Abstract
In the second part of this JACC State-of-the-Art Review, an early and sustainable preventive care plan is described for cardiometabolic-based chronic disease. This plan can improve cardiometabolic health by targeting early mechanistic events to decrease the risk for certain cardiovascular diseases (e.g., coronary heart disease, heart failure, and atrial fibrillation). Included are various prevention modalities, intensive lifestyle interventions, pharmacotherapy and cardiovascular outcome trial evidence, and bariatric/metabolic procedures. A tactical approach of implementing published clinical practice guidelines/algorithms for early behavioral, adiposity, and dysglycemia targeting is emphasized, as well as relevant educational and research implications. (J Am Coll Cardiol 2020;75:539–55) © 2020 by the American College of Cardiology Foundation.
Keywords: adipokines, adiposity, atherosclerosis, atrial fibrillation, cardiometabolic, cardiomyopathy, cardiovascular, chronic disease, dysglycemia, health, insulin, obesity, resistance, type 2 diabetes
In the first part of this JACC State-of-the-Art Review, a cardiometabolic-based chronic disease (CMBCD) model is described. This model is substantiated by scientific evidence and aggregated clinical experience, and configured with the intent to expose upstream clinical primary and metabolic drivers that are affected by modifiable risk factors and serve as interventional targets to prevent downstream cardiovascular disease (CVD). The 3 upstream primary drivers of CMBCD are genetics, environment, and behavior. The 2 upstream metabolic drivers of CMBCD are adiposity and dysglycemia. These metabolic drivers interact at the level of insulin resistance, and have been previously configured as adiposity-based chronic disease (ABCD) and dysglycemia-based chronic disease (DBCD) (1,2) (Figure 1). In contrast with practice standards that focus on downstream CVD presentations and complications, which are associated with greater disease burden and intensive therapies, the CMBCD model is designed to facilitate earlier and sustainable interventions, primarily with structured lifestyle change and judicious pharmacotherapy.
FIGURE 1. The Adiposity-, Dysglycemia-, and Cardiometabolic-Based Chronic Disease Models.
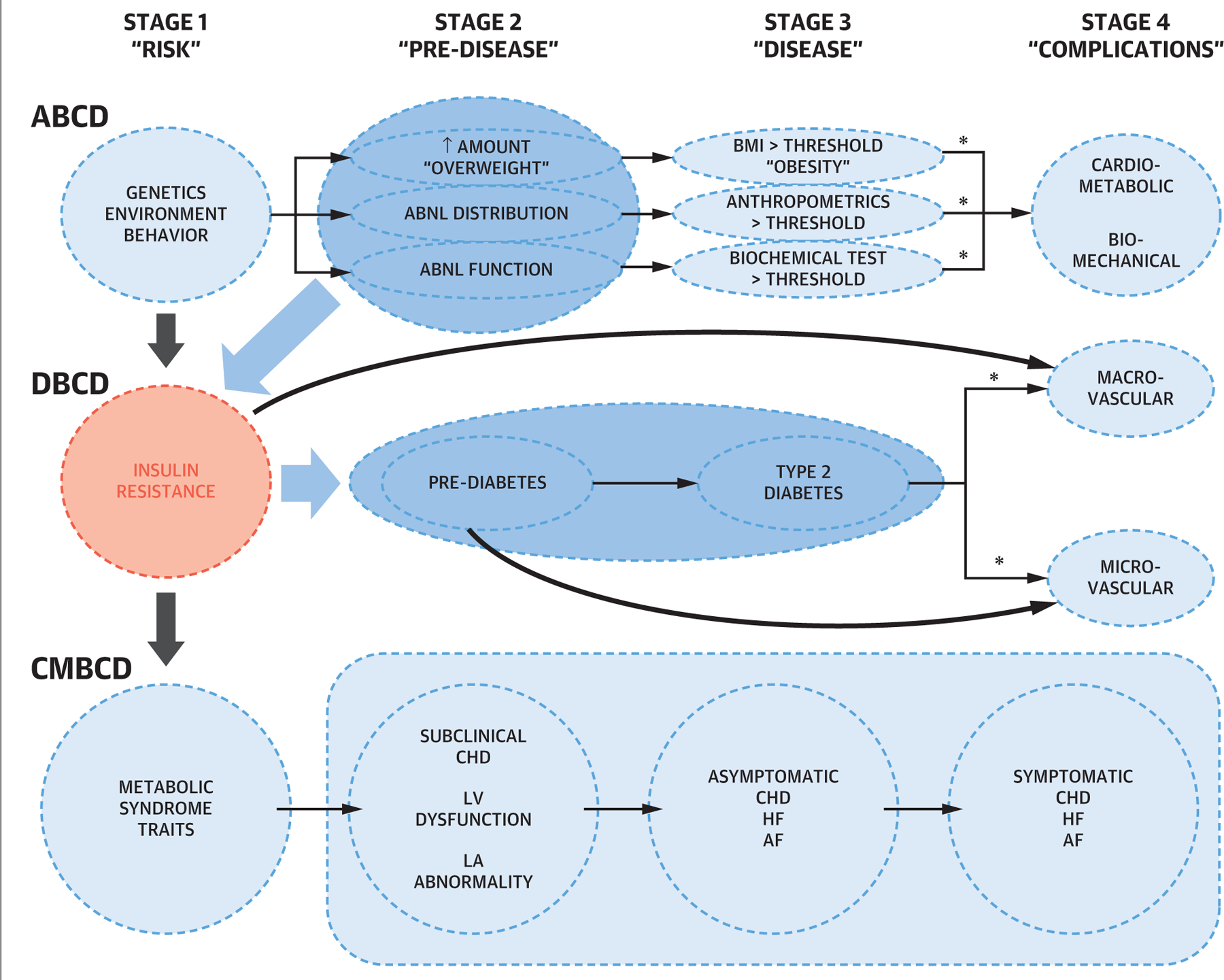
Four distinct chronic disease stages are based on a logical clustering of pathophysiological events for ABCD, DBCD, and CMBCD models. Shaded areas and arrows indicate main causal relationships: abnormal adiposity to insulin resistance to dysglycemia to cardiovascular disease. Asterisks indicate exertions of other metabolic syndrome traits. ABCD = adiposity-based chronic disease; AF = atrial fibrillation; CHD = coronary heart disease; CMBCD = cardiometabolic-based chronic disease; DBCD = dysglycemia-based chronic disease; HF = heart failure; LA = left atrial; LV = left ventricular.
The question remains: “Can targeting early metabolic events related to adiposity and dysglycemia effectively prevent later stages of CMBCD?” The answer is that success can occur by elucidating and modeling complex interactions of these primary and metabolic drivers (1–6). Prevention of disease progression and CVD events in patients with CMBCD has been challenging for multiple reasons. Care is often fragmented between cardiologists and endocrinologists, addressing the cardiovascular and metabolic dimensions of disease, respectively. Although cardiologists may be aware of the importance of metabolic factors for future CVD events, they are frequently hesitant to view management of dysglycemia and diabetes as within their scope. Finally, patients may not come to medical attention at early stages of CMBCD, where intensive prevention efforts may be most efficacious. There are 4 CMBCD stages: risk development, pre-disease, disease, and complications, which incorporate key mechanisms and lead to 3 specific CVD scenarios: coronary heart disease (CHD), heart failure (HF), and atrial fibrillation (AF). Although CHD, HF, and AF are viewed as 3 health outcomes representative of the atherosclerotic-ischemic, hemodynamic-myocardial, and electrophysiological processes frequently comorbid with CMBCD, cerebrovascular events and peripheral artery disease are important vascular outcomes outside the scope of this review. A CMBCD preventive care plan incorporates screening in stage 1, aggressive case finding in stage 2, and diagnostic testing in stages 3 and 4, followed by appropriate intervention that can slow or halt disease progression. This review focuses on adiposity and dysglycemia as drivers of risk in CMBCD. As a result, this review is limited to therapies and recent trial evidence supporting the use of newer agents to address dysglycemia and, secondarily, weight loss. Although critical to managing the risk of CVD events in CMBCD, the role of antihypertensive and lipid-lowering therapies in CMBCD is outside the scope of this review.
In the second part of this JACC State-of-the-Art Review, clinical management of CMBCD is described that concentrates on a preventive care framework. The discussion that follows is based on primordial prevention of cardiovascular risk development in the general population (stage 1), primary prevention of disease in patients at risk and with pre-disease (stage 2), secondary prevention of disease progression in patients with early disease (stage 3), and tertiary prevention of worsening symptom burden in patients with late disease (stage 4; the prevailing care plan today) (7–11). Of note, multiple prevention types, including quaternary prevention (the prevention of over-medicalization), are applied at each CMBCD stage, depending on pathophysiological targets and clinical goals.
MODIFIABLE RISKS
CORONARY HEART DISEASE.
Traditional risk factors for CHD are family history of premature disease (men age <55 years; women age <65 years), age (men age ≥45 years; women age ≥55 years), male sex, hypertension, dyslipidemia, smoking, and diabetes (12,13). Nontraditional risk factors for CHD include insulin resistance, metabolic syndrome (MetS), inflammation, autoimmune disease, human immunodeficiency status, gestational syndromes, psycho-social stressors, various social determinants, and other comorbidities (12). Another nontraditional risk factor for atherosclerosis may be somatic mutations leading to clonal hematopoiesis of indeterminate potential, which may be modifiable with cholesterol-lowering or inflammation-targeted medication (14). Validated risk scores include the American College of Cardiology (ACC)/American Heart Association (AHA) ASCVD Pooled Cohort Risk Calculator, European Systematic Coronary Risk Evaluation algorithm, QRISK calculator, Prospective Cardiovascular Münster model, and the Reynolds Risk Score. Biomarkers of risk include high-sensitivity C-reactive protein (hsCRP) and atherogenic lipid markers including lipoprotein(a). Noninvasive tests (e.g., ankle-brachial index and coronary artery calcium score) can also contribute valuable information to guide decision-making (12). However, adequately powered clinical trials validating many of these nontraditional biomarkers and tests are limited (15).
Levels of cardiorespiratory fitness also predict CHD risk (with incidence rates lowered by 20% for each maximally achieved metabolic equivalent of task unit) (16), incident coronary artery calcification (17), and CVD events (18). Cardiorespiratory fitness may also help explain discrepancies observed with metabolically healthy obese (modestly elevated body mass index [BMI] with low CHD risk) and metabolically unhealthy lean (low BMI and high CHD risk) profiles (19).
HEART FAILURE.
Various metabolic biomarkers and risk factors have been identified in HF (e.g., fasting proinsulin, apolipoprotein B/A1 ratio, serum β-carotene [20], IL-6, hsCRP, macroalbuminuria, obesity [21,22], diabetes [22], tobacco use, and HTN [22,23]). Patients with overweight or mild obesity may have lower risk for HF than those with lower or higher body weight classifications, but this obesity paradox only persists in women after adjusting for cardiorespiratory fitness and other factors, and appears to result from reverse causality (24). Leisure time and more structured physical activity improve cardiorespiratory fitness, cardiac structure and function, and HF incidence and hospitalization risk (25–27). In fact, for every metabolic equivalent of task unit increment, there is a 21% decrease in the multivariable adjusted risk for new-onset HF (28). Also, cardiorespiratory fitness, expressed as the cross product of BMI and the 6-min walk distance, is inversely proportional to HF mortality (29).
