The reducing equivalents NADH and FADH2 are essential products of the TCA that were used by the electron transport chain (ETC) to generate ATP through oxidative phosphorylation (Chandel 2020a). But an often-overlooked reducing equivalent is NADPH, which is not used for generating ATP but for biosynthesis of macromolecules. NADPH is the major reducing equivalent driving de novo synthesis of fatty acids, cholesterol, amino acids, and nucleotides. Its other major function is generation of superoxide (O2−) by NADPH oxidases (NOXs) and scavenging of H2O2 by regenerating GSH and the antioxidant protein thioredoxin (TRX). Its role in generating superoxide and scavenging H2O2 will be covered in this review, and the biosynthetic reactions coupled to NADPH will be covered in other reviews in this collection.
NADPH can be considered a high-energy molecule similar to NADH. However, the electrons of NADPH are used for biosynthesis of macromolecules and the scavenging and generation of ROS, whereas the electrons of NADH are ultimately transferred by the ETC to oxygen. Cells maintain a high-NADPH/NADP+ ratio. Many biosynthetic reactions are coupled to NADP+ + 2e− + 1H+ → NADPH to make these reactions thermodynamically favorable. Accordingly, cells have multiple sources of NADPH that are generated by cytosolic glycolytic and mitochondrial TCA-cycle intermediates. Conceptually, this makes sense because as these intermediates are used as precursors for biosynthesis of macromolecules, they concomitantly generate NADPH to drive the biosynthetic reactions thermodynamically.
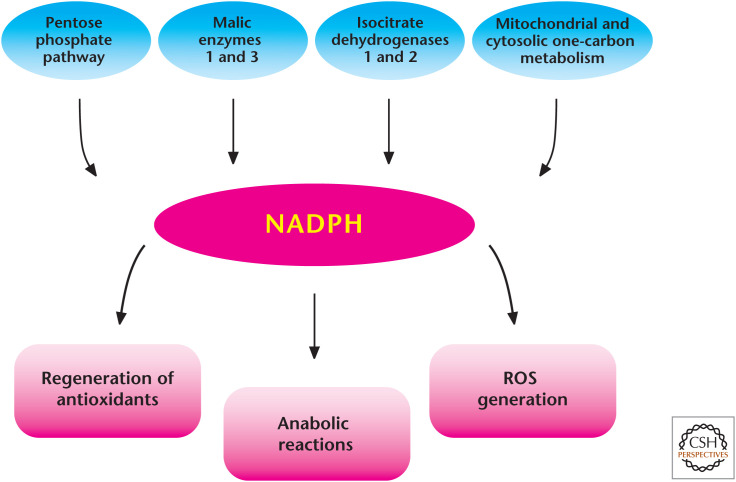
QUICK GUIDE TO NADPH (FIG. 1)
Figure 1.
Diverse roles of NADPH in metabolism. There are multiple sources that generate NADPH in the mitochondria and cytosol. NADPH is critical for many anabolic reactions and is essential to maintain antioxidant capacity in cells. NADPH can also be used to generate ROS through NADPH oxidases.
The glycolytic intermediate glucose 6-phosphate can funnel into the pentose phosphate pathway (PPP) to generate NADPH in the cytosol.
Isocitrate dehydrogenase 1 (IDH1) and 2 (IDH2) generate NADPH in the cytosol and mitochondrial matrix, respectively.
Malic enzymes 1 (ME1) and 3 (ME3) generate NADPH in the cytosol and mitochondrial matrix, respectively.
One-carbon metabolism generates NADPH in the cytosol and mitochondrial matrix.
NADPH is used for reductive biosynthesis of fatty acids, cholesterol, nucleotides, and amino acids.
NADPH is used by NOXs to generate superoxide.
NADPH is used as an electron donor to reduce oxidized GSH and TRX, thus maintaining antioxidant capacity in cells.
GLYCOLYTIC INTERMEDIATES GENERATE CYTOSOLIC NADPH THROUGH THE PENTOSE PHOSPHATE PATHWAY (PPP)
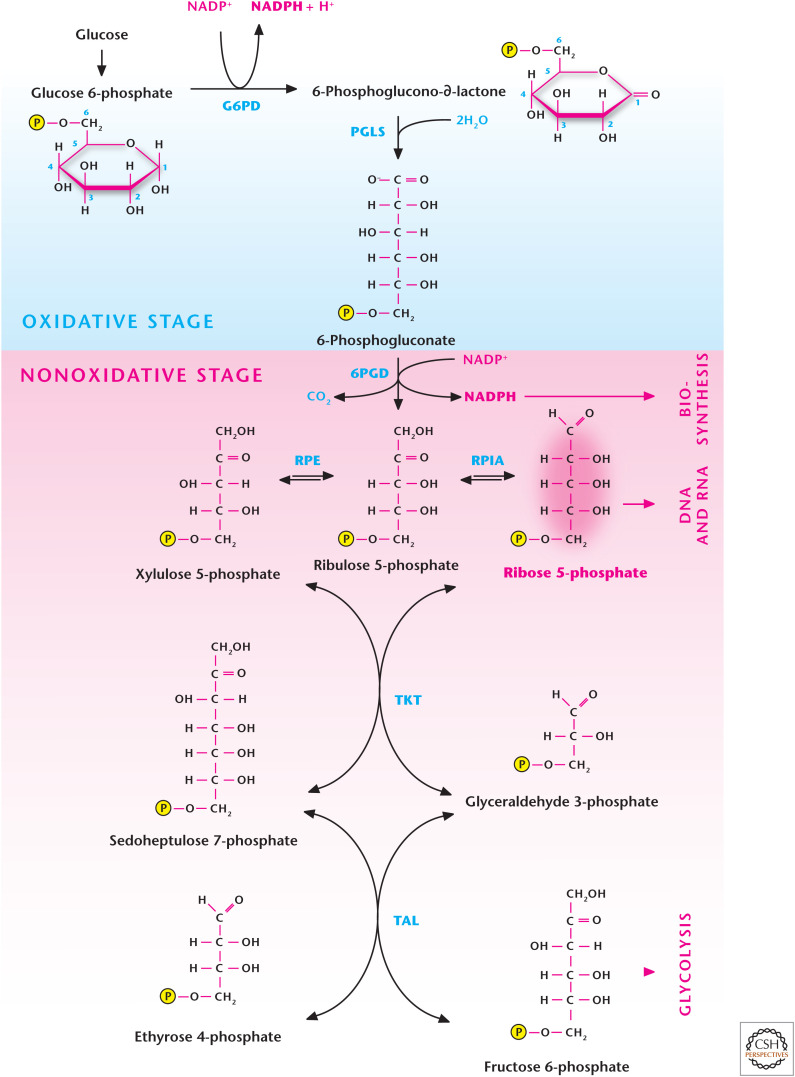
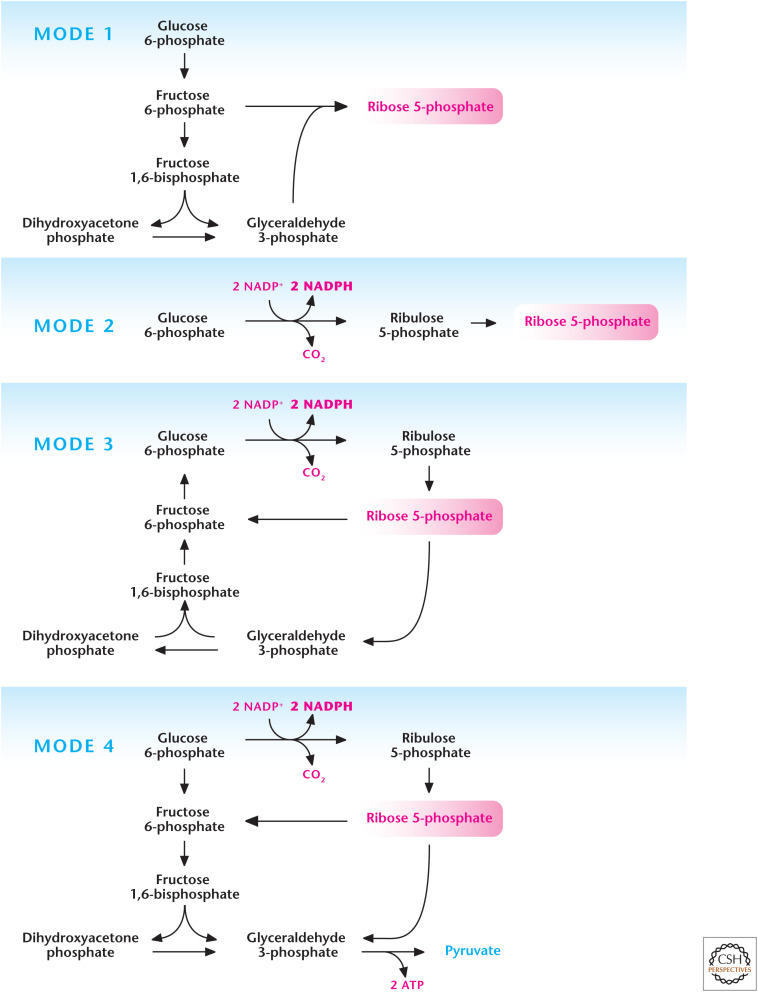
The oxidative phase of the pentose phosphate pathway (PPP), also called the phosphogluconate pathway and hexose monophosphate shunt, is one of the primary sources of cytosolic NADPH (Fig. 2). This anabolic pathway is initiated when glucose is converted into glucose 6-phosphate, which can either go down the glycolysis pathway or enter the PPP to generate NADPH. The end product of the oxidative phase of the PPP is ribulose 5-phosphate, which enters the nonoxidative phase of the PPP to generate ribose 5-phosphate, the “backbone” of nucleic acids. The metabolites generated in the nonoxidative phase of the PPP can also be converted into glycolytic intermediates to generate ATP. The flux through the oxidative phase of the PPP is dependent on the concentration of glucose 6-phosphate and the enzymes glucose 6-phosphate dehydrogenase (G6PDH) and 6-phosphogluconate dehydrogenase. Aside from generating both NADPH and ribose 5-phosphate, the PPP can operate four different modes where NADPH and/or ribose 5-phosphate is produced, depending on the cellular metabolic demands (Fig. 3). Mode 