resembled Courier font as closely as possible, retaining the number of characters/letters per string (Moore & Price, 1999). The participants were instructed to silently read words and pseudowords. Three runs were presented, both of which included 2 blocks of words, 2 blocks of pseudowords, 2 blocks of false-font strings, and 7 rest periods. During each block of 30 sec, 8 words, pseudowords, or false-font strings were presented (3.55–3.95 sec stimulus onset asynchrony, mean 3.75 sec). Rest period duration was 15 sec.
The second fMRI task was a Word learning paradigm. It was similar to that in the behavioral Experiment 1 apart from that only orthographic input was used. Due to AA’s severe phonological deficit, learning via auditory input was not attempted in the scanner. The aim of the task was to explore neural mechanisms in novel word learning during initial acquisition in AA and seven healthy females. The stimuli for this experiment consisted of 20 black-and-white line-drawings (10 novel pieces of ancient farming equipment, 10 familiar items that AA and the healthy control participants could name correctly), each shown together with its written name (2–3 syllables). Three conditions were employed in two runs: (i) Exposure to novel word–picture pairs (experimental condition) in which ten novel pictures, each coupled with a novel written name below, were presented. The participants were requested to look at each picture, covertly read the name, and try to learn the novel word–picture association. We devised six 30 sec blocks and presented three blocks in both of the two runs. In each block each novel word–picture pair was presented once (2.5 sec per item). For each block, the presentation order was randomized. The participants were exposed to each to-be-learned word–picture pair altogether six times during this experiment. (ii) Exposure to word–picture pairs familiar to the participants (control condition; e.g., picture of a flower – written word “flower”). The participants were requested to look at each picture and covertly read the name. As in the experimental condition, the ten items were presented six times across the six blocks. (iii) Rest condition with a fixation point (control condition; 20 sec, 14 blocks).
2.5. MRI scanning methods
MRI data were acquired using a Siemens Magnetom Verio 3T MRI-scanner at the Turku University Hospital. Structural images comprised a conventional high-resolution 3D T1 image [magnetization-prepared rapid-acquisition gradient echo sequence, 192 slice sagittal, repetition time (TR) = 2500 msec, echo time (TE) = 4.44 msec, inversion time = 1100 msec, flip angle = 7°, 1 mm thickness (isotropic voxels)] and a diffusion-weighted magnetic resonance imaging (DW-MRI) data set. DW-MRI data were collected using a diffusion tensor spin echo echoplanar imaging sequence [2-mm-thick slices, 70 axial slices with an isotropic voxel size of 2 mm, no gap, TR = 10,009 msec, TE = 95 msec, field of view = 256 mm, bandwidth = 1446 Hz, EPI Factor = 128, echo spacing = .78 msec]. Diffusion was measured along 30 noncollinear directions, with a single b-value of 1000 s/mm2. One run was acquired per slice and diffusion gradient direction. Functional images were collected by using a pulse-sequence sensitive to blood oxygenation level-dependent (BOLD) contrast [echo planar T2*-weighted gradient echo sequence, 32 axial slices with a 2.8 × 2.8 mm in-plane resolution, 3.5 mm slice thickness, .5 mm gap between slices, TR = 2000 msec, TE = 30 msec, flip angle = 80°]. Two MRI sessions were performed: the first one included a T1-weighted structural sequence and the resting-state fMRI paradigm, and the second one comprised of structural (T1 and DW-MRI) sequences and the two fMRI tasks. The stimuli were displayed using the Presentation software (Neurobehavioral Systems).
2.6. Diffusion tensor imaging (DTI) analysis
2.6.1. Preprocessing
Motion and eddy-current correction were performed using FMRIB’s Diffusion Toolbox (FDT), part of the FMRIB Software Library (FSL www.fmrib.ox.ac.uk/fsl/) (Smith et al., 2004; Woolrich et al., 2009). The gradient matrix was then rotated using a program kindly provided by Dr. Martin Kavec. Following this, the brain was scull stripped using FSL’s Brain Extraction Tool (Smith, 2002). Diffusion tensors were reconstructed using the linear least-squares method provided in Diffusion Toolkit (Ruopeng Wang, Van J. Wedeen, TrackVis. org, Martinos Center for Biomedical Imaging, Massachusetts General Hospital). The tensor was spectrally decomposed in order to obtain its eigenvalues and eigenvectors. The fiber direction was assumed to correspond to the principal eigenvector (the eigenvector with the largest eigenvalue). This vector was color-coded as described by Pajevic and Pierpaoli (1999) to help generate the color-coded fractional anisotropy (FA) map. FA maps were generated from the eigenvalues.
2.6.2. Deterministic tractography
Whole-brain tractography was also performed in the Diffusion Toolkit, using an interpolated streamlines algorithm with a maximum curvature threshold of 35° and a minimum FA threshold of .2. The right-sided AF was reconstructed as described in Catani et al. (2005, 2007) using TrackVis (Ruopeng Wang, Van J. Wedeen, TrackVis.org, Martinos Center for Biomedical Imaging, Massachusetts General Hospital). Briefly, on the color FA map, green (anterior-posterior) fibers lateral to the corona radiata correspond to the AF. The AF was separated into three sections on the basis of its cortical terminations using a region of interest (ROI) approach. One ROI was placed in the coronal plane, along the course of the AF near the frontal terminations, just before the path turns to meet the cortex (green on the color-coded map). The second ROI was placed in the axial plane, along the path of the AF in the posterior temporal lobe (blue on the color-coded FA map). The third ROI was placed on a sagittal slice, in the white-matter underlying the angular and supramarginal gyri. Fibers passing through the frontal and temporal ROIs belong to the direct segment, and are shown in red (see Figs. 1(C) and 3). The posterior segment (yellow) includes all fibers passing through the temporal and parietal ROIs, and the anterior segment (green) comprises those fibers that pass through the frontal and parietal ROIs. Note that the ROIs used here represent ‘obligatory passages’ for the tracts, and do not constrain the cortical projections of the tracts, which may vary from person to person (Thiebaut de Schotten et al., 2011). Although we were unable to virtually dissect a left-sided AF using deterministic tractography due to the patient’s lesion, a right-sided one was indeed dissected.
Fig. 3 –
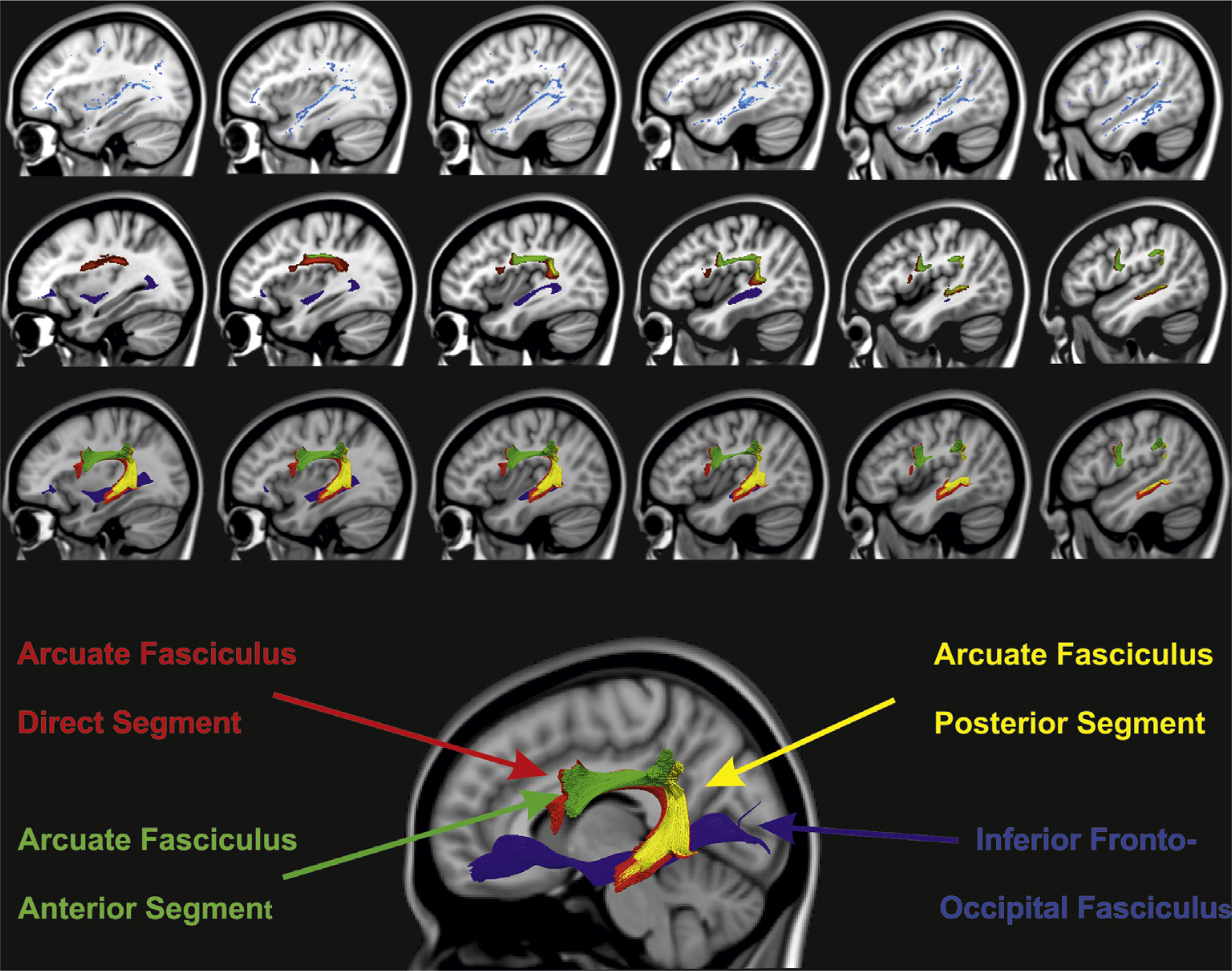
DTI comparison between AA and controls. The main image at the bottom shows the reconstruction of the left hemisphere inferior fronto-occipital fasciculus and arcuate fasciculus of the averaged control group data set: Blue color – Inferior Fronto-Occipital Fasciculus, Red – Arcuate Fasciculus direct segment, Yellow – Arcuate Fasciculus posterior segment, Green – Arcuate Fasciculus anterior segment. At the top row, depicted in blue are areas of the mean tract skeleton in which patient AA has lower fractional anisotropy than the control group (p <.01, using Crawford’s t-test). At the middle row, colored voxels represent the points at which the control group’s white-matter tracts pass through the slices shown in the top row. Finally at the bottom row, the same tracts that are shown in the middle row are now displayed in their entire 3-dimensional extent lateral (left) to the slice shown. The image shows that the IFOF and posterior and direct segments of the arcuate fasciculus ought to be affected in AA, which can be seen by comparing the top 2 rows.