ATRIAL FIBRILLATION.
There are 2 primary goals in the management of AF: symptomatic relief with rhythm control, and prevention of stroke and thromboembolic events. Weight reduction strategies can improve not only the quality of life in patients with obesity, but also the clinical burden of AF (30,31). Prevention of AF in type 2 diabetes (T2D) may also succeed with an angiotensin-converting enzyme inhibitor or angiotensin-2 receptor blocker as part of upstream therapy (32).
In the Framingham Heart Study and other cohort studies, obesity was associated with approximately 50% greater risk for AF, with each BMI unit increase conferring a 4% increased AF risk (33,34). Clinical outcomes and mortality associated with AF were also adversely affected by abnormal adiposity (35–38). Both weight loss and increased cardiorespiratory fitness correlated with improved AF outcomes (39–44). Weight loss and risk factor modification targeting sleep apnea, hypertension (HTN), hyperlipidemia, glucose intolerance, and alcohol and tobacco use are included as new recommendations for patients with overweight/obesity and AF in the 2019 AHA/ACC/Heart Rhythm Society updated guidelines for AF management (45).
PREVENTION TYPES
PRIMORDIAL PREVENTION IN STAGE 1 CMBCD.
Primordial prevention applies to stage 1 ABCD, DBCD, and CMBCD. In ABCD, primordial prevention targets potential pathophysiological events that can cause abnormalities in adiposity, including increased adipose tissue mass, that lead to insulin resistance. In DBCD, primordial prevention mitigates abnormal adiposity and insulin resistance, insulin secretory exhaustion as β-cells can no longer compensate for insulin resistance, and adverse effects of hyperglycemia that exacerbate CMBCD progression. It should not be surprising that population-based societal measures, such as improving the built environment and health messaging, as well as screening for unhealthy behaviors and lifestyles early in life, are appropriate and advised in this setting.
Lifestyle interventions on community-based cohorts have been shown to improve the metabolic health of obese children, and the longitudinal FAMILIA (Family-Based Approach in a Minority Community Integrating Systems–Biology for Promotion of Health) studies targeting children ages 3 to 5 are underway to focus on improving cardiovascular risk factors throughout the lifecycle (46,47). In a 2017 updated systematic review by the U.S. Preventive Services Task Force on behavioral counseling for healthy eating and physical activity in patients without CVD risk factors, even modest changes in self-reported healthy activities, particularly in primary care settings, were associated with significant and potentially sustainable, patient-centered, cardiometabolic outcomes (48).
Human and animal studies have demonstrated the effects of various environmental exposures (over-nutrition/obesity, undernutrition, paternal factors, toxicants, stress, physical activity, longevity, and assisted reproductive technologies) on peri-conceptional and in utero development, adiposity and dysglycemia drivers, and CVD outcomes, moving the potential preventive target (e.g., optimizing nutrition during pregnancy) to even earlier in a person’s life (49–52). Healthy eating patterns, such as the DASH (Dietary Approaches to Stop Hypertension) diet (increased whole grains, fruits and vegetables, pulses [edible seeds of legumes: beans, lentils, dry peas, chickpeas, and so on], and nuts, with moderate intake of low-fat dairy and decreased red/processed meats and sweetened beverages), are associated with improved cardiometabolic risk profiles, especially reducing hypertension in adults (53), but also preventing abnormal adiposity in children (54).
PRIMARY PREVENTION IN STAGE 2 CMBCD.
Primary prevention addresses stage 2 ABCD (progression of measurable abnormal adiposity to diagnostic thresholds), stage 2 DBCD (progression of hyperglycemia from pre-diabetes to T2D diagnostic criteria), and stage 2 CMBCD (progression of ABCD and DBCD metabolic drivers to early, detectible CVD). Initiated in 2015, ratified by health care professionals from 38 nations, and published in 2018, the Berlin Declaration proposed actionable policies for primary prevention focused on stage 2 DBCD in the context of decreasing CVD: early detection, prevention, early control, and fair access to high-quality interventions, with essential inclusion of specialist care (55).
Aggressive case finding to detect pre-disease or disease based on the presence of risk factors is appropriate in stage 2 CMBCD. For example, various imaging modalities are available for case finding that can detect abnormal adiposity amounts and distributions, correlating with MetS and CVD risk (56). This is relevant because certain interventions can mitigate the progression of ectopic adiposity to CVD, such as progressive resistance training, which reduces magnetic resonance-quantified epicardial and pericardial fat volume (57). These primary prevention concepts have been recently addressed in the 2019 ACC/AHA guidelines on CVD (58).
SECONDARY PREVENTION IN STAGE 3 CMBCD.
Individual patient-level secondary prevention strategies are implemented in stage 3 CMBCD when early CVD has been demonstrated by various diagnostic tests or a clinical event, with a goal of preventing disease progression of disease or a second event. More specifically, secondary prevention in CVD aims to reduce subsequent events and even mortality by addressing underlying pathophysiology (i.e., residual cholesterol, inflammatory, and thrombotic risks) (59). Moreover, in the CMBCD model, evidence is provided that secondary prevention also applies to specific metabolic drivers. In stage 3 ABCD, this is accomplished by decreasing the detrimental effects of abnormal adiposity on the cardiovascular system, and in stage 3 DBCD, by decreasing the detrimental effects of insulin resistance and/or hyperglycemia (pre-diabetes or T2D) on macrovascular/microvascular complications.
TERTIARY PREVENTION IN STAGE 4 CMBCD.
The majority of current preventive strategies take the form of tertiary prevention. These are interventions in the later course of disease, when symptom burdens and disability are more significant, leading to the usual patient presentation with complaints, so efforts must concentrate on management of complications to improve longevity and quality of life. Tertiary prevention addresses stage 4 ABCD (complications due to abnormal adiposity), stage 4 DBCD (vascular complications due to insulin resistance and/or hyperglycemia [pre-diabetes or T2D]), and stage 4 CMBCD (progressive and symptomatic CHD, HF, and/or AF).
QUATERNARY PREVENTION IN ALL CMBCD STAGES.
Quaternary prevention is a relatively new concept, first presented in 1986 as an intervention to identify patients with over-medicalization to deescalate these actions, avoid new but unnecessary or net harmful interventions, and overall, to behave in an ethical manner (60,61). In ABCD, DBCD, and CMBCD, quaternary prevention is effectively implemented at all stages by prioritizing early lifestyle interventions, and when necessary, judicious pharmacotherapy and procedures. Table 1 summarizes the features of the 5 prevention types discussed above.
TABLE 1.
Cardiometabolic-Based Chronic Disease Prevention Types and Features
| Primordial | Primary | Secondary | Tertiary | Quaternary |
|---|---|---|---|---|
| Entire population (especially youth) | Primordial prevention plus: | Primary prevention plus: | Secondary prevention plus: | Ethical behavior |
| Screening | At-risk population | Diagnostic testing | Noninvasive procedures | Avoid over-medicalization |
| Healthy lifestyle | Case finding | Risk stratification | Surgical procedures | Avoid action if in doubt |
| Behavioral programs | Body imaging | Inflammation focus | ↑ Cost | |
| Health promotion curricula | Specialist care | Cardiac rehabilitation | ↑ latrogenesis | |
| Family-based | Atherosclerosis focus | SGLT2i/GLP1ra | ||
| Plant-based eating pattern | Adiposity focus | Other pharmacology | ||
| High fiber | Dysglycemia focus | |||
| Minimally processed food | Metformin | |||
| Aerobic physical activity | Weight-loss medications | |||
| Progressive resistance training | Possibly SGLT2i |
GLP1ra = glucagon-like peptide 1 receptor agonist; SGLT2i = sodium-glucose cotransporter-2 inhibitors.
INTERVENTIONAL MODALITIES
INTENSIVE LIFESTYLE CHANGE.
Eating patterns and physical activity are often evaluated together as part of a comprehensive lifestyle intervention to decrease the risks associated with CMBCD. Lifestyle medicine—the nonpharmacological and nonsurgical management of chronic disease—encompasses these interventions (Table 2) (62). Achieving a healthy weight (i.e., sufficient weight loss in those with an abnormally increased BMI to prevent or reverse ABCD complications) is a critical objective of lifestyle intervention. Current guideline recommendations and summary Level of Evidence (LOE) recommendations for lifestyle medicine interventions to prevent CVD in patients with diabetes are provided in Online Table 1.
TABLE 2.
Intensive Lifestyle Interventions for Cardiometabolic-Based Chronic Disease
| Healthy eating patterns |
| Aerobic physical activity |
| Progressive resistance and strength training |
| Healthy behavior |
| Weight loss/healthy body composition |
| Sleep hygiene |
| Tobacco cessation |
| Alcohol moderation |
| Community engagement |
| Meal replacements as needed |
| Avoid social-business eating and skipping breakfast |
The Diabetes Prevention Program (n > 3,200 with pre-diabetes with about 3-year follow-up) found that randomization to an intensive lifestyle intervention, with >7% weight reduction on a low-calorie, low-fat diet and incorporating moderate intensity physical activity ≥150 min/week, was associated with approximately 60% (hazard ratio [HR]: 0.42; 95% confidence interval [CI]: 0.44 to 0.52) and 40% (HR: 0.61; 95% CI: 0.49 to 0.76) lower incidence of T2D compared with placebo and metformin, respectively (63).
The Mediterranean diets describe a set of eating patterns with distinct foods, associated with decreased cardiometabolic risk and characterized by relatively high nutrient density, fiber, mono-unsaturated and n-3 polyunsaturated fatty acid, and polyphenol content (64). Adherence with this eating pattern is associated with decreased risk for MetS and other cardiometabolic risk factors, reduced inflammation and hepatic steatosis, and improved renal function (64). In an umbrella evaluation of the Mediterranean diet eating pattern, Galbete et al. (65) affirmed that despite substantial heterogeneity of published meta-analyses, a higher adherence with a Mediterranean eating pattern was associated with lower incidence of mortality from T2D and CVD.
Vegetarian eating patterns were associated with decreased CVD risks, Framingham risk score, and development of MetS (66). A vegan eating pattern, compared with the AHA diet, was found to lower hsCRP levels in patients with CHD (67).
The largest and most extensive trial of physical activity and CVD morbidity and mortality among patients with T2D was the Look AHEAD (Action for Health in Diabetes) trial, which randomized 5,145 patients with T2D to an intensive lifestyle intervention, including caloric restriction, pre-specified caloric intake of fats and protein, meal replacements, and ≥175 min/week of moderate-intensity physical activity by week 26, or to usual care with diabetes support and education (68). Despite weight loss of nearly 9% in the intervention group, and greater improvements in fitness and CVD risk factors after nearly 10 years of follow-up, the trial was stopped early for futility to reduce CVD events (403 with intervention vs. 418 with usual care; HR: 0.95; 95% CI: 0.83 to 1.09; p = 0.51) (68).
Earlier data from the Steno-2 (Intensified Multifactorial Intervention in Patients With Type 2 Diabetes and Microalbuminuria) study randomized 160 patients with T2D and albuminuria to either conventional CVD risk factor management from their general practitioner or to multifactorial intervention over-seen by a diabetes center project team (69). The trial intervention included smoking cessation courses, restricted total and saturated fat intake, light-to-moderate exercise 3 to 5 days/week, and a stepwise intensive regimen that included more stringent control of blood glucose (target A1C <6.5%) and blood pressure (BP) (target <140/85 mm Hg for most of the study), along with lipid-lowering therapy and an angiotensin-converting enzyme inhibitor, regardless of BP (69,70). Patients randomized to the intensive treatment arm had a 53% (HR: 0.47; 95% CI: 0.24 to 0.73) reduction in the composite outcome of CVD death, nonfatal myocardial infarction (MI) or stroke, revascularization, or amputation, along with significant reductions in microvascular endpoints (69).
