Abstract
Indole-3-acetic acid, that is, auxin, is a molecule found in a broad phylogenetic distribution of organisms, from bacteria to eukaryotes. In the ancestral land plant auxin was co-opted to be the paramount phytohormone mediating tropic responses and acting as a facilitator of developmental decisions throughout the life cycle. The evolutionary origins of land plant auxin biology genes can now be traced with reasonable clarity. Genes encoding the two enzymes of the land plant auxin biosynthetic pathway arose in the ancestral land plant by a combination of horizontal gene transfer from bacteria and possible neofunctionalization following gene duplication. Components of the auxin transcriptional signaling network have their origins in ancestral alga genes, with gene duplication and neofunctionalization of key domains allowing integration of a portion of the preexisting transcriptional network with auxin. Knowledge of the roles of orthologous genes in extant charophycean algae is lacking, but could illuminate the ancestral functions of both auxin and the co-opted transcriptional network.
In the late 19th century during the birth of modern plant physiology, effort was directed toward understanding the physiochemical nature of “correlations,” that is, how different parts of plants interact with one another and with the environment to produce characteristic phenotypes. Julius Sachs proposed that “correlations” were caused by specific chemicals, not just nutritive substances, acting at low concentrations, which we would now recognize as plant hormones (Sachs 1880, 1882). In a number of simple, yet elegant experiments over the next 40 years, it was established that the effects of light and gravity were perceived at the shoot and root tips, respectively, with the stimulus subsequently communicated to distant regions where growth changes occurred (Darwin 1880), that the stimulus could pass through a wound gap filled by gelatin (Boysen Jensen 1910), and that asymmetric application to a cut shoot tip could induce the same growth changes (Paál 1919). Further, this substance could be infused from the tip into agar, which could then provide the growth substance to a decapitated shoot, a key finding that enabled biochemical determination of the growth substance, indole-3-acetic acid (IAA), that we now know as auxin (Went 1926, 1928). While most early experiments focused on angiosperms, in subsequent decades auxin was found to influence bryophyte development in a similar manner suggesting a conserved role throughout land plants (Fitting 1939; Heitz 1940; von Witsch 1940; Rousseau 1950; Halbsguth and Kohlenbach 1953; LaRue and Narayanaswami 1957). An adaptation of Went's experiment whereby chemicals infused into agar blocks applied asymmetrically to decapitated Avena plants were used to test whether various sources contained auxin activity. This test suggested that auxin, or related molecules, could be found in a remarkably wide variety of organisms including fungi, bacteria, and even mammalian (human) urine (Kögl and Kostermans 1934; Went and Thimann 1937).
During the early decades of the twentieth century, it became apparent that auxin elicited a wide variety of effects in different tissues and that auxin itself did not have specificity of action. This was first explicitly recognized by Cholodny, who noted that geotropic sensitivity to decapitated roots was restored by placing either another root tip or, alternatively, a shoot tip on the decapitated stump, thus demonstrating that auxins produced in the shoot and root tips are equivalent and that auxin acts in a context-dependent manner (Cholodny 1924). This notion was elaborated upon in subsequent years with Went and Thimann who hypothesized that “… auxins bring about some master-reaction within the cell, the results of which will be determined by the presence and amount of other factors, and by conditions of the experiment” (Went and Thimann 1937). This idea was reiterated in the previous version of this collection where auxin was described as a cellular “currency” that interacts with genetic programs that vary between cell types (Stewart and Nemhauser 2010). Likewise, auxin has been described as an “impetus” absent of specificity (Bennett and Leyser 2014). The discovery of a minimal auxin response system in the liverwort Marchantia polymorpha and the myriad of effects of auxin on its growth and development confirmed that there exists no tissue or cell-type specificity of action with respect to auxin signaling components, but rather since the origin of land plants, auxin has acted as a facilitator rather than a determiner of cell fate (Flores-Sandoval et al. 2015; Kato et al. 2015).
Recently, it has become apparent that auxin transcriptional signaling, as defined in land plants, evolved in the ancestral land plant, but other aspects of auxin biology, such as transport, are more ancient. The scope of this review will include (1) the origin and evolution of the molecular components of land plant auxin biology (Fig. 1), (2) the predicted auxin biology of the ancestral land plant, (3) how auxin was integrated into a preexisting transcriptional network, and (4) possible antecedent functions of auxin biology in algal relatives. In short, how did a ubiquitous molecule found in all domains of life become the key developmental hormone mediating tropisms and differentiation in land plants?
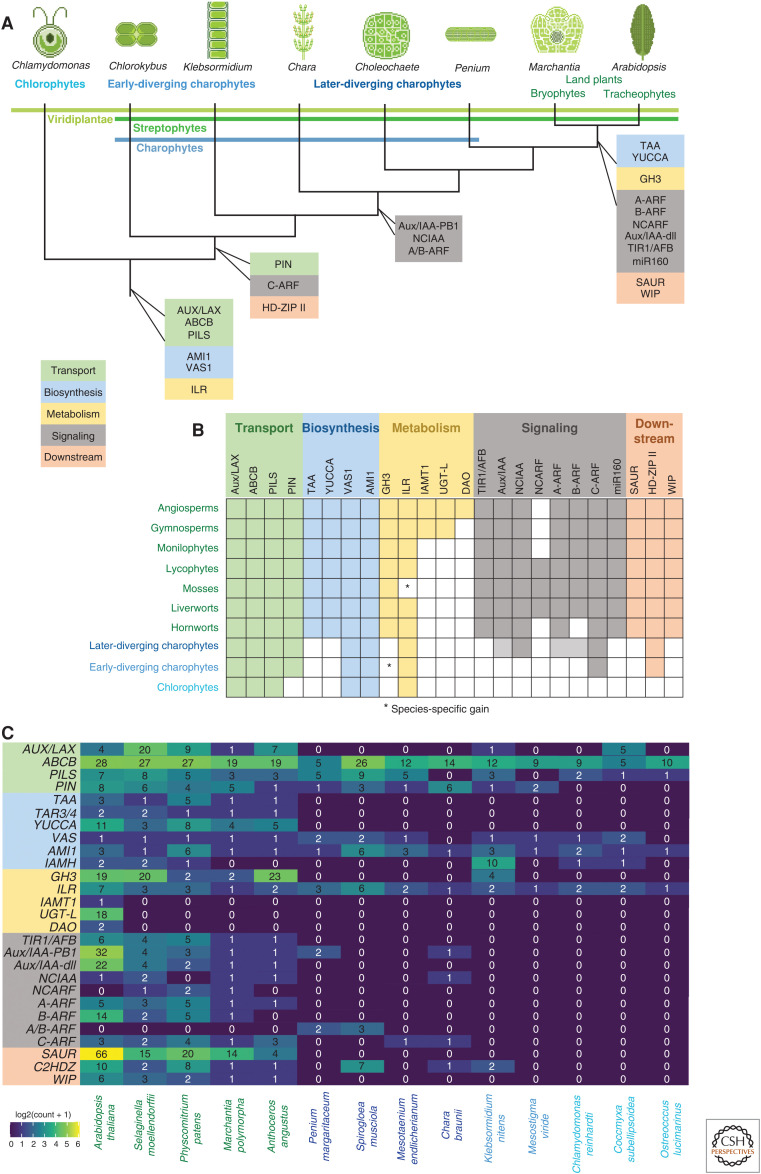
Figure 1.
The predicted evolutionary origin of auxin biology genes based on genomic and transcriptomic analyses. (A) Origin of auxin biology genes are mapped onto the current consensus of Viridiplantae relationships based on phylogenomic studies, with representative genera depicted above. The phylogenetic delineations of Viridiplantae, Streptophytes, and charophytes are indicated. (B) Distribution of auxin biology gene families across the major land plant lineages highlights lineage-specific losses and species-specific gains (*). (C) Genetic redundancy within auxin biology gene families. Heat map of gene counts for fully sequenced genomes of species. Gene counts are indicated and color coding follows a log10 transformation. Families not previously accounted for in published genome studies were cross-corroborated using tblastx and a reciprocal blast against TAIR10 (www.arabidopsis.org). E-value thresholds for both BLASTS were <1 × 10−20. Sequences were derived from GenBank (www.ncbi.nlm.nih.gov), Phytozome (phytozome.jgi.doe.gov/pz/portal.html), MarpolBase (marchantia.info), and the Klebsormidium nitens (http://www.plantmorphogenesis.bio.titech.ac.jp/~algae_genome_project/klebsormidium/) genome website. Gene families are color coded according to their proposed function in auxin biology.
ORIGIN AND EVOLUTION OF THE COMPONENTS
Auxin Biosynthesis
IAA is found widely throughout both bacteria and eukaryotes (Žižková et al. 2017; Morffy and Strader 2020). In bacteria, auxin is synthesized by plant pathogens or symbionts via independently evolved enzymes (e.g., iaaM and iaaH of Agrobacterium) that convert tryptophan (trp) into auxin via a variety of intermediaries (Kunkel and Harper 2018). In eukaryotes, biologically active auxins can be found in fungi (Chanclud and Morel 2016), brown algae (Basu et al. 2002; Sun et al. 2004; Le Bail et al. 2010), red algae (Ashen et al. 1999), and in Viridiplantae lineages, including Chlorophytes (Stirk et al. 2013; Žižková et al. 2017), charophycean algae (Sztein et al. 2000; Žižková et al. 2017), and embryophytes (Cooke et al. 2002). Whereas there is no evidence supporting an ancestral eukaryotic auxin biosynthetic pathway, that auxin triggers physiological or morphological changes in these eukaryotic lineages suggests independent recruitment of auxin for diverse biological functions (Wood and Berliner 1979; Vance 1987; Yokoya and Handro 1996; Basu et al. 2002; Jin et al. 2008; Le Bail et al. 2010; Ohtaka et al. 2017; Bogaert et al. 2019).
The genetic basis of auxin biosynthesis has been elucidated in land plants (Zhao et al. 2001; Stepanova et al. 2008; Tao et al. 2008), with the primary pathway being tryptophan-dependent: TRYPTOPHAN AMINOTRANSFERASEs (TAAs) convert trp to indole-3-pyruvic acid (IPyA) and subsequently IPyA is converted into IAA by YUCCA flavin monooxygenases (Mashiguchi et al. 2011; Stepanova et al. 2011). The origins of these two enzymes, via gene duplication of an ancestral algal gene and horizontal gene transfer from bacteria, respectively, provides compelling evidence that this auxin biosynthetic pathway is a land plant innovation (Fig. 1). The predicted control of cellular auxin homeostasis of an ancestral charophyte and the ancestral land plant are presented in Figure 2 and will be referred to in the following discussion.
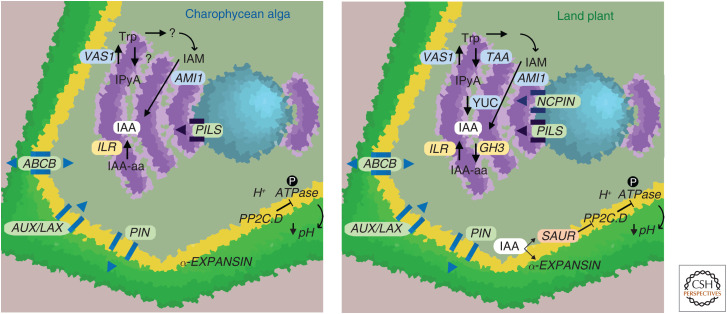
Figure 2.
Control of auxin homeostasis in charophycean algae and land plants. Biosynthesis and metabolism: In land plants, tryptophan (trp) is the substrate for TRYPTOPHAN AMINOTRANSFERASE (TAA), which produces indole-3-pyruvic acid (IPyA) that is subsequently converted to indole-3-acetic acid (IAA) by YUCCA enzymatic activity. The conversion of trp to IPyA could be mediated by a TAA/TAR34 ortholog in charophycean algae but this needs experimental corroboration. VAS1 enzymes mediating the conversion of IPyA into Trp are conserved across Streptophytes. In land plants, GH3 enzymes conjugate free IAA into amino acid conjugates (IAA-aa) and ILRs conversely hydrolyze IAA-aa back into free IAA. Whereas GH3 genes have not been found in charophytes, ILR enzymes are conserved across Streptophytes, suggesting hydrolysis of IAA-amino acid conjugates into auxin may occur in charophycean algae. Amidases that convert IAM into IAA are conserved across Streptophytes, suggestive of a possible older auxin metabolic pathway. Transport: Auxin transporters of the PIN, PILS, ABCB, and AUX/LAX protein families were present in the common ancestor of Streptophytes, implying intracellular (PILS) and intercellular (AUX/LAX, ABCB, PIN) active transport was present in the charophycean algae ancestor. In land plants, NCPINs channel auxin into the endoplasmic reticulum (ER), a role perhaps filled by PILS proteins in algae. Cell expansion: Acid growth modulated by H+ ATPases, PP2C-D phosphatases and EXPANSINs is present in charophycean algal lineages (e.g., Nitella). Given the absence of SAUR genes in charophycean algae, acid growth may not have been dependent on auxin levels in the Streptophyte ancestor. In land plants, acid growth is dependent on auxin, as IAA induces both SAUR and α-EXPANSIN transcription. SAURs inhibit PP2C-D phosphatases, allowing phosphorylation of H+ ATPases and acidification of the apoplastic environment. Acid growth in turn is dependent on the cell wall loosening activity of α-EXPANSINs. In cell diagrams, the cell wall (green), cell membrane (yellow), ER (purple), and nucleus (blue) are color coded.
