Abstract
Development of mechanically advanced tissue-engineered vascular grafts (TEVGs) from human induced pluripotent stem cell (hiPSC)-derived vascular smooth muscle cells (hiPSC-VSMCs) offers an innovative approach to replace or bypass diseased blood vessels. To move current hiPSC-TEVGs toward clinical application, it is essential to obtain hiPSC-VSMC-derived tissues under xenogeneic-free conditions, meaning without the use of any animal-derived reagents. Many approaches in VSMC differentiation of hiPSCs have been reported, although a xenogeneic-free method for generating hiPSC-VSMCs suitable for vascular tissue engineering has yet to be established. Based on our previously established standard method of xenogeneic VSMC differentiation, we have replaced all animal-derived reagents with functional counterparts of human origin and successfully derived functional xenogeneic-free hiPSC-VSMCs (XF-hiPSC-VSMCs). Next, our group developed tissue rings via cellular self-assembly from XF-hiPSC-VSMCs, which exhibited comparable mechanical strength to those developed from xenogeneic hiPSC-VSMCs. Moreover, by seeding XF-hiPSC-VSMCs onto biodegradable polyglycolic acid (PGA) scaffolds, we generated engineered vascular tissues presenting effective collagen deposition which were suitable for implantation into an immunodeficient mice model. In conclusion, our xenogeneic-free conditions for generating hiPSC-VSMCs produce cells with the comparable capacity for vascular tissue engineering as standard xenogeneic protocols, thereby moving the hiPSC-TEVG technology one step closer to safe and efficacious clinical translation.
Keywords: Human induced pluripotent stem cells, vascular smooth muscle cells, xenogeneic-free, vascular tissue engineering
Graphical Abstract

1. Introduction
There is a tremendous need for vascular grafts worldwide to treat cardiovascular diseases. Autologous vessels such as saphenous veins are commonly used as vascular grafts in clinic [1–3], while the autologous vessel segments suitable as grafts could be highly limited. Application of synthetic vascular prosthesis such as polytetrafluoroethylene (PTFE)-based grafts received significant attention, but the risks of infection and thrombosis due to the low biocompatibility of the synthetic materials could hinder the widespread of synthetic grafts [4, 5]. In comparison, tissue-engineered vascular grafts (TEVGs) may represent a promising option. TEVGs can be generated by culturing seeded cells together with biodegradable scaffolds or hydrogels to develop an engineered tissue with tubular shape. Recent techniques based on vascular tissue-derived extracellular matrix bioinks and triple-coaxial cell printing have been developed to generate cell-based TEVGs [6]. Notably, these bio-printed TEVGs could be less practical for clinical application due to the moderate mechanical stretngth. Moreover, in situ vessel formation based on resorbable synthetic grafts and host cell repopulation has shown great promise due to the ready availability and affordability of the grafts [7–9]. Notably, a considerable number of patients with dysfunctional native vascular cells, due to aging [10, 11] or diseases such as diabetes[12, 13], may not effectively replace resorbable synthetic materials with autologous vascular tissue, which may lead to suboptimal vessel function. Alternatively, mechanically robust TEVGs can be generated by culturing seeded cells onto biodegradable scaffolds under pulsatile and radial stretching [14–20]. To date, TEVGs generated by culturing human primary vascular smooth muscle cells (VSMCs) on biodegradable polyglycolic acid (PGA) scaffolds coupled with decellularization have been applied in hemodialysis access in clinical trials [17, 18]. However, human primary VSMCs display restricted accessibility, limited expandability and donor to donor functional variability [16, 21], which may lead to variable quality in TEVG production and hinder the broader application of TEVGs.
Alternatively, human induced pluripotent stem cells (hiPSCs) could be applied as a robust cell source for TEVG generation. hiPSCs present self-renewability and differentiation capacity into virtually any somatic cell type, including VSMCs [22]. Therefore, hiPSCs could serve as an accessible and unlimited reservoir from which VSMCs (hiPSC-VSMCs) of comparable quality could be derived for TEVG construction. Indeed, functional hiPSC-VSMCs have been efficiently derived for vascular tissue engineering [23–30]. Recently, implantable TEVGs (hiPSC-TEVGs) with advanced mechanical strength approaching that of saphenous veins routinely employed for vascular bypass have been generated using hiPSC-VSMCs [31], setting the stage of future application of hiPSC-TEVGs in the clinic.
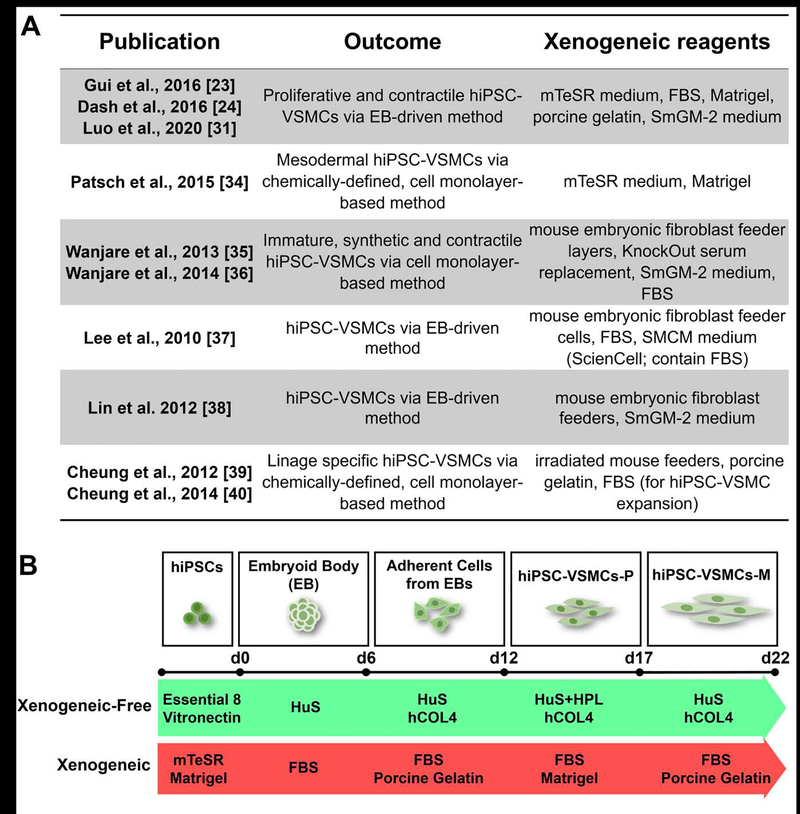
To safeguard the application of cell or tissue-based products in clinical practice, it is critical to utilize xenogeneic-free conditions to derive these cells and engineered tissue grafts, since animal-derived reagents may carry zoonoses or trigger xenogeneic immune responses of cell or tissue derivatives [32]. This xenogeneic reagent mediated inflammatory response heightens the risk of graft complications post implantation, with graft failure as the worst-case scenario. To date, primary VSMC-derived TEVGs are able to be produced under xenogeneic-free conditions using human recombinant growth factors and human serum (U.S. patent No. US9657265B2). To effectively promote the future application of hiPSC-TEVGs, a xenogeneic-free platform for generating hiPSC-VSMCs and subsequent engineered vascular tissues is required. A protocol for the establishment and expansion of hiPSCs under xenogeneic-free conditions has been developed [33]. However, current approaches of VSMC differentiation and expansion from hiPSCs remains largely dependent on the application of animal-derived components including fetal bovine serum (FBS), mouse-derived Matrigel, porcine-derived gelatin, etc. [23, 24, 26, 31, 34–40] (Fig. 1A). It is worth mentioning that chemically-defined methods for differentiating hiPSC to VSMCs have been reported [34, 39, 40]. However, in these chemically-defined methods, reagents containing animal-derived components such as Matrigel, bovine serum albumin, and mTeSR medium (xenogeneic pluripotency-promoting medium) are commonly employed to initiate the differentiation [34, 39, 40]. Additionally, expansion of hiPSC-VSMCs derived via chemically-defined methods appear to be dependent on the presence of FBS [39, 40]. Therefore, it is essential to establish a xenogeneic-free approach without utilizing any animal-derived reagents to generate functional hiPSC-VSMCs for future production of clinical grade TEVGs.
Figure 1. Strategy of deriving hiPSC-VSMCs under xenogeneic-free conditions.
(A) Representative studies of currently established methods for deriving vascular smooth muscle cells from human induced pluripotent stem cells.
(B) Schematic illustration of VSMC differentiation of hiPSCs under xenogeneic-free or xenogeneic conditions. Note that the essential reagents applied in current xenogeneic-free and previous xenogeneic methods are listed. See the Materials and Methods section and Table S1 for details of the methods. HuS, human serum; HPL, human platelet lysate; hCOL4, human collagen type 4; FBS, fetal bovine serum.
In this study, we aimed at establishing robust, xenogeneic-free conditions for deriving hiPSC-VSMCs suitable for vascular tissue engineering. We have transitioned our current method for obtaining hiPSC-VSMCs [23, 24] by applying xenogeneic-free basal media and replacing animal-derived reagents with their counterparts of human origin, including human serum, human platelet lysate, human serum albumin, human recombinant growth factors, and human extracellular matrix. By using this method, we were able to derive xenogeneic-free hiPSC-VSMCs (XF-hiPSC-VSMCs) with appropriate VSMC marker expression and contractile function. We subsequently used XF-hiPSC-VSMCs to fabricate scaffold-free engineered vascular tissues rings via cellular self-assembly and generated biodegradable scaffold-based engineered tissues under xenogeneic-free culture conditions, which further suggests the potential of XF-hiPSC-VSMCs in vascular tissue engineering. Our study establishes an efficient method for obtaining xenogeneic-free hiPSC-VSMCs feasible for vascular tissue engineering, and lays the groundwork for future manufacturing of hiPSC-TEVGs which follow Current Good Manufacturing Practices for clinical application.
2. Materials and methods
2.1. Animal use
This study was approved by the Yale University Institutional Animal Care and Use Committee. All animal care was in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Cell populations were obtained using protocols approved by the Yale University Human Investigation Committee.
2.2. Culture of human induced pluripotent stem cells (hiPSCs)
The hiPSC line utilized was originally generated from human neonatal fibroblast cells isolated from a healthy female donor using non-integrative Sendai viral particles encoding human OCT4, KLF4, SOX2, and c-MYC genes (ThermoFisher) as previously described [24]. In this study, hiPSCs were cultured under either xenogeneic or xenogeneic-free conditions.
