Abstract
Despite the increasing recognition of noroviruses as major pathogens associated with community-acquired diarrhoea in children, there are few studies from Africa. Long-term surveillance studies of rotavirus gastroenteritis in Malawian children have provided an opportunity to undertake a study of the importance and epidemiological features of norovirus infection in this population. Faecal specimens were collected from children <5 years of age admitted to hospital with acute diarrhoea, as well as from a comparison group of diarrhoea-free children, in Blantyre, Malawi between 1997 and 2007. Norovirus was detected using real-time PCR and strains genotyped by nucleotide sequence analysis. Norovirus was detected in 220/1,941 (11.3%) faecal specimens, comprising genogroup GI (1.8%), GII (9.4%) and mixed GI/GII (0.1%). The median age of children with norovirus was 6 months (range, 0–48 months). Norovirus was detected throughout the year, with peaks at the end of the rainy season (March) and towards the end of the dry season (August–November). Norovirus GII.4 was the most commonly detected genotype accounting for 70% of strains characterised, followed by GII.2 (6%), GII.6 (4%) and GII.12 (4%). Sub typing of GII.4 noroviruses demonstrated local circulation of strains prior to their subsequent detection in association with global epidemics of gastroenteritis. The prevalence of norovirus in children without diarrhoea was similar to the level in cases. This largest study to date of norovirus infection in African children indicates the potential role of paediatric surveillance in predicting the emergence of norovirus strains with global epidemic potential.
Keywords: gastroenteritis, norovirus, Malawi
INTRODUCTION
Acute gastroenteritis is a major cause of childhood mortality in the developing world. While rotavirus is firmly established as the most important cause of severe childhood diarrhoea globally, the role of norovirus is gaining increasing recognition [Patel et al., 2008]. Noroviruses comprise a genetically diverse group of non-enveloped, single stranded RNA viruses, belonging to the family Caliciviridae [Green et al., 2000]. Noroviruses are classified into five genogroups (GI–GV) on the basis of the amino acid sequence diversity of the major structural protein (VP1) [Zheng et al., 2006], with GI and GII being responsible for the main burden of human disease [Patel et al., 2008]. Noroviruses can be further classified into genotypes, with GII noroviruses divided into 21 genotypes [Hall et al., 2011]. Since 2002, genotype GII.4 has emerged as the most important cause of human disease worldwide [Siebenga et al., 2009].
Understanding of norovirus epidemiology has changed dramatically over the past few decades with the introduction of increasingly sensitive and specific diagnostic tools, especially real-time PCR [Glass et al., 2000; Lopman, 2006]. Norovirus is now recognised as the most common cause of both epidemic and sporadic acute gastroenteritis in adults in the developed world [Glass et al., 2009; Tam et al., 2012]. While the role of norovirus in sporadic paediatric gastroenteritis in industrialised countries is recognised increasingly [Pang et al., 1999; Phan et al., 2006; Iturriza-Gomara et al., 2009], understanding of the genotype distribution of norovirus infections is biased towards data from outbreaks, especially those associated with food borne transmission and healthcare settings. In addition, norovirus epidemiology in childhood sporadic gastroenteritis in the developing world remains poorly described, with very few studies from Africa.
The epidemiology of rotavirus infection has been well described in Africa [Cunliffe et al., 2010; Todd et al., 2010; Waggie et al., 2010], including as part of a rotavirus surveillance programme a 10-year investigation of rotavirus infections among hospitalised children with acute diarrhoea in Blantyre, Malawi [Cunliffe et al., 2010]. The aim of the current study was to determine, using remaining faecal specimens from this rotavirus surveillance programme: (i) the prevalence of norovirus in hospitalised children with acute severe gastroenteritis, (ii) the age distribution and seasonality of norovirus infection, and (iii) the genogroup and genotype distribution of circulating norovirus strains.
MATERIALS AND METHODS
Study Patients, Enrolment and Stool collection
The study patients comprised children <5 years of age admitted with acute gastroenteritis to the Queen Elizabeth Central Hospital, Blantyre, Malawi, from July 1997 through June 2007. The enrolment procedures have been described previously [Cunliffe et al., 2010]. Briefly, study nurses enrolled children with acute gastroenteritis (defined as the passage of ≥3 loose or watery stools in a 24-hr period for <14 days) who were admitted to the paediatric wards. Children <5 years of age hospitalised between 1997 and 1999 with acute medical illness other than gastroenteritis, for example, respiratory infections and malaria, and from whom a negative history of diarrhoea in the 2 weeks preceding admission was obtained from the parents/guardians, were enrolled as a comparison group. The comparison group was selected from the same age group (<5 years) but was not matched for age or season. A single faecal sample was collected from each child as soon as possible after admission. Ten percent faecal suspensions were prepared in phosphate buffered saline and were then stored at −80°C. Specimens were tested previously for rotavirus infection using ELISA [Cunliffe et al., 2010]. Specimens for those children admitted to hospital in which sufficient volume was remaining were further tested for norovirus.
Norovirus Detection and Strain Characterisation
Nucleic acid was extracted from 10% faecal suspensions in phosphate buffered saline using a high throughput extractor, QIAsymphony® (Qiagen, Manchester, UK). Reverse transcription of RNA was performed using random hexamers. Norovirus genogroups GI/GII were detected using a real-time PCR technique described by Kageyama et al. [2003] as modified by Amar et al. [2007]. For genotyping, a fragment of the N/S-domain of the norovirus capsid was amplified by PCR using G2SKF and G2SKR primers [Kojima et al., 2002]. Amplicons were purified using Micro-spin columns (GE Healthcare, Buckinghamshire, UK), prior to direct sequencing by a commercial company, Cogenics (Beckman Coulter, Genomics, UK).