Comparative genomics indicates the presence of a TAA/YUCCA biosynthetic pathway in the land plant ancestor (Bowman et al. 2017; Poulet and Kriechbaumer 2017; Li et al. 2018) with both TAA and YUCCA genes required for auxin production in bryophytes (Eklund et al. 2015) and Tracheophyta (Zhao et al. 2001; Stepanova et al. 2008; Tao et al. 2008). Notably, loss of this pathway in M. polymorpha results in complete loss of developmental patterning (Eklund et al. 2015) and the same is likely the case in the flowering plant Arabidopsis (Cheng et al. 2007), indicating the assembly of this pathway was critical for the evolution of land plants. Whether IPyA-synthesizing TAA genes exist in charophytes is equivocal as phylogenetic analyses place charophycean TAA-related genes as sister to two monophyletic land plant lineages (Wang et al. 2014, 2016; Romani 2017). One lineage contains embryophyte TAA orthologs encoding enzymes catalyzing the first step of auxin biosynthesis. The second lineage contains orthologs of Arabidopsis TAR3 and TAR4, whose function(s) are as-yet uncharacterized. These genes encode a conserved EGF-Alliinase protein motif that has been repeatedly lost in the TAA lineage genes (Turnaev et al. 2015; Wang et al. 2016; Romani 2017). Charophytes experienced multiple lineage-specific losses of TAA-related (TAR) genes, except in Klebsormidium (Hori et al. 2014; Nishiyama et al. 2018; Cheng et al. 2019; Jiao et al. 2020), suggesting that this gene family is not bound by strong selective pressures in charophytes. Functional characterization of charophyte TAR enzymes and cross-lineage complementation experiments could clarify the origin of the embryophyte TAA genes. Pertinent questions are whether charophyte TAR mutants are auxin-deficient and whether algal TAR genes complement embryophyte mutant TAA alleles.
Although YUCCA flavin monooxygenases were proposed to exist in Chlorophytes (Khasin et al. 2018), charophytes (Wang et al. 2014), and brown algae (Bogaert et al. 2019), exhaustive phylogenetic (Yue et al. 2012, 2014; Bowman et al. 2017; Turnaev et al. 2020), and sequence similarity network analysis (Dai et al. 2013) indicate YUCCA flavin monooxygenases are a land plant innovation acquired by horizontal gene transfer from bacteria. Thus, if charophyte TAR genes can produce IPyA, IPyA may have had an IAA-independent function in the algal ancestor. VAS1 enzymes, which convert IPyA into Trp (Zheng et al. 2013; Matsuo et al. 2020) are conserved across Streptophytes, suggesting IPyA homeostatic levels were actively regulated in the Streptophyte ancestor (Fig. 2).
In at least some embryophytes, auxin is produced from other trp-dependent derivatives (for review, see Tivendale et al. 2014; Morffy and Strader 2020) such as indole-3-acetaldoxime (IAOx), indole-3-acetonitrile (IAN), indole-3-acetamide (IAM), and tryptamine (TAM). AMIDASEs (AMI) and IAM hydrolases (IAMH) that convert IAM to auxin, are found across Streptophytes, hinting at the presence of an ancestral biosynthetic pathway (Fig. 1). This is further supported by the presence of IAM in chlorophytes and charophycean algae (Stirk et al. 2013; Žižková et al. 2017), although enzymes converting Trp to IAM and whether reaction intermediaries exist are unknown (Fig. 2). Finally, auxin may be produced in Streptophytic algae via trp-independent derivatives (Wright et al. 1991; Normanly et al. 1993) such as indole, indole-3-glycerol phosphate (Ouyang et al. 2000; Ljung 2013) or indole-3-butyric acid (Strader et al. 2011). However, these pathways contribute little to the developmental and tropic roles of auxin in embryophytes and their evolutionary origins are largely unexplored.
Auxin Metabolism
In embryophytes, auxin exists in a “free” active form or can be converted, reversibly or irreversibly, into inactive conjugates. In angiosperms, the major natural auxin, IAA, is inactivated by conjugation with amino acids or sugars, oxidation, and methylation (Fig. 3A). Amide conjugation with various amino acids (IAA-aa) is catalyzed by members of the Streptophyte-specific GRETCHEN HAGEN3 (GH3) protein family, which is divided into three subgroups (Staswick et al. 2005). In angiosperms, group II members play a major role in auxin conjugation while those of groups I and III utilize jasmonic acid (JA) or benzoate as substrates, respectively (Staswick and Tiryaki 2004; Staswick et al. 2005; Okrent et al. 2009; Chiu et al. 2018). IAA-aa are also present in various bryophytes, although the amounts and conversion rates are lower than in angiosperms (Sztein et al. 1999; Záveská Drábková et al. 2015; Kaneko et al. 2020). The moss Physcomitrium patens has two group I GH3 genes (Bierfreund et al. 2004) whose encoded proteins can conjugate various amino acids to both IAA and JA in vitro (Ludwig-Müller et al. 2009). Double mutants exhibit increased auxin sensitivity with higher free-IAA and reduced IAA-aa levels (Ludwig-Müller et al. 2009). In algal species, the amount of IAA-Asp detected was almost at the detection limit (Žižková et al. 2017) and conversion rates are even slower than in bryophytes (Sztein et al. 2000). Among charophytes, only Klebsormidium nitens has known GH3 homologs; however, phylogenetic analysis suggests the K. nitens ancestor independently obtained GH3 genes by horizontal gene transfer from bacteria (Fig. 3B).
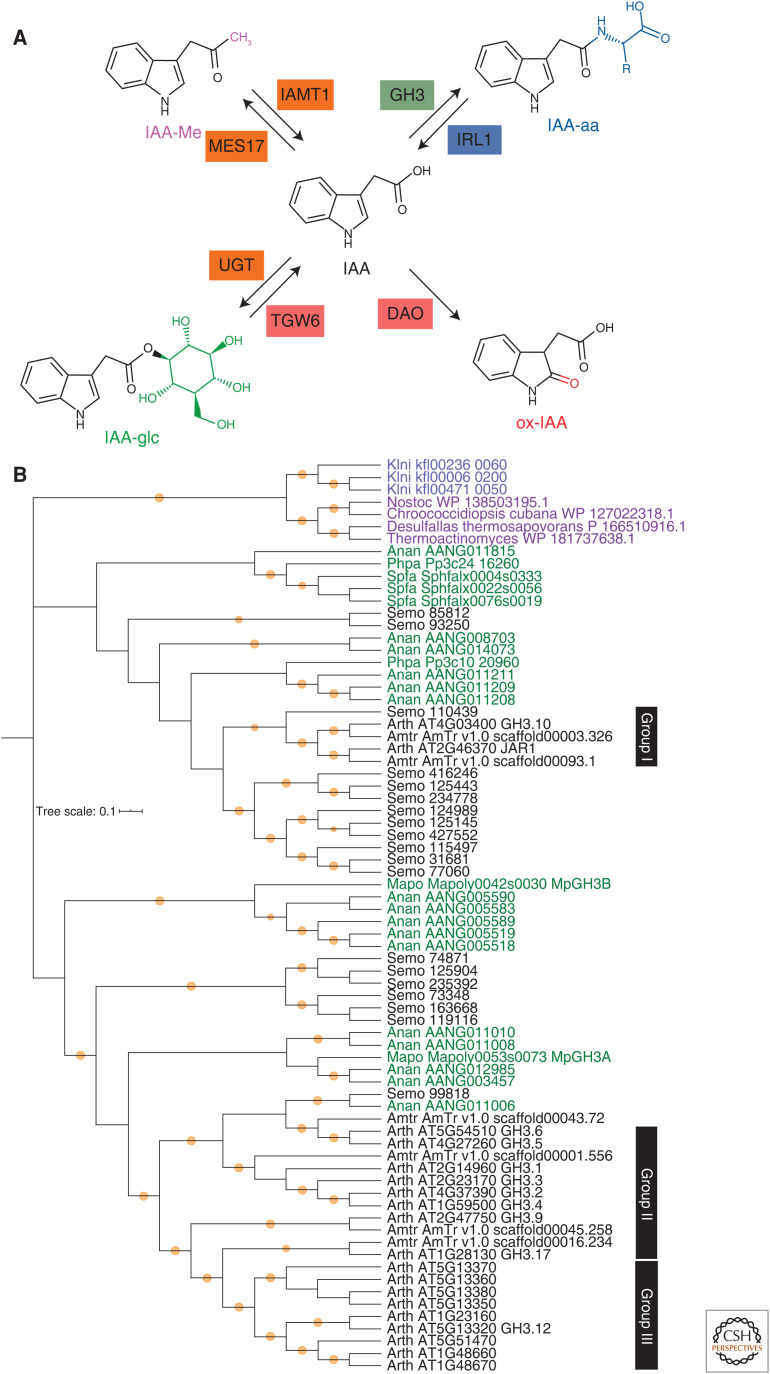
Figure 3.
Indole-3-acetic acid (IAA) is modified into several inactive forms in land plants. (A) IAA is inactivated by conjugation with amino acids (IAA-aa: blue) or sugars (IAA-glc: green), oxidation (oxIAA: red), and methylation (IAA-Me: magenta), and reactivated by enzymes indicated. The enzyme box color indicates conservation among charophycean algae + land plants (blue), land plants (green), seed plants (orange), or angiosperms (red). (B) Phylogenetic reconstruction of the GH3 protein family. The tree highlights two factors to consider when tracing the evolutionary history of genes. First, similar sequences may not be orthologous, but rather derived ultimately from independent sources (e.g., the Klebsormidium and land plant GH3 genes). Second, classifications based on analyses of sequences from only derived taxa (e.g., GH3 classes based on angiosperm sequences may fail to recognize phylogenetic diversity found outside the derived taxa). Protein sequences were collected from GenBank, Phytozome, and the Klebsormidium nitens genome website using blastp search with threshold E-value = 1.0 × 10−20, and aligned using the MUSCLE program (Edgar 2004) implemented in MEGA X (Kumar et al. 2018). Alignment positions with more than 50% gaps were removed using the Phyutility program (version 2.2.6; code.google.com/p/phyutility/downloads/list) and then sequences with more than 30% gap in the alignment were removed. The phylogenetic tree was constructed in MEGA X using an LG + G model with bootstrap replicates = 500. Orange circles indicate bootstrap values more than 0.75. Black: Tracheophytes (Arabidopsis thaliana [Arth], Amborella trichopoda [Amtr], Selaginella moerrendorffii [Semo]), green: bryophytes (Anthoceros angustus [Anan], Marchantia polymorpha [Mapo], Physcomitrium patens [Phpa], Sphagnum fallax [Spfa]), blue: K. nitens (Klni), purple: bacteria. Groups I to III are based on data in Staswick et al. (2005).