Under the xenogeneic condition, hiPSCs were maintained in mTeSR1 medium (STEMCELL Technologies) on surfaces coated with Growth Factor Reduced (GFR)-Matrigel (Corning; xenogeneic reagent derived from mouse sarcoma) with daily medium changes. Once at 80% confluency, hiPSCs were dissociated with 0.5 mM ethylenediaminetetraacetic acid (EDTA; ThermoFisher) and subcultured.
Under the xenogeneic-free condition, hiPSCs were cultured in chemically-defined, xenogeneic-free Essential 8 Medium (E8 medium; ThermoFisher) on surfaces coated with 10 μg/mL recombinant human Vitronectin XF™ (STEMCELL Technologies) with daily medium changes [33]. When cells reached 80% confluency, hiPSCs were dissociated with 0.5 mM EDTA and subcultured.
2.3. Culture of human primary vascular smooth muscle cells (VSMCs)
Primary aortic human VSMCs (Lonza) were cultured under either xenogeneic or xenogeneic-free conditions in this study, depending on terminal experiments.
Under the xenogeneic condition, primary VSMCs in a proliferative state (XG-primary VSMCs-P) within passage five were maintained in xenogeneic VSMC growth medium (medium components listed in Table S1) on surfaces coated with 0.1% (w/v) porcine skin-derived gelatin (Sigma-Aldrich). Culture medium was changed every other day. Upon reaching 80% confluency, cells were passaged using 0.05% trypsin-EDTA (ThermoFisher; xenogeneic reagent derived from porcine pancreas). To induce functional maturation (XG-primary VSMC-M), XG-primary VSMCs-P were subcultured and placed in xenogeneic VSMC maturation medium (medium components listed in Table S1) for seven days, with medium replenished every other day.
Under the xenogeneic-free condition, primary VSMCs in a proliferative state (XF-primary VSMCs-P) were maintained in xenogeneic-free VSMC growth medium (medium components listed in Table S1) on surfaces coated with 20 μg/mL human collagen type IV (hCOL4; Sigma-Aldrich). XF-primary VSMCs maintained for at least one passage but no longer than five passages were used for experiments. Culture medium was changed every other day. Upon reaching 80% confluency, cells were passaged using xenogeneic-free, chemically-defined TrypLE (ThermoFisher). To induce functional maturation (XF-primary VSMC-M), XF-primary VSMCs-P were subcultured in xenogeneic-free VSMC maturation medium (medium components listed in Table S1) for seven days, with medium changed every other day.
2.4. VSMC differentiation of hiPSCs under xenogeneic condition
Xenogeneic hiPSC-VSMCs were obtained as previously reported [31]. Briefly, hiPSCs were differentiated to VSMCs via formation of embryoid bodies (EBs). hiPSCs cultured in mTeSR medium were dissociated with 0.5 mM EDTA on day 0, plated on a low attachment 6-well plate (Corning), and cultured overnight with mTeSR medium containing 5 μM ROCK inhibitor (Calbiochem) and 1% (v/v) Matrigel to allow the formation of EBs. Then the mTeSR1 medium was progressively mixed with xenogeneic EB medium (medium components listed in Table S1) in a 2:1 ratio (volume) on day 1, 1:1 on day 2 and 1:2 on day 3. From day 4 onward, EBs were cultured with pure EB medium. On day 6, EBs were harvested and re-plated onto culture dishes coated with 0.1% porcine-derived gelatin. EBs subsequently attach to the surface and the outgrowth of differentiated cells can readily be observed. These differentiated cells were cultured in xenogeneic EB medium, with medium changed every day for six days. On day 12, EB-derived differentiated cells were dissociated with 0.05% trypsin-EDTA, re-seeded on a Matrigel-coated surface, and cultured with xenogeneic VSMC growth medium (complete Smooth muscle Growth Medium-2, SmGM-2, Lonza; medium components listed in Table S1) for 7–10 days to derive xenogeneic hiPSC-VSMCs in a proliferative state (XG-hiPSC-VSMCs-P). The medium was then changed every other day for maintenance. After reaching 80% confluency, XG-hiPSC-VSMCs-P were passaged using 0.05% trypsin-EDTA for expansion. To induce maturation (XG-hiPSC-VSMCs-M), XG-hiPSC-VSMCs-P were cultured with VSMC maturation medium (medium components listed in Table S2) on a porcine gelatin-coated surface for 7 days. The maturation medium was changed every other day.
2.5. VSMC differentiation of hiPSCs under xenogeneic-free condition
The xenogeneic-free method for obtaining hiPSC-VSMCs was based on our xenogeneic method as described above. hiPSCs maintained in E8 medium on human vitronectin-coated plates were dissociated with 0.5 mM EDTA on day 0, re-plated in low attachment 6-well plates, and cultured overnight with E8 medium containing 5 μM ROCK inhibitor and 10 μg/mL human vitronectin to generate EBs. E8 medium was progressively mixed with xenogeneic-free EB medium (medium components listed in Table S1) in 2:1 ratio (volume) on day 1, 1:1 on day 2 and 1:2 on day 3. From day 4 onward, EBs were cultured with xenogeneic-free EB medium. On day 6, EBs were harvested and re-plated onto a culture dish coated with human collagen IV. Differentiated cells outgrew from EBs and were cultured with xenogeneic-free EB medium, with medium changed daily for six days. On day 12, the differentiated cells were dissociated with TrypLE, re-seeded on human collagen IV-coated plates, and cultured with xenogeneic-free VSMC growth medium (medium components listed in Table S1) for 7–10 days to derive the xenogeneic-free hiPSC-VSMCs in a proliferative state (XF-hiPSC-VSMCs-P). The medium was then changed every other day for maintenance. Upon reaching 80% confluency, XF-hiPSC-VSMCs-P were passaged using TrypLE. To induce maturation (XF-hiPSC-VSMCs-M), XF-hiPSC-VSMCs-P were cultured with xenogeneic-free VSMC maturation medium (medium components listed in Table S1) on human collagen IV-coated surfaces for 7 days, with medium was changed every other day.
2.6. Quantitative reverse transcription PCR
RNA was extracted from cell samples and subjected to reverse transcription PCR using TRIzol RNA Isolation Kit (ThermoFisher) and iScript cDNA synthesis Kit (Bio-Rad). Quantitative polymerase chain reaction (qPCR) was completed using the IQ SYBR green supermix (Bio-Rad) following the manufacturer’s instructions. Expression of gene candidates were normalized to that of human GAPDH. Three biological replicates were completed for each gene. Primer sequences for qPCR are listed in Table S2.
2.7. TUNEL assay
Cellular viabilities in engineered vascular tissue were assessed via terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using In Situ Cell Death Detection Kit (Roche). Nuclei were counterstained with DAPI (ThermoFisher). Three biological replicates were completed for each group for analyzing the percentage of TUNEL-positive cells. All images of TUNEL staining were captured using an inverted microscope (Nikon Eclipse 80i). ImageJ software was used to quantify the percentage of TUNEL-positive apoptotic cells.
2.7. Immunostaining of cells in culture
Cells were fixed in 4% paraformaldehyde (PFA; Electron Microscopy Sciences) for 10 minutes at room temperature (RT), washed with PBS and blocked with PBST buffer (PBS with 0.3% Triton X-100 [Sigma-Aldrich]) containing 10% normal goat serum (NGS; ThermoFisher) for 30 minutes at RT. Next, cells were incubated with primary antibody in PBST containing 1% NGS at 4°C overnight. On the next day, cells were washed in PBS, and subsequently incubated with secondary antibody (1:1000 diluted in PBST containing 1% NGS) for one hour at RT in the dark. Then the cells were washed with PBS, and nuclei were counterstained with DAPI. Antibody information is listed in supplementary Table S3. Samples were observed using a fluorescent microscope (Leica). ImageJ software was used to quantify the percentage of cells positive for the marker.
2.8. Contractility assay
hiPSC-VSMCs or primary VSMCs cultured in maturation medium were treated with 1 mM carbachol (Abcam) or PBS (vehicle control) for 20 min. Images of the cell morphology were taken before and after the treatment. Changes of surface area were analyzed using ImageJ. Three independent batches of cells were used to perform the contractility assay for each treatment, and 10 cells were randomly selected in each batch for each treatment for analyzing the changes in surface area.
2.9. Fabrication of scaffold-free engineered vascular tissue rings
Polydimethylsiloxane (PDMS) molds used to make agarose cell seeding wells were produced and generously provided by Dr. Marsha Rolle, Department of Biomedical Engineering, Worcester Polytechnic Institute, Worcester, MA, as previously described [41, 42]. Wells for seeding cells were generated by casting a 2% agarose (ThermoFisher) solution in DMEM in the PDMS mold and equilibrated in xenogeneic-free or xenogeneic collagen promoting medium (medium components listed in Table S1) overnight in 6-well culture dishes. To fabricate the ring-shaped tissue, one million XF-hiPSC-VSMCs-P or XG-hiPSC-VSMCs-P cultured in the respective xenogeneic-free and xenogeneic collagen promoting medium for 5 days were mixed in 50 μL of the respective medium and added into the wells of agarose mold. After 30 minutes, the wells containing the agarose mold and cells were filled with 5 mL of the respective collagen promoting medium. Medium was replenished every other day, and the rings were harvested on day 14 for further analysis.
2.10. Histological analysis of tissue
Tissue samples were fixed in 10% Neutral Buffered Formalin (ThermoFisher) overnight and transported to Yale Pathology Tissue Services to perform sample sectioning, hematoxylin and eosin (H&E) staining and Masson’s Trichrome staining based on standard protocols. For immunostaining, tissues were fixed with 4% PFA at 4°C for three hours and submerged in PBS containing 15% (w/v) sucrose (Sigma-Aldrich) at 4°C for 12 hours. The fixed tissues were submerged in Tissue-Tek Optimal Cutting Temperature (O.C.T.) compound (Sakura Finetek) on dry ice to produce frozen blocks. Frozen blocks were sectioned at 5 μm thickness using a cryostat (Leica CM1950). The slides with tissue sections were washed in PBS for 10 minutes, blocked in PBST with 10% NGS for 30 minutes at RT, and incubated with primary antibody in PBST with 1% NGS at 4°C overnight in a humidified chamber. On the next day, sections were incubated with secondary antibody (1:1000 in PBST with 1% NGS) for one hour at RT. Nuclei were counterstained with DAPI. Samples were observed using an inverted microscope (Nikon Eclipse 80i).
