Abstract
Early studies of transmissible tumors in chickens provided evidence that viruses such as avian leukosis virus (ALV) and Rous sarcoma virus (RSV) can cause cancer in these animals. Doubts about the relevance to human tumors and failures to replicate some early work meant the field of tumor virology followed a bumpy course. Nevertheless, viruses that can cause cancers in rodents and humans were ultimately identified, and several Nobel prizes were awarded for work in this area. In this excerpt from his forthcoming book on the history of cancer research, Joe Lipsick looks back at the early history of tumor virus research, from some of the early false starts and debates, to discovery of reverse transcriptase, and identification of human papilloma virus (HPV) as the major cause of cervical cancer.
HERE A CHICK, THERE A CHICK
The early twentieth century witnessed the rise and ignoble fall of Fibiger's Nobel Prize–winning “discovery” of worms as a cause of cancer. But all was not rotten in the state of Denmark. Elsewhere at the University of Copenhagen, Vilhelm Ellerman and Oluf Bang were also testing whether cancer might be an infectious disease. In 1908 they published a paper entitled “Experimental Leukemia in Chickens.” They had found that leukemia, a cancer of the blood cells, could be transmitted from one bird to another by injection (Fig. 1). Furthermore, the causative agent was able to pass through filters too fine to permit passage of any cells, animal or bacterial. Such filterable agents, first discovered in plants, eventually became known as viruses. Ellermann and Bang had discovered avian leukosis virus (ALV), the first known tumor virus. For reasons that remain unclear, their work did not attract the attention it deserved. One criticism was that although one could transmit this leukemia by injection, there was no observable transmission from bird to bird in the absence of injection. Furthermore, not every injected animal developed the disease (∼40%), and those that did often took a while to do so (6–12 mo). There was also a concern that the increased number of white blood cells might be a physiological response to infection, rather than a true malignant proliferation. Finally, it was not then widely accepted that animals, particularly non-mammals, were good models for human disease.
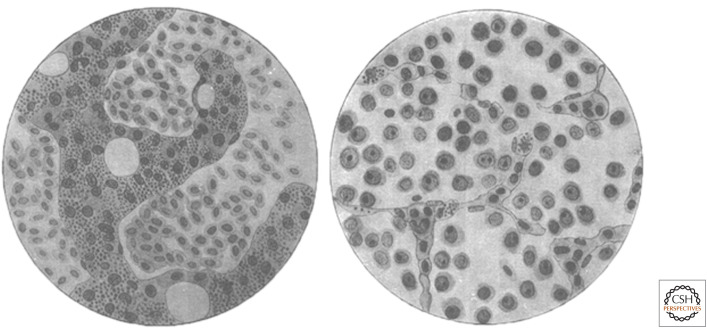
Figure 1.
Ellermann and Bang's drawings of normal chicken bone marrow (left) and leukemic chicken bone marrow (right). The normal marrow contains dense, dark trabeculae of bone. The cells in the spaces between trabeculae are nucleated erythrocytes (red blood cells). The leukemic marrow contains little bone, many immature leukocytes (white blood cells, later shown to be B lymphocytes), and very few erythrocytes. (Reprinted from Ellermann V, Bang O. 1909. Z Hygeine Infektionskrakheiten 63: 231–273.)
Two years after Ellermann and Bang had published their work, Peyton Rous at the Rockefeller Institute described a transmissible sarcoma in chickens (Fig. 2). The original tumor “was found in a barred Plymouth Rock hen of light color and pure blood” that was brought to him by a chicken breeder. Rous minced the tumor into small pieces, injected part into the other breast of the same chicken and part into two other chickens of the same brood. The original chicken died of widespread cancer 35 d later. By this time, one of the other injected chickens had also developed a palpable tumor. In his initial report Rous noted, “The tumor is at best so difficult of propagation that no attempts have been made to determine whether it can be transmitted by cell-fragments, or by cell-free derivatives.”
