Abstract
Aging-related changes to biological structures such as cardiovascular and musculoskeletal systems contribute to the development of comorbid conditions including cardiovascular disease and frailty, and ultimately lead to premature death. Although, frail older adults often demonstrate both cardiovascular and musculoskeletal comorbidities, the etiology of sarcopenia, and especially the contribution of cardiovascular aging is unclear. Aging-related vascular calcification is prevalent in older adults and is a known risk factor for cardiovascular disease and death. The effect vascular calcification has on function during aging is not well understood. Emerging findings suggest vascular calcification can impact skeletal muscle perfusion, negatively affecting nutrient and oxygen delivery to skeletal muscle, ultimately accelerating muscle loss and functional decline. The present review summarizes existing evidence on the biological mechanisms linking vascular calcification with sarcopenia during aging.
Keywords: Aging, skeletal muscle, inflammation, oxidative stress, biology of aging
Introduction
The world’s population is rapidly aging and the number of adults 65 years and older is projected to double by 2050 1. Given the aging society, frailty, a common geriatric syndrome of increased vulnerability to adverse outcomes including falls, dementia and physical disability 2, has become an important health issue 3. One of the most important factors for developing frailty is sarcopenia, a condition defined as a gradual loss of muscle mass, strength and function, assigned the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) code (M62.84) 4. Muscle mass has been reported to decline at an annual rate of 1–2% after the age of 50, with muscle strength decreasing by 1.5% per year between 50 and 60 years of age, and 3% per year thereafter 5,6. There are multiple age-related factors present in sarcopenic older adults such as denervated motor units 7, hormonal changes 8, inflammation 9, oxidative stress 10, decline in physical activity and malnutrition 11. However, there is a lack of consensus on the pathophysiological mechanism of the development of sarcopenia 12,13.
Older adults who demonstrate skeletal muscle loss, mostly have co-existing cardiovascular disease such as hypertension 14. In some studies, vascular calcification, as one of the CVD risk factors, was negatively associated with grip strength 15,16, but not with muscle mass 15,17. Age-related risk factors such as inflammation, oxidative stress, hypercholesterolemia, calcium deposition, hypertension, diabetes, renal disease and physical inactivity are associated with worsening vascular calcification as well as the loss of muscle function, strength and mass 18,19. Previous reports have shown a relationship between skeletal muscle and vascular pathology. For example, arterial stiffness was associated with limited flow volume in lower 20 and upper extremities 21, lower muscle mass 22,23 and physical function 15–17,24–28. Higher abdominal aortic calcification was also negatively associated with lower abdominal lean muscle 26,24 and positively associated with truncal fat mass 27. In the Melbourne Collaborative Cohort Study 24, lower lean muscle mass, especially in non-obese individuals, was related to the presence and severity of abdominal aortic calcification, and older women with severe abdominal aortic calcification showed a decline in handgrip strength, but not in the appendicular lean mass 15. The authors assumed that aortic calcification may be related to neuromuscular factors, which represent muscle function, rather than muscle atrophy based on the data where vascular calcification was related to muscle strength, but not with muscle mass 15.
To date, besides the clinical cross-sectional and observational studies, a pathophysiological mechanism on how vascular calcification contributes to skeletal muscle atrophy and functional loss is unclear. It is an important topic to investigate because if vascular dysfunction precedes and leads to the loss of skeletal muscle, interventional studies would target modifiable features of the vasculature to improve its function and prevent the loss of skeletal muscle in older adults. Therefore, in this article, we aim to review the pathophysiological mechanisms contributing to age-related vascular and skeletal muscle dysfunction and the available biological mechanisms through which vascular calcification can contribute to sarcopenia. The novel hypothesis is that endothelial dysfunction contributes to sarcopenia by increasing arterial calcification that restricts blood flow and muscle perfusion, which attenuate substrate delivery to skeletal muscle and contributes to atrophy and loss of function.
Literature search
A literature search for this review was conducted via the PubMed database for English-language publications on pre-clinical and clinical studies using the terms “vascular calcification” AND “skeletal muscle” (103 articles), “vascular calcification” AND “sarcopenia” OR “muscle strength” (19 articles). Two authors independently performed the database searches, screened potential studies, and reviewed the data. Discrepancies were resolved by consensus. The main focus of this article was to find relevant research articles exploring the mechanistic link between vascular calcification and sarcopenia including changes of muscle mass and function. Due to the limited literature evidence, we did not apply an age criterion in the selection process.
Biological factors contributing to vascular calcification
Vascular calcification has two categories according to the location in the vessel (mostly arteries) i.e. intimal and medial calcification. Atherosclerotic intimal calcification occurs at the innermost layer (tunica intima) 29,30. In response to the increasing shear stress during aging the intimal wall becomes thicker 31. The shear stress as a mechanical strain on the vascular wall induces an inflammatory process that leads to an atherosclerotic plaque formation and blood flow restriction with increased vulnerability to a thrombotic event 32,29. The medial calcification occurs in the middle layer (tunica media), which is composed of vascular smooth muscle cells (VSMCs), elastin, and collagen 33. VSMCs have two main functions such as contraction and synthesis of extracellular matrix proteins 34. Medial calcification is caused by elastic fiber mineralization, degeneration and osteogenic process in the VSMCs 29. Below, we specify the biological mechanisms leading to vascular calcification.
Oxidative Stress
Reactive oxygen species (ROS) are chemically reactive molecules containing oxygen with oxidizing capabilities 35. Physiological levels of ROS regulate cell growth and differentiation, but in excess as oxidative stress, well-known as an age-related factor, have detrimental effects such as senescence and apoptosis 36,37.
