Abstract
Immune checkpoint blockade has shown unprecedented and durable clinical response in a wide range of cancers. T cell immunoglobulin and mucin domain 3 (TIM3) is an inhibitory checkpoint protein that is highly expressed in tumor-infiltrating lymphocytes. In various cancers, the interaction of TIM3 and Galectin 9 (Gal9) suppresses anti-tumor immunity mediated by innate as well as adaptive immune cells. Thus, the blockade of the TIM3/Gal9 interaction is a promising therapeutic approach for cancer therapy. In addition, co-blockade of the TIM3/Gal9 pathway along with the PD-1/PD-L1 pathway increases the therapeutic efficacy by overcoming non-redundant immune resistance induced by each checkpoint. Here, we summarize the physiological roles of the TIM3/Gal9 pathway in adaptive and innate immune systems. We highlight the recent clinical and preclinical studies showing the involvement of the TIM3/Gal9 pathway in various solid and blood cancers. In addition, we discuss the potential of using TIM3 and Gal9 as prognostic and predictive biomarkers in different cancers. An in-depth mechanistic understanding of the blockade of the TIM3/Gal9 signaling pathway in cancer could help in identifying patients who respond to this therapy as well as designing combination therapies.
Keywords: TIM3/Gal9, immuno-oncology, immune checkpoint receptors, immune checkpoint blockade, immune suppression
1. INTRODUCTION
Immune checkpoint proteins are stimulatory or inhibitory regulators that play a key role in maintaining immune homeostasis and preventing the onset of autoimmunity [1]. Stimulatory checkpoint proteins like the cluster of differentiation 28 (CD28), OX40, glucocorticoid-induced tumor necrosis factor receptor (GITR), CD137, and CD27 enhances T cell functions, whereas inhibitory checkpoint proteins such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), T cell immunoglobulin and mucin domain-containing protein 3 (TIM3), B and T lymphocyte attenuator (BTLA), V-domain immunoglobulin suppressor of T cell activation (VISTA), and lymphocyte-activation gene 3 (LAG3) suppress T cell immunity [2]. Immune checkpoint proteins help in maintaining a balance between positive and negative signals mediated by T cells. In cancers, inhibitory immune checkpoints are often activated, which protect the tumor cells from immune surveillance [1]. Targeting immune checkpoint proteins is, therefore, one of the most active areas of cancer research. Particularly, checkpoint inhibitors targeting the PD-1/PD-L1 axis and CTLA-4 have been approved for different types of cancer.
Among these checkpoint proteins, TIM3 has attracted much attention and is being vigorously studied. TIM3 biology and its suppressing role of antitumor immunity upon interaction with its ligands like Galectin 9 (Gal9) [3], phosphatidylserine (Ptdser) [4], high mobility group box 1 (HMGB1), [5] and carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) [6] are being actively studied. A recent review by Wolf Y et al. focused on the four ligands of TIM3, the role of TIM3 in cancer and chronic viral infections, and ongoing clinical trials of anti-TIM3 antibodies [7]. Another review by Solinas C et al. described TIM3 biology in different immune cells, T helper cells (Th1 and Th17), regulatory T cells (Tregs), dysfunctional cytotoxic T lymphocytes (CD8+ T cells), natural killer (NK) cells, and dendritic cells (DCs) along with several anti-TIM3 antibodies in clinical trials [8]. These articles discussed the heterogeneous biological role of TIM3 in cancer and the application of TIM3 as a therapeutic target regardless of its specificity to any particular ligand. Our review focuses on the role of the TIM3/Gal9 interaction in various cancers and implications of this pathway in cancer immunotherapy. As the binding site of Gal9 on TIM3 is different from other ligands, the anti-TIM3 antibodies that have been investigated in clinical trials may not block the TIM3/Gal9 pathway. A handful of anti-TIM3 mAbs (TSR022, Sym023, ICAGN02390, BGB-A425, and MBG453) that prominently block the TIM3/Ptdser interaction are investigated in clinical trials. Among them, LY3321367 is the only anti-TIM3 mAb that partially blocks the TIM3/Gal9 interaction [9–13]. Even though Gal9 is the most relevant ligand for TIM3 [8], drugs that specifically block the TIM3/Gal9 pathway have not entered clinical trials yet. As a result, there is a great need to fully understand the TIM3/Gal9 interaction and its role in tumor pathogenesis. The current review highlights the importance of the TIM3/Gal9 pathway in cancer development, and how the blockade of this pathway can be exploited for the treatment of different types of cancer. We also discuss the potential applications of the TIM3/Gal9 pathway in other diseases along with the possibilities of using TIM3 or Gal9 as a prognostic or predictive biomarker for cancer.
2. Structure and Functions of TIM3
The immune checkpoint protein TIM3 is a negative regulator of anti-tumor immunity [14] and a member of the TIM family, which comprises of eight members, TIM1-TIM8, among which TIM1, TIM3, and TIM4 are found in humans [15]. TIM3 is a type I membrane glycoprotein expressed on terminally differentiated CD4+ T cell subsets like Th1 cells, Th17 cells, Tregs, and CD8+ T cell subset type1 CD8+ T cell (Tc1) but not on Th2 cells. It is also expressed on B cells, macrophages, DCs, NK cells, mast cells, and monocytes [8, 16–21].
TIM3 is composed of an N-terminal extracellular immunoglobulin variable (IgV) domain that has FG-CC’ loop and N-linked glycans, a mucin domain-containing sites for O-linked glycosylation, a stalk domain-containing N-linked glycans, a transmembrane domain, and a cytoplasmic domain-containing tyrosine phosphorylation motifs (Figure 1) [7, 22, 23]. There are four different ligands of TIM3 (Table 1). Binding of TIM3 with Gal9 dampens Th1-mediated immunity [3] and induces apoptosis of TIM3+ T cells [24]. TIM3 expressing T cells can recognize apoptotic cells by binding to another ligand Ptdser, which is expressed on the apoptotic cells, but cannot engulf the apoptotic cells. However, TIM3 expressing DCs, when bound to Ptdser, can recognize and clear the apoptotic bodies [25]. Another TIM3 ligand, HMGB1, upon binding to TIM3 expressed in DCs, inhibits the transportation of tumor-derived nucleic acids to the endosome, leading to the suppression of pattern recognition receptor (PPR) mediated immune response to these nucleic acids [5, 26]. However, the interaction of TIM3 expressing T cells with HMGB1 is poorly understood [27]. The interaction of CEACAM1 with TIM3 expressed on T cell suppresses T-cell effector functions and helps maintain T-cell tolerance. Among the TIM3 ligands, Gal9 binds to the N-linked glycans in the IgV domain of TIM3, while other ligands bind to the unique cleft called the FG-CC’ loop in the IgV domain [28]. Although the binding sites for Gal9 and CEACAM1 in the IgV domain are different, studies have shown that they induce the same downstream signaling events [23, 29].
Figure 1. The structure of TIM3.
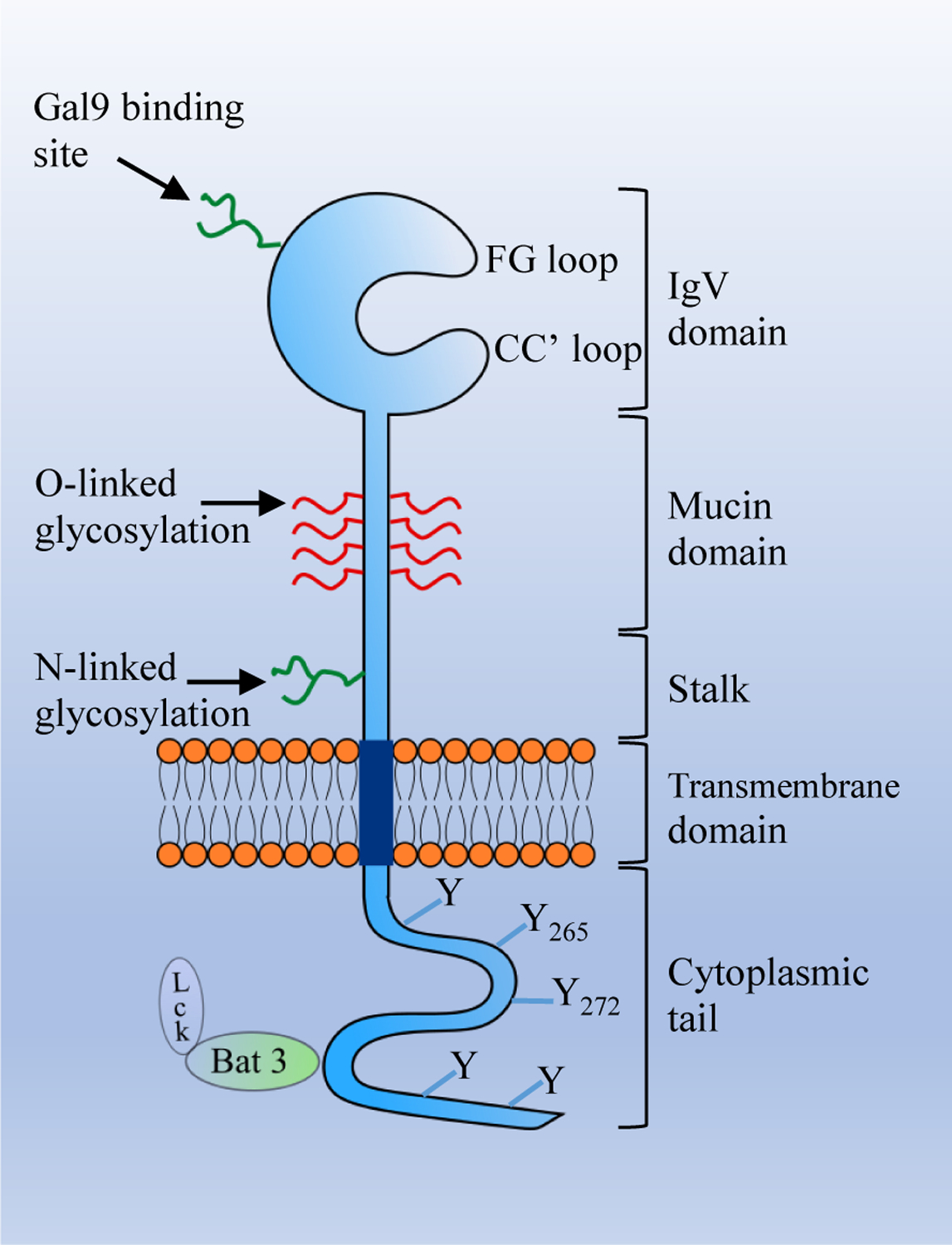
The extracellular region of T cell immunoglobulin and mucin domain-containing protein 3 (TIM3) consists of an IgV domain, a mucin domain, and a stalk domain. The transmembrane region consists of a transmembrane domain and the intracellular region consists of a cytoplasmic tail with five tyrosine residues. The IgV domain contains binding sites for its ligands. Phosphatidylserine (Ptdser), carcinoembryonic antigen-related cell adhesion molecule (CEACAM1), and high mobility group box 1 (HMGB1) bind to the FG-CC’ loop, while Gal9 binds to the N-linked glycan.
Table 1.
Receptor and Ligands for TIM3 and Gal9
| Receptor/Ligands | Biological function of Receptor-Ligand interaction | References | |
|---|---|---|---|
| TIM3 | Gal9 | In T cells: Immunosuppression, T cell apoptosis | [43] |
| In NK cells: Immunostimulatory effect | [39] | ||
| DCs: Immunostimulatory effect | [44] | ||
| Macrophages: Immunosuppression | [45] | ||
| Ptdser | Apoptotic cells recognition, and clearance of apoptotic cells (in DCs) | [25] | |
| CEACAM1 | Suppresses T cell immunity | [23, 29] | |
| HMGB1 | Inhibits tumor derived nucleic acid migration in DCs | [46] | |
| Gal9 | TIM3 | As mentioned above | |
| Dectin-1 | Macrophage dependent T cell immunity suppression | [40] | |
| 4–1BB | Enhances cytokine secretion, enhances immune response | [42] | |
| CD40 | Immunosuppressive effect | [41] | |
3. Structure and Functions of Gal9
Galectins are soluble proteins composed of a characteristic structure, called the carbohydrate recognition domain (CRD), which binds to β galactose [30, 31]. Based on the number of CRDs, galectins are subdivided into the prototype, tandem-repeat type, and chimeric-type galectins [32]. Gal9 is a member of a ‘tandem-repeat’ type of galectin superfamily that consists of two non-homologous CRDs joined by a flexible peptide linker (14 to 56 amino acids in length) that plays important roles in protein-protein interactions, membrane insertions, and presentation of the CRDs [33]. The peptide linker contains a metalloproteinase site which can assist with the secretion of Gal9 into the extracellular matrix [34, 35]. Gal9 is highly expressed in epithelial cells, endothelial cells, T cells, B cells, mast cells, and macrophages [31, 36]. Gal9 has an important function in eosinophil activation, hemagglutination and anti-metastasis, T cell apoptosis via the TIM3/Gal9 pathway, T cell homeostasis and production of cytokines, and differentiation of Tregs and Th17 cells, making Gal9 a regulator of immune responses [37].
