Abstract
Older adults are at high risk of developing cardiovascular disease (CVD). Pre-clinical studies indicate that resveratrol (RSV), a polyphenol commonly found in grapes and red wine, may help prevent development of CVD. Based on our previous reports where the 300mg and 1000mg doses appeared safe and improved psychomotor function in a dose-dependent manner, our hypothesis was that RSV would reduce biomarkers of CVD risk in overweight, but otherwise healthy older adults and that 1000mg would lower CVD biomarkers more than 300mg.
This analysis was performed on samples from older participants (65 years and older) who were randomized to a 90 day RSV treatment with 300mg (n=10), 1000mg (n=9) or placebo (n=10). We measured levels of CVD risk biomarkers i.e. oxidized low-density lipoprotein (oxLDL), soluble E-selectin-1 (sE-selectin), soluble Intercellular Adhesion Molecule-1 (sICAM-1), Soluble Vascular Cell Adhesion Molecule-1 (sVCAM-1), total plasminogen activator inhibitor (tPAI-1). Statistical significance was set at p<0.05.
Both sVCAM-1 and tPAI increased significantly more in the 1000 mg vs. 300 mg and placebo groups. Other biomarkers (300mg vs. 1000mg vs. placebo: oxLDL, sEselectin-1 and sICAM-1) followed the same trend toward higher levels in the 1000mg group compared to the 300mg and placebo groups, without reaching statistical significance.
This pilot project suggests that a higher dose of RSV may increase the levels of CVD risk biomarkers in overweight older adults. Given no change in the CVD risk biomarkers in response to a lower dose, future studies should test the effects of different doses of RSV to evaluate potential detrimental effects of higher doses on CVD biomarkers and measures of cardiovascular function in older adults at risk for CVD.
Keywords: resveratrol, older adults, clinical trial, pilot study, overweight, cardiovascular disease
Background
Age-related increase in the risk for cardiovascular disease (CVD) leads to premature death in older adults [1–3]. Structural changes to the arterial wall such as fibrosis and atherosclerotic plaque formations are indicated as a main cause of CVD and are sustained by the development of age-related increase in oxidative stress and systemic inflammation [4].
Pre-clinical studies indicated that resveratrol (RSV), a polyphenol commonly found in grapes and red wine, may improve cardiovascular health [5–8]. For example, animal studies have shown a positive effect of RSV on protection against ischemia/reperfusion (I/R) injury [5–8] and having anti-atherogenic effects by inhibiting expression of a atherosclerosis development biomarker, intercellular adhesion molecule-1–(ICAM-1), thus suppressing inflammatory mechanisms and delaying the onset of atherosclerosis [5–8].
Although RSV has been shown to improve cardiovascular function and to prevent development of CVD in animals, evidence of its effects on the cardiovascular system in humans is inconclusive. Randomized clinical trials have tested various doses in various populations, doses and treatment durations [9–12], and most of these studies have shown positive effects of RSV. For example, Wong et al. reported improved endothelial function, but not blood pressure in obese adults (40–75 years old) after 6 weeks of supplementation with 75mg of RSV per day [9]. Timmers et al., reported that 150mg of RSV per day for 30 days decreased systolic blood pressure, improved glucose and fat metabolism [13]. In another study, 4-week supplementation with 1000–2000mg of RSV per day in participants with impaired glucose metabolism, resulted in improved insulin sensitivity and post-prandial glucose stabilization [14]. Not all studies however have shown positive effects. For example, van der Made et al., found no change in metabolic risk markers for cardiovascular health (apoA-I, apoB100, HDL, LDL, TG, glucose and insulin), endothelial function and inflammation after 4 weeks of supplementation with 150mg of RSV per day in overweight adults (45–70 years old) [11]
Overweight, but otherwise healthy older adults 65 years and older are at high risk of developing CVD, however, evidence of the effects of RSV treatment on cardiovascular health in this population is currently lacking. Therefore, clinical trials are needed in this more homogenous population at risk for CVD to investigate the potentially beneficial effects of RSV on cardiovascular health in humans, for which we chose blood biomarkers of early atherosclerotic plaque formation. Based on our previous reports where the 300mg and 1000mg doses appeared safe and improved psychomotor function in a dose-dependent manner [15, 16], our hypothesis was that RSV reduces biomarkers of CVD risk in overweight, but otherwise healthy older adults and that 1000mg would lower CVD biomarkers more than 300mg.