1 primarily generates ribose 5-phosphate by conversion of glucose 6-phosphate into fructose 6-phosphate and glyceraldehyde 3-phosphate, which generate ribose 5-phosphate through reversal of transaldolase and transketolase reactions in the nonoxidative stage of PPP. Mode 2 produces a balance of both NADPH and ribose 5-phosphate through the canonical oxidative and nonoxidative stages of the PPP. Mode 3 produces primarily NADPH and occurs in postmitotic cells that have a limited need for DNA but require high amounts of cytosolic NADPH, such as adipose or liver tissue, for fatty acid synthesis. In this case, the oxidative phase of the PPP generates two molecules of NADPH and one molecule of ribose 5-phosphate. The latter is converted into glycolytic intermediates fructose 6-phosphate and glyceraldehyde 3-phosphate by transketolase and transaldolase. The glycolytic intermediates are converted into glucose 6-phosphate by the gluconeogenic pathway. Mode 4 generates NADPH, and the ribose 5-phosphate is converted into the glycolytic intermediates fructose 6-phosphate and glyceraldehyde 3-phosphate, which enter the glycolytic pathway to generate ATP rather than the gluconeogenic pathway to produce glucose 6-phosphate.
Figure 2.
The pentose phosphate pathway (PPP). Glucose conversion to glucose 6-phosphate by G6PD in step 1 of glycolysis allows for the funneling of the latter metabolite down the glycolytic pathway or into the PPP. The oxidative stage of the PPP generates NADPH, whereas the nonoxidative stage generates ribose 5-phosphate, which can be used for DNA and RNA synthesis. G6PD, glucose 6-phosphate hydrogenase; PGLS, 6-phosphogluconolactonase; 6PGD, 6-phosphogluconate dehydrogenase; RPE, ribulose 5-phosphate-3-epimerase; RPIA, ribose 5-phosphate isomerase A; TKT, transketolase; TAL, transaldolase.
Figure 3.
Different modes of the pentose phosphate pathway (PPP). The PPP can operate in different modes depending on cellular requirements of NADPH and ribose 5-phosphate. Mode 1 primarily generates ribose 5-phosphate by funneling glycolytic intermediates into the nonoxidative stage of PPP. Mode 2 produces balance of both NADPH and ribose 5-phosphate. Mode 3 produces primarily NADPH as the ribose 5-phosphate is funneled into glycolysis to go back into the oxidative stage of PPP for further generation of NADPH. Mode 4 generates NADPH and the ribose 5-phosphate is funneled into glycolysis to generate ATP. (Adapted, with permission, from Berg et al. 2012, © W.H. Freeman and Company.)
Cells have developed multiple mechanisms to decrease the flux through glycolysis, thereby increasing glucose 6-phosphate levels. For example, certain neurons will degrade the positive regulator of glycolysis 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3, thus funneling glucose 6-phosphate into the PPP. Neurons are exquisitely sensitive to oxidative stress and require high levels of NADPH to generate antioxidant defense systems regulated by GSH and Trx. Neurons can meet their ATP demand by mitochondrial oxidative phosphorylation and so are willing to compromise their glycolytic ATP generation to produce copious amounts of NADPH for antioxidant defense. Proliferating cells also have multiple mechanisms to ensure they can balance flux through glycolysis and the PPP (Chandel 2020b).
Although the PPP is an important source of NADPH, individuals can survive with mutations in the oxidative phase of this pathway. (The next section covers other major sources of cytosolic PPP.) The most common human enzyme defect estimated to be carried by 400 million people is glucose 6-phosphate dehydrogenase (G6PD) deficiency (see Box 1). The oxidative phase of the PPP is the only source of NADPH in red blood cells, which are devoid of mitochondria. As a result, these cells become very sensitive to oxidative stress, and anemia can develop because of damaged red blood cells.
BOX 1.
