Abstract
Hematopoietic stem and progenitor cells (HSPC) are crucial in the maintenance of lifelong production of all blood cells. These stem cells are highly regulated to maintain homeostasis through a delicate balance between quiescence, self-renewal and differentiation. However, this balance is altered during the recovery after HSPC transplantation. Transplantation efficacy can be limited by inadequate hematopoietic stem cell number, poor homing, low level of engraftment, or limited self-renewal. As recent evidence indicates that estrogens are involved in regulating hematopoiesis, we sought to examine whether natural estrogens (estrone or E1, estradiol or E2, estriol or E3 and estetrol or E4) modulate human HSPC. Our results show that human HSPC subsets express estrogen receptors, and that signaling is activated by E2 and E4 on these cells. Additionally, these natural estrogens cause different effects on human progenitors in vitro. We found that both E2 and E4 expand human HSPC. However, E4 was the best tolerated estrogen and promoted cell cycling of human hematopoietic progenitors. Furthermore, we found that E2 and, more significantly, E4 doubled human hematopoietic engraftment in immunodeficient mice without altering other HSPC properties. Finally, the impact of E4 on promoting human hematopoietic engraftment in immunodeficient mice might be mediated through the regulation of mesenchymal stromal cells in the bone marrow niche. Collectively, our data demonstrate that E4 is well tolerated and enhances human reconstitution in immunodeficient mice directly, by modulating human hematopoietic progenitor properties, and indirectly, by interacting with the bone marrow niche. This might have particular relevance for improving hematopoietic recovery after myeloablative conditioning, especially when limited numbers of HSPC are available.
Introduction
Hematopoietic stem cells (HSC) are a rare cell population resident in the bone marrow (BM) of adult mammals and are at the top of a hierarchy of progenitors that become progressively restricted to several or a single blood lineage. HSC are capable of self-renewal and multipotent differentiation to all blood cell lineages,1 and are crucial for the maintenance of lifelong production of all blood cells. They are homeostatically regulated through a delicate balance between quiescence, self-renewal and differentiation. Although HSC divide infrequently, they are activated to proliferate in response to BM injury to re-establish homeostasis.2 Transplantation of hematopoietic stem and progenitor cells (HSPC) is routinely used to reconstitute hematopoiesis after myeloablative regimens to treat leukemia or hematopoietic genetic diseases. However, the efficacy of HSPC transplantation can be limited by inadequate cell numbers, poor homing, low engraftment, or differentiation stress of the HSPC. Different approaches have been attempted to solve these problems, such as using different sources of HSPC,3-5 ex vivo expansion of HSPC6-10 or stimulating the HSPC by accessory molecules11,12 or cells.13 However, these approaches require a profound understanding of HSPC regulation and how the properties of the cells can be boosted to maximize their efficacy at reconstituting a patient’s blood system after HSPC transplantation.1,14
Estrogen is the primary female sex hormone and, apart from its known role in the reproductive system, it is responsible for controlling many cellular and molecular processes, including growth and differentiation. Estrogens act through genomic or nuclear signaling and non-genomic or membrane- initiated steroid signaling (MISS), modulating intracellular second messengers.15 The four major naturallyoccurring estrogens in women are estrone (E1), estradiol (E2), estriol (E3) and estetrol (E4). E1 is the predominant estrogen in postmenopausal women. E2 is considered the active estrogen during the estrous cycle. E3 and E4 are synthesized during pregnancy by the placenta and fetal liver, respectively, but their physiological roles are essentially unknown.16
Recent evidence indicates that E2 is involved in regulating the proliferation and lineage commitment of HSC,17 although the studies are few and their results are sometimes contradictory. E2 treatment was able to specifically increase the number of vascular HSC, but the long-term repopulating capacity of the HSC was limited.18 Additionally, this E2 was shown to promote the cell cycle of HSC and multipotent progenitors (MPP) and increase erythroid differentiation in females, also during pregnancy.19 Furthermore, E2 favors hematopoietic regeneration through the activation of telomerase activity20-22 and the stimulation of the unfolded protein response on mouse HSC, which sustains protein homeostasis to favor hematopoietic regeneration.23 In contrast, tamoxifen, whose active metabolite (4-hydroxytamoxifen) acts as an estrogen receptor antagonist, reduces the number of MPP and short-term HSC but activates the proliferation of long-term HSC.24 In addition, E2 might modulate HSC indirectly through activating BM mesenchymal stromal cells (MSC). E2 treatment has been described to activate MSC osteogenic differentiation and also promotes the secretion of granulocyte-macrophage colonystimulating factor and interleukin 6, which increased the number of HSC by modulating their niche.25 Therefore, estrogen-mediated regulation of HSPC can also indirectly change the HSC BM niche. For that reason, a full understanding of the role of estrogens in HSC regulation is essential in order to be able to further develop the clinical potential of these hormones.