Norovirus strains were genotyped by phylogenetic analysis against sequences of prototype strains representative of the different norovirus genotypes available in Genbank (data not shown). Nucleotide sequences were analysed by multiple alignment and phylogeny according to the neighbor-joining method [Saitou and Nei, 1987] in the ClustalW software package [Thompson et al., 1994]. In addition, GII.4 strains were subtyped further using a web-based norovirus typing tool available through Noronet (http://www.rivm.nl/en/Topics/Topics/N/NoroNet/Databases/The_norovirus_typingtool).
RESULTS
Prevalence of Norovirus
From the primary study [Cunliffe et al., 2010], faecal specimens from 2,458 children hospitalised with diarrhoea were collected, of which 32.1% tested positive for rotavirus [Cunliffe et al., 2010]. Of these, a total of 1,941/2,458 (79%) faecal specimens were available for norovirus testing. The prevalence of norovirus was 11.3% (220 patients), comprising GI 1.8% (35 patients), GII 9.4% (183 patients) and mixed GI/GII 0.1% (2 patients). Norovirus prevalence fluctuated between years, from 5.5% in 2005 to 19.3% in 2000 (Table I). The proportion of norovirus positive samples which also contained rotavirus was 1.5% (29 patients). For the 2-year period from 1997 to 1999 during which specimens from both diarrhoea patients and healthy controls were selected, norovirus was detected in 11.9% (89/746) of diarrhoea patients. In 505 faecal samples obtained from children without diarrhoea, the prevalence of norovirus was 11.8% (60 patients), comprising GI 0.9% (5 patients), GII 10.7% (54 patients) and mixed GI/GII 0.2% (1 patient). There was no apparent difference in the Ct value for cases (median 30; IQR 29–31) compared to controls (median 29; IQR 27–29).
TABLE I.
Norovirus Genogroups and Genotypes, by Year of Collection
| Year of collection | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | 1997 | 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | Total |
| GI.3 | 1 | 1 | 1 | 1 | 4 | |||||||
| GI.5 | 2 | 2 | ||||||||||
| GI.7 | 1 | 5 | 3 | 9 | ||||||||
| GI.9 | 1 | 1 | ||||||||||
| GI.11 | 1 | 1 | ||||||||||