2.11. Mechanical evaluation of engineered vascular tissue rings
Maximum modulus, maximum tensile stress and failure strain of the engineered vascular tissue rings generated from XF-hiPSC-VSMCs-P or XG-hiPSC-VSMCs-P were evaluated using an Instron 5960 microtester (Instron) equipped with a 10 N load cell as described previously [42]. Two stainless steel pins were respectively anchored to the actuator and the load cell, and tissue rings were mounted to the hooks of these two pins. Tissue rings were pre-stretched to 10% strain for three cycles and then increasingly stretched until failure to determine the maximum strain and ultimate tensile strength. Tissue stress was calculated by normalizing tensile strength to cross-sectional area (A =2 π r2). Accordingly, the strain-stress plot of each tissue ring was plotted, and maximum stress, failure strain and maximum modulus of each tissue ring was then calculated. Three independent batches of engineered vascular tissue rings from XF-hiPSC-VSMCs-P or XG-hiPSC-VSMCs-P were analyzed.
2.12. Culturing hiPSC-VSMCs on 5 mm x 5 mm PGA scaffolds
Nonwoven-PGA polymer mesh (0.3 mm × 150 mg/cc, 20 cm × 30 cm sheet, BIOFELT) scaffold was cut into 5 mm × 5 mm squares as described previously [43]. PGA squares were treated with 1.0 N NaOH (SigmaAldrich) for 1 minute, washed in distilled water, sterilized with 70% ethanol for 30 minutes, and air-dried under sterile conditions overnight. On the next day, PGA squares were treated with human collagen type IV (20ul/mL, SigmaAldrich; xenogeneic-free condition) or 0.1% porcine skin-derived gelatin (SigmaAldrich; xenogeneic condition) at 37°C for 1 hour, air-dried for 2–3 hours under sterile conditions, and moved into a 24-well low attachment dish (Corning).
Prior to generation of engineered vascular tissues, XF-hiPSC-VSMCs-P or XG-hiPSC-VSMCs-P cultured in the xenogeneic-free or xenogeneic collagen promoting medium for 5 days were harvested using TrypLE or 0.05% Trypsin-EDTA, respectively. Cells were then resuspended in collagen promoting medium at the density of 10 million cells/mL. 40 μl of the cell suspension was dropped onto the PGA mesh and then incubated at 37°C and 5% CO2 for one hour to allow the cells to adhere. The wells were then filled with 1 mL of xenogeneic-free or xenogeneic collagen promoting medium. The medium was changed every other day for 21 days, and then the tissues were harvested for further analysis or implantation.
2.13. Hydroxyproline assay
Collagen content weight of cultured cells or engineered tissues was determined by converting the measured weight of hydroxyproline in each sample as described previously [44]. The weight of collagen content was calculated as 10 times the weight of hydroxyproline. The cell numbers in experimental group of cultured cells were determined by cell counting using a separated well of hiPSC-VSMCs in the same batch of culture under the same culture condition, and the total collagen amount was normalized to the average cell numbers to obtain the collagen content per cell of each sample. Three biological replicates were completed for each group.
2.14. Implantation of engineered tissues into immunodeficient mice
Subcutaneous implantation of engineered vascular tissues into Rag2/Il2rg Double Knockout mice were completed as previously described [42]. The animals were anesthetized with isoflurane, and animal surgery was performed under stereomicroscope (Leica, Model MST34). The engineered tissue was subcutaneously implanted into the groin, and then the animals were recovered from surgery. After 14 days post-operation, the mice were sacrificed, and the tissues were explanted and cut into two parts: one part fixed with 10% Neutral Buffered Formalin and transferred to Yale Pathology Tissue Services in 70% ethanol for paraffin embedding and histological analysis (H&E staining and Masson’s Trichrome staining), the other part fixed with 4% PFA for producing frozen blocks for immunostaining. PGA meshes without seeding cells were cultured in xenogeneic-free collagen promoting medium for 14 days as a blank control. Implantation was independently repeated on three mice for engineered tissues derived from XF-hiPSC-VSMCs-P or XG-hiPSC-VSMCs-P or blank controls.
2.15. Statistical analysis
Numerical data were reported in the format of mean ± S.E.M from three independent experiments. All graph illustrations and statistical analyses were completed using GraphPad Prism6. Unpaired two-tailed Student’s t-test was performed to determine the significance of difference between controls and experimental groups. One-way or two-way ANOVA was used for comparison among multiple groups when appropriate. P values less than 0.05 were considered statistically significant.
3. Results
3.1. Derivation of hiPSC-VSMCs under xenogeneic-free conditions
For therapeutic application of hiPSC-VSMCs, it is critically important to derive hiPSC-VSMCs under xenogeneic-free conditions. As all the currently available hiPSC-VSMC derivation protocol involve the use of animal products [23, 24, 26, 31, 34–40] (Fig. 1A), we set to generate xenogeneic-free hiPSC-VSMCs (XF-hiPSC-VSMCs), as illustrated in Fig. 1B, by replacing all animal-derived reagents from our previous method of hiPSC-VSMCs derivation [23, 24] with analogous reagents of human origin (details see Materials and Methods and Table S1). In brief, undifferentiated hiPSCs were cultured on human vitronectin-coated surfaces with xenogeneic-free Essential 8 (E8) medium (Fig. 1B). hiPSCs were then dissociated and subjected to embryoid body (EB) formation during a 6-day-floating suspension culture with progressive transition from E8 medium to xenogeneic-free EB medium containing 10% human serum (HuS) (Fig. 1B). Subsequently, EBs were plated onto human collagen type IV (hCOL4)-coated culture dishes and maintained in xenogeneic-free EB medium for another 6 days, which allowed differentiated cells to grow out of the EBs. On day 12, these differentiated cells were dissociated with xenogeneic-free TrypLE, harvested, seeded onto a hCOL4-coated surface, and cultured with xenogeneic-free VSMC growth medium, which was composed of xenogeneic-free smooth muscle basal medium (Lonza), 5% (v/v) HuS, 5% (v/v) human platelet lysate (HPL), human recombinant FGF2, EGF and insulin. This combination of reagents allows the generation of XF-hiPSC-VSMCs in a proliferative state (XF-hiPSC-VSMCs-P, Fig. 1B). To generate mature xenogeneic-free hiPSC-VSMCs (XF-hiPSC-VSMCs-M), XF-hiPSC-VSMCs-P were further cultured on hCOL4-coated surfaces with maturation medium containing xenogeneic-free DMEM medium, 1% (v/v) HuS, and human recombinant TGF-β1 (Fig. 1B).
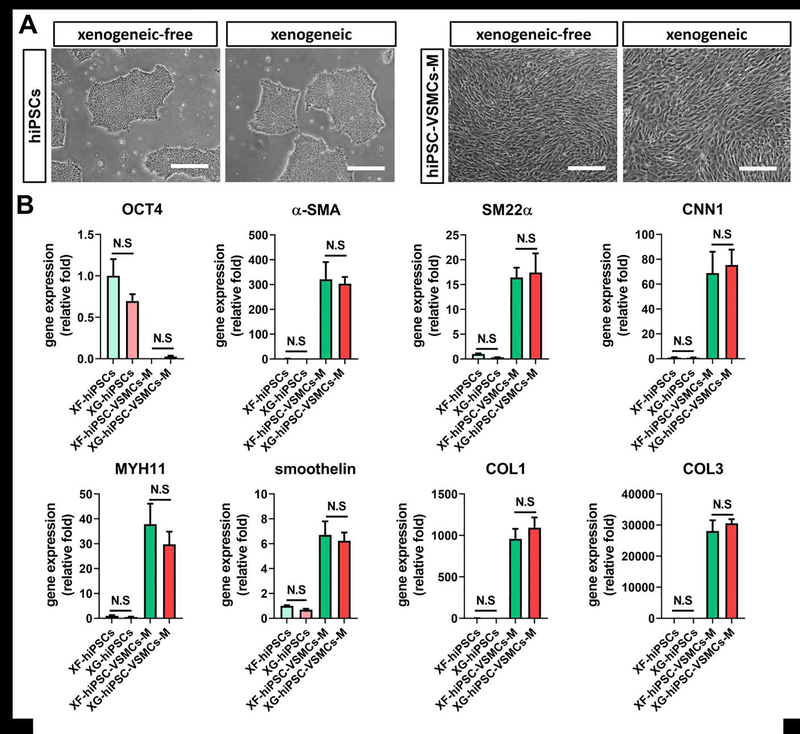
In comparison to the xenogeneic counterparts, xenogeneic-free hiPSCs and hiPSC-VSMCs-M displayed comparable morphologies (Fig. 2A). qPCR results suggested that XF-hiPSC-VSMCs-M displayed significantly downregulated pluripotency gene (OCT4) and upregulated expression levels of VSMC-specific genes (α-smooth muscle actin [α-SMA], transgelin [SM22α], calponin1 [CNN1], smooth muscle myosin heavy chain [MYH11], smoothelin, collagen type 1 [COL1] and collagen type 3 [COL3]) compared with those of XF-hiPSCs (Fig. 2B). Moreover, no significant difference was observed in expression of pluripotency and VSMC-specific genes between xenogeneic-free and xenogeneic hiPSCs or hiPSC-VSMCs-M (Fig. 2B). These results demonstrate that xenogeneic-free conditions can effectively induce VSMC lineage specification of hiPSCs.
Figure 2. Generation of hiPSC-VSMCs under xenogeneic-free conditions.
Results suggested that the hiPSC-VSMCs could be efficiently generated under xenogeneic-free conditions with comparable morphology and gene expression profiles to those under xenogeneic conditions.
(A) Representative images of hiPSCs and mature hiPSC-VSMCs under xenogeneic-free or xenogeneic conditions. Scale bar: 200 μm.
