Abstract
Pomegranate phenolics have been reported to exert skin beneficial effects but their mechanisms of action remain unclear. Herein, we investigated a standardized commercial pomegranate extract (PE; Pomella®) and its phenolics including punicalagin (PA), ellagic acid (EA), and urolithin A (UA) for their protective effects against hydrogen peroxide (H2O2)-induced oxidative stress and cytotoxicity in human keratinocyte HaCaT cells. PE, PA, and EA reduced the production of H2O2-induced ROS in HaCaT cells by 1.03-, 1.37-, and 2.67-fold, respectively. PE, PA, and UA increased the viability of H2O2-stimulated HaCaT cells by 89.9, 94.9, and 90.0%, respectively. PE, PA, and UA reduced apoptotic cell populations by 3.39, 7.11, and 8.26%, respectively. In addition, PE, PA and UA decreased H2O2-stimulated caspase-3 level by 2.31-, 2.06-, and 2.68-fold, respectively. The ameliorative effects of this PE and its phenolics against the H2O2-induced oxidative stress and cytotoxicity in keratinocytes support their utilization as natural cosmeceuticals for skin health.
Keywords: Pomegranate, phenolics, keratinocytes, oxidative stress, cytotoxicity, skin protection
1. Introduction
Epidermis, the outermost layer and protective barrier of skin, is mainly composed of keratinocytes (80–95%) (Baroni et al., 2012; Feingold, 2007). Keratinocytes are constantly exposed to many harmful stimuli including extrinsic insults (e.g. UV-exposure, smoking, and pollutants) (D’Orazio, Jarrett, Amaro-Ortiz, & Scott, 2013; Rinnerthaler, Bischof, Streubel, Trost, & Richter, 2015) and intrinsic stresses (e.g. toxins, oxidation, and glycation) (Gkogkolou & Bohm, 2012). Both extrinsic and intrinsic factors can lead to cellular oxidative stress through the formation of reactive oxygen species (ROS) including superoxide anion radical (O2•−), and hydroxyl radical (OH•−) in keratinocytes (Farage, Miller, Elsner, & Maibach, 2008; Poljsak, Dahmane, & Godic, 2012). Although the generation of ROS is a biologically inevitable process and cells have developed complex antioxidant mechanisms, oxidative stress occurs when ROS levels exceed the antioxidant capacity of cells. Excessive production of ROS can impair the integrity of the structure of keratinocytes, which further leads to the loss of cellular functions and eventually causes cell death. Compromised keratinocytes are directly associated with the skin aging process and many aging-related skin symptoms including dehydration (Sengupta et al., 2010), irritation (Lawrence, Dickson, & Benford, 1997), laxity (Yaar & Gilchrest, 2001), and the formation of wrinkles (Blume-Peytavi et al., 2016). Therefore, the use of natural product antioxidant interventions, including dietary phenolics, to counteract ROS-induced oxidative stress in keratinocytes has attracted research interest for cosmeceutical and/or dermatological applications (Filip et al., 2011; Rahman, Biswas, & Kirkham, 2006).
Previous published studies using in vitro and in vivo models support the skin protective effects of bioactive compounds from pomegranate (Punica granatum) fruit. A pomegranate fruit extract was reported to show antioxidant effects in immortalized human keratinocytes by decreasing UVB-induced cytotoxicity and intracellular glutathione content (Zaid, Afaq, Syed, Dreher, & Mukhtar, 2007). Similarly, a pomegranate fruit extract [standardized to 30% punicalagins (PA), a characteristic ellagitannin in pomegranate] showed protective effects against UVA- and UVB-induced damage in human fibroblasts by reducing inflammatory and oxidative stresses (Pacheco-Palencia, Mertens-Talcott, & Talcott, 2008). A pomegranate fruit extract was also reported to show photochemopreventive effects in normal human keratinocytes against UVA-mediated activation of transcription factor (signal transducer and activator of transcription 3; STAT3), protein kinase B (Akt), and extracellular signal-regulated kinase and the UVB-mediated activation of mitogen-activated protein kinase (MAPK) and nuclear factor kappa-light-chain-enhancer of activated B (NF-κB) pathways (Syed et al., 2006). The photochemopreventive effects of that pomegranate extract were further supported by an in vivo study wherein it’s oral delivery (by dissolving pomegranate extract in drinking water; 0.2%, wt/vol) protected mouse skin from UVB radiation by mediating the UVB-induced signaling pathways (Khan, Syed, Pal, Mukhtar, & Afaq, 2012). Moreover, the skin protective effects of pomegranate phenolics are supported by a double-blind and placebo-controlled human clinical trial (Kasai, Yoshimura, Koga, Arii, & Kawasaki, 2006), in which the oral consumption of an ellagic acid (EA)-enriched pomegranate extract showed ameliorative effect against UV radiation-induced sun burn at a dosage of 100 mg/day. In addition, this pomegranate extract also showed inhibitory effects of pigmentation in human skin cells (Kasai et al., 2006). Interestingly, the gut microbial metabolites of pomegranate ellagitannins, namely, urolithins, have also been reported to show skin lightening effects by reducing the melanin production in murine melanoma cells (Wang, Chang, Hsu, & Su, 2017). However, the protective effects of pomegranate fruit extracts, their constituent phenolics (such as punicalagins and ellagic acid), and their gut microbial metabolites (such as urolithin A; UA) against hydrogen peroxide (H2O2)-induced oxidative stress in human keratinocytes HaCaT cells and their mechanisms of action remain unclear.
Our laboratory has initiated a program to systematically investigate the phytochemical composition of pomegranate and has identified over 100 phenolic compounds from various aerial parts of pomegranate (Liu & Seeram, 2018; Yuan et al., 2012; Yuan, Wan, Ma, & Seeram, 2013). In addition, our group has investigated a standardized pomegranate fruit extract (PE; Pomella®) and its phenolics, PA, EA and urolithins, for their neuroprotective effects against Alzheimer’s disease (Yuan et al., 2016) and their anti-neuroinflammatory (DaSilva et al., 2017) and anti-glycation (Liu et al., 2014) effects. Herein, using human HaCaT keratinocytes, we investigated the skin protective effects of this PE and its phenolics (PE, EA and UA) by evaluating their capacity to reduce H2O2-induced ROS production and toxicity, as well as their potential mechanisms of action by investigating their anti-apoptosis and -necrosis effects, and effects on caspases-3/7, −8, and −9 enzymes. The ameliorative effects of the pomegranate phenolics in human keratinocytes support their utilization as natural cosmeceuticals for skin health.
2. Materials and Methods
2.1. Chemicals and reagents
Pomegranate fruit extract (PE; Pomella®) was kindly provided by Verdure Sciences (Noblesville, IN, USA). The PE was standardized to punicalagins (PA; c.a. 30%) and ellagic acid (EA; c.a. 2.3%) and has been extensively chemically characterized by our laboratory (Yuan et al., 2012; Yuan, Wan, Ma, & Seeram, 2013; Ahmed et al., 2014; Yuan et al., 2016). PE contains a total polyphenol content (as gallic acid equivalents) of 61.5% (Ahmed et al., 2014). PA and EA were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Urolithin A (3,8-dihydroxy-6H-dibenzo [b, d] pyran-6-one; UA) was synthesized in our laboratory using a reported protocol (Yuan et al., 2013). Hoechst 33342, crystal violet, propidium iodide (PI), dimethyl sulfoxide (DMSO), hydrogen peroxide (H2O2), and 2’,7’-dichlorouorescin diacetate (DCFDA) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). CellTiter-Glo® (CTG) 2.0 assay kit was purchased from Promega (Fitchburg, WI, USA). Alexa Fluor® 488 Annexin V/Dead cell apoptosis kit was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Caspase-Glo® assay kits for caspase- 3/7, −8, and −9 were purchased from Promega (Fitchburg, WI, USA).