| GI.14 | 1 | 1 | 2 | |||||||||
| GII.2 | 3 | 1 | 2 | 6 | ||||||||
| GII.3 | 1 | 2 | 3 | |||||||||
| GII.4 | 12 | 16 | 22 | 4 | 2 | 1 | 2 | 3 | 2 | 3 | 67 | |
| GII.6 | 1 | 2 | 1 | 4 | ||||||||
| GII.7 | 1 | 1 | ||||||||||
| GII.10 | 1 | 1 | 1 | 3 | ||||||||
| GII.11 | 1 | 1 | ||||||||||
| GII.12 | 2 | 1 | 1 | 4 | ||||||||
| GII.13 | 1 | 1 | ||||||||||
| GII.15 | 1 | 2 | 3 | |||||||||
| GII.16 | 2 | 1 | 3 | |||||||||
| GII.4/GI.5 | 1 | 1 | ||||||||||
| Total genotyped | 8 | 16 | 25 | 36 | 8 | 3 | 2 | 2 | 6 | 7 | 3 | 116 |
| Total NoV-pos (%) | 17 (11.0) | 30 (9.5) | 42 (15.2) | 57 (19.3) | 18 (14.3) | 8 (6.8) | 7 (9.6) | 9 (6.3) | 9 (5.5) | 16 (8.6) | 7 (7.9) | 220 (11.3) |
| Total tested | 154 | 316 | 276 | 296 | 126 | 118 | 73 | 143 | 165 | 185 | 89 | 1,941 |
Age Distribution of Children With Norovirus Infection
Norovirus infections peaked in children 3–5 months of age, and the median age of children with norovirus infection was 6 months (range, <1–48 months), similar to that of children with rotavirus gastroenteritis in the same population (median 7 months; range, <1–41 months; Fig. 1).
Fig. 1.
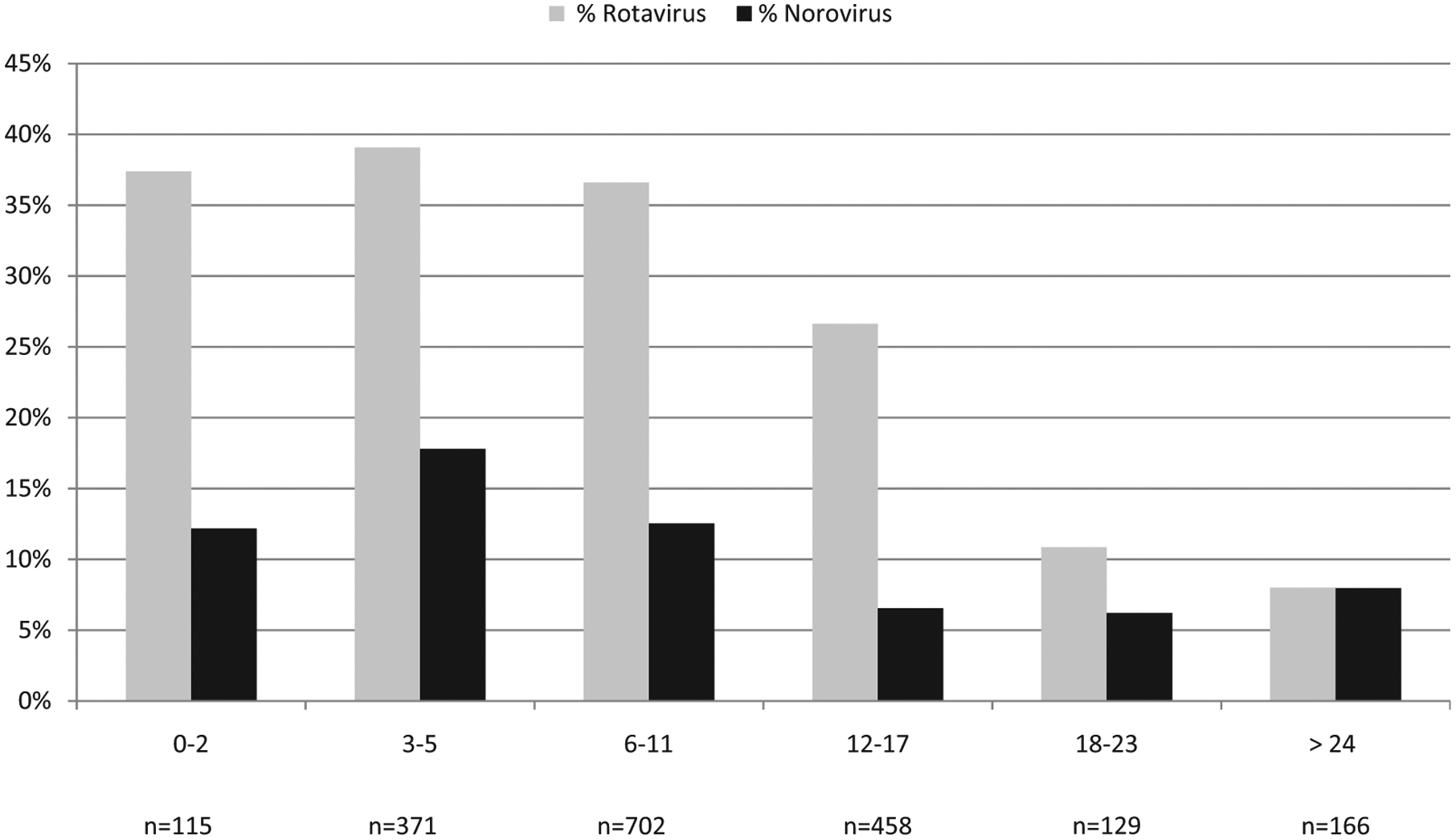
Age distribution (months) of hospitalised children with norovirus and rotavirus gastroenteritis at the Queen Elizabeth Central Hospital, Blantyre, Malawi, 1997–2007.
Seasonality of Norovirus Infection
Norovirus prevalence was analysed by month for the duration of the study and compared with the monthly detection of rotavirus (Fig. 2). Norovirus circulated throughout the year with increased detection towards the end of the dry season (August through November) and the end of the wet season (February through March), peaking in March. This is in contrast to the seasonality of rotavirus infection in Malawi, the detection rate of which is characteristically highest during the dry season (May through August).
Fig. 2.
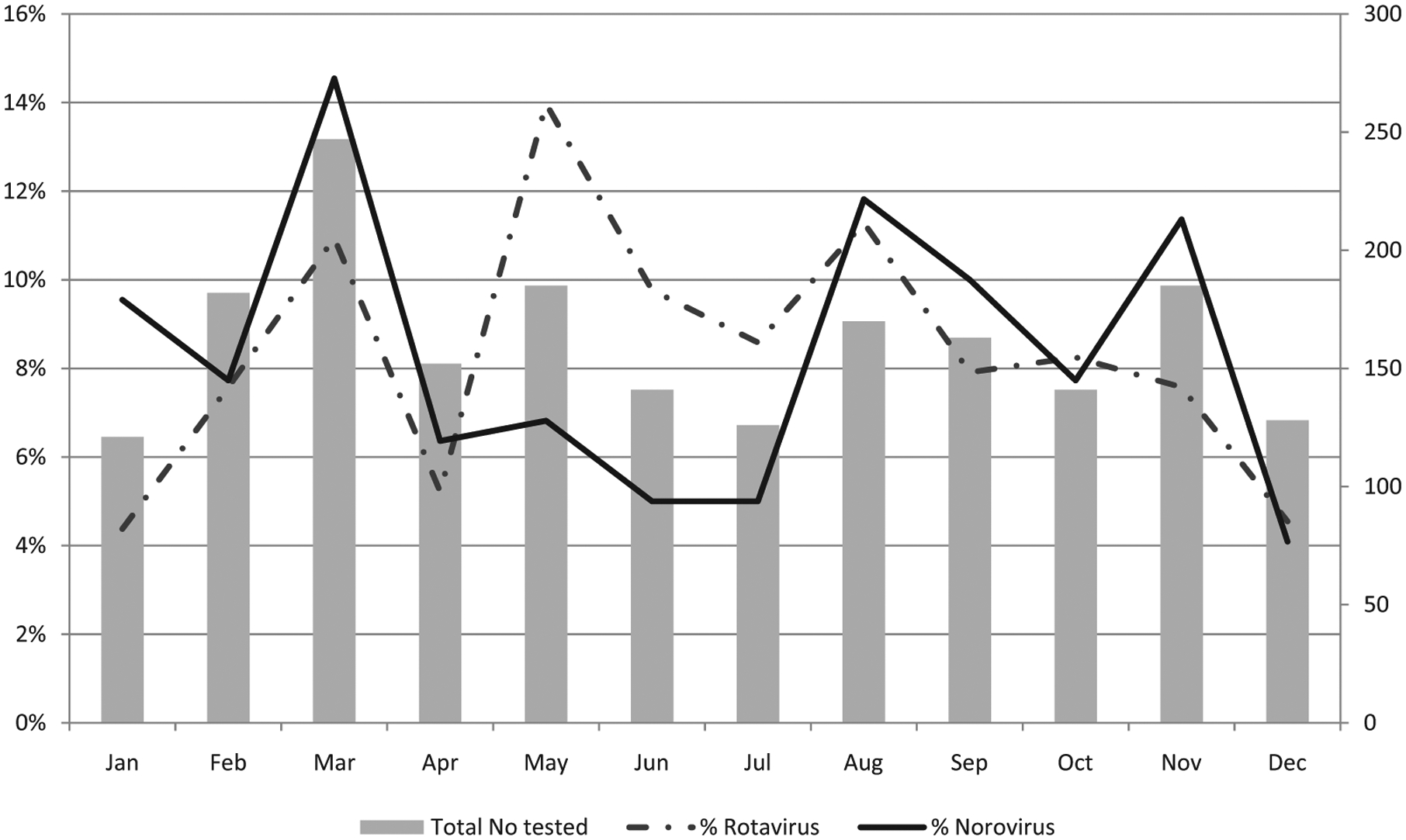
Seasonality of norovirus and rotavirus in hospitalised children with acute gastroenteritis at the Queen Elizabeth Central Hospital, Blantyre, Malawi, 1997–2007 (each month was combined for the 10 years).