One should note here a methodological limitation related to the deterministic tractography analysis. The use of standard single tensor model to estimate fiber orientation does not provide an accurate reconstruction of fiber orientation in regions of intravoxel fiber-crossing. Tractograms may be greatly improved by using advanced high-angular resolution diffusion imaging approaches which can capture multiple fibers directions within the same imaging voxel.
The exploratory approach to reconstruct the average tracts of the control group (Fig. 3) was performed as follows. First, each participant’s diffusion tensor field was separated into its six independent components. Each of the six tensor component maps were then non-linearly registered to 1 × 1 × 1 mm MNI-152 space using the warps created during TBSS processing (see below). This process was repeated for each participant and the mean of each component was created. These mean tensor components were then used to create the mean diffusion tensor at each voxel. Tractography was then performed on this mean data set using the same steps as above. The inferior fronto-occipital fasciculus (e.g., Forkel et al., 2012; Lawes et al., 2008; Powell et al., 2006) was reconstructed by placing ROIs in the external/extreme capsule and in the occipital lobe (Catani & Thiebaut de Schotten, 2008).
2.6.3. Probabilistic tractography
Probability distributions on fiber direction were calculated at each voxel using methods described by Behrens, Johansen-Berg, et al. (2003, Behrens, Woolrich, et al., 2003) using FSL. Fiber-crossing probabilistic tractography was then performed from voxels within seed masks. Statistically significant clusters of activation from the fMRI analysis were used as seed regions. In order to elucidate the connections from AA’s left hippocampal region activated during the word learning task, we linearly registered this area to the participant’s FA image, and then used this region as a seed for tractography. This same functional region was also non-linearly registered to the native DW-MRI space of each of the seven control participants using a series of linear and non-linear transformations implemented with FSL’s FLIRT and FNIRT (Jenkinson, Bannister, Brady, & Smith, 2002; www.fmrib.ox.ac.uk/fsl/). This region was then used as a seed region for tractography in each of the seven healthy control participant’s native DTI space. All probabilistic tractography was performed in the participant’s native MRI space, with linear transformations between that and the fMRI and T1-weighted image spaces used in order to move seed regions and reconstructed tracts between the three spaces (Jenkinson et al., 2002).
Due to our inability to reconstruct AA’s left parieto-frontal section of the AF using deterministic tractography, as an exploratory approach, we then used the left frontal region found to be activated by AA during the reading task as a seed region for probabilistic tractography. We then restricted the visualized tracts to those paths that entered a region in the left inferior parietal lobe that was functionally connected to the left frontal region during the same task.
2.6.4. Voxel-based analysis of FA
In order to compare patient AA’s FA values to those of the control group in a voxel-by-voxel manner we used Tract Based Spatial Statistics (TBSS).
The TBSS of FA images is implemented in the FSL software package (www.fmrib.ox.ac.uk/fsl, Version 5.1), and the analysis was carried out according to the standard procedure described in detail by Smith et al. (2006). In particular, both AA’s and each participant FA map were non-linearly registered to the participant found to have the most “typical” brain (i.e., the participant whose image minimizes the amount of warping required to register each other participants’ image to it) and then the entire aligned data set was linearly registered to 1 × 1 × 1 mm MNI-152 space (Smith et al., 2006). AA’s brain lesion was taken into account by including a binary mask of the lesion, which excludes the signal under the masked area in the calculation of the transformations used during normalization (Brett, Leff, Rorden, & Ashburner, 2001). All registrations were then visually inspected and no noticeable misalignment was seen. Next, a mean FA volume was created and used to generate an FA skeleton that corresponds to the center of the major white-matter tracts. Each participant’s FA data were mapped onto the skeleton by searching within that participant’s normalized FA volume perpendicular to the overlaid mean skeleton, and assigning the highest FA value within the search space to the relevant voxel in the skeleton.
Moreover, in order to illustrate the white-matter tracts that are likely to be affected by AA’s lesion, we reconstructed and overlaid the fiber tracts that should pass through the lesioned area from an average data set constructed from the control group’s data. In detail, after motion and eddy-current related distortions were corrected in the diffusion data and the individual FA maps were created, we affinely registered them to the MNI standard space 1 mm image individually, using FSL. Then, the individual transformations were applied to the diffusion corrected data set for each control participant, and the bvecs were rotated correspondingly. Finally, both the seven 4D normalized datasets and the corresponding bvecs were concatenated (giving 210 virtual diffusion ‘directions’ with seven b0 images) and were run through the normal tractography and dissection processing (see Fig. 3). This reconstructed average tract was not used in any statistical calculation and serves solely to better visualize the tracts that were likely affected by the lesioned area.
2.7. fMRI analysis
2.7.1. Preprocessing
Functional data were analyzed using standard procedures implemented in the Statistical Parameter Mapping software (SPM8, Wellcome Department of Imaging Neuroscience, University College, London, UK, www.fil.ion.ucl.ac.uk/spm/). The preprocessing included realignment, segmentation, normalization and smoothing. Unified segmentation (Ashburner & Friston, 2005) with medium regularization and cost function masking (CFM) was applied (Andersen, Rapcsak, & Beeson, 2010; Brett et al., 2001; Ripollés et al., 2012). CFM does not take into account the abnormal tissue when calculating the normalization transformations. For this purpose, a binary mask of the lesioned tissue was defined in the structural T1 image using the MRIcron software package (Rorden & Brett, 2000) (http://www.cabiatl.com/mricro/mricron/index.html).
2.7.2. Main contrasts of interest
In the first fMRI task, Reading, the (words + pseudowords) versus false-font contrast was explored. For this contrast a Lateralization Index (LI) was calculated. LI ranges between 1 for extreme left-lateralized activations and −1 for absolute right lateralization patterns. An LI greater than .2 means that at least 60% of the activation is on the left side, while an LI lower than −.2 means that at least 60% of the activation is on the right hemisphere. LIs between .2 and −.2 are considered bilateral. LIs were calculated using the LI-toolbox (Wilke & Lidzba, 2007) in its bootstrapped version.
The second fMRI task, Learning novel word–picture pairs, was used to explore the neural mechanisms of word learning during initial encoding. The main contrast reported in the present analysis is learning novel words versus rest condition. We were particularly interested in whether the hippocampal and parahippocampal regions showed increased activation when AA tried to learn new word–picture pairs, providing evidence of a “binding” process. Thus the contrast novel word–picture pairs versus rest was examined at a whole-brain level and also using ROI approach, focusing on bilateral hippocampal and parahippocampal regions. The toolbox Wfu pickatlas for SPM was applied to generate this ROI (Maldjian, Laurienti, Kraft, & Burdette, 2003).
2.7.3. Statistical analysis of fMRI data
The statistical evaluation of the fMRI data were based on a least-square estimation using the general linear model by modeling the different conditions with a box-car regressor waveform convolved with a canonical hemodynamic response function (Friston et al., 1998). Confounding factors from head movement (six parameters obtained from realignment) were also included in the model. Thus, a block-related design matrix was created including the conditions of interest (Learning novel word–picture pairs: novel word–picture pairs, familiar word–picture pairs, and rest; Reading (words + pseudowords): words, pseudowords, false-font, and rest). After model estimation, main effects for each condition were calculated. For the control group, main contrasts of interest were taken into a second-level analysis to obtain group activations.
Main fMRI results for both tasks for AA and the control group were reported thresholded at p < .001 uncorrected for multiple comparisons at the whole-brain level with a cluster extent of at least 10 contiguous voxels. For the sake of clarity, a less constrained threshold was used for the figures (specified in each figure legend). In order to compare AA’s activations, right AF volume and FA, and whole-brain skeleton FA with those of the control group, a modified version of the independent samples t-test, which accounts for the limited size of the control group, was used (Crawford & Garthwaite, 2004; Crawford & Howell, 1998).
3. Results
3.1. Structural DTI results
Using a deterministic fiber tracking algorithm tractography in order to examine AF connections, we found that AA had all three sections intact in her right hemisphere (see Fig. 1(C)) but, the direct segment of the AF and the posterior part of the indirect segment were disconnected in AA’s left hemisphere. Although we were unable to reconstruct the left-sided AF using deterministic tractography, likely due to a loss in FA following the infarct, we were able to visualize left frontoparietal pathways corresponding to the anterior section of the left AF using probabilistic tractography. Hence, the anterior part of the indirect segment of the left AF was likely preserved (Fig. 1(B)). However, this statement has to be interpreted with caution because both the presence of disruptions in anatomical connections or axonal degeneration in the lesioned region and the potential artifacts produced by the implanted clip, may reduce the strength of the evidence for the affected track.
A voxel-based (using TBSS) FA comparison of white-matter integrity between AA and a group of seven healthy control participants indicated a significant reduction in FA in left superior and middle temporal, inferior parietal and inferior frontal gyri in AA (see Fig. 3). Visual comparison with tracts from the average control dataset (Fig. 3) implicated damage to the left AF (dorsal system) and tracts passing through the external/extreme capsule (ventral system including the inferior fronto-occipital fasciculus – IFOF) (Saur et al., 2008). However, the lower FA in the inferior frontal and external/extreme capsule regions may not be reliable due to a metallic clip affecting signal in these areas locally (see Fig. 1(A)). Statistical comparison of the right-sided AF between AA and controls revealed no significant differences (all p > .2) in FA or tract volume (see Table 2).
Table 2.