2.2. Cell culture and sample preparation
Human keratinocyte HaCaT cells were purchased from the American Type Culture Collection (ATCC, Rockville, USA) and maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Life Technologies, Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum (FBS) (Life Technologies, Gaithersburg, MD, USA) at 37 °C in the presence of 5% CO2 at constant humidity. Test samples were dissolved in DMSO as stock solution and then diluted with cell culture medium to the desired concentrations (6.25–100 μg/mL for PE and 6.25–100 μM for the purified compounds; DMSO% < 0.1%).
2.3. Measurement of reactive oxygen species (ROS)
HaCaT cells were seeded in 96-well plates at 5×103 cells per well for 12 h and then incubated with test samples (at aforementioned concentrations) for 12 h. Next, medium was removed and cells were washed twice with phosphate-buffered saline (PBS). Medium containing fluorescent probe (DCFDA; 20 μM) was then added to the cells and incubated for 20 min. Cells were then were treated with 100 μL H2O2 (at concentrations of 50, 100, 200, 400, and 800 μM) for 1 h followed by measuring fluorescence intensity of each well with excitation and emission wavelengths of 485 and 525 nm, respectively, using a Spectramax M2 plate reader (Molecular Devices, Sunyvale, CA, USA).
2.4. Measurement of H2O2-induced cytotoxicity in HaCaT cells
The viability of H2O2-treated HaCaT cells was measured by a CTG 2.0 assay using a previously reported method (Ma et al., 2016; Ma et al., 2018). HaCaT cells were seeded in 96-well plates at 5×103 cells per well and allowed to attach for 12 h. Media was then removed and 100 μL of H2O2 (at concentrations of 50, 100, 200, 400, and 800 μM) was added and incubated for 24 h. Next, CTG 2.0 reagent (100 μL) was added in each well and shaken at 200 rpm for 2 min on an orbital shaker. The plate was then kept at room temperature for 10 min after which luminescence intensity was recorded using a Spectramax M2 plate reader. Morphological analysis was conducted to evaluate cell damage with crystal violet staining method. Cells were fixed in 70% ethanol for 15 min after which medium was removed. Staining solution (0.05% w/v) was added to each well and incubated for 20 min. Then the staining solution was removed and cells were washed with PBS for three times. The morphological changes of cells were observed by an EVOS Cell Imaging System (Invitrogen, Waltham, MA, USA).
2.5. Detection of apoptosis and necrosis (flow cytometry assay)
HaCaT cells were seeded in 6-well plates at 0.3×106 cells per well and allowed to attach for 12 h followed by treatment of test samples (at aforementioned concentrations) for 6 h. The medium was then removed and cells were washed twice with PBS. Next, cells were treated with H2O2 (at 200 μM) and incubated for 24 h. Detection of apoptosis was performed according to the previously reported method (Zhang et al., 2017). Cells were then harvested and suspended in 500 μL of binding buffer containing 5 μL of FITC-labeled Annexin-V and 5 μL of propidium iodide (PI) followed by incubation in the dark for 15 min. Then the population of apoptotic and necrotic cells were measured by flow cytometry (BD FACSCalibur, San Jose, CA, USA) and data were analyzed using software FlowJo (LLC, Ashland, Oregon, USA).
2.6. Hoechst 33342 and propidium iodide PI double staining
HaCaT cells were seeded in 24 well plates at 0.5×105 cells per well and allowed to attach for 12 h followed by treatment of test samples (at aforementioned concentrations) for 6 h. Medium were then removed and cells were washed twice with PBS. Next, 300 μL of fresh medium containing H2O2 (200 μM) were added to cells and incubated for 24 h. Medium were then removed and cells were washed twice with PBS. Hoechst 33342 staining buffer (300 μL) was added to the cells and incubated for 30 min in darkness. Next, PI staining buffer (300 μL) was added to the cells and incubated for 30 min in darkness. The morphological changes of the nucleus of the cells were observed by an EVOS Cell Imaging System with a fluorescence microscope (Invitrogen, Waltham, MA, USA).
2.7. Measurements of caspases −3/7, −8, and −9 levels
HaCaT cells were seeded in 96-well plates at 5×103 cells per well and allowed to attach for 12h. Cells were then treated with test samples (at aforementioned concentrations) for 6 h. Media were then removed and cells were washed twice with PBS. Next, 100 μL of fresh medium containing H2O2 (200 μM) was added and incubated for 24 h followed by addition of 100 uL of caspase-Glo reagent (for measurement of caspases-3/7, −8, and −9, separately). Plates were then incubated at room temperature for 30 min and the luminescence intensity of each well was read using a Spectramax M2 plate reader.
2.8. Statistical analysis
Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software, La Jolla, CA, USA). Data are expressed as the mean value ± standard deviation (S.D.) obtained from triplicates of experiments. The significance of differences was determined using a two-way analysis of variance (ANOVA) followed by a post hoc Student-Newman–Keuls multiple comparison test (SNK). P < 0.05, P < 0.01, or P < 0.001 was determined to be significant.
3. Results and Discussion
3.1. Hydrogen peroxide (H2O2) induces the production of ROS and cytotoxicity in HaCaT cells
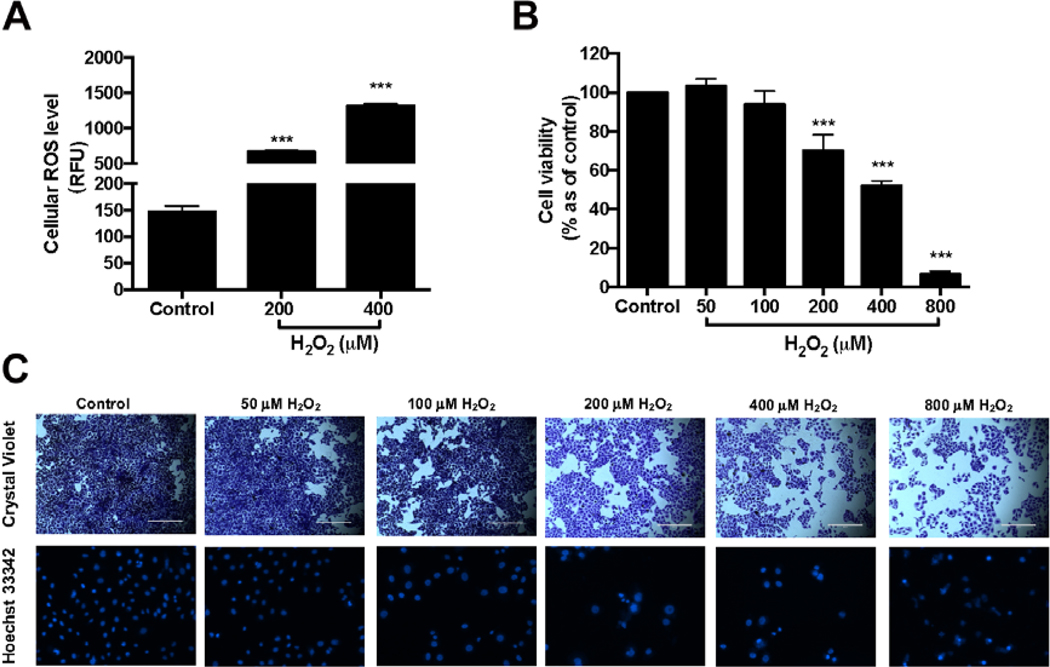
Hydrogen peroxide (H2O2) is a common ROS produced in keratinocytes and its toxic effects were examined in human keratinocytes HaCaT cells. Treatment of H2O2 (200 and 400 μM) significantly elevated the production of ROS in HaCaT cells by 4.58-, and 9.01-fold, respectively (Fig. 1A). In addition, H2O2 (200, 400, and 800 μM) significantly induced cytotoxicity by decreasing the viability of HaCaT cells to 70.0, 51.8, and 6.6%, respectively (Fig. 1B). The toxic effects of H2O2 in HaCaT cells were confirmed by morphological analyses using crystal violet and Hoechst 33342 staining methods (Fig 1C). The nucleus of cells treated with H2O2 (200–800 μM) had irregular shapes as compared to the cells without H2O2 treatment suggesting that treatments of H2O2 (>200 μM) induced oxidative stress and caused cell death in HaCaT cells.