Norovirus Genotyping
Among 220 norovirus positive samples, sufficient DNA to enable genotype determination by sequence analysis was obtained from 116 samples (53%; Table I). Among GI noroviruses (n = 19), the most commonly detected genotype was GI.7 (n = 9 [47%]), followed by GI.3 (n = 4 [21%]) and GI.5/GI.14 (n = 2 for each, [11%]). Among GII noroviruses (n = 96), GII.4 was by far the most commonly detected genotype (n = 67 [70%]), followed by GII.2 (n = 6 [6%]) and jointly GII.6 and GII.12 with 4 (4%) each (Table I).
Genotype GII.4 norovirus was first detected in 1998 and was the predominant genotype every year thereafter with the exception of 2005 when GI.7 and GII.4 were detected in equal numbers. Genotype GII.2 norovirus disappeared after 1998, remerging in 2006 as the second most predominant strain along with GII.15. Other predominant genotypes circulating during this period included GI.7, which was the second most predominant in 2000, and GI.3 which was second from 2002 and joint first with GII.4 in 2003 (Table I).
Phylogenetic analysis of the sequences derived from the GII.4 stains from Malawi showed that they clustered chronologically (data not shown). Strains homologous to the reference GII.4 strains associated with large epidemics of norovirus worldwide were circulating in Malawi several years prior to their identification as global epidemic strains (Table II).
TABLE II.
Norovirus GII.4 Variant Strains Detected in Malawi Between 1997 and 2007
| Strain | Year of detection | Genotype/variant | Full name of reference Strain | Ref. strain accesion number |
|---|---|---|---|---|
| MW1-229 | 01/1998 | II.4|1996 | 408/97003012/1996/FL | AF080558 |
| MW1-243 | 01/1998 | II.4|1996 | 408/97003012/1996/FL | AF080558 |
| MW1-314 | 02/1998 | II.4|1996 | 408/97003012/1996/FL | AF080558 |
| MW1-329 | 02/1998 | II.4|1996 | 408/97003012/1996/FL | AF080558 |
| MW1-522 | 09/1998 | II.4|Camb | Camberwell/1994 | AF145896 |
| MW1-665 | 02/1999 | II.4|2002 | Farmington Hills/02/US | AY502023 |
| MW1-679 | 02/1999 | II.4|Camb | Camberwell/1994 | AF145896 |
| MW1-684 | 03/1999 | II.4|1996 | 408/97003012/1996/FL | AF080558 |
| MW1-756 | 04/1999 | II.4|Camb | Camberwell/1994 | AF145896 |
| MW1-777 | 04/1999 | II.4|1996 | 408/97003012/1996/FL | AF080558 |
| MW1-805 | 05/1999 | II.4|Camb | Camberwell/1994 | AF145896 |
| MW1-848 | 06/1999 | II.4|Camb | Camberwell/1994 | AF145896 |
| MW2-002 | 07/1999 | II.4|2002 | Farmington Hills/02/US | AY502023 |
| MW2-023 | 08/1999 | II.4|2002 | Farmington Hills/02/US | AY502023 |
| MW2-287 | 04/2000 | II.4|2002/CN | Houston/TCH186/02/US | EU310927 |
| MW2-338 | 05/2000 | II.4|2002 | Farmington Hills/02/US | AY502023 |
| MW2-365 | 06/2000 | II.4|2002/CN | Houston/TCH186/02/US | EU310927 |
| MW2-378 | 06/2000 | II.4|2004 | GII.4/04/NL | AY883096 |
| MW2-382 | 06/2000 | II.4|2002/CN | Houston/TCH186/02/US | EU310927 |
| MW2-491 | 09/2000 | II.4|2002/CN | Houston/TCH186/02/US | EU310927 |
| MW2-510 | 09/2000 | II.4|2002/CN | Houston/TCH186/02/US | EU310927 |
| MW2-515 | 09/2000 | II.4|2002 | Farmington Hills/02/US | AY502023 |
| MW2-636 | 03/2001 | II.4|2006b | DenHagg89/2006/NL | EF126963 |
| MW2-670 | 05/2001 | II.4|2002 | Farmington Hills/02/US | AY502023 |
| MW2-742 | 09/2001 | II.4|2002 | Farmington Hills/02/US | AY502023 |
| MW2-751 | 09/2001 | II.4|2004 | GII.4/04/NL | AY883096 |
| MW2-833 | 03/2002 | II.4|2008 | Apeldoorn317/2007/NL | AB445395 |
| MW2-1020 | 08/2003 | II.4|2003 | Norovirus Hu/Chiba/04-1050/2005/JP | AB220921 |
| MW2-1069 | 02/2004 | II.4|2002 | Farmington Hills/02/US | AY502023 |
| MW2-1199 | 11/2004 | II.4|2003 | Norovirus Hu/Chiba/04-1050/2005/JP | AB220921 |
| MW2-1218 | 01/2005 | II.4|2004 | GII.4/04/NL | AY883096 |
| MW2-1226 | 01/2005 | II.4|2004 | GII.4/04/NL | AY883096 |
| QEC-014 | 08/2005 | II.4|2006a | Yerseke38/2006/NL | EF126963 |
| QEC-264 | 10/2006 | II.4|Camb | Camberwell/1994 | AF145896 |
| QEC-309 | 01/2007 | II.4|2002 | Farmington Hills/02/US | AY502023 |
| QEC-350 | 03/2007 | II.4|2002 | Farmington Hills/02/US | AY502023 |
According to the Typing Scheme Used by Noronet (http://www.rivm.nl/en/Topics/Topics/N/NoroNet/Databases/The_norovirus_typingtool). Strains associated with global epidemics are highlighted in boldface.