Here, we have examined the impact of natural estrogens on human HSPC. Ex vivo, E2 and E4 treatment expanded human HSPC and, more importantly, the administration of E4 to immunodeficient mice previously transplanted with human HSPC enhanced the level of engraftment of human hematopoietic cells.
Methods
Human cord blood-CD34+ cell samples and bone marrow mesenchymal stromal cells
Umbilical cord blood samples (CB) from healthy donors were provided by the Centro de Transfusión de la Comunidad de Madrid. All samples were collected with written consent and agreement from the Centro de Transfusión de la Comunidad de Madrid‘s institutional review board (number PKDEFIN [SAF2017-84248-P]). Mononuclear cells were obtained by fractionation in Ficoll-hypaque according to the manufacturer’s recommendations (GE Healthcare). Purified CB-CD34+ cells were obtained using a MACS CD34 Micro-Bead kit (Miltenyi Biotec). Cells were frozen in 10% dimethyl sulfoxide solution and stored in liquid nitrogen until their use.
Mononuclear cells from human BM were obtained by Ficoll- Paque Plus density gradient separation from heparinized BM samples obtained from healthy donors after informed consent. All the procedures were in accordance with the Helsinki Declaration of 1975, and its revision in 2000. Samples were cultured at 1.6×105 cells/cm2 in MesenCult medium plus supplements for human cells (Stemcell Technologies). After 24 h, nonadherent cells were discarded. Fresh medium was added and replaced twice a week. At 80% confluence, adherent cells were trypsinized, washed, and seeded at 4×103 cells/cm2. In all the experiments, BM-MSC were used at passages 5 to 8.
Hematopoietic cell transplant protocol in immunodeficient mice
All the mice were kept under standard pathogen-free conditions in the animal facility of CIEMAT. All animal experiments were performed in compliance with European and Spanish legislation and institutional guidelines. The protocol was approved by Consejeria de Medio Ambiente y Ordenación del Territorio (protocol number PROEX 078/15).
CB-CD34+ cells were administered through the tail vein of female or male NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice sublethally irradiated the day before the transplant with 1.5 Gy. Three days later, the animals were treated with vehicle (olive oil) or daily doses of either E2 or E4 (2 g of estrogen per day) intraperitoneally for 4 days. Four months after transplantation, the mice were sacrificed and BM was collected from the long bones of these animals. Additionally, when analysis of the hematopoietic niche was involved, the long bones were flushed, cut into small pieces and crushed before being digested with 200 U/mL collagenase IV/2 g/mL DNaseI in Hanks balanced salt solution at 37°C for 45 min. Human engraftment was analyzed by flow cytometry (LSR Fortessa; BD). The cells were stained with hCD45-APCCy7 and hCD3-APC (BioLegend), hCD45- FITC, hCD33-PE, hCD19-FITC and hCD235a-FITC (Beckman Coulter), hCD34-Pecy5 (Immunotech), hCD38-PE, hCD90- APC, mCD45.1-PE, mCD45.1-Biotin and Ter119-Biotin (BD), mCD140a-APC (Pdgfra-APC, eBiosience) and mCD144-PE (VECadherin- PE, eBiosience). DAPI-positive cells were excluded from the analysis. FlowJo software was used for the analyses.