Fig. 1.
Effects of H2O2 on viability of HaCaT cells and ROS production in HaCaT cells (A). HaCaT cells were incubated with H2O2 at concentrations of 200 and 400 μM and then incubated with DMEM containing 20 μM DCFDA. Fluorescence intensity of each well was measured at excitation and emission wavelength of 485 and 525 nm, respectively. HaCaT cells were treated with H2O2 at concentrations of 50, 10, 200, 400, and 800 μM. Cell viability was measured by using CTG 2.0 assay (B). Representative images of HaCaT cells exposed to H2O2 for 24 h and then stained with crystal violet and Hoechst 33342 (C). Significance was defined as compared with control group: ***P < 0.001. The values presented are the means ± S.D.
Although excessive accumulation of H2O2 is harmful for skin cells, studies have also showed that moderate levels of ROS is important for cellular signaling and proliferation (Ray, Huang, & Tsuji, 2012). Therefore, the effects of H2O2 at various concentrations on HaCaT cells were evaluated, first. Our results from the cell viability and staining assays were in agreement with published data (Ray et al., 2012) showing that at lower concentrations (50 and 100 μM), treatment of H2O2 was non-toxic; however, H2O2 at 200 μM or higher concentrations caused significantly cytotoxicity. Therefore, H2O2 at 200 μM, was used as an inducer of oxidative stress and cytotoxicity in HaCaT cells for further experiments.
3.2. PE, PA, and EA reduce H2O2-induced ROS production in HaCaT cells
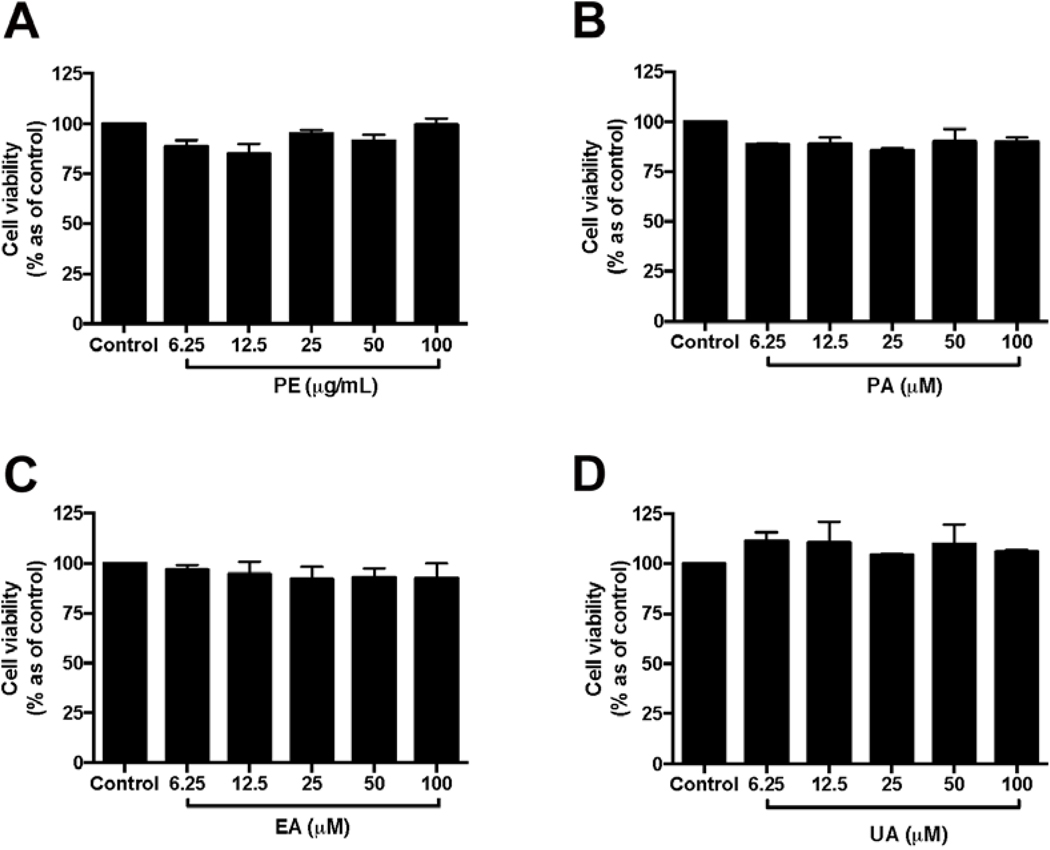
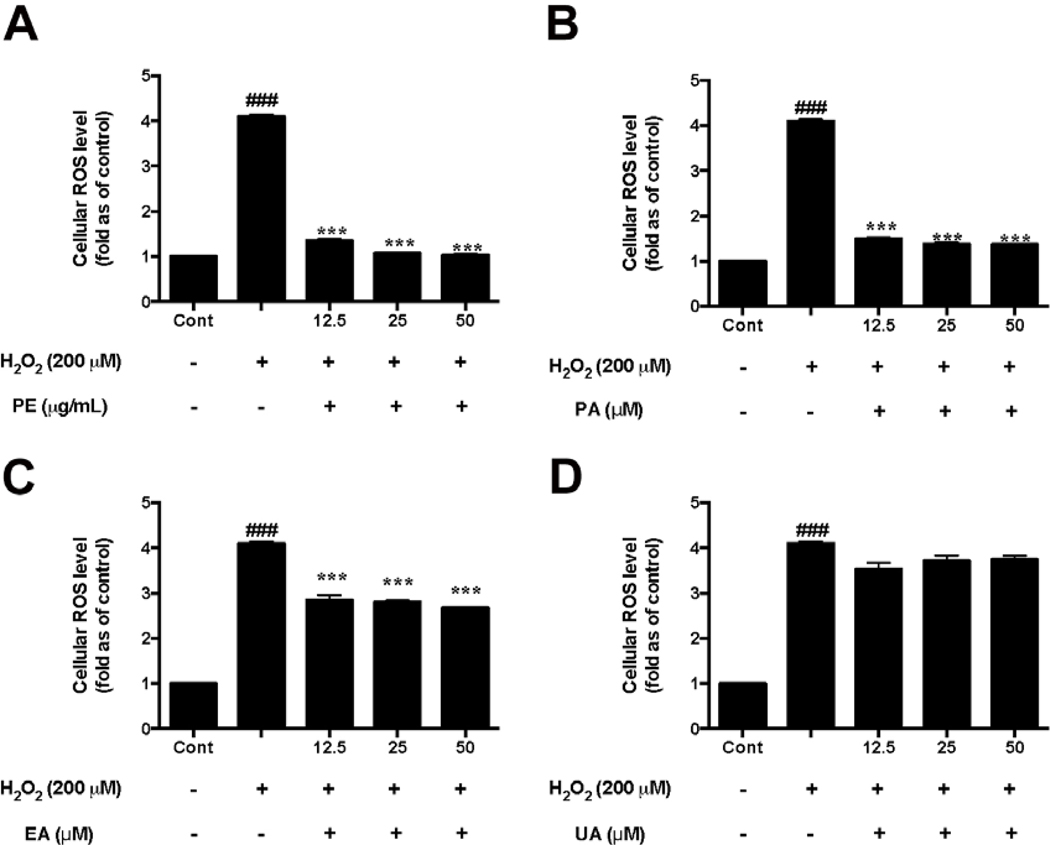
Prior to the investigation of the protective effects of PE and the pure pomegranate phenolics on HaCaT cells, the non-toxic concentrations of test samples were determined. PE (6.25–100 μg/mL; Fig. 2A) and its phenolics including PA, EA, and UA (6.25–100 μM; Fig. 2B–D, respectively) did not induce significant cytotoxicity in HaCaT cells. Therefore, PE (at 12.5, 25, and 50 μg/mL), PA, EA, and UA (at 12.5, 25, and 50 μM) were selected for the evaluation of their protective effects in HaCaT cells. Next, the protective effects of PE and the pomegranate phenolics against oxidative stress in HaCaT cells was evaluated. Treatment of PE (12.5, 25, and 50 μg/mL) significantly reduced the production of H2O2-induced ROS in HaCaT cells by 1.36-, 1.07-, and 1.03-fold, respectively, as compared to the H2O2-treated group (Fig. 3A). In addition, PA and EA (12.5, 25, and 50 μM) reduced H2O2-indcued ROS production by 1.50-, 1.38-, 1.37-fold and 2.85-, 2.80-, and 2.67-fold, respectively, as compared to the H2O2-treated group (Fig. 3B and C). Although a trend was observed for UA, the reduction of ROS production was not significant at the test concentrations (Fig. 3D).