Various studies have focused on the nutritional component of intensive lifestyle intervention. Recently, the results of the DiRECT (Primary Care-Led Weight Management for Remission of Type 2 Diabetes) trial were published demonstrating 1-year (71) and 2-year (72) effects of total dietary replacement (825 to 853 kcal/day for 3 to 5 months and then stepped food reintroduction over 2 to 8 weeks, with structured support), compared with best practice, in patients with T2D. At 1 year, 24% of subjects in the treatment arm lost >15 kg (compared with none in the control arm), and 46% had T2D remission (compared with only 4% in the control arm) (71). Remission of T2D was related to the amount of weight lost (71). These significant beneficial effects affirm the need for structured lifestyle change in all patients with T2D and abnormal adiposity to prevent the event cascade that defines CMBCD progression.
Although moderate alcohol use (≤1 drink/day for women and 1 to 2 drinks/day for men) may be part of a healthy lifestyle, evidence for cardiometabolic benefit is limited (73). Red wine before or with the evening meal has been associated with improved long-term cardiovascular outcomes in some observational studies. However, data from the Saku study (74) in Japan demonstrated a positive association between alcohol consumption and the incidence of both insulin resistance and impaired insulin secretion. In a recent systematic analysis of the global burden of disease for 195 countries, the level of alcohol use that minimized harm across a broad range of health outcomes was zero (75). In the totality, nonusers should not be advised to begin drinking alcohol for CVD protection.
The Look AHEAD trial results were consistent with decades old scientific evidence on the benefit of and need to assess cardiorespiratory fitness (76,77). Strength training and cardiorespiratory fitness exert a magnitude of secondary prevention benefits similar to many pharmacotherapies (78). When offering lifestyle counseling, teasing out the subtle effects of different physical activity levels, sedentary time, and sleep can be difficult. Using an isotemporal substitution analysis, Dumuid et al. (79) found that, of these variables, time spent engaging moderate-to-vigorous physical activity had the greatest beneficial impact on CVD outcomes.
PHARMACOTHERAPY.
The cardiovascular outcomes trials.
The evidence from cardiovascular outcomes trials (CVOTs) has been largely generated from the population of adults who have T2D and are at risk for atherosclerotic cardiovascular disease, and is based on the safety study experience of the newer medications.
The sodium-glucose cotransporter-2 inhibitors.
General mechanisms.
The sodium-glucose cotransporter-2 inhibitors (SGLT2i) decrease activity of the high-capacity, low-affinity SGLT2 receptor in the proximal tubule of the kidney, which is responsible for reabsorbing nearly 90% of filtered glucose (80). The activity of the SGLT2 is paradoxically increased in states of hyperglycemia, leading to enhanced glucose and sodium reabsorption (81). Four large CVOTs have been completed for SGLT2i in patients with T2D: empagliflozin (EMPA-REG OUTCOME [Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients] [82]), canagliflozin (CANVAS [CANagliflozin cardioVascular Assessment Study] [83] and CREDENCE [Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy] [84]), and dapagliflozin (DECLARE-TIMI 58 [Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events-Thrombolysis In Myocardial Infarction 58] [85]) (Table 3). Based on these CVOTs, the reduction in A1C between active and placebo arms was modest at only 0.1% to 0.6% (82–85), and therefore, is unlikely to account for the reduction in CVD events (86).
TABLE 3.
SGLT2i Cardiovascular Outcomes Trials
| EMPA-REG (82) | CANVAS Program (83) | CREDENCE(84) | DECLARE (85) | |
|---|---|---|---|---|
| Empagliflozin (n = 7,020) | Canagliflozin (n = 10,142) | Canagliflozin (n = 4,401) | Dapagliflozin (n = 17,160) | |
| Median follow-up, yrs | 3.1 | 2.4 | 2.6 | 4.2 |
| Mean age, yrs | 63 | 63 | 63 | 64 |
| Female, % | 29 | 36 | 34 | 37 |
| BMI, kg/m2, mean | 30.6 | 32.0 | 31.3 | 32.1 |
| HbA1c, % | 8.1 | 8.3 | 8.3 | 8.3 |
| Baseline eGFR, ml/min, mean | 74 | 77 | 56 | 85 |
| eGFR <60 ml/min, % | 26 | 20 | 59 | 7 |
| Prior CVD, % | 99 | 66 | 50 | 41 |
| Prior HF, % | 10 | 14 | 15 | 10 |
| 3P-MACE | 0.86 (0.74–0.99) | 0.86 (0.67–0.91) | 0.80 (0.67–0.95) | 0.93 (0.84–1.03) |
| CV death | 0.62 (0.49–0.77) | 0.87 (0.72–1.06) | 0.78 (0.61–1.00) | 0.98 (0.82–1.17) |
| Nonfatal MI | 0.87 (0.70–1.09) | 0.89 (0.73–1.09) | NA | 0.89 (0.77–1.01) |
| Nonfatal stroke | 1.18 (0.89–1.56) | 0.87 (0.69–1.09) | NA | 1.01 (0.84–1.21) |
| CV death or HHF | 0.66 (0.55–0.79) | 0.78 (0.67–0.91) | 0.69 (0.57–0.83) | 0.83 (0.73–0.95) |
| HHF | 0.65 (0.50–0.85) | 0.67 (0.52–0.87) | 0.61 (0.47–0.80) | 0.73 (0.61–0.88) |
| All-cause mortality | 0.68 (0.57–0.82) | 0.87 (0.74–1.01) | 0.83 (0.68–1.02) | 0.93 (0.82–1.04) |
| Renal events* | 0.61 (0.53–0.70) | 0.60 (0.47–0.77) | 0.70 (0.59–0.82) | 0.53 (0.43–0.66) |
Values in parenthesis are HR (95% CI).
Definition varied across trials.
Values in bold are statistically significant at p < 0.05.
3P-MACE = 3-point major adverse cardiac events; BMI = body mass index; CV = cardiovascular; CANVAS = CANagliflozin cardioVascular Assessment Study; CREDENCE = Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy; CVD = cardiovascular disease; DECLARE-TIMI = Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events-Thrombolysis In Myocardial Infarction; eGFR = estimated glomerular filtration rate; EMPA-REG = Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients; HbA1c = hemoglobin A1c; HF = heart failure; HHF = hospitalization for heart failure; MI = myocardial infarction; NA = not available.
Analysis and comparisons of CVOT evidence.
Notably, all 4 of these CVOT were noninferior compared with placebo for cardiovascular safety (82–85). Additionally, EMPA-REG OUTCOME, CANVAS, and CREDENCE demonstrated reductions in a 3-point major adverse cardiac event (MACE) (CVD death, nonfatal MI, or nonfatal stroke) (82–84). DECLARE-TIMI 58 had 2 primary endpoints: a 3-point MACE and a 2-item composite of CVD death or hospitalization for heart failure (HHF) (85). In contrast to EMPA-REG OUTCOME and CANVAS, the 3-point composite MACE was not met in DECLARE-TIMI 58 (HR: 0.93; 95% CI: 0.84 to 1.03; p-superiority = 0.17) (85). However, in DECLARE-TIMI 58, there was a nearly 20% relative risk reduction in 1 of the primary endpoints—CVD death or HHF (HR: 0.83; 95% CI: 0.73 to 0.95; p-superiority = 0.005)—driven by a nearly 30% reduction in HHF (HR: 0.73; 95% CI: 0.61 to 0.88) (85). Consistent reductions in HHF and renal outcomes were observed in all 4 SGLT2i CVOT, but due to the pre-specified hierarchical testing plan in CANVAS, estimates for secondary outcomes were considered nonsignificant and exploratory only (83). Most recently, among 4,744 patients with heart failure and an ejection fraction <40%, dapagliflozin reduced heart failure or death from cardiovascular causes regardless of the presence or absence of diabetes (87).
Safety concerns with SGLT2i.
The SGLT2i were well tolerated in the CVOT with some notable, albeit infrequent, exceptions. Genital infections were more common with SGLT2i than with placebo in all 4 trials (82–85). However, these infections infrequently (<1%) resulted in study drug discontinuation (82–85). Importantly, CANVAS identified amputations and fractures, captured as adverse events of special interest, as new safety concerns at the time (83). Increased fractures or amputations have not been consistently demonstrated with either empagliflozin or dapagliflozin (82,85). Of note, results from a recent search of the U.S. Food and Drug Administration Adverse Event Reporting System supported current warnings that SGLT2i use is associated with increased risk for euglycemic ketoacidosis (88).
The glucagon-like peptide 1 receptor agonists.
General mechanisms.
The incretin pathway plays a key role in the physiological response to oral glucose intake (89). Glucagon-like peptide 1 is an incretin polypeptide secreted by the distal intestinal L cells in response to oral nutrient ingestion, having several downstream effects prior to its degradation by dipeptidyl peptidase-4 (90).
At present, all but 1 glucagon-like peptide 1 receptor agonist (GLP1ra) approved for the treatment of T2D are administered subcutaneously, and differ in structure and duration of effect (91), the exception being oral semaglutide (92). Exenatide and lixisenatide are derived from exogenous Gila monster venom, whereas the remaining 4 commercially available GLP1ra are modifications of the endogenous molecule. Large CVOT have been completed for 6 of the GLP1ra, all of which demonstrated noninferiority compared with placebo (Table 4) (93–98). To date, liraglutide, semaglutide, albiglutide, and dulaglutide (but not exenatide and lixisenatide) each demonstrated superiority in reducing MACE compared with placebo (93–98). However, only liraglutide has demonstrated reductions in both CVD and all-cause mortality (94).
TABLE 4.
