Abstract
Throughout plant development, vascular cells continually form from within a population of seemingly equivalent cells. Vascular cells connect end to end to form continuous strands, and vascular strands connect at both or either end to form networks of exquisite complexity and mesmerizing beauty. Here we argue that experimental evidence gained over the past few decades implicates the plant hormone auxin—its production, transport, perception, and response—in all the steps that lead to the patterned formation of the plant vascular system, from the formation of vascular cells to their connection into vascular networks. We emphasize the organizing principles of the cell- and tissue-patterning process, rather than its molecular subtleties. In the picture that emerges, cells compete for an auxin-dependent, cell-polarizing signal; positive feedback between cell polarization and cell-to-cell movement of the polarizing signal leads to gradual selection of cell files; and selected cell files differentiate into vascular strands that drain the polarizing signal from the neighboring cells. Although the logic of the patterning process has become increasingly clear, the molecular details remain blurry; the future challenge will be to bring them into razor-sharp focus.
AUXIN AND THE PLANT VASCULAR SYSTEM
In most multicellular organisms, water, nutrients, and signals are transported by tissue networks. In animals, this essential transport function is distributed over separate tissue networks—for example, the nervous, circulatory, and respiratory systems. By contrast, in plants, all transport functions are performed by the only tissue network: the vascular system.
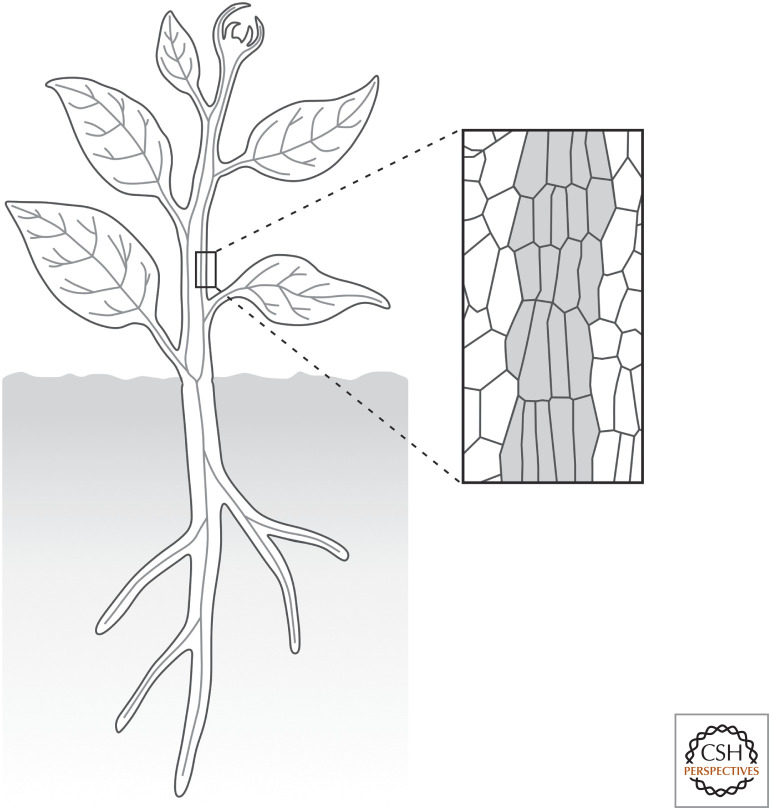
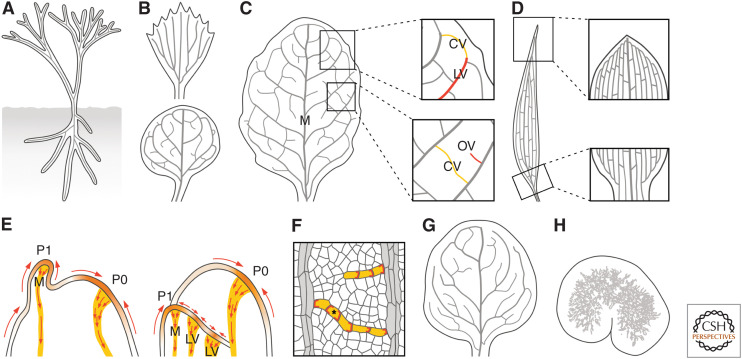
The plant vascular system is a network of vascular strands that connect the different parts of an organ and the different organs of a plant (Fig. 1; Esau 1965). Vascular strands are bundles of files of vascular cells that are elongated along the file and connected at their ends (Fig. 1). Vascular strands are named differently in different organs: veins in flat organs like leaves, petals, sepals, and cotyledons; vascular bundles in the stem; and vascular cylinder or stele in the root.
Figure 1.
The plant vascular system. (Left) The plant vascular system is a network of vascular strands (gray lines) that connect the different parts of an organ and the different organs of a plant. (Right) Vascular strands are bundles of files of vascular cells (gray fill) that are elongated along the file and connected at their ends.
The relation between the plant hormone auxin and vascular development is old (e.g., Kraus et al. 1936) and varied (e.g., Snow 1935; Camus 1949; Jacobs 1952; Wangermann 1967). Here we discuss evidence that suggests that auxin controls the patterned formation of the vascular system at all the system's organization levels: the cells’, the strands’, and the network's. We will emphasize the “syntax” of such control, rather than its “semantics.” This latter has been reviewed recently and comprehensively elsewhere (e.g., Jouannet et al. 2015; De Rybel et al. 2016; Etchells et al. 2016; Cho et al. 2017; Ramachandran et al. 2017; Ruonala et al. 2017; Anne and Hardtke 2018; Fischer et al. 2019; Fukuda and Ohashi-Ito 2019).
AUXIN SIGNALING AND THE FORMATION OF THE FIRST VASCULAR CELLS
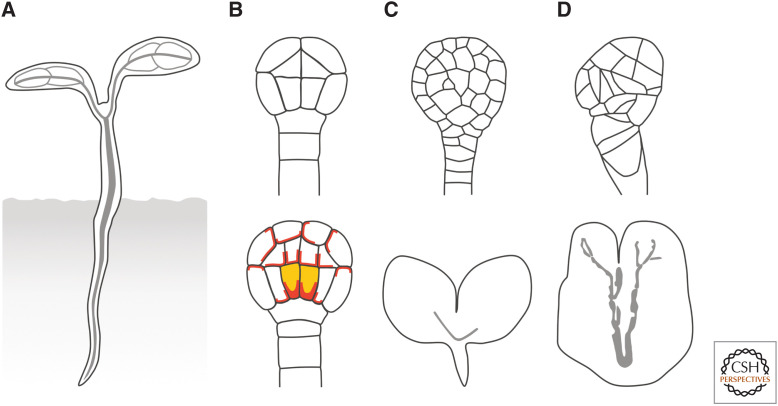
Much of a seedling can be seen as a cylinder with a vascular strand in its center (Fig. 2A). The formation of this cylinder coincides with the formation of the first vascular cells in the Arabidopsis globular embryo (Fig. 2A; Scheres et al. 1994; De Rybel et al. 2014; Yoshida et al. 2014; Gooh et al. 2015). However, expression of vascular-specific markers suggests that the identity of these first vascular cells had been specified earlier, at the dermatogen stage (Fig. 2B; Smit et al. 2020).
Figure 2.
Auxin signaling and the formation of the first vascular cells. (A) Much of the seedling is a cylinder with a vascular strand (gray line) in its center. (B) The localization of PIN1 (red) is polarized in the first vascular cells (yellow fill) of the globular embryo (bottom), which originate from the periclinal, asymmetric division of the lower inner cells in the dermatogen embryo (top). (C) The lower inner cells in the dermatogen embryos of auxin signaling mutants fail to divide periclinally and asymmetrically, leading to vascularless globular embryos (top) and seedlings in which the vascularized cylinder is replaced by a vascularless cone (bottom). (D) Auxin-signaling-unrelated mutants whose cells divide along random planes (top) form vascular strands nonetheless (bottom).
The Arabidopsis dermatogen embryo is composed of 16 cells: eight outer cells, which are the precursors of the epidermis, and eight inner cells, which are the precursors of all the other tissue types (Fig. 2B; Mansfield and Briarty 1991; De Rybel et al. 2014; Yoshida et al. 2014; Gooh et al. 2015). These eight inner cells will divide periclinally and asymmetrically, and the resulting four larger, innermost cells in the basal half of the globular embryo will become the first vascular cells (Fig. 2B; Esau 1965).
Available evidence suggests that the formation of the seedling cylinder and the vascular strand in its center depend on auxin signaling. Indeed, dermatogen embryos of auxin signaling mutants express vascular-specific markers abnormally—if at all—and the eight inner cells in these mutant embryos fail to divide periclinally and asymmetrically and to form the first vascular cells in globular embryos (Fig. 2C; Berleth and Jurgens 1993; Hardtke and Berleth 1998; Hamann et al. 1999, 2002; Hobbie et al. 2000; Hellmann et al. 2003; Dharmasiri et al. 2005; Yoshida et al. 2014; Smit et al. 2020). Most of these mutant embryos develop into seedlings in which the vascularized cylinder is replaced by a vascularless cone (Fig. 2C). This defect characterizes mutants in auxin perception or response but also mutants in auxin production and embryos developed in the presence of auxin antagonists (Hadfi et al. 1998; Dharmasiri et al. 2003, 2007; Cheng et al. 2007; Stepanova et al. 2008; Thomas et al. 2009).
Among such mutants, defects are most severe in mutants lacking the function of the MONOPTEROS/AUXIN RESPONSE FACTOR5 (MP hereafter) gene of Arabidopsis, which encodes a transcription factor that regulates auxin-responsive gene expression (Berleth and Jurgens 1993; Ulmasov et al. 1997, 1999; Hardtke and Berleth 1998; Mattsson et al. 2003), and in mutants with a stabilized variant of the otherwise short-lived MP-inhibitor INDOLE-3-ACETIC ACID12/BODENLOS (Hamann et al. 1999, 2002; Hardtke et al. 2004; Dharmasiri et al. 2005; Weijers et al. 2005, 2006; Schlereth et al. 2010; Lau et al. 2011; Garrett et al. 2012; Krogan et al. 2012). Similar defects also characterize mutants in the EMBRYO DEFECTIVE30/GNOM (GN hereafter) gene of Arabidopsis (Mayer et al. 1993; Shevell et al. 1994; Busch et al. 1996; Yoshida et al. 2014), which encodes a regulator of membrane trafficking that controls auxin signaling through unknown pathways (Mayer et al. 1993; Wolters et al. 2011; Verna et al. 2019).
That these mutants fail to form the first vascular cells seems to result from the inability of the lower inner cells of the dermatogen embryo to divide periclinally and asymmetrically, suggesting that auxin signaling promotes such asymmetric cell division (Hamann et al. 1999; Yoshida et al. 2014). However, vascular cells—although abnormally shaped—still form in mutant embryos in which cells divide along random planes (Fig. 2D; Torres-Ruiz and Jurgens 1994; Yoshida et al. 2014)—random planes that presumably include those which in auxin signaling mutants are correlated with failure to form vascular cells. Furthermore, auxin application to various tissues induces the formation of vascular strands even in the absence of cell division (Fig. 3A; see, e.g., Sinnott and Bloch 1944, 1945; Sachs 1969). Therefore, the vascular-differentiation-promoting influence of auxin does not seem to necessarily depend on its cell-division-orienting activity.
Figure 3.