Fig. 2.
Effects of PE and pomegranate phenolics on viability of HaCaT cells. HaCaT cells were incubated with PE (A), PA (B), EA (C), and UA (D) for 24 h. Cell viability was measured by CTG 2.0 assay. Values are presented as means ± S.D. from three replicates.
Fig. 3.
Effects of PE and pomegranate pheonlics on ROS production in HaCaT cells exposed to H2O2. HaCaT cells were incubated with PE (A), PA (B), EA (C), and UA (D) for 12 h before H2O2 induction. Cells were incubated with DMEM containing 20 μM DCFDA after medium removal. Fluorescence intensity of each well was measured at excitation and emission wavelength of 485 and 525 nm, respectively. Significances were defined as compared with control group: ###P < 0.01; compared with the H2O2-treated group: ***P< 0.001. Values are presented as means ± S.D. from three replicates.
Our results showed that PE and its phenolics including PA and EA had promising antioxidant effects against H2O2 in HaCaT cells. This is in agreement with a previously reported study showing that that PA attenuated ROS production in primary human epidermal keratinocytes stimulated by an airborne particulate matter (Seok, Lee, Kim, & Boo, 2018). In addition, EA has also been reported to show protective effects in HaCaT cells against UVA-mediated oxidative stress by regulation of antioxidant genes such as HO-1 and Nrf-2 (Hseu et al., 2012). However, to date, the effects of urolithins, the gut microbial metabolites of ellagitannins, on HaCaT cells have not been reported. In this study, we observed that compared to PA and EA, UA had lower antioxidant effects which is agreement with a previous study reporting that the antioxidant capacities of urolithins were less potent than ellagitannins in a panel of biochemical assays (Ito, 2011). Similar to our observations, a study showed that EA but not UA reduced H2O2-induced oxidative stress in human neuroblastoma SH-SY5Y cells (Gonzalez-Sarrias, Nunez-Sanchez, Tomas-Barberan, & Espin, 2017). However, it should be noted that urolithins have been reported to show cellular antioxidant effects in other cell types including bladder T24 cells and human liver carcinoma HepG2 cells (Qiu et al., 2013; Wang et al., 2015). This may due to differences in the redox activities of urolithins which could be influenced by different bioassays systems (Kallio et al., 2013). Therefore, further studies are warranted to elucidate the mechanisms of antioxidant effects of urolithins in skin cells.
3.3. PE, PA, and UA reduce H2O2-induced cytotoxicity in HaCaT cells
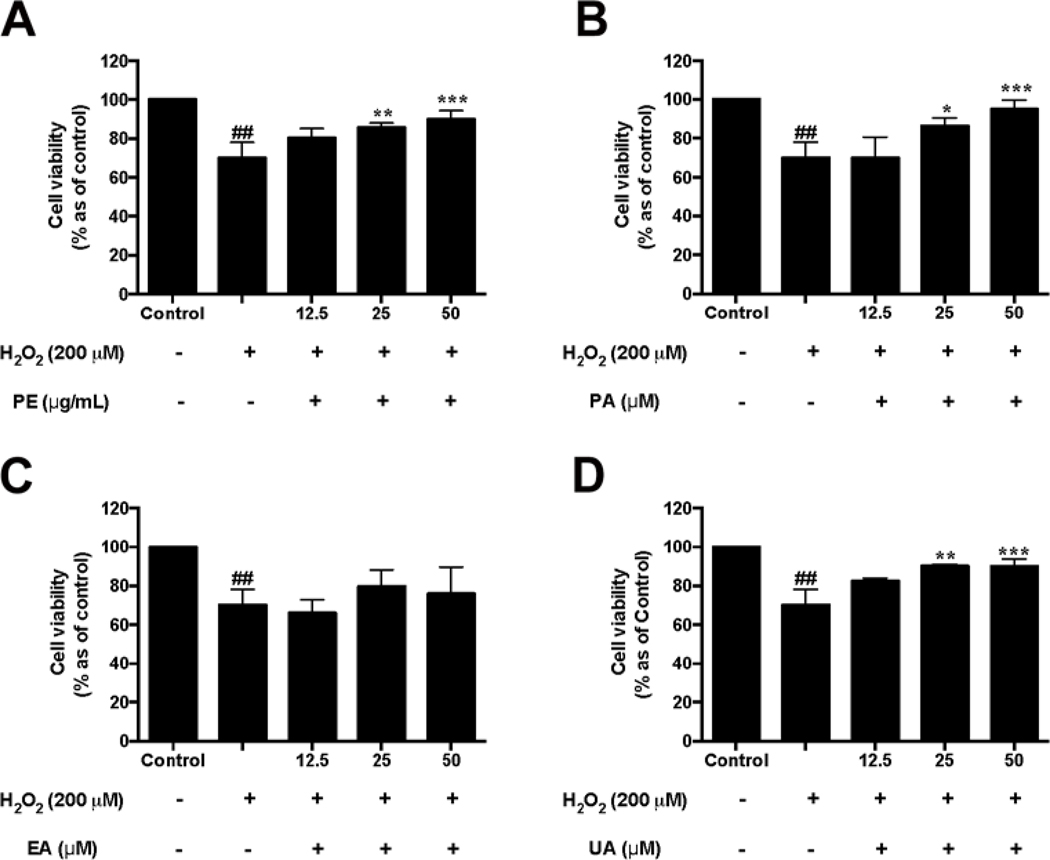
The protective effects of pomegranate phenolics against H2O2-induced toxicity in HaCaT cells were evaluated. Treatment with PE (25 and 50 μg/mL) significantly reduced H2O2-induced cytotoxicity by increasing the viability of HaCaT cells by 85.6% and 89.9%, respectively, as compared to the H2O2-treated group (Fig. 4A). Treatments of PA (25 and 50 μM) and UA (25 and 50 μM) also significantly inhibited H2O2-induced cell death by 86.4% and 94.9% (Fig. 4B), and 90.0% and 90.0%, (Fig. 4D), respectively, as compared to the H2O2-treated group. However, EA (12.5–50 μM) did not increase the viability of H2O2-treated HaCaT cells (Fig. 4C). Although UA did not show potent antioxidant effects in the aforementioned assay, it protected HaCaT cells against oxidative stress in HaCaT cells. This may possibly be explained since urolithins may exert cytoprotective effects by different mechanisms. For example, urolithins have been reported to regulate cell proliferation by as endocrine-disrupting molecules with estrogenic and/or antiestrogenic activities (Larrosa, González-Sarrías, García-Conesa, Tomás-Barberán, & Espín, 2006). UA has also been reported to increase the viability of neonatal rat cardiomyocytes in a myocardial ischemia/reperfusion injury model through regulation of PI3K/Akt pathway (Tang et al., 2017). In addition, our group reported that urolithins increased the cell viability of differentiated human neuronal SH-SY5Y cells by down-regulating the levels of inflammatory stress and related cytokines (DaSilva et al., 2017). However, the underlying mechanism(s) of the protective effects of urolithins in skin cells in the current study are not clear warranting further investigations.