GLP1ra Cardiovascular Outcomes Trials
| ELIXA (93) | LEADER (94) | SUSTAIN-6 (95) | EXSCEL (96) | HARMONY (97) | REWIND (98) | PIONEER 6 (92) | |
|---|---|---|---|---|---|---|---|
| Lixisenatide (n = 6,068) | Liraglutide (n = 9,340) | Semaglutide (n = 3,297) | Exenatide QW (n = 14,752) | Albiglutide QW (n = 9,463) | Dulaglutide QW (n = 9,901) | Oral Semaglutide (n = 3,183) | |
| Median follow-up, yrs | 2.1 | 3.8 | 2.1 | 3.2 | 1.6 | 5.4 | 1.3 |
| Mean age, yrs | 60 | 64 | 54 | 62 | 64 | 66 | 66 |
| Female, % | 30 | 36 | 39 | 38 | 31 | 46 | 32 |
| BMI, kg/m2, mean | 30.2 | NA | NA | NA | 32.3 | 32.3 | 32.3 |
| HbA1c, % | 7.7 | 8.7 | 8.7 | 8.1 | 8.8 | 7.3 | 8.2 |
| Baseline eGFR, ml/min, mean | 76 | 75 | 75 | 76 | 79 | 75 | 74 |
| eGFR <60 ml/min, % | 23 | 23 | 28.5 | 18 | 23 | 22 | 27 |
| Prior CVD, % | 100 | 81 | 83 | 73 | 100 | 32 | 85 |
| Prior HF, % | 22 | 18 | 24 | 16 | 20 | 9 | 12 |
| 3P-MACE | 1.02 (0.89–1.17) | 0.87 (0.78–0.97) | 0.74 (0.58–0.95) | 0.91 (0.83–1.00) | 0.78 (0.68–0.90) | 0.88 (0.79–0.99) | 0.79 (0.51–1.11) |
| CV death | 0.98 (0.78–1.22) | 0.78 (0.66–0.93) | 0.98 (0.65–1.48) | 0.88 (0.76–1.02) | 0.93 (0.73–1.19) | 0.91 (0.78–1.06) | 0.49 (0.27–0.92) |
| Nonfatal MI | 1.03 (0.87–1.22) | 0.86 (0.73–1.00) | 0.74 (0.51–1.08) | 0.95 (0.84–1.09) | 0.75 (0.61–0.90)** | 0.96 (0.79–1.16) | 1.18 (0.73–1.90) |
| Nonfatal stroke | 1.12 (0.79–1.58) | 0.86 (0.71–1.06) | 0.61 (0.38–0.99) | 0.86 (0.70–1.07) | 0.86 (0.66–1.14) | 0.76 (0.61–0.95) | 0.74 (0.35–1.57) |
| All-cause mortality | 0.94 (0.78–1.13) | 0.85 (0.74–0.97) | 1.05 (0.74–1.50) | 0.86 (0.77–0.97) | 0.95 (0.79–1.16) | 0.90 (0.80–1.01) | 0.51 (0.31–0.84) |
| HHF | 0.96 (0.75–1.23) | 0.87 (0.73–1.05) | 1.11 (0.77–1.61) | 0.94 (0.78–1.13) | NA | 0.93 (0.77–1.12) | 0.86 (0.48–1.55) |
| Renal events* | 0.81 (0.66–0.99) | 0.78 (0.67–0.92) | 0.64 (0.46–0.88) | 0.85 (0.73–0.98) | NA | 0.85 (0.77–0.93) | NA |
Values in parenthesis are HR (95% CI).
Values in bold are statistically significant at p < 0.05.
Definition varied across trials.
Hazard ratio for fatal or non-fatal MI.
ELIXA = Evaluation of Cardiovascular Outcomes in Patients With Type 2 Diabetes After Acute Coronary Syndrome During Treatment With AVE0010; EXSCEL = Exenatide Study of Cardiovascular Event Lowering Trial; HARMONY = Albiglutide and Cardiovascular Outcomes in Patients With Type 2 Diabetes and Cardiovascular Disease; LEADER = Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results; PIONEER-6 = Cardiovascular Safety of Oral Semaglutide in Subjects With Type 2 Diabetes; REWIND = Researching cardiovascular events with a Weekly INcretin in Diabetes; SUSTAIN-6 = Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes; other abbreviations as in Table 3.
Weight reduction.
The GLP1ra lowered weight significantly more than placebo, with semaglutide 1.0 mg/week lowering body weight up to nearly 4 kg more than placebo (95). Overall, the weight loss associated with GLP1ra use is more than the 0.8 to 2.0 kg weight loss observed in the SGLT2i CVOT (82–85). The weight loss associated with GLP1ra is likely due to multiple mechanisms, including reduced caloric intake, versus the glycosuric caloric loss, and other effects associated with SGLT2i use (99).
Analysis of CVOT evidence.
There have been 6 completed GLP1ra CVOT with nearly all patients (70% to 100%) having CVD (93–98). Of these, only LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) (liraglutide [94]), SUSTAIN-6 (Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes) (semaglutide [95]), and HARMONY (Albiglutide and Cardiovascular Outcomes in Patients With Type 2 Diabetes and Cardiovascular Disease) (albiglutide [97]) demonstrated superiority of the GLP1ra for the composite 3-point MACE outcome. In addition, the REWIND (Researching cardiovascular events with a Weekly INcretin in Diabetes) trial investigated weekly dulaglutide on CVD outcomes, and is more representative of a typical, middle-aged general medicine practice population, with an international scope, primarily women, and a higher proportion (69%) of patients without prior CVD (100). REWIND randomized 9,901 participants to receive dulaglutide or placebo. During a median follow-up of 5.4 years, the primary composite outcome occurred in 12.0% in the dulaglutide group, compared with 13.4% in the placebo group (HR: 0.88; 95% CI: 0.79 to 0.99; p = 0.026) (98). Recently, in PIONEER-6 (Cardiovascular Safety of Oral Semaglutide in Subjects With Type 2 Diabetes), oral semaglutide demonstrated cardiovascular noninferiority to placebo but not superiority, indicating cardiovascular safety and possible efficacy for oral GLP1ra for reduction of cardiovascular events in T2D (92).
The reasons other GLP1ra, such as lixisenatide or exenatide, did not demonstrate superiority compared with placebo for reductions in MACE were possibly due to differences in enrolled patient populations, pharmacokinetics, and composite outcome definitions (86). The main results from REWIND indicating superiority over placebo for CVD event reduction support a class effect of GLP1ra improving outcomes for patients with T2D and CMBCD.
Safety concerns with GLP1ra.
The LEADER and SUSTAIN-6 trials demonstrated potential side effects associated with GLP1ra use, including the risk of retinopathy and acute gallstone disease (86). Other gastrointestinal adverse effects, including nausea/vomiting, have been reported in CVOT with liraglutide (94) and semaglutide (95); albiglutide was associated with more diarrhea but not nausea/vomiting (97), and the occurrence of adverse gastrointestinal events was significantly more common with dulaglutide than placebo (98).
Nuanced decision-making based on CVOT.
To date, all SGLT2i and GLP1ra CVOT data pertain to T2D, and initiating these agents is currently part of T2D comprehensive care, requiring long-term follow-up. The SGLT2i and GLP1ra CVOT also afford the opportunity to compare events in patients with T2D, with and without established CVD. In a recent systematic review and meta-analysis, Zelniker et al. (101) demonstrated that the benefits of SGLT2i were observed in patients with established CVD (p for interaction = 0.05). This meta-analysis studied patients across EMPA-REG, CANVAS, and DECLARE-TIMI 58, with the risk factor–only populations composed of patients from CANVAS and DECLARE-TIMI 58 (101).
Tables 5 and 6 summarize the evidence for SGLT2i and GLP1ra benefits on the presence or absence of established CVD and demonstrated that a reduction in 3-point MACE was only definitive in those with established CVD (a secondary prevention effect). In addition, SGLT2i reduced the risk of CVD death or HHF by 23% (HR: 0.77; 95% CI: 0.71 to 0.84; p < 0.0001), with a similar benefit in patients with and without established CVD, and with and without a history of HF (both primary and secondary prevention effects) (101).
TABLE 5.
Heterogeneity of SGLT2i on Primary Outcome 3-Point MACE*
TABLE 6.
Heterogeneity of GLP1ra on Primary Outcome 3-Point MACE*
| Established CVD | CVD Risk Factors | p Value for Interaction | |
|---|---|---|---|
| HR (95% Cl) | HR (95% Cl) | ||
| ELIXA (93) | 1.02 (0.89–1.17) | NA | |
| LEADER (94) | 0.83 (0.74–0.93) | 1.20 (0.86–1.67) | 0.04 |
| SUSTAIN-6 (95) | 0.72 (0.55–0.93) | 1.00 (0.41–2.46) | 0.49 |
| EXSCEL (96) | 0.90 (0.82–1.00) | 0.99 (0.77–1.28) | 0.50 |
| HARMONY (97) | 0.78 (0.68–0.90) | NA | |
| REWIND (98) | 0.87 (0.74–1.02) | 0.87 (0.74–1.02) | 0.92 |
In these recent CVOTs, it is worth noting that background therapy with statins, aspirin, or metformin was not mandated per trial protocol, but approximately 70% to 90% of participants across the SGTL2i and GLP1ra CVOTs were recorded as receiving these agents depending on study participant characteristics. Notably, this percentage is likely greater than recorded use of these agents in adults with T2D, with and without CVD treated in the community (102–104).
Other agents.
Metformin generally occupies a position as first-line pharmacotherapy for primary prevention in stage 2 DBCD (pre-diabetes) or secondary prevention in stage 3 DBCD (T2D), but this is being reconsidered in patients with high-risk CMBCD, such as with HF (where metformin is associated with decreased mortality by retrospective analysis [105], but no demonstrable benefit based on meta-analyses [106]).
The dipeptidyl peptidase-4 inhibitors (DPP4i) augment levels of endogenously produced GLP1 and have also been studied in CVOT involving patients with T2D. Potential targets of DPP4i mirror those of GLP1ra in CMBCD. To date, 5 DPP4i CVOT have been completed—SAVOR-TIMI 53 (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients With Diabetes Mellitus-Thrombolysis In Myocardial Infarction 53) (107), EXAMINE (EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome) (108), TECOS (Evaluating Cardiovascular Outcomes with Sitagliptin) (109), CARMELINA (CArdiovascular safety and Renal Microvascular outcomE study with LINAgliptin) (110), and CAROLINA (CARdiovascular Outcome study of LINAgliptin versus glimepiride in patients with type 2 diabetes) (111)—in which saxagliptin, alogliptin, sitagliptin, linagliptin, and linagliptin versus glimepiride, respectively, were noninferior for MACE, but none satisfying superiority to placebo criteria in reducing CVD events (Table 7).
TABLE 7.
DPP4i Cardiovascular Outcomes Trials*
| SAVOR-TIMI 53 (107) | EXAMINE (108) | TECOS (109)** | CARMELINA (110) | CAROLINA (111) | |
|---|---|---|---|---|---|
| Saxagliptin (n = 16,492) | Alogliptin (n = 5,380) | Sitagliptin (n = 14,523) | Linagliptin (n = 6,979) | Linagliptin vs. Glimepiride (n = 6,033) | |
| Median follow-up, yrs | 2.1 | 1.5 | 3.0 | 2.2 | 5.9 |
| Mean age, yrs | 65 | 61 | 65 | 66 | 64 |
| Female, % | 33 | 32 | 29 | 37 | 40 |
| BMI, kg/m2, mean | 31.2 | 28.7 | 30.2 | 31.4 | 30.1 |
| HbA1c, % | 8.0 | 8.0 | 7.2 | 8.0 | 7.2 |
| Baseline eGFR, ml/min, mean | 73 | 71 | 75 | 55 | 77 |
| eGFR <6O ml/min, % | <50 ml/min: 16 | 29 | NA | 62 | 19 |
| Prior CVD, % | 78 | 100 | 100 | 57 | 42 |
| Prior HF, % | 13 | 28 | 18 | 27 | 4 |
| 3P-MACE | 1.00 (0.89–1.12) | 0.96 (≤1.16) | 0.99 (0.89–1.10) | 1.02 (0.89–1.17) | 0.98 (0.84–1.13) |
| CV death | 1.03 (0.87–1.22) | 0.85 (0.66–1.10) | 1.03 (0.89–1.19) | 0.96 (0.81–1.14) | 1.00 (0.81–1.24) |
| Nonfatal MI | 0.95 (0.80–1.12) | 1.08 (0.88–1.33) | 0.95 (0.81–1.11) | 1.15 (0.91–1.40) | 1.01 (0.80–1.28) |
| Nonfatal stroke | 1.11 (0.88–1.39) | 0.95 (≤ 1.14) | 0.97 (0.79–1.19) | 0.88 (0.63–1.23) | 0.86 (0.66–1.12) |
| CV death or HHF | NA | 1.00 (0.82–1.20) | 1.02 (0.90–1.15) | NA | 1.00 (0.84–1.20) |
| HHF | 1.27 (1.07–1.51) | 1.07 (0.79–1.46) | 1.00 (0.83–1.20) | 0.90 (0.74–1.08) | 1.21 (0.92–1.59) |
| All-cause mortality | 1.11 (0.96–1.27) | 0.88 (0.71–1.09) | 1.01 (0.90–1.14) | 0.98 (0.84–1.13) | 0.91 (0.78–1.06) |
| Renal events* | 1.08 (0.88–1.3) | NA | NA | 0.98 (0.82–1.18) | NA |
Values in parenthesis are HR (95% CI).