Additionally, the hCD45+ population from primary mice was sorted in an Influx Cell Sorter (BD) and 1x106 hCD45+ cells were transplanted into sublethally irradiated female secondary NSG recipients. Four months later, the animals were sacrificed and analyzed as previously described.
Results
Engraftment of human cord blood CD34+ cells is favored in female immunodeficient mice
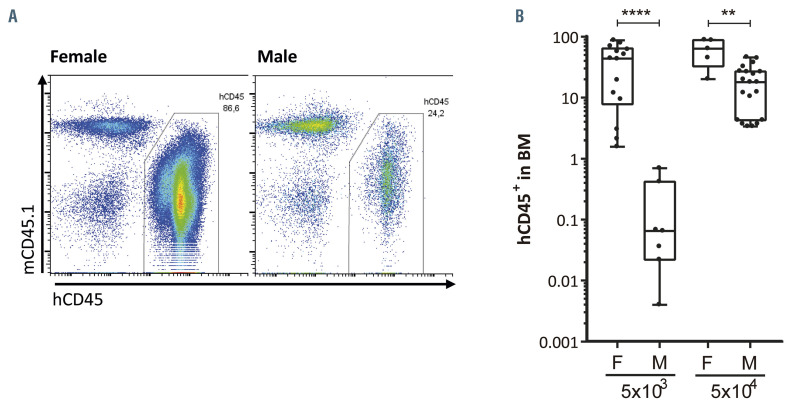
It has been previously described that the engraftment of highly purified human HSC (Lin-CD34+CD38- CD90+CD45RA-) is improved when these cells are transplanted into immunodeficient female recipients, as compared to male recipients.26 To investigate whether this enhanced engrafting potential in female recipients was also present in CB-CD34+ cells, we transplanted different amounts of HSPC into sublethally irradiated animals. As occurred when highly purified HSC were transplanted, we observed higher engraftment of human HSPC in female NSG animals than in their male counterparts (Figure 1A). Four months after the transplantation of 5x104 CB-CD34+ cells, human engraftment in mouse BM was 61.06±26.07% (mean ± standard deviation) in female mice and 18.94±13.93% in male mice. Interestingly, this impairment in engrafting potential in males was even greater when only 5x103 CB-CD34+ cells were transplanted (38.74±30.42% BM cells were of human origin in female animals versus only 0.19±0.27% in male animals) (Figure 1B). Therefore, engraftment of human cells was from 3.2- to >200-fold greater in female recipients than in male recipients when 5x104 or 5x103 CB-CD34+ cells, respectively, were transplanted. Additionally, there were no differences in the percentages of myeloid, B, T cells or HSPC (hCD34+, hCD34+hCD38- and hCD34+hCD38-hCD90+ cells) between the human engrafted cells (Online Supplementary Figure S1). These data highlight the importance of the gender of the NSG mouse recipients to facilitate the engraftment of human HSPC.
Human hematopoietic stem and progenitor cell subsets expressed both ESR1 and ESR2
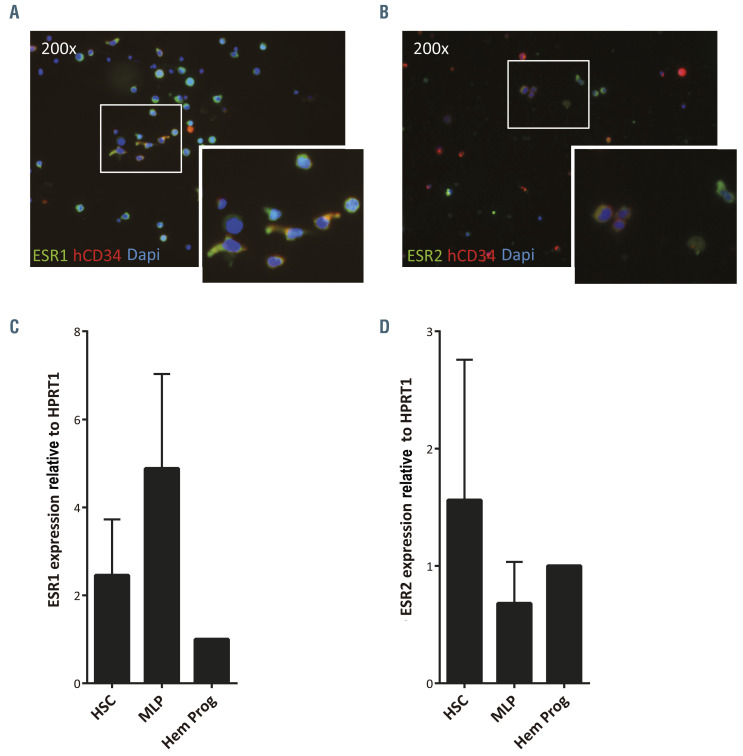
To understand the potential role of sex hormones in the observed differences of human hematopoietic engraftment between male and female recipient mice, we analyzed the expression of the two main estrogen receptors, ESR1 and ESR2, in CB-CD34+ cells. As shown by immunostaining analysis (Figure 2A and B), most CD34+ cells were positive for ESR1, while ESR2 staining was dimmer in CD34+ cells (Figure 2A and B; Online Supplementary Figure S2A). Additionally, to investigate the differential expression of these receptors in the hematopoietic progenitors, different populations of HSPC, such as HSC/MPP (CD34+CD38-CD45RA-), multilymphoid