Fig. 4.
Effects of PE and pomegranate phenolics on cell viability of HaCaT cells exposed to H2O2. HaCaT cells were incubated with PE (A), PA (B), EA (C), and UA (D) for 12 h before H2O2 induction. Cell viability was measured by using CTG 2.0 assay. Significances were defined as compared with control group: ##P < 0.01; compared with the H2O2-treated group: *P< 0.05, **P< 0.01, ***P< 0.001. Values are presented as means ± S.D. from three replicates.
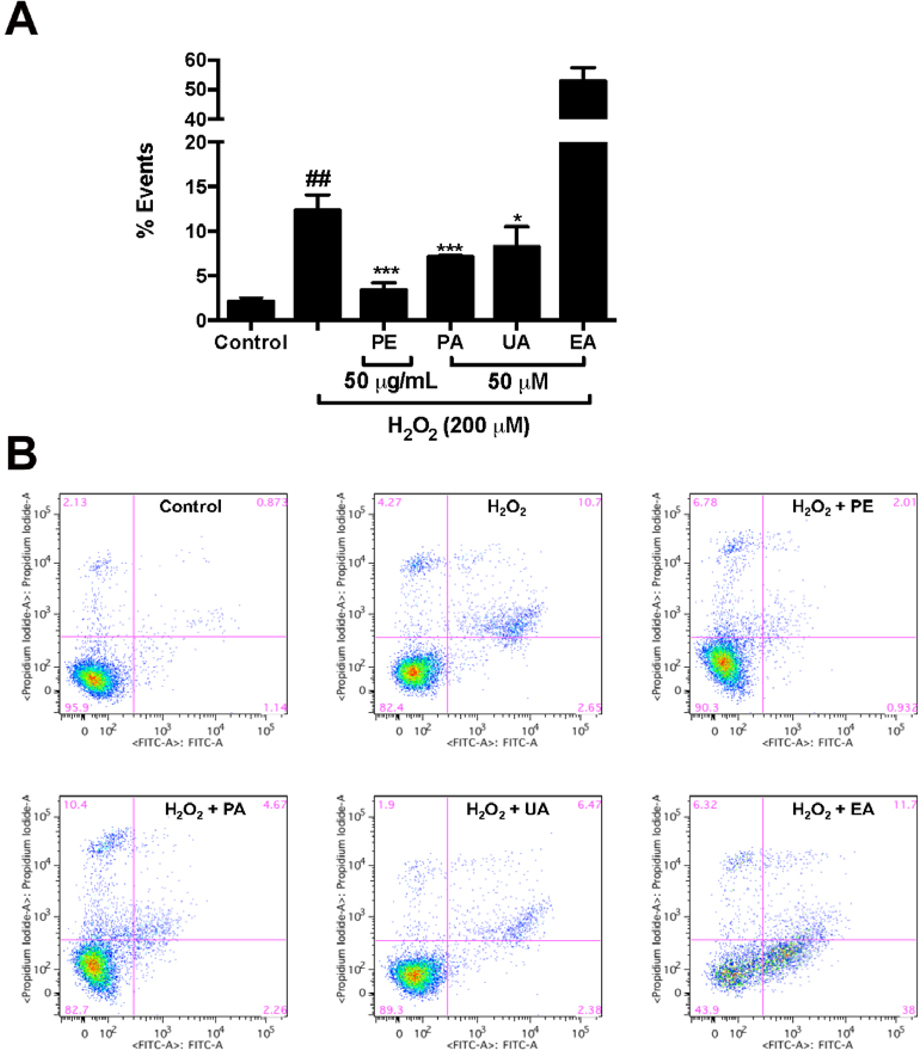
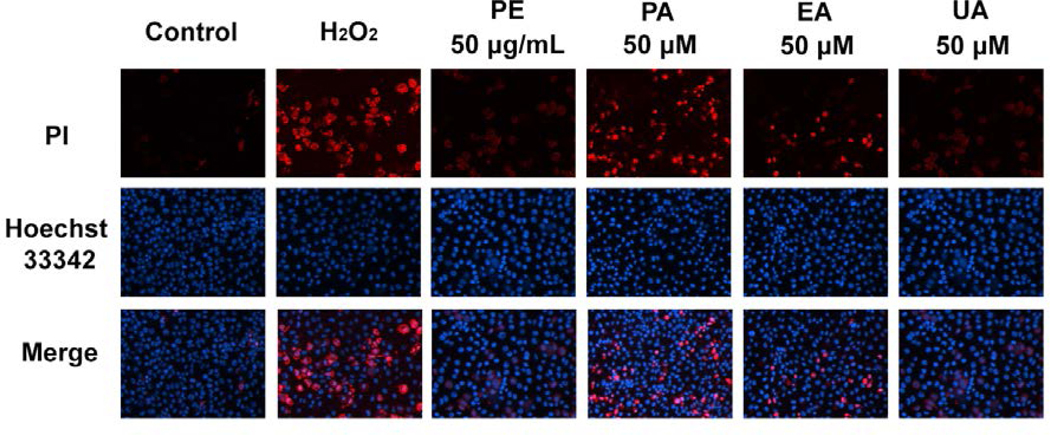
3.4. PE, PA, and UA show protective effects against H2O2-induced apoptosis and necrosis in HaCaT cells
The protective effects of pomegranate phenolics against H2O2-induced cytotoxicity were further evaluated by analyses of the apoptotic and necrotic cell population. The treatment of H2O2 increased the percentage of apoptotic cells including early- and late-apoptotic cells by 2.65% and 10.7%, respectively, as compared to the H2O2-untreated control group (Fig. 5). Treatments of PE (50 μg/mL), PA, and UA (50 μM) significantly decreased the apoptotic cell population by 3.39%, 7.11%, and 8.26%, respectively, as compared to the H2O2-treated group (Fig. 5). Interestingly, treatment of EA (50 μM) increased the apoptotic cell populations by 52.9 % as compared to the H2O2-treated group (Fig. 5). The anti-necrosis effects of pomegranate phenolics in HaCaT cells were further evaluated by morphological analyses using Hoechst 33342 and PI staining. Treatment of H2O2 significantly increased the number of PI positive cells as compared to the H2O2-untreated control cells, suggesting that H2O2 induced HaCaT cell necrosis (Fig. 6). The treatment of PE (50 μg/mL) significantly reduced the number of PI positive cells, compared to the H2O2-treated cells, indicating that PE attenuated the H2O2 -induced necrosis in HaCaT cells. The treatment of PA, EA, and UA (50 μM) also reduced the number of PI positive cells as compared to the H2O2-treated cells. Among the pomegranate phenolics, UA showed the highest anti-necrotic effects in HaCaT cells. Flow cytometry analyses showed that PE and the pomegranate phenolics had different effects in the H2O2-stimulated HaCaT cells. PA and UA showed anti-apoptotic effects in HaCaT cells while EA increased the population of apoptotic cells. This is in agreement with the observation from the CTG assay in which PA and UA, but not EA, increased the viability of H2O2-treated cells. This is not surprising since it has been well established that EA shows anti-proliferative effects in several cell lines by modulations of apoptosis-related pathways (Chung et al., 2013; Wang et al., 2016; Zhao et al., 2017). In addition, PE (which contains both PA and EA) showed anti-apoptotic effects suggesting that the pomegranate phenolics may exert cytoprotective effects against H2O2-induced oxidative stress in an additive and/or synergistic manner. Notably, apart from PA and EA, our group reported that over seventy phenolic compounds, which may also show antioxidant effects, have also been identified from the PE, a standardized pomegranate extract (Pomella®), used in this study (Liu & Seeram, 2018). Thus, it is possible that other compounds present in PE may also contribute to its overall cytoprotective effects in HaCaT cells.