G6PD—NOW AND THEN
The X-linked G6PD deficiency is the most common genetic enzyme disorder in humans, affecting an estimated 400 million people worldwide. Its distribution in northern Africa, southern Asia, the Mediterranean countries, and Middle Eastern regions matches that of high-malaria (Plasmodium falciparum–caused) prevalence. G6PD deficiency provides some protection against malaria, as do the thalassemias and sickle cell disease also common in these areas, suggesting an evolutionary benefit. G6PD converts NADP+ to NADPH in the PPP and this protects red blood cells from oxidative stress. The lack of NADPH in G6PD deficiency results in the failure to reduce H2O2 and ROS, oxidation of hemoglobin to methemoglobin, and the membrane damage that results in hemolysis. These G6PD-deficient red blood cells, when parasitized by the malaria parasite, are more readily phagocytosed by macrophages.
The most common manifestation of G6PD deficiency is hemolytic anemia, which is triggered by infection and certain drugs. But, perhaps the strangest is favism, a severe hemolytic reaction to eating or inhaling the pollen of the broad or fava bean, Vicia faba. Although favism occurs only in people with G6PD deficiency, not all people with this deficiency suffer favism.
Fava beans, apparently, have been known since Neolithic times, but stories of avoiding the ingestion of fava beans date back to ancient Greece and the Pythagoreans. The reasons vary, but many believed that these beans contained the souls of the dead and that the black spot on the hilum of the bean was associated with death. Aristotle tells of Pythagoras (ca. 570–495 BCE) refusing to eat fava beans, and others tell stories of Pythagoras refusing to walk through fields of fava beans. Although there are no contemporary accounts, later historians tell of Pythagoras being pursued by his enemies and refusing to cross a bean field. As described by the 3rd century historian Diogenes Laertius, “sooner than trample on it [i.e., a bean field], he endured to be slain at the cross-roads by the men of Acragas.”
Modern reports of favism date from the mid-19th century when some G6PD-deficient patients presented with jaundice. Fava beans contain glycosidic compounds, including vicine and covicine, that in the presence of G6PD deficiency result in increased susceptibility of erythrocytes to H2O2 and other ROS, and ultimately hemolysis. G6PD is the major source of NADPH in red blood cells; thus lack of this enzyme makes them highly susceptible to ROS-induced damage.
TCA-CYCLE INTERMEDIATES GENERATE CYTOSOLIC AND MITOCHONDRIAL NADPH
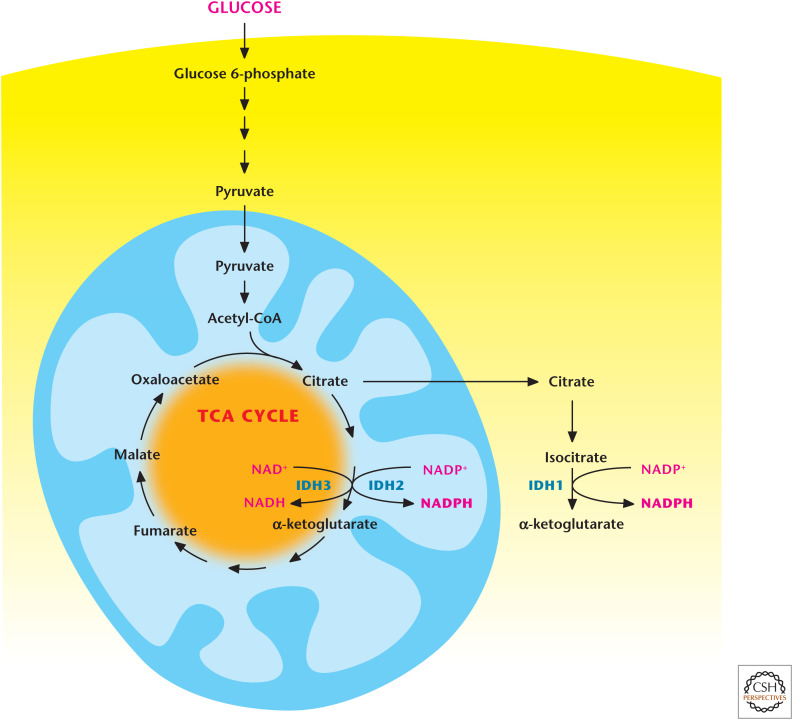
Isocitrate and malate are two TCA-cycle metabolites that generate NADPH through mitochondrial and cytosolic forms of isocitrate dehydrogenases (IDH1 and IDH2) and malic enzymes (ME1 and ME3), respectively. IDH2 in the mitochondrial matrix converts isocitrate to α-ketoglutarate to generate NADPH from NADP+. Note that isocitrate dehydrogenase 3 (IDH3) converts isocitrate to α-ketoglutarate to generate NADH from NAD+. IDH1 in the cytoplasm converts isocitrate to α-ketoglutarate to generate NADPH from NADP+. Cytoplasmic pools of isocitrate are generated from citrate by cytosolic aconitase 1. The generation of NADPH by IDH1 requires citrate export from the mitochondria (Fig. 4) in exchange for malate imported into the mitochondria. The mitochondrial malate is used for exchange of cytoplasmic α-ketoglutarate; therefore, this cycle produces no net change in the cytoplasmic malate pool.
Figure 4.
Isocitrate dehydrogenases 1 and 2 produce NAPDH. Cytosolic isocitrate dehydrogenase 1 (IDH1) and mitochondrial isocitrate dehydrogenase 2 (IDH2) produce NADPH through conversion of isocitrate into α-ketoglutarate. Note that mitochondrial isocitrate dehydrogenase 3 (IDH3) generates NADH by converting isocitrate into α-ketoglutarate.
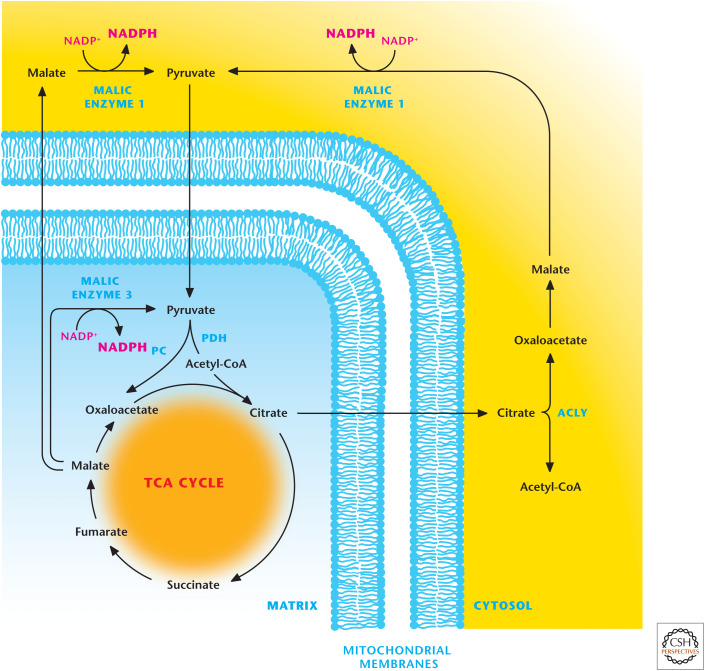
The citrate pool in the cytoplasm can also be converted into oxaloacetate and acetyl-CoA by ATP-citrate lyase (ACLY). The acetyl-CoA can be used for protein acetylation or fatty acid synthesis. Malate dehydrogenase converts the oxaloacetate into malate, which is subsequently converted into pyruvate by ME1 to generate NADPH (Fig. 5). The pyruvate generated is transported into the mitochondria to enter the TCA cycle to generate citrate. ME2 can generate NADPH by converting mitochondrial matrix pool of malate into pyruvate that can reenter the TCA cycle.