Values in bold are statistically significant at p < 0.05.
Definition varied across trials.
CV death, nonfatal MI and nonfatal stroke was a pre-specified secondary composite outcome in TECOS.
SAVOR-TIMI 53 = Saxagliptin Assessment of Vascular Outcomes Recorded in Patients With Diabetes Mellitus-Thrombolysis In Myocardial Infarction 53; EXAMINE = EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome; TECOS = Trial Evaluating Cardiovascular Outcomes with Sitagliptin; CARMELINA = CArdiovascular safety and Renal Microvascular outcomE study with LINAgliptin; CAROLINA = CARdiovascular Outcome study of LINAgliptin versus glimepiride in patients with type 2 diabetes; other abbreviations as in Table 3.
Hypertriglyceridemia is a MetS trait that can affect the progression of CMBCD. In REDUCE-IT (Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial) (112,113), patients with persistently elevated triglycerides in the setting of atherosclerosis (71%) or diabetes (29%), and despite statin dosing, experienced decreased first, subsequent, and total ischemic CVD events with icosapent ethyl, 2 g twice daily, compared with placebo control subjects.
Obesity medications used with lifestyle interventions produce greater weight loss and durable effects when compared with lifestyle alone (114). Five U.S. Food and Drug Administration–approved medications are currently available for the chronic treatment of obesity: orlistat, lorcaserin, phentermine/topiramate ER, naltrexone ER/bupropion ER, and liraglutide 3 mg. These medications are effective in treating multiple manifestations of CMBCD including improvements in glycemia, lipids, and BP (114). Phentermine/topiramate ER (115) and liraglutide 3 mg (116) have been shown to prevent progression to T2D by w80% in patients with pre-diabetes at baseline over the 2- to 3-year course of these studies. A CVOT for lorcaserin, primarily designed as a noninferiority trial, demonstrated that metabolic benefits were not accompanied by an increase in MACE (117). Cardioprotection against MACE was demonstrated in high-risk patients with T2D at the lower 1.8 mg/day dose of liraglutide (94) and at a dose of 1.0 mg/week of semaglutide (95).
BARIATRIC SURGERY.
Bariatric surgery primarily targets weight loss in patients with obesity, but can also target cardiometabolic risk factors under the designation of metabolic surgery. Two key studies (118,119) that focused on T2D endpoints with bariatric surgery found not only significant reductions in A1C and reduction in T2D medications, but also reductions in HTN, dyslipidemia, inflammation, symptom burden, and health care costs (120). However, there are no CVOT data available for patients undergoing bariatric surgery.
Coronary heart disease.
Bariatric surgery with significant and sustained weight loss is associated with decreased future rates of MI and mortality (121). This may be related, in part, to decreased insulin resistance and inflammation (decreased hsCRP), increased adiponectin, and improved arterial remodeling markers after bariatric surgery (122).
Heart failure.
Based on the Swedish National Patient Registry (123) and analysis of the Swedish Obese Subjects study (124), patients undergoing bariatric surgery have decreased risk of HF post-operatively. Based on 2 large Scandinavian nationwide registries (125), and a retrospective analysis of data from the United States (126), Roux-en-Y gastric bypass was found to be associated with significant reductions in HF incidence, post-operatively. Prior bariatric surgery with successful weight loss has also been associated with better inpatient outcomes (e.g., mortality and length of stay) among those patients with HF (127,128).
Atrial fibrillation.
Unfortunately, the effects of bariatric procedures on AF are inconclusive, with divergent results stemming from different populations and risk profiles studied. From 2008 to 2012, the prevalence of AF among patients undergoing bariatric surgery was 1.9%, primarily in the older population and in men, but not influenced by BMI (129). Although bariatric procedures may be associated with decreased incident AF (130,131), possibly associated with attenuated P-wave dispersions (132), these procedures have also been found to be associated with increased risk of AF requiring emergency room visits or hospitalization for up to 2 years post-operatively (133). Nevertheless, in a retrospective observational cohort study of patients with severe obesity, Donnellan et al. (134) found a lower AF recurrence rate after ablation, performed a mean of 22 months after bariatric surgery, compared with those not having bariatric surgery. These effects have been reviewed (135) and the role of bariatric surgery to decrease CMBCD risk has been incorporated into clinical practice guidelines (CPG) recommendations (136).
IMPLEMENTING THE CMBCD PREVENTION CARE PLAN
In the CMBCD preventive care plan presented here (Central Illustration), strategic targets corresponding to mechanistic events are identified, and then, tactically, published CPG/clinical practice algorithms (CPAs) are selected and implemented by the clinician. Table 8 provides the references for many of the current CPG/CPAs that are relevant for each of these targets and can be used to implement the CMBCD preventive care plan (45,102,114,137–161).
CENTRAL ILLUSTRATION. Cardiometabolic Prevention Care Plan.
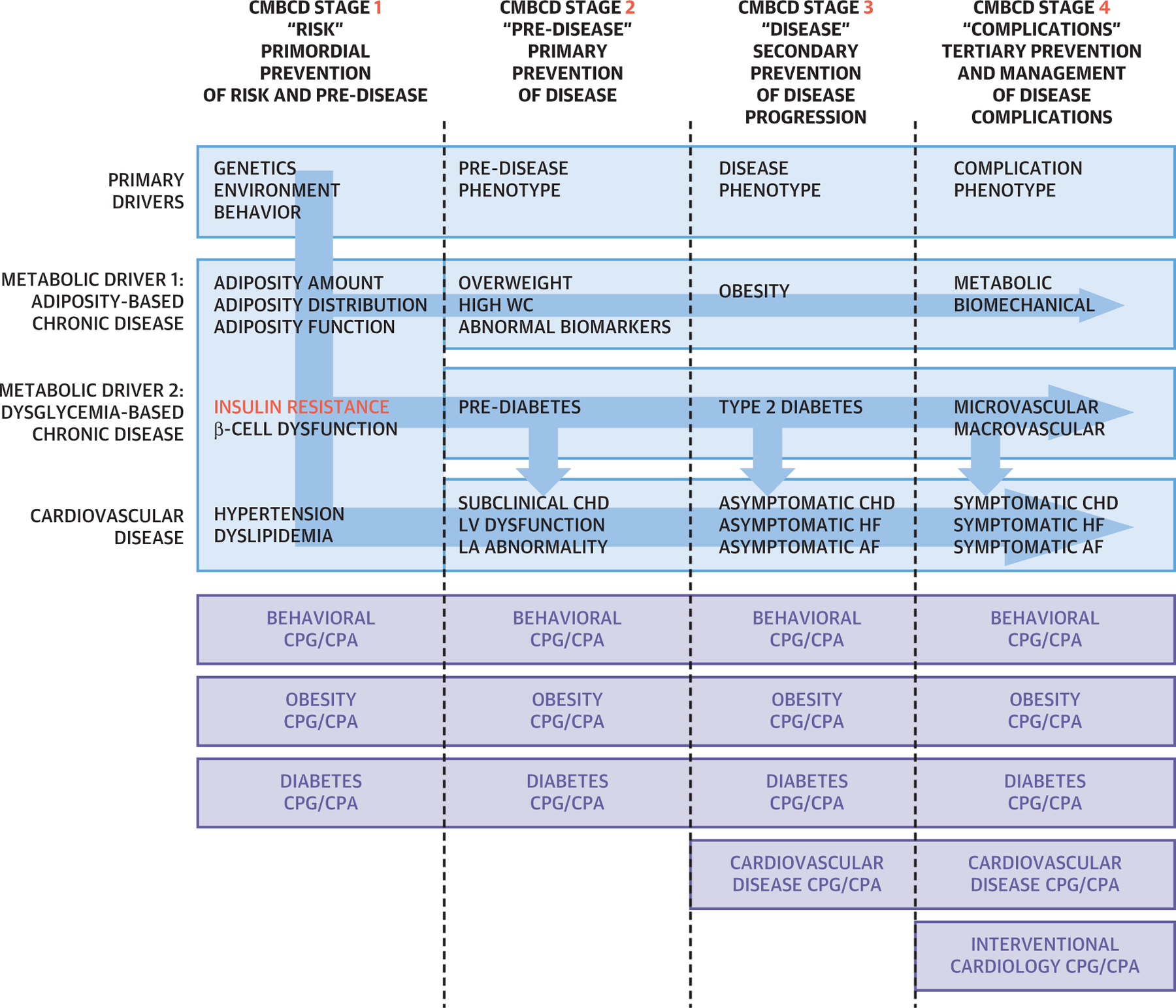
The 4 stages of cardiometabolic-based chronic disease (CMBCD) are depicted at the top. In CMBCD stage I (first column), adiposity, dysglycemia, and other metabolic drivers act concurrently and independently to produce cardiovascular disease. For cardiovascular pre-disease (bottom row, second column), subclinical coronary heart disease is suspected by interrogating 3 vascular beds: increased coronary calcium score, 3-dimensional carotid ultrasound, and ankle-brachial index; left ventricular dysfunction by echocardiography; and left atrial abnormality by electrocardiogram and echocardiogram. Stages are currently defined by artificial boundaries, determined by thresholds of detectability, and positioned along a continuum. Blue arrows indicate complex causal relationships among drivers and stages. Insulin resistance (red) occupies a critical integrating pathophysiological role. Preventive care modalities are depicted in the bottom purple panel and represented by the most current evidence-based clinical practice guidelines and clinical practice algorithm applicable for the specific CMBCD stage and targets. Dotted lines are demarcations between stages in column headings. AF = atrial fibrillation; CHD = coronary heart disease; CI = confidence interval; CMBCD = cardiometabolic-based chronic disease; CPA = clinical practice algorithm; CPG = clinical practice guidelines; HF = heart failure; LA = left atrial; LV = left ventricular; wc = waist circumference.
TABLE 8.