Methods
Participants
This study was performed on stored plasma samples of the randomized study of overweight older men and women [15]. In brief, the key inclusion criteria were the following: (1) males and females aged 65 years and above; (2) body mass index (BMI) of 25–34.9 kg/m2); (3) self-reported ability to walk 1 mile and (4) willingness and ability to give informed consent. The methods of this study were published previously and are described elsewhere [15].
RSV supplementation
In brief, participants were randomized into one of three groups (300mg, 1000mg per day or placebo). Participants in both the treatment and placebo conditions were instructed to consume one oral capsule twice daily, immediately following breakfast and dinner. The product was provided by Reserveage Organics and contained Wildcrafted Japanese Knotweed Extract (Polygonum cuspidatum) (root and rhizome) (standardized to contain 500 mg of trans-Resveratrol), Organic French Whole Red Wine Grape (Vitis vinifera) (skin, seeds, fruit, stem, vine), certified Organic Muscadine Whole Red Grape (Vitis rotundifolia) (skin and seed), quercetin and other organic polyphenols. The placebo capsules consisted of microcrystalline cellulose. This study was performed in a double-blind fashion. The full description of the treatment and the products were described previously [15].
Blood collection and analyses
Venous blood was collected at baseline and 90-day follow-up, and it was processed and stored in a −80C frozen for further laboratory analyses.
Specifically, (oxidized low-density lipoprotein (oxLDL), soluble E-selectin-1 (sE-selectin), soluble Intercellular Adhesion Molecule-1 (sICAM-1), Soluble Vascular Cell Adhesion Molecule-1 (sVCAM-1), total plasminogen activator inhibitor (tPAI-1) were measured via a multiplex, magnetic bead-based immunoassay (MILLIPLEX® map; EMD Millipore, Billerica, MA, USA) in plasma. The samples were run in triplicate. The multiplex immunoassay panels were analyzed on a MILLIPLEX® Analyzer 3.1x PONENT System (Luminex®200™) and data analysis performed through the MILLIPLEX® Analyst software. The inter-assay coefficient of variation was <15% [17]. tPAI-1 is mostly produced by adipose tissue and vascular endothelial cells, and is strongly associated with the metabolic syndrome and CVD [18]. Cellular adhesion molecules, sVCAM-1, sICAM-1 and eSelectin-1, are strongly associated with the metabolic syndrome and CVD, and oxLDL is associated with atherosclerotic plaque destabilization [19].
Statistical analyses
The primary outcomes of interest were the changes in the levels of CVD risk biomarkers at Day 90 from baseline. Each outcome was summarized by mean ± standard error. To compare the changes of biomarkers for different groups, an analysis of covariance model was used by adjusting for the baseline measurements. In the analysis of covariance model, partial eta-squared statistics were used to measure the effect size of the difference between the three dose groups. All analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC) and R 3.4.1.
Results
Participants
Samples of thirty-two older (73.2 ± 2.1 yrs.) participants were considered and 29 were available for this analysis. In brief, the main overall participant’s baseline characteristics were as follows: height (164.05 ± 10.52 cm), weight (76.5 ± 15.5 kg), BMI (28.3 ± 4.5 kg/m2), waist circumference (97.9 ± 14.5 cm) resting radial pulse (66.6 ± 9.9 bpm) systolic blood pressure (131.6 ± 13.3 mmHg) and diastolic blood pressure (76.2 ± 9.1 mmHg). These characteristics were not different between the randomized treatment and placebo groups. Demographics, other baseline characteristics and CVD biomarker levels are presented in Table 1. Baseline levels of sVCAM-1 and sE-selectin-1 were significantly higher in the placebo group than in either the 300 mg/day or 1000 mg/day RSV group.
Table 1.