Fig. 5.
Effects of PE and pomegranate phenolics on apoptosis of HaCaT cells induced by H2O2. The apoptotic cell populations (annexin V+/PI− and annexin V+/PI+) of HaCaT cells with or without treatments of PE, PA, EA, and UA were quantified by gated patterns in double stains (A). HaCaT cells stained with annexin V-FITC/PI and assayed by flow cytometry (B). Significances were defined as compared with control group: ##P < 0.001; compared with the H2O2-treated group: *P < 0.05, ***P < 0.001.
Fig. 6.
Effects of PE and pomegranate phenolics on necrosis of HaCaT cells induced by H2O2. Fluorescence microscopy shows representative pictures of HaCaT cells treated with PE, PA, EA, and UA. HaCaT cells were stained with Hoechst 33342 and PI.
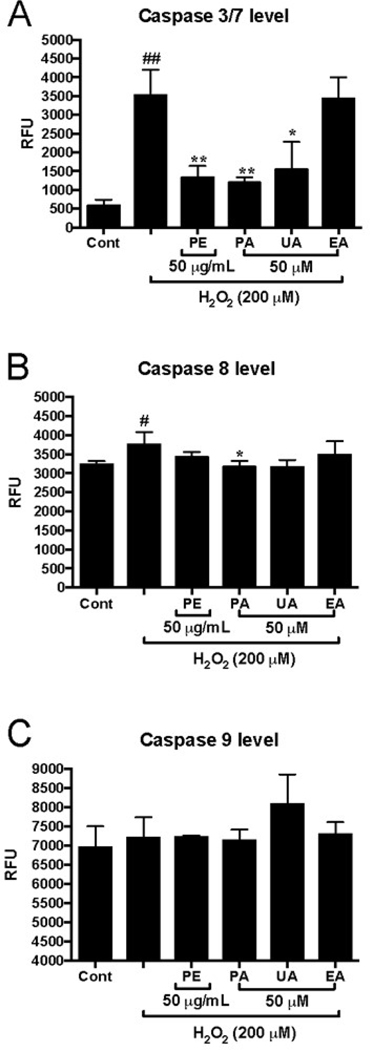
3.5. PE, PA, and UA inhibit Caspase-3 content of HaCaT cells induced by H2O2
To further investigate the mechanisms of the anti-apoptotic effects of pomegranate phenolics, their effects on the levels of apoptosis-associated enzymes including caspases −3/7, −8, and −9, were evaluated in HaCaT cells. Treatment of H2O2 (200 μM) stimulated HaCaT cells and increased the levels of capases-3/7 and −8 by 6.1- and 1.2-fold, respectively, as compared to the H2O2-untreated control group (Fig. 7A and B). PE (50 μg/mL), PA, and UA (50 μM) significantly reduced the levels of caspases-3/7 by 2.31-, 2.07-, and 2.68-fold, respectively, as compared to the H2O2-treated group (Fig. 7A). Although the pomegranate phenolics showed a trend in reducing the levels of caspase-8, only PA (50 μM) significantly decreased the levels of capase-8 by 98.2% as compared to the H2O2-treated group (Fig. 7B). Treatments of H2O2 and the pomegranate phenolics did not affect the level of capase-9 in HaCaT cells (Fig. 7C). Caspases are a family of cysteine proteases that regulate programmed cell death processes including apoptosis, pyroptosis, and necroptosis (McIlwain, Berger, & Mak, 2015). Down-regulation of caspases activities is a plausible mechanism to prolong the life of skin cells against oxidative stress (He, Huang, Block, Hong, & Chignell, 2005; Yang et al., 2015). Our data showed that PE, PA, and UA decreased the levels of H2O2-activated caspases 3/7 in HaCaT cells. This is in agreement with a previous study showing that PE reduced the gene expression of caspase 3 in SKU-1064 human skin fibroblast cellsafter exposure to UV radiation (Pacheco-Palencia, Noratto, Hingorani, Talcott, & Mertens-Talcott, 2008). In addition, our group has reported that urolithins mitigated H2O2-induced apoptosis by reducing the levels of caspases-3/7 and −9 in murine microglia BV-2 and human neuronal SH-SY5Y cells (DaSilva et al., 2017). However, in the current study, PE and its phenolics did not reduce the levels of caspase-9 in HaCaT cells, suggesting that the pomegranate phenolics, especially urolithins, may display anti-apoptotic and -necrotic effects in various cell lines via modulation of different pathways. Further studies are warranted to elucidate the mechanism(s) of pomegranate phenolics on skin cells.
Fig. 7.
Effects of PE and pomegranate phenolics on cellular caspase 3/7 (A), caspase 8 (B) and caspase 9 (C) in HaCaT cells exposed to H2O2. HaCaT cells were incubated with PE, PA, EA, and UA for 12 h before H2O2 induction. Significances were defined as compared with control group: ##P < 0.01; compared with H2O2-treated group: *P< 0.05, **P< 0.01, ***P< 0.001. Values are presented as means ± S.D. from three replicates.
Although it is common that natural products from dietary supplements are used topically for skin health, consumable applications of dietary supplements have also been developed for dermatological and cosmetic purposes (Szyszkowska, Lepecka-Klusek, Kozlowicz, Jazienicka, & Krasowska, 2014). The beneficial effects of oral consumption of PE on skin has also been supported by a human clinical study (Adhami, Khan, & Mukhtar, 2009), however, the underlying mechanisms remained unclear. Overall, our study supports the possibility that pomegranate phenolics may exert protective effects in human HaCaT skin cells by reducing oxidative stress. A limitation of the current study is that the bioavailability of pomegranate phenolics in skin cells and tissue were not explored but this will be pursued in our future studies.
In summary, pomegranate phenolics showed protective effects against H2O2-induced oxidative stress and cytotoxicity in human keratinocytes HaCaT cells. Pomegranate phenolics protected keratinocytes by reducing H2O2-induced ROS production and cytotoxicity. In addition, pomegranate phenolics decreased H2O2-induced apoptotic cell population and down-regulated the level of caspases-3/7 in HaCaT cells. These results suggest that pomegranate phenolics may be utilized as natural antioxidants for cosmeceutical applications for skin health.
Acknowledgements
Data were acquired from instruments located at the University of Rhode Island in the RI-INBRE core facility obtained from Grant P20GM103430 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). C.L. was supported by a teaching assistantship from College of Pharmacy, the University of Rhode Island. H.G. was supported by a funding from China Scholarship Council (201708210229). D.L. was supported by grants from the Department of Education of Guangdong Province (2016KCXTD005; 2017KSYS010). The plant material was kindly provided by Mr Ajay Patel, Verdure Sciences (Noblesville, IN, USA).