(B) qRT-PCR analysis of relative mRNA transcript amounts of pluripotency marker (OCT4) and VSMC contractile (α-SMA, SM22α, CNN1, MYH11 and smoothelin) and ECM (COL1 and COL3) markers in xenogeneic-free or xenogeneic hiPSCs (XF-hiPSCs and XG-hiPSCs) and mature hiPSC-VSMCs (XF-hiPSC-VSMCs-M and XG-hiPSC-VSMCs-M). Values in the y-axis represent fold changes relative to human GAPDH expression. Gene expression in each group was normalized to that of in XF-hiPSCs (One-way ANOVA with Tukey’s multiple comparisons test; Mean values and S.E.M indicated by the error bars are shown; n=3; N.S: not significant).
3.2. Characterization of XF-hiPSC-VSMCs
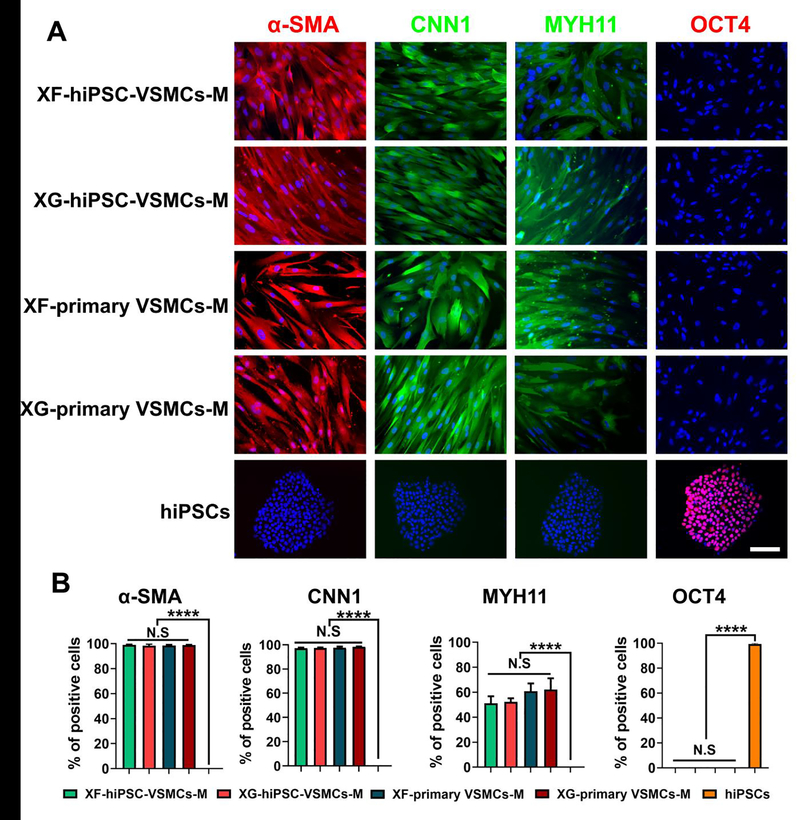
To characterize these XF-hiPSC-VSMCs-M, we immunostained them to evaluate the presence of critical VSMC markers. XF-hiPSC-VSMCs-M displayed expression of VSMC contractile markers (α-SMA, CNN1, and MYH11), the levels of which appeared to be comparable to those of both mature human primary VSMCs cultured under xenogeneic-free or xenogeneic conditions and mature XG-hiPSC-VSMCs (Fig. 3A–B and S1). Moreover, no hiPSC-VSMCs and primary VSMCs expressed the pluripotency marker OCT4, while hiPSCs were OCT4-positive but negative for VSMC markers (Fig. 3A–B and S1). Additionally, to test if the potential batch-to-batch variation of human serum might affect VSMC differentiation, we generated XF-hiPSC-VSMCs using three batches of human serum, respectively. Results showed that the expression levels of critical VSMC markers of the XF-hiPSC-VSMCs from three batches of differentiation were comparable to each other (Fig. S2A and B). These data suggested that our method of deriving XF-hiPSC-VSMCs was minimally or not affected by the potential batch to batch variation of human serum and that XF-hiPSC-VSMCs displayed the characteristic VSMC marker expression profile.
Figure 3. Marker expression of XF-hiPSC-VSMCs.
Results suggested that XF-hiPSC-VSMCs presented comparable VSMC marker expression profiles to those of XG-hiPSC-VSMCs and human primary VSMCs.
(A) Immunostaining of VSMC (α-SMA, CNN1, and MYH11) and pluripotency (OCT4) markers in XF-hiPSC-VSMCs-M, XG-hiPSC-VSMCs-M, XF-primary VSMCs-M and XG-primary VSMCs-M in maturation stages, and undifferentiated xenogeneic-free hiPSCs. DNA (nuclear) was counterstained by DAPI. Scale bar: 200 μm.
(B) The percentage of cells (XF-hiPSC-VSMCs-M, XG-hiPSC-VSMCs-M, XF-primary VSMCs-M, XG-primary VSMCs-M and xenogeneic-free hiPSCs) positive for VSMC (α-SMA, CNN1, and MYH11) and pluripotency (OCT4) markers from immunostaining (One-way ANOVA with Tukey’s multiple comparisons test; Mean values and S.E.M indicated by error bars are shown; n=3; ****: p<0.0001; N.S: not significant).
Next, we investigated the contractile function of XF-hiPSC-VSMCs. Mature hiPSC-VSMCs or primary VSMCs generated under xenogeneic-free or xenogeneic conditions were treated with vasoconstrictor (carbachol) or vehicle only (PBS) for 20 minutes, and the decrease of cell surface area was measured (Fig. 4A). The percentage of decreased surface area of XF-hiPSC-VSMCs-M treated with carbachol (20.7% ± 1.3%) was significantly higher than that of XF-hiPSC-VSMCs-M treated with vehicle (2.4% ± 1.4%) while comparable to that of the carbachol-treated XG-hiPSC-VSMCs-M (21.3% ± 0.6%), XF-primary VSMCs-M (17.7% ± 0.7%), and XG-primary VSMCs-M (24.2% ± 4.4%) (Fig. 4B). These results suggested that XF-hiPSC-VSMCs were functionally contractile when exposed to vasoconstrictive signals.
Figure 4. Contractility analysis of XF-hiPSC-VSMCs.
Results suggested that XF-hiPSC-VSMCs displayed comparable contractililty to those of XG-hiPSC-VSMCs and human primary VSMCs.
(A) Contractility of XF-hiPSC-VSMCs-M, XG-hiPSC-VSMCs-M, XF-primary VSMCs-M and XG-primary VSMCs-M in maturation stages in response to 1 mM carbachol (before and after 20 minutes incubation). Representative cells were outlined by the blue and red lines to indicate the surface areas before and after carbachol treatment, respectively. Scale bar: 200 μm.
(B) Quantification of reduced cell area of xenogeneic-free or xenogeneic hiPSC-VSMCs-M and human primary VSMCs-M in response to 1 mM carbachol or vehicle control (PBS) (One-way ANOVA with Tukey’s multiple comparisons test; Mean values and S.E.M indicated by the error bars are shown; n=3; ***: p < 0.001; ****: p < 0.0001; N.S: not significant).
3.3. Determination of xenogeneic-free collagen promoting medium for vascular tissue engineering
Collagen production by seeded cells is essential to achieve strong mechanical properties in engineered vascular tissues. Therefore, we developed medium to enhance collagen synthesis by XF-hiPSC-VSMCs for vascular tissue engineering (collagen promoting medium). Our previous study has suggested that XG-hiPSC-VSMCs-P primed with short term culture in DMEM medium containing 10% fetal bovine serum (xenogeneic priming medium) could significantly enhance the expression of collagen and contractile genes without reducing the proliferation rate in comparison to those cultured in regular xenogeneic VSMC growth medium (complete SmGM-2 medium) [31]. Based on this, we chose to include in the xenogeneic-free priming medium DMEM medium containing 5% (v/v) HuS and 5% (v/v) HPL without the addition of ectopic growth factors. We further established the xenogeneic-free collagen promoting medium by supplementing the xenogeneic-free priming medium with a series of collagen promoting reagents (ascorbic acid, proline, glycine, alanine, insulin, CuSO4, and TGF-β1; medium components listed in Table S1) [14].
Next, we cultured XF-hiPSC-VSMCs-P in xenogeneic-free VSMC growth medium, xenogeneic-free priming medium or xenogeneic-free collagen promoting medium, and XG-hiPSC-VSMCs-P in xenogeneic collagen promoting medium as control, for 6 days. Results revealed that XF-hiPSC-VSMCs-P grown in xenogeneic-free collagen promoting medium presented higher expression levels of COL1, α-SMA and CNN1 and significantly more abundant collagen deposition than those grown in xenogeneic-free VSMC growth or priming medium, while levels were comparable to those of the xenogeneic control (Fig. 5A–C). Moreover, no significant difference of growth rate was observed between groups (Fig. 5D). Additionally, there was no significant difference in proliferation rates and collagen synthesis capacities of XF-hiPSC-VSMCs derived from three different batches of human serum when cultured in collagen-promoting medium (Fig. S2C and D). These data indicated that xenogeneic-free collagen promoting medium was able to effectively stimulate collagen deposition by XF-hiPSC-VSMCs without reducing their proliferation and that XF-hiPSC-VSMCs were minimally or not affected by potential batch variation of human serum.
Figure 5. Determining xenogeneic-free collagen promoting medium.
Results suggested that xenogeneic-free collagen promoting medium could lead to robust VSMC marker expression, collagen synthesis, and proliferation of XF-hiPSC-VSMCs.
(A) Immunostaining of VSMC contractile (α-SMA and CNN1) and ECM (COL1) markers in XF-hiPSC-VSMCs-P cultured in xenogeneic-free VSMC growth medium, priming medium (DMEM supplemented with 5% human serum and 5% human platelet lysate) or collagen promoting medium for 6 days, and XG-hiPSC-VSMCs-P cultured in xenogeneic collagen promoting medium for 6 days (see Table S1 for detailed media components). DNA (nuclear) was counterstained by DAPI. Scale bar: 200 μm.
(B) qRT-PCR analysis of relative mRNA transcript amounts of VSMC contractile (α-SMA and CNN1) and extracellular matrix (COL1) genes in XF-hiPSC-VSMCs cultured in xenogeneic-free VSMC growth medium, priming medium (DMEM supplemented with 5% human serum and 5% human platelet lysate) or collagen promoting medium, and XG-hiPSC-VSMCs-P cultured in xenogeneic collagen promoting medium. Values in the y-axis represent fold changes relative to human GAPDH expression. Gene expression in each group was normalized to that of in XF-hiPSC-VSMCs-P cultured in xenogeneic-free VSMC growth medium (One-way ANOVA with Tukey’s multiple comparisons test; Mean values and S.E.M indicated by the error bars are shown; n=3; *: p < 0.05; **: p < 0.01; ***: p < 0.001; N.S: not significant).