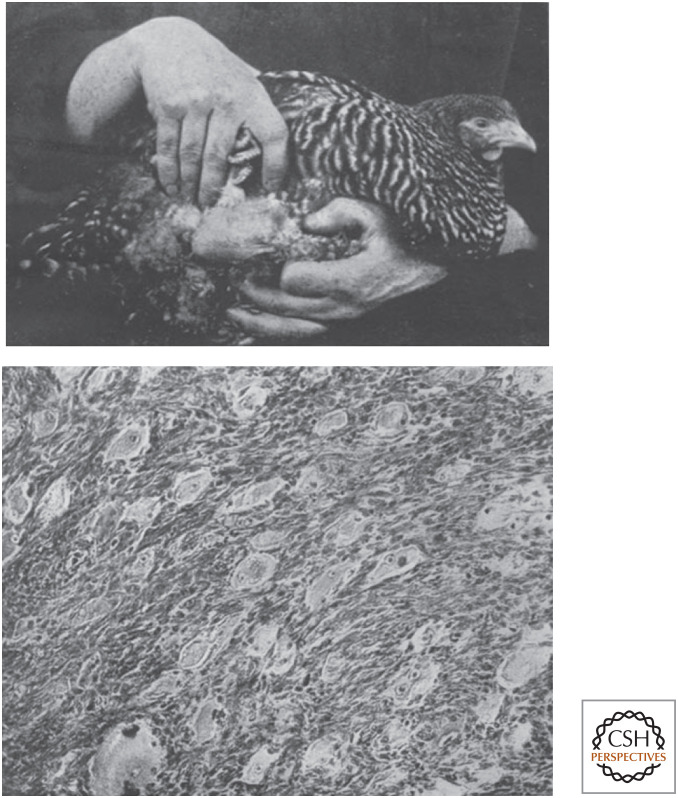
Figure 2.
The origins of Rous sarcoma virus. (Top) A sarcoma caused by injection of fragments of the transmissible tumor. (Bottom) Histopathologic evidence of a sarcoma invading into muscle. (Reprinted from Rous P. 1910. J Exp Med 12: 696–705.)
However, within a year Rous had found that by passage from chicken to chicken, the transmissible tumor had become increasingly aggressive and was now capable of metastasizing. By 1911 he was able to follow in the footsteps of Bang and Ellermann and transmit this cancer by a filterable agent. This virus eventually became known as Rous sarcoma virus (RSV). As before, the work was not generally accepted as proof that cancer could be caused by an infectious agent. On the contrary, physicians were spending considerable time and effort trying to disabuse the public of the view that human cancer was infectious. This mistaken belief often resulted in the shunning or even the quarantine of patients afflicted with cancer. In search of better career prospects, Rous stopped working on RSV a few short years after publishing his landmark paper in 1911.
Extracts of chicken tumors from Rous's laboratory did make it across the pond to England, only to become entangled in a rather Dickensian tale. It began with an unusual proposition made to a studious railway stationmaster named William Bullock. If Bullock would agree to take a wealthy but childless benefactor's name, he would be left a small fortune with which he could attend medical school. Thus, was he reborn as William Gye. He enrolled at Edinburgh University, pursued a career in cancer research, and eventually was able to repeat the experiments of Rous. Taking things one step further, he then found that he could amplify the infectious material in vitro using fragments of chicken embryos. He also claimed to have isolated similar infectious agents in tumors from mice, from rats, and from humans, all of which could cause tumors in chickens.
Gye then collaborated with J.E. Barnard, a wealthy hatter and amateur microscopist, to obtain what they believed were images of the infectious cancer virus particles. These studies were published in 1925 as back-to-back papers in The Lancet. Not surprisingly, this work attracted wide attention in the popular press, helping to again fuel fears about the infectious nature of human cancer. Ultimately, none of this work stood the test of time, except for Gye's replication of Rous's work on chicken viruses. Gye went on to become a very successful cancer research administrator, eventually serving as the Director of the Imperial Cancer Research Fund Laboratories at Mill Hill.
The field of tumor virology itself also followed a rather bumpy course. Although additional tumor viruses were isolated from chickens in Japan and elsewhere, critics harped on the lack of evidence for similar viruses in mammals. In 1933 Richard Shope at the Rockefeller Institute identified a virus capable of causing papillomas (warts) in rabbits. Rous himself propagated and studied this virus for many years. A few years later John Bittner discovered a transmissible mammary cancer in mice that was caused by a milk-borne virus that became known as mouse mammary tumor virus (MMTV). In the early 1950s, Ludwik Gross identified two more mouse tumor viruses, a murine leukemia virus and a polyoma virus, which caused many different types of cancer. Tumor viruses were then identified in a variety of other mammals, including rats, cats, cows, and monkeys.