Footnotes
Declarations of interest:
none
Ethics statement:
Research did not include any human subjects and animal experiments
References
- Adhami VM, Khan N, & Mukhtar H. (2009). Cancer chemoprevention by pomegranate: laboratory and clinical evidence. Nutrition and Cancer, 61(6), 811–815. doi: 10.1080/01635580903285064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed HA, Subaiea GM, Eid A, Li L, Seeram NP, & Zawia NH (2014). Pomegranate extract modulates processing of amyloid-β precursor protein in an aged Alzheimer’s disease animal model. Current Alzheimer Research, 11(9), 834–843. [PubMed] [Google Scholar]
- Baroni A, Buommino E, De Gregorio V, Ruocco E, Ruocco V, & Wolf R. (2012). Structure and function of the epidermis related to barrier properties. Clinics in Dermatology, 30(3), 257–262. doi: 10.1016/j.clindermatol.2011.08.007 [DOI] [PubMed] [Google Scholar]
- Blume-Peytavi U, Kottner J, Sterry W, Hodin MW, Griffiths TW, Watson RE, et al. (2016). Age-associated skin conditions and diseases: current perspectives and future options. Gerontologist, 56 Suppl 2, S230–242. doi: 10.1093/geront/gnw003 [DOI] [PubMed] [Google Scholar]
- Chung YC, Lu LC, Tsai MH, Chen YJ, Chen YY, Yao SP, et al. (2013). The inhibitory effect of ellagic acid on cell growth of ovarian carcinoma cells. Evidence-Based Complementary and Alternative Medicine, 2013, 306705. doi: 10.1155/2013/306705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Orazio J, Jarrett S, Amaro-Ortiz A, & Scott T. (2013). UV radiation and the skin. International Journal of Molecular Sciences, 14(6), 12222–12248. doi: 10.3390/ijms140612222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaSilva NA, Nahar PP, Ma H, Eid A, Wei Z, Meschwitz S, et al. (2017). Pomegranate ellagitannin-gut microbial-derived metabolites, urolithins, inhibit neuroinflammation in vitro. Nutritional Neuroscience, 1–11. doi: 10.1080/1028415X.2017.1360558 [DOI] [PubMed] [Google Scholar]
- Farage MA, Miller KW, Elsner P, & Maibach HI (2008). Intrinsic and extrinsic factors in skin ageing: a review. International Journal of Cosmetic Science, 30(2), 87–95. doi: 10.1111/j.1468-2494.2007.00415.x [DOI] [PubMed] [Google Scholar]
- Feingold KR (2007). Thematic review series: skin lipids. The role of epidermal lipids in cutaneous permeability barrier homeostasis. Journal of Lipid Research, 48(12), 2531–2546. doi: 10.1194/jlr.R700013-JLR200 [DOI] [PubMed] [Google Scholar]
- Filip A, Daicoviciu D, Clichici S, Mocan T, Muresan A, & Postescu ID (2011). Photoprotective effects of two natural products on ultraviolet B-induced oxidative stress and apoptosis in SKH-1 mouse skin. Journal of Medicinal Food, 14(7–8), 761–766. doi: 10.1089/jmf.2010.0142 [DOI] [PubMed] [Google Scholar]
- Gkogkolou P, & Bohm M. (2012). Advanced glycation end products: Key players in skin aging? Dermatoendocrinology, 4(3), 259–270. doi: 10.4161/derm.22028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Sarrias A, Nunez-Sanchez MA, Tomas-Barberan FA, & Espin JC (2017). Neuroprotective effects of bioavailable polyphenol-derived metabolites against oxidative stress-induced cytotoxicity in human neuroblastoma SH-SY5Y cells. Journal of Agricultural and Food Chemistry, 65(4), 752–758. doi: 10.1021/acs.jafc.6b04538 [DOI] [PubMed] [Google Scholar]
- He YY, Huang JL, Block ML, Hong JS, & Chignell CF (2005). Role of phagocyte oxidase in UVA-induced oxidative stress and apoptosis in keratinocytes. Journal of Investigative Dermatology, 125(3), 560–566. doi: 10.1111/j.0022-202X.2005.23851.x [DOI] [PubMed] [Google Scholar]
- Hseu YC, Chou CW, Senthil Kumar KJ, Fu KT, Wang HM, Hsu LS, et al. (2012). Ellagic acid protects human keratinocyte (HaCaT) cells against UVA-induced oxidative stress and apoptosis through the upregulation of the HO-1 and Nrf-2 antioxidant genes. Food and Chemical Toxicology, 50(5), 1245–1255. doi: 10.1016/j.fct.2012.02.020 [DOI] [PubMed] [Google Scholar]
- Ito H. (2011). Metabolites of the ellagitannin geraniin and their antioxidant activities. Planta Medica, 77(11), 1110–1115. doi: 10.1055/s-0030-1270749 [DOI] [PubMed] [Google Scholar]
- Kallio T, Kallio J, Jaakkola M, Mä M, inen P, & Virtanen V. (2013). Urolithins display both antioxidant and pro-oxidant activities depending on assay system and conditions. Journal of Agricultural and Food Chemistry, 61(45), 10720–10729. [DOI] [PubMed] [Google Scholar]
- Kasai K, Yoshimura M, Koga T, Arii M, & Kawasaki S. (2006). Effects of oral administration of ellagic acid-rich pomegranate extract on ultraviolet-induced pigmentation in the human skin. Journal of Nutritional Science and Vitaminology, 52(5), 383–388. [DOI] [PubMed] [Google Scholar]
- Khan N, Syed DN, Pal HC, Mukhtar H, & Afaq F. (2012). Pomegranate fruit extract inhibits UVB-induced inflammation and proliferation by modulating NF-kappaB and MAPK signaling pathways in mouse skin. Photochemistry and Photobiology, 88(5), 1126–1134. doi: 10.1111/j.1751-1097.2011.01063.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrosa M, González-Sarrías A, García-Conesa MT, Tomás-Barberán FA, & Espín JC (2006). Urolithins, ellagic acid-derived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities. Journal of Agricultural and Food Chemistry, 54(5), 1611–1620. [DOI] [PubMed] [Google Scholar]
- Lawrence J, Dickson F, & Benford D. (1997). Skin irritant-induced cytotoxicity and prostaglandin E2 release in human skin keratinocyte cultures. Toxicology in Vitro, 11(5), 627–631. [DOI] [PubMed] [Google Scholar]
- Liu W, Ma H, Frost L, Yuan T, Dain JA, & Seeram NP (2014). Pomegranate phenolics inhibit formation of advanced glycation endproducts by scavenging reactive carbonyl species. Food & Function, 5(11), 2996–3004. doi: 10.1039/c4fo00538d [DOI] [PubMed] [Google Scholar]
- Liu Y, & Seeram NP (2018). Liquid chromatography coupled with time-of-flight tandem mass spectrometry for comprehensive phenolic characterization of pomegranate fruit and flower extracts used as ingredients in botanical dietary supplements. Journal of Separation Science, 41(15), 3022–3033.doi: 10.1002/jssc.201800480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, DaSilva NA, Liu W, Nahar PP, Wei Z, Liu Y, et al. (2016). Effects of a standardized phenolic-enriched maple syrup extract on beta-amyloid aggregation, neuroinflammation in microglial and neuronal cells, and beta-amyloid induced neurotoxicity in Caenorhabditis elegans. Neurochemical Research, 41(11), 2836–2847. doi: 10.1007/s11064-016-1998-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Johnson SL, Liu W, DaSilva NA, Meschwitz S, Dain JA, et al. (2018). Evaluation of polyphenol anthocyanin-enriched extracts of blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry for free radical scavenging, reactive carbonyl species trapping, anti-glycation, anti-beta-amyloid aggregation, and microglial neuroprotective effects. International Journal of Molecular Sciences, 19(2). doi: 10.3390/ijms19020461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwain DR, Berger T, & Mak TW (2015). Caspase functions in cell death and disease. Cold Spring Harbor Perspectives in Biology, 7(4). doi: 10.1101/cshperspect.a026716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco-Palencia LA, Mertens-Talcott S, & Talcott ST (2008). Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from Acai (Euterpe oleracea Mart.). Journal of Agricultural and Food Chemistry, 56(12), 4631–4636. doi: 10.1021/jf800161u [DOI] [PubMed] [Google Scholar]