Oxidative stress results in endothelial cell apoptosis, monocyte adhesion, and inactivation of nitric oxide (NO), which enhances endothelium-dependent vasorelaxation 38. Apoptotic bodies, calcifying membrane bound matrix vesicles that result from apoptosis, could become a site of calcification in blood vessels 39. Oxidative stress also involves disturbance of inorganic phosphate homeostasis resulting in vascular calcification through promotion of the p65 nuclear translocation 40. Hyperphosphatemia-induced VSMCs senescence and osteochondrogenesis result in calcification of VSMCs 41. Hyperphosphatemia and calcium-phosphate products cause a progressive increase in calcium deposition in arteries 42.
Generation of ROS is central to progression of inflammation in blood vessels 43. For example, ROS activate the nuclear factor kappa-light-chain-enhancer of activated B cell (NF-κβ) 44, a transcription factor, which increases proinflammatory cytokine production 45.
Inflammation
Inflammation plays a detrimental role in the vascular calcification process 46–53. For example, inflammatory cells such as macrophages release cytokines such as interleukin (IL)-1, IL-6, and tumor necrosis factor alpha (TNF)-α, which induce VSMCs apoptosis and osteogenic differentiation resulting in mineral deposition in arterial plaques 54. Circulatory inflammatory cytokines contribute to increasing calcification through TNF-α and calcium phosphate 54. In particular, internalization of calcium phosphate crystals into vacuoles of macrophages trigger secretion of inflammatory cells such as TNF-α and IL-6 54. In the pro-inflammatory state, TNF-α reduces the level of matrix Gla-protein (MGP), which is an inhibitor of vascular mineralization secreted by chondrocytes and VSMCs in the arterial tunica media 55. TNF-α activates the bone morphogenetic protein (BMP)-2, a potent bone anabolic factor in VSMCs contributing to vascular calcification 56. In in vitro studies, TNF-α induced differentiation of VSMCs to osteoblast-like morphology enhancing matrix mineralization 57, and increasing calcium deposits 58,59. TNF-α also stimulates IL-6 production 49. IL-6 is also involved in inhibiting MGP 50,51, which results in activation of BMP-2, which increases osteogenesis and contributes to arterial calcification 47,48.
Hormonal Dysregulation
Chronic inflammation associated with aging is also related to insulin resistance, defined as a decrease in tissue response to hormonal insulin stimulation 60. Insulin resistance causes reduction in NO bioavailability and increases generation of ROS, which result in oxidative stress and endothelial dysfunction 61. Insulin resistance is also associated with glucotoxicity, lypotoxicity, and inflammation that also contribute to endothelial dysfunction 62.
Dysregulation of other hormone levels during aging affects vascular walls as well. For example, estrogen enhances the production of the transforming growth factor-β (TGF-β), which has antioxidant and anti-TNF-α effects, leading to suppression of ROS over-production as well as has anti-inflammatory effects 63,64. Estrogen was also reported to prevent oxidative stress in ovariectomized rats through an increase in NO synthesis, which mediated vasodilation 65.
Growth hormone (GH) has been reported to increase inducible nitric oxide synthase (NOS) expression, which catalyzes the production of NO from L-arginine 66. NO showed an inhibitory effect on calcification of VSMCs through the TGF-β signaling 66. GH replacement therapy showed an improvement of endothelial function and reduction of oxidative stress 67 through increased NO release in endothelial cell 68. The effect of GH on improved endothelial function is mediated by the insulin like growth factor-1 (IGF-1) 69,70. Chronic inflammation during aging reduced expression of IGF-1 in VSMCs in a rat model 71, and decreased expression of IGF-1 triggers VSMCs’ apoptosis resulting in atherosclerosis, plaque instability, and rupture 72.
Contribution of vascular calcification to the development of sarcopenia
A common cause of vascular calcification is endothelial dysfunction induced by chronic inflammation and oxidative stress 73, which leads to endothelial cell apoptosis, monocyte adhesion, and decreased production of NO 38. NO is reported to regulate blood flow to skeletal muscle at both rest and during dynamic exercise, thus inactivation of NO could impair the blood flow to the muscle 74. Inflammation and oxidative stress-induced microcalcification and hormonal dysregulation such as insulin resistance may be mechanistically contributing factors to skeletal muscle atrophy by reduced capillary microcirculation, reduced nutrient and oxygen delivery, and thus impaired muscle protein synthesis 75, increased protein breakdown 76, mitochondrial dysfunction and apoptosis 77.
Skeletal muscle is a major site that absorbs glucose through the insulin-responsive glucose transporter type 4 (GLUT4), and decreased expression of GLUT4 in insulin resistance reduces glucose uptake from the blood stream 78,79. Therefore, sufficient available capillary surface area of trans-endothelial transport of insulin is needed 80. Insulin is delivered into the capillaries and crosses the endothelial barrier 80. In support of this, obese male mice treated with high-fat diet had a 15% reduction in endothelial insulin transport, which was associated with a 45% reduction in the density of endothelial vesicles (putative vehicles for the endothelial insulin transport) in skeletal muscle capillaries that increased systemic hyperinsulinemia, which is a cardiometabolic risk factor 81.
A well-functioning endothelium is essential for insulin-induced endothelial nitric oxide synthase (eNOS) phosphorylation to increase NO bioavailability, and thus capillary perfusion 80,82. In mice, reduction of eNOS phosphorylation reduced insulin-induced glucose uptake by skeletal muscle, and restored phosphorylation of eNOS in the endothelial cells improved capillary recruitment and perfusion 82. Endothelial dysfunction with intimal calcification could disturb this process through attenuation of capillary recruitment and reduced insulin delivery 83. Insulin resistance lowered suppression of phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling leading to activation of caspase-3 and the ubiquitin-proteasome proteolytic pathway causing muscle protein degradation 84. Decreased uptake of glucose from the capillaries decreases the energy production by lower adenosine triphosphate (ATP) production, lipid oxidation 85, and protein synthesis in skeletal muscle 85,86. Additionally, capillary rarefaction and lower angiogenesis with endothelial dysfunction and microcalcification impair the capillary diffusion capacity of oxygen, nutrients and hormones to skeletal muscle during contractions 87. Lower angiogenesis during aging is due to reduced expression of the vascular endothelial growth factor (VEGF) and lower sensitivity of the endothelial cells to VEGF due to the dysregulated nicotinamide adenine nucleotide (NAD) metabolome 88,89. Due to vascular inflammation, one of the causes of endothelial dysfunction and vascular calcification, cytokines stimulate lymph production (lymphangiogenesis) for the removal of tissue fluid and inflammatory cells 90. However, aging-related lymphatic dysfunction due to low NO in the lymphatic vessels leads to poor drainage, which causes fluid retention (edema) and accumulation of inflammatory cells, and thus a persisting pro-inflammatory process 91,92.