Among amino acid conjugates, IAA-Ala and IAA-Leu inhibit root elongation similar to IAA and are thus hydrolyzable in Arabidopsis (Bartel and Fink 1995; LeClere et al. 2002; Rampey et al. 2004), suggesting their contribution as storage forms. Hydrolysis of IAA-aa is mediated by M20D peptidase family members IAA–LEUCINE RESISTANT1 (ILR1) and its homologs, ILR-LIKEs, and IAA-ALANINE RESISTANT3 (Bartel and Fink 1995; Davies et al. 1999; LeClere et al. 2002). ILR1 homologs are found broadly (Fig. 1), but not ubiquitously, throughout Streptophytes and even cyanobacteria (Campanella et al. 2014, 2018, 2019). Three P. patens genes have activity against a variety of auxin-aa conjugates (Campanella et al. 2019), but their higher sequence similarity and codon usage to bacterial genes rather than Streptophyte genes, and absence of homologs in 42 other mosses suggest a loss of the plant ILR1 gene in the ancestral moss and independent acquisition in P. patens by horizontal gene transfer. M. polymorpha has a single plant-like ILR1 homolog (MpILR1), but MpILR1 has weak hydrolase activity in comparison with seed plant ILRs (Campanella et al. 2018). Notably, MpILR1 does not hydrolyze either of the two M. polymorpha auxin conjugates found under normal conditions, IAA-Val and IAA-Gly (Sztein et al. 1999; Campanella et al. 2018), suggesting MpILR1 may be disconnected with active regulation of free IAA. Thus, while the ancestral land plant likely possessed GH3-mediated amino acid conjugation (Fig. 2), its role might have been primarily inactivation rather than maintaining an accessible auxin pool. This scenario is consistent with earlier ideas of a progressively increasing complexity in the control of auxin homeostasis within land plants (Sztein et al. 2000; Cooke et al. 2002).
Other IAA conjugates have been characterized in angiosperms, and while such conjugates are also found in bryophytes and algae, genes encoding the relevant angiosperm enzymes are not conserved among earlier diverging lineages (Fig. 3). For example, IAA inactivation to 2-oxindole-3-acetic acid (oxIAA) is a major IAA oxidation pathway in seed plants (Östin et al. 1998) and is mediated in angiosperms by DIOXYGENASE FOR AUXIN OXIDATION (DAO) (Zhao et al. 2013; Porco et al. 2016; Zhang et al. 2016). Whereas various bryophytes accumulate oxIAA, and oxIAA has been detected in various algal lineages (Záveská Drábková et al. 2015; Žižková et al. 2017), phylogenetic analysis indicates DAO homologs are limited to angiosperms (Takehara et al. 2020). Likewise, ester-linked IAA–sugar conjugates (e.g., IAA-glucose) have been identified in angiosperms and bryophytes, although amounts in bryophytes are limited (Korasick et al. 2013; Záveská Drábková et al. 2015). Production of IAA-glucose in angiosperms is mediated by group L UDP glucosyltransferases (UGTs) (Jackson et al. 2001; Li et al. 2001; Jin et al. 2013), which are found only in seed plants (Wilson and Tian 2019). Enzymes hydrolyzing IAA-glucose have been identified in rice and wheat, but related genes appear monocot specific. Finally, methylated IAA (MeIAA), a presumed storage form, is detected in angiosperms (Korasick et al. 2013). In Arabidopsis, MeIAA is produced by the SABATH family enzyme IAA CARBOXYLMETHYLTRANSFERASE 1 (IAMT1) (Zubieta et al. 2003; Qin et al. 2005), with IAMT1 orthologs limited to seed plants. As most studies have approached the question from an angiosperm perspective, investigations in bryophytes are required to ascertain the involvement of these and other possible conjugates in auxin homeostasis.
Auxin Transport
Polar auxin transport (PAT)—the unidirectional intercellular flow of auxin between cells—has been reported in Tracheophytes (Okada et al. 1991), mosses (Poli et al. 2003), liverworts (Maravolo 1976), and Chara (Klämbt et al. 1992; Boot et al. 2012). Auxin concentrations resulting from PAT is contingent on the activity of influx, efflux, and intracellular carriers, with PAT-generated auxin gradients translated into transcriptional changes that determine many land plant morphogenetic processes.
The AUX/LAX protein family constitutes the only dedicated auxin influx carrier yet characterized in plants and was originally identified by auxin resistance of aux mutants (Maher and Martindale 1980). AUX/LAX proteins are plasma membrane (PM) localized (Swarup et al. 2004) and resemble amino acid permeases (Bennett et al. 1996). AUX/LAX homologs are present throughout the Viridiplantae (Figs. 1 and 2). Pending functional analyses, this suggests that the unicellular Viridiplantae ancestor had the potential to actively import environmental auxin and that multicellular charophytes and embryophytes retained and co-opted AUX/LAX proteins to regulate auxin flow within the plant body.
Streptophyte-specific PIN proteins act as auxin efflux carriers and have been classified into either canonical or noncanonical by virtue of their central hydrophilic domain or loop length, although this is a functional not a phylogenetic distinction (Petrásek et al. 2006; Bennett et al. 2014; Vosolsobe et al. 2020). Canonical PINs, with long loops, are PM localized, while noncanonical PINs, with short loops, are localized to the endoplasmic reticulum (ER) membrane (Ding et al. 2012). Canonical PIN proteins may be polarized to reside on only specific cell faces, providing the basis for directional auxin transport (Naramoto 2017; Béziat and Kleine-Vehn 2018). Embryophyte noncanonical PINs evolved multiple times from an ancestral canonical PIN, and given their capacity to transport auxin analogs or conjugates, they are proposed to act as homeostatic modulators of auxin metabolites (Ding et al. 2012; Bennett et al. 2014). Whereas canonical PINs have been considered the ancestral type of land plant PIN (Bennett et al. 2014), phylogenetic analyses with charophyte sequences as outgroups are equivocal. Charophyte PIN proteins are not simply classified as canonical or noncanonical, but a Klebsormidium PIN can act as an auxin efflux carrier when heterologously expressed in angiosperms, suggesting that PIN-mediated PAT occurred in a Streptophyte ancestor (Skokan et al. 2019). An independent expansion of PIN gene family members in Chara braunii (Nishiyama et al. 2018) is correlated with the presence of PAT in other Chara species (Klämbt et al. 1992; Boot et al. 2012).
The ancient PIN-LIKES (PILS) protein family, likely present in the unicellular Viridiplantae ancestor (Fig. 1), is distantly related to the PIN family, sharing a transmembrane domain topology but with low amino acid sequence conservation (Barbez et al. 2012; Vosolsobe et al. 2020). PILS are proposed to canalize auxin into the ER (Fig. 2), thus dampening nuclear responses (Béziat et al. 2017; Feraru et al. 2019).
The ATP-binding cassette subfamily-B/multidrug resistant/P-glycoprotein (ABCB/MDR/PGP) subfamily B (ABCB) proteins also act as auxin efflux carriers in angiosperms (Noh et al. 2001; Blakeslee et al. 2007; Wu et al. 2010), with some also showing facultative auxin influx capabilities (Kamimoto et al. 2012). The ABCB subfamily is ancient, with members throughout Viridiplantae, with ABCB proteins having diverse functions, and thus functional studies in algae are required to authenticate any auxin-transport capabilities.
In summary, protein families with the capacity to import (AUX/LAX), export (ABCB), and sequester (PILS) auxin were present in the Viridiplantae ancestor, suggesting that ancestral unicellular green algae may have had the capacity to respond to fluctuating concentrations of auxin or auxin-like compounds in their environment. The evolution of these three protein families could have been an adaptation to excess auxin in the environment, if auxin was used as a nutrient or a quorum-sensing signal in unicellular algae (Vosolsobe et al. 2020). In contrast, PIN-mediated auxin efflux originated in the freshwater Streptophyte ancestor, with PAT evolving before the evolution of the canonical land plant auxin biosynthesis and signaling pathways, perhaps presaging the evolution of morphological complexity in this lineage.
Auxin Transcriptional Signaling
The current land plant auxin signaling model (Fig. 4) stems from four decades of experimental work in flowering plants, although it is an ancestral land plant innovation derived from both ancient and neofunctionalized genes. The conservation of the auxin signaling gene families across land plants has been corroborated by multiple genomic studies (Rensing et al. 2008; Banks et al. 2011; De Smet et al. 2011; Finet et al. 2013; Bowman et al. 2017; Mutte et al. 2018; Li et al. 2020; Zhang et al. 2020) and experimental studies in P. patens (Prigge et al. 2010; Lavy et al. 2016) and M. polymorpha (Flores-Sandoval et al. 2015; Kato et al. 2015, 2017). The M. polymorpha genome harbors single copies of each of the auxin signaling genes implying that this is the likely ancestral condition in land plants (Flores-Sandoval et al. 2015; Kato et al. 2015; Bowman et al. 2017). Most auxin signaling components have undergone diversification in both Tracheophytes and mosses, but have remained mostly single copy in liverworts and hornworts (Bowman et al. 2017; Li et al. 2020; Zhang et al. 2020).
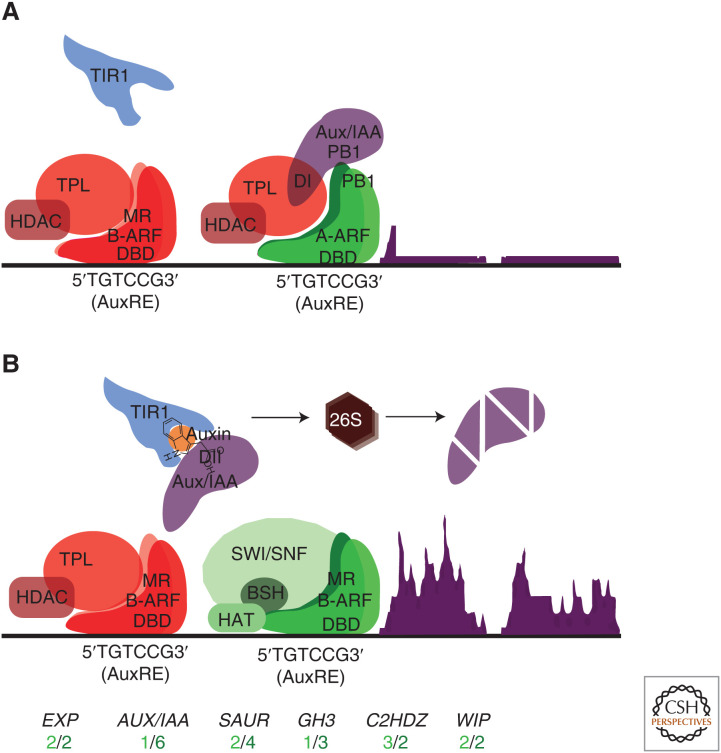
Figure 4.
Nuclear auxin signaling in land plants. (A) In the absence of nuclear auxin, AUXIN/INDOLE-3-ACETIC ACID (AUX/IAA) repressor proteins interact with A-ARF proteins via a shared carboxy-terminal protein–protein interaction domain called Phox/Bem1 (PB1) (Kim et al. 1997; Korasick et al. 2014; Nanao et al. 2014) and recruit members of the TOPLESS (TPL) family of corepressors via an EAR-like domain I (DI). TPL proteins, in turn, recruit histone deacetylases forming a repressive complex (Tiwari et al. 2001, 2004; Szemenyei et al. 2008). B-ARF proteins also interact with TOPLESS corepressors and can compete with A-ARFs for Auxin Response Elements (AuxREs) in the promoters of auxin-inducible genes (Ulmasov et al. 1997; Kato et al. 2020). (B) Auxin is perceived in the nucleus by TRANSPORT INHIBITOR RESPONSE1/AUXIN SIGNALING F-BOX (TIR1/AFB) proteins. TIR1/AFBs are ubiquitin ligases by virtue of their amino-terminal F-box domain and recognize auxin in the pockets formed in their leucine-rich repeats (LRRs). Auxin “glues” TIR1 to the degron domain II (DII) of AUX/IAA proteins and ubiquitinates AUX/IAAs for degradation in the 26S proteasome, releasing A-ARF proteins from the TPL complex (Gray et al. 2001; Dharmasiri et al. 2005; Kepinski and Leyser 2005; Tan et al. 2007). This allows activation of downstream targets via recruitment of SWI/SNF ATPases, which in turn recruit BUSHY (BSH) proteins allowing HISTONE ACETYL TRANSFERASES (HATs) to promote an open chromatin state and further recruitment of activating transcription factors (Gray et al. 2001; Dharmasiri et al. 2005; Kepinski and Leyser 2005; Wu et al. 2015). In this scenario, A-ARF targets can still be inhibited by B-ARFs when both are coexpressed. Putative ancestral land plant targets of the auxin signaling pathway are shown. The number of species of bryophytes (light green) and Tracheophytes (dark green) where at least one member of each gene family is auxin-inducible is indicated (Abel and Theologis 1996; Catalá et al. 2000; Esmon et al. 2006; Lavy et al. 2016; Kato et al. 2017; Mutte et al. 2018).