FA and volume values for direct and indirect (anterior and posterior) segments of the arcuate fasciculus (AF) reconstructed using deterministic tractography in healthy controls and AA. FA, Fractional Anisotropy; Vol, volume of tract; –, failure in reconstruction of the tract using deterministic tractography.
| Participant | Left direct |
Left anterior |
Left posterior |
Right direct |
Right anterior |
Right posterior |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vol | FA | Vol | FA | Vol | FA | Vol | FA | Vol | FA | Vol | FA | |
| Control 1 | 6.58 | .49 | 7.07 | .46 | 4.21 | .49 | 6.16 | .50 | 2.42 | .43 | 2.99 | .43 |
| Control 2 | 5.62 | .45 | 3.98 | .43 | 4.91 | .45 | 4.88 | .50 | 4.54 | .44 | 3.98 | .47 |
| Control 3 | 5.78 | .55 | 4.22 | .51 | .57 | .51 | – | – | 6.46 | .48 | 1.82 | .55 |
| Control 4 | 9.12 | .50 | 4.78 | .50 | .57 | .45 | 5.05 | .54 | 5.46 | .47 | 2.87 | .47 |
| Control 5 | 3.02 | .51 | 4.29 | .49 | 11.4 | .52 | 5.58 | .49 | 1.57 | .42 | .46 | .42 |
| Control 6 | 8.25 | .50 | 6.48 | .46 | 1.95 | .46 | 7.34 | .54 | 5.75 | .46 | 3.37 | .46 |
| Control 7 | 8.58 | .51 | 1.24 | .48 | 3.42 | .51 | 6.81 | .52 | 2.92 | .47 | 1.98 | .47 |
| AA | – | – | – | – | – | – | 6.35 | .47 | 4.78 | .46 | 1.14 | .45 |
3.2. Behavioral word learning performance
To test the claim that the left dorsal speech processing pathway is crucial in word learning (Hickok & Poeppel, 2007; Rodríguez-Fornells et al., 2009), we probed acquisition and maintenance of new active vocabulary in AA and five healthy age-matched controls. The learning curves on spontaneous naming (Fig. 2(B)) showed rapid acquisition and good long-term maintenance of the novel words in the five controls. AA’s word learning was somewhat slower than in the control group (Yates’ corrected χ2 = 6.67, df = 1, p < .01; responses summed over all learning sessions up to post-training), although she did eventually achieve a 100% naming accuracy. Not unexpectedly, the controls varied in their word learning speed. AA’s learning did not differ statistically from the slowest normal learner (Yates’ corrected χ2 = .18, df = 1, p = .67). Moreover, AA’s long-term maintenance up to 6 months post-training was comparable to that of the controls (Fisher’s exact test, one-tailed, p = .31, responses summed over the maintenance period). In fact, only one of the five healthy controls showed as high overall maintenance as AA. AA’s self-report suggested that seeing the novel word in written form was crucial for her learning. To verify this, we conducted a similar, short-term word learning experiment (behavioral Experiment 2) with novel word–picture pairs separately for written-only and auditory-only input. The learning curves in Fig. 2(C) show clearly that it was indeed the written-only input that resulted in superior learning in AA.
We also tested whether AA’s remarkable word learning ability through orthographic input translated into a clinically relevant treatment for her persistent word-finding difficulty (behavioral Experiment 3; Fig. 2(D)). A post-training picture naming test after 18 days of training showed successful acquisition of all the 42 trained items, and AA performed well also during the 9-weeks follow-up. The training effect was item-specific with no generalization to the 21 untrained control items. Naming in sentence context (using action images that differed from the object-only images used in training) was also at a high level post-training, showing that AA’s word learning ability was not limited to the training context or to the specific stimulus pictures used. Rather, the learned words were available to her in connected speech, too.
3.3. Functional brain imaging with MRI
3.3.1. Reading task
To shed light on the neural substrates of AA’s well-preserved word learning ability through the written modality, we carried out two fMRI experiments (fMRI Experiments 1 and 2) with AA and seven control participants. We first aimed to unveil the neural network involved in word reading in AA, the modality she used for learning new words. Reading words and pseudowords activated a network comprising spared regions in the left hemisphere (medial-inferior occipital and fusiform gyrus (FG) as well as middle and superior frontal gyrus (SFG)), but also extensive activation of the right hemisphere (Fig. 4(A) and Table 3). In contrast, the healthy control participants activated a strongly left-lateralized network in the same task (see Fig. 4(B) and Table 4).
Fig. 4 –
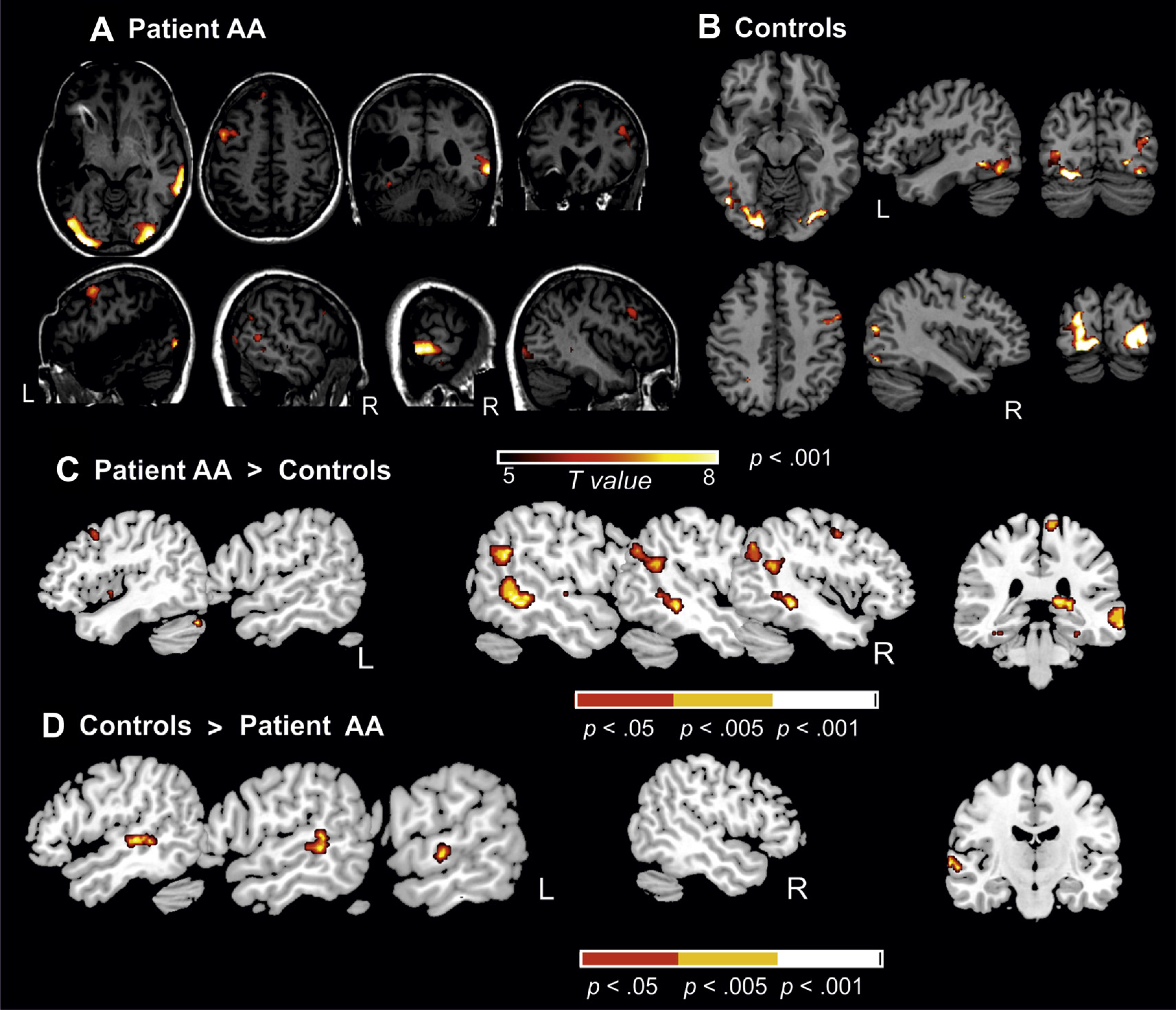
Brain substrates of reading: (word + pseudoword) versus false-font contrast. (A) Main results for this reading contrast for AA (p < .001, uncorrected, n = 20 voxels spatial extent). Sagittal, axial and coronal views of the functional contrast superimposed on AA’s structural MRI image in standard stereotactic space (see Table 3 for the peak values and coordinates). (B) Main results for the same reading contrast in the control group (see peak coordinates at Table 4). (C) Contrast showing the comparison between AA against the control group (using a modified t-test according to Crawford & Howell, 1998) (three different p-values uncorrected at whole-brain level are depicted for the sake of visualization). Notice the large increase in activation in the right middle temporal gyrus (MTG) and angular region in AA when compared to the control group. (D) The reverse contrast is depicted showing the significant increase in activation in the control group when compared to AA in this particular case in the left hemisphere MTG region.
Table 3.
Brain activation during reading and word learning: Main fMRI results from AA. All peak values reported were significant at p < .001 (uncorrected) at voxel level, 10 voxels spatial extent. Peak coordinates of each cluster are reported in MNI coordinates.
| Side | Cluster size (voxels) | t value | Peak coordinate |
|||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Reading task | ||||||
| (Word + pseudoword) versus false-font | ||||||
| MOG* | L | 1246 | 5.42 | −34 | −90 | −4 |
| MOG* | R | 930 | 4.35 | 48 | −75 | 2 |
| MTG* | R | 742 | 5.21 | 66 | −46 | −8 |
| PG/MFG/IFG* | L | 655 | 4.45 | −46 | 4 | 44 |
| IFG/MFG* | R | 245 | 4.08 | 42 | 20 | 32 |
| FG* | L | 52 | 3.97 | −34 | −44 | −22 |
| SMG* | R | 70 | 3.86 | 48 | −54 | 20 |
| MeFG* | L | 39 | 3.81 | −8 | 44 | 46 |
| Word learning task | ||||||
| Novel word–picture pairs versus rest (whole brain) | ||||||
| MOG* | L | 842 | 6.26 | −46 | −78 | 4 |
| Cuneus* | R | 48 | 4.59 | 22 | −98 | −6 |
| MFG* | L | 63 | 3.86 | −22 | 42 | −8 |
| MTG* | R | 12 | 3.67 | 70 | −40 | −6 |
| Novel word–picture pairs versus rest (hippocampal ROI) | ||||||
| Hippocampus | L | 11 | 3.34 | −34 | −32 | −10 |
FDR-Corrected at peak level (p < .05); MOG, Middle Occipital Gyrus; MTG, Middle Temporal Gyrus; IFG, Inferior Frontal Gyrus; MeFG, Medial Frontal Gyrus; MFG, Middle Frontal Gyrus; PG, Precentral Gyrus; FG, Fusiform Gyrus; SMG, Supramarginal Gyrus.