Four receptors with an affinity for Gal9 have been reported (Table 1). Among the receptors, TIM3 is a fully characterized receptor for Gal9 [3]. The TIM3/Gal9 interaction shows inhibitory or stimulatory effects in the immune system depending on the cell type that expresses TIM3. In normal physiology, TIM3 expressed on T cell subsets (Th1, Th17, and Tc1) and macrophages show inhibitory effects upon Gal9 binding, whereas TIM3 expressed on NK cells and DCs show stimulatory effects when it binds to Gal9 [38, 39]. The other receptors for Gal9 are Dectin-1, 4–1BB, and CD40. Dectin-1 is highly expressed on macrophages and exerts an immunosuppressive effect after binding to Gal9 [40]. Similarly, the Gal9/CD40 interaction is known to prevent the proliferation and survival effects of CD40 on CD4loCD40+ effector T cells [41]. Gal9 binds to 4–1BB (an activating immune checkpoint protein) and promotes the clustering of 4–1BB, enhancing the proinflammatory pathway and immune response. [42].
4. Physiological functions of the TIM3/Gal9 pathway
4.1. TIM3/Gal9 pathway in adaptive immunity
Unlike other immune checkpoint proteins such as PD-1, CTLA-4, TIGIT, and LAG-3, which have an inhibitory motif in their cytoplasmic tail, TIM3 has five tyrosine residues in its cytoplasmic tail. Of the five tyrosine residues, Y265 and Y272 (Y256 and Y263 in mice) play an important role in TIM3 signal transduction [47]. While the exact molecular mechanism of TIM3 signaling is not fully elucidated, it has been demonstrated that before TIM3 ligand engagement, the tyrosine residues present in the intracellular tail of TIM3 are necessary for the augmentation of T cell receptor (TCR) activation suggesting the coupling of TIM3 and TCR pathways [48].
TIM3 when not bound to any of its ligands, its cytoplasmic tail containing Y256 and Y263, interacts with HLA-B associated transcript 3 (Bat3), [49] which facilitates the recruitment of catalytically activated Lck [7], a member of the Src family protein kinase (Figure 1). The formed Lck clusters phosphorylate the CD3 domain of TCR, leading to the recruitment and activation of protein kinase Zap 70 [50], which phosphorylates the linker adaptor protein (LAT) [51, 52]. Phosphorylated LAT recruits and activates the signaling effector phospholipase Cγ1 (PLCγ1), which participates in the production of second messengers like inositol 1,4,5-triphosophate (IP3) and diacylglycerol (DAG) [53, 54]. These second messengers subsequently activate NFAT, MEK/ERK, and NF-kB pathways, resulting in T cell response and modulation, cell proliferation, and production of interleukin-2 (IL-2), Tumor Necrosis Factor (TNFα), and interferon γ (IFNγ) [53, 55] (Figure 2A).
Figure 2. Schematics of the TIM3/Gal9 signaling pathway in T cells.
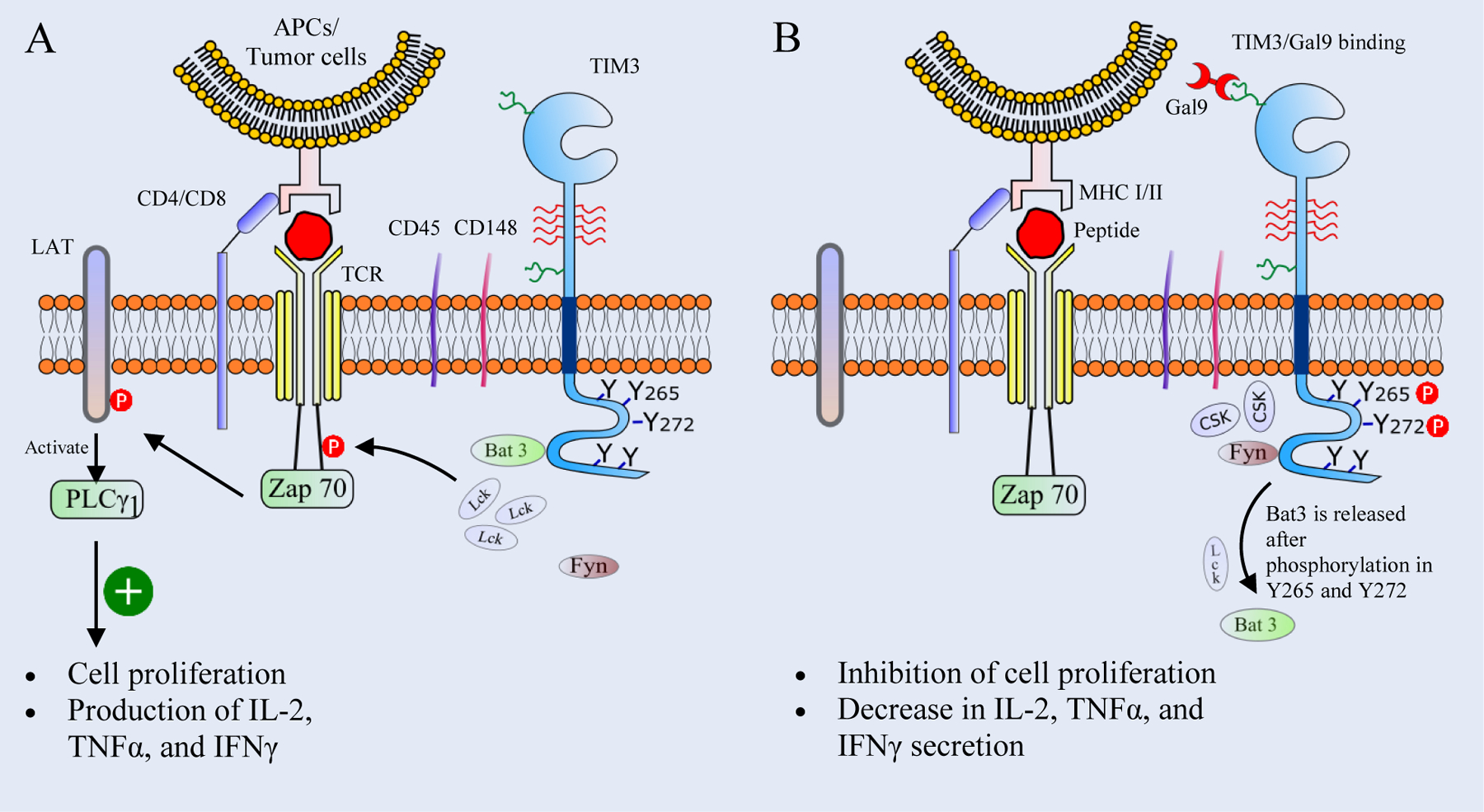
(A) In an unbound state, the cytoplasmic tail of T cell immunoglobulin and mucin domain-containing protein 3 (TIM3) binds to HLA-B associated transcript 3 (Bat3) which helps to recruit the tyrosine kinase Lck. Lck clusters then phosphorylate the CD3 domain of TCR, leading to the recruitment of the tyrosine kinase Zap 70. Zap 70 then phosphorylates linker adaptor protein (LAT), which activates the phospholipase Cγ1 (PLCγ1) to produce second messengers like Inositol trisphosphate (IP3) and diacylglycerol (DAG), leading to the production of cytokines and T cell proliferation. (B) Upon the TIM3/Gal9 interaction, Y265 and Y272 in the cytoplasmic tail of TIM3 are phosphorylated, resulting in the release of Bat3 and Lck. The tyrosine kinase Fyn is recruited to the cytoplasmic tail of TIM3 and helps in the clustering of C-terminal c-Src kinase (Csk). Csk clusters promote phosphorylation of the C-terminal tyrosine of Lck and inhibit its catalytic activity. Subsequently this leads to the inhibition of T cell proliferation and suppression of the production of IL-2, TNFα, and IFNγ.
Interaction between TIM3 and Gal9 induces phosphorylation of the cytoplasmic tail at Y265 and Y272, [47] resulting in the release of bound Bat3 from the cytoplasmic tail [49] (Figure 2B). Bat3-released TIM3 binds to Fyn, one of the Src family protein kinases that share the same binding site with Bat3 [49]. Fyn recruitment in the cytoplasmic tail of TIM3 [7] phosphorylates the phosphoprotein associated with glycosphingolipid microdomains 1 (PAG), which promotes the recruitment of the C-terminal c-Src kinase (Csk). Csk then phosphorylates the C-terminal tyrosine of Lck, which negatively regulates the Lck activity and leads to the inhibition of T cell functions [56–58]. Davidson et al. have reported that PAG-associated Fyn is involved in increasing the Ca++ flux intracellularly, which weakens the TCR signaling and leads to T cell anergy [59]. Thus, the TIM3/Gal9 signaling pathway mediates events like inhibition of T cell proliferation, reduction in cytokine production and potentially resulting in T cell death.
The TIM3/Gal9 interaction also affects proximal TCR signaling. The conserved tyrosine residues present in the cytoplasmic tail of TIM3 are also responsible for proximal signaling events. The TIM3/Gal9 interaction enhances the clustering of tyrosine receptor phosphatases CD45 and CD148 present in the immunological synapse [43]. The receptor phosphatase CD45 dephosphorylates the tyrosine residues Y505 and Y394 of Lck and reduces its catalytic activity [60]. Similarly, CD148 hypophosphorylates LAT and PLCγ1, leading to the inhibition of the downstream T cell functions [61]. In conclusion, the TIM3/Gal9 interaction inhibits immunity mediated by Th1, Th17, and Tc1 cells through inhibiting the production of IFNγ, TNFα, and IL-2 (Figure 3A, 3B and 3D) (Table 2).
Figure 3: The role of the TIM3/Gal9 interaction on different T cell subsets.
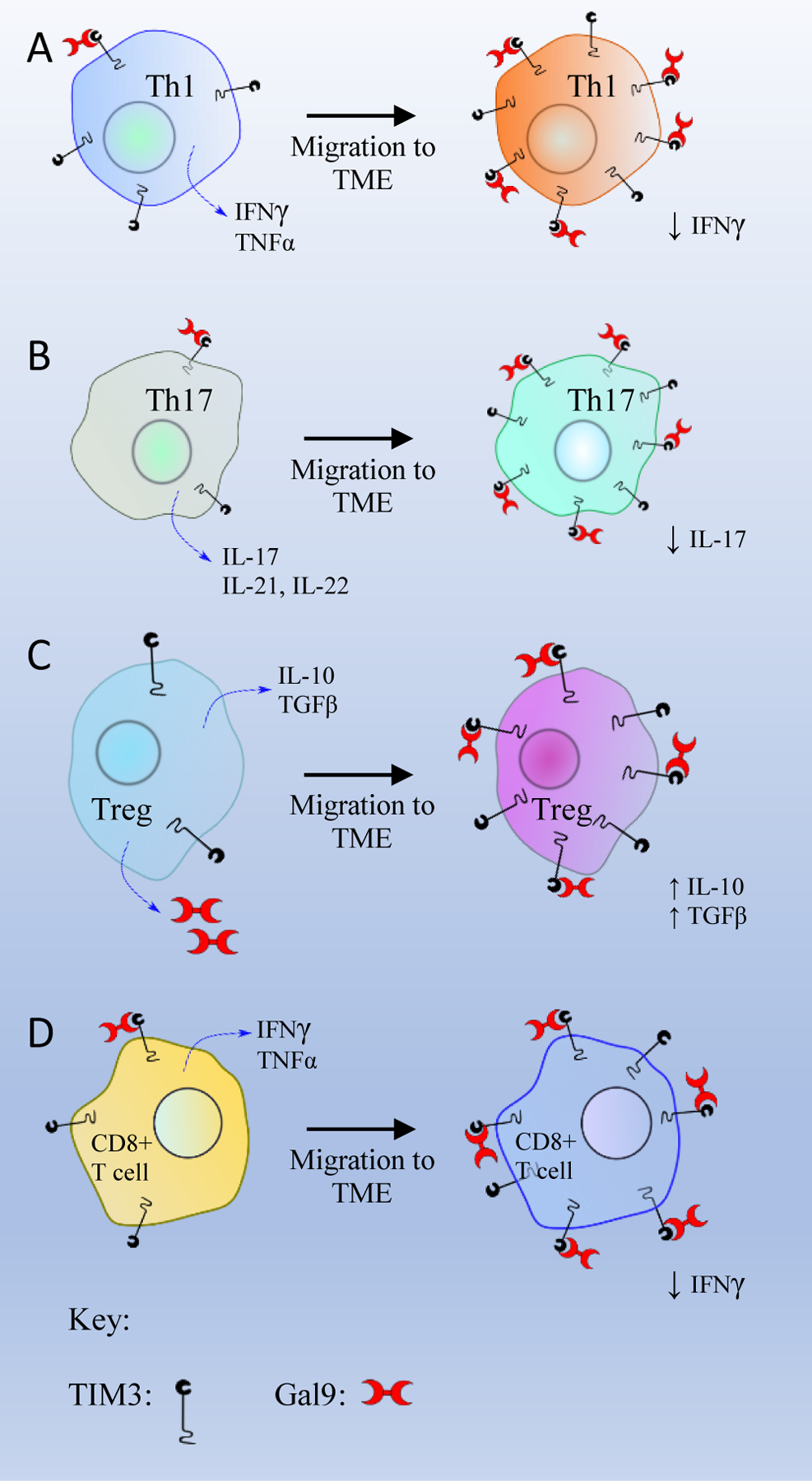
(A) T helper 1 cell (Th1), (B) T helper 17 cell (Th17), (C) T regulatory cells (Tregs), and (D) CD8+ T cell.