DISCUSSION
This 10-year study is the largest to date using molecular detection methods to examine the role of human noroviruses in community-acquired paediatric gastroenteritis in hospitalised African children. A norovirus detection rate of 11.3% is demonstrated in this population, with 83.1% of infections comprising genogroup GII. These data confirm norovirus as the second most commonly identified virus in hospitalised Malawian children with acute gastroenteritis after rotavirus, which was detected previously in 32.1% of children [Cunliffe et al., 2010].
These results are consistent with a global systematic review in which the pooled norovirus detection rate was 12% in hospitalised children <5 years of age with acute gastroenteritis [Patel et al., 2008]. Several smaller studies have examined norovirus infections using RT-PCR in hospitalised children in the African region; these include Malawi where a detection rate of 6.5% was reported using RT-PCR of the polymerase gene [Dove et al., 2005], Ghana (15.9%), Tunisia (16.2%), South Africa (14.3%), Botswana (22%), Libya (13.8%), Cameron (29%), Tanzania (14%) and Nigeria (25%) [Armah et al., 2006; Sdiri-Loulizi et al., 2009; Mans et al., 2010; Mattison et al., 2010; Abugalia et al., 2011; Ayukekbong et al., 2011; Moyo et al., 2011; Oluwatoyin Japhet et al., 2012]. This study documented mixed norovirus and rotavirus infections in 1.5% of the cases; elsewhere, dual rotavirus and norovirus infections have been reported in 0.2–11% of children hospitalised with gastroenteritis [Dove et al., 2005; Monica et al., 2007; Victoria et al., 2007; Iturriza-Gomara et al., 2008; Onishi et al., 2008; Sdiri-Loulizi et al., 2009]. Differences in rotavirus and norovirus seasonality may account for the relatively low rate of co-infections in this study.
The norovirus detection rate in the asymptomatic comparison group did not differ from that in children with symptoms of gastroenteritis. Norovirus detection rates ranging between 0% and 31% have been reported in studies that included diarrhoea free children [Bon et al., 1999; Pang et al., 1999; O’Ryan et al., 2000; de Wit et al., 2001a,b; Oh et al., 2003; Parashar et al., 2004; Amar et al., 2007; Monica et al., 2007; Mattison et al., 2010]. In most studies the prevalence of norovirus was significantly higher in the cases than in the controls, however, in a small study from Botswana [Mattison et al., 2010] the rate of detection of norovirus was higher in asymptomatic than in symptomatic children, whilst in another study in Cameroon norovirus infections were detected always in the presence of another enteric virus co-infection [Ayukekbong et al., 2011]. The reasons for the finding of similar rates of norovirus detection in children with and without diarrhoea are unclear and raise questions about the role of noroviruses in diarrhoeal disease particularly in this setting. These findings may be explained by a combination of truly asymptomatic infections, incomplete recall of diarrhoeal symptoms from parents and/or persistent viral shedding from resolved acute norovirus gastroenteritis episodes. In order to better interpret the presence of norovirus in controls, and ultimately their etiologic role in cases, studies are required that prospectively follow children to determine how asymptomatic infection is acquired and how immunity to norovirus develops amongst children in lower income settings.
Most of the globally available data on norovirus strain diversity are from outbreak settings involving adults and the elderly in developed nations. Studies examining community-acquired norovirus infections in children are much fewer in number, but have highlighted a greater diversity of norovirus strains than in adult-predominant outbreak settings [Monica et al., 2007; Iturriza-Gomara et al., 2009]. The predominant norovirus genogroup identified in this study was GII, the most prevalent genogroup worldwide, accounting for 96% of all non-outbreak-associated infections in children ≤18 years over the past decade [Hoa Tran et al., 2013]. Among GII noroviruses in Malawi, genotypes detected most commonly over the 10 years of study were GII.4, GII.2 and jointly GII.6/GII.12. Globally over the past decade after GII.4, the genotype most commonly described has been GII.3 [Hoa Tran et al., 2013], which was a relatively uncommon genotype in this study, appearing only once in 1997 and twice in 1999. However, the predominance of genotypes GII.2 and GII.6 has been described in children ≤18 years in several other countries during the past decade including Japan, Brazil, South Africa and Finland [Phan et al., 2005; Ferreira et al., 2010; Mans et al., 2010; Puustinen et al., 2011].
Phylogenetic analysis demonstrated that in Malawi genotype GII.4 stains clustered largely according to the year of detection (data not shown), as described elsewhere [Lindesmith et al., 2008]. Sub-typing showed that the strain reported to be associated with large norovirus epidemics in 2002 in Europe and the US [Lopman et al., 2004; Widdowson et al., 2004] was circulating in Malawi as early as 1999. The detection of this GII.4 strain also coincided with a peak in norovirus detection in the years 1999 and 2000, which reached 19%, as well as with the predominance of GII.4 strains (>60% of the norovirus strains). Also, other GII.4 norovirus strains associated with global epidemics in 2004 [Bull et al., 2006], 2006 [Gallimore et al., 2007; Siebenga et al., 2007] and in 2008 [Belliot et al., 2010] were detected in Malawi as early as in 2000, 2001 and 2002, respectively. Of note, the detection of a pandemic GII.4 strain in children in northern Africa a year prior to its widespread detection in association with outbreaks in 2004 has been reported previously [Sdiri-Loulizi et al., 2009].