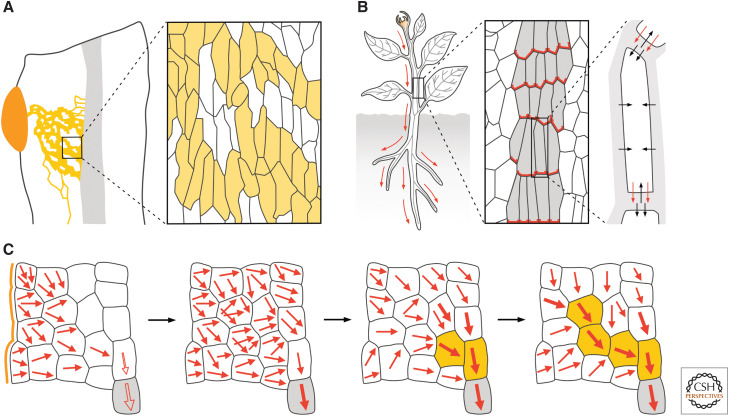
Auxin transport and vascular strand formation. (A) (Left) The application of auxin (orange) to mature plant tissues induces the differentiation of vascular cells in continuous files to form vascular strands (yellow lines) that connect the applied auxin to the preexisting vascular strands (gray fill) basal to the auxin application site. (Right) In the vascular strands formed in response to auxin application, vascular cells (yellow fill) are not aligned along the file like in Figure 1, but along the axis of the tissue. (B) (Left) Auxin (orange) is mainly produced in immature shoot organs and transported (red arrows) toward the roots through vascular strands (gray lines). (Middle) Apicobasal, polar auxin transport results from the localization of auxin efflux carriers of the PIN family (red) at the basal plasma membrane of vascular cells (gray fill). (Right) Auxin efflux carriers are required for auxin to leave the cell (red arrows) because auxin is mostly negatively charged inside the cell; by contrast, in the extracellular space, auxin is to a larger extent uncharged and can thus diffuse into the cell (black arrows). (C) The auxin canalization hypothesis assumes that auxin diffuses from the auxin application site (orange line) toward the preexisting vascular strands in the organ (gray fill). The positive feedback between auxin movement (red arrows) and localization of auxin efflux carriers to the site where auxin leaves the cell would gradually polarize auxin transport (thicker arrows). This would occur first in the cells connected to the preexisting vasculature (gray fill), which are still polarized along the original, apicobasal polarity of the tissue (empty red arrows) and thus orient auxin movement toward themselves. Increased auxin transport polarity, capacity, or velocity in the selected cells would lead to vascular differentiation (yellow fill) and drain auxin from neighboring cells, thus inhibiting their differentiation. The process would continue until a vascular strand formed that connected the applied auxin to the preexisting vascular strands basal to the auxin application site. Black arrows connect successive stages of auxin-induced vascular-strand formation.
AUXIN TRANSPORT AND VASCULAR STRAND FORMATION
The functional unit of the vascular system is the vascular strand, whose formation seems to depend on auxin transport. This conclusion is suggested by the result of experiments in which auxin is applied to mature plant tissues: Auxin application leads to the formation of continuous files of vascular cells that connect the applied auxin to the preexisting vascular strands basal to the auxin application site (Fig. 3A; Kraus et al. 1936; Jost 1942; Jacobs 1952; Sachs 1968; Linh et al. 2018), and this polar formation of vascular strands in response to auxin application is prevented by inhibitors of polar auxin transport (Roberts 1960; Thompson and Jacobs 1966), suggesting that it depends on the ability of the responding tissue to transport auxin polarly.
Indeed, although auxin is mainly produced in immature shoot organs such as leaf and flower primordia (Thimann and Skoog 1934; Avery 1935), it is transported toward the roots through vascular strands (Fig. 3B; Went 1928; Wangermann 1974). This apicobasal, polar auxin transport is thought to result from the localization of auxin efflux carriers at the basal end of auxin-transporting cells (Fig. 3B; Rubery and Sheldrake 1974; Raven 1975). Indeed, the weak acid auxin is mostly negatively charged inside the cell and can thus efficiently leave the cell only through plasma-membrane-localized auxin efflux carriers (Fig. 3B). Although the mechanism of action is still unclear (for review, see Barbosa et al. 2018), available evidence suggests that these auxin efflux carriers are encoded by PIN-FORMED (PIN) genes (for review, see Adamowski and Friml 2015). Computational simulations of this model suggest that it can account for both polar auxin transport (Mitchison 1980b) and the polar formation of vascular strands in response to auxin application, provided that auxin movement through a cell positively feeds back on the localization of auxin efflux carriers to the site where auxin leaves the cell, as proposed by the auxin canalization hypothesis (Sachs 1981, 1991, 2000).
The auxin canalization hypothesis assumes that the applied auxin—or an auxin-dependent signal that is experimentally indistinguishable from auxin—initially diffuses from the auxin application site toward the preexisting vascular strands in the organ (Fig. 3C; Sachs 1981, 1991, 2000). In the cells between the application site and the preexisting vascular strands, auxin efflux carriers would initially be localized to all the different sections of the plasma membrane (i.e., nonpolarly). By contrast, in the preexisting vascular strands, auxin efflux carriers would be localized along the original apicobasal, auxin transport polarity of the tissue. Therefore, the preexisting vascular strands would efficiently remove any auxin that reached them by diffusion from the auxin application site, thereby acting as auxin sinks that orient toward themselves auxin movement in the neighboring cells and polarize the localization of auxin efflux carriers in those same cells (Fig. 3C). The resulting induction of polar auxin transport in the cells neighboring the preexisting vascular strands would be gradually enhanced by the positive feedback between auxin movement and localization of auxin efflux carriers proposed by the auxin canalization hypothesis. By draining auxin increasingly more efficiently and polarly, the cells neighboring the preexisting vascular strands would in turn induce polar localization of auxin efflux carriers and polar auxin transport in the cells above them and inhibit the same processes in the lateral neighbors (Fig. 3C). The repetition of these steps would eventually lead to preferential auxin transport through single cell files—the “canals” the hypothesis refers to—that would connect the applied auxin to the preexisting vascular strands basal to the auxin application site and that would differentiate into vascular strands (Fig. 3C). During this process, any random polarization in the localization of auxin efflux carriers would be stabilized by the positive feedback between auxin movement and localization of auxin efflux carriers proposed by the auxin canalization hypothesis and lead to random deviations in the course of the selected cell files and from the shortest route for auxin transport.
The plasma membrane localization of PIN1 auxin efflux carriers of Arabidopsis marks the presumed auxin efflux side of cells (Petrasek et al. 2006; Wisniewska et al. 2006). Therefore, the polarity of auxin transport can be inferred from the localization of PIN1 to the plasma membrane. Auxin application to mature plant tissues induces the formation of broad expression domains of nonpolarly localized PIN1 that connect the applied auxin to the preexisting vascular strands (Sauer et al. 2006; Mazur et al. 2016). Over time, the broad PIN1-expression domains (PEDs hereafter) become restricted to sites of auxin-induced vascular strand formation, and PIN1 localization becomes polarized: In the cells along the PEDs’ midline, PIN1 localization becomes polarized toward the preexisting vascular strands basal to the site of auxin application; in the cells flanking the PEDs’ midline, PIN1 localization becomes polarized toward the domains' midline. Eventually, narrow PEDs mark the sites of auxin-induced vascular strand formation, and polar PIN1 localization in the vascular strands formed in response to auxin application suggests auxin transport away from the applied auxin and toward the preexisting vascular strands basal to the auxin application site. Both the restriction of broad PEDs and the polarization of PIN1 localization during auxin-induced vascular strand formation initiate and proceed away from the preexisting vascular strands and are consistent with predictions of the auxin canalization hypothesis.
Consistent with predictions of the auxin canalization hypothesis is also the observation that auxin application fails to induce vascular strand formation in pin1 mutants or plants treated with auxin transport inhibitors (Mazur et al. 2020). This observation is, however, unexpected because auxin application to shoot tips of pin1 mutants or wild-type plants grown in the presence of auxin transport inhibitors leads to the formation of whole leaves, including their veins (Reinhardt et al. 2000, 2003). This finding is not the only apparent inconsistency between assumptions or predictions of the auxin canalization hypothesis and experimental observations (Bennett et al. 2014; Runions et al. 2014; Ravichandran et al. 2020). For example, the auxin canalization hypothesis assumes that auxin readily diffuses out of the cells (Sachs 1981), but auxin diffusion out of the cell is unfavored over diffusion into the cell by nearly two orders of magnitude (Runions et al. 2014).
The auxin canalization hypothesis also assumes that cells can measure net auxin transport across their plasma membranes (Sachs 1969). No biological mechanism is known that allows cells to measure net auxin transport directly (Mitchison 1980a, 1981; Kramer 2009; Cieslak et al. 2015). However, cells could use the concentration of auxin, of auxin-bound auxin carriers, or of substances produced or consumed during auxin transport to measure auxin influx and efflux separately, which would indirectly provide a measure of net auxin transport (Mitchison 1980a; Coen et al. 2004; Cieslak et al. 2015). Alternatively, cells could measure intracellular auxin gradients, which depend on auxin transport: Auxin concentration would be higher where auxin enters the cell and lower near auxin efflux carriers, where auxin leaves the cell (Mitchison 1981; Kramer 2009). Even though these mechanisms await experimental testing, they are both plausible; instead, that auxin controls polarization of PIN1 localization by inhibiting PIN1 deallocation from the section of the plasma membrane from which auxin leaves the cell can be ruled out (Paponov et al. 2019).
Finally, the auxin canalization hypothesis assumes that developing vascular strands effortlessly connect to preexisting vascular strands, an assumption that seems to be justified by experimental observations (Sachs 1968) but that cannot be easily reproduced by computational simulations (Bayer et al. 2009; Smith and Bayer 2009). This limitation is overcome in plants that have two PIN1 proteins: one that broadly polarizes auxin transport toward preexisting vascular strands, and the other that restricts the broad auxin transport domains to narrow sites of vascular strand formation (O'Connor et al. 2014, 2017). In plants like Arabidopsis that have only one PIN1 protein, instead, a hypothetical substance has been proposed to diffuse from preexisting vascular strands and polarize PIN1 localization in developing vascular strands toward preexisting vascular strands (Bayer et al. 2009; Smith and Bayer 2009).
Despite the apparent limitations, vascular strand formation in response to auxin application seems to be an expression of auxin transport whose essence is captured by the auxin canalization hypothesis; can we say the same of the vascular strands that form during normal development?
AUXIN TRANSPORT AND VEIN FORMATION
Just like auxin application to mature plant tissues, auxin application to developing leaves leads to the formation of continuous files of vascular cells that connect the applied auxin to the preexisting veins basal to the auxin application site (Scarpella et al. 2006; Sawchuk et al. 2007; Verna et al. 2019). During both auxin-induced and normally occurring vein formation, PIN1 expression is initiated in broad domains of leaf inner cells connected to preexisting veins (Fig. 4A; Scarpella et al. 2006; Wenzel et al. 2007; Marcos and Berleth 2014). In the cells of those broad PEDs, PIN1 is initially nonpolarly localized (Carraro et al. 2006; Scarpella et al. 2006; Wenzel et al. 2007; Lee et al. 2009; Marcos and Berleth 2014; Johnston et al. 2015). Over time, however, the broad PEDs become restricted to sites of vein formation, and PIN1 localization becomes polarized: In the cells along the PEDs’ midline, PIN1 localization becomes polarized toward preexisting veins; in the cells flanking the PEDs’ midline, PIN1 localization becomes polarized toward the domains' midline (Fig. 4A). Both the restriction of broad PEDs and the polarization of PIN1 localization initiate and proceed away from preexisting veins and are delayed by auxin transport inhibition. And both auxin transport inhibition and higher auxin levels lead to the formation of broader PEDs, but given time even these broader PEDs eventually become restricted to sites of vein formation (Aloni et al. 2003; Mattsson et al. 2003; Hay et al. 2006; Scarpella et al. 2006; Wenzel et al. 2007).
Figure 4.