Participant Demographic and Health Information at Baseline.
| Resveratrol | Placebo | P-value | ||
|---|---|---|---|---|
| 300 mg/d (n = 12) | 1000 mg/d (n = 10) | (n = 10) | ||
| Age (years) | 73.17±2.08 | 73.60±2.53 | 73.30±2.06 | 0.97 |
| Caucasian, n (%) | 12 (100%) | 10 (100%) | 9 (90%) | 0.32 |
| Females, n (%) | 6 (50%) | 5 (50%) | 5 (50%) | 1.00 |
| BMI (kg/m2) | 29.84±0.60 | 29.03±1.00 | 29.74±0.62 | 0.88 |
| Mini-Mental State Exam Score | 27.67±0.63 | 28.80±0.29 | 27.70±0.47 | 0.17 |
| Depression Score (CES-D) | 7.33±1.33 | 6.80±2.02 | 6.60±2.04 | 0.75 |
| Excellent or good health, n (%) | 8 (67%) | 9 (90%) | 9 (90%) | 0.27 |
| Hypertension, n (%) | 5 (45%) | 2 (20%) | 7(70%) | 0.08 |
| Baseline blood CVD biomarkers | n=10 | n=9 | n=10 | |
| sVCAM-1 (ng/ml) | 1066.6±251.8 | 953.2±262.1 | 1230.2±209.7 | 0.05 |
| sICAM-1 (ng/ml) | 161.8±38.7 | 146.0±63.6 | 171.0±29.8 | 0.50 |
| sE-selectin-1 (ng/ml) | 26.7±13.2 | 28.0±7.3 | 47.5±23.2 | 0.01 |
| oxLDL (U/L) | 51.0±12.2 | 58.1±13.2 | 51.3±12.8 | 0.41 |
| tPAI-1 (ng/ml) | 102.2±21.2 | 104.0±28.6 | 97.1±32.0 | 0.86 |
CES-D=Center for Epidemiologic Studies Depression; BMI=body mass index; CVD=cardiovascular disease; sICAM-1=soluble Intercellular adhesion molecule-1; oxLDL= oxidized low-density lipoprotein; s E-selectin= soluble e-selectin; tPAI-1=total plasminogen activator inhibitor.
Cardiovascular disease risk biomarkers
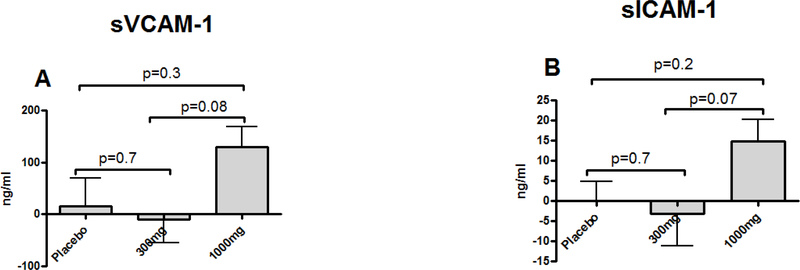
Changes in sVCAM-1 300mg vs. 1000mg vs. placebo: (−10.4±43.8 ng/ml vs. 130.2±38.2 ng/ml vs. 15.1±51.4 ng/ml) and tPAI-1 300mg vs. 1000mg vs. placebo (−1.6±5.1 ng/ml vs. 15.2±3.5 ng/ml vs. 4.9±5.9 ng/ml) indicate significantly higher levels in the 1000mg group compared to the 300mg and placebo groups (Figure 1 B and E). Other biomarkers (300mg vs. 1000mg vs. placebo: oxLDL (−1.2±2.1 vs. 3.1±0.8 vs. 1.5±2.2 U/L), sEselectin-1 (−1.9±3.9 vs. 7.8±6.5 vs. −3.2±6.5 ng/ml) and sICAM-1 (−3.2±7.9 vs. 14.8±5.4 vs. 0.1±4.7 ng/ml) followed the same trend toward higher levels in the 1000mg group compared to the 300mg and placebo groups, without reaching statistical significance (Figure 1 A, C and D). Three-group ANCOVA test comparisons for sVCAM-1, sICAM-1, oxLDL, eSelectin-1, tPAI-1 change from baseline levels between 300mg 1000mg and placebo groups did not reach statistical significance, but the effect sizes (η2) comparing the groups showed the largest effects between the 300 and 1000mg treatment groups for all CVD biomarkers except of sE-selectin-1.
