Sickle cell disease (SCD), an inherited red blood cell disorder caused by homozygous or compound heterozygous inheritance of mutations in the b-globin gene, affects 1 in 365 African Americans.1 SCD is considered an immunodeficient state2 and infectious complications are a major contributor to the morbidity and mortality.3 Newborn screening, penicillin prophylaxis, and pneumococcal vaccination have led to reductions in sepsis-related mortality and > 95% of children with SCD living in highresource settings now survive into adulthood.4 Despite these measures, high rates of hospitalizations in SCD patients are characterized by fevers5 and 14–18% of deaths are attributed to infectious causes in contemporary SCD cohorts.3
Antimicrobial-resistant infections are a global public health crisis associated with high rates of morbidity, health-related costs, and death in the general population.6 Penicillin prophylaxis may be leading to changes in antimicrobial resistance patterns in SCD. In a cohort of SCD children, 71% of whom were receiving penicillin prophylaxis, nasopharyngeal carriage of penicillin-resistant Streptococcus pneumoniae was observed in 55% of isolates. 7 Antimicrobial resistance patterns in SCD adults are less clear and their impact on survival are not well understood.
We conducted a longitudinal study to i) identify risk factors for multidrug resistant (MDR) infections in adults with SCD, ii) compare antimicrobial resistance patterns to 16,000 propensity score-matched African Americans, and iii) determine the association of MDR infections on survival in SCD adults enrolled in a prospective registry at the University of Illinois at Chicago (UIC).
We analyzed 320 SCD patients receiving medical care at UIC Hospital between January 1, 2017 and April 14, 2020. The protocol was approved by the Institutional Review Board and all subjects provided written informed consent.
Baseline demographic, clinical, and laboratory variables were obtained at each patient’s first outpatient visit during the study period from the Cerner Medical Systems. Central venous catheterizations and diabetes diagnosis were queried using pertinent 9th and 10th Procedure Coding system editions of the International Classification of Disease (ICD-9-PCS and ICD-10-PCS) during the study period (Online Supplementary Table S1). Pneumococcal conjugate vaccine (PCV13) and pneumococcal polysaccharide vaccine (PPSV23) administration were queried during the 5 years prior to and including the study period. A blood culture contaminant was defined as a blood culture isolating coagulase-negative Staphylococcus, Bacillus species, Corynebacterium species, Propionibacterium species, or Streptococcus viridans group in less than 50% of simultaneously ordered cultures or in only one positive culture result.8 Organisms were considered to be multidrug resistant if the isolate organism was non-susceptible to three or more categories of antibiotics according to the European Center for Disease Prevention and Control (ECDC) and the Centers for Disease Control and Prevention (CDC) guidelines.9
Between January 1, 2017 and April 14, 2020, 34,612 adults with culture data and race classified as “black” or “African American” were identified through the UIC Cerner Medical Systems. Those with an ICD-9/10 code of SCD were excluded from this cohort. The African American cohort of 16,000 patients used in our analyses were chosen by propensity score, matched to the SCD cohort at a 50:1 ratio for age, sex, and follow-up time using the Matchit R analytic software package by the nearest-neighbor matching algorithm.10
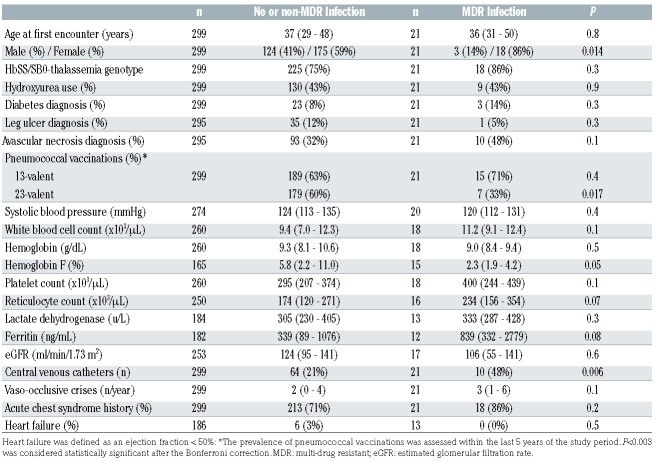
Table 1.