An adenovirus isolated from human tissue was shown to cause cancer in rodents in 1962, but was ultimately found not to be a cause of human cancer. Simian vacuolating virus 40 (SV40), a contaminant discovered in cells used to produce polio vaccine, was also shown to cause cancer in rodents in 1962. However, there has been a lack of convincing evidence that SV40 causes human cancer, despite much effort.
Finally, in 1964 Michael Epstein and Yvonne Barr published evidence of the first human tumor virus. They had discovered a herpes-like virus in the lymphoblasts of patients with Burkitt's lymphoma, a cancer endemic in children in tropical Africa. Epstein–Barr virus was later shown to cause both mononucleosis (a benign proliferation of B lymphocytes) and a form of nasopharyngeal cancer endemic in certain regions of China. On the heels of the discovery of the first human tumor virus, Rous was finally awarded the Nobel Prize in Physiology or Medicine in 1966 for his discovery of a chicken sarcoma virus 55 years earlier.
THE HUMAN CONDITION
Rising political pressure in the 1970s caused President Richard Nixon to announce a “war on cancer.” Ironically, much of this pressure came from Mary Lasker, a champion of medical research whose husband Albert Lasker had created advertising campaigns that greatly increased the popularity of cigarettes (Fig. 3). Viruses turned out to be important causes of cancer in domesticated animals, such as chickens, laboratory mice, house cats, and cattle. However, despite the expenditure of much effort and many dollars, viruses were not found to cause the majority of human cancers. There were a number of highly publicized false leads, derisively referred to as “rumor viruses.” These reports were most often a result of contamination of human cells by animal viruses in the laboratory. The Special Virus Cancer Program, a directed medical research effort within the National Cancer Institute (NCI), had been charged with the discovery of new human cancer viruses. The program began in 1964, grew to consume substantial resources, and was eventually discontinued in the late 1970s. In part this was due to criticism from scientists outside the walls of the NCI, who favored peer-reviewed research directed by independent individual investigators rather than a centralized bureaucracy that managed large research contracts.
Figure 3.
The Laskers’ contributions to cancer dissemination (top) and to political pressure for increasing spending on research for a cancer cure (bottom, advertisement in The Washington Post). (Top, From the collection of Stanford Research Into the Impact of Tobacco Advertising [tobacco.stanford.edu]; bottom, https://profiles.nlm.nih.gov/spotlight/tl/catalog/nlm:nlmuid-101584665X20-doc.)
Eventually other human tumor viruses were discovered. Most of these viruses cause relatively rare types of cancer (e.g., HTLV-I causes lymphoid cancers of the skin; HTLV-II causes a rare form of leukemia; HHV-8 causes Kaposi's sarcoma; MCV causes Merkel cell cancer). One notable exception is a family of human papilloma viruses (HPVs) very similar to those discovered in rabbits by Shope. Two such viruses (HPV16 and HPV18) were shown by Harald zur Hausen in the 1980s to be the major cause of cancer of the uterine cervix in women. HPV also causes head and neck cancers and anogenital cancers. These observations led to a Nobel Prize in Physiology or Medicine for zur Hausen in 2008, and to the creation of preventive HPV vaccines, the first of which was approved by the FDA for clinical use in 2006.
In addition to viruses that appear to directly cause human cancer, certain viruses and other infectious agents appear to cause cancer in part via the response of the host to infection. For example, infection with hepatitis B and C viruses is strongly associated with cancer of the liver, infection with Helicobacter pylori bacteria is strongly associated with cancer of the stomach, and infection with Schistosoma haematobium is strongly associated with bladder cancer in some countries. Hepatitis B virus and H. pylori have been shown to encode proteins that can promote cell proliferation. However, these cancers all appear to require repeated cycles of infection, chronic inflammation, and tissue repair, resulting in the continued proliferation of cells that have also been exposed to environmental carcinogens. The result is an unholy alliance of three old rivals—the irritation theory, the germ theory, and the mutagen theory of cancer.