The median age of children with norovirus was 6 months, similar to children with rotavirus (7 months), with both viruses being most common in the 3–5-month age group. In a study of Libyan children with acute gastroenteritis, the median age of norovirus and rotavirus infection was 10 months, with peak detection rate between 6 and 11 months of age [Abugalia et al., 2011], and in South Africa norovirus infections were only detected in children <2 years of age. In industrialised countries, evidence points to a later age of acquisition of norovirus, with the prevalence of infection peaking in children 1–4 years old in England and the Netherlands [de Wit et al., 2001b; Amar et al., 2007; Iturriza-Gomara et al., 2008, 2009]; however, these studies describe norovirus infections in general practice or the community rather than in hospitalised children with gastroenteritis, which may contribute to the differences in age distribution between studies.
These data show that norovirus and rotavirus infections co-circulate in this population year round; however there was a divergence in peak seasonality with rotavirus infections peaking during the dry season and norovirus during the wet season. In other African nations a variety of patterns of seasonality have been reported. In Ghanaian children norovirus peaked together with rotavirus during the dry season, with norovirus absent during the wet season [Armah et al., 2006], and in Libyan children rotavirus peaked in the cool winter months and norovirus during the hot summer months. Data from temperate countries also indicate year round circulation of noroviruses, but with peaks in cooler winter months [Mounts et al., 2000]. The factors which influence norovirus seasonality appear complex, and probably involve the interaction of multiple variables including host factors, climate and human behaviour such as diet and leisure pursuits.
These findings highlight the value of systematic norovirus surveillance in community-acquired gastroenteritis in children, as this may provide a more efficient way to monitor the emergence of variant strains before they cause large epidemics. Additionally, with the introduction of rotavirus vaccine in the national childhood immunisation schedule in Malawi in October 2012, these results provide an epidemiological baseline to assess any impact of rotavirus vaccination on the prevalence of norovirus. As norovirus vaccines become available in the future, monitoring strain diversity in children will prove important to allow inclusion of relevant strains for the formulation of effective norovirus vaccines ahead of the epidemic spread of emerging strains including GII.4 [Parra et al., 2012].
ACKNOWLEDGMENTS
Dr Eamonn Trainor is an NIHR-funded Academic Clinical Fellow in Medical Microbiology.
REFERENCES
- Abugalia M, Cuevas L, Kirby A, Dove W, Nakagomi O, Nakagomi T, Kara M, Gweder R, Smeo M, Cunliffe N. 2011. Clinical features and molecular epidemiology of rotavirus and norovirus infections in Libyan children. J Med Virol 83:1849–1856. [DOI] [PubMed] [Google Scholar]
- Amar CF, East CL, Gray J, Iturriza-Gomara M, Maclure EA, McLauchlin J. 2007. Detection by PCR of eight groups of enteric pathogens in 4,627 faecal samples: Re-examination of the English case-control Infectious Intestinal Disease Study (1993–1996). Eur J Clin Microbiol Infect Dis 26:311–323. [DOI] [PubMed] [Google Scholar]
- Armah GE, Gallimore CI, Binka FN, Asmah RH, Green J, Ugoji U, Anto F, Brown DW, Gray JJ. 2006. Characterisation of norovirus strains in rural Ghanaian children with acute diarrhoea. J Med Virol 78:1480–1485. [DOI] [PubMed] [Google Scholar]
- Ayukekbong J, Lindh M, Nenonen N, Tah F, Nkuo-Akenji T, Bergstrom T. 2011. Enteric viruses in healthy children in Cameroon: Viral load and genotyping of norovirus strains. J Med Virol 83:2135–2142. [DOI] [PubMed] [Google Scholar]
- Belliot G, Kamel AH, Estienney M, Ambert-Balay K, Pothier P. 2010. Evidence of emergence of new GGII.4 norovirus variants from gastroenteritis outbreak survey in France during the 2007-to-2008 and 2008-to-2009 winter seasons. J Clin Microbiol 48:994–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bon F, Fascia P, Dauvergne M, Tenenbaum D, Planson H, Petion AM, Pothier P, Kohli E., 1999. Prevalence of group A rotavirus, human calicivirus, astrovirus, and adenovirus type 40 and 41 infections among children with acute gastroenteritis in Dijon, France. J Clin Microbiol 37:3055–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull RA, Tu ET, McIver CJ, Rawlinson WD, White PA. 2006. Emergence of a new norovirus genotype II.4 variant associated with global outbreaks of gastroenteritis. J Clin Microbiol 44:327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunliffe NA, Ngwira BM, Dove W, Thindwa BD, Turner AM, Broadhead RL, Molyneux ME, Hart CA. 2010. Epidemiology of rotavirus infection in children in Blantyre, Malawi, 1997–2007. J Infect Dis 202:S168–S174. [DOI] [PubMed] [Google Scholar]
- de Wit MA, Koopmans MP, Kortbeek LM, van Leeuwen NJ, Bartelds AI, van Duynhoven YT. 2001a. Gastroenteritis in sentinel general practices, The Netherlands. Emerg Infect Dis 7:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit MA, Koopmans MP, Kortbeek LM, Wannet WJ, Vinje J, van Leusden F, Bartelds AI, van Duynhoven YT. 2001b. Sensor, a population-based cohort study on gastroenteritis in the Netherlands incidence and etiology. Am J Epidemiol 154: 666–674. [DOI] [PubMed] [Google Scholar]