Dysregulation of other hormones, diminished during aging, may be detrimental for muscle metabolism and contractile performance by modulating blood flow to and within the skeletal muscle 93. For example, in older mice with restricted blood supply, IGF-1 expression was reduced in skeletal muscle 94, which could diminish a protective effect of IGF-1 against age-related loss of muscle mass 95. In particular, binding of IGF-1 on its receptor in skeletal muscle 96 activates the PI3K/Akt pathway 97. Akt stimulates protein synthesis through the mammalian target of rapamycin (mTOR) 97. Akt inactivates phosphorylation of the forkhead box transcription factor (FoxO) resulting in a decrease of proteolysis 98. Through these mechanisms, GH and IGF-1 stimulate protein synthesis and attenuate muscle breakdown, and decreased GH and IGF-1 levels during aging have negative impact on muscle mass 99.
Additionally, testosterone and estrogen induce relaxation of vessels through increased NO production through the PI3K/Akt pathway 100 and improved endothelial function 101, thus this effect is impaired with aging-related decline of these hormones. In systematic reviews and meta-analyses, testosterone showed strong effects on maintaining muscle mass and a modest to minimal effects on muscle strength and physical performance, especially with low serum levels 102. For example, diminished endogenous production of testosterone in mice by orchidectomy induced suppression of the IGF-1/Akt pathway resulting in a reduction of muscle mass and function 103. When treated with testosterone, IGF-1/Akt pathway was activated and restored muscle mass and function 103. Loss of estrogen in female mice lead to a decrease in skeletal muscle satellite cells and lower muscle regeneration 104. Others reported that reduction of estrogen might cause a reduction of skeletal muscle IGF-1 in ovariectomized rats and it was reversed by an estrogen treatment 105. Estrogen replacement therapy reversed ovariectomy-induced muscle contractile and myosin dysfunction in mice 106–108. Although, testosterone and estrogen have an effect on both vascular and skeletal muscle function, they have not been studied together to investigate a mediating role of the vascular dysfunction inducing skeletal muscle dysfunction.
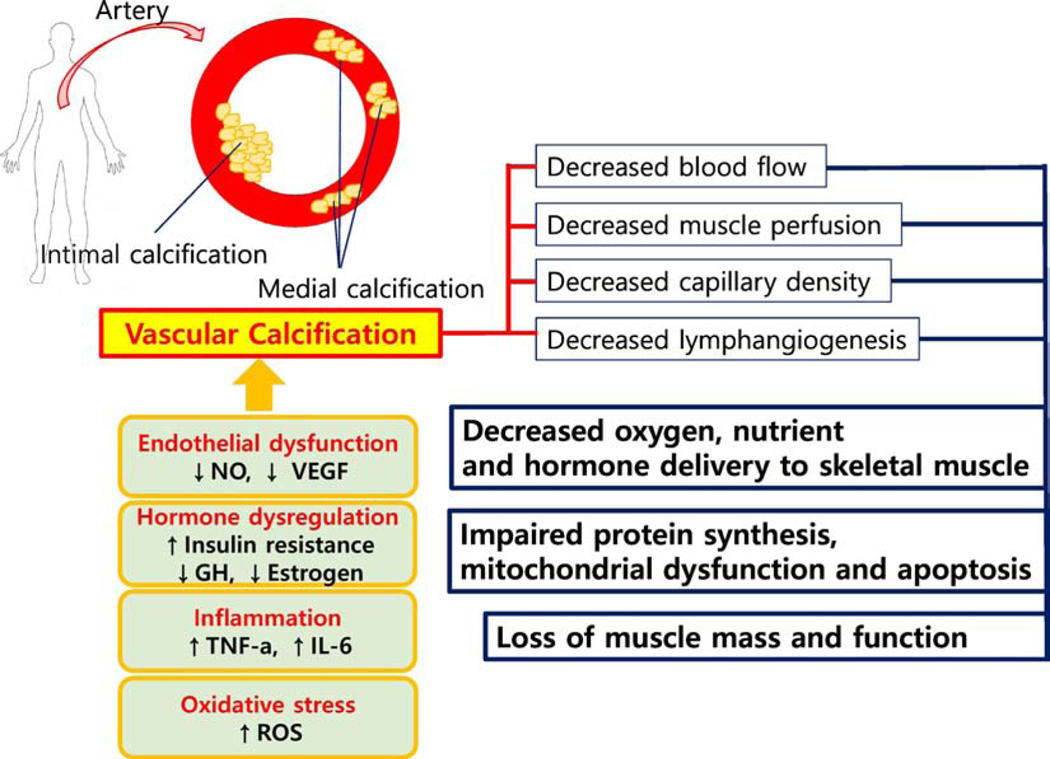
Taken together, based on this growing body of evidence, vascular impairments due to age-related inflammation, oxidative stress and dysregulated hormonal system impair endothelial function leading to arterial calcification in the peripheral and skeletal muscle capillaries that restrict the delivery of substrates needed for muscle regeneration and hypertrophy. Figure 1 demonstrates a simplified conceptual model of vascular calcification contributing to sarcopenia.
Figure 1. Schematic interaction between Vascular calcification and Muscular Atrophy.