THE RISE OF THE QUANTS
In the mid-twentieth century, a number of physicists turned their attention from physics to biology. A particularly influential book called What Is Life? by Erwin Schrödinger proposed that genetic information might be contained within a chemical form. This idea spurred a group of physicists-turned-biologists to focus their attention on simpler and simpler genetic systems that could be studied by quantitative methods. The result was the Phage Group led by Max Delbrück, Alfred Hershey, and Salvador Luria. By studying the viruses of bacteria (bacteriophage or phage), they and their colleagues were able to deduce many of the basic principles of molecular biology. A by-product of their efforts was the development of methods for quantitative virology, largely based on the plaque assay first described by Félix d'Hérelle in 1917.
Similar advances in animal virology required the development of methods for studying viruses in systems simpler than a whole animal. In the early twentieth century, Alexis Carrel, a Nobel Prize–winning French surgeon, developed and publicized rather complex and somewhat mystical methods for culturing fragments of tissue in the laboratory. In 1928 Carrel reported that he could use these cultures to propagate RSV. He also claimed to have kept a continuous culture of embryonic chicken heart cells alive for decades in his laboratory at the Rockefeller Institute. His work attracted the attention of Charles “Lucky Lindy” Lindbergh, who with Carrel sought a path to physical immortality (Fig. 4). Eventually Carrel's immortal chicken heart experiments failed to be repeated by others. Most likely, new cells had been continually added via the embryo extracts used to “feed” the cultures. Carrel later returned to Europe, where he became an advocate of eugenics for the Vichy government in Nazi-occupied France, thus sharing sympathies with Lindbergh.
Figure 4.
Charles Lindbergh, Alexis Carrel, and the quest for immortality. (Image from the National Portrait Gallery.)
The fields of animal cell culture and animal virology progressed slowly until the 1940s, when recurrent polio epidemics spurred intense interest in animal (including human) virology. In 1948, John Enders and his colleagues succeeded in propagating poliovirus in cultures of human embryonic tissue fragments. Their work led to the intensely competitive development of polio vaccines by Jonas Salk and Albert Sabin. Based on the work of Enders, in 1954 Renato Delbecco and Marguerite Vogt developed a plaque assay for poliovirus that was similar to the method used to study the viruses that had lysed bacteria. At the same time, Harry Eagle at the National Institutes of Health (NIH) was systematically determining the requirements for animal cell growth. The result was a defined medium that, when supplemented with relatively small amounts of animal serum, permitted the reproducible growth of animal cells in culture without the need for embryo extracts or plasma clots. Together these powerful methods were then applied to the quantitative study and isolation of mutants of an ever-increasing number of animal viruses, including other important human pathogens like influenza.
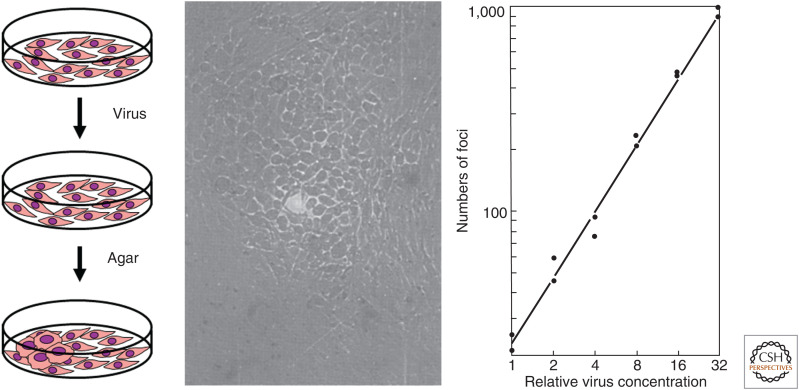
But what about tumor viruses? How could one study viruses that caused cells to proliferate rather than die? The key insight came from Howard Temin and Harry Rubin, a graduate student and a postdoctoral fellow working in Dulbecco's laboratory. They infected dishes of adherent fibroblasts from chicken embryos with different dilutions of RSV, layered agar over the cultures, and then watched and waited. Following infection with very dilute stocks of virus, distinct patches of transformed cells appeared (Fig. 5). Normal fibroblasts are flat, spindly cells that stop proliferating once they touch one another (contact inhibition). By contrast, RSV-transformed fibroblasts round up and keep proliferating, eventually forming small mounds of cells (transformed foci) that are very refractile when seen through a phase-contrast microscope. The agar overlay was an important modification of the method described two years earlier by Manaker and Groupé, because it greatly decreased secondary foci caused by subsequent rounds of infection or by detachment and diffusion of transformed cells. Once again using the logic of the Phage School, Temin and Rubin were able to rapidly and readily quantitate stocks of a tumor virus in a far easier fashion than had been possible with assays in whole animals or chicken eggs. Temin and Rubin observed a linear relationship between the concentration of the virus and the number of foci that extended over a thousandfold range. These results implied that infection with a single viral particle was sufficient to cause the oncogenic transformation of a normal cell. Therefore, by isolating virus from a single focus formed at a low concentration of virus, one could isolate a biological clone that had arisen from a single virus particle.