Ultimately, changes in transcriptional patterns depend upon AUXIN RESPONSE FACTORs (ARFs), only some of which are integrated into the auxin signaling system (Fig. 5). The ancestral land plant possessed three ARF genes, A, B, and C (Finet et al. 2013), and a noncanonical ARF (NCARF) derived via gene duplication of the A-ARF and lacking a DNA-binding domain (DBD) (Flores-Sandoval et al. 2018; Mutte et al. 2018). The ancestral land plant harbored two evolutionarily related genes encoding the Aux/IAA repressor of A-ARFs and a noncanonical Aux/IAA (NCIAA), both lacking a DBD (Flores-Sandoval et al. 2018; Mutte et al. 2018). ARFs possess an amino-terminal B3-type DBD (Ulmasov et al. 1997) that mediates dimerization and binding to two palindromically positioned AuxREs (Boer et al. 2014) and a carboxy-terminal Phox/Bem1 (PB1) protein–protein interaction domain (Kim et al. 1997; Korasick et al. 2014; Nanao et al. 2014). In between, the middle region (MR) confers activator or repressor identity (Ulmasov et al. 1999; Kato et al. 2015, 2020). A-ARFs have a glutamine-rich MR, function as transcriptional activators, and are generally subject to Aux/IAA regulation (Fig. 4). B- and C-ARFs are transcriptional repressors (Ulmasov et al. 1999; Kato et al. 2015, 2020) able to form complexes with TPL corepressors (Martin-Arevalillo et al. 2017, 2019; Choi et al. 2018) and act auxin independently (Piya et al. 2014; Flores-Sandoval et al. 2018; Mutte et al. 2018; Kato et al. 2020). B-ARFs can inhibit A-ARF activity via target competition, as their B3 domains are interchangeable (Kato et al. 2020). Hornworts sequenced to date lack B-ARFs (Li et al. 2020; Zhang et al. 2020), suggesting unknown mechanisms are responsible for auxin-independent A-ARF target repression in this lineage. NCARFs function as positive regulators of auxin responses in M. polymorpha but these genes have been lost in hornworts and Euphyllophytes (Mutte et al. 2018). NCIAAs are found across land plants, preferentially interacting with C-ARFs (Piya et al. 2014) with protein accumulation in response to auxin mediated via phosphorylation by MITOGEN-ACTIVATED PROTEIN KINASE 14 (MPK14) (Lv et al. 2020). Thus, the ancestral land plant possessed a complex network of ARF and ARF-related proteins (Fig. 5), with only the Aux/IAA and A-ARF proteins integrated with auxin signaling, with the remainder being auxin-independent (C-ARF) or indirectly associated (NCARF, B-ARF).
Figure 5.
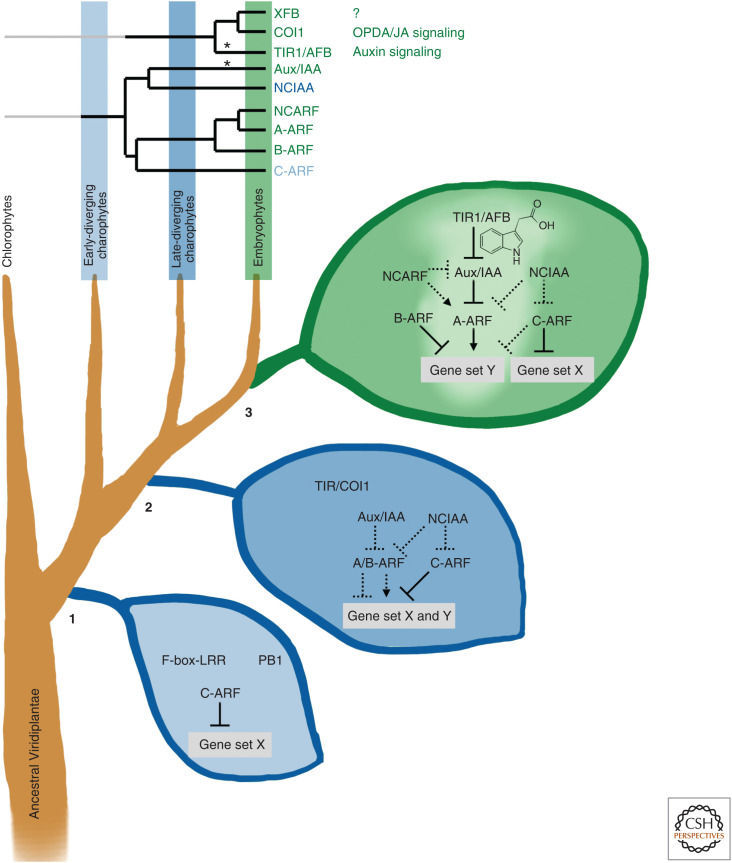
Evolutionary scenario of auxin signaling assembly. At the advent of Streptophyte evolution (1) C-type ARF transcription factors were established by combining preexisting small protein domains in ancestral Streptophyte. Prior to this time, proteins containing an F-box with a leucine-rich repeat (LRR) and others with PB1 domains were present but not functionally connected with each other. In the common ancestor of later-diverging charophytes and embryophytes (2; see Fig. 1 for phylogenetic delineations), an A/B-type ARF evolved, via gene duplication of the C-type ARF followed by neofunctionalization. The A/B-ARF and C-ARF transcription factors might have shared the target genes. In addition, two types of Aux/IAA-related PB1-containing proteins lacking the B3 DNA-binding domain (DBD) evolved, also likely via a gene duplication of the ancestral C-ARF followed by DBD loss and a further gene duplication. These proteins could act as negative regulators of the C-ARF and A/B-ARF proteins via their ability to heterodimerize but not bind to DNA. Thus, a complex network of related transcription factors with multiple levels of cross-regulation had evolved in the common ancestor of later-diverging charophytes and embryophytes. However, while the ancestral protein that would give rise to TIR1/AFB and COI1 was present, it is likely that it did not perceive either auxin or OPDA/JA, and its relationship, if any, to the ARF transcriptional network is unknown. After the divergence of the ancestral land plant from the most closely related extant charophycean algal lineage (3), the A/B-ARF gene gave rise to activator (A-ARF) and repressor (B-ARF) genes via gene duplication. In addition, a gene duplication of the A-ARF produced a paralog, NCARF, that subsequently lost its DBD. Gene duplication also generated dedicated receptors for auxin (TIR1/AFB) and OPDA/JA (COI1), along with a third as-yet-uncharacterized F-box protein (XFB). The TIR1/AFB protein became integrated with the Aux/IAA-ARF transcriptional pathway. This was accomplished by the acquisition of dII in an Aux/IAA protein and amino acid changes in the TIR1/AFB protein to form a binding pocket between the two proteins for auxin (noted by *). In addition, distinct target specification differences between A/B-ARF and C-ARF proteins progressed, perhaps driven by the auxin-dependent versus auxin-independent action of the two classes of ARF. Thus, in the ancestral land plant, the ARF transcriptional network was expanded with the activity of a core part of the network now integrated with auxin, while a portion of the network, including the ancestral C-ARF, remained auxin independent.
Across Streptophytes, conservation of auxin signaling genes is fragmented (Hori et al. 2014; Wang et al. 2020) and comparative genomic studies suggest that the complete canonical pathway only exists in land plants (Bowman et al. 2017; Mutte et al. 2018; Nishiyama et al. 2018; Cheng et al. 2019; Jiao et al. 2020). Whereas the Aux/IAA and NCIAA lineages have not been found in the genomes of early diverging charophytes (e.g., Mesostigma, Chlorokybus, and Klebsormidium) (Hori et al. 2014; Wang et al. 2020), ARFs have been found in Chlorokybus and Mesostigma, suggesting that the Aux/IAA and NCIAA lineages could have evolved from ancestral ARFs via loss of the B3-DBD domain and it might be presumed that they act as negative regulators in the charophyte transcriptional network (Fig. 5). Despite the early origin of Aux/IAA proteins, the acquisition of functional domains I and II in the ancestral land plant was an important neofunctionalization allowing part of the network to become auxin modulated (De Smet et al. 2011; Flores-Sandoval et al. 2018; Mutte et al. 2018; Nishiyama et al. 2018; Jiao et al. 2020). A further key innovation occurred in the ancestral TIR1 gene. Charophyte genomes have F-box orthologs equally related to TIR1 and the COI1 OPDA/JA receptor proteins, and although these orthologs are predicted to form three-dimensional structures similar to either TIR1 or COI1, they lack key residues that allow binding to either auxin or OPDA/JA (Bowman et al. 2017; Monte et al. 2018; Mutte et al. 2018). Thus, coevolution of Aux/IAA domain II and specific amino acids in TIR1 was the key innovation in the evolution of auxin as a molecular glue between the two proteins and the integration of auxin into a preexisting transcriptional network.
Although ARFs have undergone multiple lineage-specific losses in charophytes (Hori et al. 2014), two types of ARFs (A/B and C-ARFs) were present in the common ancestor of later diverging charophytes and embryophytes (Flores-Sandoval et al. 2018; Mutte et al. 2018), while the Streptophyte ancestor only had C-ARFs (Martin-Arevalillo et al. 2019; Wang et al. 2020), suggesting C-ARFs may be ancestral. A Chlorokybus C-ARF can bind land plant AuxREs and interact with TPL in vitro, suggesting ARFs originated as transcriptional repressors (Martin-Arevalillo et al. 2019). Charophyte C-ARFs lack miR160-binding sites, suggesting the miR160/C-ARF regulatory loop is a land plant innovation (Mallory et al. 2005; Flores-Sandoval et al. 2018). Known charophyte A/B ARFs are sister to land plant A- and B-ARFs, whose divergence can be dated to the ancestral land plant, and their identity as activators or repressors is uncertain.
The evolution of the auxin signaling pathway is the product of the rewiring of a preexisting transcriptional network to respond to a novel ligand, providing an outstanding example of exaptation within the green lineage of life. Understanding how this novel pathway was used to pattern key features of the ancestral land plant still requires further functional studies in charophytes as well in Tracheophytes with free-living haploid and diploid generations. To date, tropisms, cell differentiation, and cell expansion (Fig. 2) represent likely biological processes controlled by auxin in the common ancestor of all land plants. While not explored here, the parallel evolution of the auxin and OPDA/JA signaling pathways from a common ancestral F-box protein, and potentially using common ancestral GH3-conjugating enzymes, deserves further investigation.
Downstream of Auxin Signaling
Whereas auxin transcriptional signaling is conserved throughout land plants, the question of whether there are universal, and hence ancestral targets, has only been addressed recently. The land plant auxin signaling pathway attains a steady state through multiple negative feedback loops involving A-ARFs inactivation via auxin-dependent AUX/IAA transcription (Abel et al. 1995; Abel and Theologis 1996) and the reduction of free IAA levels via down-regulation of YUCCA biosynthetic enzymes (Mutte et al. 2018) and up-regulation of GH3-conjugating enzymes (Hagen et al. 1991; Abel and Theologis 1996). While YUCCA down-regulation is consistent across embryophytes, auxin-dependent AUX/IAA transcription and GH3 activation have not been detected across all bryophyte lineages (Mutte et al. 2018).
Genes encoding two transcriptional repressors (Steindler et al. 1999; Jones and Dolan 2017; Ren et al. 2018), the CLASS-II HOMEODOMAIN LEUCINE ZIPPER (C2HDZ) and the WIP-DOMAIN C2H2 zinc fingers, are consistently up-regulated by auxin across land plants (Sawa et al. 2002; Crawford et al. 2015; Mutte et al. 2018). C2HDZ genes as targets evolved in the ancestral land plant as charophyte orthologs (Romani et al. 2018) are not auxin up-regulated (Mutte et al. 2018). It is unknown whether these transcription factors regulate auxin signaling through feedback effects or, alternatively, control conserved developmental or physiological responses.
SAUR (SMALL AUXIN UP-REGULATED RNA) genes are a distinct auxin-induced gene class (McClure et al. 1989) whose products do not influence feedback regulation, but rather promote cell expansion via acid growth (Chae et al. 2012; Spartz et al. 2017). Auxin-mediated SAUR gene up-regulation is observed widely in embryophytes, although it may have been lost in liverworts (Mutte et al. 2018). SAUR proteins promote acidification of the apoplast via inhibition of D-type PPC (PP2C.D) phosphatases, which in turn modulate H+ ATPases (Cosgrove 1998; Spartz et al. 2014; Stortenbeker and Bemer 2019; Wong et al. 2019). α-EXPANSINs are another auxin-inducible protein family (Lavy et al. 2016; Kato et al. 2017; Mutte et al. 2018) potentially acting downstream of the SAUR/PP2C.D/AHA module. Whereas acid growth exists in Nitella (Métraux and Taiz 1977; Métraux et al. 1980), SAUR protein-mediated acid growth is a land plant innovation (Fig. 2).