NO: nitric oxide, VEGF: vascular endothelial growth factor, ROS: reactive oxygen species, TNF-α: Tumor necrosis factor, IL-6: interleukin-6; GH: growth hormone.
Conclusion
Vascular calcification induced by age-related biological processes such as oxidative stress, inflammation and hormonal dysregulation impair endothelial function. Based on the literature evidence, we hypothesize that endothelial dysfunction increases arterial calcification that restricts blood flow and muscle perfusion, which attenuate substrate delivery to skeletal muscle and contribute to atrophy and loss of function. Human studies are warranted to experimentally investigate the causal relationship between vascular function and sarcopenia and identify interventions targeting diminished vascular function to prevent the development of sarcopenia. Studying various exercise modalities will be among these research areas to better understand how exercise can overcome the vascular limitations to improve skeletal muscle function and prevent sarcopenia.
Clinical Implications
Clinically, assessments of vascular calcification can be performed frequently as Standard-of-Care. Currently, when vascular calcification is detected, risk stratification for atherosclerotic CVD (ASCVD) is needed. Assessment of CV risk factors such as serum lipids, presence of diabetes, blood pressure and smoking history becomes essential for considering long-term risk of ASCVD 109. Clinicians often pursue additional testing such as coronary artery calcium testing for further risk stratification and often will be more aggressive with adding statin therapy in these patients. With the current emerging evidence that vascular dysfunction may precede and be a culprit of sarcopenia, future clinical studies may confirm that vascular calcification is not only a predictor of vascular disease, but may also be an important predictor of muscle loss and increased risk of mobility disability.
Potential Interventions
If prospective studies support this proposed mechanistic hypothesis, then future interventions targeting sarcopenia and/or functional decline should focus on improving vascular function in parallel to physical function. For example, interventions such as high-intensity exercise training and resistance training with blood flow restriction may be appropriate interventions to improve endothelial function, muscle capillary perfusion, and thus more efficiently improve muscle function and strength110–113.
However, vascular dysfunction may also be a limiting factor in an effective adaptation to exercise 114. In particular, preclinical evidence has shown that muscle capillary perfusion and angiogenesis are diminished during aging and are the limiting factor in supplying muscle with nutrients and oxygen to induce an adaptive process of muscle strength and function. For example, age-related dysregulation of the NAD metabolome may be a reason of a diminished vascular remodeling response to exercise 89,115. Reversed vascular function and improved angiogenesis by the restored NAD metabolome, improved exercise performance and skeletal muscle function 89. Therefore, future pharmacological and lifestyle interventions, and its combinations are warranted to target not only improvements of endothelial function, but also angiogenesis to maintain skeletal muscle function and strength.
Highlights.
Aging is associated with inflammation, oxidative stress and hormonal changes, which may contribute to vascular calcification.
Age-related vascular calcification leads to arterial stiffness, which may decrease muscle perfusion.
Aging-related vascular calcification and muscle loss not only share common pathogenesis, but vascular calcification may precede and contribute to sarcopenia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mathers CD, Stevens GA, Boerma T, White RA, Tobias MI. Causes of international increases in older age life expectancy. The Lancet. 2015;385(9967):540–548. [DOI] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2001;56(3):M146–M157. [DOI] [PubMed] [Google Scholar]
- 3.Ofori-Asenso R, Chin KL, Mazidi M, et al. Global incidence of frailty and prefrailty among community-dwelling older adults: a systematic review and meta-analysis. JAMA network open. 2019;2(8):e198398–e198398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vellas B, Fielding R, Bens C, et al. Implications of ICD-10 for sarcopenia clinical practice and clinical trials: report by the International Conference on Frailty and Sarcopenia Research Task Force. The Journal of frailty & aging. 2018;7(1):2–9. [DOI] [PubMed] [Google Scholar]
- 5.von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. Journal of cachexia, sarcopenia and muscle. 2010;1(2):129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keller K, Engelhardt M. Strength and muscle mass loss with aging process. Age and strength loss. Muscles, ligaments and tendons journal. 2013;3(4):346. [PMC free article] [PubMed] [Google Scholar]
- 7.Kung TA, Cederna PS, van der Meulen JH, Urbanchek MG, Kuzon WM Jr, Faulkner JA. Motor unit changes seen with skeletal muscle sarcopenia in oldest old rats. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2014;69(6):657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamel HK, Maas D, Duthie EH. Role of hormones in the pathogenesis and management of sarcopenia. Drugs & aging. 2002;19(11):865–877. [DOI] [PubMed] [Google Scholar]
- 9.Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. The American journal of medicine. 2006;119(6):526. e529–526. e517. [DOI] [PubMed] [Google Scholar]
- 10.Brioche T, Lemoine-Morel S. Oxidative stress, sarcopenia, antioxidant strategies and exercise: molecular aspects. Current pharmaceutical design. 2016;22(18):2664–2678. [DOI] [PubMed] [Google Scholar]