Auxin and vein formation. (A) In rounded leaves, PIN1 localization in epidermal cells of the leaf margin (red arrows) becomes polarized toward sites of leaf lateral growth (orange). Epidermal convergence points of PIN1 polarity are associated with sites of leaf lateral growth and broad PEDs in the inner tissue that become restricted to sites of lateral vein (LV) formation (yellow). Over time, PIN1 localization becomes polarized (red arrows) in the cells of these broad domains: In the cells along the PEDs’ midline, PIN1 localization becomes polarized toward preexisting veins (gray); in the cells flanking PEDs’ midline, PIN1 localization becomes polarized toward the domains' midline. Both the restriction of broad PEDs and the polarization of PIN1 localization initiate and proceed away from preexisting veins (gray), in which PIN1 localization is polarized (red arrows). Broad PEDs in the inner tissue that become restricted to sites of minor vein (MV) formation (yellow) branch from preexisting veins (gray). Over time, PIN1 localization becomes polarized (red arrows) in the cells of these broad domains: In the cells along the PEDs’ midline, PIN1 localization becomes polarized toward preexisting veins (gray); in the cells flanking PEDs’ midline, PIN1 localization becomes polarized toward the domains' midline. Both the restriction of broad PEDs and the polarization of PIN1 localization initiate and proceed away from preexisting veins, in which PIN1 localization is polarized (red arrows). Broad MV-associated PEDs can gradually disappear instead of becoming restricted and polarized. Initially, MV-associated PEDs connect to preexisting veins at one end only (“open” PEDs), but they can extend to connect to preexisting veins at both ends (“closed” PEDs). Open PEDs have a single polarity; closed PEDs have two opposite polarities, which are connected by a “bipolar” cell: a cell in which PIN1 localization is polarized at both ends (see also Fig. 6F). Each vein loop forms from the fusion of LV-associated PEDs and MV-associated closed PEDs. Black arrows connect successive stages of leaf development. (B–D) In wild-type (B) and auxin-transport-inhibited (C) leaves, vascular cells are connected end-to-end and aligned along the vein. By contrast, in leaves in which both auxin transport and auxin signaling are inhibited (D), veins are replaced by clusters of vascular cells that are randomly oriented.
Many of these observations can be accounted for by the positive feedback between auxin movement and localization of auxin efflux carriers proposed by the auxin canalization hypothesis (Mitchison 1980a, 1981; Sachs 1981, 1991, 2000; Rolland-Lagan and Prusinkiewicz 2005). But if the auxin canalization hypothesis were truly to account for vein formation in response to auxin, leaves of pin mutant plants or wild-type plants grown in the presence of auxin transport inhibitors or lacking PIN proteins should form no veins (Rolland-Lagan and Prusinkiewicz 2005). However, this prediction seems to be unsupported by experimental evidence: Leaves of wild-type plants grown in the presence of auxin transport inhibitors do form veins; and as in normally grown plants, the veins of auxin-transport-inhibited plants are oriented along the apicobasal axis of the leaf, and their vascular cells are elongated along the vein and connected at their ends (Fig. 4B,C; Sieburth 1999; Verna et al. 2019). Veins with these same features also form in leaves of Arabidopsis plants lacking all the PIN proteins with vascular function (Verna et al. 2019). Therefore, that auxin-induced vascular-strand formation depends on auxin transport (i.e., the core prediction of the auxin canalization hypothesis) seems to be unsupported by experimental evidence. Moreover, unlike auxin application to mature plant tissues—but like auxin application to shoot apices—pin mutant leaves can still respond to auxin application by forming veins that connect to preexisting veins basal to the auxin application site (Verna et al. 2019). This observation seems to suggest that vein formation in pin mutants still depends on auxin, but how?
AUXIN SIGNALING AND VEIN FORMATION
The residual vein-formation activity in auxin-transport-inhibited leaves turns out to depend, at least in part, on auxin signaling. That auxin signaling controls vein formation has long been known. Indeed, in auxin-signaling-inhibited leaves, fewer veins form, and veins are often incompletely differentiated; yet in those veins, vascular cells are still elongated along the vein and connected at their ends (Przemeck et al. 1996; Hardtke and Berleth 1998; Candela et al. 1999; Alonso-Peral et al. 2006; Strader et al. 2008; Esteve-Bruna et al. 2013; Verna et al. 2019; Mazur et al. 2020). Instead, inhibition of auxin signaling, either because of growth in the presence of auxin signaling inhibitors or because of mutation in auxin receptors or their regulators, leads to entirely new vein defects in auxin-transport-inhibited leaves (Verna et al. 2019).
In the leaves of plants in which both auxin transport and signaling are inhibited, the end-to-end alignment of vascular cells oriented along the vein is replaced by the formation of clusters of randomly oriented vascular cells (Fig. 4D; Verna et al. 2019). Although hypotheses have been proposed (for a recent review, see Ravichandran et al. 2020), it is currently unclear how auxin signaling, which takes place in the nucleus and is thus inherently nonpolar (for review, see Leyser 2018), would control, in the absence of polar auxin transport, the polar propagation of the auxin signal.
AUXIN AND THE FORMATION OF CONTINUOUS VASCULAR STRANDS
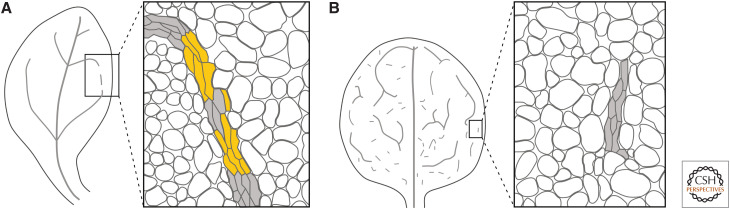
If vascular strand formation depended on the cell-to-cell transport of an auxin-dependent signal, vascular strands would always be “continuous” (i.e., they would connect to other vascular strands at least at one of their two ends). Yet vascular strands that fail to satisfy this requirement—vascular “fragments”—have been observed in leaves of wild types and mutants (e.g., Pray 1955a,b; Lersten 1965; Herbst 1971; Berleth and Jurgens 1993; Carland et al. 1999; Deyholos et al. 2000; Koizumi et al. 2000; Steynen and Schultz 2003; Sawa et al. 2005). Such vascular fragments are of two types.
Vascular fragments of the first type, including those observed in auxin signaling mutants, are composed of files of mature vascular cells that are connected by files of immature vascular cells (Fig. 5A; Pray 1955a,b; Lersten 1965; Herbst 1972; Przemeck et al. 1996; Hardtke and Berleth 1998; Mähönen et al. 2006; Scacchi et al. 2010; Truernit et al. 2012; Ruiz Sola et al. 2017). Because the identification of immature vascular cells can be problematic (Esau 1943), vascular strands of this type have often been interpreted as fragmented when they are instead incompletely differentiated.
Figure 5.
Auxin and the formation of continuous veins. (A) Vein fragments of the first type are composed of files of mature vascular cells (gray fill) that are connected by files of immature vascular cells (yellow fill). (B) Vein fragments of the second type are composed of files of vascular cells (gray fill) that are separated by nonvascular cells (white fill). Vein fragments of this type originate from continuous PEDs within which some cells terminate PIN1 expression and differentiate into nonvascular cells.
Vascular fragments of the second type are composed of files of vascular cells that are separated by nonvascular cells (Fig. 5B; Herbst 1972; Carland et al. 1999; Deyholos et al. 2000; Koizumi et al. 2000). Vascular fragments of this type originate from continuous PEDs within which some cells terminate PIN1 expression and differentiate into nonvascular cells (Scarpella et al. 2006; Naramoto et al. 2009). Therefore, both types of vascular fragments are continuous, at least at formative stages, and thus compatible with a vein-formation mechanism that depends on the cell-to-cell transport of an auxin-dependent signal.
Vascular fragments of the first type have been observed in the leaf and the seedling cylinder (Pray 1955a,b; Lersten 1965; Herbst 1972; Przemeck et al. 1996; Mähönen et al. 2006; Scacchi et al. 2010; Truernit et al. 2012; Rodriguez-Villalon et al. 2014, 2015; Ruiz Sola et al. 2017; Marhava et al. 2018). However, no vascular fragments of the second type have ever been observed in the seedling cylinder, even in those mutants with such vascular fragments in their leaves. This observation suggests that the function of the genes that control the formation of continuous veins in the leaf is not required for the continuity of the vascular strand in the seedling cylinder. But how could that be?
PIN1 localization is already polarized in the first vascular cells that form in the globular embryo (Steinmann et al. 1999). These first vascular cells are stem cells, and as such they continually divide into cells with unequal fates: One cell will maintain the stem cell population; the other will extend the vascular strand in the cylinder of the embryo during embryogenesis and of the seedling root upon germination (Scheres et al. 1994; van den Berg et al. 1997; Aida et al. 2004; Yoshida et al. 2014). Unlike in the embryo, in the leaf PIN1 localization is initially nonpolar, and PIN1-expressing cells do not behave like vascular stem cells. In developing leaves of both wild type and mutants with vascular fragments of the second type, PEDs are initially continuous. In wild type, over time, PIN1 localization becomes polarized (Scarpella et al. 2006; Wenzel et al. 2007; Marcos and Berleth 2014). By contrast, in mutants with vascular fragments of the second type, PIN1 expression is terminated in some of the cells in a PED before PIN1 localization becomes polarized in any of the cells in the domain (Scarpella et al. 2006; Naramoto et al. 2009; Verna et al. 2019). Therefore, it is possible that the function of the genes that control the formation of continuous veins in the leaf is required for the polarization of PIN1 localization, and that it is the lack of such polarization that leads to PED fragmentation. If so, the function of the genes that control the continuity of the veins in the leaf would not be required during the extension of the vascular strand in the embryo and seedling cylinder because polar PIN1 localization would need to be maintained and propagated during such extension and not established like in leaf vein formation. In support of this interpretation, available evidence suggests that distinct mechanisms control the polarization of PIN1 localization and the maintenance of such polar localization (Kleine-Vehn et al. 2011; Łangowski et al. 2016).
AUXIN AND VASCULAR NETWORK FORMATION
Leaf Vein Networks
Just like a seedling can be seen as a vascularized cylinder, early, leafless plants can be seen as two systems of branched vascularized cylinders: one above ground and one below ground (Fig. 6A; Fairon-Demaret and Li 1993). And even flat organs such as leaves can be seen, at least at early stages of their development, as vascularized cylinders (Mattsson et al. 1999; Kang and Dengler 2004; Scarpella et al. 2004). However, during their development, flat-organ primordia soon lose their cylindrical shape by expanding laterally to acquire their distinctive flattened shape, a process that coincides with the formation of branched systems of veins that largely mirror the shape of the leaf (von Ettinghausen 1861; Ash et al. 1999; Dengler and Kang 2001). These vein networks are said to be “open” if all the veins connect to other veins at only one end or “closed” if at least some veins connect to other veins at both ends (Fig. 6B; Roth-Nebelsick et al. 2001; Verna et al. 2015).
Figure 6.