Table 2.
The mechanisms and physiological functions of TIM3/Gal9 in different immune cells
| TIM3 expression in immune cells | Mechanisms | Physiological functions | References |
|---|---|---|---|
| Th1, Th17 and Tc1 cells | ● Inhibits Lck mediated phosphorylation of TCR ● Cytokines production: TNFα, IFNγ and IL-17 |
● Decrease in cell proliferation, migration, ● T cell apoptosis |
[43, 80] |
| T regs | ● Increased production of IL-10 and TGFβ | ● Enhancement of suppressive effects of Tregs ● Proliferation of Tregs |
[63] |
| NK cells | ● Increase IFNγ production in non-tumor cells ● Decrease in cytokine release by dNKs |
● Enhances innate immune response ● Maintains pregnancy |
[39, 72] |
| Dendritic cells | ● Synergizes with LPS/TLR4 increasing TNFα release | ● Enhances innate immune response | [18] |
| Monocytes | ● Cross talk with TLR4 ● Decreases TNFα, and IL-6 |
● Suppresses innate immunity | [45, 73] |
| Macrophages | ● Trans association inhibits TLR4 mediated cytokine production, (decreases TNFα and IL-6 levels) ● Cis association enhances TLRs |
● Downregulate innate immunity against diseased condition. ● Increase secretion of Gal9 |
[81] |
In Tregs, numerous studies have reported the downstream effects of the TIM3/Gal9 interaction, but the exact intermediate steps of this pathway are yet to be explored. The TIM3/Gal9 interaction is believed to maintain and regulate Tregs function and development. One study has shown that TIM3/Gal9 ligation results in overexpression of cytokines like IL-10 and TGFβ, which facilitates the proliferation of CD4+CD25+Foxp3+ Treg cells. The proliferation of Tregs then suppresses the activity of effector T cells [62]. A study by Yan et al. has shown that TIM3 expression is significantly higher in CD4+Foxp3+ T cells (70%) as compared to CD4+Foxp3− T cells (20%). They also found that Foxp3 is expressed in tumor-infiltrating TIM3+CD4+ T cells which exhibit Treg properties in the tumor microenvironment [63]. A similar study using CT26 cells demonstrated that 50% of CD4+Foxp3+ T cells express TIM3, whereas only 10% of CD4+Foxp3− TILs express TIM3. In line with this in vitro study, an in vivo study showed that TIM3+ Tregs produced two-fold production of IL-10 and had twice the immune-suppressive activity compared to TIM3− Tregs in B16 melanoma and CT26 colon carcinoma [64].
Additionally, Tregs also express Gal9 (Figure 3C), which may bind to TIM3 expressed on Th1/Th17 cells, and suppress T cell-mediated immunity and induce apoptosis of Th1/Th17 cells [65, 66]. In tumor-associated Tregs, TIM3 is overexpressed as a marker of infiltrating Tregs [63]. TIM3+ Tregs are prominent in the tumor nest but are significantly low in peripheral blood. The TIM3/Gal9 interaction was found to enhance the immunosuppressive function of Tregs and participate in the development of allograft tolerance [67, 68]. The TIM3/Gal9 interaction enhances the immunosuppressive activity of Tregs in the tumor microenvironment (TME) by increasing the secretion of IL-10 and TGFβ.
4.2. TIM3/Gal9 pathway in innate immunity
The expression of TIM3 on innate immune cells like NK cells, DCs, macrophages, and monocytes is prominent (Table 2). For example, TIM3 is highly expressed in mature CD56dim CD16+ NK cells and acts as a maturation marker [69]. Even though the role of the TIM3/Gal9 pathway in NK cells is still not fully understood, Jost et al. has shown that Gal9 binding to TIM3 present on NK cells has a stimulatory effect when NK cells are pre-treated with cytokines IL-12, IL-15 and IL-18. But constant exposure to Gal9 leads to the downregulation of TIM3 on NK cells (Figure 4A) [70]. In addition, Gleason et al. reported that coupling of TIM3 and Gal9 increases IFNγ production in NK92 NK cells, suggesting that TIM3 is an NK-cell coreceptor to enhance IFNγ production [39]. In contrast, during pregnancy, the TIM3/Gal9 pathway plays an important role in the differentiation of NK cells to decidual NK (dNK)-like cells to promote fetal tolerance by maintaining the anti-inflammatory function of dNK cells [71]. The binding of Gal9 to TIM3 expressed on dNK cells inhibits lipopolysaccharide (LPS)-induced production of pro-inflammatory cytokines and perforin, thus maintaining a normal pregnancy [72]. In addition, the TIM3/Gal9 pathway in NK cells also has been reported in other diseases. For instance, blockade of the TIM3/Gal9 interaction leads to increased IFNγ production resulting in increased cytotoxicity of NK cells in chronic hepatitis B virus infection [19]. Under normal physiological condition, the binding of Gal9 to TIM3 present on NK cells induces immune-stimulatory activity, whereas, in diseased conditions like cancers or viral infections, the TIM3/Gal9 interaction leads to overactivation of NK cells, resulting in exhaustion of NK cells. The possible reason could be that the TIM3/Gal9 pathway may crosstalk with other pathways like IL-2/IL-2 Receptor (IL-2R) and LPS/Toll-like Receptor 4 (TLR4) to produce immune-stimulatory or inhibitory effects.
Figure 4. Schematics of the TIM3/Gal9 pathway in innate immune cells.
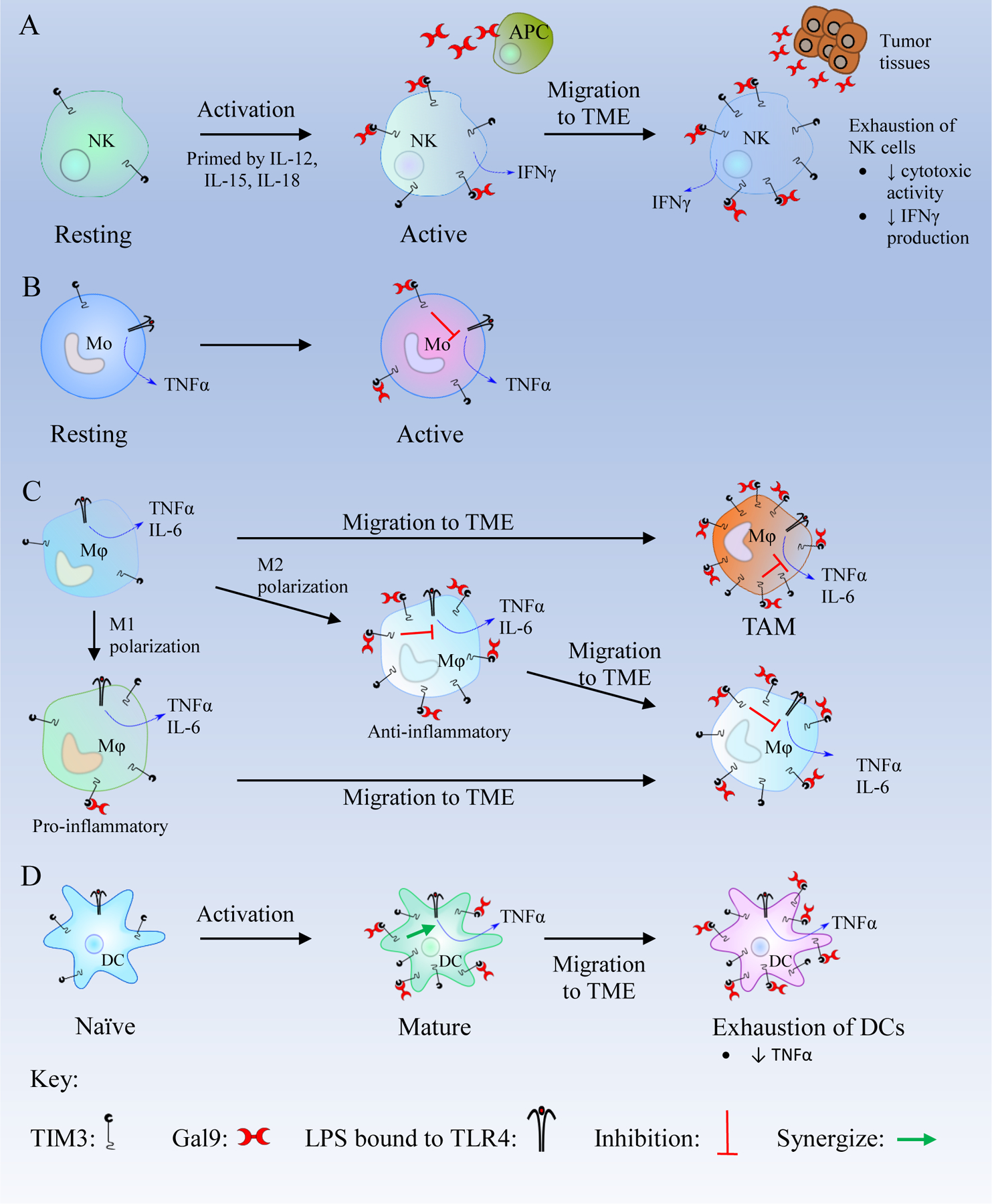
(A) Resting natural killer (NK) cells become active in the presence of IL-12, IL-15, and IL-18 and express more T cell immunoglobulin and mucin-containing protein 3 (TIM3). In active NK cells, TIM3/Galectin 9 (Gal9) binding enhances interferon γ (IFNγ) secretion. After migration to the tumor microenvironment (TME), NK cells are exposed to high levels of Gal9 which leads to their exhaustion.
(B) Active monocytes (Mo) express a high number of TIM3 receptors. The TIM3/Gal9 interaction inhibits the Lipopolysaccharide (LPS)/Toll like receptor 4 (TLR4) pathway and decreases the secretion of TNFα. (C) Macrophages (Mφ) in the TME overexpress TIM3. Macrophages are polarized to M1 and M2 phenotypes. M1 macrophages are pro-inflammatory and express a low level of TIM3. M1 macrophages can migrate to the TME and may transform to M2 macrophages. M2 macrophages are anti-inflammatory and express high levels of TIM3. TIM3/Gal9 binding in M2 macrophages inhibits LPS/TLR4 pathway and decreases the production of inflammatory cytokines like Tumor Necrosis Factor α (TNFα), interleukin-6 (IL-6), and IL-12. After migrating to the TME, M2 macrophages overexpress TIM3, and the TIM3/Gal9 binding leads to the inhibition of the LPS/TLR4 pathway to suppress the immune response. (D) Mature DCs express a high level of TIM3, and the TIM3/Gal9 binding synergizes with the LPS/TLR4 pathway which produces TNFα. In Dendritic cells (DCs) within the TME, the TIM3/Gal9 interaction suppresses CXCL9 expression, thus inhibiting innate immunity and promote tumor growth.
In monocytes and macrophages (M/Mϕ), Gal9 bound TIM3 crosstalks with TLR4 receptors, hence increasing the production of IL-10 but reducing the production of cytokines like TNFα, IL-6, and IL-12 (Figure 4B and 4C) [45, 73]. Similar findings were reported in human CD14+M/Mϕ, TIM3 acts as a brake that controls TLR4 and TLR7, thus inhibiting TLR-mediated IL-2 production [44]. In active monocytes, the inhibition of LPS/TLR4 pathway is found to be reversed after treatment with mAbs that block the TIM3/Gal9 interaction [74]. Zhang and colleagues demonstrated that the TIM3/Gal9 interaction inhibits the function of macrophages by decreasing the production of inflammatory mediators. Furthermore, TIM3/Gal9 binding, in an autocrine fashion, regulates M1/M2 polarization via LPS stimulation. Short term LPS exposure in macrophages upregulates TIM3/Gal9 signaling, subsequently inhibiting M1 polarization. Conversely, long term LPS stimulation results in downregulation of TIM3/Gal9 pathway, resulting in inhibition of M2 polarization [75]. The upregulation of TIM3 expression is correlated with M2 polarization while downregulation is correlated with the M1 polarization of macrophages (Figure 4C). However, a study by Ma et al. reported a different finding regarding the TIM3/Gal9 interaction on M/Mϕ. Trans ligation of Gal9 with TIM3 negatively regulates M/Mϕ and decreases the TLR-mediated IL-12 expression, whereas cis ligation of Gal9 with TIM3 increases the expression of IL-12 and IL-23 via STAT3 phosphorylation resulting in enhanced inflammatory responses [76].