- 11.Doherty TJ. Invited review: aging and sarcopenia. Journal of applied physiology. 2003;95(4):1717–1727. [DOI] [PubMed] [Google Scholar]
- 12.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age and ageing. 2019;48(1):16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walston JD. Sarcopenia in older adults. Current opinion in rheumatology. 2012;24(6):623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han P, Yu H, Ma Y, et al. The increased risk of sarcopenia in patients with cardiovascular risk factors in Suburb-Dwelling older Chinese using the AWGS definition. Scientific reports. 2017;7(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez AJ, Lewis JR, Scott DS, et al. Aortic Calcification is Associated with Five-Year Decline in Handgrip Strength in Older Women. Calcif Tissue Int. 2018;103(6):589–598. [DOI] [PubMed] [Google Scholar]
- 16.Den Ouden M, Schuurmans M, Arts E, et al. Atherosclerosis and physical functioning in older men, a longitudinal study. The journal of nutrition, health & aging. 2013;17(1):97–104. [DOI] [PubMed] [Google Scholar]
- 17.Everson-Rose SA, Mendes de Leon CF, Roetker NS, Lutsey PL, Alonso A. Subclinical cardiovascular disease and changes in self-reported mobility: multi-ethnic study of atherosclerosis. The Journals of Gerontology: Series A. 2017;73(2):218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Semba RD, Ferrucci L, Sun K, et al. Oxidative stress and severe walking disability among older women. The American journal of medicine. 2007;120(12):1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karwowski W, Naumnik B, Szczepański M, Myśliwiec M. The mechanism of vascular calcification–a systematic review. Medical science monitor: international medical journal of experimental and clinical research. 2012;18(1):RA1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki E, Kashiwagi A, Nishio Y, et al. Increased arterial wall stiffness limits flow volume in the lower extremities in type 2 diabetic patients. Diabetes care. 2001;24(12):2107–2114. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell GF, Lacourciere Y, Arnold JMO, Dunlap ME, Conlin PR, Izzo JL Jr. Changes in aortic stiffness and augmentation index after acute converting enzyme or vasopeptidase inhibition. Hypertension. 2005;46(5):1111–1117. [DOI] [PubMed] [Google Scholar]
- 22.Abbatecola AM, Chiodini P, Gallo C, et al. Pulse wave velocity is associated with muscle mass decline: Health ABC study. Age. 2012;34(2):469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sampaio RAC, Sewo Sampaio PY, Yamada M, et al. Arterial stiffness is associated with low skeletal muscle mass in J apanese community- dwelling older adults. Geriatrics & gerontology international. 2014;14:109–114. [DOI] [PubMed] [Google Scholar]
- 24.Rodríguez AJ, Scott D, Khan B, et al. Low relative lean mass is associated with increased likelihood of abdominal aortic calcification in community-dwelling older Australians. Calcified tissue international. 2016;99(4):340–349. [DOI] [PubMed] [Google Scholar]
- 25.Park J, Park H. Muscle strength and carotid artery flow velocity is associated with increased risk of atherosclerosis in adults. Cardiology journal. 2017;24(4):385–392. [DOI] [PubMed] [Google Scholar]
- 26.Jensky NE, Criqui MH, Wright CM, Wassel CL, Alcaraz JE, Allison MA. The association between abdominal body composition and vascular calcification. Obesity. 2011;19(12):2418–2424. [DOI] [PubMed] [Google Scholar]
- 27.Alexandersen P, Tankó LB, Bagger YZ, Jespersen J, Skouby SO, Christiansen C. Associations between aortic calcification and components of body composition in elderly men. Obesity. 2006;14(9):1571–1578. [DOI] [PubMed] [Google Scholar]
- 28.Abizanda PS, Paterna GM, Martín ES, Casado LM, López EJ, Martínez ES. Subclinial atherosclerosis as a predictor of functional limitation at one year in high-functioning older adults: the Albacete study. Revista espanola de geriatria y gerontologia. 2010;45(3):125–130. [DOI] [PubMed] [Google Scholar]
- 29.Amann K. Media calcification and intima calcification are distinct entities in chronic kidney disease. Clinical Journal of the American Society of Nephrology. 2008;3(6):1599–1605. [DOI] [PubMed] [Google Scholar]
- 30.Mackey RH, Venkitachalam L, Sutton-Tyrrell K. Calcifications, arterial stiffness and atherosclerosis. In: Atherosclerosis, Large Arteries and Cardiovascular Risk. Vol 44. Karger Publishers; 2007:234–244. [DOI] [PubMed] [Google Scholar]
- 31.Orlandi A, Mauriello A, Marino B, Spagnoli LG. Age-related modifications of aorta and coronaries in the rabbit: a morphological and morphometrical assessment. Archives of gerontology and geriatrics. 1993;17(1):37–53. [DOI] [PubMed] [Google Scholar]
- 32.Gofman JW, Lindgren F, Elliott H, et al. The role of lipids and lipoproteins in atherosclerosis. Science. 1950;111(2877):166–186. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez AJ, Scott D, Ebeling PR. Exploring the Links Between Common Diseases of Ageing—Osteoporosis, Sarcopenia and Vascular Calcification. Clinical Reviews in Bone and Mineral Metabolism. 2019;17(1):1–23. [Google Scholar]
- 34.Metz RP, Patterson JL, Wilson E. Vascular smooth muscle cells: isolation, culture, and characterization. In: Cardiovascular Development. Springer; 2012:169–176. [DOI] [PubMed] [Google Scholar]
- 35.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxidants & redox signaling. 2014;20(7):1126–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2000;279(6):L1005–L1028. [DOI] [PubMed] [Google Scholar]
- 37.Griffith B, Pendyala S, Hecker L, Lee PJ, Natarajan V, Thannickal VJ. NOX enzymes and pulmonary disease. Antioxidants & redox signaling. 2009;11(10):2505–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42(6):1075–1081. [DOI] [PubMed] [Google Scholar]