AUXIN BIOLOGY OF THE ANCESTRAL LAND PLANT AND ANTECEDENT FUNCTIONS IN THE ALGAL ANCESTOR
Knowledge of the phylogenetic distribution of genes encoding the critical components of auxin biology allows the prediction of the auxin biology network in the ancestral plants and, further, functional analyses spanning the phylogenetic spectrum of extant land plants has provided insight into the functionality of the ancestral network. Using these criteria, the ancestral land plant is predicted to have possessed a system similar to that existing in M. polymorpha, with solitary paralogs of most components (Flores-Sandoval et al. 2015; Kato et al. 2015; Bowman et al. 2017; Mutte et al. 2018). Two most critical innovations shaped changes in auxin biology during the transition from an ancestral alga to land plants and form the basis of how auxin became a key land plant hormone. First, the acquisition via HGT from a bacterium of a YUCCA gene enabled assembly of a new auxin biosynthetic pathway (Yue et al. 2014; Bowman et al. 2017). Second, the integration of auxin into a subsection of a preexisting transcriptional network, which was also expanded in the ancestral land plant, established the response output of the pathway to be modulated by the presence of auxin (Bowman et al. 2017; Flores-Sandoval et al. 2018; Mutte et al. 2018). Two auxin-mediated responses may be common to all land plants. First, tropic growth responses are intimately connected with auxin biology throughout land plants and presumably this was the case in the ancestral land plants. Second, auxin biology is also critical for almost every characterized developmental process to proceed appropriately. In M. polymorpha, loss of the TAA/YUCCA auxin biosynthetic pathway results in a complete loss of differentiation with plants being composed of an amorphous mass of dividing cells (Eklund et al. 2015). Whereas complete loss of the TAA/YUCCA pathway has not been achieved in angiosperms due to a high level of genetic redundancy, various multiple mutant combinations result in a spectrum of developmental defects (for review, see Zhao 2018). With respect to development, one plausible hypothesis is that the ancestral algal transcriptional network controlled the balance between meristematic (i.e., cell division) and differentiated states and that this came under the influence of auxin in the ancestral land plant (Flores-Sandoval et al. 2018). In this scenario, the role of auxin as a facilitator rather than a specifier follows naturally, with specific tissue-/cell-type specification dependent upon the milieu of other transcription factors present in the cells transitioning from cell division to differentiation.
While there are credible (and testable) hypotheses concerning the role(s) of auxin in the ancestral land plants, the situation is enigmatic in the algal ancestor and in extant charophytes. There are two components to consider: (1) What was the role of the ancestral transcriptional network? One hypothesis was presented above. (2) What was the function of auxin in these algae? Development of charophyte models amenable to genome editing would facilitate addressing these questions, albeit the latter question may require specific knowledge of any auxin biosynthetic pathways in these organisms. Answers to these two questions could illuminate why auxin was co-opted from an as-yet-unknown ancestral role into a molecule critical for most aspects of land plant development.
ACKNOWLEDGMENTS
The authors acknowledge funding from The Australian Research Council (DP160100892 and DP170100049 to J.L.B.), the Japan Society for the Promotion of Science (standard postdoctoral fellowship P19381 to E.F.S. and KAKENHI through Grant No. 19K23751 to H.K.).
Footnotes
Editors: Dolf Weijers, Karin Ljung, Mark Estelle, and Ottoline Leyser
Additional Perspectives on Auxin Signaling available at www.cshperspectives.org
REFERENCES
- Abel S, Theologis A. 1996. Early genes and auxin action. Plant Physiol 111: 9–17. 10.1104/pp.111.1.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel S, Nguyen MD, Theologis A. 1995. The PS-IAA4/5-like family of early auxin-inducible mRNAs in Arabidopsis thaliana. J Mol Biol 251: 533–549. 10.1006/jmbi.1995.0454 [DOI] [PubMed] [Google Scholar]
- Ashen JB, Cohen JD, Goff LJ. 1999. GC-SIM-MS detection and quantification of free indole-3-acetic acid in bacterial galls on the marine alga Prionitis lanceolata (rhodophyta). J Phycology 35: 493–500. 10.1046/j.1529-8817.1999.3530493.x [DOI] [Google Scholar]
- Banks JA, Nishiyama T, Hasebe M, Bowman JL, Gribskov M, dePamphilis C, Albert VA, Aono N, Aoyama T, Ambrose BA, et al. 2011. The Selaginella genome identifies genetic changes associated with the evolution of vascular plants. Science 332: 960–963. 10.1126/science.1203810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbez E, Kubeš M, Rolčík J, Béziat C, Pěnčík A, Wang B, Rosquete MR, Zhu J, Dobrev PI, Lee Y, et al. 2012. A novel putative auxin carrier family regulates intracellular auxin homeostasis in plants. Nature 485: 119–122. 10.1038/nature11001 [DOI] [PubMed] [Google Scholar]
- Bartel B, Fink GR. 1995. ILR1, an amidohydrolase that releases active indole-3-acetic acid from conjugates. Science 268: 1745–1748. 10.1126/science.7792599 [DOI] [PubMed] [Google Scholar]
- Basu S, Sun H, Brian L, Quatrano RL, Muday GK. 2002. Early embryo development in Fucus distichus is auxin sensitive. Plant Physiol 130: 292–302. 10.1104/pp.004747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett T, Leyser O. 2014. The auxin question: a philosophical overview. In Auxin and its role in plant development (ed. Zažímalová E, Petrášek J, Benková E), pp. 3–19. Springer, Vienna. [Google Scholar]
- Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, Schulz B, Feldmann KA. 1996. Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science 273: 948–950. 10.1126/science.273.5277.948 [DOI] [PubMed] [Google Scholar]
- Bennett T, Brockington SF, Rothfels C, Graham SW, Stevenson D, Kutchan T, Rolf M, Thomas P, Wong GK, Leyser O, et al. 2014. Paralogous radiations of PIN proteins with multiple origins of noncanonical PIN structure. Mol Biol Evol 31: 2042–2060. 10.1093/molbev/msu147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béziat C, Kleine-Vehn J. 2018. The road to auxin-dependent growth repression and promotion in apical hooks. Curr Biol 28: R519–R525. 10.1016/j.cub.2018.01.069 [DOI] [PubMed] [Google Scholar]
- Béziat C, Barbez E, Feraru MI, Lucyshyn D, Kleine-Vehn J. 2017. Light triggers PILS-dependent reduction in nuclear auxin signalling for growth transition. Nat Plants 3: 17105. 10.1038/nplants.2017.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierfreund NM, Tintelnot S, Reski R, Decker EL. 2004. Loss of GH3 function does not affect phytochrome-mediated development in a moss, Physcomitrella patens. J Plant Physiol 161: 823–835. 10.1016/j.jplph.2003.12.010 [DOI] [PubMed] [Google Scholar]
- Blakeslee JJ, Bandyopadhyay A, Lee OR, Mravec J, Titapiwatanakun B, Sauer M, Makam SN, Cheng Y, Bouchard R, Adamec J, et al. 2007. Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis. Plant Cell 19: 131–147. 10.1105/tpc.106.040782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer DR, Freire-Rios A, van den Berg WA, Saaki T, Manfield IW, Kepinski S, Lopez-Vidrieo I, Franco-Zorrilla JM, de Vries SC, Solano R, et al. 2014. Structural basis for DNA binding specificity by the auxin-dependent ARF transcription factors. Cell 156: 577–589. 10.1016/j.cell.2013.12.027 [DOI] [PubMed] [Google Scholar]
- Bogaert KA, Blommaert L, Ljung K, Beeckman T, De Clerck O. 2019. Auxin function in the brown alga Dictyota dichotoma. Plant Physiol 179: 280–299. 10.1104/pp.18.01041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boot KJ, Libbenga KR, Hille SC, Offringa R, van Duijn B. 2012. Polar auxin transport: an early invention. J Exp Bot 63: 4213–4218. 10.1093/jxb/ers106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman JL, Kohchi T, Yamato KT, Jenkins J, Shu S, Ishizaki K, Yamaoka S, Nishihama R, Nakamura Y, Berger F, et al. 2017. Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell 171: 287–304.e15. 10.1016/j.cell.2017.09.030 [DOI] [PubMed] [Google Scholar]
- Boysen Jensen P. 1910. Über die Leitung des phototropischen Reizes in Avena-keimpflanzen [About the conduction of the phototropic stimulus in Avena seedlings]. Ber Dtsch Bot Ges 28: 118–120. [Google Scholar]
- Campanella JJ, Zaben N, Enriquez D, Smalley JV, Ludwig-Müller J. 2014. An enzymatic analysis of loblolly pine and sitka spruce auxin conjugate hydrolases and evolutionary implications, pp. 79–88. International Society for Horticultural Science (ISHS), Leuven, Belgium. [Google Scholar]
- Campanella JJ, Kurdach S, Bochis J, Smalley JV. 2018. Evidence for exaptation of the Marchantia polymorpha M20D peptidase MpILR1 into the tracheophyte auxin regulatory pathway. Plant Physiol 177: 1595–1604. 10.1104/pp.18.00543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella JJ, Kurdach S, Skibitski R, Smalley JV, Desind S, Ludwig-Müller J. 2019. Evidence for the early evolutionary loss of the M20D auxin amidohydrolase family from mosses and horizontal gene transfer from soil bacteria of cryptic hydrolase orthologues to Physcomitrella patens. J Plant Growth Regulation 38: 1428–1438. 10.1007/s00344-019-09945-6 [DOI] [Google Scholar]
- Catalá C, Rose JK, Bennett AB. 2000. Auxin-regulated genes encoding cell wall-modifying proteins are expressed during early tomato fruit growth. Plant Physiol 122: 527–534. 10.1104/pp.122.2.527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae K, Isaacs CG, Reeves PH, Maloney GS, Muday GK, Nagpal P, Reed JW. 2012. Arabidopsis SMALL AUXIN UP RNA63 promotes hypocotyl and stamen filament elongation. Plant J 71: 684–697. 10.1111/j.1365-313X.2012.05024.x [DOI] [PubMed] [Google Scholar]
- Chanclud E, Morel JB. 2016. Plant hormones: a fungal point of view. Mol Plant Pathol 17: 1289–1297. 10.1111/mpp.12393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Dai X, Zhao Y. 2007. Auxin synthesized by the YUCCA flavin monooxygenases is essential for embryogenesis and leaf formation in Arabidopsis. Plant Cell 19: 2430–2439. 10.1105/tpc.107.053009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Xian W, Fu Y, Marin B, Keller J, Wu T, Sun W, Li X, Xu Y, Zhang Y, et al. 2019. Genomes of subaerial Zygnematophyceae provide insights into land plant evolution. Cell 179: 1057–1067.e14. 10.1016/j.cell.2019.10.019 [DOI] [PubMed] [Google Scholar]
- Chiu LW, Heckert MJ, You Y, Albanese N, Fenwick T, Siehl DL, Castle LA, Tao Y. 2018. Members of the GH3 family of proteins conjugate 2,4-D and dicamba with aspartate and glutamate. Plant Cell Physiol 59: 2366–2380. [DOI] [PubMed] [Google Scholar]
- Choi HS, Seo M, Cho HT. 2018. Two TPL-binding motifs of ARF2 are involved in repression of auxin responses. Front Plant Sci 9: 372. 10.3389/fpls.2018.00372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholodny N. 1924. Über die hormonale Wirkung der Organspitze bei der geotropischen Krümmung [About the hormonal effect of the organ tip in the geotropic curvature]. Ber Dtsch Bot Ges 42: 356–362. [Google Scholar]
- Cooke TJ, Poli D, Sztein AE, Cohen JD. 2002. Evolutionary patterns in auxin action. Plant Mol Biol 49: 319–338. 10.1023/A:1015242627321 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. 1998. Molecular regulation of plant cell wall extensibility. Gravit Space Biol Bull 11: 61–70. [PubMed] [Google Scholar]