Figure 5.
Malic enzymes 1 and 3 produce NADPH. Mitochondrial malic enzyme 3 can generate NADPH by converting malate into pyruvate. Malate can also exit from the TCA cycle where it is converted into pyruvate by malic enzyme 1, resulting in the production of NADPH. The TCA-cycle intermediate citrate can be transported into the cytosol where it is converted into acetyl-CoA and oxaloacetate by ATP-citrate lyase (ACLY). The latter is converted into malate to produce NADPH by malic enzyme 1. PC, pyruvate carboxylase; PDH, pyruvate dehydrogenase.
CYTOSOLIC AND MITOCHONDRIAL ONE-CARBON METABOLISM GENERATES NADPH
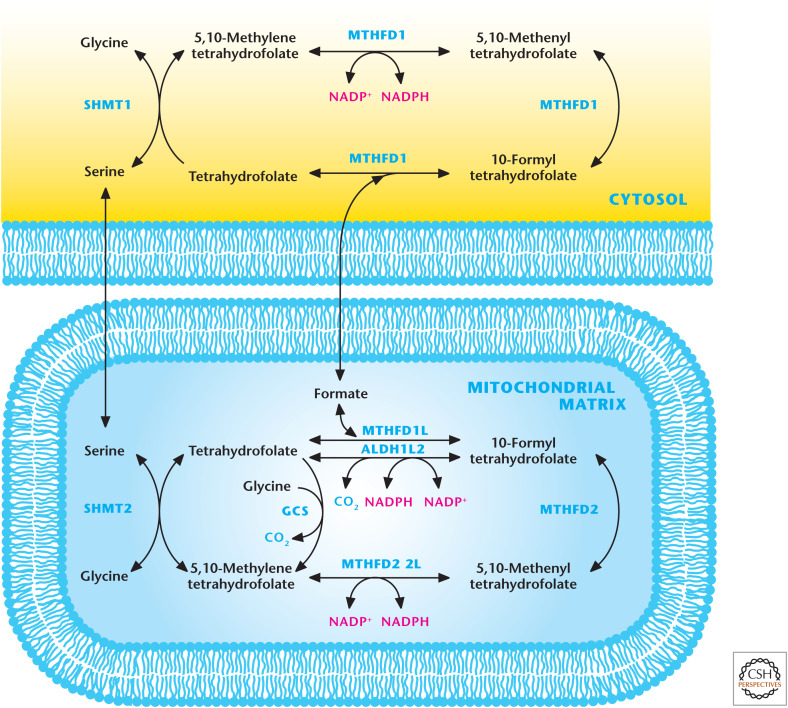
One-carbon metabolism integrates the amino acids serine and glycine into the folate and methionine cycles that participate in many diverse biological outputs, including nucleotide synthesis (see Chandel 2020c) and methylation reactions involved in epigenetics (see Chandel 2020d). In this metabolism, a carbon unit from serine or glycine is transferred to tetrahydrofolate (THF) to form 5,10-methylene-THF. Recent studies have highlighted the up-regulation and necessity of one-carbon metabolism for tumor cell proliferation, thus making this metabolic pathway a viable therapeutic target (see Chandel 2020b). One-carbon metabolism comprises parallel cytosolic and mitochondrial pathways (Fig. 6). Although there is restricted exchange between the cytoplasmic and mitochondrial pools of THF molecules, these compartments are metabolically connected by transport of the one-carbon donors (serine, glycine, and formate) across the mitochondrial membranes. Recent studies indicate that the one-carbon metabolism is a significant source of NADPH in the mitochondria and, to a lesser extent, in the cytoplasm. These NADPH-generating reactions are catalyzed by MTHFD1 in the cytosol and MTHFD1L, MTHFD2L, and ALDH1L2 in the mitochondrial matrix compartment (Fig. 6).
Figure 6.
Mitochondrial and cytosolic one-carbon metabolism generates NADPH. The one-carbon amino acids serine and glycine can feed into folate cycle to generate NADPH. The cytosolic enzyme MTHFD1 and mitochondrial matrix enzymes MTHFD1L, MTHFD2L, and ALDH1L2 generate NADPH. One-carbon metabolism is a significant source of NADPH in the mitochondria. GCS, glycine cleavage system.
THIOL-DEPENDENT REDOX SIGNALING
Other reviews in this subject collection cover the essential role of NADPH in multiple anabolic reactions to generate lipids, nucleotides, and amino acids. As mentioned earlier, a major role of NADPH is to detoxify ROS, which are intracellular chemical species that contain oxygen (O2) and are reactive toward lipids, proteins, and DNA. ROS include O2−, H2O2, and hydroxyl radicals (OH−) (Fig. 7). ROS are more chemically reactive than O2 and are able to trigger various biological events. Each ROS has different intrinsic chemical properties that dictate their reactivity and preferred biological targets. O2− is produced during oxidative metabolism by the one-electron reduction of molecular O2. O2− is rapidly converted by SODs into H2O2, which can impinge on cellular signaling by oxidizing thiols within proteins and altering their function (Fig. 8). The concentration of H2O2 associated with signaling is likely in the low-nanomolar range. Unlike O2−, H2O2 can readily diffuse through membranes, making it an ideal intracellular signaling molecule. ROS have been traditionally thought of as toxic metabolic byproducts that cause cellular damage. However, studies throughout the past decade have highlighted the role of H2O2 in cell signaling. H2O2 is detoxified to water by glutathione peroxidases (GPXs), peroxiredoxins (PRXs), and catalase. In the presence of ferrous or cuprous ions, H2O2 can become a hydroxyl radical, which is very reactive and causes oxidation of lipids, proteins, and DNA, resulting in damage to the cell.
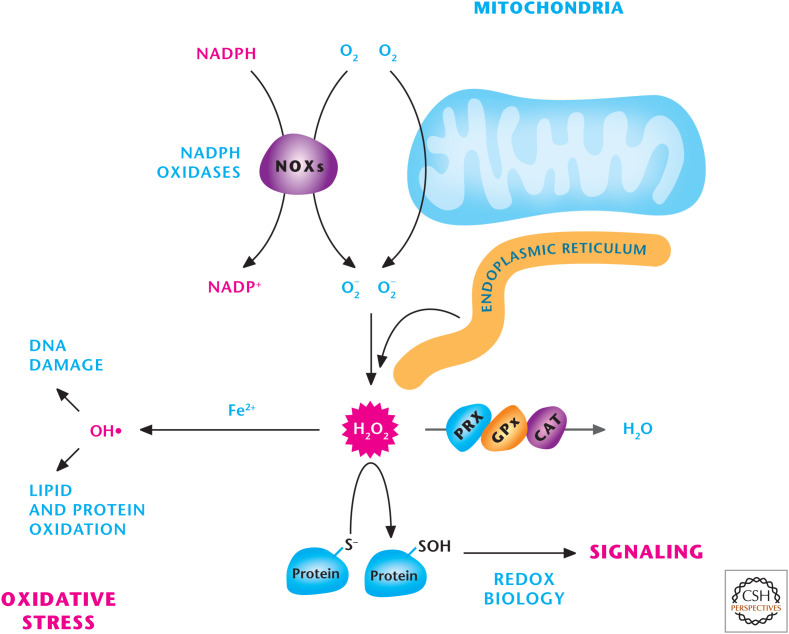
Figure 7.