(C) Collagen weight per XF- or XG-hiPSC-VSMCs-P cultured in variant media via a hydroxyproline assay (One-way ANOVA with Tukey’s multiple comparisons test; Mean values and S.E.M indicated by the error bars are shown; n=3; ****: p < 0.0001; N.S: not significant).
(D) Proliferation rates of XF- or XG-hiPSC-VSMCs-P cultured in various media for 6 days. Paired two-way ANOVA showed that there was no interaction between the time for cell counting and cell/culture conditions. The cell number significantly increased along with the increase of culturing time, while there was no significant difference between the groups of cell/culture conditions within each day (Mean values and S.E.M indicated by the error bars are shown; N.S: not significant).
3.4. Generation of engineered tissue rings using xenogeneic-free hiPSC-VSMCs
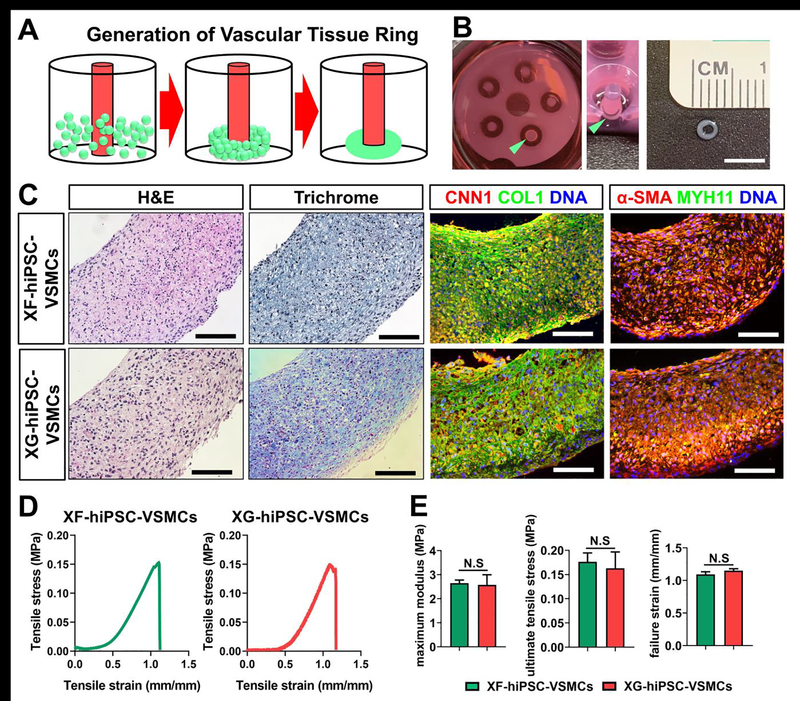
We next investigated the capacity of XF-hiPSC-VSMCs to form engineered tissue in vitro. We first used XF-hiPSC-VSMCs-P expanded in xenogeneic-free collagen promoting medium and generated scaffold-free engineered tissue rings [45]. To generate the ring-shaped tissues, XF-hiPSC-VSMCs or XG-hiPSC-VSMCs were seeded in agarose molds, allowed to form ring-shaped tissues via self-assembly, and cultured in xenogeneic-free or xenogeneic collagen promoting medium for 14 days, respectively (Fig. 6A–B). The engineered tissue rings derived from either XF-hiPSC-VSMCs or XG-hiPSC-VSMCs appeared to present comparable levels of cellularization, collagen accretion, and expression of VSMC contractile and ECM markers (Fig. 6C).
Figure 6. Fabrication of scaffold-free vascular tissue rings using XF-hiPSC-VSMCs.
Results suggested that XF-hiPSC-VSMCs could be used to develop scaffold-free vascular tissue rings with comparable histological and mechanical properties to those from XG-hiPSC-VSMCs.
(A) Illustrative scheme of the method used to establish vascular tissue rings from XF-hiPSC-VSMCs-P. Scale bar: 5 mm.
(B) Morphology of engineered tissue rings from XF-hiPSC-VSMCs-P. The tissue ring in a well of the agarose mold from above (left image), at a 45-degree angle (middle image) and the size of the ring were shown (right image). Rings are indicated by the light green arrows.
(C) H&E staining, Masson’s trichrome staining and immunofluorescent staining (CNN1, COL1, α-SMA and MYH11) of the engineered vascular tissue rings made from XF- or XG-hiPSC-VSMCs-P under xenogeneic-free or xenogeneic conditions, respectively. DNA (nuclear) was counterstained by DAPI in immunostaining. Scale bar: 100 mm.
(D) Representative stress-strain plots of engineered vascular tissue rings made from XF- or XG-hiPSC-VSMCs-P under xenogeneic-free or xenogeneic conditions, respectively.
(E) Mechanical parameters (maximum modulus, ultimate tensile stress, and failure strain) were compared between the engineered vascular tissue rings made from XF- or XG-hiPSC-VSMCs-P under xenogeneic-free or xenogeneic conditions, respectively (Two-tailed paired Student’s T-test; Mean values and S.E.M indicated by the error bars are shown; N.S: not significant).
We subsequently evaluated the mechanical properties of these engineered tissue rings using an Instron mechanical testing system. We obtained the stress-strain plots of each engineered tissue ring (representative stress-strain plots shown in Fig. 6D) and derived their mechanical parameters, including maximum modulus, maximum stress and failure strain. No significant difference of these mechanical parameters was observed between the engineered tissue rings derived from XF-hiPSC-VSMCs and those derived from XG-hiPSC-VSMCs (Fig. 6E). These results indicated that, comparable to the XG-hiPSC-VSMCs, XF-hiPSC-VSMCs were suitable for application in engineering tissues.
3.5. Generation and implantation of xenogeneic-free engineered vascular tissues using XF-hiPSC-VSMCs and biodegradable scaffolds
We have previously generated xenogeneic hiPSC-derived TEVGs by seeding XG-hiPSC-VSMCs onto biodegradable polyglycolic acid (PGA) scaffolds [31]. Therefore, it is critical to likewise determine the compatibility of XF-hiPSC-VSMCs in generating engineered vascular tissues on PGA scaffolds. As previously described [31], we applied small units of PGA scaffolds (5 mm x 5 mm squares of mesh) seeded with XF-hiPSC-VSMCs-P expanded in xenogeneic-free collagen promoting medium for 5 days (Fig. S3), and cultured these scaffolds in xenogeneic-free collagen promoting medium for 21 days to allow the formation of engineered tissues (Fig. 7A). Xenogeneic engineered vascular tissues were also developed as a control by using XG-hiPSC-VSMCs and xenogeneic collagen promoting medium (Fig. 7B and S3).
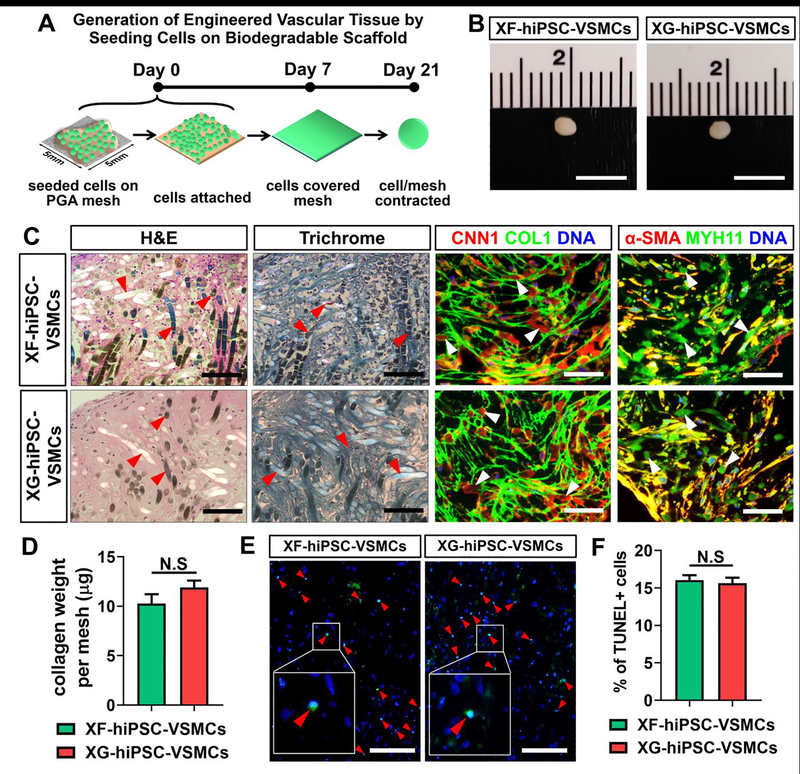
Figure 7. Generation of engineered tissues from culturing XF-hiPSC-VSMCs on biodegradable polyglycolic acid scaffolds.
Results suggested that XF-hiPSC-VSMCs could be cultured on biodegradable PGA scaffold to develop engineered vascular tissues with comparable collagen depsotion and histological properties to those from XG-hiPSC-VSMCs.
(A) Schematic illustration for developing tissue patches from XF-hiPSC-VSMCs-P grown on PGA scaffolds. Scale bar: 5 mm.
(B) Morphology of the engineered tissues developed from XF- or XG-hiPSC-VSMCs-P grown on PGA scaffolds (day 21).
(C) H&E staining, Masson’s trichrome staining and immunofluorescent staining (CNN1, COL1, α-SMA and MYH11) of the engineered tissues made from XF- or XG-hiPSC-VSMCs-P under xenogeneic-free or xenogeneic conditions, respectively. DNA (nuclear) was counterstained by DAPI in immunostaining. Red and white arrow heads indicate PGA remnants. Scale bar: 100 mm.
(D) Collagen weight per mesh of engineered tissue made from XF- or XG-hiPSC-VSMCs-P under xenogeneic-free or xenogeneic conditions, respectively, via hydroxyproline assay (Two-tailed paired Student’s T-test; Mean values and S.E.M indicated by the error bars are shown; N.S: not significant).