Implementation of Clinical Practice Guidelines for Targeted Early and Sustainable Prevention of CMBCD*
| CMBCD Target | Timing by CMBCD Stage† | Clinical Practice Guideline Topics (Ref. #) |
|---|---|---|
| Behavioral | Early: 1 > 2 > 3 > 4 | Weight loss (137) |
| Healthy eating and physical activity (138) | ||
| Modifiable cardiovascular risk factors (139) | ||
| Obesity | Early: 1 > 2 > 3 > 4 | Comprehensive (114) |
| Pharmacotherapy (140) | ||
| Type 2 diabetes (141) | ||
| Cardiovascular risk (142,143) | ||
| Diabetes | Early: 1 > 2 > 3 > 4 | Comprehensive (144) |
| Nutrition (148) | ||
| Algorithm (145) | ||
| Cardiovascular (102,145,149) | ||
| Hyperglycemia (146) | ||
| Comorbidities (150) | ||
| Lifestyle (147) | ||
| Transcultural (151) | ||
| Cardiovascular disease | Late: 3 > 4 | Hypertension (152) |
| Coronary heart disease (153–155) | ||
| Heart failure (156–158) | ||
| Atrial fibrillation (45) | ||
| Cardiovascular disease | Late:4 | Coronary revascularization (159) complications |
| Ventricular arrhythmias and sudden death (160) | ||
| Advanced heart failure and transplantation (161) |
See Central Illustration for schematic representation. For primary drivers, there are no evidence-based clinical practice guidelines/clinical practice algorithms relevant for health care professionals on genetic or environmental interventions.
Timing for preventive care actions should be as early as possible. Behavioral, obesity, and diabetes targets can still be addressed at all stages. Cardiovascular disease with or without complications are addressed in later stages. Stage 1 is “risk”; stage 2 is “pre-disease”; stage 3 is “disease”; and stage 4 is “complications.”
CONCLUSIONS
In parts 1 and 2 of this JACC State-of-the-Art Review, a new model for CMBCD is presented with an emphasis on early and sustainable prevention with consequent optimization of cardiometabolic health. The purpose of this new pathophysiological construct is to provide a template for the implementation of specific preventive strategies to improve outcomes.
If this new CMBCD model is to be accepted and disseminated, then an appropriate infrastructure should be established to optimize implementations for populations and individuals. The implementation Science Work Group of the National Heart, Lung, and Blood Institute addressed this issue with respect to CPG and found that potential strategies include educational outreach, multifaceted interventions, and more research (162). Educational initiatives will also need to focus on lifestyle medicine, the interactions with cardiometabolic pathophysiology, and suitable implementation tactics that can be incorporated into formal training programs, such as a preventive cardiology fellowship, clinic, and inpatient service (163,164).
Supplementary Material
HIGHLIGHTS.
The CMBCD prevention care plan is designed to implement early, sustainable, and multimodality protocols for all patients with CVD.
Behavior, adiposity, insulin resistance, dysglycemia, and other MetS traits are the principal targets of the CMBCD preventive care plan for CHD, HF, and AF.
Structured lifestyle change involving healthy eating patterns and physical activity, judicious use of cardioprotective pharmacotherapy based on cardiovascular outcome trial data, and bariatric procedures are emphasized.
Acknowledgments
Dr. Mechanick has received honoraria from Abbott Nutrition International. Dr. Farkouh has received research grants from Amgen, Novartis, and Novo Nordisk. Dr. Newman has received research grants from the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (K23HL125991); and has received honoraria from Creative Educational Concepts. Dr. Garvey has served on ad hoc advisory boards for Sanofi, Novo Nordisk, Boehringer Ingelheim, Gilead, Amgen, BOYDSense, and the American Medical Group Association; and has conducted research sponsored by the University of Alabama at Birmingham funded by Sanofi, Merck/Pfizer, Novo Nordisk, AstraZeneca, and Lexicon.
ABBREVIATIONS AND ACRONYMS
- ABCD
adiposity-based chronic disease
- AF
atrial fibrillation
- AHA
American Heart Association
- BMI
body mass index
- BP
blood pressure
- CHD
coronary heart disease
- CI
confidence interval
- CMBCD
cardiometabolic-based chronic disease
- CPA
clinical practice algorithm
- CPG
clinical practice guidelines
- CVD
cardiovascular disease
- CVOT
cardiovascular outcomes trials
- DBCD
dysglycemia-based chronic disease
- DPP4i
dipeptidyl peptidase-4 inhibitor(s)
- GLP1ra
glucagon-like peptide 1 receptor agonist
- HF
heart failure
- hsCRP
high-sensitivity C-reactive protein
- MACE
major adverse cardiac events
- MET
metabolic equivalent of task
- MetS
metabolic syndrome
- MI
myocardial infarction
- PA
physical activity
- SGLT2i
sodium-glucose cotransporter-2 inhibitors
- T2D
type 2 diabetes
Footnotes
APPENDIX For a supplemental table, please see the online version of this paper.
REFERENCES
- 1.Mechanick JI, Hurley DL, Garvey WT. Adiposity-based chronic disease as a new diagnostic term: American Association of Clinical Endocrinologists and the American College of Endocrinology position statement. Endocr Pract 2017;23:372–8. [DOI] [PubMed] [Google Scholar]
- 2.Mechanick JI, Garber AJ, Grunberger G, et al. Dysglycemia-based chronic disease: an American Association of Clinical Endocrinologists position statement. Endocr Pract 2018;24:995–1011. [DOI] [PubMed] [Google Scholar]
- 3.Mechanick JI, Zhao S, Garvey WT. The adipokine-cardiovascular-lifestyle network. J Am Coll Cardiol 2016;68:1787–803. [DOI] [PubMed] [Google Scholar]
- 4.Mechanick JI, Zhao S, Garvey WT. Leptin, an adipokine with central importance in the global obesity problem. Glob Heart 2018;13:113–27. [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner C, Spath-Blass V, Niederkofler V, et al. A novel network-based approach for discovering dynamic metabolic biomarkers in cardiovascular disease. PLoS ONE 2018;13: e0208953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diez D, Wheelock AM, Goto S, et al. The use of network analyses for elucidating mechanisms in cardiovascular disease. Mol Biosys 2010;6: 289–304. [DOI] [PubMed] [Google Scholar]
- 7.Arps K, Pallazola VA, Cardoso R, et al. Clinician’s guide to the updated ABCs of cardiovascular disease prevention: a review part 1. Am J Med 2019; 132:e569–80. [DOI] [PubMed] [Google Scholar]
- 8.Newman JD, Schwartzbard AZ, Weintraub HS, et al. Primary prevention of cardiovascular disease in diabetes mellitus. J Am Coll Cardiol 2017;70: 883–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peñalvo JL, Santos-Beneit G, Sotos-Prieto M, et al. The SI! Program for cardiovascular health promotion in early childhood: a cluster-randomized trial. J Am Coll Cardiol 2015;66: 1525–34. [DOI] [PubMed] [Google Scholar]
- 10.Karunathilake SP, Ganegoda GU. Secondary prevention of cardiovascular diseases and application of technology for early diagnosis. Biomed Res Internat 2018;2018:5767864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norma AH, Tesser CD. Quaternary prevention: a balanced approach to demedicalisation. Br J Gen Pract 2019;69:28–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khambhati J, Allard-Ratick M, Dhindsa D, et al. The art of cardiovascular risk assessment. Clin Cardiol 2018;41:677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenbilt PD. Extreme atherosclerotic cardiovascular disease (ASCVD) risk recognition. Curr Diab Rep 2019;19:61. [DOI] [PubMed] [Google Scholar]
- 14.Jaiswal S, Natarajan P, Silver AJ, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med 2017;377:111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin JS, Evans CV, Johnson E, et al. Nontraditional risk factors in cardiovascular disease risk assessment. JAMA 2018;320:281–97. [DOI] [PubMed] [Google Scholar]
- 16.Gander JC, Sui X, Hébert JR, et al. Association of cardiorespiratory fitness with coronary heart disease in asymptomatic men. Mayo Clin Proc 2015;90:1372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee C-D, Jacobs DR, Hankinson A, et al. Cardiorespiratory fitness and coronary artery calcification in young adults: the CARDIA study. Atherosclerosis 2009;203:263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zafrir B, Azaiza M, Gaspar T, et al. Low cardiorespiratory fitness and coronary artery calcification: complementary cardiovascular risk predictors in asymptomatic type 2 diabetics. Atherosclerosis 2015;241:634–40. [DOI] [PubMed] [Google Scholar]
- 19.Kokkinos P, Faselis C, Myers J, et al. Cardiorespiratory fitness and the paradoxical BMI-mortality risk Association in Male Veterans. Mayo Clin Proc 2014;89:754–62. [DOI] [PubMed] [Google Scholar]
- 20.Ingelsson E, Arnlov J, Sundstrom J, et al. Novel metabolic risk factors for heart failure. J Am Coll Cardiol 2005;46:2054–60. [DOI] [PubMed] [Google Scholar]
- 21.Bahrami H, Bluemke DA, Kronmal R, et al. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol 2008;51:1775–83. [DOI] [PubMed] [Google Scholar]
- 22.Uijl A, Koudstaal S, Vaartjes I, et al. Risk for heart failure: the opportunity of prevention with the American Heart Association’s Life’s Simple 7. J Am Coll Cardiol HF 2019;7:637–47. [DOI] [PubMed] [Google Scholar]
- 23.Ohkuma T, Komorita Y, Peters SAE, et al. Diabetes as a risk factor for heart failure in women and men: a systematic review and meta-analysis of 47 cohorts including 12 million individuals. Diabetologia 2019;62:1550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vest AR, Wu Y, Hachamovitch R, Young JB, Cho L. The heart failure overweight/obesity survival paradox: the missing sex link. J Am Coll Cardiol HF 2015;3:917–26. [DOI] [PubMed] [Google Scholar]
- 25.Pandey A, LaMonte M, Klein L, et al. Relationship between physical activity, body mass index, and risk of heart failure. J Am Coll Cardiol 2017;69:1129–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berry JD, Pandey A, Gao A, et al. Physical fitness and risk for heart failure and coronary artery disease. Circ Heart Fail 2013;6:627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandey A, Allen NB, Ayers C, et al. Fitness in young adulthood and long-term cardiac structure and function. J Am Coll Cardiol HF 2017;5:347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan H, Kunutsor S, Rauramaa R, et al. Cardiorespiratory fitness and risk of heart failure: a population-based follow-up study. Eur J Heart Fail 2014;16:180–8. [DOI] [PubMed] [Google Scholar]
- 29.Zafrir B, Salman N, Amir O. Joint impact of body mass index and physical capacity on mortality in patients with systolic heart failure. Am J Cardiol 2014;113:1217–21. [DOI] [PubMed] [Google Scholar]
- 30.Pathak RK, Middeldorp ME, Meredith M, et al. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol 2015;65:2159–69. [DOI] [PubMed] [Google Scholar]
- 31.Abed HS, Wittert GA, Leong DP, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA 2013;310:2050–60. [DOI] [PubMed] [Google Scholar]
- 32.Lin Y, Li H, Lan X, et al. Mechanism of and therapeutic strategy for atrial fibrillation associated with diabetes mellitus. ScientificWorldJournal 2013;2013:209428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wanahita N, Messerli FH, Bangalore S, et al. Atrial fibrillation and obesity-results of a meta-analysis. Am Heart J 2008;155:310–5. [DOI] [PubMed] [Google Scholar]
- 34.Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA 2004;292:2471–7. [DOI] [PubMed] [Google Scholar]
- 35.Badheka AO, Rathod A, Kizilbash MA, et al. Influence of obesity on outcomes in atrial fibrillation: yet another obesity paradox. Am J Med 2010;123:646–51. [DOI] [PubMed] [Google Scholar]
- 36.Inoue H, Kodani E, Atarashi H, et al. Impact of body mass index on the prognosis of Japanese patients with non-valvular atrial fibrillation. Am J Cardiol 2017;118:215–21. [DOI] [PubMed] [Google Scholar]