Table 4.
Brain activation during reading and word learning: Main fMRI results from the controls. All peak values reported were significant at p < .001 (uncorrected) at voxel level, 10 voxels spatial extent. Peak coordinates of each cluster are reported in MNI coordinates.
| Side | Cluster size (voxels) | t value | Peak coordinate |
|||
|---|---|---|---|---|---|---|
| x | y | Z | ||||
| Reading task | ||||||
| (Word + pseudoword)versus false-font | ||||||
| MOG* | R | 998 | 21.60 | 20 | −92 | −6 |
| MOG/LG* | L | 1660 | 21.20 | −24 | −86 | 14 |
| PG/MFG* | L | 14 | 7.77 | −50 | 14 | 46 |
| PG/MFG* | R | 64 | 7.50 | 54 | 2 | 44 |
| SPL* | L | 30 | 7.40 | −26 | −54 | 46 |
| Word learning task | ||||||
| Novel word–picture pairs versus rest (whole brain) | ||||||
| L FG/R MOG/L MOG* | L | 7825 | 38.54 | −42 | −66 | −14 |
| L | 27.83 | −26 | −84 | 16 | ||
| R | 25.79 | 36 | −78 | −16 | ||
| PG/MFG* | L | 146 | 16.25 | −50 | 0 | 40 |
| IFO* | L | 30 | 12.55 | −40 | 48 | −16 |
| PG/MFG* | R | 146 | 10.05 | 38 | 8 | 62 |
| FG* | L | 119 | 8.35 | −34 | −36 | −26 |
| MFO* | R | 22 | 8.13 | 24 | 46 | −20 |
| SPL* | L | 45 | 7.13 | −36 | −52 | 50 |
| Novel word–picture pairs versus rest (hippocampal ROI) | ||||||
| Hippocampus* | L | 10 | 8.79 | −18 | −28 | −6 |
| Hippocampus* | L | 13 | 7.20 | −30 | −20 | −14 |
| Hippocampus* | R | 14 | 6.93 | 26 | −34 | 2 |
FDR-Corrected at peak level (p < .05); PG, Precentral Gyrus; MOG, Middle Occipital Gyrus; MFG, Middle Frontal Gyrus; LG Lingual Gyrus; SPL, Superior Parietal Lobe; IFO Inferior Fronto-Orbital Gyrus. FG, Fusiform Gyrus; MFO Middle Fronto-Orbital Gyrus.
A direct statistical comparison between AA and the control group shows AA’s significantly increased reading-related activations in the right middle temporal and angular gyri (Fig. 4(C) and Table 5). On the other hand, the reverse comparison showed that the controls activated significantly more the left middle temporal and lingual gyri while reading (Fig. 4(D)). AA’s overt reading of the test items was probed one day after the fMRI registration. She read all the real words accurately. Concerning the pseudowords, her accuracy rate was 65% with a strict criterion and 90% when allowing for a one-phoneme error. The control participants read all items accurately.
Table 5.
Direct contrasts between the controls and AA on the reading and learning task. All peak values reported were significant at p < .001 (uncorrected) at voxel level, 10 voxels spatial extent except for the comparison between AA > Controls for the Learning task. The latter is reported at p < .01, since no significant voxels were found at p < .001 with 10 voxels spatial extent. Peak coordinates of each cluster are reported in MNI coordinates.
| Side | Cluster size (mm3) | t value | Peak coordinate |
|||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Reading task | ||||||
| (Word + pseudoword) versus false-font | ||||||
| Controls > patient AA | ||||||
| MTG | L | 17 | 6.44 | −52 | −40 | 2 |
| LG | L | 44 | 8.52 | −12 | −68 | 6 |
| Patient AA > Controls | ||||||
| SFG* | L | 80 | 10.46 | −14 | 30 | 54 |
| MTG* | R | 187 | 9.17 | 68 | −40 | 0 |
| ANG | R | 20 | 8.52 | 56 | −54 | 24 |
| Word learning task | ||||||
| Novel word–picture pairs versus rest (whole brain) | ||||||
| Controls > patient AA | ||||||
| MFG* | R | 84 | 18.24 | 46 | 48 | 6 |
| STG | L | 48 | 12.03 | −46 | −4 | 0 |
| Hippocampus | R | 74 | 10.95 | 22 | −18 | −14 |
| HG/STG | R | 44 | 9.24 | 42 | −22 | 14 |
| Insula | R | 64 | 8.51 | 40 | 20 | −6 |
| Patient AA > Controls (p < .01) | ||||||
| FG/ITG | R | 67 | 6.92 | 30 | −4 | −42 |
| Hippocampus | L | 29 | 6.42 | −32 | −32 | 10 |
| MTG | L | 62 | 5.93 | −52 | −64 | −2 |
| MTG | R | 33 | 4.37 | 54 | −48 | 0 |
FDR-Corrected at peak level (p < .05); SFG, Superior Frontal Gyrus; MTG, Middle Temporal Gyrus; STG, Superior Temporal Gyrus; ANG, Angular Gyrus; LG, Lingual Gyrus; MFG, Middle Frontal Gyrus; HG, Heschl Gyrus; ITG Inferior Temporal Gyrus. FG, Fusiform Gyrus.
Finally, in order to ensure that the differences between AA and controls were not driven e.g., by pseudoword reading only, we explored Beta values separately for words and pseudowords in two of the areas that were more strongly activated by AA (the right MTG and the left STG). These two areas showed a significant increase of activation both for words and pseudowords in our patient, as compared to the control group (right MTG for pseudowords t = 16.7 and for words t = 14.17; left SFG for pseudowords t = 4.9567 and for words t = 5.78). All the corresponding Crawford t-test comparisons between the patient and the control group were highly significant (p < .0001).
To look more closely at these functional hemispheric differences between AA and the control group, we computed a LI for reading-related activation patterns. Here we used a ROI approach including the superior, middle and inferior temporal gyri (see Maldjian et al., 2003). All control participants showed a left-lateralized LI (mean + .43, range from −.15 to +.79), except one participant being bilateral (a right-handed female like the rest of the group). On the contrary, AA’s LI (−.89) differed statistically significantly from that of the controls (t = 3.84, p < .005, using Crawford’s t-test) and showed that at least 90% of her temporal lobe activation during the reading task was lateralized to her intact right temporal lobe.
Given the left-sided inferior occipito-temporal activation during reading in AA, we searched for possible connections between this area and the other left hemisphere regions that she activated during reading. Using probabilistic DW-MRI-tractography with the areas activated in the fMRI reading analysis in AA acting as seed regions, no such connections could be reconstructed.
3.3.2. Word learning task
In the second fMRI experiment, AA and the healthy control group attempted to learn novel written word–picture pairs in a similar way as was done in the behavioral word learning training sessions reported above. For this task, we expected to see increased activation in the medial temporal regions (hippocampal and parahippocampal areas/spared in AA), as these brain areas have been shown to be important for initial word learning (Davis & Gaskell, 2009; Meinzer et al., 2010) and associative learning (Squire, Stark, & Clark, 2004).
Fig. 5(A) and (B) depicts the activation patterns for AA and the control group in the learning task. The word learning task activated a large network comprising the middle occipital regions, FG, hippocampus, middle and precentral frontal gyri, and superior temporal lobe (see Tables 3 and 4). As we expected, both AA and the healthy control participants showed significant hippocampal activation increases bilaterally when trying to learn pairs of novel words and pictures (Fig. 5(D); Tables 3 and 4). This is in line with previous functional imaging studies on word learning in healthy individuals (Breitenstein et al., 2005; Davis & Gaskell, 2009; Mestres-Missé, Rodríguez-Fornells, & Münte, 2010). The direct statistical comparison between AA and the control group (see Fig. 5(C)) showed significantly increased activation in the right middle temporal gyrus (MTG) region that also emerged during reading, as well as in the right inferior temporal – FG region. Moreover, the learning-related left posterior hippocampal activation tended to be larger in AA than in the control group (see Table 5). The opposite comparison (the controls vs AA) showed increased activations in the left superior temporal region that was lesioned in AA, as well as in right medial temporal and also frontal regions (see Table 5). Note that the short exposure rate in the fMRI (2.5 sec per item in the present experiment vs 12 sec in Experiment 1) precluded any overt learning of the novel names in AA. The healthy control participants learned to name one to five of the novel items spontaneously.