- Dove W, Cunliffe NA, Gondwe JS, Broadhead RL, Molyneux ME, Nakagomi O, Hart CA. 2005. Detection and characterization of human caliciviruses in hospitalized children with acute gastroenteritis in Blantyre, Malawi. J Med Virol 77:522–527. [DOI] [PubMed] [Google Scholar]
- Ferreira MS, Victoria M, Carvalho-Costa FA, Vieira CB, Xavier MP, Fioretti JM, Andrade J, Volotao EM, Rocha M, Leite JP, Miagostovich MP. 2010. Surveillance of norovirus infections in the state of Rio De Janeiro, Brazil 2005–2008. J Med Virol 82:1442–1448. [DOI] [PubMed] [Google Scholar]
- Gallimore CI, Iturriza-Gomara M, Xerry J, Adigwe J, Gray JJ. 2007. Inter-seasonal diversity of norovirus genotypes: Emergence and selection of virus variants. Arch Virol 152:1295–1303. [DOI] [PubMed] [Google Scholar]
- Glass RI, Noel J, Ando T, Fankhauser R, Belliot G, Mounts A, Parashar UD, Bresee JS, Monroe SS. 2000. The epidemiology of enteric caliciviruses from humans: A reassessment using new diagnostics. J Infect Dis 181:S254–S261. [DOI] [PubMed] [Google Scholar]
- Glass RI, Parashar UD, Estes MK. 2009. Norovirus gastroenteritis. N Engl J Med 361:1776–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KY, Ando T, Balayan MS, Berke T, Clarke IN, Estes MK, Matson DO, Nakata S, Neill JD, Studdert MJ, Thiel HJ. 2000. Taxonomy of the caliciviruses. J Infect Dis 181:S322–S330. [DOI] [PubMed] [Google Scholar]
- Hall RL, Jones JL, Herwaldt BL. 2011. Surveillance for laboratory-confirmed sporadic cases of cyclosporiasis–United States 1997–2008. MMWR Surveill Summ 60:1–11. [PubMed] [Google Scholar]
- Hoa Tran TN, Trainor E, Nakagomi T, Cunliffe NA, Nakagomi O. 2013. Molecular epidemiology of noroviruses associated with acute sporadic gastroenteritis in children: Global distribution of genogroups, genotypes and GII.4 variants. J Clin Virol 56: 269–277. [DOI] [PubMed] [Google Scholar]
- Iturriza-Gomara M, Simpson R, Perault AM, Redpath C, Lorgelly P, Joshi D, Mugford M, Hughes CA, Dalrymple J, Desselberger U, Gray J. 2008. Structured surveillance of infantile gastroenteritis in East Anglia, UK: Incidence of infection with common viral gastroenteric pathogens. Epidemiol Infect 136:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturriza-Gomara M, Elliot AJ, Dockery C, Fleming DM, Gray JJ. 2009. Structured surveillance of infectious intestinal disease in pre-school children in the community: ‘The Nappy Study’. Epidemiol Infect 137:922–931. [DOI] [PubMed] [Google Scholar]
- Kageyama T, Kojima S, Shinohara M, Uchida K, Fukushi S, Hoshino FB, Takeda N, Katayama K. 2003. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J Clin Microbiol 41:1548–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Kageyama T, Fukushi S, Hoshino FB, Shinohara M, Uchida K, Natori K, Takeda N, Katayama K. 2002. Genogroup-specific PCR primers for detection of Norwalk-like viruses. J Virol Methods 100:107–114. [DOI] [PubMed] [Google Scholar]
- Lindesmith LC, Donaldson EF, Lobue AD, Cannon JL, Zheng DP, Vinje J, Baric RS. 2008. Mechanisms of GII.4 norovirus persistence in human populations. PLoS Med 5:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopman B. 2006. Noroviruses: Simple detection for complex epidemiology. Clin Infect Dis 42:970–971. [DOI] [PubMed] [Google Scholar]
- Lopman B, Vennema H, Kohli E, Pothier P, Sanchez A, Negredo A, Buesa J, Schreier E, Reacher M, Brown D, Gray J, Iturriza M, Gallimore C, Bottiger B, Hedlund KO, Torven M, von Bonsdorff CH, Maunula L, Poljsak-Prijatelj M, Zimsek J, Reuter G, Szucs G, Melegh B, Svennson L, van Duijnhoven Y, Koopmans M. 2004. Increase in viral gastroenteritis outbreaks in Europe and epidemic spread of new norovirus variant. Lancet 363:682–688. [DOI] [PubMed] [Google Scholar]
- Mans J, de Villiers JC, du Plessis NM, Avenant T, Taylor MB. 2010. Emerging norovirus GII.4 2008 variant detected in hospitalised paediatric patients in South Africa. J Clin Virol 49:258–264. [DOI] [PubMed] [Google Scholar]
- Mattison K, Sebunya TK, Shukla A, Noliwe LN, Bidawid S. 2010. Molecular detection and characterization of noroviruses from children in Botswana. J Med Virol 82:321–324. [DOI] [PubMed] [Google Scholar]
- Monica B, Ramani S, Banerjee I, Primrose B, Iturriza-Gomara M, Gallimore CI, Brown DW, M F, Moses PD, Gray JJ, Kang G. 2007. Human caliciviruses in symptomatic and asymptomatic infections in children in Vellore, South India. J Med Virol 79:544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounts AW, Ando T, Koopmans M, Bresee JS, Noel J, Glass RI. 2000. Cold weather seasonality of gastroenteritis associated with Norwalk-like viruses. J Infect Dis 181:S284–S287. [DOI] [PubMed] [Google Scholar]
- Moyo SJ, Gro N, Matee MI, Kitundu J, Myrmel H, Mylvaganam H, Maselle SY, Langeland N. 2011. Age specific aetiological agents of diarrhoea in hospitalized children aged less than five years in Dar es Salaam, Tanzania. BMC Pediatr 11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DY, Gaedicke G, Schreier E. 2003. Viral agents of acute gastroenteritis in German children: Prevalence and molecular diversity. J Med Virol 71:82–93. [DOI] [PubMed] [Google Scholar]