- 39.Proudfoot D, Skepper JN, Hegyi L, Bennett MR, Shanahan CM, Weissberg PL. Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circulation research. 2000;87(11):1055–1062. [DOI] [PubMed] [Google Scholar]
- 40.Zhao M-M, Xu M-J, Cai Y, et al. Mitochondrial reactive oxygen species promote p65 nuclear translocation mediating high-phosphate-induced vascular calcification in vitro and in vivo. Kidney international. 2011;79(10):1071–1079. [DOI] [PubMed] [Google Scholar]
- 41.Takemura A, Iijima K, Ota H, et al. Sirtuin 1 retards hyperphosphatemia-induced calcification of vascular smooth muscle cells. Arteriosclerosis, thrombosis, and vascular biology. 2011;31(9):2054–2062. [DOI] [PubMed] [Google Scholar]
- 42.Giachelli CM. The emerging role of phosphate in vascular calcification. Kidney international. 2009;75(9):890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Byon CH, Javed A, Dai Q, et al. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. Journal of Biological Chemistry. 2008;283(22):15319–15327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gloire G, Legrand-Poels S, Piette J. NF-κB activation by reactive oxygen species: fifteen years later. Biochemical pharmacology. 2006;72(11):1493–1505. [DOI] [PubMed] [Google Scholar]
- 45.Tak PP, Firestein GS. NF-κB: a key role in inflammatory diseases. The Journal of clinical investigation. 2001;107(1):7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ceneri N, Zhao L, Young BD, et al. Rac2 modulates atherosclerotic calcification by regulating macrophage interleukin-1β production. Arteriosclerosis, thrombosis, and vascular biology. 2017;37(2):328–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujio Y, Matsuda T, Oshima Y, et al. Signals through gp130 upregulate Wnt5a and contribute to cell adhesion in cardiac myocytes. FEBS letters. 2004;573(1–3):202–206. [DOI] [PubMed] [Google Scholar]
- 48.Zickler D, Luecht C, Willy K, et al. Tumour necrosis factor-alpha in uraemic serum promotes osteoblastic transition and calcification of vascular smooth muscle cells via extracellular signal-regulated kinases and activator protein 1/c-FOS-mediated induction of interleukin 6 expression. Nephrology Dialysis Transplantation. 2018;33(4):574–585. [DOI] [PubMed] [Google Scholar]
- 49.Wang Z, Castresana MR, Newman WH. NF-κB is required for TNF-α-directed smooth muscle cell migration. FEBS letters. 2001;508(3):360–364. [DOI] [PubMed] [Google Scholar]
- 50.Sun M, Chang Q, Xin M, Wang Q, Li H, Qian J. Endogenous bone morphogenetic protein 2 plays a role in vascular smooth muscle cell calcification induced by interleukin 6 in vitro. International Journal of Immunopathology and Pharmacology. 2017;30(3):227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao Y, Watson AD, Ji S, Boström KI. Heat shock protein 70 enhances vascular bone morphogenetic protein-4 signaling by binding matrix Gla protein. Circulation research. 2009;105(6):575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hess K, Ushmorov A, Fiedler J, Brenner RE, Wirth T. TNFα promotes osteogenic differentiation of human mesenchymal stem cells by triggering the NF-κB signaling pathway. Bone. 2009;45(2):367–376. [DOI] [PubMed] [Google Scholar]
- 53.Lee H-L, Woo KM, Ryoo H-M, Baek J-H. Tumor necrosis factor-α increases alkaline phosphatase expression in vascular smooth muscle cells via MSX2 induction. Biochemical and biophysical research communications. 2010;391(1):1087–1092. [DOI] [PubMed] [Google Scholar]
- 54.Nadra I, Mason JC, Philippidis P, et al. Proinflammatory activation of macrophages by basic calcium phosphate crystals via protein kinase C and MAP kinase pathways: a vicious cycle of inflammation and arterial calcification? Circulation research. 2005;96(12):1248–1256. [DOI] [PubMed] [Google Scholar]
- 55.Proudfoot D, Shanahan CM. Molecular mechanisms mediating vascular calcification: role of matrix Gla protein. Nephrology. 2006;11(5):455–461. [DOI] [PubMed] [Google Scholar]
- 56.Ikeda K, Souma Y, Akakabe Y, et al. Macrophages play a unique role in the plaque calcification by enhancing the osteogenic signals exerted by vascular smooth muscle cells. Biochemical and biophysical research communications. 2012;425(1):39–44. [DOI] [PubMed] [Google Scholar]
- 57.Tintut Y, Patel J, Parhami F, Demer LL. Tumor necrosis factor-α promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation. 2000;102(21):2636–2642. [DOI] [PubMed] [Google Scholar]
- 58.Villa-Bellosta R, Levi M, Sorribas V. Vascular smooth muscle cell calcification and SLC20 inorganic phosphate transporters: effects of PDGF, TNF-α, and Pi. Pflügers Archiv-European Journal of Physiology. 2009;458(6):1151–1161. [DOI] [PubMed] [Google Scholar]
- 59.Shioi A, Katagi M, Okuno Y, et al. Induction of bone-type alkaline phosphatase in human vascular smooth muscle cells: roles of tumor necrosis factor-α and oncostatin M derived from macrophages. Circulation research. 2002;91(1):9–16. [DOI] [PubMed] [Google Scholar]
- 60.Park MH, Kim DH, Lee EK, et al. Age-related inflammation and insulin resistance: a review of their intricate interdependency. Archives of pharmacal research. 2014;37(12):1507–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duncan ER, Crossey PA, Walker S, et al. Effect of endothelium-specific insulin resistance on endothelial function in vivo. Diabetes. 2008;57(12):3307–3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim J-a, Montagnani M, Koh KK, Quon MJ Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113(15):1888–1904. [DOI] [PubMed] [Google Scholar]
- 63.Ashcroft GS, Dodsworth J, Van Boxtel E, et al. Estrogen accelerates cutaneous wound healing associated with an increase in TGF-β1 levels. Nature medicine. 1997;3(11):1209. [DOI] [PubMed] [Google Scholar]
- 64.Das UN. Nitric oxide as the mediator of the antiosteoporotic actions of estrogen, statins, and essential fatty acids. Experimental Biology and Medicine. 2002;227(2):88–93. [DOI] [PubMed] [Google Scholar]