Sources of ROS. Mitochondria, NADPH oxidase (NOXs), and the endoplasmic reticulum are three major sources of H2O2, which can either be detoxified into H2O by peroxiredoxins (PRXs), catalase (CAT), or glutathione peroxidases (GPXs) or activate signaling pathways by oxidizing specific thiols within proteins. H2O2 in the presence of iron (Fe2+) can generate hydroxyl radical (OH−) that incurs damage to DNA, lipids, and proteins.
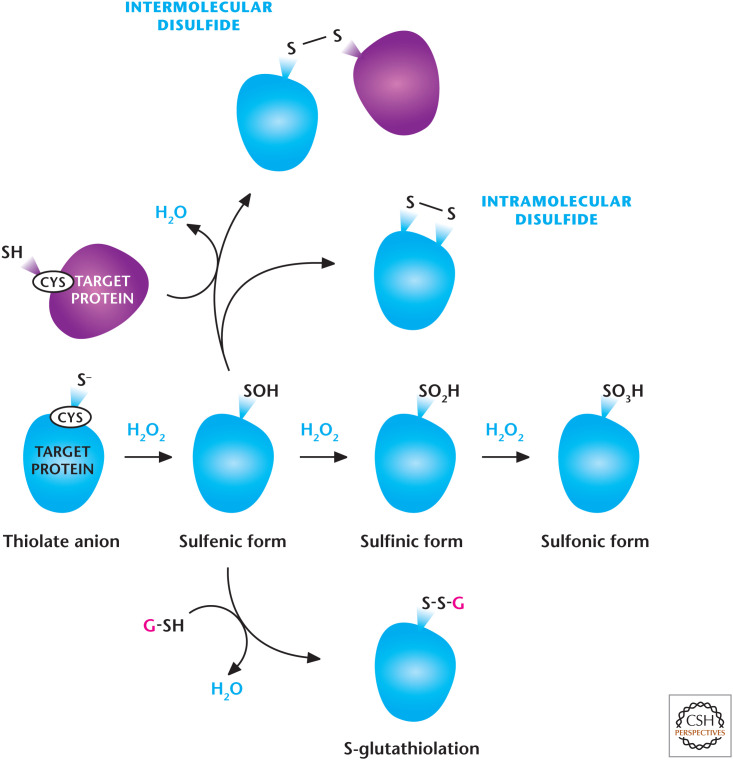
Figure 8.
H2O2-dependent signaling. H2O2 regulates signaling pathways by oxidation of thiol groups on cysteines within proteins that show a low pKa, allowing the cysteine thiol group to exist as a thiolate anion (S−). H2O2 readily oxidizes thiolate, yielding SO−. Under high concentrations of H2O2, SO− can undergo further oxidation to generate SO2− and SO3−. SO− can undergo further modifications including intra- or intermolecular disulfide bonds and S-gluthathiolation. (Modified from Finkel 2011.)
ROS levels associated with signaling comprise redox biology, whereas ROS levels associated with pathology are referred to as oxidative damage. ROS have been shown to play a causal role in various cellular events, including proliferation, differentiation, metabolic adaptation, and the regulation of adaptive and innate immunity. ROS control cellular signaling by causing reversible posttranslational modifications to proteins that regulate signaling pathways. Biological redox reactions catalyzed by H2O2 or organic hydroperoxide typically involve the oxidation of thiol groups on cysteines with a low pKa, allowing the cysteine thiol group (SH) to exist as a thiolate anion (S−). H2O2 readily oxidizes thiolate, yielding sulfenic acid (SO−). Under high concentrations of H2O2, SO− can undergo further oxidation to generate sulfinic (SO2−) and sulfonic (SO3−) acids. SO3− , generally, are irreversible oxidative modifications (Fig. 8). A common mechanism for preventing irreversible oxidation of catalytic cysteines is to incorporate the SO− intermediate into a disulfide (S–S) bond or sulfenic–amide (S–N) bond. Disulfides are formed by reaction of SO− with either an inter- or intramolecular cysteine, or with GSH. S–N bonds are formed by nucleophilic attack of the backbone nitrogen atom of the adjacent residue on SO−. The actions of cytosolic glutaredoxins (GRX) or cytosolic and mitochondrial thioredoxins (TRX1 and TRX2) restore the oxidized proteins back to their reduced state. GRXs are reduced by oxidation of GSH to disulfide glutathione (GSSG) (Fig. 8). GSH reductase regenerates GSH from GSSG by using NADPH. Oxidized TRXs are reduced by cytosolic or mitochondrial TRX reductase (TR1 or TR2) by using NADPH. Thus, NADPH is crucial in maintaining TRX and GRX in a reduced state so they can engage in cellular signaling.
Phosphatases, which control protein kinase function in the cell, are the best example of redox targets. Redox-sensitive phosphatases include PTP1B, PTEN, and mitogen-associated protein (MAP) or mitogen-activated protein kinase (MAPK) phosphatases, which can be reversibly oxidized by H2O2, inhibiting their dephosphorylation activity. A major challenge in the field is to decipher the direct targets of ROS beyond phosphatases. These targets are likely to be context dependent. However, there are many proteins that are indirectly activated by ROS, including transcription factors, such as hypoxia-inducible factor (HIF) and nuclear factor-κB (NF-κB), and kinases, like Src and AMP-activated protein kinase.
Because the levels and type of ROS dictate redox biology versus oxidative damage, the field is hampered by lack of appropriate tools to accurately measure levels and types of ROS in vitro and in vivo. There are considerable efforts to design probes that accurately measure types of ROS in different compartments of the cell. The three main production sites of ROS associated with cell signaling are mitochondria, endoplasmic reticulum (ER), and the family of NOXs. Multiple signaling pathways have been implicated in the activation of ROS at these sites, including an increase in calcium, gain of function of oncogenes, as well as changes in oxygen and nutrient levels.