Clinical and laboratory values stratified by infection status in patients with sickle cell disease.
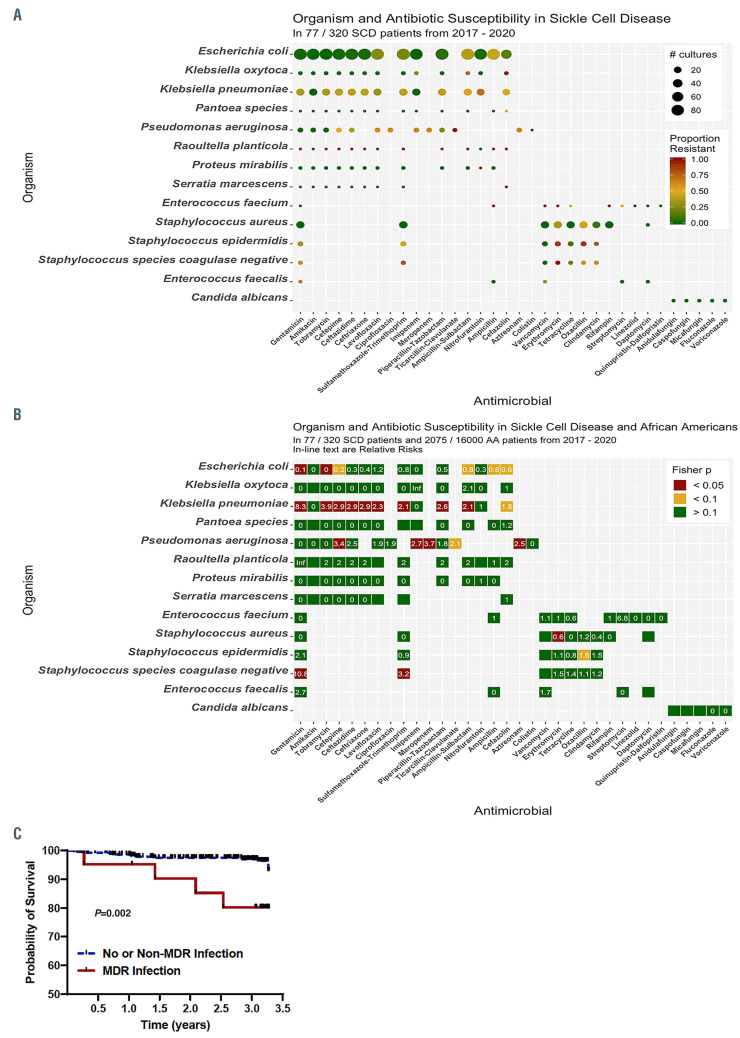
Figure 1.
Antibiotic resistance patterns and its effect on survival patterns in patients with sickle cell disease. (A) Antibiotic resistance patterns in patients with sickle cell disease (SCD); (B) a comparison of antibiotic resistance patterns in patients with SCD versus African Americans without SCD (relative risks are provided in each box); (C) survival patterns by infection status in patients with SCD.
Baseline characteristics at the time of study initiation were compared by MDR status using the Kruskal-Wallis and Chi-square or Fisher’s exact test for linear and categorical variables, respectively. Median and interquartile range (IQR) are provided. A final multivariate logistic regression model was fit by stepwise variable selection of variables with P<0.1 in the univariate analysis, adjusting for the following covariates: age, sex, sickle cell genotype, and hydroxyurea use. Fisher’s exact test was used to compare MDR status between SCD and AA patients for each specific antimicrobial-organism pair. Relative risk (RR) was calculated as the ratio of the proportional resistance between the SCD and AA groups. Survival was analyzed by MDR status using Kaplan-Meier curves and Cox Proportional Hazards models, adjusting for the following covariates: age, sex, SCD genotype, and hydroxyurea use. For patients lost to follow-up, MDR status and survival were censored at the date of last known contact. The survival time was defined as the period between January 1, 2017 and either the date of patient’s death or last known encounter up to April 14, 2020.