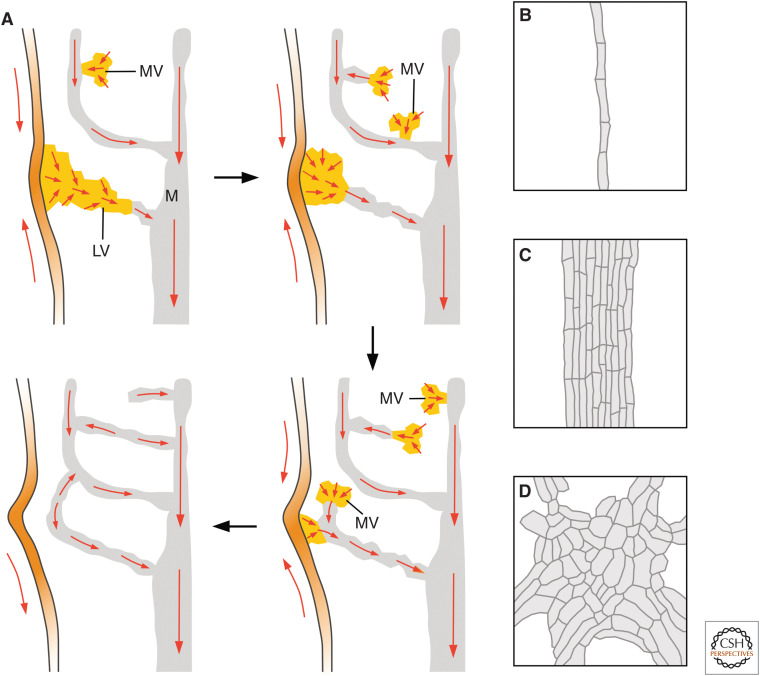
Auxin and vein network formation. (A) Early, leafless plants are systems of branched cylinders with a vascular strand (gray line) in their center. (B) Leaves of extant plants have “open” (top) or “closed” (bottom) vein networks. (C) In rounded leaves, lateral veins (LVs) branch from a single midvein (M); minor veins branch from M and LVs, and either end freely (“open” veins [OVs]; red) or connect to other veins (“closed” veins [CVs]; yellow); and vein loops form from the fusion of LVs (red) and closed minor veins (yellow) (E). (D) In elongated leaves, vein loops are compressed laterally and stretched along the leaf, such that M and LVs seem to run parallel to one another; and M and LVs are connected laterally by minor veins. (E) In epidermal cells at the shoot tip of plants with either rounded (left) or elongated (right) leaves, PIN1 localization becomes polarized (red arrows) toward sites of leaf primordium formation (orange). These epidermal convergence points of PIN1 polarity are associated with broad PEDs in the inner tissue that become restricted to sites of M formation (yellow). Over time, PIN1 localization becomes polarized (red arrows) in the cells of these broad domains: In the cells along the PEDs’ midline, PIN1 localization becomes polarized toward preexisting veins; in the cells flanking PEDs’ midline, PIN1 localization becomes polarized toward the domains' midline. Both the restriction of broad PEDs and the polarization of PIN1 localization initiate and proceed away from preexisting veins, in which PIN1 localization is polarized. P0 and P1 are successive stages of leaf primordium development. (F) The localization of PIN1 (red) in files of vascular cells (yellow) is polarized toward preexisting veins (gray; for simplicity, PIN1 expression in preexisting veins is not shown). In OVs, a single PIN1 localization polarity exists; in CVs, the two opposite PIN1 localization polarities are connected by a bipolar cell (asterisk): a cell with PIN1 at both ends. (G,H) Progressive reduction in the ability to polarize PIN1 localization during vein network formation leads to mutant vein networks with very few CVs (G); mutant vein networks in which vein fragments form along paths defined by initially continuous PEDs (Fig. 5B); or clusters of randomly oriented vascular cells, as in gn mutant leaves (H).
In rounded leaves like those of Arabidopsis, lateral veins branch from a central midvein and connect to distal veins to form vein loops; minor veins branch from midvein and loops, and either end freely or connect to other veins to form a mesh; and loops and minor veins bend near the leaf edge to give the vein network a scalloped outline (Fig. 6C; Troll 1937; Gifford and Foster 1989; Nelson and Dengler 1997). In elongated leaves of grasses like maize, vein loops are compressed laterally and stretched along the leaf, such that midvein and lateral veins seem to run parallel to one another (Fig. 6D; Troll 1937; Gifford and Foster 1989; Nelson and Dengler 1997).
Auxin and the Formation of Open Vein Networks
In both rounded and elongated leaves, localization of PIN1 proteins at the plasma membrane of epidermal cells at the shoot tip becomes polarized toward sites of leaf primordium formation (Fig. 6E; Benková et al. 2003; Reinhardt et al. 2003; Carraro et al. 2006; Scarpella et al. 2006; Bayer et al. 2009; Lee et al. 2009; Johnston et al. 2015). The resulting epidermal “convergence points” of PIN1 polarity are associated with the appearance of broad PEDs in the inner tissue that will become restricted to sites of midvein formation. Likewise, epidermal convergence points of PIN1 polarity at the leaf edge are associated with both sites of leaf lateral growth and positions of broad PEDs in the inner tissue that become restricted to sites of lateral vein formation (Fig. 4A; Hay et al. 2006; Scarpella et al. 2006; Wenzel et al. 2007). Both auxin transport inhibition or higher auxin levels reduce the distance between successive epidermal convergence points of PIN1 polarity and leaf primordia and between midvein and lateral veins (Okada et al. 1991; Bennett et al. 1995; Mattsson et al. 1999; Sieburth 1999; Reinhardt et al. 2000; Scarpella et al. 2006; Guenot et al. 2012; Sawchuk et al. 2013; Verna et al. 2019). However, the relation between convergence points of epidermal PIN1 polarity and positioning of midvein and lateral veins seems to be correlative, rather than causal. Indeed, mutants that lack such convergence points form normal vein networks (Bilsborough et al. 2011), and epidermal expression of PIN1 is neither required nor sufficient for auxin-transport-dependent vein positioning (Govindaraju et al. 2020). These observations suggest that the mechanism that controls the positioning of midvein and lateral veins depends on auxin levels and transport but is independent of epidermal convergence points of PIN1 polarity.
Auxin and the Formation of Closed Vein Networks
Unlike the broad PEDs that become restricted to sites of formation of midvein and lateral veins, those that become restricted to sites of minor vein formation in rounded leaves are not associated with epidermal convergence points of PIN1 polarity; instead, the broad PEDs that become restricted to sites of minor vein formation branch from preexisting veins (Fig. 4A; Scarpella et al. 2006; Wenzel et al. 2007; Marcos and Berleth 2014). Initially, these broad PEDs connect to preexisting veins at only one of their two ends (“open” PEDs), but they can extend to connect to other veins at both their ends (“closed” PEDs) (Fig. 4A; Scarpella et al. 2006; Wenzel et al. 2007; Marcos and Berleth 2014). The formation of such closed PEDs is promoted by auxin transport inhibition (Mattsson et al. 1999; Sieburth 1999; Verna et al. 2015). Because auxin transport inhibition delays restriction of broad PEDs and polarization of PIN1 localization, only PEDs that have yet to differentiate into highly efficient auxin transport paths can connect to preexisting veins at both ends.
But not all broad PEDs become restricted and polarized: Some gradually disappear (Fig. 4A; Marcos and Berleth 2014), suggesting that PEDs compete for a limiting amount of auxin (Sachs 2003). Therefore, how many PEDs will form at any given stage of leaf tissue development, where exactly they will form, how broad they will be, what shape they will have, and which ones will become restricted and polarized and which ones will instead gradually disappear will not only depend on positive feedback between polarization of PIN1 localization and polar auxin transport but on random variations in the local production of auxin and on the number, shape, size, position, and polarization of preexisting PEDs.
Within narrow PEDs—whether open or closed—PIN1 localization is polarized toward preexisting veins (Scarpella et al. 2006; Wenzel et al. 2007; Marcos and Berleth 2014). Therefore, open PEDs have a single PIN1 localization polarity (Fig. 6F). By contrast, closed PEDs are composed of two segments, each with a single PIN1 localization polarity, opposite to that of the other (Fig. 6F). The two opposite polarities are connected by a “bipolar” cell, a cell in which PIN1 localization is polarized at both ends (Fig. 6F).
In rounded leaves, each loop forms from the fusion of a lateral-vein-associated PED and a minor-vein-associated closed PED (Fig. 4A; Scarpella et al. 2006; Wenzel et al. 2007). Whether in elongated leaves PIN1 is expressed and localized during the formation of minor veins and loops as it is in rounded leaves is currently unknown; however, computational simulations suggest that the same vein-formation mechanism embedded in different leaf growth patterns can account for the different vein networks of elongated and rounded leaves (Runions et al. 2005; Fujita and Mochizuki 2006b).
Therefore, available evidence suggests that like vascular strand formation in response to auxin application and vein formation during normal leaf development, vein network formation is an expression of auxin transport, many aspects of which are consistent with the auxin canalization hypothesis (Mitchison 1980a, 1981; Sachs 1991, 2000; Feugier et al. 2005; Rolland-Lagan and Prusinkiewicz 2005; Feugier and Iwasa 2006; Fujita and Mochizuki 2006a; Stoma et al. 2008; Bayer et al. 2009; Smith and Bayer 2009; Alim and Frey 2010; Wabnik et al. 2010; Walker et al. 2013; Lee et al. 2014; Cieslak et al. 2015; Abley et al. 2016). Nevertheless, the auxin canalization hypothesis predicts the formation of networks of “open” veins (i.e., veins that connect to veins at only one of their two ends) (Sachs 1975; Mitchison 1980a, 1981; Rolland-Lagan and Prusinkiewicz 2005). The formation of “closed” veins (i.e., veins that connect to other veins at both their ends) has thus been proposed to result from the diffusion of a hypothetical substance from preexisting veins that allows approaching veins to connect to preexisting veins (Feugier and Iwasa 2006). This hypothesis predicts that in closed PEDs, PIN1 is polarized away from the preexisting veins to which the closed PEDs connect, when in fact in closed PEDs, PIN1 is polarized toward the preexisting veins to which the closed PEDs connect (Fig. 6F; Scarpella et al. 2006; Wenzel et al. 2007; Marcos and Berleth 2014). Therefore, this hypothesis seems to be unsupported by experimental evidence.
Alternatively, closed veins could form from veins meeting at points of peak auxin levels (Dimitrov and Zucker 2006) or from localized auxin production at precisely defined times and places (Sachs 1975, 1989; Mitchison 1980a; Aloni et al. 2003; Rolland-Lagan and Prusinkiewicz 2005; Runions et al. 2005). Both hypotheses are consistent with the observation of bipolar cells and predict peak auxin levels in these cells; however, this prediction remains to be tested experimentally.
The formation of bipolar cells seems to be very sensitive to even the partial loss of function of the auxin-signaling- and auxin-transport-dependent pathway that controls the formation of continuous veins: Mutants partially lacking the function of this pathway often fail to form bipolar cells and thus to polarize PIN1 localization along closed PEDs (Naramoto et al. 2009; Hou et al. 2010). This reduced ability to polarize PIN1 localization along closed PEDs often leads to their “opening” and thus to vein networks with very few closed veins (Fig. 6G; Steynen and Schultz 2003; Naramoto et al. 2009). Nevertheless, these mutants are still able to polarize PIN1 localization along open PEDs (Naramoto et al. 2009; Hou et al. 2010).
More severe loss of function of the pathway that controls the formation of continuous veins leads to the inability to polarize PIN1 localization even along open PEDs (Carland et al. 1999; Deyholos et al. 2000; Koizumi et al. 2000, 2005; Willemsen et al. 2003; Carland and Nelson 2004, 2009; Scarpella et al. 2006; Sieburth et al. 2006; Naramoto et al. 2009). The inability of these mutants to polarize PIN1 localization along PEDs leads to termination of PIN1 expression in some of the cells in a PED before PIN1 localization becomes polarized in any of the cells in the domain (Scarpella et al. 2006; Naramoto et al. 2009). The cells that terminate PIN1 expression differentiate into nonvascular cells, while the remaining PED fragments differentiate into vascular fragments of the second type (Fig. 5B; Carland et al. 1999; Deyholos et al. 2000; Koizumi et al. 2000, 2005; Willemsen et al. 2003; Carland and Nelson 2004, 2009; Scarpella et al. 2006; Sieburth et al. 2006; Naramoto et al. 2009; Tan et al. 2020; Xiao and Offringa 2020). Nevertheless, these vascular fragments still form along the paths where continuous veins would form in wild type, a vestige that these fragments were once connected.
Defects in vein network formation are most severe in gn mutants. In gn cotyledons and leaves, individual cells can still localize PIN1 polarly—although to a lesser extent—but they seem to have almost entirely lost the ability to coordinate between them the polarity of PIN1 localization (Steinmann et al. 1999; Verna et al. 2019). The result of this inability is the formation of clusters of randomly oriented vascular cells (Fig. 6H; Mayer et al. 1993; Steinmann et al. 1999; Geldner et al. 2004; Verna et al. 2019; Amalraj et al. 2020).