progenitors (MLP, CD34+CD38-CD45RA+) and committed hematopoietic progenitors (CD34+CD38+), were sorted out (Online Supplementary Figure S2B) and the expression of both estrogen receptors was determined by quantitative reverse transcriptase polymerase chain reaction (qRTPCR). Both ESR1 and ESR2 were expressed in HSC, MLP and in more committed hematopoietic progenitors (Figure 2C and D; Online Supplementary Figure S2C). ESR1 expression tended to be upregulated between HSC/MPP and MLP compartments to decrease again in the most committed hematopoietic progenitors (Figure 2C). In contrast, ESR2 expression seemed to follow an opposite pattern with high values in both HSC/MPP and committed hematopoietic progenitors but reduced levels in the MLP cell population (Figure 2D). In both cases, although some tendencies were observed, no statistically significant differences were documented. Like ESR1 and ESR2, the newly identified estrogen receptor, GPER1, was also detected by RT-PCR in CB-CD34+ cells from different donors (Online Supplementary Figure S2D). Consequently, human HSPC might respond to natural estrogens through any of the estrogen receptors.
Natural estrogens modified human hematopoietic stem and progenitor cells in vitro
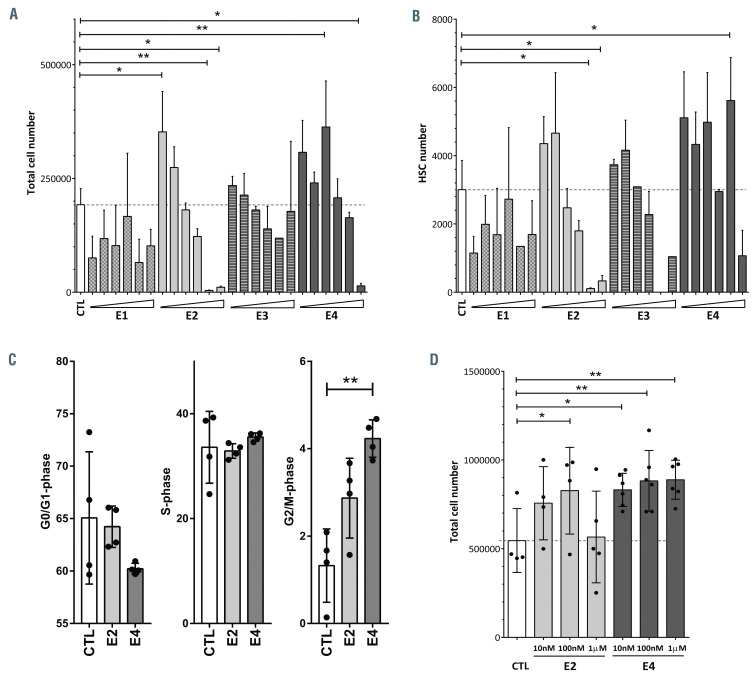
Once we had demonstrated that both estrogen receptors were expressed in HSPC, we wanted to investigate a potential direct effect of estrogens on human HSPC. We cultured CB-CD34+ cells for 4 days with a range of concentrations, from 10 nM to 500 M, of the four natural estrogens (E1, E2, E3 and E4). As shown in Figure 3A, E1 and E3 reduced the expansion of the cells in culture practically at any of the concentrations used. On the other hand, the lowest concentrations of E2 and E4 promoted the expansion of these cells, but at high doses they impaired cell growth. A similar behavior was detected when different subpopulations of hCD34+ cells were analyzed (Figure 3B; Online Supplementary Figure S3A-E). E1 prevented the expansion of hCD34+hCD38- cells (Online Supplementary Figure S3C), MLP (Online Supplementary Figure S3D), MPP (hCD34+hCD38-hCD90-hCD45RA-) (Online Supplementary Figure S3E) and most primitive HSC (hCD34+hCD38-hCD90+hCD45RA-) (Figure 3B). The data for the rest of the tested estrogens showed an apparent amplification of these primitive populations when low concentrations of the hormones were used, but at the highest concentrations, they were toxic (Figure 3B; Online Supplementary Figure S3C-E). It is important to highlight that the best tolerated estrogen was E4. Concentrations up to 10 M of E4 seemed not to be detrimental to any of these HSPC subsets, including primitive HSC. In contrast, E2 induced apoptosis