In DCs, the TIM3/Gal9 interaction synergizes with TLRs to promote inflammation [18]. Although the role of the TIM3/Gal9 interaction in DCs has not been fully elucidated, ligation of TIM3 with another ligand HMGB1 suppresses DC function. A recent study has shown that within the TME, Gal9 binds to TIM3 present on DCs, inhibiting anti-tumor immunity by suppression of the CXCL9 expression [77]. The TIM3/Gal9 interaction on DC is assumed to be in synergy with the TLRs and activates their innate immunity (Figure 4D) [18].
In summary, several studies have demonstrated the inhibitory role of TIM3/Gal9 interaction as it down-regulates Th1-mediated anti-tumor immunity as well as inhibits the autoimmune and alloimmune responses [3, 78]. On the contrary, Gal9 binding with TIM3 expressed on innate immune cells like DCs and NK cells enhances the immune response promoting inflammation. [18, 79]. These intriguing findings complicate the comprehension of the TIM3/Gal9 pathway. Further studies are necessary to understand the definitive molecular mechanism of the TIM3/Gal9 pathway in the innate immune system as well as the adaptive immune system.
5. TIM3/Gal9 pathway in cancers
Perturbation of the TIM3/Gal9 pathway results in various disease conditions like autoimmune diseases, infections, cancers, and complications of pregnancy. Numerous studies have shown increased levels of TIM3 and Gal9 in cancers including Acute Myeloid Leukemia (AML), prostate cancer, non-small cell lung cancer (NSCLC), esophageal squamous cell carcinoma (ESCC), breast cancer, glioma, head and neck cancer, colon cancer, melanoma, and lung adenocarcinoma (Table 3).
Table 3.
Roles of the TIM3/galectin9 pathway in various cancers
| Tumor type | Pathophysiology | Study nature/type | References |
|---|---|---|---|
| AML |
In AML cells ● Increase in TIM3 and Gal9 expression in AML cells. ● Autocrine TIM3/Gal9 binding facilitates self-renewal of AML cells that leads to the progression of the disease. In NK cells ● Gal9 released by AML cells binds to TIM3 present on NK cells decreasing the NK cytotoxicity activity. |
● In vitro study | [83] |
| ● Clinical study (blood samples from patients) | [85] | ||
| Prostate cancer | ● Enhanced TIM3 expression on CD4 and CD8 T cells. TIM3/gal9 binding results in suppression of anti-tumor immunity. ● TIM3 expression on T cells is related to the advancement of Prostate cancer. |
● Clinical study (Tissue specimens from patients) | [21] |
| NSCLC | ● High level of exosomal TIM3 and Gal9 present in the plasma that is correlated with the advanced stage of tumor and distant metastasis | ● Clinical study (blood samples) | [95] |
| Esophageal squamous cell cancer | ● Increased TIM3 expression indicates a poor prognosis of the disease. ● High TIM3 and low Gal9 expression shortened the overall survival of patients. |
● Clinical study (blood samples) | [96] |
| Breast cancer | ● Overexpression of TIM3 and Gal9 is seen in TILs and tumor cells. ● TIM3/Gal9 coupling leads to apoptosis of T cells leading to the suppression of host anti-tumor immunity. |
● Clinical study (human tissue samples), and ● In vitro study |
[87] |
| Glioma | ● Increased TIM3 expression on CD4 and CD8 T cells as well as high expression of Gal9 on tumor cells resulting in apoptosis of T cells. | ● Clinical study (blood samples and glioma tissues) |
[89] |
| Osteosarcoma | ● Increased expression of TIM3 and Gal9 are found in tumor-associated macrophages and T cells. | ● Clinical study (blood sample) | [74] |
| ● TIM3/Gal9 binding results in decreased production of IFNγ subsequently compromising the anti-inflammatory immune activity. | |||
| Colorectal cancer | ● Elevated TIM3 expression and binding of TIM3 with Gal9 causes exhaustion of TILs leading to tumor progression. | ● In vitro study, and ● In vivo study |
[90] |
| Gastrointestinal stromal tumors | ● Increased TIM3 expression in infiltrated NK cells and Gal9 expression seen in gastrointestinal stromal tumors. ● Interaction between TIM3/Gal9 is followed by a decrease in the cytotoxic activity of NK cells (dysfunctional NK cells). |
● Clinical study (Cancer tissue specimen) | [94] |
| Head and neck cancer | ● Increased TIM3 expression on Tregs increases the differentiation of Tregs (there is increased numbers of Tregs). ● TIM3/Gal9 binding on Tregs increases the production of inhibitory cytokines (IL-10, IL-35, perforins, granzyme A and granzyme B) ● Suppression of T cell activity is potentiated. |
● Clinical study (Head and neck squamous cell carcinoma samples), and ● In vitro study |
[92] |
| Lung adenocarcinoma | ● Increased TIM3 and Gal9 expression and their interaction result in suppression of T cell-mediated immunity. ● PD-1 is co-expressed with TIM3. Co-blockade of TIM3 and PD-1 minimizes anti-PD-1 therapy resistance. |
● In vitro study, ● In vivo study, and ● Clinical study (biopsies and effusions) |
[97] |
| Advanced melanoma | ● Increased TIM3 on T cells. ● TIM3 is co-expressed with PD-1 resulting in T cell exhaustion. |
● Ex vivo study, and ● Clinical study (blood sample) |
[80] |
| Hepatocellular carcinoma | ● Increased TIM3 and gal9 expression found on CD4 and CD8 T cells along with Kupffer cells compared to adjacent normal cells. ●TIM3/Gal9 binding decreases the number of TILs consequently suppressing the T cell mediated immunity. |
● Clinical study (tumor samples) | [88] |
| Human papilloma virus positive oropharyngeal head and neck squamous cell carcinoma | ● Increased TIM3 expression on monocytes and Gal9 expression by CD4 T cells observed. ● Exogenous as well as endogenous Gal9 binding with TIM3 leads to suppression of anti-tumor immune response. |
● Clinical study (blood samples) | [91] |
5.1. Hematological malignancy
Unlike healthy white blood cells, primary human AML cells and primary human CD34+ cells express latrophilin 1 (LPHN1) on their surface. LPHN1 binds to its receptor fibronectin leucine-rich repeat transmembrane protein (FLRT3) and activates the PKCα and mTOR pathways, which help in synthesis and secretion of TIM3 and Gal9 [82]. Intracellularly synthesized Gal9 lacks the signal peptide that is responsible for Gal9 secretion. TIM3 is known to help traffic Gal9 from the intracellular region to the extracellular region, which is facilitated by the SNARE complex [83]. The released TIM3 and Gal9 interact with each other and may result in a conformational change of Gal9. This conformational change enhances the binding ability of the unbound CRD of Gal9 to interact with TIM3 present in NK cells [84]. This interaction of Gal9 with TIM3 on NK cells leads to the inhibition of the cytotoxic effect of NK cells and their ability to kill AML cells [82]. Similarly, overexpression of TIM3 and Gal9 has also been reported in leukemic stem cells (LSCs) compared to normal healthy stem cells [85]. The in vitro study performed in CD34+ AML cells has shown that TIM3/Gal9 interaction via an autocrine loop on CD34+ AML cells activates the NF-kB and Beta-catenin pathways [85]. Activation of these pathways is believed to play an important role in the self-renewal of human AML cells decreasing patient survival [86]. However, these signaling pathways are not active in normal HSCs. It suggests that the blockade of TIM3/Gal9 coupling could be a potential therapy for myeloid malignancies and to prevent the transformation of preleukemic disease to AML [85].
5.2. Solid malignancies
As the immunoinhibitory role of TIM3 when it binds to Gal9 is well known, blockade of TIM3 is a promising therapeutic approach for a wide range of cancers. For example, increased expression of TIM3 on T cells and Gal9 on tumor cells is observed in solid tumors such as breast cancer, prostate cancer, glioma, head and neck cancer, osteosarcoma, NSCLC, and ESCC.
In breast cancer cells, the expressions of TIM3 and Gal9 were high compared to healthy tissues. Latrophilin isoforms LPHN2 and LPHN3, which are expressed in breast cancer tissues, are responsible for the secretion of Gal9. Binding of FLRT3 to LPHN2/LPHN3 facilitates the secretion of Gal9 via the phospholipase C/phosphokinase C pathway. In various solid and liquid tumors, the FLRT3/LPHN/TIM3/Gal9 pathway is believed to be involved in the translocation of TIM3 and Gal9 onto the cellular surface [87].
Recent studies have shown the overexpression of TIM3 on TILs and Gal9 on tumor cells, and their expression is correlated with the advancement of prostate cancer [21, 87]. The increased level of TIM3 expression on CD4+ T cells and CD8+ T cells were associated with a higher Gleason score and higher preoperative prostate-specific antigen (PSA) levels, suggesting TIM3 as an indicator of prostate cancer progression [21]. Similarly, increased expression of TIM3 on T cells and Gal9 on Kupffer cells was observed in hepatocellular carcinoma [88].
In glioma, there is a high expression of TIM3 and Gal9 compared to healthy brain cells. High TIM3 expression is associated with WHO grade II-IV glioma and is related to the progression of glioma. In an ex vivo study, TIM3 expression was found to be lower in healthy peripheral blood mononuclear cells (PBMCs), but TIM3 and Gal9 levels were elevated in TILs in glioma. As the severity of glioma progresses from Grade II/III to Grade IV, TIM3 expression on CD4+ and CD8+ T cells and Gal9 expression in tumors increase [89]. Likewise, in colorectal cancer, an in vitro study demonstrated that the treatment with anti-TIM3 antibody significantly decreased the Gal9-induced apoptosis of TILs, and an in vivo study showed that the use of anti-TIM3 antibody blocked the TIM3/Gal9 pathway, increasing the activity of cyclophosphamide in a CT26 mouse colon tumor model [90].
In human papilloma virus-positive oropharyngeal head and neck squamous cell carcinoma, overexpression of TIM3 on monocytes and Gal9 on CD4 T cells were observed [91]. Another study reported that the TIM3/Gal9 interaction suppresses anti-tumor immunity in head and neck cancer. The expression of TIM3 and Gal9 in the tumor stroma is positively correlated with the expression of Foxp3 (a Treg marker), CD68, and CD163 (a macrophage marker) [92]. Hence, there is an increase in the level of Tregs and M2 macrophages. The expression of TIM3 on CD4+CD25+Foxp3+ Treg cells results in the higher secretion of inhibitory cytokines IL-10 and TGFβ [93], perforins, granzyme A, and granzyme G [64]. Treatment with an anti-TIM3 mAb (RMT3–23) in a HNSCC mouse model reduced the number of CD4+CD25+Foxp3+ Treg cells and increased the percentage of CD8+ T cells, leading to high production of IFNγ [92]. Evidences have been reported that TIM3 blockade in early-stage tumors inhibits the immune suppressive function of TIM3+ Tregs while TIM3 blockade in advanced-stage tumors restores CD8+ T cell function [64, 92].
Similarly, higher expression of TIM3 and Gal9 was observed in tumor-associated macrophages isolated from osteosarcoma patients compared to non-cancer patients [74]. As in other cancers, there is a diminution of IFNγ production after the TIM3/Gal9 interaction in osteosarcoma, and the blockade of this interaction enhances the production of cytokines from CD4+ cells [74]. Like in other tumors, there is enhanced expression of TIM3 in TILs and Gal9 expression in gastrointestinal stomal tumors. After infiltration of NK cells into the tumor cells, NK cells express TIM3 whereas gastrointestinal stromal tumor cells express Gal9. When TIM3 and Gal9 interact, anti-tumor immunity is suppressed [94].
The level of exosomal TIM3 and Gal9 was found to be significantly higher in the plasma of NSCLC patients than in the plasma of healthy donor [95]. The increased expression of TIM3 and Gal9 correlates with tumor size, tumor metastasis as well as progression to advanced stages of NSCLC [95]. Also, in patients suffering from ESCC, a better survival rate post-operation was seen in tumor samples with low TIM3 expression whereas increased TIM3 expression showed poor survival rate [96]. The binding of TIM3 with Gal9 results to the suppression of anti-tumor immune response. Therefore, the blockade of TIM3 and Gal9 can be used as a therapeutic strategy for the treatment of various cancer.
5.3. Co-targeting TIM3/Gal9 and PD-1/PD-L1 pathways
Co-expression of TIM3 and PD-1 in cancers like colorectal cancer [98], mammary cancer [99], lung cancer [100], melanoma [80], and AML [101] have been reported. Co-expression of TIM3 and PD-1 on TILs decreases the production of IL-2, TNFα, and IFNγ, resulting in dysfunction or impairment of T cells in the tumors [24, 80]. Immunotherapy treatment with anti-PD-1/PD-L1, along with an antibody that blocks TIM3, has been found to be beneficial in various chemotherapy-resistant cancers. In a clinical study, combination therapy of Selinexor and HiDAC (high dose cytarabine) + Mitoxantrone in AML patients showed overexpression of PD-1 and TIM3 in patients in the total failure group as compared to patients in the complete remission group. Chemotherapy resistance was found in cancers where immune checkpoint proteins like TIM3 and PD-1 are involved [102]. Increased expression of PD-1 and its ligand PD-L1 along with TIM3 and its ligand Gal9 could be the reason for the failure of the combination chemotherapy. The use of immunotherapy that intervene with TIM3/Gal9 and PD-1/PD-L1 together with chemotherapy could diminish the drug resistance. Expression of Gal9 and PDL1 is prominent in AML cells, and the coupling of these ligands with their respective receptors on T cells leads to T cells dysfunction. Thus, the co-expression of TIM3 and PD-1 on T cells is an indication of exhausted T cells [101, 103]. Therefore, blocking either the TIM3/Gal9 pathway or the PD-1/PD-L1 pathway alone does not slow the progression of AML [101]. The combined blockade of two different immune checkpoints like TIM3/Gal9 and PD-1/PD-L1 could restore T cell function.