- Crawford BC, Sewell J, Golembeski G, Roshan C, Long JA, Yanofsky MF. 2015. Plant development. genetic control of distal stem cell fate within root and embryonic meristems. Science 347: 655–659. 10.1126/science.aaa0196 [DOI] [PubMed] [Google Scholar]
- Dai X, Mashiguchi K, Chen Q, Kasahara H, Kamiya Y, Ojha S, DuBois J, Ballou D, Zhao Y. 2013. The biochemical mechanism of auxin biosynthesis by an Arabidopsis YUCCA flavin-containing monooxygenase. J Biol Chem 288: 1448–1457. 10.1074/jbc.M112.424077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin CR. 1880. The power of movement in plants. Murray, London. [Google Scholar]
- Davies RT, Goetz DH, Lasswell J, Anderson MN, Bartel B. 1999. IAR3 encodes an auxin conjugate hydrolase from Arabidopsis. Plant Cell 11: 365–376. 10.1105/tpc.11.3.365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet I, Voß U, Lau S, Wilson M, Shao N, Timme RE, Swarup R, Kerr I, Hodgman C, Bock R, et al. 2011. Unraveling the evolution of auxin signaling. Plant Physiol 155: 209–221. 10.1104/pp.110.168161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Estelle M. 2005. The F-box protein TIR1 is an auxin receptor. Nature 435: 441–445. 10.1038/nature03543 [DOI] [PubMed] [Google Scholar]
- Ding Z, Wang B, Moreno I, Dupláková N, Simon S, Carraro N, Reemmer J, Pěnčík A, Chen X, Tejos R, et al. 2012. ER-localized auxin transporter PIN8 regulates auxin homeostasis and male gametophyte development in Arabidopsis. Nat Commun 3: 941. 10.1038/ncomms1941 [DOI] [PubMed] [Google Scholar]
- Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32: 1792–1797. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund DM, Ishizaki K, Flores-Sandoval E, Kikuchi S, Takebayashi Y, Tsukamoto S, Hirakawa Y, Nonomura M, Kato H, Kouno M, et al. 2015. Auxin produced by the indole-3-pyruvic acid pathway regulates development and gemmae dormancy in the liverwort Marchantia polymorpha. Plant Cell 27: 1650–1669. 10.1105/tpc.15.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon CA, Tinsley AG, Ljung K, Sandberg G, Hearne LB, Liscum E. 2006. A gradient of auxin and auxin-dependent transcription precedes tropic growth responses. Proc Natl Acad Sci 103: 236–241. 10.1073/pnas.0507127103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feraru E, Feraru MI, Barbez E, Waidmann S, Sun L, Gaidora A, Kleine-Vehn J. 2019. PILS6 is a temperature-sensitive regulator of nuclear auxin input and organ growth in Arabidopsis thaliana. Proc Natl Acad Sci 116: 3893–3898. 10.1073/pnas.1814015116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finet C, Berne-Dedieu A, Scutt CP, Marlétaz F. 2013. Evolution of the ARF gene family in land plants: old domains, new tricks. Mol Biol Evol 30: 45–56. 10.1093/molbev/mss220 [DOI] [PubMed] [Google Scholar]
- Fitting H. 1939. Untersuchungen über den einfluss von licht und dunkelheit auf die entwicklung von moosen. I. Die brutkörper der Marchantieen [Investigations into the influence of light and darkness on the development of mosses. I. The gemmae of the Marchantieen]. Jahrb Wiss Bot 88: 633–722. [Google Scholar]
- Flores-Sandoval E, Eklund DM, Bowman JL. 2015. A simple auxin transcriptional response system regulates multiple morphogenetic processes in the liverwort Marchantia polymorpha. PLoS Genet 11: e1005207. 10.1371/journal.pgen.1005207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Sandoval E, Eklund DM, Hong SF, Alvarez JP, Fisher TJ, Lampugnani ER, Golz JF, Vázquez-Lobo A, Dierschke T, Lin SS, et al. 2018. Class C ARFs evolved before the origin of land plants and antagonize differentiation and developmental transitions in Marchantia polymorpha. New Phytol 218: 1612–1630. 10.1111/nph.15090 [DOI] [PubMed] [Google Scholar]
- Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M. 2001. Auxin regulates SCFTIR1-dependent degradation of AUX/IAA proteins. Nature 414: 271–276. 10.1038/35104500 [DOI] [PubMed] [Google Scholar]
- Hagen G, Martin G, Li Y, Guilfoyle TJ. 1991. Auxin-induced expression of the soybean GH3 promoter in transgenic tobacco plants. Plant Mol Biol 17: 567–579. 10.1007/BF00040658 [DOI] [PubMed] [Google Scholar]
- Halbsguth W, Kohlenbach HW. 1953. Einige versuche über die wirkung von heteroauxin auf die symmetrieentwicklung der brutkörperkeimlinge von Marchanta polymorpha L. [Some experiments on the effect of heteroauxin on polar development of the gemmae of Marchanta polymorpha L.]. Planta 42: 349–366. 10.1007/BF01928191 [DOI] [Google Scholar]
- Heitz E. 1940. Die Polaritat keimender Moossporen [The polarity of germinating moss spores]. Verh Schweiz Naturforsch Ges 120: 168–170. [Google Scholar]
- Hori K, Maruyama F, Fujisawa T, Togashi T, Yamamoto N, Seo M, Sato S, Yamada T, Mori H, Tajima N, et al. 2014. Klebsormidium flaccidum genome reveals primary factors for plant terrestrial adaptation. Nat Commun 5: 3978. 10.1038/ncomms4978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson RG, Lim EK, Li Y, Kowalczyk M, Sandberg G, Hoggett J, Ashford DA, Bowles DJ. 2001. Identification and biochemical characterization of an Arabidopsis indole-3-acetic acid glucosyltransferase. J Biol Chem 276: 4350–4356. 10.1074/jbc.M006185200 [DOI] [PubMed] [Google Scholar]
- Jiao C, Sorensen I, Sun X, Sun H, Behar H, Alseekh S, Philippe G, Palacio Lopez K, Sun L, Reed R, et al. 2020. The Penium margaritaceum genome: hallmarks of the origins of land plants. Cell 181: 1097–1111.E12. 10.1016/j.cell.2020.04.019 [DOI] [PubMed] [Google Scholar]
- Jin Q, Scherp P, Heimann K, Hasenstein KH. 2008. Auxin and cytoskeletal organization in algae. Cell Biol Int 32: 542–545. 10.1016/j.cellbi.2007.11.005 [DOI] [PubMed] [Google Scholar]
- Jin SH, Ma XM, Han P, Wang B, Sun YG, Zhang GZ, Li YJ, Hou BK. 2013. UGT74D1 is a novel auxin glycosyltransferase from Arabidopsis thaliana. PLoS ONE 8: e61705. 10.1371/journal.pone.0061705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones VA, Dolan L. 2017. MpWIP regulates air pore complex development in the liverwort Marchantia polymorpha. Development 144: 1472–1476. 10.1242/dev.144287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimoto Y, Terasaka K, Hamamoto M, Takanashi K, Fukuda S, Shitan N, Sugiyama A, Suzuki H, Shibata D, Wang B, et al. 2012. Arabidopsis ABCB21 is a facultative auxin importer/exporter regulated by cytoplasmic auxin concentration. Plant Cell Physiol 53: 2090–2100. 10.1093/pcp/pcs149 [DOI] [PubMed] [Google Scholar]
- Kaneko S, Cook SD, Aoi Y, Watanabe A, Hayashi KI, Kasahara H. 2020. An evolutionarily primitive and distinct auxin metabolism in the lycophyte Selaginella moellendorffii. Plant Cell Physiol 61: 1724–1732. 10.1093/pcp/pcaa098 [DOI] [PubMed] [Google Scholar]
- Kato H, Ishizaki K, Kouno M, Shirakawa M, Bowman JL, Nishihama R, Kohchi T. 2015. Auxin-mediated transcriptional system with a minimal set of components is critical for morphogenesis through the life cycle in Marchantia polymorpha. PLoS Genet 11: e1005084. 10.1371/journal.pgen.1005084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Kouno M, Takeda M, Suzuki H, Ishizaki K, Nishihama R, Kohchi T. 2017. The roles of the sole activator-type auxin response factor in pattern formation of Marchantia polymorpha. Plant Cell Physiol 58: 1642–1651. 10.1093/pcp/pcx095 [DOI] [PubMed] [Google Scholar]
- Kato H, Mutte SK, Suzuki H, Crespo I, Das S, Radoeva T, Fontana M, Yoshitake Y, Hainiwa E, van den Berg W, et al. 2020. Design principles of a minimal auxin response system. Nat Plants 6: 473–482. 10.1038/s41477-020-0662-y [DOI] [PubMed] [Google Scholar]
- Kepinski S, Leyser O. 2005. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435: 446–451. 10.1038/nature03542 [DOI] [PubMed] [Google Scholar]
- Khasin M, Cahoon RR, Nickerson KW, Riekhof WR. 2018. Molecular machinery of auxin synthesis, secretion, and perception in the unicellular chlorophyte alga Chlorella sorokiniana UTEX 1230. PLoS ONE 13: e0205227. 10.1371/journal.pone.0205227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Harter K, Theologis A. 1997. Protein–protein interactions among the Aux/IAA proteins. Proc Natl Acad Sci 94: 11786–11791. 10.1073/pnas.94.22.11786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klämbt D, Knauth B, Dittmann I. 1992. Auxin dependent growth of rhizoids of Chara globularis. Physiol Plant 85: 537–540. 10.1111/j.1399-3054.1992.tb05823.x [DOI] [Google Scholar]
- Kögl F, Kostermans GFR. 1934. Hetero-auxin als Stoffwechselprodukt niederer pflanzlicher Organismen. Isolierung aus Hefe. 13. Mitteilung über pflanzliche Wachstumsstoffe [Hetero-auxin as a metabolic product of lower plants. Isolation from yeast. 13. Communication on Vegetable Growth Substances]. Hoppe-Seyler’s Z Physiol Chem 228: 113–121. 10.1515/bchm2.1934.228.3-6.113 [DOI] [Google Scholar]
- Korasick DA, Enders TA, Strader LC. 2013. Auxin biosynthesis and storage forms. J Exp Bot 64: 2541–2555. 10.1093/jxb/ert080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korasick DA, Westfall CS, Lee SG, Nanao MH, Dumas R, Hagen G, Guilfoyle TJ, Jez JM, Strader LC. 2014. Molecular basis for AUXIN RESPONSE FACTOR protein interaction and the control of auxin response repression. Proc Natl Acad Sci 111: 5427–5432. 10.1073/pnas.1400074111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35: 1547–1549. 10.1093/molbev/msy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel BN, Harper CP. 2018. The roles of auxin during interactions between bacterial plant pathogens and their hosts. J Exp Bot 69: 245–254. 10.1093/jxb/erx447 [DOI] [PubMed] [Google Scholar]
- LaRue CD, Narayanaswami S. 1957. Auxin inhibition in the liverwort Lunularia. New Phytol 56: 61–70. 10.1111/j.1469-8137.1957.tb07449.x [DOI] [Google Scholar]
- Lavy M, Prigge MJ, Tao S, Shain S, Kuo A, Kirchsteiger K, Estelle M. 2016. Constitutive auxin response in Physcomitrella reveals complex interactions between Aux/IAA and ARF proteins. eLife 5: e13325. 10.7554/eLife.13325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bail A, Billoud B, Kowalczyk N, Kowalczyk M, Gicquel M, Le Panse S, Stewart S, Scornet D, Cock JM, Ljung K, et al. 2010. Auxin metabolism and function in the multicellular brown alga Ectocarpus siliculosus. Plant Physiol 153: 128–144. 10.1104/pp.109.149708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeClere S, Tellez R, Rampey RA, Matsuda SP, Bartel B. 2002. Characterization of a family of IAA-amino acid conjugate hydrolases from Arabidopsis. J Biol Chem 277: 20446–20452. 10.1074/jbc.M111955200 [DOI] [PubMed] [Google Scholar]
- Li Y, Baldauf S, Lim EK, Bowles DJ. 2001. Phylogenetic analysis of the UDP-glycosyltransferase multigene family of Arabidopsis thaliana. J Biol Chem 276: 4338–4343. 10.1074/jbc.M007447200 [DOI] [PubMed] [Google Scholar]
- Li FW, Brouwer P, Carretero-Paulet L, Cheng S, de Vries J, Delaux PM, Eily A, Koppers N, Kuo LY, Li Z, et al. 2018. Fern genomes elucidate land plant evolution and cyanobacterial symbioses. Nat Plants 4: 460–472. 10.1038/s41477-018-0188-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li FW, Nishiyama T, Waller M, Frangedakis E, Keller J, Li Z, Fernandez-Pozo N, Barker MS, Bennett T, Blázquez MA, et al. 2020. Anthoceros genomes illuminate the origin of land plants and the unique biology of hornworts. Nat Plants 6: 259–272. 10.1038/s41477-020-0618-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung K. 2013. Auxin metabolism and homeostasis during plant development. Development 140: 943–950. 10.1242/dev.086363 [DOI] [PubMed] [Google Scholar]