(E) TUNEL staining of the engineered tissues from XF- or XG-hiPSC-VSMCs-P under xenogeneic-free or xenogeneic conditions, respectively. DNA (nuclear) was counterstained by DAPI. Red arrow heads indicate the TUNEL-positive apoptotic cells. Scale bar: 100 μm.
(F) Quantification of percentage of TUNEL-positive cells in engineered tissues from XF- or XG-hiPSC-VSMCs-P under xenogeneic-free or xenogeneic conditions, respectively (Two-tailed paired Student’s T-test; Mean values and S.E.M indicated by the error bars are shown; N.S: not significant).
After 21 days of culture, opaque, oval-shaped engineered vascular tissues were obtained from both xenogeneic-free and xenogeneic groups (Fig. 7B). Histological analysis revealed that these tissues were comparably cellularized and collagen-enriched, and they displayed similar expression of VSMC contractile and ECM markers including α-SMA, CNN1, MYH11 and COL1 (Fig. 7C). Results of the hydroxyproline assay suggested that xenogeneic-free and xenogeneic engineered tissues produced comparable amounts of collagen content (Fig. 7D). Moreover, xenogeneic-free and xenogeneic engineered tissues presented similar ratios of cell viability as revealed by the results of the TUNEL assay (Fig. 7E and 7F). Collectively, engineered vascular tissues developed from XF-hiPSC-VSMCs and xenogeneic-free culture conditions presented comparable cellularity, maintenance of VSMC phenotype and collagenous matrix deposition to those of the xenogeneic counterparts. These data suggested that XF-hiPSC-VSMCs may be suitable for future vascular tissue engineering under xenogeneic-free conditions.
Furthermore, we subcutaneously engrafted these engineered vascular tissues into Rag2/Il2rg double knockout, immune-deficient mice for two weeks to assess maintenance of VSMC characteristics of engineered tissues in vivo (Fig. 8A and 8B). Plain PGA scaffolds maintained in xenogeneic-free collagen promoting medium for 21 days without seeding cells were engrafted as blank controls. Histological analysis suggested that the explanted tissues developed from both XF-hiPSC-VSMCs and XG-hiPSC-VSMCs maintained cellularization and collagen deposition and displayed minimal or no PGA remnants (Fig. 8C). Moreover, XF-hiPSC-VSMCs within explants displayed the presence of human leukocyte antigen-A (HLA-A), a human-specific surface marker, as well as VSMC contractile markers, including α-SMA, CNN1 and MYH11, at levels similar to those of xenogeneic engineered tissues (Fig. 8C). In contrast, the plain scaffold control displayed infiltration of host, HLA-A-negative cells with low levels of collagen deposition and low expression levels of most contractile markers. Significant amounts of PGA remnants were also observed in the plain scaffold control (Fig. 8C). These results further suggested that XF-hiPSC-VSMCs are capable of generating engineered vascular tissues on biodegradable PGA scaffolds, and maintain the VSMC characteristics both in vitro and in vivo.
Figure 8. Engraftment of engineered tissues from culturing XF-hiPSC-VSMCs on PGA scaffolds.

Results suggested that engineered vascular tissues developed using XF-hiPSC-VSMCs presented similar histological properties to those from XG-hiPSC-VSMCs after subcutaneous implantation into immune-deficient mouse model.
(A) Schematic illustration for subcutaneously engrafting engineered tissue from XF-hiPSC-VSMCs-P grown on PGA scaffolds. Scale bar: 5 mm.
(B) Morphology of the explanted engineered tissues on day 14 post-engraftment.
(C) H&E staining, Masson’s trichrome staining and immunofluorescent staining (CNN1, human leukocyte antigen-A [HLA-A], α-SMA and MYH11) of the explanted engineered tissues made from XF- or XG-hiPSC-VSMCs-P under xenogeneic-free or xenogeneic conditions, respectively, or blank control PGA meshes without seeded cells. DNA (nuclear) was counterstained by DAPI in immunostaining. Red arrow heads indicate PGA remnants. White lines indicate the border between the engrafted and host tissues. Scale bar: 100 μm.
4. Discussion
To our knowledge, this is the first report on the establishment of a xenogeneic-free approach to derive functional hiPSC-VSMCs suitable for scaffold-assisted vascular tissue engineering. Application of hiPSC-VSMCs generated under standard, xenogeneic conditions have led to promising progress in the generation of hiPSC-based tissue engineered vascular grafts (hiPSC-TEVGs) with advanced mechanical strength as an innovative future therapy [31]. However, use of xenogeneic conditions for generating the hiPSC-derived cells and subsequent tissue engineering could impede the human clinical performance of these hiPSC-TEVGs due to the potential risks of zoonoses leading to xenogeneic immune responses. Herein, we have established an efficient method to derive hiPSC-VSMCs under xenogeneic-free conditions. These XF-hiPSC-VSMCs presented a comparable marker expression profile and functional contractility to those of human primary VSMCs and hiPSC-VSMCs obtained under standard xenogeneic conditions. Importantly, XF-hiPSC-VSMCs displayed similar potential for generating engineered vascular tissues to that of standard XG-hiPSC-VSMCs. Therefore, our study has created a promising foundation for generating hiPSC-TEVGs under completely xenogeneic-free conditions for future vascular clinical intervention.
Application of reagents with animal origins in cell culture may raise the risks of xenogeneic immune responses and zoonoses transmission. A number of clinical trials have already reported xenogeneic immune responses cells in human patients triggered by engraftment of cells cultured in medium containing animal-derived reagents such as bovine serum [46–48]. Moreover, potential transmission of zoonoses, especially bovine spongiform encephalopathy (BSE, or known as prion diseases), from animal reagents to human recipients has been emphasized, due to the absenceof available test or treatment of BSE as suggested by United States Department of Agriculture (USDA, https://www.usda.gov/topics/animals/bse-surveillance-information-center) and United States Food and Drug Administration (FDA, https://www.fda.gov/animal-veterinary/animal-health-literacy/all-about-bse-mad-cow-disease). It is therefore important to avoid using animal-derived reagent in cell or tissue culture for human clinical application.
To our knowledge, xenogeneic reagents have been applied in most reported methods for obtaining VSMCs from hiPSCs, which has made these methods suboptimal for producing cell derivatives for potential clinical application (Fig. 1A). It is worth noting that a xenogeneic-free, chemically defined method for producing VSMCs from human embryonic stem cell (hESC-VSMCs) expanded on human vitronectin has been previously reported [49]. It has been suggested that chemically defined conditions could lead to the low proliferation rates of hiPSC-VSMCs [31, 39]. Since vascular tissue engineering requires the seeded cells to promptly propagate in the porous supporting scaffolds, the chemically defined, xenogeneic-free hESC-VSMCs in this report [49] might be less proliferative and suboptimal for scaffold-assited vascular tissue fabrication. Moreover, the feasibility of these hESC-VSMCs for tissue engineering has yet to be evaluated. In our current study to develop a completely xenogeneic-free method of deriving hiPSC-VSMCs suitbale for vascular tissue engineering, we significantly modified our standard xenogeneic hiPSC-VSMC differentiation protocol. Various reagents of human origin have been used to replace those originally derived from animals [31] (Fig. 1B and Table S1). In particular, xenogeneic-free replacement of fetal bovine serum (FBS) was critical, considering the vital roles of FBS in initiating hiPSC differentiation, expansion of hiPSC-VSMCs, and vascular tissue engineering [31, 35, 39, 40]. We determined that employing a combination of human serum and human platelet lysate was a suitable cocktail to effectively replace FBS. This finding is consistent with the application of both human serum and human platelet lysate as substitutes for FBS in cell expansion, tissue engineering, and clinical therapies [32, 50, 51]. Development of a serum-free, chemically-defined culture medium has also been suggested as an alternative method for replacing FBS due to its potentially stable performance. While the previously reported serum-free, chemically-defined culture medium allows for differentiation of hiPSC-VSMCs [39], it does not support the necessary, scalable expansion of hiPSC-VSMCs, and FBS is required for cellular proliferation. The requirement of FBS for hiPSC-VSMC expansion and the use of mouse embryonic fibroblast (MEF) feeder layer for hiPSC growth in the above protocol [39] make it suboptimal for therapeutic application. In contrast, our currently reported, completely xenogeneic-free hiPSC-VSMC derivation approach employs all reagents of human origin and produces VSMCs exhibiting suitable characteristics for vascular tissue engineering, thereby providing a foundation for future therapeutic application of hiPSC-VSMCs for treatment of vascular disorders.
Future efforts will be warranted to further improve and promote the application of our current xenogeneic-free method for deriving hiPSC-VSMCs. We will determine essential components to develop serum-free, chemically defined, xenogeneic-free conditions for efficiently deriving hiPSC-VSMCs with sufficient proliferative capacity for vascular tissue engineering. Moreover, we will use XF-hiPSC-VSMCs to develop TEVGs under complete xenogeneic-free culture conditions and evaluatetheir in vivo performance in small and large animal vessel engraftment models [16, 31]. A series of systematic research will be performed to determine the optimal biochemical and biomechanical culture conditions to generate xenogeneic-free hiPSC-TEVGs with advanced mechanical strength. It has been reported that mechnanically robust TEVGs derived from our previous xenogeneic hiPSC-VSMCs did not generate mature extracellular elastin (ELN) fibers [23, 31], which are important for tissue structural integrity and elasticity. Consistent with our previous study [31], XF-hiPSC-VSMCs readily expressed ELN at a similar level to that in XG-hiPSC-VSMCs when cultured in the collagen promoting medium, though appreciable mature extracellular ELN fibers were not observed (Fig. S4). Our group is currently exploring various approaches to enhance mature ELN fiber formation by culturing XF-hiPSC-VSMCs in reagents that promote the ELN expression (e.g. TGF-β1 and miR-29a inhibitor) [52, 53], augment ELN fiber assembly through enhancing the buildup of other essential ECM proteins that interact with ELN and stabilize the fiber formation (e.g. BMP4 or LTBP4) [54, 55], and decrease proteoglycans that inhibit ELN fiber formation (e.g. versican and decorin) [54, 56]. We will also generate hiPSCs from a donor’s somatic cells under xenogeneic-free conditions for further VSMC differentiation and vascular tissue development in the future [57]. Additionally, we will process all cells and tissues according to current good manufacturing practice (cGMP)-compliant protocols. Eventually we will generate immunocompatible, human leukocyte antigen-engineered “universal” hiPSC lines to obtain minimal or non-immunogenic hiPSC-VSMCs for vascular tissue engineering under xenogeneic-free conditions [58–60], which could lead to clinical grade, allogeneic, immunocompatible hiPSC-TEVGs as an innovative therapy for any patient in the future.