- Oluwatoyin Japhet M, Adeyemi Adesina O, Famurewa O, Svensson L, Nordgren J. 2012. Molecular epidemiology of rotavirus and norovirus in Ile-Ife, Nigeria: High prevalence of G12P[8] rotavirus strains and detection of a rare norovirus genotype. J Med Virol 84:1489–1496. [DOI] [PubMed] [Google Scholar]
- Onishi N, Hosoya M, Matsumoto A, Imamura T, Katayose M, Kawasaki Y, Hashimoto O, Hayashi A, Ishiko H, Suzuki H. 2008. Molecular epidemiology of norovirus gastroenteritis in Soma, Japan, 2001–2003. Pediatr Int 50:65–69. [DOI] [PubMed] [Google Scholar]
- O’Ryan ML, Mamani N, Gaggero A, Avendano LF, Prieto S, Pena A, Jiang X, Matson DO. 2000. Human caliciviruses are a significant pathogen of acute sporadic diarrhea in children of Santiago, Chile. J Infect Dis 182:1519–1522. [DOI] [PubMed] [Google Scholar]
- Pang XL, Joensuu J, Vesikari T. 1999. Human calicivirus-associated sporadic gastroenteritis in Finnish children less than two years of age followed prospectively during a rotavirus vaccine trial. Pediatr Infect Dis J 18:420–426. [DOI] [PubMed] [Google Scholar]
- Parashar UD, Li JF, Cama R, DeZalia M, Monroe SS, Taylor DN, Figueroa D, Gilman RH, Glass RI. 2004. Human caliciviruses as a cause of severe gastroenteritis in Peruvian children. J Infect Dis 190:1088–1092. [DOI] [PubMed] [Google Scholar]
- Parra GI, Bok K, Taylor R, Haynes JR, Sosnovtsev SV, Richardson C, Green KY. 2012. Immunogenicity and specificity of norovirus Consensus GII.4 virus-like particles in monovalent and bivalent vaccine formulations. Vaccine 30:3580–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MM, Widdowson MA, Glass RI, Akazawa K, Vinje J, Parashar UD. 2008. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis 14:1224–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan TG, Nguyen TA, Kuroiwa T, Kaneshi K, Ueda Y, Nakaya S, Nishimura S, Nishimura T, Yamamoto A, Okitsu S, Ushijima H. 2005. Viral diarrhea in Japanese children: Results from a one-year epidemiologic study. Clin Lab 51:183–191. [PubMed] [Google Scholar]
- Phan TG, Takanashi S, Kaneshi K, Ueda Y, Nakaya S, Nishimura S, Sugita K, Nishimura T, Yamamoto A, Yagyu F, Okitsu S, Maneekarn N, Ushijima H. 2006. Detection and genetic characterization of norovirus strains circulating among infants and children with acute gastroenteritis in Japan during 2004–2005. Clin Lab 52:519–525. [PubMed] [Google Scholar]
- Puustinen L, Blazevic V, Salminen M, Hamalainen M, Rasanen S, Vesikari T. 2011. Noroviruses as a major cause of acute gastroenteritis in children in Finland, 2009–2010. Scand J Infect Dis 43:804–808. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. 1987. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. [DOI] [PubMed] [Google Scholar]
- Sdiri-Loulizi K, Ambert-Balay K, Gharbi-Khelifi H, Sakly N, Hassine M, Chouchane S, Guediche MN, Pothier P, Aouni M. 2009. Molecular epidemiology of norovirus gastroenteritis investigated using samples collected from children in Tunisia during a four-year period: Detection of the norovirus variant GGII.4 Hunter as early as January2003. J Clin Microbiol 47:421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenga JJ, Vennema H, Renckens B, de Bruin E, van der Veer B, Siezen RJ, Koopmans M. 2007. Epochal evolution of GGII.4 norovirus capsid proteins from 1995 to 2006. J Virol 81:9932–9941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenga JJ, Vennema H, Zheng DP, Vinje J, Lee BE, Pang XL, Ho EC, Lim W, Choudekar A, Broor S, Halperin T, Rasool NB, Hewitt J, Greening GE, Jin M, Duan ZJ, Lucero Y, O’Ryan M, Hoehne M, Schreier E, Ratcliff RM, White PA, Iritani N, Reuter G, Koopmans M. 2009. Norovirus illness is a global problem: Emergence and spread of norovirus GII.4 variants, 2001–2007. J Infect Dis 200:802–812. [DOI] [PubMed] [Google Scholar]
- Tam CC, Rodrigues LC, Viviani L, Dodds JP, Evans MR, Hunter PR, Gray JJ, Letley LH, Rait G, Tompkins DS, O’Brien SJ. 2012. Longitudinal study of infectious intestinal disease in the UK (IID2 study): Incidence in the community and presenting to general practice. Gut 61:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd S, Page NA, Duncan Steele A, Peenze I, Cunliffe NA. 2010. Rotavirus strain types circulating in Africa: Review of studies published during 1997–2006. J Infect Dis 202:S34–S42. [DOI] [PubMed] [Google Scholar]
- Victoria M, Carvalho-Costa FA, Heinemann MB, Leite JP, Miagostovich M. 2007. Prevalence and molecular epidemiology of noroviruses in hospitalized children with acute gastroenteritis in Rio de Janeiro, Brazil, 2004. Pediatr Infect Dis J 26: 602–606. [DOI] [PubMed] [Google Scholar]
- Waggie Z, Hawkridge A, Hussey GD. 2010. Review of rotavirus studies in Africa: 1976–2006. J Infect Dis 202:S23–S33. [DOI] [PubMed] [Google Scholar]
- Widdowson MA, Cramer EH, Hadley L, Bresee JS, Beard RS, Bulens SN, Charles M, Chege W, Isakbaeva E, Wright JG, Mintz E, Forney D, Massey J, Glass RI, Monroe SS. 2004. Outbreaks of acute gastroenteritis on cruise ships and on land: Identification of a predominant circulating strain of norovirus-United States, 2002. J Infect Dis 190:27–36. [DOI] [PubMed] [Google Scholar]
- Zheng DP, Ando T, Fankhauser RL, Beard RS, Glass RI, Monroe SS. 2006. Norovirus classification and proposed strain nomenclature. Virology 346:312–323. [DOI] [PubMed] [Google Scholar]