Based on their biochemical function and cellular localization, proteins in the pathway that controls the formation of continuous veins have been proposed to localize PIN1 to or retain it in the plasma membrane, polarize PIN1 localization, or maintain such polar localization (e.g., Geldner et al. 2004; Koizumi et al. 2005; Sieburth et al. 2006; Kleine-Vehn et al. 2008; Carland and Nelson 2009; Naramoto et al. 2009, 2010; Prabhakaran Mariyamma et al. 2018). However, the function of these proteins seems to entail more than the control of auxin transport and to include at least the control of auxin signaling. Indeed, defects in mutants in the pathway that controls the formation of continuous veins cannot be phenocopied by mutation in PIN genes or growth in the presence of auxin transport inhibitors; instead, those defects are reproduced in plants in which both auxin transport and signaling are compromised (Verna et al. 2019). Although it is currently unclear how proteins in the pathway that control the formation of continuous veins control auxin signaling, the most parsimonious account is that such proteins control the polar localization of proteins with vein formation function that are produced in response to auxin signaling.
CONCLUDING REMARKS
The past 20 years have witnessed unprecedented advances in our understanding of the role of auxin in the patterned formation of the vascular system; however, the very same research that has led to such advancement has also exposed unexpected gaps in our current knowledge. For example, a role for auxin signaling in vein positioning had been unsuspected because the fewer and incompletely differentiated veins of auxin signaling mutants still form in the same positions as they would in wild type. It now turns out that functions of auxin signaling in vein positioning had been obscured by nonhomologous redundancy with auxin transport. Furthermore, it also turns out that auxin signaling and auxin transport had been eclipsing each other's functions in the end-to-end alignment of vascular cells oriented along the vein. However, how precisely auxin transport and signaling control all those processes remains unclear. These and other questions will have to be addressed in future research, and as past research has taught us, even more surprises are awaiting ahead.
ACKNOWLEDGMENTS
We thank Przemek Prusinkiewicz for helpful comments on the manuscript. We apologize to colleagues whose work could not be included because of space constraints. Our research is supported by Discovery Grants of the Natural Science and Engineering Research Council of Canada (Grants NSERC RGPIN-2016-04736 and NSERC RGPAS 492872-2016).
Footnotes
Editors: Dolf Weijers, Karin Ljung, Mark Estelle, and Ottoline Leyser
Additional Perspectives on Auxin Signaling available at www.cshperspectives.org
REFERENCES
- Abley K, Sauret-Güeto S, Marée AF, Coen E. 2016. Formation of polarity convergences underlying shoot outgrowths. eLife 5: e18165. 10.7554/eLife.18165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamowski M, Friml J. 2015. PIN-dependent auxin transport: action, regulation, and evolution. Plant Cell 27: 20–32. 10.1105/tpc.114.134874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aida M, Beis D, Heidstra R, Willemsen V, Blilou I, Galinha C, Nussaume L, Noh YS, Amasino R, Scheres B. 2004. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 119: 109–120. 10.1016/j.cell.2004.09.018 [DOI] [PubMed] [Google Scholar]
- Alim K, Frey E. 2010. Quantitative predictions on auxin-induced polar distribution of PIN proteins during vein formation in leaves. Eur Phys J E Soft Matter 33: 165–173. 10.1140/epje/i2010-10604-5 [DOI] [PubMed] [Google Scholar]
- Aloni R, Schwalm K, Langhans M, Ullrich CI. 2003. Gradual shifts in sites of free-auxin production during leaf-primordium development and their role in vascular differentiation and leaf morphogenesis in Arabidopsis. Planta 216: 841–853. 10.1007/s00425-002-0937-8 [DOI] [PubMed] [Google Scholar]
- Alonso-Peral MM, Candela H, del Pozo JC, Martinez-Laborda A, Ponce MR, Micol JL. 2006. The HVE/CAND1 gene is required for the early patterning of leaf venation in Arabidopsis. Development 133: 3755–3766. 10.1242/dev.02554 [DOI] [PubMed] [Google Scholar]
- Amalraj B, Govindaraju P, Krishna A, Lavania D, Linh NM, Ravichandran SJ, Scarpella E. 2020. GAL4/GFP enhancer-trap lines for identification and manipulation of cells and tissues in developing Arabidopsis leaves. Dev Dyn 249: 1127–1146. 10.1002/dvdy.181 [DOI] [PubMed] [Google Scholar]
- Anne P, Hardtke CS. 2018. Phloem function and development—biophysics meets genetics. Curr Opin Plant Biol 43: 22–28. 10.1016/j.pbi.2017.12.005 [DOI] [PubMed] [Google Scholar]
- Ash A, Ellis B, Hickey LJ, Johnson K, Wilf P, Wing S. 1999. Manual of leaf architecture. Leaf Architecture Working Group, Smithsonian Institution, Washington, DC. [Google Scholar]
- Avery GS Jr. 1935. Differential distribution of a phytohormone in the developing leaf of nicotiana, and its relation to polarized growth. Bull Torrey Bot Club 62: 313–330. 10.2307/2480818 [DOI] [Google Scholar]
- Barbosa ICR, Hammes UZ, Schwechheimer C. 2018. Activation and polarity control of PIN-FORMED auxin transporters by phosphorylation. Trends Plant Sci 23: 523–538. 10.1016/j.tplants.2018.03.009 [DOI] [PubMed] [Google Scholar]
- Bayer EM, Smith RS, Mandel T, Nakayama N, Sauer M, Prusinkiewicz P, Kuhlemeier C. 2009. Integration of transport-based models for phyllotaxis and midvein formation. Genes Dev 23: 373–384. 10.1101/gad.497009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J. 2003. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602. 10.1016/S0092-8674(03)00924-3 [DOI] [PubMed] [Google Scholar]
- Bennett SRM, Alvarez J, Bossinger G, Smyth DR. 1995. Morphogenesis in pinoid mutants of Arabidopsis thaliana. Plant J 8: 505–520. 10.1046/j.1365-313X.1995.8040505.x [DOI] [Google Scholar]
- Bennett T, Hines G, Leyser O. 2014. Canalization: what the flux. Trends Genet 30: 41–48. 10.1016/j.tig.2013.11.001 [DOI] [PubMed] [Google Scholar]
- Berleth T, Jurgens G. 1993. The role of the monopteros gene in organizing the basal body region of the Arabidopsis embryo. Development 118: 575–587. [Google Scholar]
- Bilsborough GD, Runions A, Barkoulas M, Jenkins HW, Hasson A, Galinha C, Laufs P, Hay A, Prusinkiewicz P, Tsiantis M. 2011. Model for the regulation of Arabidopsis thaliana leaf margin development. Proc Natl Acad Sci 108: 3424–3429. 10.1073/pnas.1015162108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch M, Mayer U, Jurgens G. 1996. Molecular analysis of the Arabidopsis pattern formation of gene GNOM: gene structure and intragenic complementation. Mol Gen Genet 250: 681–691. [DOI] [PubMed] [Google Scholar]
- Camus G. 1949. Recherches sur le role des bourgeons dans les phenomenes de morphogenese [Research on the role of buds in the phenomenon of morphogenesis]. Rev Cytol Biol Veg 11: 1–199. [Google Scholar]
- Candela H, Martı´nez-Laborda A, Micol JL. 1999. Venation pattern formation in Arabidopsis thaliana vegetative leaves. Dev Biol 205: 205–216. 10.1006/dbio.1998.9111 [DOI] [PubMed] [Google Scholar]
- Carland FM, Nelson T. 2004. Cotyledon vascular pattern2-mediated inositol (1,4,5) triphosphate signal transduction is essential for closed venation patterns of Arabidopsis foliar organs. Plant Cell 16: 1263–1275. 10.1105/tpc.021030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carland F, Nelson T. 2009. CVP2- and CVL1-mediated phosphoinositide signaling as a regulator of the ARF GAP SFC/VAN3 in establishment of foliar vein patterns. Plant J 59: 895–907. 10.1111/j.1365-313X.2009.03920.x [DOI] [PubMed] [Google Scholar]
- Carland FM, Berg BL, FitzGerald JN, Jinamornphongs S, Nelson T, Keith B. 1999. Genetic regulation of vascular tissue patterning in Arabidopsis. Plant Cell 11: 2123–2137. 10.1105/tpc.11.11.2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraro N, Forestan C, Canova S, Traas J, Varotto S. 2006. ZmPIN1a and ZmPIN1b encode two novel putative candidates for polar auxin transport and plant architecture determination of maize. Plant Physiol 142: 254–264. 10.1104/pp.106.080119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Dai X, Zhao Y. 2007. Auxin synthesized by the YUCCA flavin monooxygenases is essential for embryogenesis and leaf formation in Arabidopsis. Plant Cell 19: 2430–2439. 10.1105/tpc.107.053009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Dang TV, Hwang I. 2017. Emergence of plant vascular system: roles of hormonal and non-hormonal regulatory networks. Curr Opin Plant Biol 35: 91–97. 10.1016/j.pbi.2016.11.013 [DOI] [PubMed] [Google Scholar]
- Cieslak M, Runions A, Prusinkiewicz P. 2015. Auxin-driven patterning with unidirectional fluxes. J Exp Bot 66: 5083–5102. 10.1093/jxb/erv262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen E, Rolland-Lagan AG, Matthews M, Bangham JA, Prusinkiewicz P. 2004. The genetics of geometry. Proc Natl Acad Sci 101: 4728–4735. 10.1073/pnas.0306308101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengler N, Kang J. 2001. Vascular patterning and leaf shape. Curr Opin Plant Biol 4: 50–56. 10.1016/S1369-5266(00)00135-7 [DOI] [PubMed] [Google Scholar]
- De Rybel B, Adibi M, Breda AS, Wendrich JR, Smit ME, Novák O, Yamaguchi N, Yoshida S, Van Isterdael G, Palovaara J, et al. 2014. Plant development. Integration of growth and patterning during vascular tissue formation in Arabidopsis. Science 345: 1255215. 10.1126/science.1255215 [DOI] [PubMed] [Google Scholar]
- De Rybel B, Mähönen AP, Helariutta Y, Weijers D. 2016. Plant vascular development: from early specification to differentiation. Nat Rev Mol Cell Biol 17: 30–40. 10.1038/nrm.2015.6 [DOI] [PubMed] [Google Scholar]
- Deyholos MK, Cordner G, Beebe D, Sieburth LE. 2000. The SCARFACE gene is required for cotyledon and leaf vein patterning. Development 127: 3205–3213. [DOI] [PubMed] [Google Scholar]
- Dharmasiri S, Dharmasiri N, Hellmann H, Estelle M. 2003. The RUB/Nedd8 conjugation pathway is required for early development in Arabidopsis. EMBO J 22: 1762–1770. 10.1093/emboj/cdg190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, Ehrismann JS, Jürgens G, Estelle M. 2005. Plant development is regulated by a family of auxin receptor F Box proteins. Dev Cell 9: 109–119. 10.1016/j.devcel.2005.05.014 [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Weijers D, Karunarathna N, Jurgens G, Estelle M. 2007. AXL and AXR1 have redundant functions in RUB conjugation and growth and development in Arabidopsis. Plant J 52: 114–123. 10.1111/j.1365-313X.2007.03211.x [DOI] [PubMed] [Google Scholar]