Evidence from previous studies and the ongoing clinical trial with combined immunotherapy targeting TIM3/Gal9 and PD-1/PD-L1 (NCT03066648) in myeloid leukemia provides insight into the use of immune checkpoint inhibitors in combination therapy [104]. It also suggests that co-blockade of different immune checkpoint proteins reduces drug resistance in tumors and restores the immunity of T cells against tumors. For example, the combination of anti-PD-L1 antibody or bintrafusp alpha (an anti-PD-L1 fusion peptide) with M6903 (an anti-human TIM3 antibody) increase the activity of M6903 [105]. Furthermore, in an in-vivo study of lung adenocarcinoma, Koyama et al. have shown that upregulation of TIM3 on TILs and Gal9 on KRAS-mutant tumors with anti-PD-1 resistance, proposing the idea that other immune checkpoint proteins could be expressed in tumors [97]. In an ex vivo study in advanced melanoma, expressions of TIM3 and PD-1 were found to be upregulated in NY-ESO-1-specific CD8+ T cells indicating the exhaustion of T cells. TIM3+PD-1+ NY-ESO-1–specific CD8+ T cells suppress the T cell-mediated immunity more significantly than TIM3− PD-1+ NY-ESO-1 CD8+ T cells and TIM3− PD-1− NY-ESO-1 CD8+ T cells. Upon TIM3/Gal9 binding, the TIM3+ PD-1+ NY-ESO-1-specific CD8+ T cells suppress the T cell immunity by decreasing the production of IFNγ, TNFα, and IL-2. TIM3 and PD-1 overexpression on T cells are prone to a higher percentage of dysfunctional T cells [80]. Elevated expression of TIM3 on T cells and Gal9 on Kupffer cells were observed in hepatocellular carcinoma leading to the decrease in effector T cell functions. Also, the suppression of anti-tumor immunity is displayed by the TIM3/Gal9 and B7-H (PD-L1)/PD-1 axis in hepatocellular carcinoma [88]. These studies suggest that the combined blockade of TIM3/Gal9 and PD-1/PD-L1 might restore CD4+ and CD8+ T cell function and thus be a breakthrough in the field of cancer immunotherapy. Blocking TIM3/Gal9 pathway along with the anti-PD-1 therapy would reduce the chances of drug resistance and improve the efficacy of anti-PD-1 therapy in lung cancer. In line with previous studies, a clinical study by Limagne et al. also supports the idea of combination immunotherapy [106]. Because of anti-PD-1 resistance, only about one-fourth of patients responded to the antiPD-1 drug Nivolumab, approved for use in NSCLC [100]. Limagne et al. have demonstrated the possible underlying mechanism for the resistance to anti-PD-1 therapy in NSCLC and the resistance may be due to upregulation of TIM3/Gal9 and other immune checkpoint proteins. The primary and secondary resistance to Nivolumab could be due to increased TIM3 expression on lymphoid cells and increased production of Gal9 on monocytic MDSCs which diminishes CD8+ T cell function. Genetic mutations like neoantigen removal or mutation adapter proteins like PTEN, JAK/STAT, or HLA genes also have been found to cause immunotherapy resistance [107, 108].
Principally, the TIM3/Gal9 and PD-1/PD-L1 pathway are important immune checkpoint pathways responsible for the pathogenesis of several carcinomas. Inhibiting TIM3/Gal9 binding along with PD-1/PD-L1 axis could be a valuable approach for the treatment of different cancers.
6. TIM3/Gal9 pathway in other diseases
6.1. Autoimmune diseases
In addition to cancers, TIM3/Gal9 interaction has also been documented in autoimmune diseases (multiple sclerosis, rheumatoid arthritis, and Type 1 diabetes), viral infections, and protozoal infections. In a clinical study, patients with multiple sclerosis were treated with glatiramer or IFNβ. Increased TIM3 expression was observed in both glatiramer and IFNβ-treated patients compared to untreated patients, suggesting the recovery and maintenance of a basal level of TIM3 [109]. TIM3/Gal9 interaction causes T cell exhaustion and death, which decreases autoreactive T cells and improves autoimmune diseases. Type 1 diabetes is an autoimmune disease, characterized by activation of innate immunity, proliferation of autoreactive CD4+ and CD8+ T cells that may damage β cells [110]. Gal9 activates DCs to induce the production of TNFα, and these activated DCs play a crucial role in the activation of Th1 and autoimmune response. Treating patients with RMT3–23 (anti-TIM3 mAb) resulted in decreased production of TNFα by DCs and inhibits the downstream Th1 functions. This result demonstrates that the TIM3/Gal9 pathway blockade on DCs could be a potential therapeutic target in type 1 diabetes mellitus [110, 111].
Significant downregulation of TIM3 and Gal9 is found in peripheral blood cells in patients suffering from thyroid-associated ophthalmopathy (TAO), an autoimmune disease. It is thought that Th1, and Th17 play a role in the pathogenesis of TAO [112]. mRNA expression of TIM3 and Gal9 was found to be significantly lower in TAO patients compared to healthy subjects. In an in vitro study, treatment using Gal9 blocking antibody blocked TIM3/Gal9 downstream function and resulted in increased IFNγ, TNFα, and IL-17 levels showing TIM3/Gal9 has a negative correlation with disease severity. The TIM3/Gal9 pathway suppresses T cell-mediated cytokine release in TAO fibroblasts, suppresses the Akt/NFKB pathway, and alleviates TAO [113]. The TIM3/Gal9 interaction has shown to suppress inflammation and immune responses in TAO.
6.2. Other diseases
In chronic hepatitis B infection, higher TIM3 expression on NK cells was observed. TIM3/Gal9 binding inhibits the cytotoxic function of NK cells, leading to the compromised antiviral immunity of the host. Blockade of the TIM3/Gal9 axis helped restore the cytotoxic function of NK cells [19]. Another study in a hepatitis B virus mouse model showed that TIM3/Gal9 coupling lowered the INFγ production and led to the exhaustion of CD8+ T cells. This resulted in the inhibition of antiviral immunity of infiltrating CD8+ T cells. [114]. Also, plasma endotoxin and the cytokine IL-10 are responsible for overexpression of TIM3 and PD-1 on acute alcoholic hepatitis leading to the suppression of host immunity [115]. Co-blockade of TIM3 and PD-1 pathways have shown to generate a synergistic effect in anti-viral immunity of host against hepatitis B virus and chronic hepatitis B infection [116].
In a murine malaria model, the number of Gal9- and TIM3-positive cells are increased in the lungs, liver, and spleen, suggesting an important role of the TIM3/Gal9 pathway in the damage of these organs [117, 118]. In a clinical study, the plasma level of Gal9 was found higher in severe malaria patients compared to uncomplicated cases. Moreover, Gal9 is associated with the severity of malaria. It is postulated that Gal9 inhibits immune response in malaria patients by binding to TIM3 [119]. As a result, malarial infection could be treated by blocking the TIM3/Gal9 pathway. In a PbANKA-infected mouse model, TIM3/Gal9 pathway blockade with anti-TIM3 antibody showed an increase in the mRNA level of cytokines, including IFNγ, TNFα, IL-10, and IL-4. This finding suggests that antibody-mediated blockade of TIM3 contributes to the therapeutic clearance of malaria infection [120].
7. TIM3 and Gal9 as potential biomarkers
The discovery and identification of noninvasive biomarkers help identify cancer in the early stages and develop treatment strategies as well. Identification of non-invasive biomarkers would be beneficial to patients as they could bypass invasive surgical procedures like biopsies. Numerous ongoing studies aim to identify the relevant biomarkers to predict the sensitivity, potency, and efficacy of the immune checkpoint therapy; however, the perplexing mechanism of immune checkpoints make biomarker identification more challenging. While the role of TIM3 and Gal9 in cancer development has been recognized and widely studied, TIM3 and Gal9 as biomarkers are still being explored. We have summarized the recent studies on the possible use of TIM3 and Gal9 as prognostic and predictive biomarkers in various diseases.
High TIM3 expression on tumor-infiltrating T cells and increased Gal9 level in tumor cells in the TME are associated with numerous tumors. For example, high levels of plasma exosomal TIM3 and Gal9 are related to advanced tumor stages, large tumor size, and metastasis in NSCLC [95]. Upregulated Gal9 expression on tumor cells and TIM3 expression on CD8+ T cells are observed in gastric cancer [121], glioma [89], chronic lymphocytic leukemia [122], and esophageal squamous cell carcinoma [96]. Increased infiltration of TIM3+ T cells in tumors and increased Gal9 expression by tumor cells are inversely proportional to overall survival in cancer patients. The presence of tumor DNA in blood circulation has been reported in various cancers [123, 124]. Isolation of tumor DNA from blood samples to assess DNA methylation or histone distributions of TIM3 and Gal9 genes could be used for prognostic or diagnostic purposes. A study performed in primary breast cancer patients to examine the promoter CpG profile in various immune checkpoint receptor genes found TIM3 promoter to be hypomethylated, whereas the gene body of TIM3 was hypermethylated. In addition, the distribution of repressive histone H3k27me3 was also found to be reduced in the TIM3 promoter region [125]. Similarly, in melanoma patients, DNA methylation of the TIM3 and Gal9 promoter region was inversely correlated with the mRNA expression of TIM3 and Gal9. mRNA expression of TIM3 and Gal9 is positively correlated with tumor infiltrating leucocyte fraction, but DNA methylation of TIM3 and Gal9 gene is negatively correlated with infiltrating leucocyte fraction in the tumor environment [126].
In addition to cancers, TIM3 and Gal9 expressions are prominent in other diseases like systemic lupus erythematosus, antiphospholipid syndrome [127], allograft rejection dysfunction [128], endometriosis [129], and, hepatitis C [130]. Gal9 levels in the serum can be used clinically as a stable biomarker in detecting IFN signature in systemic lupus erythematosus and antiphospholipid syndrome [127]. Li et al. showed that the serum levels of soluble TIM3 (sTIM3) and soluble Gal9 (sGal9) were found to be elevated in patients with allograft rejection, making sTIM3 and sGal9 promising biomarker for detection of allograft rejection dysfunction [128]. Elevated Gal9 levels were observed in the serum in endometriosis patients. Recently, a pilot study suggested that Gal9 is a promising biomarker for the diagnosis of endometriosis with a sensitivity of 94% and a specificity of 93.75% [129]. By contrast, a meta-analysis showed that CA125, a gold standard biomarker for endometriosis, only exhibit a sensitivity of 52 % and a specificity of 93% [131]. These results indicate that Gal9 could be a sensitive biomarker of endometriosis and other gynecological disorders. Additionally, increased Gal9 level is found in the blood of patients with hepatitis C infection, making it a useful predictive biomarker for hepatitis C [130]. Although the TIM3/Gal9 pathway is not fully understood, the higher level of sTIM3 and sGal9 is observed in the plasma during cancers and other diseases which can be exploited as potential biomarkers.
8. Conclusion and Perspective
Altogether, in CD4+ and CD8+ T cells, the TIM3/Gal9 interaction results in reduced production of cytokines (IFNγ, IL-2 and TNFα), inhibits T cell proliferation, and induces apoptosis of T cells, resulting in suppression of Th1 like immunity. The TIM3/Gal9 interaction is believed to have stimulatory or inhibitory activity in innate immunity depending on the type of innate immune cells. This pathway is critical for maintaining the immunity against self-antigens and important for preventing the onset of autoimmunity. In the TME, the interaction of TIM3 with its ligand Gal9 is believed to inhibit both adaptive and innate immunity, which favors tumor progression. Although there are perplexing physiological discoveries on the stimulatory and inhibitory effect of the TIM3/Gal9 pathway on different types of immune cells, these findings suggest that the TIM3/Gal9 pathway is involved in the pathogenesis of various types of cancers. Collective studies have highlighted the inhibitory role of the TIM3/Gal9 signaling pathway in the progression of tumors to their advanced stage. The inhibition of TIM3 interaction with Gal9 could be a potential therapeutic approach for the treatment of various cancers. In addition, monotherapy using a single immune checkpoint inhibitor is likely to develop resistance. As a result, co-blockade of more than one immune checkpoint protein, such as blocking the TIM3/Gal9 and PD-1/PD-L1 pathways simultaneously, reduces the possibility of developing drug resistance. More studies are needed to unravel the complete molecular mechanism of the TIM3/Gal9 pathway to broaden its therapeutic applications for cancer therapy.