Fig. 5 –
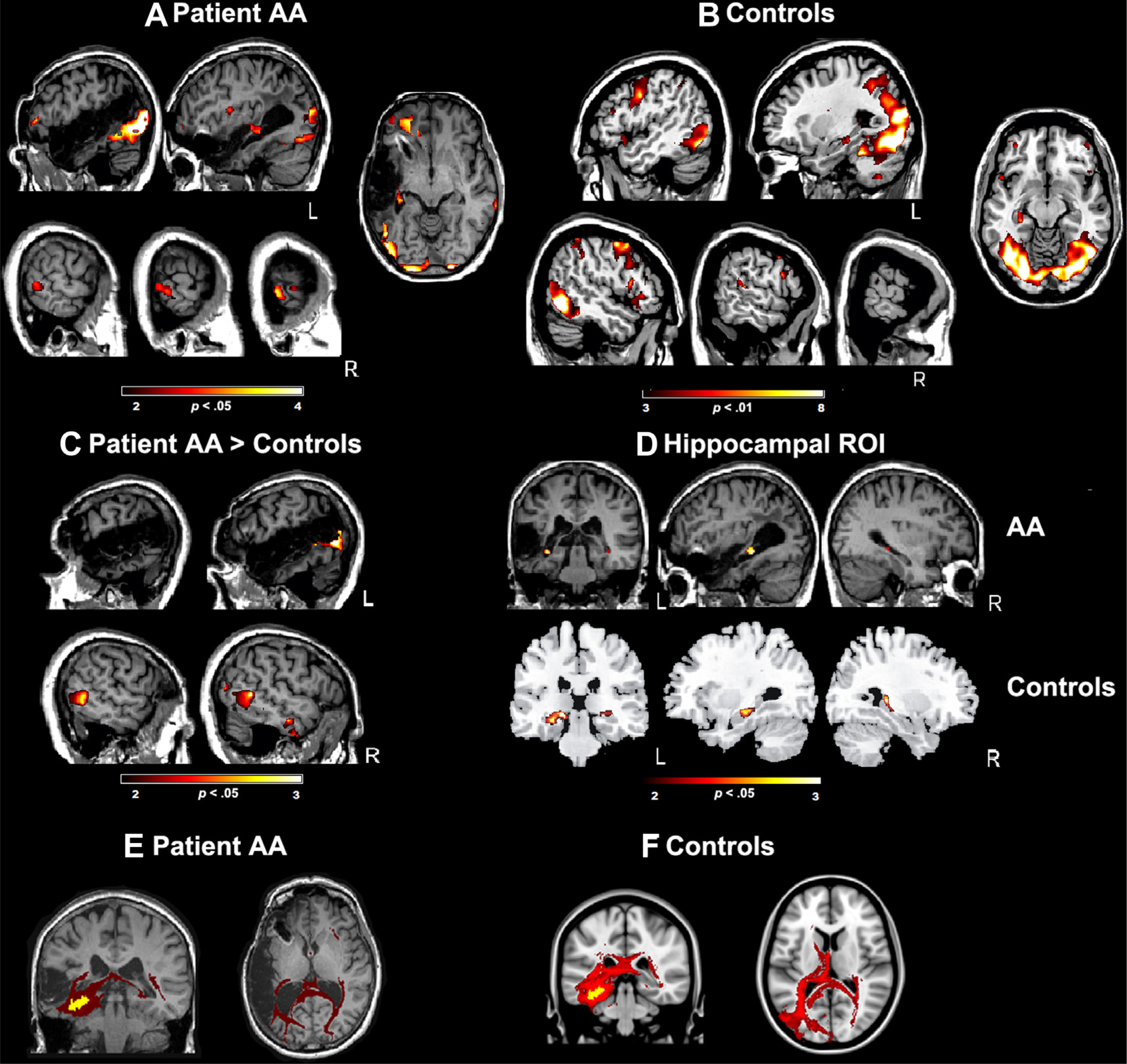
Brain substrates of word learning. The figure depicts the contrast learning novel word–picture pairs versus rest. (A) Main contrast for AA, showing the activations in the medial occipital cortex, cuneus, middle temporal gyrus (right) and middle frontal gyrus (left) (see Table 3) (p < .05, n = 10 voxels, uncorrected). (B) Same contrast depicted for the control group, showing a larger amount of activation when compared to AA (see Table 4) (p < .01, n = 10 voxels, uncorrected). (C) Contrast between AA and the control group, showing the significant increase of activation in AA in the middle temporal gyrus as well as in other regions (see Table 5) (p < .05, n = 10 voxels, uncorrected, using Crawford’s t-test). (D) Results of the ROI analysis overlaid on AA’s structural MRI image in standard stereotactic space focused on hippocampal areas is also shown at p < .05 (uncorrected) both for AA (top row) and the control group (bottom row) (see Tables 3 and 4). (E) AA’s interhemispheric tracts. Coronal and axial views of the reconstructed fiber pathway using the left hippocampal functional activation as a seed point in native MRI space. (F) The interhemispheric tracts of the healthy control participants. The image shows voxels where connections to the same seed region as in (E) were found in at least 3 of the 7 control participants.
4. Discussion
In the present case study, we probed novel word learning in chronic aphasia resulting from damage to the left-hemispheric language network including the dorsal speech processing pathway. While AA’s lesion encompassed more than just the AF, the presence of a disconnection of the AF allowed us to test the current theoretical view on the importance of this white-matter pathway in learning to produce new words (Hickok & Poeppel, 2007; Rodríguez-Fornells et al., 2009; Schultze et al., 2012). Surprisingly, AA was able to acquire and maintain novel words on par with controls despite her damage to the left dorsal pathway, challenging the current view on the crucial importance of the AF in word learning. For the explanation of this preserved ability that we provide below, the most central feature of this learning ability is the fact that it was limited to the written modality.
The results obtained through structural and functional MRI imaging clearly indicate the importance of AA’s right hemisphere in sustaining this unexpected ability to learn words through reading. First, her right hemisphere, including the AF, was intact. Second, using DTI-tractography, we were unable to observe preserved connections that could mediate between AA’s reading-related left inferior temporo-occipital activations and other left-hemispheric regions activated during reading. Third, AA showed greater reading-related activation than control participants in the right middle temporal and inferior parietal areas, and also greater activation in the right MTG during a word–picture learning task with written object names. In Fig. 6 we depict the overlap between AA’s reading-related right-hemisphere activations and the reconstructed right AF. This overlap is expected considering that interregional white-matter fiber connections constrain the flow of information and the influence that one brain region can exert over others (Behrens & Johansen-Berg, 2005; Johansen-Berg, 2010). Thus the right-hemispheric regions activated in AA when compared to control participants (the MTG, angular gyrus (ANG) and premotor and prefrontal cortex) most probably communicated via the intact right AF.
Fig. 6 –
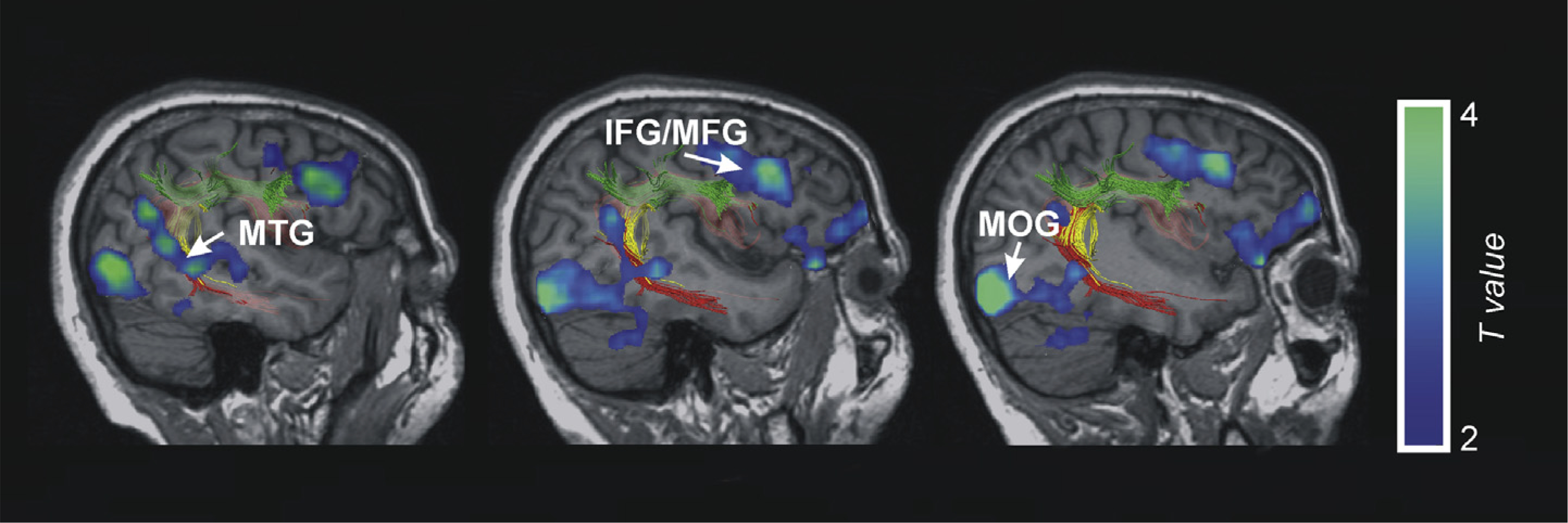
Reading effects. This figure shows the fMRI results of the contrast of word and pseudowords compared to the false-font condition (t-score overlays). Superimposed we depict the DTI-Tractography sagittal views of the three part arcuate fasciculus on AA’s right hemisphere in native MRI space. MOG, Middle Occipital Gyrus; MTG, Middle Temporal Gyrus; IFG, Inferior Frontal Gyrus; MFG, Middle Frontal Gyrus.
Increased right-hemispheric activity in aphasia during resting state or language performance is by no means a new finding. Several previous functional neuroimaging studies have reported right-sided activation increases in individuals with aphasia, but their role in the recovery process in these patients is unclear. According to some studies, right-hemispheric recruitment reflects compensatory processes that are important for aphasia recovery either on short or long-term (e.g., Abo et al., 2004; Berthier, Lambon Ralph, Pujol, & Green, 2012; Blasi et al., 2002; Crosson et al., 2005; Leff et al., 2002; Ohyama et al., 1996; Raboyeau et al., 2008; Saur et al., 2006), while others have considered these activation increases as ineffective or even detrimental to aphasia recovery (e.g., Heiss & Thiel, 2006; Naeser et al., 2004; Postman-Caucheteux et al., 2010; Richter, Miltner, & Straube, 2008). These controversies relate to variability in lesion location and extent, time post-onset, and the type of language tasks used (e.g., Sebastian & Kiran, 2011), emphasizing the need for case-by-case analyses. The recent investigation by Turkeltaub et al. (2012) adds to the discussion by providing evidence of both interfering and facilitative right-hemispheric activation in the same aphasic participant.