- Pacheco-Palencia LA, Noratto G, Hingorani L, Talcott ST, & Mertens-Talcott SU (2008). Protective effects of standardized pomegranate (Punica granatum L.) polyphenolic extract in ultraviolet-irradiated human skin fibroblasts. Journal of Agricultural and Food Chemistry, 56(18), 8434–8441. doi: 10.1021/jf8005307 [DOI] [PubMed] [Google Scholar]
- Poljsak B, Dahmane RG, & Godic A. (2012). Intrinsic skin aging: the role of oxidative stress. Acta Dermatovenerol Alp Pannonica Adriat, 21(2), 33–36. [PubMed] [Google Scholar]
- Qiu Z, Zhou B, Jin L, Yu H, Liu L, Liu Y, et al. (2013). In vitro antioxidant and antiproliferative effects of ellagic acid and its colonic metabolite, urolithins, on human bladder cancer T24 cells. Food and Chemical Toxicology, 59, 428–437. doi: 10.1016/j.fct.2013.06.025 [DOI] [PubMed] [Google Scholar]
- Rahman I, Biswas SK, & Kirkham PA (2006). Regulation of inflammation and redox signaling by dietary polyphenols. Biochemical Pharmacology, 72(11), 1439–1452. doi: 10.1016/j.bcp.2006.07.004 [DOI] [PubMed] [Google Scholar]
- Ray PD, Huang BW, & Tsuji Y. (2012). Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cellular Signalling, 24(5), 981–990. doi: 10.1016/j.cellsig.2012.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinnerthaler M, Bischof J, Streubel MK, Trost A, & Richter K. (2015). Oxidative stress in aging human skin. Biomolecules, 5(2), 545–589. doi: 10.3390/biom5020545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta A, Lichti UF, Carlson BA, Ryscavage AO, Gladyshev VN, Yuspa SH, et al. (2010). Selenoproteins are essential for proper keratinocyte function and skin development. PLoS One, 5(8), e12249. doi: 10.1371/journal.pone.0012249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok JK, Lee JW, Kim YM, & Boo YC (2018). Punicalagin and (−)-epigallocatechin-3-gallate rescue cell viability and attenuate inflammatory responses of human epidermal keratinocytes exposed to airborne particulate matter PM10. Skin Pharmacology and Physiology, 31(3), 134–143. doi: 10.1159/000487400 [DOI] [PubMed] [Google Scholar]
- Syed DN, Malik A, Hadi N, Sarfaraz S, Afaq F, & Mukhtar H. (2006). Photochemopreventive effect of pomegranate fruit extract on UVA-mediated activation of cellular pathways in normal human epidermal keratinocytes. Photochemistry and Photobiology, 82(2), 398–405. doi: 10.1562/2005-06-23-RA-589 [DOI] [PubMed] [Google Scholar]
- Szyszkowska B, Lepecka-Klusek C, Kozlowicz K, Jazienicka I, & Krasowska D. (2014). The influence of selected ingredients of dietary supplements on skin condition. Advances in Dermatology and Allergology, 31(3), 174–181. doi: 10.5114/pdia.2014.40919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Mo Y, Li Y, Zhong Y, He S, Zhang Y, et al. (2017). Urolithin A alleviates myocardial ischemia/reperfusion injury via PI3K/Akt pathway. Biochemical and Biophysical Research Communications, 486(3), 774–780. doi: 10.1016/j.bbrc.2017.03.119 [DOI] [PubMed] [Google Scholar]
- Wang D, Chen Q, Liu B, Li Y, Tan Y, & Yang B. (2016). Ellagic acid inhibits proliferation and induces apoptosis in human glioblastoma cells. Acta Cirurgica Brasleira, 31(2), 143–149. doi: 10.1590/S0102-865020160020000010 [DOI] [PubMed] [Google Scholar]
- Wang ST, Chang WC, Hsu C, & Su NW (2017). Antimelanogenic effect of urolithin A and urolithin B, the colonic metabolites of ellagic acid, in B16 melanoma cells. Journal of Agricultural and Food Chemistry, 65(32), 6870–6876. doi: 10.1021/acs.jafc.7b02442 [DOI] [PubMed] [Google Scholar]
- Wang Y, Qiu Z, Zhou B, Liu C, Ruan J, Yan Q, et al. (2015). In vitro antiproliferative and antioxidant effects of urolithin A, the colonic metabolite of ellagic acid, on hepatocellular carcinomas HepG2 cells. Toxicology In Vitro, 29(5), 1107–1115. doi: 10.1016/j.tiv.2015.04.008 [DOI] [PubMed] [Google Scholar]
- Yaar M, & Gilchrest BA (2001). Skin aging: postulated mechanisms and consequent changes in structure and function. Clinics in Geriatric Medicine, 17(4), 617–630, v. [DOI] [PubMed] [Google Scholar]
- Yang H, Liu C, Zhang YQ, Ge LT, Chen J, Jia XQ, et al. (2015). Ilexgenin A induces B16-F10 melanoma cell G1/S arrest in vitro and reduces tumor growth in vivo. International Immunopharmacology, 24(2), 423–431. doi: 10.1016/j.intimp.2014.12.040 [DOI] [PubMed] [Google Scholar]
- Yuan T, Ding Y, Wan C, Li L, Xu J, Liu K, et al. (2012). Antidiabetic ellagitannins from pomegranate flowers: inhibition of alpha-glucosidase and lipogenic gene expression. Organic Letters, 14(20), 5358–5361. doi: 10.1021/ol302548c [DOI] [PubMed] [Google Scholar]
- Yuan T, Ma H, Liu W, Niesen DB, Shah N, Crews R, et al. (2016). Pomegranate’s neuroprotective effects against Alzheimer’s disease are mediated by urolithins, its ellagitannin-gut microbial derived metabolites. ACS Chemical Neuroscience, 7(1), 26–33. doi: 10.1021/acschemneuro.5b00260 [DOI] [PubMed] [Google Scholar]
- Yuan T, Wan C, Ma H, & Seeram NP (2013). New phenolics from the flowers of Punica granatum and their in vitro alpha-glucosidase inhibitory activities. Planta Medica, 79(17), 1674–1679. doi: 10.1055/s-0033-1350925 [DOI] [PubMed] [Google Scholar]
- Zaid MA, Afaq F, Syed DN, Dreher M, & Mukhtar H. (2007). Inhibition of UVB‐mediated oxidative stress and markers of photoaging in immortalized HaCaT keratinocytes by pomegranate polyphenol extract POMx. Photochemistry and Photobiology, 83(4), 882–888. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Yang H, Sun WD, Wang J, Zhang BY, Shen YJ, et al. (2018). Ethanol extract of Ilex hainanensis Merr. exhibits anti-melanoma activity by induction of G1/S cell-cycle arrest and apoptosis. Chinese Journal of Integrative Medicine, 24(1), 47–55. doi: 10.1007/s11655-017-2544-8 [DOI] [PubMed] [Google Scholar]
- Zhao J, Li G, Bo W, Zhou Y, Dang S, Wei J, et al. (2017). Multiple effects of ellagic acid on human colorectal carcinoma cells identified by gene expression profile analysis. International Journal of Oncology, 50(2), 613–621. doi: 10.3892/ijo.2017.3843 [DOI] [PubMed] [Google Scholar]