of HSPC at high doses (Online Supplementary Figure S3F and G), as previously described for this estrogen and tamoxifen.24,27 However, only human HSPC cultured in the presence of the highest concentration of E4 showed some induction of apoptosis. Furthermore, we analyzed the cell cycle of CB-CD34+ cells after 4 days of culture in the presence of 100 nM E2 or E4. Estrogens, particularly E4, induced an increment of cells in G2/M phase (Figure 3C; Online Supplementary Figure S3H), which might explain the tendency of these two estrogens to expand human hematopoietic progenitors.
Figure 1.
Human hematopoietic stem and progenitor cells show superior hematopoietic engraftment in female NSG mice than in male ones. (A) Representative flow cytometry analyses of human engraftment of 5x104 umbilical cord blood CD34+ (CB-CD34+) cells into sublethally irradiated female (left panel) and male (right panel) NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice 4 months after transplantation. (B) Percentage of human hematopoietic cells, hCD45+, in the bone marrow of female (F) or male (M) animals transplanted with 5x103 or 5x104 CB-CD34+cells. Data were obtained from six independent biological replicates and are presented by dots and box-plots that represent the interquartile range (p75, upper edge; p25, lower edge; p50, midline; p95, line above the box; and p5, line below the box). Statistical significance was analyzed by the Mann-Whitney U test; **P<0.01 and ****P<0.001.
Figure 2.
Human hematopoietic stem and progenitor cells express estrogen receptors. (A) Representative immunofluorescent image of umbilical cord blood CD34+ (CB-CD34+) cells stained with anti-ESR1 (green), anti-hCD34 (red) and DAPI (blue). The insert, showing ESR1+ CD34+ cells (marked with arrows), is an enlargement of the white boxed area. (B) Representative immunofluorescent image of CB-CD34+ cells stained with anti-ESR2 (green), anti-hCD34 (red) and DAPI (blue). The insert, showing ESR2+ CD34+ cells (marked with arrows), is an enlargement of the white boxed area. (C) Qualitative real-time polymerase chain reaction (qRT-PCR) analysis of ESR1 expression of sorted hematopoietic stem cells/multipotent rogenitors (HSC/MPP: hCD34+hCD38-hCD45RA-), multilymphoid progenitors (MLP: hCD34+hCD38-hCD45RA+) and committed hematopoietic progenitors (Hem Prog: hCD34+hCD38+). (D) qRT-PCR analysis of ESR2 expression of sorted HSC/MPP, MLP and hematopoietic progenitors. Data were obtained from three biological replicates and are presented as the mean ± standard deviation. Statistical significance was analyzed by one-way analysis of variance with the Fisher least significant difference test: no significant differences were found.