Figure 1.
Comparison of mean differences (90-Day follow-up - baseline) of cardiovascular disease risk biomarkers between RSV treatment groups (300mg and 1000mg) and placebo.
sVCAM-1= soluble vascular cell adhesion molecule-1 (A); sICAM-1=soluble Intercellular adhesion molecule-1 (B); oxLDL= oxidized low-density lipoprotein (C); s E-selectin= soluble e-selectin (D); tPAI-1=total plasminogen activator inhibitor (E). Data presented as mean of difference ± standard error.
Despite the changes being non-significant, there were large effect sizes for sICAM-1, sVCAM-1, oxLDL and tPAI-1, suggesting there is a difference between the treatments and the reason we did not detect statistical significance was because of the small sample size (Table 2).
Table 2.
Cardiovascular disease risk biomarker levels group comparisons between the RSV treatment groups and placebo.
| Sample size | Mean of difference (90-Day - baseline) | 3-Group Comparison | Partial eta-squared | |||||
|---|---|---|---|---|---|---|---|---|
| 300mg | 1000mg | Placebo | 300mg | 1000mg | Placebo | P-value | η2 | |
| sVCAM-1 | 10 | 9 | 10 | −10.4 ± 43.7 | 130.2 ± 38.2 | 15.1 ± 55.4 | 0.209 | 0.118 |
| sICAM-1 | 10 | 9 | 10 | −3.2 ± 7.9 | 14.8 ± 5.4 | 0.1 ± 4.7 | 0.173 | 0.131 |
| oxLDL | 10 | 9 | 10 | −1.2 ± 2.1 | 3.1 ± 2.3 | 1.5 ± 2.2 | 0.232 | 0.110 |
| sESelectin-1 | 10 | 9 | 10 | −1.9 ± 3.9 | 7.8 ± 6.5 | −3.2 ± 6.5 | 0.460 | 0.060 |
| tPAI-1 | 10 | 9 | 10 | −1.6 ± 5.1 | 15.3 ± 3.5 | 4.9 ± 5.9 | 0.074 | 0.188 |
sVCAM-1= soluble vascular cell adhesion molecule-1; sICAM-1=soluble Intercellular adhesion molecule-1; oxLDL= oxidized low-density lipoprotein; s E-selectin= soluble e-selectin; tPAI-1=total plasminogen activator inhibitor; η2= effect size of the difference between the three dose groups; 3-group comparison = analysis of covariance (ANCOVA) model was used by adjusting for the baseline measurements. Data presented as mean of difference ± standard error.
Discussion
In contrast to our hypotheses, the main finding of this pilot study was that 1000 mg/day of RSV elevated two CVD disease risk blood biomarkers (sICAM-1 and tPAI-1), which are known to indicate increased atherosclerosis development [18–20]. Additionally, three other CVD risk biomarkers (oxLDL, sE-selectin and sICAM-1) showed trends to be higher in the 1000 mg/day dose group compared to the 300mg dose group or placebo. However, no detrimental effect has been observed at the 300 mg/day dose. This preliminary evidence suggests that higher doses of RSV may not be beneficial for cardiovascular health in this population of overweight, but otherwise healthy older adults and that further investigation is needed to determine the risks/benefits associated with different doses of RSV for cardiovascular health in this population.