- Dimitrov P, Zucker SW. 2006. A constant production hypothesis guides leaf venation patterning. Proc Natl Acad Sci 103: 9363–9368. 10.1073/pnas.0603559103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esau K. 1943. Origin and development of primary vascular tissues in seed plants. Botanical Review 9: 125–206. 10.1007/BF02872466 [DOI] [Google Scholar]
- Esau K. 1965. Plant anatomy. Wiley, New York. [Google Scholar]
- Esteve-Bruna D, Pérez-Pérez JM, Ponce MR, Micol JL. 2013. Incurvata13, a novel allele of AUXIN RESISTANT6, reveals a specific role for auxin and the SCF complex in Arabidopsis embryogenesis, vascular specification, and leaf flatness. Plant Physiol 161: 1303–1320. 10.1104/pp.112.207779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchells JP, Smit ME, Gaudinier A, Williams CJ, Brady SM. 2016. A brief history of the TDIF-PXY signalling module: balancing meristem identity and differentiation during vascular development. New Phytol 209: 474–484. 10.1111/nph.13642 [DOI] [PubMed] [Google Scholar]
- Fairon-Demaret M, Li CS. 1993. Lorophyton goense gen. et sp. nov. from the Lower Givetian of Belgium and a discussion of the Middle Devonian Cladoxylopsida. Rev Palaeobot Palynol 77: 1–22. 10.1016/0034-6667(93)90052-V [DOI] [Google Scholar]
- Feugier FG, Iwasa Y. 2006. How canalization can make loops: a new model of reticulated leaf vascular pattern formation. J Theor Biol 243: 235–244. 10.1016/j.jtbi.2006.05.022 [DOI] [PubMed] [Google Scholar]
- Feugier FG, Mochizuki A, Iwasa Y. 2005. Self-organization of the vascular system in plant leaves: inter-dependent dynamics of auxin flux and carrier proteins. J Theor Biol 236: 366–375. 10.1016/j.jtbi.2005.03.017 [DOI] [PubMed] [Google Scholar]
- Fischer U, Kucukoglu M, Helariutta Y, Bhalerao RP. 2019. The dynamics of cambial stem cell activity. Annu Rev Plant Biol 70: 293–319. 10.1146/annurev-arplant-050718-100402 [DOI] [PubMed] [Google Scholar]
- Fujita H, Mochizuki A. 2006a. Pattern formation of leaf veins by the positive feedback regulation between auxin flow and auxin efflux carrier. J Theor Biol 241: 541–551. 10.1016/j.jtbi.2005.12.016 [DOI] [PubMed] [Google Scholar]
- Fujita H, Mochizuki A. 2006b. The origin of the diversity of leaf venation pattern. Dev Dyn 235: 2710–2721. 10.1002/dvdy.20908 [DOI] [PubMed] [Google Scholar]
- Fukuda H, Ohashi-Ito K. 2019. Vascular tissue development in plants. Curr Top Dev Biol 131: 141–160. 10.1016/bs.ctdb.2018.10.005 [DOI] [PubMed] [Google Scholar]
- Garrett JJ, Meents MJ, Blackshaw MT, Blackshaw LC, Hou H, Styranko DM, Kohalmi SE, Schultz EA. 2012. A novel, semi-dominant allele of MONOPTEROS provides insight into leaf initiation and vein pattern formation. Planta 236: 297–312. 10.1007/s00425-012-1607-0 [DOI] [PubMed] [Google Scholar]
- Geldner N, Richter S, Vieten A, Marquardt S, Torres-Ruiz RA, Mayer U, Jurgens G. 2004. Partial loss-of-function alleles reveal a role for GNOM in auxin transport-related, post-embryonic development of Arabidopsis. Development 131: 389–400. 10.1242/dev.00926 [DOI] [PubMed] [Google Scholar]
- Gifford EM, Foster AS. 1989. Morphology and evolution of vascular plants. W.H. Freeman, New York. [Google Scholar]
- Gooh K, Ueda M, Aruga K, Park J, Arata H, Higashiyama T, Kurihara D. 2015. Live-cell imaging and optical manipulation of Arabidopsis early embryogenesis. Dev Cell 34: 242–251. 10.1016/j.devcel.2015.06.008 [DOI] [PubMed] [Google Scholar]
- Govindaraju P, Verna C, Zhu T, Scarpella E. 2020. Vein patterning by tissue-specific auxin transport. Development 147: dev187666. 10.1242/dev.187666 [DOI] [PubMed] [Google Scholar]
- Guenot B, Bayer E, Kierzkowski D, Smith RS, Mandel T, Žádníková P, Benková E, Kuhlemeier C. 2012. PIN1-independent leaf initiation in Arabidopsis. Plant Physiol 159: 1501–1510. 10.1104/pp.112.200402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadfi K, Speth V, Neuhaus G. 1998. Auxin-induced developmental patterns in Brassica juncea embryos. Development 125: 879–887. [DOI] [PubMed] [Google Scholar]
- Hamann T, Mayer U, Jurgens G. 1999. The auxin-insensitive bodenlos mutation affects primary root formation and apical-basal patterning in the Arabidopsis embryo. Development 126: 1387–1395. [DOI] [PubMed] [Google Scholar]
- Hamann T, Benkova E, Baurle I, Kientz M, Jurgens G. 2002. The Arabidopsis BODENLOS gene encodes an auxin response protein inhibiting MONOPTEROS-mediated embryo patterning. Genes Dev 16: 1610–1615. 10.1101/gad.229402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke CS, Berleth T. 1998. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J 17: 1405–1411. 10.1093/emboj/17.5.1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke CS, Ckurshumova W, Vidaurre DP, Singh SA, Stamatiou G, Tiwari SB, Hagen G, Guilfoyle TJ, Berleth T. 2004. Overlapping and non-redundant functions of the Arabidopsis auxin response factors MONOPTEROS and NONPHOTOTROPIC HYPOCOTYL 4. Development 131: 1089–1100. 10.1242/dev.00925 [DOI] [PubMed] [Google Scholar]
- Hay A, Barkoulas M, Tsiantis M. 2006. ASYMMETRIC LEAVES1 and auxin activities converge to repress BREVIPEDICELLUS expression and promote leaf development in Arabidopsis. Development 133: 3955–3961. 10.1242/dev.02545 [DOI] [PubMed] [Google Scholar]
- Hellmann H, Hobbie L, Chapman A, Dharmasiri S, Dharmasiri N, del Pozo C, Reinhardt D, Estelle M. 2003. Arabidopsis AXR6 encodes CUL1 implicating SCF E3 ligases in auxin regulation of embryogenesis. EMBO J 22: 3314–3325. 10.1093/emboj/cdg335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst D. 1971. Disjunct foliar veins in Hawaiian Euphorbias. Science 171: 1247–1248. 10.1126/science.171.3977.1247 [DOI] [PubMed] [Google Scholar]
- Herbst D. 1972. Ontogeny of foliar venation in Euphorbia forbesii. Am J Bot 59: 843–850. 10.1002/j.1537-2197.1972.tb10159.x [DOI] [Google Scholar]
- Hobbie L, McGovern M, Hurwitz LR, Pierro A, Liu NY, Bandyopadhyay A, Estelle M. 2000. The axr6 mutants of Arabidopsis thaliana define a gene involved in auxin response and early development. Development 127: 23–32. [DOI] [PubMed] [Google Scholar]
- Hou H, Erickson J, Meservy J, Schultz EA. 2010. FORKED1 encodes a PH domain protein that is required for PIN1 localization in developing leaf veins. Plant J 63: 960–973. 10.1111/j.1365-313X.2010.04291.x [DOI] [PubMed] [Google Scholar]
- Jacobs WP. 1952. The role of auxin in differentiation of xylem around a wound. Am J Bot 39: 301–309. 10.1002/j.1537-2197.1952.tb14277.x [DOI] [Google Scholar]
- Johnston R, Leiboff S, Scanlon MJ. 2015. Ontogeny of the sheathing leaf base in maize (Zea mays). New Phytol 205: 306–315. 10.1111/nph.13010 [DOI] [PubMed] [Google Scholar]
- Jost L. 1942. Über gefässbrücken. Zeitsch Bot 38: 161–215. [Google Scholar]
- Jouannet V, Brackmann K, Greb T. 2015. (Pro)cambium formation and proliferation: Two sides of the same coin? Curr Opin Plant Biol 23: 54–60. 10.1016/j.pbi.2014.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Dengler N. 2004. Vein pattern development in adult leaves of Arabidopsis thaliana. Int J Plant Sci 165: 231–242. 10.1086/382794 [DOI] [Google Scholar]
- Kleine-Vehn J, Dhonukshe P, Sauer M, Brewer PB, Wiśniewska J, Paciorek T, Benková E, Friml J. 2008. ARF GEF-dependent transcytosis and polar delivery of PIN auxin carriers in Arabidopsis. Curr Biol 18: 526–531. 10.1016/j.cub.2008.03.021 [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn J, Wabnik K, Martinière A, Łangowski L, Willig K, Naramoto S, Leitner J, Tanaka H, Jakobs S, Robert S, et al. 2011. Recycling, clustering, and endocytosis jointly maintain PIN auxin carrier polarity at the plasma membrane. Mol Syst Biol 7: 540. 10.1038/msb.2011.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi K, Sugiyama M, Fukuda H. 2000. A series of novel mutants of Arabidopsis thaliana that are defective in the formation of continuous vascular network: calling the auxin signal flow canalization hypothesis into question. Development 127: 3197–3204. [DOI] [PubMed] [Google Scholar]
- Koizumi K, Naramoto S, Sawa S, Yahara N, Ueda T, Nakano A, Sugiyama M, Fukuda H. 2005. VAN3 ARF-GAP-mediated vesicle transport is involved in leaf vascular network formation. Development 132: 1699–1711. 10.1242/dev.01716 [DOI] [PubMed] [Google Scholar]
- Kramer EM. 2009. Auxin-regulated cell polarity: an inside job? Trends Plant Sci 14: 242–247. 10.1016/j.tplants.2009.02.005 [DOI] [PubMed] [Google Scholar]
- Kraus EJ, Brown NA, Hamner KC. 1936. Histological reactions of bean plants to indoleacetic acid. Botanical Gazette 98: 370–420. 10.1086/334646 [DOI] [Google Scholar]
- Krogan NT, Ckurshumova W, Marcos D, Caragea AE, Berleth T. 2012. Deletion of MP/ARF5 domains III and IV reveals a requirement for Aux/IAA regulation in Arabidopsis leaf vascular patterning. New Phytol 194: 391–401. 10.1111/j.1469-8137.2012.04064.x [DOI] [PubMed] [Google Scholar]
- Łangowski L, Wabnik K, Li H, Vanneste S, Naramoto S, Tanaka H, Friml J. 2016. Cellular mechanisms for cargo delivery and polarity maintenance at different polar domains in plant cells. Cell Discov 2: 16018. 10.1038/celldisc.2016.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S, De Smet I, Kolb M, Meinhardt H, Jürgens G. 2011. Auxin triggers a genetic switch. Nat Cell Biol 13: 611–615. 10.1038/ncb2212 [DOI] [PubMed] [Google Scholar]
- Lee BH, Johnston R, Yang Y, Gallavotti A, Kojima M, Travençolo BA, Costa LF, Sakakibara H, Jackson D. 2009. Studies of aberrant phyllotaxy1 mutants of maize indicate complex interactions between auxin and cytokinin signaling in the shoot apical meristem. Plant Physiol 150: 205–216. 10.1104/pp.109.137034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SW, Feugier FG, Morishita Y. 2014. Canalization-based vein formation in a growing leaf. J Theor Biol 353: 104–120. 10.1016/j.jtbi.2014.03.005 [DOI] [PubMed] [Google Scholar]
- Lersten N. 1965. Histogenesis of leaf venation in Trifolium wormskioldii (Leguminosae). Am J Bot 52: 767–774. 10.1002/j.1537-2197.1965.tb07247.x [DOI] [Google Scholar]
- Leyser O. 2018. Auxin signaling. Plant Physiol 176: 465–479. 10.1104/pp.17.00765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linh NM, Verna C, Scarpella E. 2018. Coordination of cell polarity and the patterning of leaf vein networks. Curr Opin Plant Biol 41: 116–124. 10.1016/j.pbi.2017.09.009 [DOI] [PubMed] [Google Scholar]