- 37.Pandey A, Gersh BJ, McGuire DK, et al. Association of body mass index with care and outcomes in patients with atrial fibrillation: results from the ORBIT-AF registry. J Am Coll Cardiol EP 2016;2: 355–63. [DOI] [PubMed] [Google Scholar]
- 38.Sandhu RK, Ezekowitz J, Andersson U, et al. The “obesity paradox” in atrial fibrillation: observations from the ARISTOTLE (Apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation) trial. Eur Heart J 2016; 37:2869–78. [DOI] [PubMed] [Google Scholar]
- 39.Thompson PD. Physical fitness, physical activity, exercise training, and atrial fibrillation. J Am Coll Cardiol 2015;66:997–9. [DOI] [PubMed] [Google Scholar]
- 40.Mozaffarian D, Furberg CD, Psaty BM, et al. Physical activity and incidence of atrial fibrillation in older adults. Circulation 2008;118:800–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drca N, Wolk A, Jensen-Urstad M, et al. Physical activity is associated with a reduced risk of atrial fibrillation in middle-aged and elderly women. Heart 2015;101:1627–30. [DOI] [PubMed] [Google Scholar]
- 42.Qureshi WT, Alirhayim Z, Blaha MJ, et al. Cardiorespiratory fitness and risk of incident atrial fibrillation results from the Henry Ford exercise testing (FIT) project. Circulation 2015;131: 1827–34. [DOI] [PubMed] [Google Scholar]
- 43.Khan H, Kella D, Rauramaa R, et al. Cardiorespiratory fitness and atrial fibrillation: a population-based follow-up study. Heart Rhythm 2015;12:1424–30. [DOI] [PubMed] [Google Scholar]
- 44.Pathak RK, Elliott A, Middeldorp ME, et al. Impact of CARDIOrespiratory FITness on arrhythmia recurrence in obese individuals with atrial fibrillation the CARDIO-FIT study. J Am Coll Cardiol 2015;66:985–96. [DOI] [PubMed] [Google Scholar]
- 45.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. J Am Coll Cardiol 2019;74:104–32. [DOI] [PubMed] [Google Scholar]
- 46.Wylie-Rosett J, Groisman-Perelstein AE, Diamantis PM, et al. Embedding weight management into safety-net pediatric primary care: randomized controlled trial. Int J Behav Nutr Phys Act 2018;15:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bansilal S, Vedanthan R, Kovacic JC, et al. Rationale and design of Family-Based Approach in a Minority Community Integrating Systems–Biology for Promotion of Health (FAMILIA). Am Heart J 2017;187:170–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patnode CD, Evans CV, Senger CA, et al. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults without known cardiovascular disease risk factors: updated systematic review for the U.S. Preventive Services Task Force. Evidence Synthesis, Number 152. AHRQ Publication No. 15-05222-EF-1,2017. [PubMed] [Google Scholar]
- 49.Fleming TP, Watkins A, Velazquez MA, et al. Origins of lifetime health around the time of conception: causes and consequences. Lancet 2018;391:1842–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Preston JD, Reynolds LJ, Pearson KJ. Developmental origins of health span and life span: a mini-review. Gerontol 2018;64:237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olson M, Chambers M, Shaibi G. Pediatric markers of adult cardiovascular disease. Curr Pediatr Rev 2017;13:255–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Horn L, Vincent E, Perak AM. Preserving cardiovascular health in young children: beginning healthier by starting earlier. Curr Atheroscler Rep 2018;20:26. [DOI] [PubMed] [Google Scholar]
- 53.Fung TT, Chiuve SE, McCullough ML, et al. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 2008;168:713–20. [DOI] [PubMed] [Google Scholar]
- 54.Farhadnejad H, Asghari G, Mirmiran P, et al. Dietary approach to stop hypertension diet and cardiovascular risk factors among 10- to 18-year-old infividuals. Obesity 2018;13:185–94. [DOI] [PubMed] [Google Scholar]
- 55.Ceriello A, Gavin JR, Boulton AJM, et al. The Berlin Declaration: a call to action to improve early actions related to type 2 diabetes. How can specialist care help? Diab Res Clin Pract 2018;139: 392–9. [DOI] [PubMed] [Google Scholar]
- 56.Kim SR, Lerman LO. Diagnostic imaging in the management of patients with metabolic syndrome. Translat Res 2018;194:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fernandez-del-Valle M, Gonzales JU, Kloiber S, et al. Effects of resistance training on MRI-derived epicardial fat volume and arterial stiffness in women with obesity: a randomized pilot study. Eur J Appl Physiol 2018;118:1231–40. [DOI] [PubMed] [Google Scholar]
- 58.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease. J Am Coll Cardiol 2019;74:e177–232.30894318 [Google Scholar]
- 59.Kalbacher D, Waldeyer C, Blankenberg S, et al. Beyond conventional secondary prevention in coronary artery disease—what to choose in the era of CANTOS, COMPASS, FOURIER, ODYSSEY and PEGASIS-TIMI 54? A review on contemporary literature. Ann Transl Med 2018;6:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Norman AH, Tesser CD. Quaternary prevention: a balanced approach to demedicalisation. Br J Gen Pract 2019;69:28–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martins C, Godycki-Cwirko M, Heleno B, et al. Quaternary prevention: reviewing the concept. Eur J Gen Pract 2018;24:106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mechanick JI, Kushner RF. Why lifestyle medicine? In: Mechanick JI, Kushner RF, editors. Lifestyle Medicine–A Manual for Clinical Practice. New York: Springer, 2016:1–8. [Google Scholar]
- 63.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Russo MA, Perrone GA, Polese M, et al. Molecular nutrient targeting with Mediterranean diets. In: Mechanick JI, Via MA, Zhao S, editors. Molecular Nutrition. Washington, DC: Endocrine Press, 2015:63–79. [Google Scholar]
- 65.Galbete C, Schwingshackl L, Schwedhelm C, et al. Evaluating Mediterranean diet and risk of chronic disease in cohort studies: an umbrella review of meta-analyses. Eur J Epidemiol 2018;33: 909–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Navarro JCA, Antoniazzi L, Oki AM, et al. Prevalence of metabolic syndrome and Framingham Risk Score in apparently healthy vegetarian and omnivorous men. Arq Bras Cardiol 2018;110: 430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shah B, Newman JD, Woolf K, et al. Anti-inflammatory effects of a vegan diet versus the American Heart Association-recommended diet in coronary artery disease trial. J Am Heart Assoc 2018;7:e011367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wing RR, Bolin P, Brancati FL, et al. , for the Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gaede P, Vedel P, Parving H, et al. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet 1999;353: 617–22. [DOI] [PubMed] [Google Scholar]
- 70.Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003; 348:383–93. [DOI] [PubMed] [Google Scholar]
- 71.Lean MEJ, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet 2018;391:541–51. [DOI] [PubMed] [Google Scholar]
- 72.Lean MEJ, Leslie WS, Barnes AC, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol 2019;7:344–55. [DOI] [PubMed] [Google Scholar]
- 73.Tatsumi Y, Morimoto A, Asayama K, et al. Association between alcohol consumption and incidence of impaired insulin secretion and insulin resistance in Japanese: The Saku study. Diab Res Clin Pract 2018;135:11–7. [DOI] [PubMed] [Google Scholar]
- 74.O’Keefe EL, DiNicolantonio JJ, O’Keefe JH, et al. Alcohol and CV health: Jekyll and Hyde J-curves. Prog Cardiovasc Dis 2018;61:68–75. [DOI] [PubMed] [Google Scholar]
- 75.GBD 2016 Alcohol Collaborators. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018;392: 1015–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blair SN, Kohl HW III., Paffenbarger RS Jr., et al. Physical fitness and all-cause mortality: a prospective study of healthy men and women. JAMA 1989;262:2395–401. [DOI] [PubMed] [Google Scholar]
- 77.Blair Kennedy A, Lavie CJ, Blair SN. Fitness or fatness. Which is more important? JAMA 2018; 319:231–2. [DOI] [PubMed] [Google Scholar]
- 78.Naci H, Ioannidis JPA. Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. BMJ 2013;347:f5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dumuid D, Lewis LK, Olds TS, et al. Relationships between older adults’ use of time and cardio-respiratory fitness, obesity and cardiometabolic risk: a compositional isotemporal substitution analysis. Maturitas 2018;110:104–10. [DOI] [PubMed] [Google Scholar]
- 80.Gallo LA, Wright EM, Vallon V. Probing SGLT2 as a therapeutic target for diabetes: basic physiology and consequences. Diab Vasc Dis Res 2015; 12:78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rahmoune H, Thompson PW, Ward JM, et al. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes 2005;54:3427–34. [DOI] [PubMed] [Google Scholar]
- 82.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373: 2117–28. [DOI] [PubMed] [Google Scholar]
- 83.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–57. [DOI] [PubMed] [Google Scholar]
- 84.Percovic V, Jardine MJ, Neal B, et al. , for the CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–306. [DOI] [PubMed] [Google Scholar]
- 85.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–57. [DOI] [PubMed] [Google Scholar]
- 86.Newman JD, Vani AK, Aleman JO, et al. The changing landscape of diabetes therapy for cardiovascular risk reduction: JACC state-of-the-art review. J Am Coll Cardiol 2018;72:1856–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 88.Blau JE, Tella SH, Taylor SI, et al. Ketoacidosis associated with SGLT2 inhibitor treatment: analysis of FAERS data. Diabetes Metab Res Rev 2017; 33:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Perley M, Kipnis D. Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic subjects. J Clin Invest 1967;46: 1954–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Deacon C. Circulation and degradation of GIP and GLP-1. Horm Metab Res 2004;36:761–5. [DOI] [PubMed] [Google Scholar]
- 91.Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diab Care 2011;34 Suppl 2:S279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2019; 381:841–51. [DOI] [PubMed] [Google Scholar]
- 93.Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015;373: 2247–57. [DOI] [PubMed] [Google Scholar]
- 94.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marso SP, Bain SC, Consoli A, et al. , for the SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–44. [DOI] [PubMed] [Google Scholar]
- 96.Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017; 377:1228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 2018;392:1519–29. [DOI] [PubMed] [Google Scholar]
- 98.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomized placebo-controlled trial. Lancet 2019;13:121–30. [DOI] [PubMed] [Google Scholar]
- 99.Heerspink H, Perkins BA, Fitchett DH, et al. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus. Circulation 2016; 134:752–72. [DOI] [PubMed] [Google Scholar]
- 100.Gerstein HC, Colhoun HM, Dagenais GR, et al. Design and baseline characteristics of participants in the Researching cardiovascular events with a Weekly INcretin in Diabetes (REWIND) trial on the cardiovascular effects of dulaglutide. Diabetes Obes Metab 2018;20:42–9. [DOI] [PubMed] [Google Scholar]
- 101.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019;393: 31–9. [DOI] [PubMed] [Google Scholar]
- 102.American Diabetes Association. Cardiovascular disease and risk management: standards of medical care in diabetes–2019. Diabetes Care 2019;42 Suppl 1:S103–23. [DOI] [PubMed] [Google Scholar]
- 103.Ali MK, Bullard KM, Saaddine JB, et al. Achievement of goals in U.S. diabetes care, 1999–2010. N Engl J Med 2013;368:1613–24. [DOI] [PubMed] [Google Scholar]