Our results suggest that the 300mg dose did not change the CVD risk biomarkers compared to the 1000mg are in line with the evidence from randomized clinical trials and a potential of lower doses of RSV to have favorable effects. For example, a clinical trial in older patients (65 years and older) with peripheral artery disease reported that a 6-month supplementation with a lower (125mg) and a higher dose (500mg) did not produce statistically significant improvements in physical function (i.e. greater distance in the 6-min walk test), however the lower dose group tended to reach a greater distance post-treatment compared to a higher dose [10]. Although preclinical studies have shown that lower doses of RSV can be beneficial for cardiovascular health and high doses can have negative effects such as cytotoxicity in endothelial cells and increased apoptosis [21], clinical studies however, have not demonstrated negative effects of higher doses of RSV. For example, Crandall et al. reported that 4-weeks of RSV supplementation improved insulin sensitivity and post-prandial glucose control in older adults, but there was no dose-dependent effect on glucose control between the 1000mg, 1500mg 2000mg doses [14]. On the other hand, Pollack et al., found that 6 weeks of RSV supplementation at doses between 2000 mg and 3000mg per day in older adults with impaired glucose regulation may have beneficial effects on vascular function, but not on glucose metabolism or insulin sensitivity. In this study, the 3000mg daily dose in the initial 9 participants was lowered to 2000mg for the rest of the cohort due to gastrointestinal side effects [22].
We have presented the results on CVD biomarkers where the increase in tPAI-1 levels in the 1000mg group was significantly greater than in the 300mg group. In case of sVCAM-1, sICAM-1 and oxLDL the levels were trending to be increased in the 1000mg group, except for E-Selectin-1. The effect sizes were considered large in case of sVCAM-1, sICAM-1, oxLDL and with tPAI-1 having the largest effect size, suggesting the 1000mg dose had a larger effect than the 300mg or placebo in elevating these biomarkers. Although these biomarkers are considered to be associated with the atherosclerotic process, the results should be treated with caution as each of these biomarkers has a specific functions in the atherosclerotic process, which could also be a reason why the changes in some of these biomarkers were not statistically significant [23]. For example, tPAI-1 is categorized as an acute phase reactant, an inflammatory cytokine, and is produced mostly by endothelial cells and is mainly stored in platelets. tPAI-1 is mainly involved in the intravascular fibrinolysis, which promotes the prothrombotic state [24]. Adhesion molecules (sICAM-1 and sVCAM-1) may indicate an extent of endothelial cell activation and oxLDL may be associated with atherosclerotic foam formation [23]. These results warrant further research the effects of different doses of RSV to evaluate potential detrimental effects of higher doses on CVD biomarkers and measures of cardiovascular function in obese, rather healthy older adults.
Our results are novel because we show that a higher dose, which is considered safe in other studies, produced an increase in CVD biomarker levels compared to no change in the lower dose or placebo. However, our results on the increased levels of CVD risk biomarkers need to be treated with caution. Although most literature suggest that increased levels of CVD blood biomarkers are associated with the development of CVD, the higher levels of these biomarkers may also be the result of the tissue repair process and tissue restructuring, during which pro-inflammatory processes and free radical formation are present [25–27]. These results warrant further studies involving objective measures of cardiovascular function to test the potential clinical benefits of higher doses of RSV.
The strengths of this pilot study are: first, there were no differences in baseline characteristics among participants in the three treatment groups; second, the individuals were randomized to one of the treatment groups or placebo in equal numbers; third, this study was conducted in a double-blind fashion; and fourth, we used reliable laboratory measures for cardiovascular biomarker assessments.
This study also had some limitations. Given the pilot nature of the study, the sample size was small. In this limited sample randomization did not equalize the mean baseline levels of the CVD biomarkers and so the results need to be treated with caution despite the fact that the results were statistically corrected for the baseline levels. Additionally, we chose only CVD risk biomarkers that were representative of mostly vascular health and atherosclerosis development, and we did not perform functional measures of the cardiac and vascular function to better assess the clinical value of the RSV treatment. Reported biomarkers of CVD risk are associated with ongoing inflammatory process that may be linked to the reparatory process or to atherosclerosis [25–27]. Although we did not examine the effects of RSV on systemic pro-inflammatory cytokines in the present study, RSV was not found to produce significant reductions in IL-6 and CRP levels in previous clinical trials in our recent meta-analysis of the effects of natural compounds on systemic inflammation [28]. Finally, we cannot rule out the potential effect of the other non-RSV compounds, such as Wildcrafted Japanese Knotweed Extract (Polygonum cuspidatum), Organic French Whole Red Wine Grape (Vitis vinifera), certified Organic Muscadine Whole Red Grape (Vitis rotundifolia), quercetin and other organic polyphenols, which were present in the study product. It is worth noting, however, that these other compounds were present in a very low amount compared to RSV.