Previously, E2 was described to have a positive role in enhancing both CB-CD34+ cell proliferation and in vitro hematopoietic progenitor potential after more than week of in vitro treatment.28 Hence, we cultured human HSPC in the presence of the lowest and best tolerated doses of E2 or E4 for 8 days. We detected a significant expansion of human progenitors with E4 at all the concentrations tested (Figure 3D). A similar effect was identified with 100 nM E2. The better tolerance of E4 over E2 was confirmed, since all the tested concentrations of E4 were non-toxic to CB-CD34+ cells (Figure 3D). The in vitro functionality of the estrogen-treated HSPC was assessed with colonyforming unit (CFU) assays. We did not observe any differences among the groups in CFU numbers or CFU types (Online Supplementary Figure S3I).
In order to assess which estrogen receptor was involved in the effect of these molecules in human HSPC, the cell cycle of CB-CD34+ was determined in the presence of these two estrogens together with either ESR1 antagonist (MPP), ESR2 antagonist (PHTPP) or GPER1 antagonist (G15). Treatment with E2 or E4 alone tended to increase the percentage of cells in S/G2/M-phase as previously described; however, the addition of ESR2 antagonist seemed to block the increase of cells in S/G2/M-phase induced by E4 (Online Supplementary Figure S3J). Less clearly, ESR1 and GPER1 antagonists seemed to reduce the number of cells in S/G2/M-phase in E2-treated HSPC. We also assessed the expression of ESR1 and ESR2 in human HSPC cultured with estrogens for 4 days by immunofluorescence analysis (Online Supplementary Figure S3K and L). While ESR1 fluorescence intensity increased slightly but significantly with 100 nM of E2 or E4 (Online Supplementary Figure S3K and M), ESR2 expression was significantly increased in the presence of both E2 and E4 (Online Supplementary Figure S3L and N). Moreover, estrogen treatment increased the percentage of human HSPC showing a polarized localization of ESR1 at the membrane (Online Supplementary Figure S3K and O). Furthermore, the treatment with estrogens enhanced the percentage of hCD34+ cells with cytoplasmic localization of ESR2 (Online Supplementary Figure S3L and P).
Figure 3.
Natural estrogens affect human hematopoietic stem and progenitor cells differently. (A) Total number of estrogen-treated hematopoietic stem and progenitor cells (HSPC) after 4 days in culture. Different concentrations (10 nM, 100 nM, 1 M, 10 M, 100 M and 500 M) of the natural estrogens (E1, E2, E3 and E4) were used. (B) Total number of hematopoietic stem cells (HSC: hCD34+hCD38-hCD90+hCD45RA-) after 4 days in culture. Different concentrations (10 nM, 100 nM, 1 M, 10 M, 100 M and 500 M) of the natural estrogens (E1, E2, E3 and E4) were used. (C) Cell cycle analysis of HSPC treated with 100 nM E2 or E4. G0/G1-phase (left panel), S-phase (middle panel) and G2/M-phase (right panel). (D) Total cell number of estrogen-treated HSPC after 8 days in culture. Data were obtained from three to five biological replicates and are presented as mean ± standard deviation. Statistical significance was analyzed by one-way analysis of variance with the Fisher least significant difference test: *P<0.05 and **P<0.01.
Collectively, these data indicate that natural estrogens regulate human HSPC through the signaling of estrogen receptors.