Baseline characteristics of the SCD and AA cohort were similar for age, sex, and follow-up time (Online Supplementary Table S2). Between January 1, 2017 and April 14, 2020, we observed 176 non-recurrent positive cultures, defined as not occurring within 30 days of a previous culture with an identical organism and source,11 in 77 of 320 (24.1%) SCD patients (Online Supplementary Table S3). The total infection rate in SCD patients was 205.0 infections per 1,000-person-years. We observed 3,968 non-recurrent positive cultures in 2,075 of 16,000 (13.0%) AA patients, for a total infection rate of 92.8 infections per 1,000-person-years (P<0.001).
An MDR infection was observed in 27.3% (21 of 77) of SCD and 33.3% (692 of 2,075) of AA patients (P=0.3) with an infection. Baseline differences in the SCD patients by MDR status are provided in Table 1. There were no SCD adults on penicillin prophylaxis during the study period. Female sex (OR 5.9, 95% Confidence Interval [CI]: 1.6–21.7; P=0.007) and central venous catheter placement (OR 4.3, 95%CI: 1.6–11.5; P=0.004) were independently associated with an increased MDR infection risk and PPSV23 vaccination was associated with a reduced risk (OR 0.2, 95%CI: 0.1–0.6; P=0.003), adjusting for age, SCD genotype and hydroxyurea use.
Antimicrobial resistance patterns for antimicrobialorganism pairs in SCD patients are provided in Figure 1A and Online Supplementary Table S4. Antimicrobial resistance was observed in 11.4% (135 of 1,050) of unique Escherichia coli infections, including 26.1% (23 of 88 tested) resistant to levofloxacin, 22.7% (20 of 88 tested) to sulfamethoxazole-trimethoprim, and 2.4% (2 of 82 tested) to nitrofurantoin. Klebsiella pneumoniae and Pseudomonas aeruginosa were highly resistant to several antibiotic groups, with the exception of amikacin and imipenem for Klebsiella pneumoniae and amikacin for Pseudomonas aeruginosa. Oxacillin-resistant Staphylococcus aureus was observed in 53.8% of infections. Both coagulase- negative Staphylococcus epidermidis and Staphylococcus species were highly resistant to most antibiotic groups except for vancomycin. We observed only one Staphylococcus pneumoniae infection, which was resistant to penicillin.
A comparison of resistance patterns between SCD and AA patients are provided in Figure 1B. Escherichia coli infections were less commonly resistant, while Klebsiella pneumoniae and Pseudomonas aeruginosa infections were typically more resistant to antibiotics in SCD versus AA patients.
During the 3-year study period, 14 of 320 (4.4%) SCD patients died; ten of 299 (3.3%) without and four of 21 (19.0%) with an. MDR infection (Figure 1C). Sepsis was a contributing factor to the cause of mortality in eight of ten deaths with a known etiology. Developing an MDR infection was an independent risk factor for death (Hazard Ratio [HR] 4.9, 95%CI: 1.5–16.4; P=0.009), adjusting for age, sex, SCD genotype and hydroxyurea use.