AA initially suffered from global aphasia, including an inability to read words. However, she had recovered in this respect, and it seems plausible that the strongly activated right temporal and parietal regions provided a compensatory route for reading. Split-brain studies and visual half-field experiments in normals have revealed basic reading abilities in the right hemisphere (Taylor & Regard, 2003). Recent evidence from normals suggests that the right hemisphere can access not only orthographic but also (rudimentary) phonological input codes for words, albeit the left hemisphere is needed for phonological output (Halderman, 2011). While AA’s left temporal damage had resulted in the loss of phonological input/output connections needed for word learning through the auditory modality, her right-sided receptive abilities carried compensatory potential through orthography. Interestingly, a recent fMRI study with normals by Seghier et al. (2008) revealed two parallel neural pathways in reading of familiar words, one encompassing the left anterior occipito-temporal and left ventral frontal regions, and the other one left posterior occipito-temporal and right inferior parietal regions. The latter pathway could be related to AA’s preserved word learning ability via reading. Evidence for compensatory right-hemisphere participation in reading has also been observed in deep dyslexia associated with extensive left hemisphere lesions and aphasia (e.g., Coltheart, 2000), epileptic patients undergoing surgical removal of the left anterior temporal areas (Noppeney, Price, Duncan, & Koepp, 2005), and in “hyperlexic” reading in autism Turkeltaub et al., 2004).
Our combined structural–functional imaging results and the literature on reading-related neural systems provide important clues to AA’s compensatory pathways for written word learning. The structures subserving visual word recognition (fusiform gyri including the left “visual word form area”) were intact and activated during AA’s reading. The fusiform gyri and the left and right hippocampi are interconnected (see Fig. 5(E) and (F); Epelbaum et al., 2008; Smith et al., 2009), enabling interhemispheric information transfer from the left-sided visual word form area to its right-sided homolog. Through these interhemispheric connections and right-sided perisylvian tracts, right temporal and parietal regions could be involved in reading in AA. The fusiform gyri would also provide access to the hippocampal regions needed for encoding novel word–picture associations (Davis & Gaskell, 2009; Smith et al., 2009).
The present study focused on active vocabulary learning, which has been related to the dorsal language pathway function. We did not explore e.g., AA’s acquisition of novel lexical-semantic representations that would presumably normally engage the ventral pathway (Duffau et al., 2005; Saur et al., 2008, 2010; Wong, Chandrasekaran, Garibaldi, & Wong, 2011). It has been suggested that the ventral pathway plays a compensatory role when the dorsal pathway is unavailable during normal development (Yeatman, Dougherty, Ben-Shachar, & Wandell, 2012), or because of a lesion (Rauschecher et al., 2009) or during special language task constraints (Lopez-Barroso et al., 2011). Note that AA’s left ventral pathway (IFOF) was damaged as well, but the presence of the clip precludes any firm conclusions concerning the complete absence of structural-functional integrity in this pathway. For the present learning task, AA needed to establish and maintain oral production for the novel words. Oral production is strongly left-lateralized (Laine & Martin, 2006). It probably continued to be subserved by the spared left inferior frontal gyrus (IFG) in AA, albeit the present MRI evidence is not fully conclusive due to the aneurysm clip close by that introduced small distortions to MRI signal (Khursheed, Rohlffs, Suzuki, Kim, & Ellmore, 2011). AA’s reading-related activations suggest that the connection from reading to oral production was established through a route from the right temporo-parietal areas to the right frontal premotor regions, with intercallosal transfer to the left premotor areas and further to Broca’s area. Interestingly, a TMS study by Meister et al. (2006) found that in individuals with aphasia, right motor cortex excitability increased during single-word reading. In normals, this effect was seen in the left hemisphere only. This indicates a compensatory laterality shift in the links between frontal/motor functions, reading and oral output following left-hemisphere damage and aphasia.
While we account for AA’s preserved modality-specific learning ability by compensatory functional changes following her extensive left temporal lesion, one could ask whether some peculiar premorbid features could play a role here. Bilateral language lateralization is not likely as AA was strongly right-handed and became chronically aphasic after her left-hemispheric insult. Furthermore, the micro- and macroanatomical features of her right-sided AF fell within the boundaries of the age-matched control group. Recent research suggests that more bilateral distributions of the direct temporo-frontal segment of the AF seem to be beneficial for verbal memory in adults (Catani et al., 2007) as well as in female children (Yeatman et al., 2011), and less lateralized individuals with aphasia seem to recover better from unilateral lesions because the contralateral compensation (Pizzamiglio, Mammucari, & Razzano, 1985) (see Everts et al., 2010, for a similar compensatory pattern in infants with epilepsy). Thus AA’s gender might have played some role here: aphasia recovery could be more effective in females as they show a bilateral representation of the direct temporo-frontal segment of the AF more often than men (Catani et al., 2007).
The present case has important theoretical repercussions by indicating that in a literate, the left dorsal temporo-frontal stream (Hickok & Poeppel, 2007; Rodríguez-Fornells et al., 2009; Schulze et al., 2012) is not the only neural pathway that can mediate new active vocabulary acquisition. AA had a previously hidden avenue for word learning via orthography, enabling a high level of learning and long-term maintenance on par with that of controls. FMRI identified the compensatory role of the intact right hemisphere in her reading and visual word learning, as well as the involvement of the spared medial temporal regions needed for associative learning (Squire et al., 2004). We were not in a position to explore the emergence of the right hemisphere pathway after her CVA as at the time of the present experiments, AA was several years post-onset and had already recovered reading abilities. Finally, AA’s learning ability translated into an effective treatment for her word-finding difficulties with familiar words.
A surprising aspect of this case study is the fact that while many individuals with aphasia can re-learn words through intensive training (e.g., Laine & Martin, 2006), near perfect acquisition and long-term maintenance (up to 6months post-training) of novel active vocabulary has not been reported in aphasia before. Indeed, only one out of five control participants was able to show a similar retention rate to AA at the retest 6 months post-training. Our measure of vocabulary learning, spontaneous naming of the trained items, was a very demanding one for individuals with aphasia who (as AA) typically suffer from anomia. As noted earlier, novel word learning tasks tap directly into word learning mechanisms as they are free from the confound of cue-based memory retrieval that may operate in re-learning of previously familiar but currently inaccessible words. AA’s seemingly unique word learning ability may also reflect the relative lack of research on novel language learning in aphasia. Our case thus prompts a closer look at the learning potential via preserved input (and output) modalities in individuals suffering from acquired language disorders. Reciprocally, further case-by-case analyses should clarify exactly why re-learning of language remains impossible in many individuals with aphasia, even if their lesion spares several critical neural elements.
Acknowledgments
This work was supported by the Finnish Graduate School in Language Studies LANGNET [L.T.]; the Academy of Finland [135688 to M.L.]; the Spanish Government [MICINN, PSI2011-29219 to A.R.F]; and the Catalan Government [Generalitat de Catalunya, 2009 SGR 93 to A.R.F.]. We are grateful to Teemu Laine for his aid with the MRI experiments, Flavio Dell’Acqua for helpful discussions, and the three anonymous reviewers for their comments.
Footnotes
Conflict of interest
The authors declare no competing financial interests.
REFERENCES
- Abo M, Senoo A, Watanabe S, Miyano S, Doseki K, Sasaki N, et al. (2004). Language-related brain function during word repetition in post-stroke aphasics. NeuroReport, 15(12), 1891–1894. [DOI] [PubMed] [Google Scholar]
- Aboitiz F (2012). Gestures, vocalizations, and memory in language origins. Frontiers in Evolutionary Neuroscience, 4, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SM, Rapcsak SZ, & Beeson PM (2010). Cost function masking during normalization of brains with focal lesions: still a necessity? NeuroImage, 53(1), 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, & Friston KJ (2005). Unified segmentation. NeuroImage, 26(3), 839–851. [DOI] [PubMed] [Google Scholar]
- Behrens TE, & Johansen-Berg H (2005). Relating connectional architecture to grey matter function using diffusion imaging. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 360(1457), 903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, et al. (2003). Noninvasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature Neuroscience, 6(7), 750–757. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, et al. (2003). Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magnetic Resonance in Medicine, 50(5), 1077–1088. [DOI] [PubMed] [Google Scholar]
- Berthier ML, Lambon Ralph MA, Pujol J, & Green C (2012). Arcuate fasciculus variability and repetition: the left sometimes can be right. Cortex, 48(2), 133–143. [DOI] [PubMed] [Google Scholar]
- Blasi V, Young AC, Tansy AP, Petersen SE, Snyder AZ, & Corbetta M (2002). Word retrieval learning modulates right frontal cortex in patients with left frontal damage. Neuron, 36(1), 159–170. [DOI] [PubMed] [Google Scholar]