Furthermore, overexpression of TIM3 or Gal9 in the TME and elevated serum levels of TIM3 or Gal9 can be used as a predictive or prognostic biomarker to help the optimization and selection of appropriate therapeutic options in cancers. The identification of TIM3 and Gal9 as biomarkers in the serum could accelerate the development of non-invasive and early-stage cancer diagnoses.
Highlights:
Gal9 is an important ligand of TIM3.
The TIM3/Gal9 interaction is known to suppress the adaptive immune system.
Increased levels of TIM3 and Gal9 are evident in various cancers.
Blockade of TIM3/Gal9 pathway can be exploited for the treatment of different cancers.
Co-blockade of TIM3/Gal9 pathway with PD-1/PD-L1interaction overcomes the resistance against immune therapy.
Increased serum levels of TIM3 and Gal9 could serve as a potential biomarker for cancer diagnosis.
Acknowledgements
This work is partially supported by the awards (R01CA231099 and R01AA021510) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare that they have no competing interests.
Declaration of interests
⊠The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Darvin P, Toor SM, Sasidharan Nair V, Elkord E, Immune checkpoint inhibitors: recent progress and potential biomarkers, Exp Mol Med, 50 (2018) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pardoll DM, The blockade of immune checkpoints in cancer immunotherapy, Nat Rev Cancer, 12 (2012) 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK, The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity, Nat Immunol, 6 (2005) 1245–1252. [DOI] [PubMed] [Google Scholar]
- [4].Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH, TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity, Immunol Rev, 235 (2010) 172–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, Fujioka Y, Ohba Y, Gorman JV, Colgan JD, Hirashima M, Uede T, Takaoka A, Yagita H, Jinushi M, Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1, Nat Immunol, 13 (2012) 832–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Huang YH, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, Dougan SK, Petersen BS, Melum E, Pertel T, Clayton KL, Raab M, Chen Q, Beauchemin N, Yazaki PJ, Pyzik M, Ostrowski MA, Glickman JN, Rudd CE, Ploegh HL, Franke A, Petsko GA, Kuchroo VK, Blumberg RS, CEACAM1 regulates TIM-3-mediated tolerance and exhaustion, Nature, 517 (2015) 386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wolf Y, Anderson AC, Kuchroo VK, TIM3 comes of age as an inhibitory receptor, Nat Rev Immunol, (2019). [DOI] [PMC free article] [PubMed]
- [8].Solinas C, De Silva P, Bron D, Willard-Gallo K, Sangiolo D, Significance of TIM3 expression in cancer: From biology to the clinic, Semin Oncol, 46 (2019) 372–379. [DOI] [PubMed] [Google Scholar]
- [9].Lindsted T, Gad M, Grandal MV, Frölich C, Bhatia VK, Gjetting T, Lantto J, Horak ID, Kragh M, Koefoed K, Pedersen MW, Abstract 5629: Preclinical characterization of Sym023 a human anti-TIM3 antibody with a novel mechanism of action, Cancer Research, 78 (2018) 5629–5629. [Google Scholar]
- [10].Zhang T, Xue L, Zhang J, Liu Q, Ma J, Zhang Y, Shi Y, Hou H, Peng H, Liu N, Zhang Y, Song X, Li Y, Song J, Wang L, Wei M, Li K, Abstract 2628: BGB-A425: a humanized anti-human Tim-3 antibody that exhibits strong immune cell activation, Cancer Research, 77 (2017) 2628–2628. [Google Scholar]
- [11].Haidar JN, Antonysamy S, Mathew S, Wu L, Zhang Y, Kearins MC, Shen L, Sauder JM, Schaer D, Driscoll KE, Kalos M, Abstract 2753: The molecular basis of blocking the TIM-3 checkpoint with the LY3321367 mAb in cancer immunotherapy, Cancer Research, 79 (2019) 2753–2753. [Google Scholar]
- [12].Murtaza A, Laken H, Correia J.d.S., McNeeley P, Altobell L, Zhang J-G, Vancutsem PM, Wilcoxen KM, Jenkins D, Discovery of TSR-022, a novel, potent anti-human TIM-3 therapeutic antibody, European Journal of Cancer, 69 (2016). [Google Scholar]
- [13].Waight J, Iyer P, Breous-Nystrom E, Riordan C, Findeis M, Underwood D, Connolly J, Sanicola-Nadel M, Nastri H, Scherle P, Hollis G, Huber R, Stein R, Dijk M.v., Wilson NS, Abstract 3825: INCAGN02390, a novel antagonist antibody that targets the co-inhibitory receptor TIM-3, Cancer Research, 78 (2018) 3825–3825. [Google Scholar]
- [14].Anderson AC, Tim-3, a negative regulator of anti-tumor immunity, Curr Opin Immunol, 24 (2012) 213–216. [DOI] [PubMed] [Google Scholar]
- [15].Cheng L, Ruan Z, Tim-3 and Tim-4 as the potential targets for antitumor therapy, Hum Vaccin Immunother, 11 (2015) 2458–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liu F, Liu Y, Chen Z, Tim-3 expression and its role in hepatocellular carcinoma, J Hematol Oncol, 11 (2018) 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fernandez-Santamaria R, Palomares F, Salas M, Dona I, Bogas G, Ariza A, Rodriguez-Nogales A, Plaza-Seron MC, Mayorga C, Torres MJ, Fernandez TD, Expression of the Tim3-galectin-9 axis is altered in drug-induced maculopapular exanthema, Allergy, 74 (2019) 1769–1779. [DOI] [PubMed] [Google Scholar]
- [18].Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, Chandwaskar R, Karman J, Su EW, Hirashima M, Bruce JN, Kane LP, Kuchroo VK, Hafler DA, Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells, Science, 318 (2007) 1141–1143. [DOI] [PubMed] [Google Scholar]
- [19].Ju Y, Hou N, Meng J, Wang X, Zhang X, Zhao D, Liu Y, Zhu F, Zhang L, Sun W, Liang X, Gao L, Ma C, T cell immunoglobulin- and mucin-domain-containing molecule-3 (Tim-3) mediates natural killer cell suppression in chronic hepatitis B, J Hepatol, 52 (2010) 322–329. [DOI] [PubMed] [Google Scholar]
- [20].Nakae S, Iikura M, Suto H, Akiba H, Umetsu DT, Dekruyff RH, Saito H, Galli SJ, TIM-1 and TIM-3 enhancement of Th2 cytokine production by mast cells, Blood, 110 (2007) 2565–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Piao Y, Jin X, Analysis of Tim-3 as a therapeutic target in prostate cancer, Tumour Biol, 39 (2017) 1010428317716628. [DOI] [PubMed] [Google Scholar]
- [22].Friedlaender A, Addeo A, Banna G, New emerging targets in cancer immunotherapy: the role of TIM3, ESMO Open, 4 (2019) e000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Das M, Zhu C, Kuchroo VK, Tim-3 and its role in regulating anti-tumor immunity, Immunol Rev, 276 (2017) 97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC, Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity, J Exp Med, 207 (2010) 2187–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].DeKruyff RH, Bu X, Ballesteros A, Santiago C, Chim YL, Lee HH, Karisola P, Pichavant M, Kaplan GG, Umetsu DT, Freeman GJ, Casasnovas JM, T cell/transmembrane, Ig, and mucin-3 allelic variants differentially recognize phosphatidylserine and mediate phagocytosis of apoptotic cells, J Immunol, 184 (2010) 1918–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lin Y, Zhang L, Cai AX, Lee M, Zhang W, Neuberg D, Canning CM, Soiffer RJ, Alyea EP, Ritz J, Hacohen N, Means TK, Wu CJ, Effective posttransplant antitumor immunity is associated with TLR-stimulating nucleic acid-immunoglobulin complexes in humans, J Clin Invest, 121 (2011) 1574–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dolina JS, Braciale TJ, Hahn YS, Liver-primed CD8+ T cells suppress antiviral adaptive immunity through galectin-9-independent T-cell immunoglobulin and mucin 3 engagement of high-mobility group box 1 in mice, Hepatology, 59 (2014) 1351–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhu C, TIM-3 and its regulatory role in immune responses, Current topics in microbiology and immunology, (2010). [DOI] [PubMed]
- [29].Du W, Yang M, Turner A, Xu C, Ferris RL, Huang J, Kane LP, Lu B, TIM-3 as a Target for Cancer Immunotherapy and Mechanisms of Action, Int J Mol Sci, 18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fujihara S, Mori H, Kobara H, Rafiq K, Niki T, Hirashima M, Masaki T, Galectin-9 in cancer therapy, Recent Pat Endocr Metab Immune Drug Discov, 7 (2013) 130–137. [DOI] [PubMed] [Google Scholar]
- [31].Lai JH, Luo SF, Wang MY, Ho LJ, Translational Implication of Galectin-9 in the Pathogenesis and Treatment of Viral Infection, Int J Mol Sci, 18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chou FC, Chen HY, Kuo CC, Sytwu HK, Role of Galectins in Tumors and in Clinical Immunotherapy, Int J Mol Sci, 19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lu M, Tian X, Yang X, Yuan C, Ehsan M, Liu X, Yan R, Xu L, Song X, Li X, The N- and C-terminal carbohydrate recognition domains of Haemonchus contortus galectin bind to distinct receptors of goat PBMC and contribute differently to its immunomodulatory functions in host-parasite interactions, Parasit Vectors, 10 (2017) 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sideras K, de Man RA, Harrington SM, Polak WG, Zhou G, Schutz HM, Pedroza-Gonzalez A, Biermann K, Mancham S, Hansen BE, Bart Takkenberg R, van Vuuren AJ, Pan Q, Ijzermans JNM, Sleijfer S, Sprengers D, Dong H, Kwekkeboom J, Bruno MJ, Circulating levels of PD-L1 and Galectin-9 are associated with patient survival in surgically treated Hepatocellular Carcinoma independent of their intra-tumoral expression levels, Sci Rep, 9 (2019) 10677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chabot S, Kashio Y, Seki M, Shirato Y, Nakamura K, Nishi N, Nakamura T, Matsumoto R, Hirashima M, Regulation of galectin-9 expression and release in Jurkat T cell line cells, Glycobiology, 12 (2002) 111–118. [DOI] [PubMed] [Google Scholar]
- [36].Oomizu S, Arikawa T, Niki T, Kadowaki T, Ueno M, Nishi N, Yamauchi A, Hattori T, Masaki T, Hirashima M, Cell surface galectin-9 expressing Th cells regulate Th17 and Foxp3+ Treg development by galectin-9 secretion, PLoS One, 7 (2012) e48574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Moar P, Tandon R, Galectin-9 as a biomarker of disease severity, Cell Immunol, 361 (2021) 104287. [DOI] [PubMed] [Google Scholar]
- [38].Nagahara K, Arikawa T, Oomizu S, Kontani K, Nobumoto A, Tateno H, Watanabe K, Niki T, Katoh S, Miyake M, Nagahata S, Hirabayashi J, Kuchroo VK, Yamauchi A, Hirashima M, Galectin-9 increases Tim-3+ dendritic cells and CD8+ T cells and enhances antitumor immunity via galectin-9-Tim-3 interactions, J Immunol, 181 (2008) 7660–7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gleason MK, Lenvik TR, McCullar V, Felices M, O’Brien MS, Cooley SA, Verneris MR, Cichocki F, Holman CJ, Panoskaltsis-Mortari A, Niki T, Hirashima M, Blazar BR, Miller JS, Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9, Blood, 119 (2012) 3064–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Daley D, Mani VR, Mohan N, Akkad N, Ochi A, Heindel DW, Lee KB, Zambirinis CP, Pandian GSB, Savadkar S, Torres-Hernandez A, Nayak S, Wang D, Hundeyin M, Diskin B, Aykut B, Werba G, Barilla RM, Rodriguez R, Chang S, Gardner L, Mahal LK, Ueberheide B, Miller G, Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance, Nat Med, 23 (2017) 556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Vaitaitis GM, Wagner DH Jr., Galectin-9 controls CD40 signaling through a Tim-3 independent mechanism and redirects the cytokine profile of pathogenic T cells in autoimmunity, PLoS One, 7 (2012) e38708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Madireddi S, Eun SY, Lee SW, Nemcovicova I, Mehta AK, Zajonc DM, Nishi N, Niki T, Hirashima M, Croft M, Galectin-9 controls the therapeutic activity of 4–1BB-targeting antibodies, J Exp Med, 211 (2014) 1433–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Clayton KL, Haaland MS, Douglas-Vail MB, Mujib S, Chew GM, Ndhlovu LC, Ostrowski MA, T cell Ig and mucin domain-containing protein 3 is recruited to the immune synapse, disrupts stable synapse formation, and associates with receptor phosphatases, J Immunol, 192 (2014) 782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhang Y, Ma CJ, Wang JM, Ji XJ, Wu XY, Moorman JP, Yao ZQ, Tim-3 regulates pro- and anti-inflammatory cytokine expression in human CD14+ monocytes, J Leukoc Biol, 91 (2012) 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yang X, Jiang X, Chen G, Xiao Y, Geng S, Kang C, Zhou T, Li Y, Guo X, Xiao H, Hou C, Wang R, Lin Z, Li X, Feng J, Ma Y, Shen B, Li Y, Han G, T cell Ig mucin-3 promotes homeostasis of sepsis by negatively regulating the TLR response, J Immunol, 190 (2013) 2068–2079. [DOI] [PubMed] [Google Scholar]