- Ludwig-Müller J, Jülke S, Bierfreund NM, Decker EL, Reski R. 2009. Moss (Physcomitrella patens) GH3 proteins act in auxin homeostasis. New Phytol 181: 323–338. 10.1111/j.1469-8137.2008.02677.x [DOI] [PubMed] [Google Scholar]
- Lv B, Yu Q, Liu J, Wen X, Yan Z, Hu K, Li H, Kong X, Li C, Tian H, et al. 2020. Non-canonical AUX/IAA protein IAA33 competes with canonical AUX/IAA repressor IAA5 to negatively regulate auxin signaling. EMBO J 39: e101515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher EP, Martindale SJ. 1980. Mutants of Arabidopsis thaliana with altered responses to auxins and gravity. Biochem Genet 18: 1041–1053. 10.1007/BF00484337 [DOI] [PubMed] [Google Scholar]
- Mallory AC, Bartel DP, Bartel B. 2005. MicroRNA-directed regulation of Arabidopsis AUXIN RESPONSE FACTOR17 is essential for proper development and modulates expression of early auxin response genes. Plant Cell 17: 1360–1375. 10.1105/tpc.105.031716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maravolo NC. 1976. Polarity and localization of auxin movement in the hepatic, Marchantia polymorpha. Am J Bot 63: 526–531. 10.1002/j.1537-2197.1976.tb11841.x [DOI] [Google Scholar]
- Martin-Arevalillo R, Nanao MH, Larrieu A, Vinos-Poyo T, Mast D, Galvan-Ampudia C, Brunoud G, Vernoux T, Dumas R, Parcy F. 2017. Structure of the Arabidopsis TOPLESS corepressor provides insight into the evolution of transcriptional repression. Proc Natl Acad Sci 114: 8107–8112. 10.1073/pnas.1703054114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Arevalillo R, Thévenon E, Jégu F, Vinos-Poyo T, Vernoux T, Parcy F, Dumas R. 2019. Evolution of the auxin response factors from charophyte ancestors. PLoS Genet 15: e1008400. 10.1371/journal.pgen.1008400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashiguchi K, Tanaka K, Sakai T, Sugawara S, Kawaide H, Natsume M, Hanada A, Yaeno T, Shirasu K, Yao H, et al. 2011. The main auxin biosynthesis pathway in Arabidopsis. Proc Natl Acad Sci 108: 18512–18517. 10.1073/pnas.1108434108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo S, Miyatake K, Endo M, Urashimo S, Kawanishi T, Negoro S, Shimakoshi S, Fukuoka H. 2020. Loss of function of the Pad-1 aminotransferase gene, which is involved in auxin homeostasis, induces parthenocarpy in Solanaceae plants. Proc Natl Acad Sci 117: 12784–12790. 10.1073/pnas.2001211117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure BA, Hagen G, Brown CS, Gee MA, Guilfoyle TJ. 1989. Transcription, organization, and sequence of an auxin-regulated gene cluster in soybean. Plant Cell 1: 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métraux JP, Taiz L. 1977. Cell wall extension in Nitella as influenced by acids and ions. Proc Natl Acad Sci 74: 1565–1569. 10.1073/pnas.74.4.1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métraux JP, Richmond PA, Taiz L. 1980. Control of cell elongation in Nitella by endogenous cell wall pH gradients: multiaxial extensibility and growth studies. Plant Physiol 65: 204–210. 10.1104/pp.65.2.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte I, Ishida S, Zamarreño AM, Hamberg M, Franco-Zorrilla JM, García-Casado G, Gouhier-Darimont C, Reymond P, Takahashi K, García-Mina JM, et al. 2018. Ligand-receptor co-evolution shaped the jasmonate pathway in land plants. Nat Chem Biol 14: 480–488. 10.1038/s41589-018-0033-4 [DOI] [PubMed] [Google Scholar]
- Morffy N, Strader LC. 2020. Old town roads: routes of auxin biosynthesis across kingdoms. Curr Opin Plant Biol 55: 21–27. 10.1016/j.pbi.2020.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutte SK, Kato H, Rothfels C, Melkonian M, Wong GK, Weijers D. 2018. Origin and evolution of the nuclear auxin response system. eLife 7: e33399. 10.7554/eLife.33399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanao MH, Vinos-Poyo T, Brunoud G, Thévenon E, Mazzoleni M, Mast D, Lainé S, Wang S, Hagen G, Li H, et al. 2014. Structural basis for oligomerization of auxin transcriptional regulators. Nat Commun 5: 3617. 10.1038/ncomms4617 [DOI] [PubMed] [Google Scholar]
- Naramoto S. 2017. Polar transport in plants mediated by membrane transporters: focus on mechanisms of polar auxin transport. Curr Opin Plant Biol 40: 8–14. 10.1016/j.pbi.2017.06.012 [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Sakayama H, de Vries J, Buschmann H, Saint-Marcoux D, Ullrich KK, Haas FB, Vanderstraeten L, Becker D, Lang D, et al. 2018. The Chara genome: secondary complexity and implications for plant terrestrialization. Cell 174: 448–464.e24. 10.1016/j.cell.2018.06.033 [DOI] [PubMed] [Google Scholar]
- Noh B, Murphy AS, Spalding EP. 2001. Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell 13: 2441–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normanly J, Cohen JD, Fink GR. 1993. Arabidopsis thaliana auxotrophs reveal a tryptophan-independent biosynthetic pathway for indole-3-acetic acid. Proc Natl Acad Sci 90: 10355–10359. 10.1073/pnas.90.21.10355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtaka K, Hori K, Kanno Y, Seo M, Ohta H. 2017. Primitive auxin response without TIR1 and Aux/IAA in the charophyte alga Klebsormidium nitens. Plant Physiol 174: 1621–1632. 10.1104/pp.17.00274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y. 1991. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 3: 677–684. 10.2307/3869249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okrent RA, Brooks MD, Wildermuth MC. 2009. Arabidopsis GH3.12 (PBS3) conjugates amino acids to 4-substituted benzoates and is inhibited by salicylate. J Biol Chem 284: 9742–9754. 10.1074/jbc.M806662200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Östin A, Kowalyczk M, Bhalerao RP, Sandberg G. 1998. Metabolism of indole-3-acetic acid in Arabidopsis. Plant Physiol 118: 285–296. 10.1104/pp.118.1.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang J, Shao X, Li J. 2000. Indole-3-glycerol phosphate, a branchpoint of indole-3-acetic acid biosynthesis from the tryptophan biosynthetic pathway in Arabidopsis thaliana. Plant J 24: 327–334. 10.1046/j.1365-313x.2000.00883.x [DOI] [PubMed] [Google Scholar]
- Paál A. 1919. Über phototropische reizleitung [About phototropic stimulus conduction]. Jahrb Wiss Bot 58: 406–458. [Google Scholar]
- Petrásek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertová D, Wisniewska J, Tadele Z, Kubes M, Covanová M, et al. 2006. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312: 914–918. 10.1126/science.1123542 [DOI] [PubMed] [Google Scholar]
- Piya S, Shrestha SK, Binder B, Stewart CN Jr, Hewezi T. 2014. Protein–protein interaction and gene co-expression maps of ARFs and Aux/IAAs in Arabidopsis. Front Plant Sci 5: 744. 10.3389/fpls.2014.00744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli D, Jacobs M, Cooke TJ. 2003. Auxin regulation of axial growth in bryophyte sporophytes: its potential significance for the evolution of early land plants. Am J Bot 90: 1405–1415. 10.3732/ajb.90.10.1405 [DOI] [PubMed] [Google Scholar]
- Porco S, Pěnčík A, Rashed A, Voß U, Casanova-Sáez R, Bishopp A, Golebiowska A, Bhosale R, Swarup R, Swarup K, et al. 2016. Dioxygenase-encoding AtDAO1 gene controls IAA oxidation and homeostasis in Arabidopsis. Proc Natl Acad Sci 113: 11016–11021. 10.1073/pnas.1604375113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulet A, Kriechbaumer V. 2017. Bioinformatics analysis of phylogeny and transcription of TAA/YUC auxin biosynthetic genes. Int J Mol Sci 18: 1791. 10.3390/ijms18081791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge MJ, Lavy M, Ashton NW, Estelle M. 2010. Physcomitrella patens auxin-resistant mutants affect conserved elements of an auxin-signaling pathway. Curr Biol 20: 1907–1912. 10.1016/j.cub.2010.08.050 [DOI] [PubMed] [Google Scholar]
- Qin G, Gu H, Zhao Y, Ma Z, Shi G, Yang Y, Pichersky E, Chen H, Liu M, Chen Z, et al. 2005. An indole-3-acetic acid carboxyl methyltransferase regulates Arabidopsis leaf development. Plant Cell 17: 2693–2704. 10.1105/tpc.105.034959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampey RA, LeClere S, Kowalczyk M, Ljung K, Sandberg G, Bartel B. 2004. A family of auxin-conjugate hydrolases that contributes to free indole-3-acetic acid levels during Arabidopsis germination. Plant Physiol 135: 978–988. 10.1104/pp.104.039677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G, Li L, Huang Y, Wang Y, Zhang W, Zheng R, Zhong C, Wang X. 2018. GhWIP2, a WIP zinc finger protein, suppresses cell expansion in Gerbera hybrida by mediating crosstalk between gibberellin, abscisic acid, and auxin. New Phytol 219: 728–742. 10.1111/nph.15175 [DOI] [PubMed] [Google Scholar]
- Rensing SA, Lang D, Zimmer AD, Terry A, Salamov A, Shapiro H, Nishiyama T, Perroud PF, Lindquist EA, Kamisugi Y, et al. 2008. The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 319: 64–69. 10.1126/science.1150646 [DOI] [PubMed] [Google Scholar]
- Romani F. 2017. Origin of TAA genes in charophytes: new insights into the controversy over the origin of auxin biosynthesis. Front Plant Sci 8: 1616. 10.3389/fpls.2017.01616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani F, Reinheimer R, Florent SN, Bowman JL, Moreno JE. 2018. Evolutionary history of HOMEODOMAIN LEUCINE ZIPPER transcription factors during plant transition to land. New Phytol 219: 408–421. 10.1111/nph.15133 [DOI] [PubMed] [Google Scholar]
- Rousseau J. 1950. Action de l'acide indol beta-acétique sur les propagules de Marchantia polymorpha et Lunularia cruciata [Action of indol beta-acetic acid on the gemmae of Marchantia polymorpha and Lunularia cruciata]. C R Hebd Séances Acad Sci 230: 675–676. [Google Scholar]
- Sachs Jv. 1880. Stoff und Form der Pflanzenorgane. I [Substance and shape of plant organs. I]. Arbeiten des Botanischen Instituts in Wurzburg 1: 452–488. [Google Scholar]
- Sachs Jv. 1882. Stoff und Form der Pflanzenorgane. II [Substance and shape of plant organs. II]. Arbeiten des Botanischen Instituts in Wurzburg 2: 689–718. [Google Scholar]
- Sawa S, Ohgishi M, Goda H, Higuchi K, Shimada Y, Yoshida S, Koshiba T. 2002. The HAT2 gene, a member of the HD-Zip gene family, isolated as an auxin inducible gene by DNA microarray screening, affects auxin response in Arabidopsis. Plant J 32: 1011–1022. 10.1046/j.1365-313X.2002.01488.x [DOI] [PubMed] [Google Scholar]
- Skokan R, Medvecká E, Viaene T, Vosolsobě S, Zwiewka M, Müller K, Skůpa P, Karady M, Zhang Y, Janacek DP, et al. 2019. PIN-driven auxin transport emerged early in Streptophyte evolution. Nat Plants 5: 1114–1119. 10.1038/s41477-019-0542-5 [DOI] [PubMed] [Google Scholar]