E2 and E4 increased the number of human hematopoietic stem and progenitor cells in an in vitro model of human hematopoietic niche
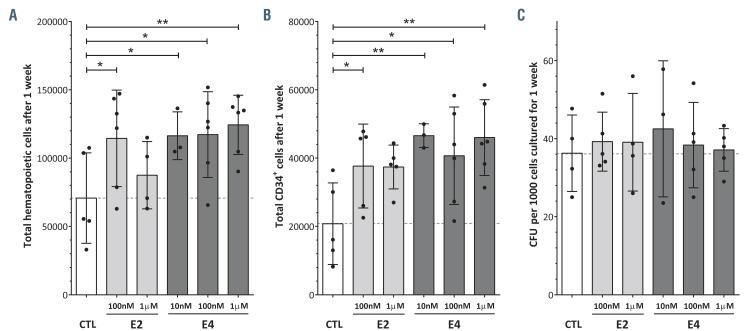
Subsequently, we investigated the indirect effect of E2 and E4 on HSPC in an in vitro model of the human hematopoietic niche. CB-CD34+ cells were co-cultured on an irradiated human BM-MSC layer in the presence of 100 nM or 1 M of E2 and E4 (Online Supplementary Figure S4A). We analyzed the expansion of the hematopoietic cells in two ways: (i) after 1 week of co-culture (Figure 4), and (ii) after 4 weeks of co-culture with the estrogen present only during the first week (Online Supplementary Figure S4C and D). From 10 nM to 1 M of E4 and the lowest concentration of E2 increased the hematopoietic cells in the culture in the first week of co-culture (Figure 4A). Likewise, the number of hCD34+ cells in the co-culture was significantly higher following treatment with E4 or 10 nM E2 than in the control group (Figure 4B; Online Supplementary Figure S4B). However, we could not detect significant differences in the functionality of the hCD34+ cells in CFU assays (Figure 4C). Furthermore, the effect of these two estrogens on the expansion of human hematopoietic cells or hCD34+ cells did not seem to be enhanced after 4 weeks in co-culture with an initial single dose (Online Supplementary Figure S4C and D). Consequently, the positive effect of E2 and E4 on HSPC also occurs in an in vitro model of the human hematopoietic niche.
Figure 4.
The impact of E2 and E4 on hematopoietic stem and progenitor cells in an in vitro model of the human hematopoietic niche. (A) Total hematopoietic cells after 1 week of co-culture with human bone marrow mesenchymal stromal cells (BM-MSC) in the presence of estrogens. (B) Total hCD34+ cells after 1 week of co-culture with human BM-MSC in the presence of estrogens. (C) Colony-forming units (CFU) derived from hematopoietic stem and progenitor cells after 1 week of co-culture with human BM-MSC in the presence of estrogens. Data were obtained from three to six biological replicates and are presented as the mean ± standard deviation. The statistical significance was analyzed by one-way analysis of variance with the Fisher least significant difference test: *P<0.05 and **P<0.01.
E2 and E4 boosted human hematopoietic engraftment in immunodeficient mice
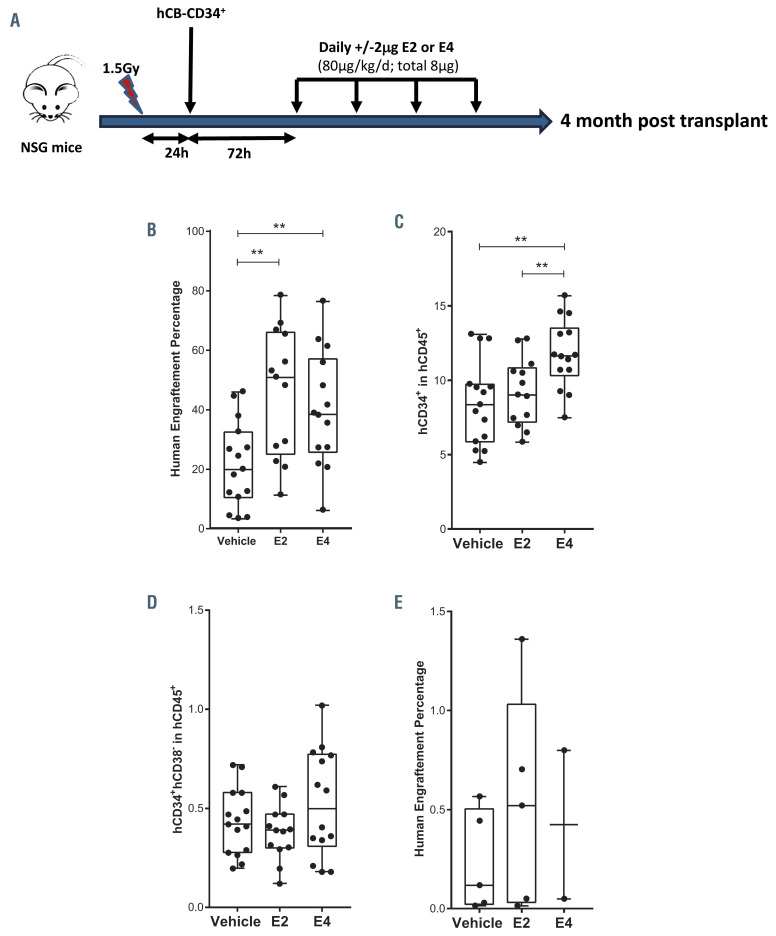
To better evaluate the impact of E2 and E4 on the properties of HSPC, we transplanted 5x104 human CB-CD34+ cells into sublethally irradiated male NSG mice, in order to avoid any additional effects of endogenous estrogens of female recipient mice, and 3 days later treated the animals with vehicle or daily low doses of either E2 or E4 (2 g of estrogen per day) for 4 days (Figure 5A). Human hematopoietic engraftment was evaluated in the mouse BM by FACS analysis 4 months after transplantation (Online Supplementary Figure S5A). Surprisingly, the human hematopoietic contribution was significantly higher in the estrogen-treated animals than in vehicle-treated ones (Figure 5B; Online Supplementary Figure S5A). None of the estrogens