In the era of penicillin prophylaxis, we observed higher rates of infection but a similar prevalence of MDR infections in SCD adults compared to AA. This suggests that penicillin prophylaxis may be altering colonization and infection patterns but not drug resistance patterns for gram-positive infections in SCD adults. Vaccination with PPSV23 reduces exposure to antimicrobial drugs and is associated with less resistance to erythromycin, trimethoprim-sulfamethoxazole, and cephalosporins in the general population.12We observed high rates of resistance to trimethoprim-sulfamethoxazole and erythromycin in gram-positive bacterial infections. Furthermore, vaccination with PPSV23 was associated with a 5-fold lower risk of having an MDR infection. In the general population, approximately 20–67% of central-line associated infections are MDR infections.13 In our cohort, central venous catheter line placement, which is often required for pain management or for exchange transfusion therapy in SCD, was associated with a 4-fold greater risk of developing an MDR infection. Alternative strategies for administering pain medications, such as inhaled routes, and better implementation of protective measures, such as the use of chlorhexidine for skin preparation, avoiding femoral vein catheters, and the use of antiseptic barrier caps,14 may help reduce MDR infection rates in SCD. The association of female sex with a higher MDR infection rate may be due to the increased risk for urinary tract infections in females. Other potential biological differences, such as the effects of sex hormones and X-chromosome genes on immune response regulation, may also be contributing to the observed differences. 15
Limitations of our study include being a single-center study and not taking into account the specific antimicrobial therapies used to treat the infections. Investigating the effects of socioeconomic status and health behavior may highlight modifiable risk factors to improve vaccination rates in SCD. Health care utilization is a likely contributor to developing antibiotic resistance, and therapies that reduce hospitalizations may be another approach to reduce MDR infections. Future strategies to reduce the spread of antibiotic resistance, such as greater implementation of PPSV23 and strategies to reduce central venous catheter placement, may help decrease the morbidity and early mortality observed in SCD.
Supplementary Material
Funding Statement
Funding: the project described was supported by the National Institutes of Health through grants R03 HL-146788, and R01 HL- 153161 (SLS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med. 2010;38(4 Suppl):S512-521. [DOI] [PubMed] [Google Scholar]
- 2.Battersby AJ, Knox-Macaulay HH, Carrol ED. Susceptibility to invasive bacterial infections in children with sickle cell disease. Pediatr Blood Cancer. 2010;55(3):401-406. [DOI] [PubMed] [Google Scholar]
- 3.Darbari DS, Wang Z, Kwak M, et al. Severe painful vaso-occlusive crises and mortality in a contemporary adult sickle cell anemia cohort study. PLoS One. 2013;8(11):e79923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood. 2010; 115(17):3447-3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogovik AL, Friedman JN, Persaud J, Goldman RD. Bacterial blood cultures in children with sickle cell disease. Am J Emerg Med. 2010; 28(4):511-514. [DOI] [PubMed] [Google Scholar]
- 6.CDC. Antibiotic resistance threats in the United States. www.cdcgov/DrugResistance/Biggest-Threatshtml. 2019. [Google Scholar]
- 7.Daw NC, Wilimas JA, Wang WC, et al. Nasopharyngeal carriage of penicillin-resistant Streptococcus pneumoniae in children with sickle cell disease. Pediatrics. 1997;99(4):e7. [DOI] [PubMed] [Google Scholar]
- 8.Chulamokha L, Scholand SJ, Riggio JM, Ballas SK, Horn D, DeSimone JA. Bloodstream infections in hospitalized adults with sickle cell disease: a retrospective analysis. Am J Hematol. 2006; 81(10):723-728. [DOI] [PubMed] [Google Scholar]
- 9.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268-281. [DOI] [PubMed] [Google Scholar]
- 10.Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42(8):1-28. [Google Scholar]
- 11.Woudt SHS, de Greeff SC, Schoffelen AF, Vlek ALM, Bonten MJM. Infectious Diseases Surveillance Information System-Antimicrobial Resistance Study Group. Antibiotic resistance and the risk of recurrent bacteremia. Clin Infect Dis. 2018;66(11):1651-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). 1997. Apr 4;46(RR-8):1-24. [PubMed] [Google Scholar]
- 13.Burnham JP, Rojek RP, Kollef MH. Catheter removal and outcomes of multidrug-resistant central-line-associated bloodstream infection. Medicine. 2018;97(42):e12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaftari AM, Hachem R, Jiang Y, et al. Changing epidemiology of catheter-related bloodstream infections in cancer patients. Infect Control Hosp Epidemiol. 2018;39(6):727-729. [DOI] [PubMed] [Google Scholar]
- 15.van Lunzen J, Altfeld M. Sex differences in infectious diseases-common but neglected. J Infect Dis. 2014;209(Suppl 3):S79-80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.