- Spartz AK, Ren H, Park MY, Grandt KN, Lee SH, Murphy AS, Sussman MR, Overvoorde PJ, Gray WM. 2014. SAUR inhibition of PP2C-D phosphatases activates plasma membrane H+-ATPases to promote cell expansion in Arabidopsis. Plant Cell 26: 2129–2142. 10.1105/tpc.114.126037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spartz AK, Lor VS, Ren H, Olszewski NE, Miller ND, Wu G, Spalding EP, Gray WM. 2017. Constitutive expression of Arabidopsis SMALL AUXIN UP RNA19 (SAUR19) in tomato confers auxin-independent hypocotyl elongation. Plant Physiol 173: 1453–1462. 10.1104/pp.16.01514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Tiryaki I. 2004. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 16: 2117–2127. 10.1105/tpc.104.023549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Serban B, Rowe M, Tiryaki I, Maldonado MT, Maldonado MC, Suza W. 2005. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 17: 616–627. 10.1105/tpc.104.026690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steindler C, Matteucci A, Sessa G, Weimar T, Ohgishi M, Aoyama T, Morelli G, Ruberti I. 1999. Shade avoidance responses are mediated by the ATHB-2 HD-zip protein, a negative regulator of gene expression. Development 126: 4235–4245. [DOI] [PubMed] [Google Scholar]
- Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie DY, Doležal K, Schlereth A, Jürgens G, Alonso JM. 2008. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133: 177–191. 10.1016/j.cell.2008.01.047 [DOI] [PubMed] [Google Scholar]
- Stepanova AN, Yun J, Robles LM, Novak O, He W, Guo H, Ljung K, Alonso JM. 2011. The Arabidopsis YUCCA1 flavin monooxygenase functions in the indole-3-pyruvic acid branch of auxin biosynthesis. Plant Cell 23: 3961–3973. 10.1105/tpc.111.088047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Nemhauser JL. 2010. Do trees grow on money? Auxin as the currency of the cellular economy. Cold Spring Harb Perspect Biol 2: a001420. 10.1101/cshperspect.a001420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirk WA, Ördög V, Novák O, Rolčík J, Strnad M, Bálint P, van Staden J. 2013. Auxin and cytokinin relationships in 24 microalgal strains. J Phycol 49: 459–467. 10.1111/jpy.12061 [DOI] [PubMed] [Google Scholar]
- Stortenbeker N, Bemer M. 2019. The SAUR gene family: the plant's toolbox for adaptation of growth and development. J Exp Bot 70: 17–27. 10.1093/jxb/ery332 [DOI] [PubMed] [Google Scholar]
- Strader LC, Wheeler DL, Christensen SE, Berens JC, Cohen JD, Rampey RA, Bartel B. 2011. Multiple facets of Arabidopsis seedling development require indole-3-butyric acid-derived auxin. Plant Cell 23: 984–999. 10.1105/tpc.111.083071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Basu S, Brady SR, Luciano RL, Muday GK. 2004. Interactions between auxin transport and the actin cytoskeleton in developmental polarity of Fucus distichus embryos in response to light and gravity. Plant Physiol 135: 266–278. 10.1104/pp.103.034900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R, Kargul J, Marchant A, Zadik D, Rahman A, Mills R, Yemm A, May S, Williams L, Millner P, et al. 2004. Structure–function analysis of the presumptive Arabidopsis auxin permease AUX1. Plant Cell 16: 3069–3083. 10.1105/tpc.104.024737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szemenyei H, Hannon M, Long JA. 2008. TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science 319: 1384–1386. 10.1126/science.1151461 [DOI] [PubMed] [Google Scholar]
- Sztein AE, Cohen JD, de la Fuente IG, Cooke TJ. 1999. Auxin metabolism in mosses and liverworts. Am J Bot 86: 1544–1555. 10.2307/2656792 [DOI] [PubMed] [Google Scholar]
- Sztein AE, Cohen JD, Cooke TJ. 2000. Evolutionary patterns in the auxin metabolism of green plants. Int J Plant Sci 161: 849–859. 10.1086/317566 [DOI] [Google Scholar]
- Takehara S, Sakuraba S, Mikami B, Yoshida H, Yoshimura H, Itoh A, Endo M, Watanabe N, Nagae T, Matsuoka M, et al. 2020. A common allosteric mechanism regulates homeostatic inactivation of auxin and gibberellin. Nat Commun 11: 2143. 10.1038/s41467-020-16068-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Calderon-Villalobos LI, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N. 2007. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 446: 640–645. 10.1038/nature05731 [DOI] [PubMed] [Google Scholar]
- Tao Y, Ferrer JL, Ljung K, Pojer F, Hong F, Long JA, Li L, Moreno JE, Bowman ME, Ivans LJ, et al. 2008. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133: 164–176. 10.1016/j.cell.2008.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tivendale ND, Ross JJ, Cohen JD. 2014. The shifting paradigms of auxin biosynthesis. Trends Plant Sci 19: 44–51. 10.1016/j.tplants.2013.09.012 [DOI] [PubMed] [Google Scholar]
- Tiwari SB, Wang XJ, Hagen G, Guilfoyle TJ. 2001. AUX/IAA proteins are active repressors, and their stability and activity are modulated by auxin. Plant Cell 13: 2809–2822. 10.1105/tpc.010289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari SB, Hagen G, Guilfoyle TJ. 2004. Aux/IAA proteins contain a potent transcriptional repression domain. Plant Cell 16: 533–543. 10.1105/tpc.017384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnaev II, Gunbin KV, Afonnikov DA. 2015. Plant auxin biosynthesis did not originate in charophytes. Trends Plant Sci 20: 463–465. 10.1016/j.tplants.2015.06.004 [DOI] [PubMed] [Google Scholar]
- Turnaev II, Gunbin KV, Suslov VV, Akberdin IR, Kolchanov NA, Afonnikov DA. 2020. The phylogeny of class B flavoprotein monooxygenases and the origin of the YUCCA protein family. Plants (Basel) 9: 1092. 10.3390/plants9091092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Hagen G, Guilfoyle TJ. 1997. ARF1, a transcription factor that binds to auxin response elements. Science 276: 1865–1868. 10.1126/science.276.5320.1865 [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Hagen G, Guilfoyle TJ. 1999. Activation and repression of transcription by auxin-response factors. Proc Natl Acad Sci 96: 5844–5849. 10.1073/pnas.96.10.5844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance BD. 1987. Phytohormone effects on cell division in Chlorella pyrenoidosa Chick (TX-7-11-05) (Chlorellaceae). J Plant Growth Regulation 5: 169–173. 10.1007/BF02087185 [DOI] [Google Scholar]
- von Witsch H. 1940. Versuch über den Einfluss von Wuchsstoff auf Wachstum und Entwicklung von Calypogeia trichomanes [Experiment on the influence of growth substances on the growth and development of Calypogeia trichomanes]. Planta 30: 664–672. 10.1007/BF01917045 [DOI] [Google Scholar]
- Vosolsobe S, Skokan R, Petrasek J. 2020. The evolutionary origins of auxin transport: what we know and what we need to know. J Exp Bot 71: 3287–3295. 10.1093/jxb/eraa169 [DOI] [PubMed] [Google Scholar]
- Wang C, Liu Y, Li SS, Han GZ. 2014. Origin of plant auxin biosynthesis in charophyte algae. Trends Plant Sci 19: 741–743. 10.1016/j.tplants.2014.10.004 [DOI] [PubMed] [Google Scholar]
- Wang C, Li SS, Han GZ. 2016. Commentary: plant auxin biosynthesis did not originate in charophytes. Front Plant Sci 7: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Li L, Li H, Sahu SK, Wang H, Xu Y, Xian W, Song B, Liang H, Cheng S, et al. 2020. Genomes of early-diverging streptophyte algae shed light on plant terrestrialization. Nat Plants 6: 95–106. 10.1038/s41477-019-0560-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Went FW. 1926. On growth-accelerating substances in the coleoptile of Avena sativa. Proc K Ned Akad Wet 30: 10–19. [Google Scholar]
- Went FW. 1928. Wuchsstoff und Wachstum [Growth substances and development]. Recueil Trav Bot Néerl 24: 1–116. [Google Scholar]
- Went FW, Thimann KV. 1937. Phytohormones. Macmillan, New York. [Google Scholar]
- Wilson AE, Tian L. 2019. Phylogenomic analysis of UDP-dependent glycosyltransferases provides insights into the evolutionary landscape of glycosylation in plant metabolism. Plant J 100: 1273–1288. 10.1111/tpj.14514 [DOI] [PubMed] [Google Scholar]
- Wong JH, Spartz AK, Park MY, Du M, Gray WM. 2019. Mutation of a conserved motif of PP2C.D phosphatases confers SAUR immunity and constitutive activity. Plant Physiol 181: 353–366. 10.1104/pp.19.00496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood NL, Berliner MD. 1979. Effects of indoleacetic acid on the desmid Micrasterias thomasiana. Plant Sci Lett 16: 285–289. 10.1016/0304-4211(79)90040-3 [DOI] [Google Scholar]
- Wright AD, Sampson MB, Neuffer MG, Michalczuk L, Slovin JP, Cohen JD. 1991. Indole-3-acetic acid biosynthesis in the mutant maize orange pericarp, a tryptophan auxotroph. Science 254: 998–1000. 10.1126/science.254.5034.998 [DOI] [PubMed] [Google Scholar]
- Wu G, Cameron JN, Ljung K, Spalding EP. 2010. A role for ABCB19-mediated polar auxin transport in seedling photomorphogenesis mediated by cryptochrome 1 and phytochrome B. Plant J 62: 179–191. 10.1111/j.1365-313X.2010.04137.x [DOI] [PubMed] [Google Scholar]
- Wu MF, Yamaguchi N, Xiao J, Bargmann B, Estelle M, Sang Y, Wagner D. 2015. Auxin-regulated chromatin switch directs acquisition of flower primordium founder fate. eLife 4: e09269. 10.7554/eLife.09269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoya NS, Handro W. 1996. Effects of auxins and cytokinins on tissue culture of Grateloupia dichotoma (Gigartinales, Rhodophyta). Hydrobiologia 326: 393–400. 10.1007/BF00047837 [DOI] [Google Scholar]
- Yue J, Hu X, Sun H, Yang Y, Huang J. 2012. Widespread impact of horizontal gene transfer on plant colonization of land. Nat Commun 3: 1152. 10.1038/ncomms2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue J, Hu X, Huang J. 2014. Origin of plant auxin biosynthesis. Trends Plant Sci 19: 764–770. 10.1016/j.tplants.2014.07.004 [DOI] [PubMed] [Google Scholar]
- Záveská Drábková L, Dobrev PI, Motyka V. 2015. Phytohormone profiling across the bryophytes. PLoS ONE 10: e0125411. 10.1371/journal.pone.0125411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Lin JE, Harris C, Campos Mastrotti Pereira F, Wu F, Blakeslee JJ, Peer WA. 2016. DAO1 catalyzes temporal and tissue-specific oxidative inactivation of auxin in Arabidopsis thaliana. Proc Natl Acad Sci 113: 11010–11015. 10.1073/pnas.1604769113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Fu XX, Li RQ, Zhao X, Liu Y, Li MH, Zwaenepoel A, Ma H, Goffinet B, Guan YL, et al. 2020. The hornwort genome and early land plant evolution. Nat Plants 6: 107–118. 10.1038/s41477-019-0588-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y. 2018. Essential roles of local auxin biosynthesis in plant development and in adaptation to environmental changes. Annu Rev Plant Biol 69: 417–435. 10.1146/annurev-arplant-042817-040226 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Christensen SK, Fankhauser C, Cashman JR, Cohen JD, Weigel D, Chory J. 2001. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291: 306–309. 10.1126/science.291.5502.306 [DOI] [PubMed] [Google Scholar]
- Zhao Z, Zhang Y, Liu X, Zhang X, Liu S, Yu X, Ren Y, Zheng X, Zhou K, Jiang L, et al. 2013. A role for a dioxygenase in auxin metabolism and reproductive development in rice. Dev Cell 27: 113–122. 10.1016/j.devcel.2013.09.005 [DOI] [PubMed] [Google Scholar]
- Zheng Z, Guo Y, Novák O, Dai X, Zhao Y, Ljung K, Noel JP, Chory J. 2013. Coordination of auxin and ethylene biosynthesis by the aminotransferase VAS1. Nat Chem Biol 9: 244–246. 10.1038/nchembio.1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žižková E, Kubeš M, Dobrev PI, Přibyl P, Šimura J, Zahajská L, Záveská Drábková L, Novák O, Motyka V. 2017. Control of cytokinin and auxin homeostasis in cyanobacteria and algae. Ann Bot 119: 151–166. 10.1093/aob/mcw194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta C, Ross JR, Koscheski P, Yang Y, Pichersky E, Noel JP. 2003. Structural basis for substrate recognition in the salicylic acid carboxyl methyltransferase family. Plant Cell 15: 1704–1716. 10.1105/tpc.014548 [DOI] [PMC free article] [PubMed] [Google Scholar]