- 65.Hernández I, Delgado JL, Díaz J, et al. 17β-Estradiol prevents oxidative stress and decreases blood pressure in ovariectomized rats. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2000;279(5):R1599–R1605. [DOI] [PubMed] [Google Scholar]
- 66.Kanno Y, Into T, Lowenstein CJ, Matsushita K. Nitric oxide regulates vascular calcification by interfering with TGF-β signalling. Cardiovascular research. 2008;77(1):221–230. [DOI] [PubMed] [Google Scholar]
- 67.Evans L, Davies J, Anderson R, et al. The effect of GH replacement therapy on endothelial function and oxidative stress in adult growth hormone deficiency. European Journal of Endocrinology. 2000;142(3):254–262. [DOI] [PubMed] [Google Scholar]
- 68.Thum T, Tsikas D, Frölich JC, Borlak J. Growth hormone induces eNOS expression and nitric oxide release in a cultured human endothelial cell line. FEBS letters. 2003;555(3):567–571. [DOI] [PubMed] [Google Scholar]
- 69.Kimbrough TD, Shernan S, Ziegler TR, Scheltinga M, Wilmore DW. Insulin-like growth factor-I response is comparable following intravenous and subcutaneous administration of growth hormone. Journal of Surgical Research. 1991;51(6):472–476. [DOI] [PubMed] [Google Scholar]
- 70.Werner H, Weinstein D, Bentov I. Similarities and differences between insulin and IGF-I: structures, receptors, and signalling pathways. Archives of physiology and biochemistry. 2008;114(1):17–22. [DOI] [PubMed] [Google Scholar]
- 71.Anwar A, Zahid A, Scheidegger K, Brink M, Delafontaine P. Tumor necrosis factor-α regulates insulin-like growth factor-1 and insulin-like growth factor binding protein-3 expression in vascular smooth muscle. Circulation. 2002;105(10):1220–1225. [DOI] [PubMed] [Google Scholar]
- 72.Okura Y, Brink M, Zahid AA, Anwar A, Delafontaine P. Decreased expression of insulin-like growth factor-1 and apoptosis of vascular smooth muscle cells in human atherosclerotic plaque. Journal of Molecular and Cellular Cardiology. 2001;33(10):1777–1789. [DOI] [PubMed] [Google Scholar]
- 73.Incalza MA, D’Oria R, Natalicchio A, Perrini S, Laviola L, Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascular pharmacology. 2018;100:1–19. [DOI] [PubMed] [Google Scholar]
- 74.Hickner R, Fisher J, Ehsani A, Kohrt W. Role of nitric oxide in skeletal muscle blood flow at rest and during dynamic exercise in humans. American Journal of Physiology-Heart and Circulatory Physiology. 1997;273(1):H405–H410. [DOI] [PubMed] [Google Scholar]
- 75.Toth MJ, Matthews DE, Tracy RP, Previs MJ. Age-related differences in skeletal muscle protein synthesis: relation to markers of immune activation. American Journal of Physiology-Endocrinology and Metabolism. 2005;288(5):E883–E891. [DOI] [PubMed] [Google Scholar]
- 76.McClung JM, Judge AR, Powers SK, Yan Z. p38 MAPK links oxidative stress to autophagy-related gene expression in cachectic muscle wasting. American Journal of Physiology-Cell Physiology. 2010;298(3):C542–C549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jaiswal N, Maurya CK, Arha D, et al. Fructose induces mitochondrial dysfunction and triggers apoptosis in skeletal muscle cells by provoking oxidative stress. Apoptosis. 2015;20(7):930–947. [DOI] [PubMed] [Google Scholar]
- 78.Gaster M, Staehr P, Beck-Nielsen H, Schrøder HD, Handberg A. GLUT4 is reduced in slow muscle fibers of type 2 diabetic patients: is insulin resistance in type 2 diabetes a slow, type 1 fiber disease? Diabetes. 2001;50(6):1324–1329. [DOI] [PubMed] [Google Scholar]
- 79.Mueckler M. Insulin resistance and the disruption of Glut4 trafficking in skeletal muscle. The Journal of clinical investigation. 2001;107(10):1211–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vincent MA, Clerk LH, Rattigan S, Clark MG, Barrett EJ. Active role for the vasculature in the delivery of insulin to skeletal muscle. Clinical and experimental pharmacology and physiology. 2005;32(4):302–307. [DOI] [PubMed] [Google Scholar]
- 81.Williams IM, McClatchey PM, Bracy DP, Bonner JS, Valenzuela FA, Wasserman DH. Transendothelial Insulin Transport is Impaired in Skeletal Muscle Capillaries of Obese Male Mice. Obesity (Silver Spring). 2020;28(2):303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kubota T, Kubota N, Kumagai H, et al. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell metabolism. 2011;13(3):294–307. [DOI] [PubMed] [Google Scholar]
- 83.Muniyappa R, Iantorno M, Quon MJ. An integrated view of insulin resistance and endothelial dysfunction. Endocrinology and metabolism clinics of North America. 2008;37(3):685–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang X, Hu Z, Hu J, Du J, Mitch WE. Insulin resistance accelerates muscle protein degradation: Activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology. 2006;147(9):4160–4168. [DOI] [PubMed] [Google Scholar]
- 85.Cleasby ME, Jamieson PM, Atherton PJ. Insulin resistance and sarcopenia: mechanistic links between common co-morbidities. J Endocrinol. 2016;229(2):R67–R81. [DOI] [PubMed] [Google Scholar]
- 86.Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proceedings of the National Academy of Sciences. 2003;100(13):7996–8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barnouin Y, McPhee JS, Butler-Browne G, et al. Coupling between skeletal muscle fiber size and capillarization is maintained during healthy aging. J Cachexia Sarcopenia Muscle. 2017;8(4):647–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tang K, Breen EC, Gerber HP, Ferrara NM, Wagner PD. Capillary regression in vascular endothelial growth factor-deficient skeletal muscle. Physiol Genomics. 2004;18(1):63–69. [DOI] [PubMed] [Google Scholar]