- 104.Newman JD, Berger JS, Ladapo JA. Underuse of medications and lifestyle counselling to prevent cardiovascular disease in patients with diabetes. Diabetes Care 2019;42:e75–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Retwinski A, Kosmalski M, Crespo-Leiro M, et al. The influence of metformin and the presence of type 2 diabetes mellitus on mortality and hospitalization in patients with heart failure. Kardiol Pol 2018;76:1336–43. [DOI] [PubMed] [Google Scholar]
- 106.Packer M Is metformin beneficial for heart failure in patients with type 2 diabetes? Diab Res Clin Pract 2018;136:168–70. [DOI] [PubMed] [Google Scholar]
- 107.Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–26. [DOI] [PubMed] [Google Scholar]
- 108.White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369:1327–35. [DOI] [PubMed] [Google Scholar]
- 109.Green JB, Bethel MA, Armstrong PW, et al. , for the TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:232–42. [DOI] [PubMed] [Google Scholar]
- 110.Rosenstock J, Perkovic V, Johansen OE, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: The CARMELINA Randomized Clinical Trial. JAMA 2019;321: 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rosenstock J, Kahn SE, Johansen OE, et al. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: The CAROLINA Randomized Clinical Trial. JAMA 2019. September 19 [E-pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380: 11–22. [DOI] [PubMed] [Google Scholar]
- 113.Bhatt DL, Steg PG, Miller M, et al. Effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J Am Coll Cardiol 2019;73:2791–802. [DOI] [PubMed] [Google Scholar]
- 114.Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for comprehensive medical care of patients with obesity. Endocr Pract 2016; 22 Suppl 3:1–203. [DOI] [PubMed] [Google Scholar]
- 115.Garvey WT, Ryan DH, Henry R, et al. Prevention of type 2 diabetes in subjects with prediabetes and metabolic syndrome treated with phentermine and topiramate extended-release. Diabetes Care 2014;37:912–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.le Roux CW, Astrup A, Fujioka K, et al. , for the SCALE Obesity Prediabetes NN8022-1839 Study Group. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet 2017;389: 1399–409. [DOI] [PubMed] [Google Scholar]
- 117.Bohula EA, Wiviott SD, McGuire DK, et al. , for the CAMELLIA–TIMI 61 Steering Committee and Investigators. Cardiovascular safety of lorcaserin in overweight or obese patients. N Engl J Med 2018;379:1107–17. [DOI] [PubMed] [Google Scholar]
- 118.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015;386:964–73. [DOI] [PubMed] [Google Scholar]
- 119.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes: 5-year outcomes. N Engl J Med 2017; 376:641–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Neovius M, Narbro K, Keating C, et al. Healthcare costs during 15 years after bariatric surgery for patients with different baseline glucose status. Lancet Diabetes Endocrinol 2015; 3:855–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vogel JA, Franklin BA, Zalesin KC, et al. Reduction in predicted coronary heart disease risk after substantial weight reduction after bariatric surgery. Am J Cardiol 2007;99: 222–6. [DOI] [PubMed] [Google Scholar]
- 122.Domienik-Karlowicz J, Rymarczyk Z, Dzikowska-Diduch O, et al. Emerging markers of atherosclerosis before and after bariatric surgery. Obes Surg 2015;25:486–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Persson CE, Bjorck L, Lagergren J, et al. Risk of heart failure in obese patients with and without bariatric surgery in Sweden – a registry-based study. J Cardiac Fail 2017;23:530–7. [DOI] [PubMed] [Google Scholar]
- 124.Jamaly S, Carlsson L, Peltonen M, et al. Surgical obesity treatment and the risk of heart failure. Eur Heart J 2019;40:2131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sundstrom J, Bruze G, Ottosson J, et al. Weight loss and heart failure. Circulation 2017;135: 1577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Benotti PN, Wood GC, Carey DJ, et al. Gastric bypass surgery produces a durable reduction in cardiovascular disease risk factors and reduces the long-term risks of congestive heart failure. J Am Heart Assoc 2017;6:e005126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Han H, Zhu T, Guo Y, et al. Impact of prior bariatric surgery on outcomes of hospitalized patients with heart failure: a population-based study. Surg Obes Rel Dis 2019;15:469–77. [DOI] [PubMed] [Google Scholar]
- 128.Aleassa EM, Khorgami Z, Kindel TL, et al. Impact of bariatric surgery on heart failure mortality. Surg Obes Rel Dis 2019;15:1189–96. [DOI] [PubMed] [Google Scholar]
- 129.Shoemaker MB, Gidfar S, Pipilas DC, et al. Prevalence and predictors of atrial fibrillation among patients undergoing bariatric surgery. Obes Surg 2014;24:611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jamaly S, Carlsson L, Peltonen M, et al. Bariatric surgery and the risk of new-onset atrial fibrillation in Swedish obese subjects. J Am Coll Cardiol 2016;68:2497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lynch KT, Mehaffey JH, Hawkins RB, et al. Bariatric surgery reduces incidence of atrial fibrillation: a propensity score-matched analysis. Surg Obes Rel Dis 2019;15:279–87. [DOI] [PubMed] [Google Scholar]
- 132.Yilmaz M, Altin C, Tekin A, et al. Assessment of atrial fibrillation and ventricular arrhythmia risk after bariatric surgery by P wave/QT interval dispersion. Obes Surg 2018;28:932–8. [DOI] [PubMed] [Google Scholar]
- 133.Shimada YJ, Tsugawa Y, Carmargo CA, et al. Effect of bariatric surgery on emergence department visits and hospitalizations for atrial fibrillation. Am J Cardiol 2017;120:947–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Donnellan E, Wazni OM, Kanj M, et al. Association between pre-ablation bariatric surgery and atrial fibrillation recurrence in morbidly obese patients undergoing atrial fibrillation ablation. Europace 2019;21:1476–83. [DOI] [PubMed] [Google Scholar]
- 135.Pareek M, Schauer PR, Kaplan LM, et al. Metabolic surgery: weight loss, diabetes, and beyond. J Am Coll Cardiol 2018;17:670–87. [DOI] [PubMed] [Google Scholar]
- 136.Mechanick JI, Apovian C, Brethauer S, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures–2019 update: cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Endocr Practice 2019. November 4 [E-pub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 137.US Preventive Services Task Force. Behavioral weight loss interventions to prevent obesity-related morbidity and mortality in adults. JAMA 2018;320:1163–71. [DOI] [PubMed] [Google Scholar]
- 138.US Preventive Services Task Force. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults without cardiovascular risk factors. JAMA 2017;318:167–74. [DOI] [PubMed] [Google Scholar]
- 139.Spring B, Ockene JK, Gidding SS, et al. Better population health through behavior change in adults–a call to action. Circulation 2013;128: 2169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2015;100:342–62. [DOI] [PubMed] [Google Scholar]
- 141.American Diabetes Association. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes–2019. Diabetes Care 2019;42:S81–9. [DOI] [PubMed] [Google Scholar]
- 142.Kotsis V, Jordan J, Micic D, et al. Obesity and cardiovascular risk: a call for action from the European Society of Hypertension Working Group of Obesity, Diabetes and the High-risk Patient and European Association for the Study of Obesity: part A: mechanisms of obesity induced hypertension, diabetes and dyslipidemia and practice guidelines for treatment. J Hypertens 2018;36: 1427–40. [DOI] [PubMed] [Google Scholar]
- 143.Bittner V The new 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease. Circulation 2019. March 17 [E-pub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 144.Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology–clinical practice guidelines for developing a diabetes mellitus comprehensive care plan–2015. Endocr Pract 2015;21 Suppl 1: 1–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 executive summary. Endocr Pract 2019;25:69–100. [DOI] [PubMed] [Google Scholar]
- 146.Davies MJ, D’Alessio Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018;41:2669–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.American Diabetes Association. Lifestyle management: standards of medical care in diabetes–2019. Diabetes Care 2019;42 Suppl 1: S46–60. [DOI] [PubMed] [Google Scholar]
- 148.Dyson PA, Twenefour D, Breen C, et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet Med 2018;35:541–7. [DOI] [PubMed] [Google Scholar]
- 149.Rosenzweig JL, Bakris GL, Berglund LF, et al. Primary prevention of ASCVD and T2D in patients at metabolic risk: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2019; 104:3939–85. [DOI] [PubMed] [Google Scholar]
- 150.American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes–2019. Diabetes Care 2019;42 Suppl 1: S34–45. [DOI] [PubMed] [Google Scholar]
- 151.Mechanick JI, Davidson JA, Fergus IV, et al. Transcultural diabetes care in the United States – a position statement by the American Association of Clinical Endocrinologists. Endocr Pract 2019;25: 729–65. [DOI] [PubMed] [Google Scholar]
- 152.Reboussin DM, Allen NB, Griswold ME, et al. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. J Am Coll Cardiol 2018;71:2176–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lloyd-Jones DM, Morris PB, Minissian MB, et al. 2017 focused update of the 2016 ACC Expert Consensus Decision Pathway on the Role of Non-Statin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk. J Am Coll Cardiol 2017;70:1785–822. [DOI] [PubMed] [Google Scholar]
- 154.Jellinger PS, Handelsman Y, Rosenbilt PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract 2017;23 Suppl 2:1–87. [DOI] [PubMed] [Google Scholar]
- 155.US Preventive Services Task Force. Risk Assessment for cardiovascular disease with nontraditional risk factors. JAMA 2018;320: 272–80. [DOI] [PubMed] [Google Scholar]
- 156.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure. J Am Coll Cardiol 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 157.Dunlay SM, Givertz MM, Aguilar D, et al. Type 2 diabetes mellitus and heart failure. Circulation 2019;139:e1–13.30615505 [Google Scholar]
- 158.Bozkurt B, Aguilar D, Deswal A, et al. Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure. Circulation 2016;134:e535–78. [DOI] [PubMed] [Google Scholar]
- 159.Patel MR, Calhoon JH, Dehmer GJ, et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with acute coronary syndromes. J Am Coll Cardiol 2017;69:2212–41. [DOI] [PubMed] [Google Scholar]
- 160.Kusumoto FM, Bailey KR, Chaouki AS, et al. Systematic review for the 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. J Am Coll Cardiol 2018;72:1653–76. [DOI] [PubMed] [Google Scholar]
- 161.Jessup M, Drazner MH, Book W, et al. 2017 ACC/AHA/HFSA/ISHLT/ACP advanced training statement on advanced heart failure and transplant cardiology (revision of the ACCF/AHA/ACP/HFSA/ISHLT 2010 clinical competence statement on management of patients with advanced heart failure and cardiac transplant). J Am Coll Cardiol 2017;69:2977–3001. [DOI] [PubMed] [Google Scholar]
- 162.Chan WV, Pearson TA, Bennett GC, et al. ACC/AHA special report: clinical practice guideline implementation strategies: a summary of systematic reviews by the NHLBI Implementation Science Work Group. J Am Coll Cardiol 2017;69: 1076–92. [DOI] [PubMed] [Google Scholar]
- 163.Shapiro MD, Fazio S. Setting the agenda for preventive cardiology. Circ Res 2017;121:211–3. [DOI] [PubMed] [Google Scholar]
- 164.Saeed A, Dabhadkar K, Virani SS, et al. Cardiovascular disease prevention: training opportunities, the challenges, and future directions. Curr Atheroscler Rep 2018;20:35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