- Breitenstein C, Jansen A, Deppe M, Foerster AF, Sommer J, Wolbers T, et al. (2005). Hippocampus activity differentiates good from poor learners of a novel lexicon. NeuroImage, 25(3), 958–968. [DOI] [PubMed] [Google Scholar]
- Breitenstein C, Kamping S, Jansen A, Schomacher M, & Knecht S (2004). Word learning can be achieved without feedback: implications for aphasia therapy. Restorative Neurology and Neuroscience, 22(6), 445–458. [PubMed] [Google Scholar]
- Brett M, Leff AP, Rorden C, & Ashburner J (2001). Spatial normalization of brain images with focal lesions using cost function masking. NeuroImage, 14(2), 486–500. [DOI] [PubMed] [Google Scholar]
- Catani M, Allin MPG, Husain M, Pugliese L, Mesulam MM, Murray RM, et al. (2007). Symmetries in human brain language pathways correlate with verbal recall. Proceedings of the National Academy of Sciences of the United States of America, 104(43), 17163–17168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Jones DK, & ffytche DH (2005). Perisylvian language networks of the human brain. Annals of Neurology, 57(1), 8–16. [DOI] [PubMed] [Google Scholar]
- Catani M, & Mesulam M (2008). The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex, 44(8), 953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, & Thiebaut de Schotten M (2008). A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex, 44(8), 1105–1132. [DOI] [PubMed] [Google Scholar]
- Coltheart M (2000). Deep dyslexia is right-hemisphere reading. Brain and Language, 71(2), 299–309. [DOI] [PubMed] [Google Scholar]
- Crawford JR, & Garthwaite PH (2004). Statistical methods for single-case studies in neuropsychology: comparing the slope of a patient’s regression line with those of a control sample. Cortex, 40(3), 533–548. [DOI] [PubMed] [Google Scholar]
- Crawford JR, & Howell DC (1998). Regression equations in clinical neuropsychology: an evaluation of statistical methods for comparing predicted and obtained scores. Journal of Clinical and Experimental Neuropsychology, 20(5), 755–762. [DOI] [PubMed] [Google Scholar]
- Crosson B, Moore AB, Gopinath K, White KD, Wierenga CE, Gaiefsky ME, et al. (2005). Role of the right and left hemispheres in recovery of function during treatment of intention in aphasia. Journal of Cognitive Neuroscience, 17(3), 392–406. [DOI] [PubMed] [Google Scholar]
- Damasio H, & Damasio AR (1980). The anatomical basis of conduction aphasia. Brain, 103(2), 337–350. [DOI] [PubMed] [Google Scholar]
- Davis MH, & Gaskell MG (2009). A complementary systems account of word learning: neural and behavioural evidence. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 364(1536), 3773–3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Renzi E, & Faglioni P (1978). Normative data and screening power of a shortened version of the Token Test. Cortex, 14(1), 41–49. [DOI] [PubMed] [Google Scholar]
- De Renzi E, & Nichelli P (1975). Verbal and non-verbal short-term memory impairment following hemispheric damage. Cortex, 11(4), 341–354. [DOI] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Mandonnet E, Peruzzi P, Tzourio-Mazoyer N, & Capelle L (2005). New insights into the anatomo-functional connectivity of the semantic system: a study using cortico-subcortical electrostimulations. Brain, 128(Pt 4), 797–810. [DOI] [PubMed] [Google Scholar]
- Epelbaum S, Pinel P, Gaillard R, Delmaire C, Perrin M, Dupont S, et al. (2008). Pure alexia as a disconnection syndrome: new diffusion imaging evidence for an old concept. Cortex, 44(8), 962–974. [DOI] [PubMed] [Google Scholar]
- Everts R, Harvey AS, Lillywhite L, Wrennall J, Abbott DF, Gonzalez L, et al. (2010). Language lateralization correlates with verbal memory performance in children with focal epilepsy. Epilepsia, 51(4), 627–638. [DOI] [PubMed] [Google Scholar]
- Fernández-Miranda JC, Rhoton AL, Álvarez-Linera J, Kakizawa Y, Choi C, & de Oliveira EP (2008). Three-dimensional microsurgical and tractographic anatomy of the white matter of the human brain. Neurosurgery, 62(6 Suppl. 3), 989–1028. [DOI] [PubMed] [Google Scholar]
- Forkel SJ, Thiebaut de Schotten M, Kawadler JM, Dell’acqua F, Danek A, & Catani M (2012. September 20). The anatomy of fronto-occipital connections from early blunt dissections to contemporary tractography. Cortex. E-pub ahead of print, 2012. [DOI] [PubMed] [Google Scholar]
- Freed DB, Marshall RC, & Nippold MA (1995). Comparison of personalized cueing and provided cueing on the facilitation of verbal labeling by aphasic subjects. Journal of Speech and Hearing Research, 38(5), 1081–1090. [DOI] [PubMed] [Google Scholar]
- Freedman ML, & Martin RC (2001). Dissociable components of short-term memory and their relation to long-term learning. Cognitive Neuropsychology, 18(3), 193–226. [DOI] [PubMed] [Google Scholar]
- Fridriksson J, Kjartansson O, Morgan PS, Hjaltason H, Magnusdottir S, Bonilha L, et al. (2010). Impaired speech repetition and left parietal lobe damage. Journal of Neuroscience, 30(33), 11057–11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, & Turner R (1998). Event-related fMRI: characterizing differential responses. NeuroImage, 7(1), 30–40. [DOI] [PubMed] [Google Scholar]
- Grossman M, & Carey S (1987). Selective word-learning deficits in aphasia. Brain and Language, 32(2), 306–324. [DOI] [PubMed] [Google Scholar]
- Gupta P, Martin N, Abbs B, Schwartz M, & Lipinski J (2006). New word learning in aphasic patients: dissociating phonological and semantic components. Brain and Language, 99(1–2), 8–9. [Google Scholar]
- Halderman LK (2011). Evidence for right hemisphere phonology in a backward masking task. Brain and Language, 119(3), 232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss WD, & Thiel A (2006). A proposed regional hierarchy in recovery of poststroke aphasia. Brain and Language, 98(1), 118–123. [DOI] [PubMed] [Google Scholar]
- Hickok G, & Poeppel D (2007). The cortical organization of speech processing. Nature Reviews Neuroscience, 8(5), 393–402. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister PR, Brady JM, & Smith SM (2002). Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17(2), 825–841. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H (2010). Behavioural relevance of variation in white matter microstructure. Current Opinion in Neurology, 23(4), 351–358. [DOI] [PubMed] [Google Scholar]
- Karttunen T, & Renvall K (2005). An auditory lexical decision task. Helsinki: Institute of Behavioural Sciences, University of Helsinki; (unpublished). [Google Scholar]
- Khursheed F, Rohlffs F, Suzuki S, Kim DH, & Ellmore TM (2011). Artifact quantification and tractography from 3 T MRI after placement of aneurysm clips in subarachnoid hemorrhage patients. BMC Medical Imaging, 11, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kümmerer D, Hartwigsen G, Kellmeyer P, Glauche V, Mader I, Klöppel S, et al. (2013). Damage to ventral and dorsal language pathways in acute aphasia. Brain, 136(Pt 2), 619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laganaro M, Di Pietro M, & Schnider A (2006). What does recovery from anomia tell us about the underlying impairment: the case of similar anomic patterns and different recovery. Neuropsychologia, 44(4), 534–545. [DOI] [PubMed] [Google Scholar]
- Laine M, Koivuselkä-Sallinen P, Hänninen R, & Niemi J (1997). Bostonin Nimentätesti [The Finnish version of the Boston Naming Test]. Helsinki: Psykologien Kustannus. [Google Scholar]
- Laine M, Kujala P, Niemi J, & Uusipaikka E (1992). On the nature of naming difficulties in aphasia. Cortex, 28(4), 537–554. [DOI] [PubMed] [Google Scholar]
- Laine M, & Martin N (2006). Anomia: Theoretical and clinical aspects. Hove, UK: Psychology Press. [Google Scholar]
- Laine M, Niemi J, Koivuselkä-Sallinen P, & Tuomainen J (1997). Bostonin Diagnostinen Afasiatutkimus [The standardized Finnish version of the Boston Diagnostic Aphasia Examination]. Helsinki: Psykologien Kustannus. [Google Scholar]
- Laine M, & Salmelin R (2010). Neurocognition of new word learning in the native tongue: lessons from the ancient farming equipment paradigm. Language Learning, 60(Suppl. s2), 25–44. [Google Scholar]
- Laine M, Schmied W, & Trefzer K (1998). Category-specific word–picture matching test [for research purposes only]. Turku: Department of Behavioural Sciences and Philosophy, University of Turku; (unpublished). [Google Scholar]
- Lawes IN, Barrick TR, Murugam V, Spierings N, Evans DR, Song M, et al. (2008). Atlas-based segmentation of white matter tracts of the human brain using diffusion tensor tractography and comparison with classical dissection. NeuroImage, 39(1), 62–79. [DOI] [PubMed] [Google Scholar]
- Leff A, Crinion J, Scott S, Turkheimer F, Howard D, & Wise R (2002). A physiological change in the homotopic cortex following left posterior temporal lobe infarction. Annals of Neurology, 51(5), 553–558. [DOI] [PubMed] [Google Scholar]
- Lopez-Barroso D, de Diego-Balaguer R, Cunillera T, Càmara E, Münte TF, & Rodríguez-Fornells A (2011). Language learning under working memory constraints correlates with microstructural differences in the ventral language pathway. Cerebral Cortex, 21(12), 2742–2750. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, & Burdette JH (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19(3), 1233–1239. [DOI] [PubMed] [Google Scholar]
- Marchina S, Zhu LL, Norton A, Zipse L, Wan CY, & Schlaug G (2011). Impairment of speech production predicted by lesion load of the left arcuate fasciculus. Stroke, 42(8), 2251–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall RC, Freed D, & Karow CM (2001). Learning of subordinate category names by aphasic subjects: a comparison of deep and surface-level training methods. Aphasiology, 15(6), 585–598. [Google Scholar]
- Marshall RC, Neuburger SI, & Phillips DS (1992). Effects of facilitation and cueing on labelling of ‘novel’ stimuli by aphasic subjects. Aphasiology, 6(6), 567–583. [Google Scholar]
- Martin N, & Saffran EM (1999). Effects of word processing and short-term memory deficits on verbal learning: evidence from aphasia. International Journal of Psychology, 34(5/6), 339–346. [Google Scholar]
- McClelland JL, McNaughton BL, & O’Reilly RC (1995). Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychological Review, 102(3), 419–457. [DOI] [PubMed] [Google Scholar]
- McGrane H (2006). An investigation into the ability of adults with post-stroke aphasia to learn new vocabulary. Doctoral dissertation. Queen Margaret University College http://www.qmu.ac.uk/ssrc/pubs/McGrane2006PhD.pdf.