Conclusions
This study suggests that higher dose of RSV increases the levels of CVD risk biomarkers in overweight, otherwise healthy older adults. These results should be interpreted with caution due to a pilot nature of this study and the unclear meaning of elevated CVD risk blood biomarkers. These results also suggest that no detrimental effects have been observed in response to the 300mg does, and there may be a level (not determined by this study) whereby RSV has detrimental effects on cardiovascular function. We cannot rule out that presence of other non-RSV compounds in a higher dose could have contributed to the adverse effects that we observed.
We suggest further investigation of lower and higher doses of RSV on measures of cardiovascular function, such as cardiac contractility, endothelial function and arterial stiffness, to better understand the clinical value of RSV on improving cardiovascular health in older adults.
Future directions
Although we need to treat our results with caution due to a small sample size, our preliminary evidence suggests that future studies warrant testing multiple doses of RSV to investigate an optimal dose-range for lowering CVD risk biomarkers in older adults at risk for CVD. Additionally, in order to test the clinical value of different lower doses of RSV, we would include baseline and follow-up non-invasive cardiovascular functional measures such as echocardiography, endothelial function and arterial stiffness.
Clinical Implications
Although biomarkers have shown persistent elevations when using the high dose of RSV, this may also be indicative of endovascular repair. The results of this study in overweight, generally healthy seniors are applicable in daily medical practice in terms of physicians being conscious about potential adverse effects of higher doses of RSV. These results, however, are not a clear-cut dismissal of the high RSV doses as a recommended supplement for older patients. Specifically, the clinical relevance of both lower and higher doses should be evaluated by using clinical measures known to be directly related to cardiovascular health, such as heart function by echocardiography and ambulatory 24-hour blood pressure. Based on the findings of this pilot study, we suggest clinicians should be cautious about recommending higher doses of RSV (1000 mg/day or higher) to patients with characteristics of the metabolic syndrome and CVD risk in older age, such as central obesity, dysglycemia, dyslipidemia and arterial hypertension.
Acknowledgement
Support was provided by the University of Florida’s McKnight Brain Institute, Claude D. Pepper Older Americans Independence Center (NIH/NIA P30AG028740), and Clinical and Translational Science Institute (NIH/NCRR UL1TR000064). Robert Mankowski is supported by the American Heart Association Career Development Award (18CDA34080001). The product for this trial was provided by Reserveage Organics.
Footnotes
Conflict of interest: none
References
- 1.Camici GG, et al. , Molecular mechanism of endothelial and vascular aging: implications for cardiovascular disease. Eur Heart J, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Kaku K, et al. , Age-related normal range of left ventricular strain and torsion using three-dimensional speckle-tracking echocardiography. J Am Soc Echocardiogr, 2014. 27(1): p. 55–64. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein BH, et al. , Usefulness of peripheral vascular function to predict functional health status in patients with Fontan circulation. Am J Cardiol, 2011. 108(3): p. 428–34. [DOI] [PubMed] [Google Scholar]
- 4.Gurovich AN, et al. , Flow-mediated dilation is associated with endothelial oxidative stress in human venous endothelial cells. Vasc Med, 2014. 19(4): p. 251–256. [DOI] [PubMed] [Google Scholar]
- 5.Zheng H, et al. , The effects of age and resveratrol on the hypoxic preconditioning protection against hypoxia-reperfusion injury: studies in rat hearts and human cardiomyocytes. Eur J Cardiothorac Surg, 2015. 48(3): p. 375–81. [DOI] [PubMed] [Google Scholar]
- 6.Marzetti E, et al. , Cellular mechanisms of cardioprotection by calorie restriction: state of the science and future perspectives. Clin Geriatr Med, 2009. 25(4): p. 715–32, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng L, et al. , Resveratrol attenuates inflammation and oxidative stress induced by myocardial ischemia-reperfusion injury: role of Nrf2/ARE pathway. Int J Clin Exp Med, 2015. 8(7): p. 10420–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Rimbaud S, et al. , Resveratrol improves survival, hemodynamics and energetics in a rat model of hypertension leading to heart failure. PLoS One, 2011. 6(10): p. e26391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong RH, et al. , Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J Hypertens, 2013. 31(9): p. 1819–27. [DOI] [PubMed] [Google Scholar]