Figure 5.
Temin and Rubin's focus assay for morphologic transformation by Rous sarcoma virus. (Left) Experimental scheme. (Center) A transformed focus visualized by phase contrast microscopy. (Right) Relationship between viral concentration and number of foci. (Center and right panels from Temin H, Rubin H. 1958. Virology 6: 669–688, with permission from Elsevier.)
PROVIRAL HERESY
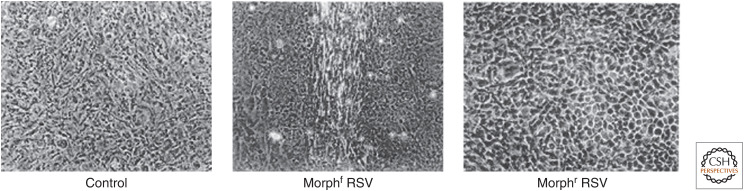
Temin continued these studies in his own laboratory. He noticed that some isolates of RSV transformed cells with a cobblestone-like morphology (round), whereas other isolates of RSV transformed cells with a spindly morphology (fusiform) (Fig. 6). Remarkably, these characteristics bred true. Cells transformed by a “round” variant of RSV and the progeny of these cells remained “round,” as did naive cells transformed by viruses isolated from “round” cells. Similarly, cells transformed by the “fusiform” variant of RSV and the progeny of those cells remained “fusiform,” as did naive cells transformed by viruses isolated from “fusiform” cells. Furthermore, the conversion of one viral variant into another occurred very rarely, if ever. These observations led Temin to propose that the transformed state was a stably inherited property of the infected cells. To account for this heritable state, he further proposed that the genetic material of the virus somehow became part of the genetic material of the cell.
Figure 6.
Cells transformed by fusiform (f) variant and round (r) morphological variants of Rous sarcoma virus. (Photomicrograph by Peter Vogt from Coffin J, et al. 1997. Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, with permission from Peter Vogt.)
There were precedents for viral integration into the host genome in the world of bacteriophage biology. In the 1920s, Eugene and Elizabeth Wollman at the Institut Pasteur in Paris had discovered a latent form of bacteriophage that did not cause cells to lyse. Sadly, the Wollmans were deported from France and perished in Auschwitz. After the end of World War II, their former colleague Andre Lwoff continued their work. By 1949 he had shown that some bacteriophage could exist stably within bacteria in a nonlytic (lysogenic) state. This latent form of the virus (prophage) could be inherited during bacterial division and later be re-activated to produce virus in the absence of any additional infection. Careful studies eventually showed that lysogenic bacteriophage DNA becomes integrated into the genomic DNA of infected bacteria. Much of this latter work was done by François Jacob and by Elie Wollman, the surviving son of Eugene and Elizabeth.
Temin was reportedly unaware of this work because Delbrück (with whom Elie Wollman had trained) and his colleagues at Caltech did not believe in lysogeny. However, by 1962 Vogt and Dulbecco had provided evidence via nucleic acid hybridization that polyoma, a lytic DNA tumor virus originally discovered in mice, was retained in a nonlytic form in oncogenically transformed hamster cells. Soon thereafter, Temin presented similar evidence for the incorporation of the RSV genome into infected cells, although others questioned his results. Temin's proposal that RSV becomes integrated into the DNA of an infected chicken cell genome as a provirus was met with intense skepticism for another reason.