- [46].Tang D, Lotze MT, Tumor immunity times out: TIM-3 and HMGB1, Nat Immunol, 13 (2012) 808–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].van de Weyer PS, Muehlfeit M, Klose C, Bonventre JV, Walz G, Kuehn EW, A highly conserved tyrosine of Tim-3 is phosphorylated upon stimulation by its ligand galectin-9, Biochem Biophys Res Commun, 351 (2006) 571–576. [DOI] [PubMed] [Google Scholar]
- [48].Lee J, Su EW, Zhu C, Hainline S, Phuah J, Moroco JA, Smithgall TE, Kuchroo VK, Kane LP, Phosphotyrosine-dependent coupling of Tim-3 to T-cell receptor signaling pathways, Mol Cell Biol, 31 (2011) 3963–3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rangachari M, Zhu C, Sakuishi K, Xiao S, Karman J, Chen A, Angin M, Wakeham A, Greenfield EA, Sobel RA, Okada H, McKinnon PJ, Mak TW, Addo MM, Anderson AC, Kuchroo VK, Bat3 promotes T cell responses and autoimmunity by repressing Tim-3-mediated cell death and exhaustion, Nat Med, 18 (2012) 1394–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].van Oers NS, Killeen N, Weiss A, Lck regulates the tyrosine phosphorylation of the T cell receptor subunits and ZAP-70 in murine thymocytes, J Exp Med, 183 (1996) 1053–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE, LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation, Cell, 92 (1998) 83–92. [DOI] [PubMed] [Google Scholar]
- [52].Lo WL, Shah NH, Ahsan N, Horkova V, Stepanek O, Salomon AR, Kuriyan J, Weiss A, Lck promotes Zap70-dependent LAT phosphorylation by bridging Zap70 to LAT, Nat Immunol, 19 (2018) 733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lovatt M, Filby A, Parravicini V, Werlen G, Palmer E, Zamoyska R, Lck regulates the threshold of activation in primary T cells, while both Lck and Fyn contribute to the magnitude of the extracellular signal-related kinase response, Mol Cell Biol, 26 (2006) 8655–8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Paz PE, Wang S, Clarke H, Lu X, Stokoe D, Abo A, Mapping the Zap-70 phosphorylation sites on LAT (linker for activation of T cells) required for recruitment and activation of signalling proteins in T cells, Biochem J, 356 (2001) 461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Gaud G, Lesourne R, Love PE, Regulatory mechanisms in T cell receptor signalling, Nat Rev Immunol, 18 (2018) 485–497. [DOI] [PubMed] [Google Scholar]
- [56].Davidson D, Zhong MC, Pandolfi PP, Bolland S, Xavier RJ, Seed B, Li X, Gu H, Veillette A, The Csk-Associated Adaptor PAG Inhibits Effector T Cell Activation in Cooperation with Phosphatase PTPN22 and Dok Adaptors, Cell Rep, 17 (2016) 2776–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Vang T, Torgersen KM, Sundvold V, Saxena M, Levy FO, Skalhegg BS, Hansson V, Mustelin T, Tasken K, Activation of the COOH-terminal Src kinase (Csk) by cAMP-dependent protein kinase inhibits signaling through the T cell receptor, J Exp Med, 193 (2001) 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bergman M, Mustelin T, Oetken C, Partanen J, Flint NA, Amrein KE, Autero M, Burn P, Alitalo K, The human p50csk tyrosine kinase phosphorylates p56lck at Tyr-505 and down regulates its catalytic activity, EMBO J, 11 (1992) 2919–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Davidson D, Schraven B, Veillette A, PAG-associated FynT regulates calcium signaling and promotes anergy in T lymphocytes, Mol Cell Biol, 27 (2007) 1960–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].D’Oro U, Ashwell JD, Cutting edge: the CD45 tyrosine phosphatase is an inhibitor of Lck activity in thymocytes, J Immunol, 162 (1999) 1879–1883. [PubMed] [Google Scholar]
- [61].Baker JE, Majeti R, Tangye SG, Weiss A, Protein tyrosine phosphatase CD148-mediated inhibition of T-cell receptor signal transduction is associated with reduced LAT and phospholipase Cgamma1 phosphorylation, Mol Cell Biol, 21 (2001) 2393–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Moorman JP, Wang JM, Zhang Y, Ji XJ, Ma CJ, Wu XY, Jia ZS, Wang KS, Yao ZQ, Tim-3 pathway controls regulatory and effector T cell balance during hepatitis C virus infection, J Immunol, 189 (2012) 755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Yan J, Zhang Y, Zhang JP, Liang J, Li L, Zheng L, Tim-3 expression defines regulatory T cells in human tumors, PLoS One, 8 (2013) e58006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Sakuishi K, Ngiow SF, Sullivan JM, Teng MW, Kuchroo VK, Smyth MJ, Anderson AC, TIM3(+)FOXP3(+) regulatory T cells are tissue-specific promoters of T-cell dysfunction in cancer, Oncoimmunology, 2 (2013) e23849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wang F, Wan L, Zhang C, Zheng X, Li J, Chen ZK, Tim-3-Galectin-9 pathway involves the suppression induced by CD4+CD25+ regulatory T cells, Immunobiology, 214 (2009) 342–349. [DOI] [PubMed] [Google Scholar]
- [66].Gupta S, Thornley TB, Gao W, Larocca R, Turka LA, Kuchroo VK, Strom TB, Allograft rejection is restrained by short-lived TIM-3+PD-1+Foxp3+ Tregs, J Clin Invest, 122 (2012) 2395–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Sehrawat S, Reddy PB, Rajasagi N, Suryawanshi A, Hirashima M, Rouse BT, Galectin-9/TIM-3 interaction regulates virus-specific primary and memory CD8 T cell response, PLoS Pathog, 6 (2010) e1000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang F, Sun J, Yang Q, Zhang X, Lu B, TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression, PLoS One, 7 (2012) e30676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ndhlovu LC, Lopez-Verges S, Barbour JD, Jones RB, Jha AR, Long BR, Schoeffler EC, Fujita T, Nixon DF, Lanier LL, Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity, Blood, 119 (2012) 3734–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Jost S, Moreno-Nieves UY, Garcia-Beltran WF, Rands K, Reardon J, Toth I, Piechocka-Trocha A, Altfeld M, Addo MM, Dysregulated Tim-3 expression on natural killer cells is associated with increased Galectin-9 levels in HIV-1 infection, Retrovirology, 10 (2013) 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hu XH, Tang MX, Mor G, Liao AH, Tim-3: Expression on immune cells and roles at the maternal-fetal interface, J Reprod Immunol, 118 (2016) 92–99. [DOI] [PubMed] [Google Scholar]
- [72].Li YH, Zhou WH, Tao Y, Wang SC, Jiang YL, Zhang D, Piao HL, Fu Q, Li DJ, Du MR, The Galectin-9/Tim-3 pathway is involved in the regulation of NK cell function at the maternal-fetal interface in early pregnancy, Cell Mol Immunol, 13 (2016) 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kerr H, Richards A, Complement-mediated injury and protection of endothelium: lessons from atypical haemolytic uraemic syndrome, Immunobiology, 217 (2012) 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Li X, Chen Y, Liu X, Zhang J, He X, Teng G, Yu D, Tim3/Gal9 interactions between T cells and monocytes result in an immunosuppressive feedback loop that inhibits Th1 responses in osteosarcoma patients, Int Immunopharmacol, 44 (2017) 153–159. [DOI] [PubMed] [Google Scholar]
- [75].Zhang W, Zhang Y, He Y, Wang X, Fang Q, Lipopolysaccharide mediates time-dependent macrophage M1/M2 polarization through the Tim-3/Galectin-9 signalling pathway, Exp Cell Res, 376 (2019) 124–132. [DOI] [PubMed] [Google Scholar]
- [76].Ma CJ, Li GY, Cheng YQ, Wang JM, Ying RS, Shi L, Wu XY, Niki T, Hirashima M, Li CF, Moorman JP, Yao ZQ, Cis association of galectin-9 with Tim-3 differentially regulates IL-12/IL-23 expressions in monocytes via TLR signaling, PLoS One, 8 (2013) e72488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].de Mingo Pulido A, Gardner A, Hiebler S, Soliman H, Rugo HS, Krummel MF, Coussens LM, Ruffell B, TIM-3 Regulates CD103(+) Dendritic Cell Function and Response to Chemotherapy in Breast Cancer, Cancer Cell, 33 (2018) 60–74 e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Sanchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA, Manlongat N, Bender O, Kamradt T, Kuchroo VK, Gutierrez-Ramos JC, Coyle AJ, Strom TB, Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance, Nat Immunol, 4 (2003) 1093–1101. [DOI] [PubMed] [Google Scholar]
- [79].Nakayama M, Akiba H, Takeda K, Kojima Y, Hashiguchi M, Azuma M, Yagita H, Okumura K, Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation, Blood, 113 (2009) 3821–3830. [DOI] [PubMed] [Google Scholar]
- [80].Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM, Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients, J Exp Med, 207 (2010) 2175–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ocana-Guzman R, Torre-Bouscoulet L, Sada-Ovalle I, TIM-3 Regulates Distinct Functions in Macrophages, Front Immunol, 7 (2016) 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Goncalves Silva I, Yasinska IM, Sakhnevych SS, Fiedler W, Wellbrock J, Bardelli M, Varani L, Hussain R, Siligardi G, Ceccone G, Berger SM, Ushkaryov YA, Gibbs BF, Fasler-Kan E, Sumbayev VV, The Tim-3-galectin-9 Secretory Pathway is Involved in the Immune Escape of Human Acute Myeloid Leukemia Cells, EBioMedicine, 22 (2017) 44–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Goncalves Silva I, Ruegg L, Gibbs BF, Bardelli M, Fruehwirth A, Varani L, Berger SM, Fasler-Kan E, Sumbayev VV, The immune receptor Tim-3 acts as a trafficker in a Tim-3/galectin-9 autocrine loop in human myeloid leukemia cells, Oncoimmunology, 5 (2016) e1195535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Nagae M, Nishi N, Murata T, Usui T, Nakamura T, Wakatsuki S, Kato R, Crystal structure of the galectin-9 N-terminal carbohydrate recognition domain from Mus musculus reveals the basic mechanism of carbohydrate recognition, J Biol Chem, 281 (2006) 35884–35893. [DOI] [PubMed] [Google Scholar]
- [85].Kikushige Y, Miyamoto T, Yuda J, Jabbarzadeh-Tabrizi S, Shima T, Takayanagi S, Niiro H, Yurino A, Miyawaki K, Takenaka K, Iwasaki H, Akashi K, A TIM-3/Gal-9 Autocrine Stimulatory Loop Drives Self-Renewal of Human Myeloid Leukemia Stem Cells and Leukemic Progression, Cell Stem Cell, 17 (2015) 341–352. [DOI] [PubMed] [Google Scholar]
- [86].Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Goktuna SI, Ziegler PK, Canli O, Heijmans J, Huels DJ, Moreaux G, Rupec RA, Gerhard M, Schmid R, Barker N, Clevers H, Lang R, Neumann J, Kirchner T, Taketo MM, van den Brink GR, Sansom OJ, Arkan MC, Greten FR, Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties, Cell, 152 (2013) 25–38. [DOI] [PubMed] [Google Scholar]
- [87].Yasinska IM, Sakhnevych SS, Pavlova L, Teo Hansen Selno A, Teuscher Abeleira AM, Benlaouer O, Goncalves Silva I, Mosimann M, Varani L, Bardelli M, Hussain R, Siligardi G, Cholewa D, Berger SM, Gibbs BF, Ushkaryov YA, Fasler-Kan E, Klenova E, Sumbayev VV, The Tim-3-Galectin-9 Pathway and Its Regulatory Mechanisms in Human Breast Cancer, Front Immunol, 10 (2019) 1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, Liu J, Shi L, Liu C, Wang G, Zou W, Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma, Hepatology, 56 (2012) 1342–1351. [DOI] [PubMed] [Google Scholar]