- Mähönen AP, Bishopp A, Higuchi M, Nieminen KM, Kinoshita K, Tormakangas K, Ikeda Y, Oka A, Kakimoto T, Helariutta Y. 2006. Cytokinin signaling and its inhibitor AHP6 regulate cell fate during vascular development. Science 311: 94–98. 10.1126/science.1118875 [DOI] [PubMed] [Google Scholar]
- Mansfield SG, Briarty LG. 1991. Early embryogenesis in Arabidopsis thaliana. II: The developing embryo. Canadian Journal of Botany 69: 461–476. 10.1139/b91-063 [DOI] [Google Scholar]
- Marcos D, Berleth T. 2014. Dynamic auxin transport patterns preceding vein formation revealed by live-imaging of Arabidopsis leaf primordia. Front Plant Sci 5: 235. 10.3389/fpls.2014.00235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhava P, Bassukas AEL, Zourelidou M, Kolb M, Moret B, Fastner A, Schulze WX, Cattaneo P, Hammes UZ, Schwechheimer C, et al. 2018. A molecular rheostat adjusts auxin flux to promote root protophloem differentiation. Nature 558: 297–300. 10.1038/s41586-018-0186-z [DOI] [PubMed] [Google Scholar]
- Mattsson J, Sung ZR, Berleth T. 1999. Responses of plant vascular systems to auxin transport inhibition. Development 126: 2979–2991. [DOI] [PubMed] [Google Scholar]
- Mattsson J, Ckurshumova W, Berleth T. 2003. Auxin signaling in Arabidopsis leaf vascular development. Plant Physiol 131: 1327–1339. 10.1104/pp.013623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer U, Buttner G, Jurgens G. 1993. Apical-basal pattern formation in the Arabidopsis embryo: studies on the role of the gnom gene. Development 117: 149–162. [Google Scholar]
- Mazur E, Benková E, Friml J. 2016. Vascular cambium regeneration and vessel formation in wounded inflorescence stems of Arabidopsis. Sci Rep 6: 33754. 10.1038/srep33754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur E, Kulik I, Hajný J, Friml J. 2020. Auxin canalization and vascular tissue formation by TIR1/AFB-mediated auxin signaling in Arabidopsis. New Phytol 226: 1375–1383. 10.1111/nph.16446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison GJ. 1980a. Model for vein formation in higher-plants. Proc Biol Sci 207: 79–109. [Google Scholar]
- Mitchison GJ. 1980b. The dynamics of auxin transport. Proc Biol Sci 209: 489–511. [Google Scholar]
- Mitchison GJ. 1981. The polar transport of auxin and vein patterns in plants. Philos Trans R Soc Lond B Biol Sci 295: 461–471. 10.1098/rstb.1981.0154 [DOI] [Google Scholar]
- Naramoto S, Sawa S, Koizumi K, Uemura T, Ueda T, Friml J, Nakano A, Fukuda H. 2009. Phosphoinositide-dependent regulation of VAN3 ARF-GAP localization and activity essential for vascular tissue continuity in plants. Development 136: 1529–1538. 10.1242/dev.030098 [DOI] [PubMed] [Google Scholar]
- Naramoto S, Kleine-Vehn J, Robert S, Fujimoto M, Dainobu T, Paciorek T, Ueda T, Nakano A, Van Montagu MCE, Fukuda H, et al. 2010. ADP-ribosylation factor machinery mediates endocytosis in plant cells. Proc Natl Acad Sci 107: 21890–21895. 10.1073/pnas.1016260107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson T, Dengler N. 1997. Leaf vascular pattern formation. Plant Cell 9: 1121–1135. 10.1105/tpc.9.7.1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor DL, Runions A, Sluis A, Bragg J, Vogel JP, Prusinkiewicz P, Hake S. 2014. A division in PIN-mediated auxin patterning during organ initiation in grasses. PLoS Comput Biol 10: e1003447. 10.1371/journal.pcbi.1003447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor DL, Elton S, Ticchiarelli F, Hsia MM, Vogel JP, Leyser O. 2017. Cross-species functional diversity within the PIN auxin efflux protein family. eLife 6: e31804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y. 1991. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 3: 677–684. 10.2307/3869249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paponov IA, Friz T, Budnyk V, Teale WD, Wüst F, Paponov M, Al-Babili S, Palme K. 2019. Natural auxin does not inhibit Brefeldin A induced PIN1 and PIN2 internalization in root cells. Front Plant Sci 10: 574. 10.3389/fpls.2019.00574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrasek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertova D, Wisniewska J, Tadele Z, Kubes M, Covanova M, et al. 2006. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312: 914–918. 10.1126/science.1123542 [DOI] [PubMed] [Google Scholar]
- Prabhakaran Mariyamma N, Clarke KJ, Yu H, Wilton EE, Van Dyk J, Hou H, Schultz EA. 2018. Members of the Arabidopsis FORKED1-LIKE gene family act to localize PIN1 in developing veins. J Exp Bot 69: 4773–4790. 10.1093/jxb/ery248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pray TR. 1955a. Foliar venation of angiosperms. II: Histogenesis of the venation of Liriodendron. Am J Bot 42: 18–27. 10.1002/j.1537-2197.1955.tb11089.x [DOI] [Google Scholar]
- Pray TR. 1955b. Foliar venation of angiosperms. IV: Histogenesis of the venation of Hosta. Am J Bot 42: 698–706. 10.1002/j.1537-2197.1955.tb10409.x [DOI] [Google Scholar]
- Przemeck GK, Mattsson J, Hardtke CS, Sung ZR, Berleth T. 1996. Studies on the role of the Arabidopsis gene MONOPTEROS in vascular development and plant cell axialization. Planta 200: 229–237. 10.1007/BF00208313 [DOI] [PubMed] [Google Scholar]
- Ramachandran P, Carlsbecker A, Etchells JP. 2017. Class III HD-ZIPs govern vascular cell fate: an HD view on patterning and differentiation. J Exp Bot 68: 55–69. 10.1093/jxb/erw370 [DOI] [PubMed] [Google Scholar]
- Raven JA. 1975. Transport of indole acetic acid in plant cells in relation to pH and electrical potential gradients, and its significance for polar IAA transport. New Phytol 74: 163–172. 10.1111/j.1469-8137.1975.tb02602.x [DOI] [Google Scholar]
- Ravichandran SJ, Linh NM, Scarpella E. 2020. The canalization hypothesis—challenges and alternatives. New Phytol 227: 1051–1059. 10.1111/nph.16605 [DOI] [PubMed] [Google Scholar]
- Reinhardt D, Mandel T, Kuhlemeier C. 2000. Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 12: 507–518. 10.1105/tpc.12.4.507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D, Pesce ER, Stieger P, Mandel T, Baltensperger K, Bennett M, Traas J, Friml J, Kuhlemeier C. 2003. Regulation of phyllotaxis by polar auxin transport. Nature 426: 255–260. 10.1038/nature02081 [DOI] [PubMed] [Google Scholar]
- Roberts LW. 1960. Experiments on xylem regeneration in stem wound responses in coleus. Botanical Gazette 121: 201–208. 10.1086/336070 [DOI] [Google Scholar]
- Rodriguez-Villalon A, Gujas B, Kang YH, Breda AS, Cattaneo P, Depuydt S, Hardtke CS. 2014. Molecular genetic framework for protophloem formation. Proc Natl Acad Sci 111: 11551–11556. 10.1073/pnas.1407337111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Villalon A, Gujas B, van Wijk R, Munnik T, Hardtke CS. 2015. Primary root protophloem differentiation requires balanced phosphatidylinositol-4,5-biphosphate levels and systemically affects root branching. Development 142: 1437–1446. 10.1242/dev.118364 [DOI] [PubMed] [Google Scholar]
- Rolland-Lagan AG, Prusinkiewicz P. 2005. Reviewing models of auxin canalization in the context of leaf vein pattern formation in Arabidopsis. Plant J 44: 854–865. 10.1111/j.1365-313X.2005.02581.x [DOI] [PubMed] [Google Scholar]
- Roth-Nebelsick A, Uhl D, Mosbrugger V, Kerp H. 2001. Evolution and function of leaf venation architecture: a review. Ann Bot 87: 553–566. 10.1006/anbo.2001.1391 [DOI] [Google Scholar]
- Rubery PH, Sheldrake AR. 1974. Carrier-mediated auxin transport. Planta 118: 101–121. 10.1007/BF00388387 [DOI] [PubMed] [Google Scholar]
- Ruiz Sola MA, Coiro M, Crivelli S, Zeeman SC, Schmidt Kjølner Hansen S, Truernit E. 2017. OCTOPUS-LIKE 2, a novel player in Arabidopsis root and vascular development, reveals a key role for OCTOPUS family genes in root metaphloem sieve tube differentiation. New Phytol 216: 1191–1204. 10.1111/nph.14751 [DOI] [PubMed] [Google Scholar]
- Runions A, Fuhrer M, Lane B, Federl P, Rolland-Lagan AG, Prusinkiewicz P. 2005. Modeling and visualization of leaf venation patterns. ACM Trans Graphics 24: 702–711. 10.1145/1073204.1073251 [DOI] [Google Scholar]
- Runions A, Smith RS, Prusinkiewicz P. 2014. Computational models of auxin-driven development. In Auxin and its role in plant development (ed. Zažímalová E. et al. ), pp. 315–357. Springer, Vienna. [Google Scholar]
- Ruonala R, Ko D, Helariutta Y. 2017. Genetic networks in plant vascular development. Annu Rev Genet 51: 335–359. 10.1146/annurev-genet-120116-024525 [DOI] [PubMed] [Google Scholar]
- Sachs T. 1968. On the determination of the pattern of vascular tissues in peas. Ann Bot 32: 781–790. 10.1093/oxfordjournals.aob.a084249 [DOI] [Google Scholar]
- Sachs T. 1969. Polarity and the induction of organized vascular tissues. Ann Bot 33: 263–275. 10.1093/oxfordjournals.aob.a084281 [DOI] [Google Scholar]
- Sachs T. 1975. Control of the differentiation of vascular networks. Ann Bot 39: 197–204. 10.1093/oxfordjournals.aob.a084930 [DOI] [Google Scholar]
- Sachs T. 1981. The control of the patterned differentiation of vascular tissues. Adv Bot Res 9: 151–262. 10.1016/S0065-2296(08)60351-1 [DOI] [Google Scholar]
- Sachs T. 1989. The development of vascular networks during leaf development. Curr Top Plant Biochem Physiol 8: 168–183. [Google Scholar]
- Sachs T. 1991. Cell polarity and tissue patterning in plants. Development 13: 83–93.1769343 [Google Scholar]
- Sachs T. 2000. Integrating cellular and organismic aspects of vascular differentiation. Plant Cell Physiol 41: 649–656. 10.1093/pcp/41.6.649 [DOI] [PubMed] [Google Scholar]
- Sachs T. 2003. Collective specification of cellular development. Bioessays 25: 897–903. 10.1002/bies.10328 [DOI] [PubMed] [Google Scholar]
- Sauer M, Balla J, Luschnig C, Wisniewska J, Reinohl V, Friml J, Benkova E. 2006. Canalization of auxin flow by Aux/IAA-ARF-dependent feedback regulation of PIN polarity. Genes Dev 20: 2902–2911. 10.1101/gad.390806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa S, Koizumi K, Naramoto S, Demura T, Ueda T, Nakano A, Fukuda H. 2005. DRP1A is responsible for vascular continuity synergistically working with VAN3 in Arabidopsis. Plant Physiol 138: 819–826. 10.1104/pp.105.061689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchuk MG, Head P, Donner TJ, Scarpella E. 2007. Time-lapse imaging of Arabidopsis leaf development shows dynamic patterns of procambium formation. New Phytol 176: 560–571. 10.1111/j.1469-8137.2007.02193.x [DOI] [PubMed] [Google Scholar]