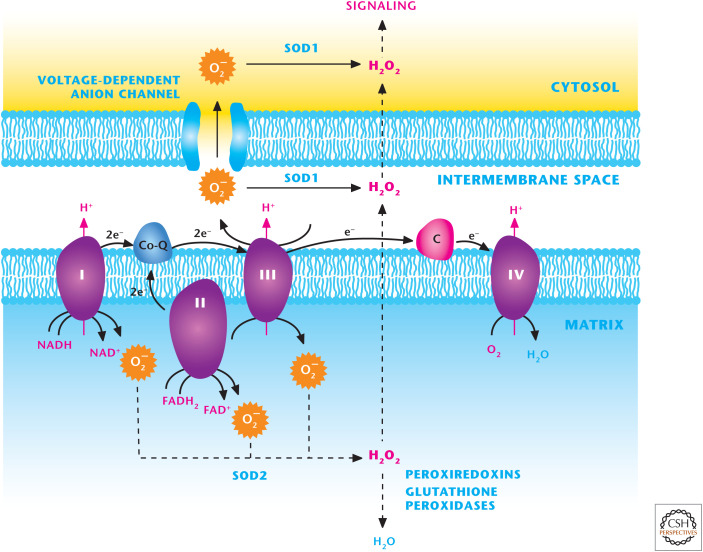
Mitochondria are major generators of ROS at eight distinct sites (see the discussion in Chandel 2020a). The three best-characterized sites within the mitochondrial respiratory chain are complexes I, II, and III, located in the inner mitochondrial membrane. These complexes generate O2− by the one-electron reduction of molecular O2. Complexes I, II, and III release O2− into the mitochondrial matrix, where SOD2 rapidly converts it into H2O2. Complex III can also generate O2− and release it into the intermembrane space where the O2− traverses through voltage-dependent anion channels (VDACs) into the cytosol and is converted into H2O2 by SOD1. Complexes I and III are considered major sites of O2− generation (Fig. 9). Other sites within mitochondria known to generate O2− that are gaining biological significance include pyruvate dehydrogenase, α-ketoglutarte dehydrogenase, glycerol 3-phosphate dehydrogenase, and proline dehydrogenase. An important function of mitochondrial-generated O2− and H2O2 is the activation of cellular signaling, which is essential for stem-cell differentiation, tissue morphogenesis, lymphocyte and macrophage activation, and metabolic adaptation under nutrient deprivation. The molecular details of how mitochondrial ROS regulate these diverse biological outcomes are currently being intensely investigated.
Figure 9.
The mitochondrial electron transport chain (ETC) generates ROS. ETC complexes I, II, and III generate O2− superoxide in the mitochondrial matrix that is rapidly converted into H2O2 by SOD2. H2O2 can traverse through the inner and outer mitochondrial membranes to activate cellular signaling. Complex III can also generate O2− superoxide into the intermembrane space where it can traverse through voltage-dependent anion channels (VDACs) into the cytosol or into H2O2 by SOD1 in the intermembrane space. Subsequently H2O2 can efflux into the cytosol to activate signaling.
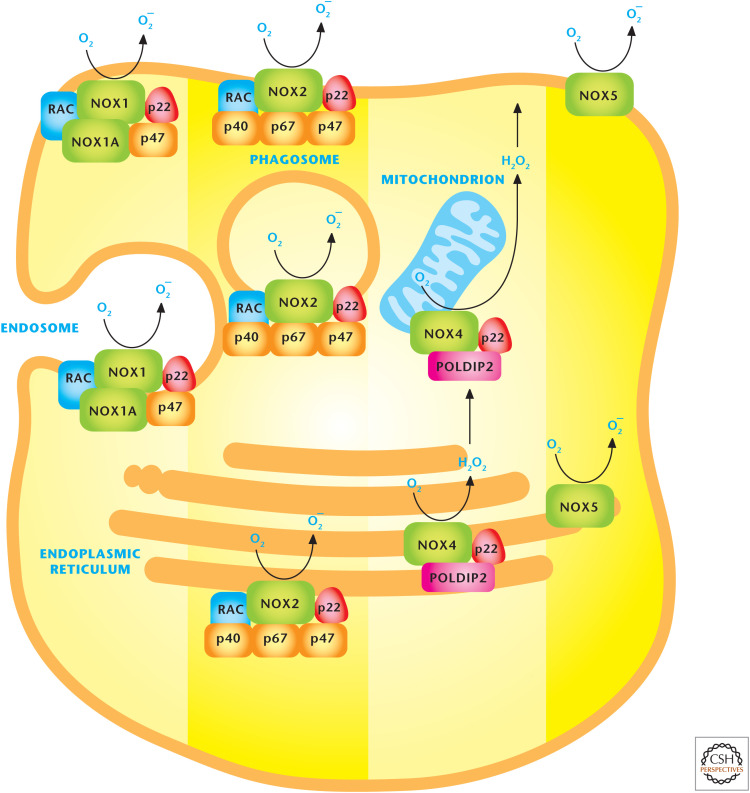
The NOX family of proteins is primarily localized to the plasma membrane; however, these proteins can be found on other membranes, including those of the ER and mitochondria. NOXs have also been implicated in cellular signaling and regulation of multiple diverse biological outcomes, including cell proliferation, cell migration, immune cell activation, and metabolic adaptation. NADPH donates electrons to the center of the NOX catalytic subunit to generate O2− through the one-electron reduction of O2 (Fig. 10). SOD1 in the cytosol converts NOX-generated O2− to H2O2. Seven membrane-bound NOX catalytic isoforms, referred to as NOX1 to NOX5, dual oxidase 1 (DUOX1), and 2 (DUOX2) have been identified with similar but distinct structural and subcellular localization characteristics. NOX catalytic subunits transfer electrons from NADPH across the membranes to molecular oxygen to generate O2−. NOX1, -2, -4, and -5 have been detected in the plasma membrane where they generate O2− into the extracellular space; O2−, subsequently, is rapidly converted into H2O2 by SOD3, which is tethered to the cell surface. NOX4 resides in the ER and mitochondrial and nuclear membranes. The facilitation of electron transport requires the small membrane-bound protein p22phox and various cytosolic NOX-regulatory subunits, which are distinct for each NOX protein. For example, NOX2 oxidase requires p22phox and the recruitment of cytosolic p47phox, p67phox, p40phox, and the GTPase Rac1. In contrast, NOX4 oxidase only has p22phox and a catalytic NOX4 subunit. NOX2 oxidase requires Rac1 to activate it optimally, whereas NOX4 oxidase is activated by the transcriptional induction of the NOX4 subunit. Although the NOX family member knockouts are remarkably normal, they are activated in pathological conditions, making them therapeutic targets. One physiological function of NOX2 in macrophages and neutrophils is bacterial killing. Patients suffering from chronic granulomatous disease, an inherited immune deficiency caused by the absence of one of the components of the NOX2, have recurrent bacterial infections and require antibiotics.
Figure 10.
NADPH oxidases (NOXs) produce ROS. The NOX family of proteins localizes to the plasma, endoplasmic reticulum, and mitochondrial membranes. NOX catalytic subunits transfer electrons from NADPH across the membranes to molecular oxygen to generate O2− superoxide or H2O2 hydrogen peroxide. (Adapted, with permission, from Drummond et al. 2011, © Macmillan.)