- Meinzer M, Mohammadi S, Kugel H, Schiffbauer H, Flöel A, Albers J, et al. (2010). Integrity of the hippocampus and surrounding white matter is correlated with language training success in aphasia. NeuroImage, 53(1), 283–290. [DOI] [PubMed] [Google Scholar]
- Meister IG, Sparing R, Foltys H, Gebert D, Huber W, Töpper R, et al. (2006). Functional connectivity between cortical hand motor and language areas during recovery from aphasia. Journal of the Neurological Sciences, 247(2), 165–168. [DOI] [PubMed] [Google Scholar]
- Mestres-Missé A, Rodríguez-Fornells A, & Münte TF (2010). Neural differences in the mapping of verb and noun concepts onto novel words. NeuroImage, 49(3), 2826–2835. [DOI] [PubMed] [Google Scholar]
- Moore CJ, & Price CJ (1999). Three distinct ventral occipitotemporal regions for reading and object naming. NeuroImage, 10(2), 181–192. [DOI] [PubMed] [Google Scholar]
- Morrow KL (2006). Patterns of cortical activation associated with novel word learning in persons with aphasia. Doctoral Dissertation. University of South Carolina. Ann Arbor: ProQuest Information and Learning Company. [Google Scholar]
- Naeser MA, Martin PI, Baker EH, Hodge SM, Sczerzenie SE, Nicholas M, et al. (2004). Overt propositional speech in chronic nonfluent aphasia studied with the dynamic susceptibility contrast fMRI method. NeuroImage, 22(1), 29–41. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Price CJ, Duncan JS, & Koepp MJ (2005). Reading skills after left anterior temporal lobe resection: an fMRI study. Brain, 128(6), 1377–1385. [DOI] [PubMed] [Google Scholar]
- Nucifora PGP, Verma R, Melhem ER, Gur RE, & Gur RC (2005). Leftward asymmetry in relative fiber density of the arcuate fasciculus. NeuroReport, 16(8), 791–794. [DOI] [PubMed] [Google Scholar]
- Ohyama M, Senda M, Kitamura S, Ishii K, Mishina M, & Terashi A (1996). Role of the nondominant hemisphere and undamaged area during word repetition in poststroke aphasics. Stroke, 27(5), 897–903. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971). The Edinburgh Inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Pajevic S, & Pierpaoli C (1999). Color schemes to represent the orientation of anisotropic tissues from diffusion tensor data: application to white matter fiber tract mapping in the human brain. Magnetic Resonance in Medicine, 42(3), 526–540. [PubMed] [Google Scholar]
- Parker GJ, Luzzi S, Alexander DC, Wheeler-Kingshott CA, Ciccarelli O, & Lambon Ralph MA (2005). Lateralization of ventral and dorsal auditory-language pathways in the human brain. NeuroImage, 24(3), 656–666. [DOI] [PubMed] [Google Scholar]
- Pizzamiglio L, Mammucari A, & Razzano C (1985). Evidence for sex differences in brain organization in recovery in aphasia. Brain and Language, 25(2), 213–223. [DOI] [PubMed] [Google Scholar]
- Postman-Caucheteux WA, Birn RM, Pursley RH, Butman JA, Solomon JM, Picchioni D, et al. (2010). Single-trial fMRI shows contralesional activity linked to overt naming errors in chronic aphasic patients. Journal of Cognitive Neuroscience, 22(6), 1299–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, Wheeler-Kingshott CA, et al. (2006). Hemispheric asymmetries in language-related pathways: a combined functional MRI and tractography study. NeuroImage, 32(1), 388–399. [DOI] [PubMed] [Google Scholar]
- Price CJ, & Devlin JT (2011). The interactive account of ventral occipitotemporal contributions to reading. Trends in Cognitive Sciences, 15(6), 246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raboyeau G, De Boissezon X, Marie N, Balduyck S, Puel M, Bézy C, et al. (2008). Right hemisphere activation in recovery from aphasia: lesion effect or function recruitment? Neurology, 70(4), 290–298. [DOI] [PubMed] [Google Scholar]
- Rauschecher AM, Deutsch GK, Ben-Shachar M, Schwartzman A, Perry LM, & Dougherty RF (2009). Reading impairment in a patient with missing arcuate fasciculus. Neuropsychologia, 47(1), 180–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renvall K, Laine M, Laakso M, & Martin N (2003). Anomia treatment with contextual priming: a case study. Aphasiology, 17(3), 305–328. [Google Scholar]
- Renvall K, & Tuomiranta LA (2010). Synonymy judgment task. Turku: Department of Behavioural Sciences and Philosophy, University of Turku; (unpublished). [Google Scholar]
- Richter M, Miltner WH, & Straube T (2008). Association between therapy outcome and right-hemispheric activation in chronic aphasia. Brain, 131(Pt 5), 1391–1401. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Glasser MF, Preuss TM, Ma X, Zhao T, Hu X, et al. (2008). The evolution of the arcuate fasciculus revealed with comparative DTI. Nature Neuroscience, 11(4), 426–428. [DOI] [PubMed] [Google Scholar]
- Ripollés P, Marco-Pallarés J, de Diego-Balaguer R, Miró J, Falip M, Juncadella M, et al. (2012). Analysis of automated methods for spatial normalization of lesioned brains. NeuroImage, 60(2), 1296–1306. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Fornells A, Cunillera T, Mestres-Missé A, & de Diego-Balaguer R (2009). Neurophysiological mechanisms involved in language learning in adults. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 364(1536), 3711–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, & Brett M (2000). Stereotaxic display of brain lesions. Behavioural Neurology, 12(4), 191–200. [DOI] [PubMed] [Google Scholar]
- Sarno MT, Silverman M, & Sands E (1970). Speech therapy and language recovery in severe aphasia. Journal of Speech and Hearing Research, 13(3), 607–623. [DOI] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kümmerer D, Kellmeyer P, Vry MS, et al. (2008). Ventral and dorsal pathways for language. Proceedings of the National Academy of Sciences of the United States of America, 105(46), 18035–18040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, et al. (2006). Dynamics of language reorganization after stroke. Brain, 129(6), 1371–1384. [DOI] [PubMed] [Google Scholar]
- Saur D, Schelter B, Schnell S, Kratochvil D, Küpper H, Kellmeyer P, et al. (2010). Combining functional and anatomical connectivity reveals brain networks for auditory language comprehension. NeuroImage, 49(4), 3187–3197. [DOI] [PubMed] [Google Scholar]
- Schultze K, Vargha-Khadem F, & Mishkin M (2012). Test of motor theory of long-term auditory memory. Proceedings of the National Academy of Sciences of the United States of America, 109(18), 7121–7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian R, & Kiran S (2011). Task-modulated neural activation patterns in chronic stroke patients with aphasia. Aphasiology, 25(8), 927–951. [Google Scholar]
- Seghier ML, Lee HL, Schofield T, Ellis CL, & Price CJ (2008). Inter-subject variability in the use of two different neuronal networks for reading aloud familiar words. NeuroImage, 42(3), 1226–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage, 31(4), 1487–1505. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. (2004). Advances in structural and functional image analysis and implementation in FSL. NeuroImage, 23(1), 208–219. [DOI] [PubMed] [Google Scholar]
- Smith CD, Lori NF, Akbudak E, Sorar E, Gultepe E, Shimony JS, et al. (2009). MRI diffusion tensor tracking of a new amygdalo-fusiform and hippocampo-fusiform pathway system in humans. Journal of Magnetic Resonance Imaging, 29(6), 1248–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CE, & Clark RE (2004). The medial temporal lobe. Annual Review of Neuroscience, 27, 279–306. [DOI] [PubMed] [Google Scholar]
- Taylor KI, & Regard M (2003). Language in the right cerebral hemisphere: contributions from reading studies. News in Physiological Sciences, 18, 257–261. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Ffytche DH, Bizzi A, Dell’Acqua F, Allin M, Walshe, et al. (2011). Atlasing location, asymmetry and inter-subject variability of white matter tracts in the human brain with MR diffusion tractography. NeuroImage, 54(1), 49–59. [DOI] [PubMed] [Google Scholar]
- Tikofsky RS (1971). Two studies of verbal learning by adult aphasics. Cortex, 7, 106–125. [DOI] [PubMed] [Google Scholar]
- Tuomiranta L, Grönholm-Nyman P, Kohen F, Rautakoski P, Laine M, & Martin N (2011). Learning and maintaining new vocabulary in persons with aphasia: two controlled case studies. Aphasiology, 25(9), 1030–1052. [Google Scholar]
- Tuomiranta L, Laine M, & Martin N (2009). Adaptation of the Temple Assessment of Language and Short-term Memory In Aphasia (TALSA) into the Finnish language. Turku: Department of Psychology and Logopedics, Abo Akademi University; (unpublished). [Google Scholar]
- Tuomiranta L, Rautakoski P, Rinne JO, Martin N, & Laine M (2012). Long-term maintenance of novel vocabulary in persons with chronic aphasia. Aphasiology, 26(8), 1053–1073. [Google Scholar]
- Turkeltaub PE, Coslett HB, Thomas AL, Faseyitan O, Benson J, Norise C, et al. (2012). The right hemisphere is not unitary in its role in aphasia recovery. Cortex, 48(9), 1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Flowers DL, Verbalis A, Miranda M, Gareau L, & Eden GF (2004). The neural basis of hyperlexic reading: an fMRI case study. Neuron, 41(1), 11–25. [DOI] [PubMed] [Google Scholar]
- Ueno T, Saito S, Rogers TT, & Lambon Ralph MA (2011). Lichtheim 2: synthesizing aphasia and the neural basis of language in a neurocomputational model of the dual dorsal-ventral language pathways. Neuron, 72(2), 385–396. [DOI] [PubMed] [Google Scholar]
- Wilke M, & Lidzba K (2007). LI-tool: a new toolbox to assess lateralization in functional MR-data. Journal of Neuroscience Methods, 163(1), 128–136. [DOI] [PubMed] [Google Scholar]
- Wong FC, Chandrasekaran B, Garibaldi K, & Wong PC (2011). White matter anisotropy in the ventral language pathway predicts sound-to-word learning success. Journal of Neuroscience, 31(24), 8780–8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, et al. (2009). Bayesian analysis of neuroimaging data in FSL. NeuroImage, 45(1), 173–186. [DOI] [PubMed] [Google Scholar]
- Yeatman JD, Dougherty RF, Ben-Shachar M, & Wandell BA (2012). Development of white matter and reading skills. Proceedings of the National Academy of Sciences of the United States of America, 109(44), E3045–E3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD, Dougherty RF, Rykhlevskaia E, Sherbondy AJ, Deutsch GK, Wandell BA, et al. (2011). Anatomical properties of the arcuate fasciculus predict phonological and reading skills in children. Journal of Cognitive Neuroscience, 23(11), 3304–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]