- 10.McDermott MM, et al. , Effect of Resveratrol on Walking Performance in Older People With Peripheral Artery Disease: The RESTORE Randomized Clinical Trial. JAMA Cardiol, 2017. 2(8): p. 902–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Made SM, Plat J, and Mensink RP, Resveratrol does not influence metabolic risk markers related to cardiovascular health in overweight and slightly obese subjects: a randomized, placebo-controlled crossover trial. PLoS One, 2015. 10(3): p. e0118393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magyar K, et al. , Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin Hemorheol Microcirc, 2012. 50(3): p. 179–87. [DOI] [PubMed] [Google Scholar]
- 13.Timmers S, et al. , Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab, 2011. 14(5): p. 612–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crandall JP, et al. , Pilot study of resveratrol in older adults with impaired glucose tolerance. J Gerontol A Biol Sci Med Sci, 2012. 67(12): p. 1307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anton SD, et al. , Safety and metabolic outcomes of resveratrol supplementation in older adults: results of a twelve-week, placebo-controlled pilot study. Exp Gerontol, 2014. 57: p. 181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anton SD, et al. , Effects of 90 Days of Resveratrol Supplementation on Cognitive Function in Elders: A Pilot Study. J Altern Complement Med, 2018. 24(7): p. 725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marzetti E, et al. , Patterns of circulating inflammatory biomarkers in older persons with varying levels of physical performance: a partial least squares-discriminant analysis approach. Front Med (Lausanne), 2014. 1: p. 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kressel G, et al. , Systemic and vascular markers of inflammation in relation to metabolic syndrome and insulin resistance in adults with elevated atherosclerosis risk. Atherosclerosis, 2009. 202(1): p. 263–71. [DOI] [PubMed] [Google Scholar]
- 19.Koenig W and Khuseyinova N, Biomarkers of atherosclerotic plaque instability and rupture. Arterioscler Thromb Vasc Biol, 2007. 27(1): p. 15–26. [DOI] [PubMed] [Google Scholar]
- 20.Mathew M, Tay E, and Cusi K, Elevated plasma free fatty acids increase cardiovascular risk by inducing plasma biomarkers of endothelial activation, myeloperoxidase and PAI-1 in healthy subjects. Cardiovasc Diabetol, 2010. 9: p. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukherjee S, Dudley JI, and Das DK, Dose-dependency of resveratrol in providing health benefits. Dose Response, 2010. 8(4): p. 478–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollack RM, et al. , Resveratrol Improves Vascular Function and Mitochondrial Number but Not Glucose Metabolism in Older Adults. J Gerontol A Biol Sci Med Sci, 2017. 72(12): p. 1703–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armstrong EJ, Morrow DA, and Sabatine MS, Inflammatory biomarkers in acute coronary syndromes: part I: introduction and cytokines. Circulation, 2006. 113(6): p. e72–5. [DOI] [PubMed] [Google Scholar]
- 24.Cesari M, Pahor M, and Incalzi RA, Plasminogen activator inhibitor-1 (PAI-1): a key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc Ther, 2010. 28(5): p. e72–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rainer PP, et al. , Cardiomyocyte-specific transforming growth factor beta suppression blocks neutrophil infiltration, augments multiple cytoprotective cascades, and reduces early mortality after myocardial infarction. Circ Res, 2014. 114(8): p. 1246–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serhan CN, et al. , Resolution of inflammation: state of the art, definitions and terms. FASEB J, 2007. 21(2): p. 325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sansbury BE and Spite M, Resolution of Acute Inflammation and the Role of Resolvins in Immunity, Thrombosis, and Vascular Biology. Circ Res, 2016. 119(1): p. 113–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Custodero C, et al. , Evidence-based nutritional and pharmacological interventions targeting chronic low-grade inflammation in middle-age and older adults: A systematic review and meta-analysis. Ageing Res Rev, 2018. 46: p. 42–59. [DOI] [PMC free article] [PubMed] [Google Scholar]