- Sawchuk MG, Edgar A, Scarpella E. 2013. Patterning of leaf vein networks by convergent auxin transport pathways. PLoS Genet 9: e1003294. 10.1371/journal.pgen.1003294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scacchi E, Salinas P, Gujas B, Santuari L, Krogan N, Ragni L, Berleth T, Hardtke CS. 2010. Spatio-temporal sequence of cross-regulatory events in root meristem growth. Proc Natl Acad Sci 107: 22734–22739. 10.1073/pnas.1014716108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpella E. 2017. The logic of plant vascular patterning. polarity, continuity and plasticity in the formation of the veins and of their networks. Curr Opin Genet Dev 45: 34–43. 10.1016/j.gde.2017.02.009 [DOI] [PubMed] [Google Scholar]
- Scarpella E, Francis P, Berleth T. 2004. Stage-specific markers define early steps of procambium development in Arabidopsis leaves and correlate termination of vein formation with mesophyll differentiation. Development 131: 3445–3455. 10.1242/dev.01182 [DOI] [PubMed] [Google Scholar]
- Scarpella E, Marcos D, Friml J, Berleth T. 2006. Control of leaf vascular patterning by polar auxin transport. Genes Dev 20: 1015–1027. 10.1101/gad.1402406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres B, Wolkenfelt H, Willemsen V, Terlouw M, Lawson E, Dean C, Weisbeek P. 1994. Embryonic origin of the Arabidopsis primary root and root-meristem initials. Development 120: 2475–2487. [Google Scholar]
- Schlereth A, Möller B, Liu W, Kientz M, Flipse J, Rademacher EH, Schmid M, Jürgens G, Weijers D. 2010. MONOPTEROS controls embryonic root initiation by regulating a mobile transcription factor. Nature 464: 913–916. 10.1038/nature08836 [DOI] [PubMed] [Google Scholar]
- Shevell DE, Leu WM, Gillmor CS, Xia G, Feldmann KA, Chua NH. 1994. EMB30 is essential for normal cell division, cell expansion, and cell adhesion in Arabidopsis and encodes a protein that has similarity to Sec7. Cell 77: 1051–1062. 10.1016/0092-8674(94)90444-8 [DOI] [PubMed] [Google Scholar]
- Sieburth LE. 1999. Auxin is required for leaf vein pattern in Arabidopsis. Plant Physiol 121: 1179–1190. 10.1104/pp.121.4.1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburth LE, Muday GK, King EJ, Benton G, Kim S, Metcalf KE, Meyers L, Seamen E, Van Norman JM. 2006. SCARFACE encodes an ARF-GAP that is required for normal auxin efflux and vein patterning in Arabidopsis. Plant Cell 18: 1396–1411. 10.1105/tpc.105.039008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnott EW, Bloch R. 1944. Visible expression of cytoplasmic pattern in the differentiation of xylem strands. Proc Natl Acad Sci 30: 388–392. 10.1073/pnas.30.12.388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnott EW, Bloch R. 1945. The cytoplasmic basis of intercellular patterns in vascular differentiation. Am J Bot 32: 151–156. 10.1002/j.1537-2197.1945.tb05100.x [DOI] [Google Scholar]
- Smit ME, Llavata-Peris CI, Roosjen M, van Beijnum H, Novikova D, Levitsky V, Sevilem I, Roszak P, Slane D, Jürgens G, et al. 2020. Specification and regulation of vascular tissue identity in the Arabidopsis embryo. Development 147: dev186130. [DOI] [PubMed] [Google Scholar]
- Smith RS, Bayer EM. 2009. Auxin transport-feedback models of patterning in plants. Plant Cell Environ 32: 1258–1271. 10.1111/j.1365-3040.2009.01997.x [DOI] [PubMed] [Google Scholar]
- Snow R. 1935. Activation of cambial growth by pure hormones. New Phytol 34: 347–360. 10.1111/j.1469-8137.1935.tb06853.x [DOI] [Google Scholar]
- Steinmann T, Geldner N, Grebe M, Mangold S, Jackson CL, Paris S, Galweiler L, Palme K, Jurgens G. 1999. Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science 286: 316–318. 10.1126/science.286.5438.316 [DOI] [PubMed] [Google Scholar]
- Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie DY, Doležal K, Schlereth A, Jürgens G, Alonso JM. 2008. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133: 177–191. 10.1016/j.cell.2008.01.047 [DOI] [PubMed] [Google Scholar]
- Steynen QJ, Schultz EA. 2003. The FORKED genes are essential for distal vein meeting in Arabidopsis. Development 130: 4695–4708. 10.1242/dev.00689 [DOI] [PubMed] [Google Scholar]
- Stoma S, Lucas M, Chopard J, Schaedel M, Traas J, Godin C. 2008. Flux-based transport enhancement as a plausible unifying mechanism for auxin transport in meristem development. PLoS Comput Biol 4: e1000207. 10.1371/journal.pcbi.1000207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader LC, Monroe-Augustus M, Bartel B. 2008. The IBR5 phosphatase promotes Arabidopsis auxin responses through a novel mechanism distinct from TIR1-mediated repressor degradation. BMC Plant Biol 8: 41. 10.1186/1471-2229-8-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S, Zhang X, Kong W, Yang XL, Molnár G, Vondráková Z, Filepová R, Petrášek J, Friml J, Xue HW. 2020. The lipid code-dependent phosphoswitch PDK1-D6PK activates PIN-mediated auxin efflux in Arabidopsis. Nat Plants 6: 556–569. 10.1038/s41477-020-0648-9 [DOI] [PubMed] [Google Scholar]
- Thimann KV, Skoog F. 1934. On the inhibition of bud development and other functions of growth substance in Vicia faba. Proc R Soc Lond B 114: 317–339. 10.1098/rspb.1934.0010 [DOI] [Google Scholar]
- Thomas CL, Schmidt D, Bayer EM, Dreos R, Maule AJ. 2009. Arabidopsis plant homeodomain finger proteins operate downstream of auxin accumulation in specifying the vasculature and primary root meristem. Plant J 59: 426–436. 10.1111/j.1365-313X.2009.03874.x [DOI] [PubMed] [Google Scholar]
- Thompson NP, Jacobs WP. 1966. Polarity of IAA effect on sieve-tube and xylem regeneration in Coleus and tomato stems. Plant Physiol 41: 673–682. 10.1104/pp.41.4.673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Ruiz RA, Jurgens G. 1994. Mutations in the FASS gene uncouple pattern formation and morphogenesis in Arabidopsis development. Development 120: 2967–2978. [DOI] [PubMed] [Google Scholar]
- Troll W. 1937. Vergleichende morphologie der höhren pflanzen. Gebrüder Borntraeger, Berlin. [Google Scholar]
- Truernit E, Bauby H, Belcram K, Barthélémy J, Palauqui JC. 2012. OCTOPUS, a polarly localised membrane-associated protein, regulates phloem differentiation entry in Arabidopsis thaliana. Development 139: 1306–1315. 10.1242/dev.072629 [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Hagen G, Guilfoyle TJ. 1997. ARF1, a transcription factor that binds to auxin response elements. Science 276: 1865–1868. 10.1126/science.276.5320.1865 [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Hagen G, Guilfoyle TJ. 1999. Dimerization and DNA binding of auxin response factors. Plant J 19: 309–319. 10.1046/j.1365-313X.1999.00538.x [DOI] [PubMed] [Google Scholar]
- van den Berg C, Willemsen V, Hendriks G, Weisbeek P, Scheres B. 1997. Short-range control of cell differentiation in the Arabidopsis root meristem. Nature 390: 287–289. 10.1038/36856 [DOI] [PubMed] [Google Scholar]
- Verna C, Sawchuk MG, Linh NM, Scarpella E. 2015. Control of vein network topology by auxin transport. BMC Biol 13: 94. 10.1186/s12915-015-0208-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verna C, Ravichandran SJ, Sawchuk MG, Linh NM, Scarpella E. 2019. Coordination of tissue cell polarity by auxin transport and signaling. eLife 8: e51061. 10.7554/eLife.51061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ettinghausen C. 1861. Die Blatt-Skelette der Dikotyledonen. Kais, Kön, Hof, und Staatsdr., Vienna. [Google Scholar]
- Wabnik K, Kleine-Vehn J, Balla J, Sauer M, Naramoto S, Reinöhl V, Merks RM, Govaerts W, Friml J. 2010. Emergence of tissue polarization from synergy of intracellular and extracellular auxin signaling. Mol Syst Biol 6: 447. 10.1038/msb.2010.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker ML, Farcot E, Traas J, Godin C. 2013. The flux-based PIN allocation mechanism can generate either canalyzed or diffuse distribution patterns depending on geometry and boundary conditions. PLoS ONE 8: e54802. 10.1371/journal.pone.0054802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangermann E. 1967. The effect of the leaf on differentiation of primary xylem in the internode of Coleus blumei Benth. New Phytol 66: 747–754. 10.1111/j.1469-8137.1967.tb05442.x [DOI] [Google Scholar]
- Wangermann E. 1974. The pathway of transport of applied indolyl-acetic acid through internode segments. New Phytol 73: 623–636. 10.1111/j.1469-8137.1974.tb01288.x [DOI] [Google Scholar]
- Weijers D, Benkova E, Jäger KE, Schlereth A, Hamann T, Kientz M, Wilmoth JC, Reed JW, Jürgens G. 2005. Developmental specificity of auxin response by pairs of ARF and Aux/IAA transcriptional regulators. EMBO J 24: 1874–1885. 10.1038/sj.emboj.7600659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijers D, Schlereth A, Ehrismann JS, Schwank G, Kientz M, Jürgens G. 2006. Auxin triggers transient local signaling for cell specification in Arabidopsis embryogenesis. Dev Cell 10: 265–270. 10.1016/j.devcel.2005.12.001 [DOI] [PubMed] [Google Scholar]
- Went FW. 1928. Wuchsstoff und wachstum. Rec Trav Bot Néerland 25: 11–16. [Google Scholar]
- Wenzel CL, Schuetz M, Yu Q, Mattsson J. 2007. Dynamics of MONOPTEROS and PIN-FORMED1 expression during leaf vein pattern formation in Arabidopsis thaliana. Plant J 49: 387–398. 10.1111/j.1365-313X.2006.02977.x [DOI] [PubMed] [Google Scholar]
- Willemsen V, Friml J, Grebe M, van den Toorn A, Palme K, Scheres B. 2003. Cell polarity and PIN protein positioning in Arabidopsis require STEROL METHYLTRANSFERASE1 function. Plant Cell 15: 612–625. 10.1105/tpc.008433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewska J, Xu J, Seifertova D, Brewer PB, Ruzicka K, Blilou I, Rouquie D, Benkova E, Scheres B, Friml J. 2006. Polar PIN localization directs auxin flow in plants. Science 312: 883. 10.1126/science.1121356 [DOI] [PubMed] [Google Scholar]
- Wolters H, Anders N, Geldner N, Gavidia R, Jürgens G. 2011. Coordination of apical and basal embryo development revealed by tissue-specific GNOM functions. Development 138: 117–126. 10.1242/dev.059147 [DOI] [PubMed] [Google Scholar]
- Xiao Y, Offringa R. 2020. PDK1 regulates auxin transport and Arabidopsis vascular development through AGC1 kinase PAX. Nat Plants 6: 544–555. 10.1038/s41477-020-0650-2 [DOI] [PubMed] [Google Scholar]
- Yoshida S, Barbier de Reuille P, Lane B, Bassel GW, Prusinkiewicz P, Smith RS, Weijers D. 2014. Genetic control of plant development by overriding a geometric division rule. Dev Cell 29: 75–87. 10.1016/j.devcel.2014.02.002 [DOI] [PubMed] [Google Scholar]