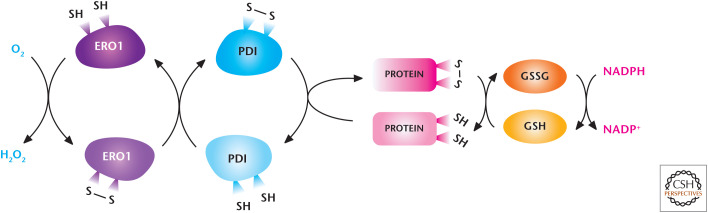
The ER also generates ROS as by-products of protein folding. The ER has a significantly more oxidized environment compared with the cytosol to allow oxidation of cysteine residues in nascent polypeptide chains to form intramolecular disulfide bonds. The enzyme protein disulfide isomerase (PDI) catalyzes this reaction by accepting electrons from thiol residues in the polypeptide chain substrate, leading to its oxidation. PDI becomes reduced and is reoxidized by oxidoreductin 1 (ERO1) (Fig. 11). FAD accepts two electrons from reduced ERO1 to reoxidize and recycle ERO1 for another round of protein disulfide formation. Subsequently, FADH2 donates its two electrons to O2 resulting in regeneration of FAD and H2O2 as the product. Thus, protein oxidation in the ER is interlinked with H2O2 generation. Cells with high needs for de novo protein synthesis, such as cancer cells, generate abundant levels of ER-generated H2O2.
Figure 11.
The endoplasmic reticulum (ER) generates ROS. The oxido-reductases PDI and ERO1 are coupled to generate H2O2 and mediate the disulfide bridge formation and protein folding of proteins. Reduced GSH reduces incorrectly placed disulfide bonds so that the protein can be recycled for correct disulfide bridge formation and folding. (Modified from Bhandary et al. 2013.)
NADPH IS USED TO DETOXIFY ROS
Given the reactivity and toxicity of ROS at high levels and that specific quantities of ROS determine various cellular signaling events, spatial and temporal regulatory strategies must exist to regulate intracellular ROS levels. O2− can damage the iron–sulfur cluster of proteins. Cells have abundant SOD1 and SOD2 to rapidly detoxify O2− to H2O2. There are ample PRXs and GPXs present in the cytosol and mitochondria that convert H2O2 or organic hydroperoxides, such as t-butyl hydroperoxide, to water or the corresponding alcohol. Catalase is abundant in peroxisomes. Thus, nature has insured us against the toxic effects of O2−, H2O2, and hydroperoxides by having abundant enzymes that can detoxify these. It is important to note that although nature has selected to have abundant antioxidants, there are always residual levels of ROS to participate in signal transduction.
Mammals have six PRXs: Prx 1, 2, and 6 are found in the cytoplasm, Prx 3 in the mitochondria, Prx 4 in the ER, and Prx 5 in multiple compartments in the cell, including mitochondria and peroxisomes. PRXs function by undergoing oxidation by H2O2 or organic hydroperoxides at an active-site redox-sensitive cysteine and, then, subsequent reduction by cytosolic and mitochondrial TRX1 and TRX2, cytosolic and mitochondrial TR1 and TR2, and NADPH.
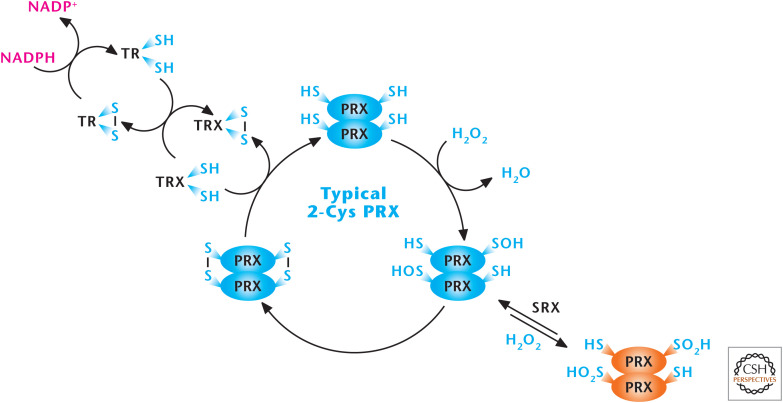
The PRX family of proteins can be divided into three classes (typical 2-Cys, atypical 2-Cys [PRX5], and 1-Cys) with respect to the mechanism by which they decompose H2O2 (Fig. 12). The typical 2-cysteine residue PRXs react with H2O2 to form a SO− intermediate, which condenses with the resolving cysteine of the adjacent PRX to form an intermolecular dimer. The oxidized PRX dimer is reduced by TRX to complete the catalytic cycle. High levels of H2O2 can also react with the SO− intermediate to form a hyperoxidized SO2−. The SO2− form of Prx is slowly reduced back to the native form of the enzyme by sulfiredoxin (SRX). The atypical 2-Cys Prxs have the same mechanism as typical 2-Cys PRXs, but they are functionally monomeric. The redox-sensitive cysteine and its corresponding resolving cysteine are located on the same polypeptide to generate an intermolecular disulfide bond. To recycle the disulfide, known atypical 2-Cys PRXs appear to use Trx as an electron donor. The 1-Cys Prxs contain the redox-sensitive cysteine without a corresponding resolving cysteine. Their mechanism of reacting with peroxide and their subsequent regeneration is not fully understood.
Figure 12.
Peroxiredoxins (PRXs) scavenge H2O2. The peroxiredoxin (PRX1–6) family of proteins can be divided into three classes (typical 2-Cys, atypical 2-Cys [PRX5], and 1-Cys). PRXs function by undergoing oxidation by H2O2 at an active-site redox-sensitive cysteine and then subsequent reduction by cytosolic and mitochondrial thioredoxins (TRX1 and TRX2), cytosolic and mitochondrial thioredoxin reductase (TR1 and TR2), and NADPH. (Modified, with permission, from D'Autréaux and Toledano 2007, © Macmillan.)
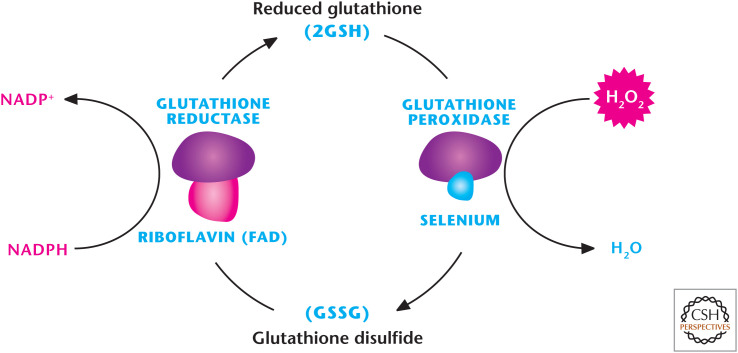
There are eight mammalian GPXs known to catalyze the reduction of H2O2 or organic hydroperoxides to water or the corresponding alcohols, respectively (Fig. 13). Reduced GSH (γ-l-glutamyl-l-cysteinyl-glycine) is used as the reducing agent. Mammalian GPX1–4 are selonproteins with a selenocysteine as the catalytic moiety that allows for a rapid reaction with peroxide or hydroperoxide and fast reducibility by GSH. This generates GSSG, often called oxidized GSH, which is reduced by GSH reductase. GPXs 5–8 use a cysteine residue that participates in detoxifying peroxides. GPX1 is ubiquitously expressed and found in both the cytosol and mitochondria. GPX2 is found in the intestinal epithelium, whereas GPX3 is secreted into plasma. GPX3 is highly expressed in adipose tissues. GPX4 is alternatively spliced and exists in three different isoforms: a cytosolic, a mitochondrial, and sperm nuclear GPX4. GPX5 and GPX6 are abundant in the epididymis and olfactory epithelium, respectively. GPX7 and GPX8 are cysteine-based with low-catalytic activity and presumed to function in the ER.