5. Conclusion
In this study, we have reported an effective xenogeneic-free method to derive hiPSC-VSMCs suitable for vascular tissue engineering. We substituted all animal-derived reagents in our standard xenogeneic VSMC differentiation approach with reagent counterparts of human origin, and ultimately generated functional XF-hiPSC-VSMCs. These XF-hiPSC-VSMCs are comparable to human primary VSMCs and XG-hiPSC-VSMCs both functionally and in their expression profile. More importantly, we have demonstrated that these XF-hiPSC-VSMCs displayed comparable feasibility to XG-hiPSC-VSMCs in the development of engineered vascular tissues via self-assembly or interaction with biodegradable PGA scaffold. Our xenogeneic-free method of deriving hiPSC-VSMCs has led to similar cellular products and comparable capacity for vascular tissue engineering compared with our standard xenogeneic protocol. We believe the outcome of this research lays a strong foundation for generating xenogeneic-free hiPSC-TEVGs of human clinical grade in the future.
Supplementary Material
Statement of Significance.
Tissue-engineered vascular grafts (TEVGs) generated from human induced pluripotent stem cell-derived vascular smooth muscle cells (hiPSC-VSMCs) represent promising vascular therapy. To move current hiPSC-TEVGs toward clinical application, it is important to generate hiPSC-TEVGs under the conditions without using any animal-derived reagents (xenogeneic-free conditions). However, completely xenogeneic-free method for deriving hiPSC-VSMCs (XF-hiPSC-VSMCs) has not been established yet. Herein, we established an efficient method for deriving functional XF-hiPSC-VSMCs. Our xenogeneic-free method for deriving hiPSC-VSMCs produce similar cell products with comparable cellular functions and capacity for vascular tissue engineering as standard xenogeneic protocols. The outcome of our research sets the foundation to generate xenogeneic-free hiPSC-TEVGs as clinical therapy in the future.
Acknowledgments
We thank the Qyang group members for their valuable feedback for this line of research. We acknowledge funding support from DOD 11959515, DOD W81XWH1910557, R01HL116705, R01HL150352, and CRMRF 15-RMB-YALE-08 (all to YQ), The American Heart Association (AHA) Postdoctoral Fellowship 19POST34450100 (to JL) and 19POST34381048 (to MHK), China Scholarships Council 201806280209 (to YL) and 201706370156 (to XS), NIH/NIGMS Medical Scientist Training Program Grant NIH 1F31HL143928-01 (to CWA), and NIH (United States) grants F31-HL143924 and T32-GM0007324 (to MWE).
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Feliciano DV, Pitfalls in the management of peripheral vascular injuries, Trauma surgery & acute care open 2(1) (2017) e000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Feliciano DV, Moore EE, West MA, Moore FA, Davis JW, Cocanour CS, Scalea TM, McIntyre RC Jr., Western Trauma Association critical decisions in trauma: evaluation and management of peripheral vascular injury, part II, The journal of trauma and acute care surgery 75(3) (2013) 391–7. [DOI] [PubMed] [Google Scholar]
- [3].Pashneh-Tala S, MacNeil S, Claeyssens F, The Tissue-Engineered Vascular Graft-Past, Present, and Future, Tissue Eng Part B Rev 22(1) (2016) 68–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Schild AF, Perez E, Gillaspie E, Seaver C, Livingstone J, Thibonnier A, Arteriovenous fistulae vs. arteriovenous grafts: a retrospective review of 1,700 consecutive vascular access cases, J Vasc Access 9(4) (2008) 231–5. [PubMed] [Google Scholar]
- [5].Shum-Tim D, Stock U, Hrkach J, Shinoka T, Lien J, Moses MA, Stamp A, Taylor G, Moran AM, Landis W, Langer R, Vacanti JP, Mayer JE Jr., Tissue engineering of autologous aorta using a new biodegradable polymer, Ann Thorac Surg 68(6) (1999) 2298–304; discussion 2305. [DOI] [PubMed] [Google Scholar]
- [6].Gao G, Kim H, Kim BS, Kong JS, Lee JY, Park BW, Chae S, Kim J, Ban K, Jang J, Park HJ, Cho DW, Tissue-engineering of vascular grafts containing endothelium and smooth-muscle using triple-coaxial cell printing, Appl Phys Rev 6(4) (2019). [Google Scholar]
- [7].Wu W, Allen RA, Wang Y, Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery, Nat Med 18(7) (2012) 1148–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Muylaert DE, van Almen GC, Talacua H, Fledderus JO, Kluin J, Hendrikse SI, van Dongen JL, Sijbesma E, Bosman AW, Mes T, Thakkar SH, Smits AI, Bouten CV, Dankers PY, Verhaar MC, Early in-situ cellularization of a supramolecular vascular graft is modified by synthetic stromal cell-derived factor-1alpha derived peptides, Biomaterials 76 (2016) 187–95. [DOI] [PubMed] [Google Scholar]
- [9].Lee KW, Gade PS, Dong L, Zhang Z, Aral AM, Gao J, Ding X, Stowell CET, Nisar MU, Kim K, Reinhardt DP, Solari MG, Gorantla VS, Robertson AM, Wang Y, A biodegradable synthetic graft for small arteries matches the performance of autologous vein in rat carotid arteries, Biomaterials 181 (2018) 67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR, Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB, Circ Res 100(11) (2007) 1659–66. [DOI] [PubMed] [Google Scholar]
- [11].Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR, Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase, Am J Physiol Heart Circ Physiol 297(1) (2009) H425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Avogaro A, Albiero M, Menegazzo L, de Kreutzenberg S, Fadini GP, Endothelial dysfunction in diabetes: the role of reparatory mechanisms, Diabetes Care 34 Suppl 2 (2011) S285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kolluru GK, Bir SC, Kevil CG, Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing, International journal of vascular medicine 2012 (2012) 918267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R, Functional arteries grown in vitro, Science 284(5413) (1999) 489–93. [DOI] [PubMed] [Google Scholar]
- [15].Syedain ZH, Meier LA, Bjork JW, Lee A, Tranquillo RT, Implantable arterial grafts from human fibroblasts and fibrin using a multi-graft pulsed flow-stretch bioreactor with noninvasive strength monitoring, Biomaterials 32(3) (2011) 714–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dahl SL, Kypson AP, Lawson JH, Blum JL, Strader JT, Li Y, Manson RJ, Tente WE, DiBernardo L, Hensley MT, Carter R, Williams TP, Prichard HL, Dey MS, Begelman KG, Niklason LE, Readily available tissue-engineered vascular grafts, Sci Transl Med 3(68) (2011) 68ra9. [DOI] [PubMed] [Google Scholar]
- [17].Lawson JH, Glickman MH, Ilzecki M, Jakimowicz T, Jaroszynski A, Peden EK, Pilgrim AJ, Prichard HL, Guziewicz M, Przywara S, Szmidt J, Turek J, Witkiewicz W, Zapotoczny N, Zubilewicz T, Niklason LE, Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: two phase 2 single-arm trials, Lancet 387(10032) (2016) 2026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kirkton RD, Santiago-Maysonet M, Lawson JH, Tente WE, Dahl SLM, Niklason LE, Prichard HL, Bioengineered human acellular vessels recellularize and evolve into living blood vessels after human implantation, Sci Transl Med 11(485) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Syedain Z, Reimer J, Schmidt J, Lahti M, Berry J, Bianco R, Tranquillo RT, 6-month aortic valve implantation of an off-the-shelf tissue-engineered valve in sheep, Biomaterials 73 (2015) 175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Syedain ZH, Graham ML, Dunn TB, O’Brien T, Johnson SL, Schumacher RJ, Tranquillo RT, A completely biological “off-the-shelf” arteriovenous graft that recellularizes in baboons, Sci Transl Med 9(414) (2017) eaan4209. [DOI] [PubMed] [Google Scholar]
- [21].Sundaram S, Niklason LE , Smooth muscle and other cell sources for human blood vessel engineering, Cells Tissues Organs 195(1–2) (2012) 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S, Induction of pluripotent stem cells from adult human fibroblasts by defined factors, Cell 131(5) (2007) 861–72. [DOI] [PubMed] [Google Scholar]
- [23].Gui L, Dash BC, Luo J, Qin L, Zhao L, Yamamoto K, Hashimoto T, Wu H, Dardik A, Tellides G, Niklason LE, Qyang Y, Implantable tissue-engineered blood vessels from human induced pluripotent stem cells, Biomaterials 102 (2016) 120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dash BC, Levi K, Schwan J, Luo J, Bartulos O, Wu H, Qiu C, Yi T, Ren Y, Campbell S, Rolle MW, Qyang Y, Tissue-Engineered Vascular Rings from Human iPSC-Derived Smooth Muscle Cells, Stem Cell Reports 7(1) (2016) 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang Y, Hu J, Jiao J, Liu Z, Zhou Z, Zhao C, Chang LJ, Chen YE, Ma PX, Yang B, Engineering vascular tissue with functional smooth muscle cells derived from human iPS cells and nanofibrous scaffolds, Biomaterials 35(32) (2014) 8960–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Xie CQ, Zhang J, Villacorta L, Cui T, Huang H, Chen YE, A highly efficient method to differentiate smooth muscle cells from human embryonic stem cells, Arterioscler Thromb Vasc Biol 27(12) (2007) e311–2. [DOI] [PubMed] [Google Scholar]