- [89].Liu Z, Han H, He X, Li S, Wu C, Yu C, Wang S, Expression of the galectin-9-Tim-3 pathway in glioma tissues is associated with the clinical manifestations of glioma, Oncol Lett, 11 (2016) 1829–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Kang CW, Dutta A, Chang LY, Mahalingam J, Lin YC, Chiang JM, Hsu CY, Huang CT, Su WT, Chu YY, Lin CY, Apoptosis of tumor infiltrating effector TIM-3+CD8+ T cells in colon cancer, Sci Rep, 5 (2015) 15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Dong J, Cheng L, Zhao M, Pan X, Feng Z, Wang D, Tim-3-expressing macrophages are functionally suppressed and expanded in oral squamous cell carcinoma due to virus-induced Gal-9 expression, Tumour Biol, 39 (2017) 1010428317701651. [DOI] [PubMed] [Google Scholar]
- [92].Liu JF, Wu L, Yang LL, Deng WW, Mao L, Wu H, Zhang WF, Sun ZJ, Blockade of TIM3 relieves immunosuppression through reducing regulatory T cells in head and neck cancer, J Exp Clin Cancer Res, 37 (2018) 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Sun H, Gao W, Pan W, Zhang Q, Wang G, Feng D, Geng X, Yan X, Li S, Tim3(+) Foxp3 (+) Treg Cells Are Potent Inhibitors of Effector T Cells and Are Suppressed in Rheumatoid Arthritis, Inflammation, 40 (2017) 1342–1350. [DOI] [PubMed] [Google Scholar]
- [94].Komita H, Koido S, Hayashi K, Kan S, Ito M, Kamata Y, Suzuki M, Homma S, Expression of immune checkpoint molecules of T cell immunoglobulin and mucin protein 3/galectin-9 for NK cell suppression in human gastrointestinal stromal tumors, Oncol Rep, 34 (2015) 2099–2105. [DOI] [PubMed] [Google Scholar]
- [95].Gao J, Qiu X, Li X, Fan H, Zhang F, Lv T, Song Y, Expression profiles and clinical value of plasma exosomal Tim-3 and Galectin-9 in non-small cell lung cancer, Biochem Biophys Res Commun, 498 (2018) 409–415. [DOI] [PubMed] [Google Scholar]
- [96].Hou N, Ma J, Li W, Zhao L, Gao Q, Mai L, T-cell immunoglobulin and mucin domain-containing protein-3 and galectin-9 protein expression: Potential prognostic significance in esophageal squamous cell carcinoma for Chinese patients, Oncol Lett, 14 (2017) 8007–8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ, Asahina H, Jones RE, Kulkarni MM, Kuraguchi M, Palakurthi S, Fecci PE, Johnson BE, Janne PA, Engelman JA, Gangadharan SP, Costa DB, Freeman GJ, Bueno R, Hodi FS, Dranoff G, Wong KK, Hammerman PS, Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints, Nat Commun, 7 (2016) 10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Kuai W, Xu X, Yan J, Zhao W, Li Y, Wang B, Yuan N, Li Z, Jia Y, Prognostic Impact of PD-1 and Tim-3 Expression in Tumor Tissue in Stage I-III Colorectal Cancer, Biomed Res Int, 2020 (2020) 5294043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Du H, Yi Z, Wang L, Li Z, Niu B, Ren G, The co-expression characteristics of LAG3 and PD-1 on the T cells of patients with breast cancer reveal a new therapeutic strategy, Int Immunopharmacol, 78 (2020) 106113. [DOI] [PubMed] [Google Scholar]
- [100].Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M, Safety, activity, and immune correlates of anti-PD-1 antibody in cancer, N Engl J Med, 366 (2012) 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, Murphy WJ, Azuma M, Anderson AC, Kuchroo VK, Blazar BR, Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia, Blood, 117 (2011) 4501–4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Dama P, Tang M, Fulton N, Kline J, Liu H, Gal9/Tim-3 expression level is higher in AML patients who fail chemotherapy, J Immunother Cancer, 7 (2019) 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Tan J, Yu Z, Huang J, Chen Y, Huang S, Yao D, Xu L, Lu Y, Chen S, Li Y, Increased PD-1+Tim-3+ exhausted T cells in bone marrow may influence the clinical outcome of patients with AML, Biomark Res, 8 (2020) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Kursunel MA, Esendagli G, A Co-Inhibitory Alliance in Myeloid Leukemia: TIM-3/Galectin-9 Complex as a New Target for Checkpoint Blockade Therapy, EBioMedicine, 23 (2017) 6–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Zhang D, Jiang F, Zaynagetdinov R, Huang H, Sood VD, Wang H, Zhao X, Jenkins MH, Ji Q, Wang Y, Nannemann DP, Musil D, Wesolowski J, Paoletti A, Bartholomew T, Derner MG, An Q, Iffland C, Halle JP, Identification and characterization of M6903, an antagonistic anti-TIM-3 monoclonal antibody, Oncoimmunology, 9 (2020) 1744921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Limagne E, Richard C, Thibaudin M, Fumet JD, Truntzer C, Lagrange A, Favier L, Coudert B, Ghiringhelli F, Tim-3/galectin-9 pathway and mMDSC control primary and secondary resistances to PD-1 blockade in lung cancer patients, Oncoimmunology, 8 (2019) e1564505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Anagnostou V, Smith KN, Forde PM, Niknafs N, Bhattacharya R, White J, Zhang T, Adleff V, Phallen J, Wali N, Hruban C, Guthrie VB, Rodgers K, Naidoo J, Kang H, Sharfman W, Georgiades C, Verde F, Illei P, Li QK, Gabrielson E, Brock MV, Zahnow CA, Baylin SB, Scharpf RB, Brahmer JR, Karchin R, Pardoll DM, Velculescu VE, Evolution of Neoantigen Landscape during Immune Checkpoint Blockade in Non-Small Cell Lung Cancer, Cancer Discov, 7 (2017) 264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Fares CM, Van Allen EM, Drake CG, Allison JP, Hu-Lieskovan S, Mechanisms of Resistance to Immune Checkpoint Blockade: Why Does Checkpoint Inhibitor Immunotherapy Not Work for All Patients?, Am Soc Clin Oncol Educ Book, 39 (2019) 147–164. [DOI] [PubMed] [Google Scholar]
- [109].Yang L, Anderson DE, Kuchroo J, Hafler DA, Lack of TIM-3 immunoregulation in multiple sclerosis, J Immunol, 180 (2008) 4409–4414. [DOI] [PubMed] [Google Scholar]
- [110].Bluestone JA, Herold K, Eisenbarth G, Genetics, pathogenesis and clinical interventions in type 1 diabetes, Nature, 464 (2010) 1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Kanzaki M, Wada J, Sugiyama K, Nakatsuka A, Teshigawara S, Murakami K, Inoue K, Terami T, Katayama A, Eguchi J, Akiba H, Yagita H, Makino H, Galectin-9 and T cell immunoglobulin mucin-3 pathway is a therapeutic target for type 1 diabetes, Endocrinology, 153 (2012) 612–620. [DOI] [PubMed] [Google Scholar]
- [112].Fang S, Huang Y, Zhong S, Zhang Y, Liu X, Wang Y, Gu P, Zhou H, Fan X, IL-17A Promotes RANTES Expression, But Not IL-16, in Orbital Fibroblasts Via CD40-CD40L Combination in Thyroid-Associated Ophthalmopathy, Invest Ophthalmol Vis Sci, 57 (2016) 6123–6133. [DOI] [PubMed] [Google Scholar]
- [113].Luo LH, Li DM, Wang YL, Wang K, Gao LX, Li S, Yang JG, Li CL, Feng W, Guo H, Tim3/galectin-9 alleviates the inflammation of TAO patients via suppressing Akt/NF-kB signaling pathway, Biochem Biophys Res Commun, 491 (2017) 966–972. [DOI] [PubMed] [Google Scholar]
- [114].Ju Y, Hou N, Zhang XN, Zhao D, Liu Y, Wang JJ, Luan F, Shi W, Zhu FL, Sun WS, Zhang LN, Gao CJ, Gao LF, Liang XH, Ma CH, Blockade of Tim-3 pathway ameliorates interferon-gamma production from hepatic CD8+ T cells in a mouse model of hepatitis B virus infection, Cell Mol Immunol, 6 (2009) 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Markwick LJ, Riva A, Ryan JM, Cooksley H, Palma E, Tranah TH, Manakkat Vijay GK, Vergis N, Thursz M, Evans A, Wright G, Tarff S, O’Grady J, Williams R, Shawcross DL, Chokshi S, Blockade of PD1 and TIM3 restores innate and adaptive immunity in patients with acute alcoholic hepatitis, Gastroenterology, 148 (2015) 590–602 e510. [DOI] [PubMed] [Google Scholar]
- [116].Nebbia G, Peppa D, Schurich A, Khanna P, Singh HD, Cheng Y, Rosenberg W, Dusheiko G, Gilson R, ChinAleong J, Kennedy P, Maini MK, Upregulation of the Tim-3/galectin-9 pathway of T cell exhaustion in chronic hepatitis B virus infection, PLoS One, 7 (2012) e47648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Liu J, Xiao S, Huang S, Pei F, Lu F, Upregulated Tim-3/galectin-9 expressions in acute lung injury in a murine malarial model, Parasitol Res, 115 (2016) 587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Xiao S, Liu J, Huang S, Lu F, Increased Gal-9 and Tim-3 expressions during liver damage in a murine malarial model, Parasitol Res, 115 (2016) 663–672. [DOI] [PubMed] [Google Scholar]
- [119].Dembele BP, Chagan-Yasutan H, Niki T, Ashino Y, Tangpukdee N, Shinichi E, Krudsood S, Kano S, Hattori T, Plasma levels of Galectin-9 reflect disease severity in malaria infection, Malar J, 15 (2016) 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Hou N, Zou Y, Piao X, Liu S, Wang L, Li S, Chen Q, T-Cell Immunoglobulin- and Mucin-Domain-Containing Molecule 3 Signaling Blockade Improves Cell-Mediated Immunity Against Malaria, J Infect Dis, 214 (2016) 1547–1556. [DOI] [PubMed] [Google Scholar]
- [121].Wang Y, Zhao E, Zhang Z, Zhao G, Cao H, Association between Tim3 and Gal9 expression and gastric cancer prognosis, Oncol Rep, 40 (2018) 2115–2126. [DOI] [PubMed] [Google Scholar]
- [122].Liang T, Wang X, Wang F, Feng E, You G, Galectin-9: A Predictive Biomarker Negatively Regulating Immune Response in Glioma Patients, World Neurosurg, 132 (2019) e455–e462. [DOI] [PubMed] [Google Scholar]
- [123].Esteller M, Sanchez-Cespedes M, Rosell R, Sidransky D, Baylin SB, Herman JG, Detection of aberrant promoter hypermethylation of tumor suppressor genes in serum DNA from non-small cell lung cancer patients, Cancer Res, 59 (1999) 67–70. [PubMed] [Google Scholar]
- [124].Wong IH, Lo YM, Zhang J, Liew CT, Ng MH, Wong N, Lai PB, Lau WY, Hjelm NM, Johnson PJ, Detection of aberrant p16 methylation in the plasma and serum of liver cancer patients, Cancer Res, 59 (1999) 71–73. [PubMed] [Google Scholar]
- [125].Sasidharan Nair V, El Salhat H, Taha RZ, John A, Ali BR, Elkord E, DNA methylation and repressive H3K9 and H3K27 trimethylation in the promoter regions of PD-1, CTLA-4, TIM-3, LAG-3, TIGIT, and PD-L1 genes in human primary breast cancer, Clin Epigenetics, 10 (2018) 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Holderried TAW, de Vos L, Bawden EG, Vogt TJ, Dietrich J, Zarbl R, Bootz F, Kristiansen G, Brossart P, Landsberg J, Dietrich D, Molecular and immune correlates of TIM-3 (HAVCR2) and galectin 9 (LGALS9) mRNA expression and DNA methylation in melanoma, Clin Epigenetics, 11 (2019) 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].van den Hoogen LL, van Roon JAG, Mertens JS, Wienke J, Lopes AP, de Jager W, Rossato M, Pandit A, Wichers CGK, van Wijk F, Fritsch-Stork RDE, Radstake T, Galectin-9 is an easy to measure biomarker for the interferon signature in systemic lupus erythematosus and antiphospholipid syndrome, Ann Rheum Dis, 77 (2018) 1810–1814. [DOI] [PubMed] [Google Scholar]
- [128].Li YM, Shi YY, Li Y, Yan L, Tang JT, Bai YJ, Wu XJ, Dai B, Zou YG, Wang LL, Soluble Tim-3 and Gal-9 are associated with renal allograft dysfunction in kidney transplant recipients: A cross-sectional study, Int Immunopharmacol, 55 (2018) 330–335. [DOI] [PubMed] [Google Scholar]
- [129].Brubel R, Bokor A, Pohl A, Schilli GK, Szereday L, Bacher-Szamuel R, Rigo J Jr., Polgar B, Serum galectin-9 as a noninvasive biomarker for the detection of endometriosis and pelvic pain or infertility-related gynecologic disorders, Fertil Steril, 108 (2017) 1016–1025 e1012. [DOI] [PubMed] [Google Scholar]
- [130].Mengshol JA, Golden-Mason L, Arikawa T, Smith M, Niki T, McWilliams R, Randall JA, McMahan R, Zimmerman MA, Rangachari M, Dobrinskikh E, Busson P, Polyak SJ, Hirashima M, Rosen HR, A crucial role for Kupffer cell-derived galectin-9 in regulation of T cell immunity in hepatitis C infection, PLoS One, 5 (2010) e9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Hirsch M, Duffy J, Davis CJ, Nieves Plana M, Khan KS, International O Collaboration to Harmonise, E. Measures for, Diagnostic accuracy of cancer antigen 125 for endometriosis: a systematic review and meta-analysis, BJOG, 123 (2016) 1761–1768. [DOI] [PubMed] [Google Scholar]