Figure 13.
Glutathione peroxidase (GPX) detoxifies H2O2. There are eight mammalian GPXs known to catalyze the reduction of H2O2 to H2O by oxidizing GSH to GSSG. GSH reductase reduces GSSH back to GSH by using NADPH as an electron donor.
The function of these antioxidant enzymes is dependent on how fast they react with H2O2 (rate constant) and the expression of enzyme. The expression of these antioxidants is transcriptionally regulated by NRF2 (NF-E2 p45-related factor). PRXs are in high abundance and, therefore, are thought to be responsible for scavenging nanomolar levels of H2O2 associated with signaling. GPXs have higher rate constants than PRXs, but are less abundant and, therefore, are likely only important at higher intracellular concentrations of H2O2 when GPXs can begin to compete with PRXs for substrate. Therefore, it is possible that PRXs are critical for turning ROS signaling off, whereas GPXs are critical for buffering high levels of ROS to bring them to a level at which the cell evades damage and can initiate signaling stress responses.
ARE ELEVATED ROS A CAUSE OR CONSEQUENCE OF PATHOLOGY?
A scientific colleague and friend once quipped, “if you don't have a mechanism just say it is ROS.” Unfortunately, there is some truth to this statement. Many studies use N-acetylcysteine (NAC) as a putative antioxidant to show ROS as the causal agent in their biological system. In fact, over the past couple of decades, aberrant intracellular ROS levels and the cell's inability to clear the oxidants have been implicated in various diseases, including cancer, neurodegenerative disease, cardiovascular disease, diabetes, and gastrointestinal disease (Box 2). But, a critical question here is whether ROS are causal or a consequence of these pathologies. Although the answer to this question is far from being answered, antioxidants are the most widely used or abused drugs worldwide. Perhaps, the most egregious is the overuse of vitamin C to prevent the common cold. The eminent chemist Linus Pauling was infatuated with the notion that megadoses of vitamin C would cure the ills of the world. He took abundant amounts of vitamin C and lived to the ripe old age of 93; he also captured the imagination of the public with his advocacy of vitamin C. However, repeatedly clinical trials have shown no efficacy of vitamin C in fighting the common cold. Yet, the myth continues, even among my own family members. Multiple clinical trials have uniformly failed to show beneficial effects of antioxidants on a variety of pathologies, including cancer. This suggests that either ROS are not causal for pathologies or we have not developed proper antioxidants. Another plausible explanation is that ROS at low levels regulate signaling to control physiological processes and adaptation to stressful conditions and, thus, antioxidants might interfere with our body's way to deal with stressful conditions. There are data to indicate in certain clinical trials that antioxidants have adverse effects, such as the NAC trial in patients suffering from the inflammatory syndrome sepsis caused by bacterial or viral infection. Further understanding of the importance of ROS in normal physiological processes and rationally designed antioxidants that do not undermine normal physiology, but might be effective under pathological conditions, are both needed.
BOX 2.
THE FREE RADICAL THEORY OF AGING
Aging is an inevitable, universal phenomenon observed in all living organisms, yet its cause is open to debate. Some argue that aging is an effect of accumulation of damage to proteins, DNA, and lipids, and others suggest certain genes preprogram aging. In the 1950s, Denham Harman proposed the “mitochondrial free radical theory of aging” as a molecular explanation for why aging occurs. This theory is that free radicals, as by-products of mitochondrial oxidative metabolism, cause cumulative cellular damage that results in overall loss of organismal fitness over time. In subsequent decades, excessive ROS production has been postulated to be a causal agent for a variety of diseases, including diabetes, cancer, inflammatory disorders, and neurodegeneration. If this theory is correct, then increasing antioxidant capacity should increase organismal life span and ameliorate these devastating diseases. Yet, a large number of clinical trials have failed to show beneficial effects of antioxidants for these pathologies, and a handful of trials have suggested that antioxidants increase mortality. Worse, antioxidants have been shown to increase the progression of certain cancers. This indicates that either we have not used the right antioxidants or the mitochondrial free radical theory has some gaps. One possibility is that mitochondrial ROS are required to maintain homeostasis and adaptation to stress. Thus, increasing antioxidant capacity would limit adaptation to stress incurred during aging. Interestingly, recent findings show that low levels of mitochondrial ROS activate stress responses that are beneficial to the organism and extend life span. Going forward, if these findings are consistently upheld, then it would make the public rethink the overuse of antioxidants as antiaging agents.
Footnotes
From the recent volume Navigating Metabolism by Navdeep S. Chandel
Additional Perspectives on Metabolism available at www.cshperspectives.org
SUGGESTED READING
*Reference is also in this collection.
- Berg JM, Tymoczko JL, Stryer L. 2012. Biochemistry, 7th ed. WH Freeman, New York. [Google Scholar]
- Bhandary B, Marahatta A, Kim HR, Chae HJ. 2012. An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int J Mol Sci 14: 434–456. 10.3390/ijms14010434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD. 2010. The sites and topology of mitochondrial superoxide production. Exp Gerontol 45: 466–472. 10.1016/j.exger.2010.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Chandel NS. 2020a. Mitochondria. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a040543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Chandel NS. 2020b. Metabolism of proliferating cells. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a040618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Chandel NS. 2020c. Nucleotide metabolism. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a040592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Chandel NS. 2020d. Signaling and metabolism. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a040600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Autréaux B, Toledano MB. 2007. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol 8: 813–824. 10.1038/nrm2256 [DOI] [PubMed] [Google Scholar]
- Drummond GR, Selemidis S, Griendling KK, Sobey CG. 2011. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov 10: 453–471. 10.1038/nrd3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T. 2011. Signal transduction by reactive oxygen species. J Cell Biol 194: 7–15. 10.1083/jcb.201102095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekimi S, Lapointe J, Wen Y. 2011. Taking a ‘good’ look at free radicals in the aging process. Trends Cell Biol 21: 569–576. 10.1016/j.tcb.2011.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A. 2008. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med 45: 1–17. 10.1016/j.freeradbiomed.2008.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CA, Parker SJ, Fiske BP, McCloskey D, Gui DY, Green CR, Vokes NI, Feist AM, Vander Heiden MG, Metallo CM. 2014. Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol Cell 55: 253–263. 10.1016/j.molcel.2014.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyssiotis CA, Son J, Cantley LC, Kimmelman AC. 2013. Pancreatic cancers rely on a novel glutamine metabolism pathway to maintain redox balance. Cell Cycle 12: 1987–1988. 10.4161/cc.25307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemer J, Bulleid N, Herrmann JM. 2009. Disulfide formation in the ER and mitochondria: two solutions to a common process. Science 324: 1284–1287. 10.1126/science.1170653 [DOI] [PubMed] [Google Scholar]
- Ristow M. 2014. Unraveling the truth about antioxidants: mitohormesis explains ROS-induced health benefits. Nat Med 20: 709–711. 10.1038/nm.3624 [DOI] [PubMed] [Google Scholar]
- Winterbourn CC, Hampton MB. 2008. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med 45: 549–561. 10.1016/j.freeradbiomed.2008.05.004 [DOI] [PubMed] [Google Scholar]