- 89.Das A, Huang GX, Bonkowski MS, et al. Impairment of an Endothelial NAD(+)-H2S Signaling Network Is a Reversible Cause of Vascular Aging. Cell. 2018;173(1):74–89 e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Csanyi G, Singla B. Arterial Lymphatics in Atherosclerosis: Old Questions, New Insights, and Remaining Challenges. J Clin Med. 2019;8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bridenbaugh EA, Nizamutdinova IT, Jupiter D, et al. Lymphatic muscle cells in rat mesenteric lymphatic vessels of various ages. Lymphat Res Biol. 2013;11(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shang T, Liang J, Kapron CM, Liu J. Pathophysiology of aged lymphatic vessels. Aging (Albany NY). 2019;11(16):6602–6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clark MG, Wallis MG, Barrett EJ, et al. Blood flow and muscle metabolism: a focus on insulin action. American Journal of Physiology-Endocrinology And Metabolism. 2003;284(2):E241–E258. [DOI] [PubMed] [Google Scholar]
- 94.Hammers DW, Matheny RW Jr, Sell C, et al. Impairment of IGF-I expression and anabolic signaling following ischemia/reperfusion in skeletal muscle of old mice. Experimental gerontology. 2011;46(4):265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ascenzi F, Barberi L, Dobrowolny G, et al. Effects of IGF- 1 isoforms on muscle growth and sarcopenia. Aging cell. 2019;18(3):e12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pandini G, Frasca F, Mineo R, Sciacca L, Vigneri R, Belfiore A. Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved. Journal of Biological Chemistry. 2002;277(42):39684–39695. [DOI] [PubMed] [Google Scholar]
- 97.Nadler ST, Stoehr JP, Rabaglia ME, Schueler KL, Birnbaum MJ, Attie AD. Normal Akt/PKB with reduced PI3K activation in insulin-resistant mice. Am J Physiol Endocrinol Metab. 2001;281(6):E1249–1254. [DOI] [PubMed] [Google Scholar]
- 98.Bois PR, Grosveld GC. FKHR (FOXO1a) is required for myotube fusion of primary mouse myoblasts. The EMBO journal. 2003;22(5):1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lamberts SW, Van den Beld AW, Van Der Lely A-J. The endocrinology of aging. Science. 1997;278(5337):419–424. [DOI] [PubMed] [Google Scholar]
- 100.Goglia L, Tosi V, Sanchez A, et al. Endothelial regulation of eNOS, PAI-1 and t-PA by testosterone and dihydrotestosterone in vitro and in vivo. Molecular human reproduction. 2010;16(10):761–769. [DOI] [PubMed] [Google Scholar]
- 101.Miller VM, Mulvagh SL. Sex steroids and endothelial function: translating basic science to clinical practice. Trends in pharmacological sciences. 2007;28(6):263–270. [DOI] [PubMed] [Google Scholar]
- 102.De Spiegeleer A, Beckwée D, Bautmans I, Petrovic M. Pharmacological interventions to improve muscle mass, muscle strength and physical performance in older people: an umbrella review of systematic reviews and meta-analyses. Drugs & aging. 2018;35(8):719–734. [DOI] [PubMed] [Google Scholar]
- 103.Ibebunjo C, Eash JK, Li C, Ma Q, Glass DJ. Voluntary running, skeletal muscle gene expression, and signaling inversely regulated by orchidectomy and testosterone replacement. American Journal of Physiology-Endocrinology and Metabolism. 2011;300(2):E327–E340. [DOI] [PubMed] [Google Scholar]
- 104.Collins BC, Arpke RW, Larson AA, et al. Estrogen Regulates the Satellite Cell Compartment in Females. Cell reports. 2019;28(2):368–381. e366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tsai W-JA, McCormick KM, Brazeau DA, Brazeau GA. Estrogen Effects on Skeletal Muscle Insulin-Like Growth Factor–1 and Myostatin in Ovariectomized Rats. Experimental biology and medicine. 2007;232(10):1314–1325. [DOI] [PubMed] [Google Scholar]
- 106.Moran AL, Nelson SA, Landisch RM, Warren GL, Lowe DA. Estradiol replacement reverses ovariectomy-induced muscle contractile and myosin dysfunction in mature female mice. Journal of applied physiology. 2007;102(4):1387–1393. [DOI] [PubMed] [Google Scholar]
- 107.Tanideh N, Sheikhani HS, Salesi M, Tamadon A, Rostamzad K, Kardeh A. Effects of endurance exercise and estrogen supplementation on the proliferation of satellite cells. Comparative Clinical Pathology. 2014;23(5):1645–1649. [Google Scholar]
- 108.Brown M, Ning J, Ferreira JA, Bogener JL, Lubahn DB. Estrogen receptor-α and-β and aromatase knockout effects on lower limb muscle mass and contractile function in female mice. American Journal of Physiology-Endocrinology and Metabolism. 2009;296(4):E854–E861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74(10):e177–e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Scott SN, Shepherd SO, Hopkins N, et al. Home-hit improves muscle capillarisation and eNOS/NAD(P)Hoxidase protein ratio in obese individuals with elevated cardiovascular disease risk. J Physiol. 2019;597(16):4203–4225. [DOI] [PubMed] [Google Scholar]
- 111.Lopes KG, Bottino DA, Farinatti P, et al. Strength training with blood flow restriction - a novel therapeutic approach for older adults with sarcopenia? A case report. Clin Interv Aging. 2019;14:1461–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cook SB, LaRoche DP, Villa MR, Barile H, Manini TM. Blood flow restricted resistance training in older adults at risk of mobility limitations. Exp Gerontol. 2017;99:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fry CS, Glynn EL, Drummond MJ, et al. Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J Appl Physiol (1985). 2010;108(5):1199–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yasuda T, Fukumura K, Uchida Y, et al. Effects of Low-Load, Elastic Band Resistance Training Combined With Blood Flow Restriction on Muscle Size and Arterial Stiffness in Older Adults. J Gerontol A Biol Sci Med Sci. 2015;70(8):950–958. [DOI] [PubMed] [Google Scholar]
- 115.Custodero C, Saini SK, Shin MJ, et al. Nicotinamide riboside-A missing piece in the puzzle of exercise therapy for older adults? Exp Gerontol. 2020;137:110972. [DOI] [PMC free article] [PubMed] [Google Scholar]