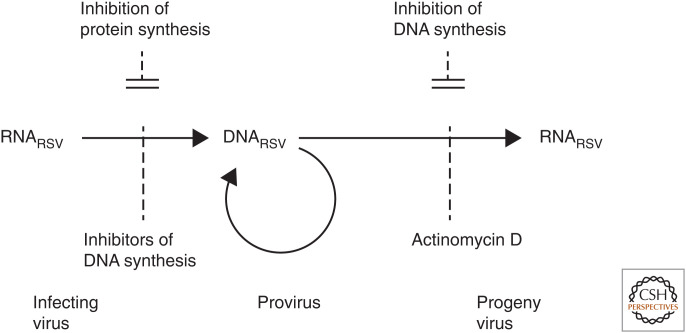
The genome of RSV was known to be composed of RNA, not DNA. Studies of other viruses like polio and influenza had provided examples of RNA serving as a template for the production of more RNA. However, it was generally believed that the flow of information from DNA to RNA to protein was unidirectional. Temin's provirus hypothesis required the heretical conversion of viral RNA into DNA prior to integration and stable inheritance as part of host cell genomic DNA. Temin used inhibitors of DNA synthesis, RNA transcription from DNA, and protein synthesis to obtain evidence consistent with his hypothesis. Viral infection required DNA synthesis, but not protein synthesis. By contrast, viral production by infected cells required RNA synthesis but not DNA synthesis (Fig. 7). Further evidence for a DNA intermediate in viral replication was provided in 1970 by the experiments of David Boettiger in the Temin laboratory. He showed that incorporation of 5-bromodeoxyuridine, a thymidine analog, into the virus caused it to become sensitive to inactivation by ultraviolet light. Similar experiments were also reported by Piero Balduzzi, John Bader, and Herbert Morgan.
Figure 7.
Experimental evidence for Temin's provirus hypothesis. Viral infection was prevented by inhibitors of DNA synthesis, but not inhibitors of protein synthesis. By contrast, virus production was prevented by an inhibitor of RNA transcription (actinomycin D), but not by inhibitors of DNA synthesis. (Reprinted from Temin H. 1972. Proc Natl Acad Sci 69: 1016–1020.)
Meanwhile, the criticism had been fierce and unrelenting. Michael Bishop, who would eventually propose his own controversial hypothesis about RSV, described his first encounter with Howard Temin at a scientific meeting in 1968 as follows:
The hypothesis had earned him little but ridicule and grief. So that summer evening, I watched with interest (and from a respectful distance) as Howard argued long into the night with skeptics and detractors. It was my first experience with a scientist who was essentially alone in his beliefs. What I witnessed was a lesson for a lifetime. The opposition to the provirus hypothesis that evening was strong, even vitriolic. In response, Howard was unfailingly patient and reasoned. He had no doubt that his hypothesis was correct, but he was open to constructive criticism, and he painstakingly tried to refute each opposing argument, even those that had no force other than their animus.
Eventually Temin proved to be right. In 1970 he and Satoshi Mizutani reported the existence of an enzyme within detergent-disrupted RSV virions that could indeed convert RNA into DNA. An RNA-dependent DNA polymerase was independently discovered in a murine leukemia virus by David Baltimore, a virologist who had also trained with Renato Dulbecco and was studying the mechanisms of replication of different types of animal viruses. The result was a scientific earthquake, similar in magnitude to that of New Madrid in 1812, which was said to have caused the Mississippi River to run backward. Because of the unprecedented reversal of flow of genetic information from RNA to DNA, this new viral enzyme became known as “reverse transcriptase.” The RNA tumor viruses that encode this enzyme became known as “retroviruses.”
Physical proof of the existence of a DNA provirus came shortly thereafter. Jan Svoboda, a talented virologist, had persisted in studying the biology of RSV behind the Iron Curtain in what was then Czechoslovakia. In the early 1960s he and his colleagues had been able to transform rat fibroblasts by coculture with RSV-infected chicken cells. However, the rat cells themselves were unable to produce infectious RSV unless fused to normal chicken cells. Although these findings supported the provirus theory, Temin himself remained skeptical of this evidence. In 1972, using the same methods and logic by which Oswald Avery and colleagues had first shown that DNA was the genetic material of bacteria, Miroslav Hill and Jana Hillova sealed the deal. They showed that purified genomic DNA from rat cells transformed by RSV could be introduced into uninfected chicken cells, resulting in the production of infectious RSV.
In 1975, Temin and Baltimore received a Nobel Prize in Physiology or Medicine for the discovery of reverse transcriptase, a prize they shared with their mentor Renato Dulbecco for his (and Marguerite Vogt's) work on polyomavirus. Along with the awarding of a Nobel Prize to Peyton Rous in 1966 for the discovery of RSV, this event prompted Peter Duesburg (a fellow retrovirologist) to quip, “One sick chicken, two Nobel prizes.” But that was hardly the end of the story.