- [27].Atchison L, Abutaleb NO, Snyder-Mounts E, Gete Y, Ladha A, Ribar T, Cao K, Truskey GA, iPSC-Derived Endothelial Cells Affect Vascular Function in a Tissue-Engineered Blood Vessel Model of Hutchinson-Gilford Progeria Syndrome, Stem Cell Reports 14(2) (2020) 325–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Atchison L, Zhang H, Cao K, Truskey GA, A Tissue Engineered Blood Vessel Model of Hutchinson-Gilford Progeria Syndrome Using Human iPSC-derived Smooth Muscle Cells, Sci Rep 7(1) (2017) 8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yang L, Geng Z, Nickel T, Johnson C, Gao L, Dutton J, Hou C, Zhang J, Differentiation of Human Induced-Pluripotent Stem Cells into Smooth-Muscle Cells: Two Novel Protocols, PLoS One 11(1) (2016) e0147155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bajpai VK, Mistriotis P, Loh YH, Daley GQ, Andreadis ST, Functional vascular smooth muscle cells derived from human induced pluripotent stem cells via mesenchymal stem cell intermediates, Cardiovasc Res 96(3) (2012) 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Luo J, Qin L, Zhao L, Gui L, Ellis MW, Huang Y, Kural MH, Clark JA, Ono S, Wang J, Yuan Y, Zhang SM, Cong X, Li G, Riaz M, Lopez C, Hotta A, Campbell S, Tellides G, Dardik A, Niklason LE, Qyang Y, Tissue-Engineered Vascular Grafts with Advanced Mechanical Strength from Human iPSCs, Cell Stem Cell 26(2) (2020) 251–261 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Astori G, Amati E, Bambi F, Bernardi M, Chieregato K, Schafer R, Sella S, Rodeghiero F, Platelet lysate as a substitute for animal serum for the ex-vivo expansion of mesenchymal stem/stromal cells: present and future, Stem Cell Res Ther 7(1) (2016) 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, Wagner R, Lee GO, Antosiewicz-Bourget J, Teng JM, Thomson JA, Chemically defined conditions for human iPSC derivation and culture, Nature methods 8(5) (2011) 424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Patsch C, Challet-Meylan L, Thoma EC, Urich E, Heckel T, O’Sullivan JF, Grainger SJ, Kapp FG, Sun L, Christensen K, Xia Y, Florido MH, He W, Pan W, Prummer M, Warren CR, Jakob-Roetne R, Certa U, Jagasia R, Freskgard PO, Adatto I, Kling D, Huang P, Zon LI, Chaikof EL, Gerszten RE, Graf M, Iacone R, Cowan CA, Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells, Nature cell biology 17(8) (2015) 994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wanjare M, Kuo F, Gerecht S, Derivation and maturation of synthetic and contractile vascular smooth muscle cells from human pluripotent stem cells, Cardiovasc Res 97(2) (2013) 321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wanjare M, Kusuma S, Gerecht S, Defining differences among perivascular cells derived from human pluripotent stem cells, Stem Cell Reports 2(5) (2014) 561–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lee TH, Song SH, Kim KL, Yi JY, Shin GH, Kim JY, Kim J, Han YM, Lee SH, Lee SH, Shim SH, Suh W, Functional recapitulation of smooth muscle cells via induced pluripotent stem cells from human aortic smooth muscle cells, Circ Res 106(1) (2010) 120–8. [DOI] [PubMed] [Google Scholar]
- [38].Lin B, Kim J, Li Y, Pan H, Carvajal-Vergara X, Salama G, Cheng T, Li Y, Lo CW, Yang L, High-purity enrichment of functional cardiovascular cells from human iPS cells, Cardiovasc Res 95(3) (2012) 327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cheung C, Bernardo AS, Trotter MW, Pedersen RA, Sinha S, Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility, Nat Biotechnol 30(2) (2012) 165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cheung C, Bernardo AS, Pedersen RA, Sinha S, Directed differentiation of embryonic origin-specific vascular smooth muscle subtypes from human pluripotent stem cells, Nat Protoc 9(4) (2014) 929–38. [DOI] [PubMed] [Google Scholar]
- [41].Gwyther TA, Hu JZ, Christakis AG, Skorinko JK, Shaw SM, Billiar KL, Rolle MW, Engineered vascular tissue fabricated from aggregated smooth muscle cells, Cells Tissues Organs 194(1) (2011) 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Luo J, Qin L, Kural MH, Schwan J, Li X, Bartulos O, Cong XQ, Ren Y, Gui L, Li G, Ellis MW, Li P, Kotton DN, Dardik A, Pober JS, Tellides G, Rolle M, Campbell S, Hawley RJ, Sachs DH, Niklason LE, Qyang Y, Vascular smooth muscle cells derived from inbred swine induced pluripotent stem cells for vascular tissue engineering, Biomaterials 147 (2017) 116–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gao J, Niklason L, Langer R, Surface hydrolysis of poly(glycolic acid) meshes increases the seeding density of vascular smooth muscle cells, J Biomed Mater Res 42(3) (1998) 417–24. [DOI] [PubMed] [Google Scholar]
- [44].Woessner JF, Determination of Hydroxyproline in Tissue and Protein Samples Containing Small Proportions of This Imino Acid, Arch Biochem Biophys 93(2) (1961) 440–&. [DOI] [PubMed] [Google Scholar]
- [45].Strobel HA, Hookway TA, Piola M, Fiore GB, Soncini M, Alsberg E, Rolle MW, Assembly of Tissue-Engineered Blood Vessels with Spatially Controlled Heterogeneities, Tissue Eng Part A 24(19–20) (2018) 1492–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Selvaggi TA, Walker RE, Fleisher TA, Development of antibodies to fetal calf serum with arthus-like reactions in human immunodeficiency virus-infected patients given syngeneic lymphocyte infusions, Blood 89(3) (1997) 776–9. [PubMed] [Google Scholar]
- [47].Mackensen A, Drager R, Schlesier M, Mertelsmann R, Lindemann A, Presence of IgE antibodies to bovine serum albumin in a patient developing anaphylaxis after vaccination with human peptide-pulsed dendritic cells, Cancer Immunol Immunother 49(3) (2000) 152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Pagan JA, Postigo I, Rodriguez-Pacheco JR, Pena M, Guisantes JA, Martinez J, Bovine serum albumin contained in culture medium used in artificial insemination is an important anaphylaxis risk factor, Fertil Steril 90(5) (2008) 2013 e17–9. [DOI] [PubMed] [Google Scholar]
- [49].Zhang J, McIntosh BE, Wang B, Brown ME, Probasco MD, Webster S, Duffin B, Zhou Y, Guo LW, Burlingham WJ, Kent C, Ferris M, Thomson JA, A Human Pluripotent Stem Cell-Based Screen for Smooth Muscle Cell Differentiation and Maturation Identifies Inhibitors of Intimal Hyperplasia, Stem Cell Reports 12(6) (2019) 1269–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Henschler R, Gabriel C, Schallmoser K, Burnouf T, Koh MBC, Human platelet lysate current standards and future developments, Transfusion 59(4) (2019) 1407–1413. [DOI] [PubMed] [Google Scholar]
- [51].Perez-Simon JA, Lopez-Villar O, Andreu EJ, Rifon J, Muntion S, Diez Campelo M, Sanchez-Guijo FM, Martinez C, Valcarcel D, Canizo CD, Mesenchymal stem cells expanded in vitro with human serum for the treatment of acute and chronic graft-versus-host disease: results of a phase I/II clinical trial, Haematologica 96(7) (2011) 1072–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kahari VM, Olsen DR, Rhudy RW, Carrillo P, Chen YQ, Uitto J, Transforming growth factor-beta up-regulates elastin gene expression in human skin fibroblasts. Evidence for post-transcriptional modulation, Lab Invest 66(5) (1992) 580–8. [PubMed] [Google Scholar]
- [53].Zhang P, Huang A, Ferruzzi J, Mecham RP, Starcher BC, Tellides G, Humphrey JD, Giordano FJ, Niklason LE, Sessa WC, Inhibition of microRNA-29 enhances elastin levels in cells haploinsufficient for elastin and in bioengineered vessels--brief report, Arterioscler Thromb Vasc Biol 32(3) (2012) 756–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Tojais NF, Cao A, Lai YJ, Wang L, Chen PI, Alcazar MAA, de Jesus Perez VA, Hopper RK, Rhodes CJ, Bill MA, Sakai LY, Rabinovitch M, Codependence of Bone Morphogenetic Protein Receptor 2 and Transforming Growth Factor-beta in Elastic Fiber Assembly and Its Perturbation in Pulmonary Arterial Hypertension, Arterioscler Thromb Vasc Biol 37(8) (2017) 1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Noda K, Dabovic B, Takagi K, Inoue T, Horiguchi M, Hirai M, Fujikawa Y, Akama TO, Kusumoto K, Zilberberg L, Sakai LY, Koli K, Naitoh M, von Melchner H, Suzuki S, Rifkin DB, Nakamura T, Latent TGF-beta binding protein 4 promotes elastic fiber assembly by interacting with fibulin-5, Proc Natl Acad Sci U S A 110(8) (2013) 2852–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Huang R, Merrilees MJ, Braun K, Beaumont B, Lemire J, Clowes AW, Hinek A, Wight TN, Inhibition of versican synthesis by antisense alters smooth muscle cell phenotype and induces elastic fiber formation in vitro and in neointima after vessel injury, Circ Res 98(3) (2006) 370–7. [DOI] [PubMed] [Google Scholar]
- [57].Baghbaderani BA, Tian X, Neo BH, Burkall A, Dimezzo T, Sierra G, Zeng X, Warren K, Kovarcik DP, Fellner T, Rao MS, cGMP-Manufactured Human Induced Pluripotent Stem Cells Are Available for Pre-clinical and Clinical Applications, Stem Cell Reports 5(4) (2015) 647–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Deuse T, Hu X, Gravina A, Wang D, Tediashvili G, De C, Thayer WO, Wahl A, Garcia JV, Reichenspurner H, Davis MM, Lanier LL, Schrepfer S, Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients, Nat Biotechnol 37(3) (2019) 252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gornalusse GG, Hirata RK, Funk SE, Riolobos L, Lopes VS, Manske G, Prunkard D, Colunga AG, Hanafi LA, Clegg DO, Turtle C, Russell DW, HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells, Nat Biotechnol 35(8) (2017) 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Xu H, Wang B, Ono M, Kagita A, Fujii K, Sasakawa N, Ueda T, Gee P, Nishikawa M, Nomura M, Kitaoka F, Takahashi T, Okita K, Yoshida Y, Kaneko S, Hotta A, Targeted Disruption of HLA Genes via CRISPR-Cas9 Generates iPSCs with Enhanced Immune Compatibility, Cell Stem Cell 24(4) (2019) 566–578 e7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.