Footnotes
From the forthcoming volume Stalking the Enemy Within: A History of Cancer Research, by Joseph Lipsick
Additional Perspectives on A History of Cancer Research available at www.cshperspectives.org
SUGGESTED READING
The Phage School, Cell Culture, and the Birth of Quantitative Animal Virology
- Dulbecco R, Vogt M. 1954. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med 99: 167–182. 10.1084/jem.99.2.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle H. 1955. Nutrition needs of mammalian cells in tissue culture. Science 122: 501–514. 10.1126/science.122.3168.501 [DOI] [PubMed] [Google Scholar]
- Judson HF. 1996. The eighth day of creation: makers of the revolution in biology. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Skloot R. 2010. The immortal life of Henrietta Lacks. Crown/ Random House, New York. [Google Scholar]
- Watson JD, Stent GS, Cairns J. 2000. Phage and the origins of molecular biology. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
The Discovery and Assay of RNA Tumor Viruses
- Coffin JM, Hughes SH, Varmus HE editors. 1997. Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed] [Google Scholar]
- Ellermann V, Bang O. 1909. Experimental leukemia in chickens II. Z Hygiene Infektionskrakheiten 63: 231–272. [English translation available in Some classics in experimental oncology (ed. Shimkin M), NIH Publication No 80-2120 (1980)]. 10.1007/BF02227892 [DOI] [Google Scholar]
- Rous P. 1910. A transmissible avian neoplasm (sarcoma of the common fowl). J Exp Med 12: 696–705. 10.1084/jem.12.5.696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rous P. 1911. Transmission of a malignant growth by means of a cell-free filtrate. J Am Med Assoc 56: 198. [Google Scholar]
- Temin HM, Rubin H. 1958. Characteristics of an assay for Rous sarcoma virus and Rous sarcoma cells in tissue culture. Virology 6: 669–688. 10.1016/0042-6822(58)90114-4 [DOI] [PubMed] [Google Scholar]
The Discovery of Reverse Transcriptase
- Baltimore D. 1970. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature 226: 1209–1211. 10.1038/2261209a0 [DOI] [PubMed] [Google Scholar]
- Temin HM, Mizutani S. 1970. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature 226: 1211–1213. 10.1038/2261211a0 [DOI] [PubMed] [Google Scholar]
The Provirus Hypothesis
- Baltimore D. 1995. Thinking about Howard Temin. Genes Dev 9: 1303–1307. 10.1101/gad.9.11.1303 [DOI] [PubMed] [Google Scholar]
- Cooper GM, Temin RG, Sugden B editors. 1995. The DNA provirus: Howard Temin's scientific legacy. ASM Press, Washington, DC. [Google Scholar]
- Hill M, Hillova J. 1972. Virus recovery in chicken cells tested with rous sarcoma cell DNA. Nat New Biol 237: 35–39. 10.1038/newbio237035a0 [DOI] [PubMed] [Google Scholar]
- Svoboda J. 2008. The turns of life and science. Adv Cancer Research 99: 1–32. 10.1016/S0065-230X(07)99001-6 [DOI] [PubMed] [Google Scholar]
- Temin HM. 1976. The DNA provirus hypothesis. Science 192: 1075–1080. 10.1126/science.58444 [DOI] [PubMed] [Google Scholar]
The Search for Human Tumor Viruses
- Epstein MA, Achong BG, Barr YM. 1964. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet 283: 702–703. 10.1016/S0140-6736(64)91524-7 [DOI] [PubMed] [Google Scholar]
- Javier RT, Butel JS. 2008. The history of tumor virology. Cancer Res 68: 7693–7706. 10.1158/0008-5472.CAN-08-3301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PS, Chang Y. 2010. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat Rev Cancer 10: 878–889. 10.1038/nrc2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade N. 1971. Special virus cancer program: travails of a biological moonshot. Science 174: 1306–1311. 10.1126/science.174.4016.1306 [DOI] [PubMed] [Google Scholar]
- Zur Hausen H. 2009. The search for infectious causes of human cancer: where and why? Virology 392: 1–10. 10.1016/j.virol.2009.06.001 [DOI] [PubMed] [Google Scholar]