altered the normal distribution of human hematopoietic lineages within the hCD45+ population (Online Supplementary Figure S5B-D). More importantly, E4 administration significantly enhanced the hCD34+ cell population in male NSG mice (Figure 5C). No increase in the presence of the more primitive compartment, hCD34+hCD38-, was observed (Figure 5D). To explore the impact of the estrogen treatment on the long-term HSC, secondary transplants were performed. One million hCD45+ cells, purified from the BM of the primary recipients, were transplanted into sublethally irradiated female NSG mice. As shown in Figure 5E, the estrogen-treated human hematopoietic cells maintained their long-term engraftment potential without any observable problem in human hematopoietic reconstitution or any abnormal proliferation. This led us to conclude that these two estrogens, particularly E4, enhance in vivo human hematopoietic engraftment in male immunodeficient mice.
To study this finding in more depth, we transplanted limited numbers of human HSPC (5x103 CB-CD34+ cells/mouse), into male NSG mice, which were subsequently treated with vehicle, E2 or E4 as previously described. The percentage of mice positive for human engraftment, defined as animals in which hCD45+ cells constituted more than 0.1% of the cells in the mouse BM 4 months after transplantation, tended to increase after estrogen treatment (Online Supplementary Figure S5E). Moreover, the human hematopoietic chimerism of the positive animals seemed to be higher in the group treated with E4 than in the group given the vehicle (Online Supplementary Figure S5F). So, E2 and E4 might be able to improve the engraftment of human HSPC even when a very limited number of cells are transplanted.
To explore whether the enhancement of engraftment mediated by estrogens occurred in female recipients as well, we repeated the transplant of this very low number of CB-CD34+ cells into sublethally irradiated female NSG nice. As shown in Online Supplementary Figure S5G, human engraftment 4 months after transplantation tended to increase in the female animals treated with either of the two estrogens, although differences between groups were not statistically significant. The percentages of hematopoietic progenitors within the human population did not show larger differences between vehicle- and estrogentreated animals (Online Supplementary Figure S5H). Consequently, there is no clear effect of E2 or E4 on the engraftment of human HSPC in female animals.
Figure 5.
E2 and E4 enhance human engraftment in immunodeficient male mice. (A) Experimental scheme of transplantation of human hematopoietic stem and progenitor cells (HSPC) into immunodeficient mice. Sublethally irradiated NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice were transplanted with human umbilical cord blood CD34+ (CBCD34+) cells, and 3 days later the animals were treated with vehicle or with daily low doses of either E2 or E4 (2 g of estrogen per day) for 4 days. Four months after transplantation, human hematopoietic engraftment was evaluated in the mouse bone marrow. (B) Percentage of hCD45+ cells in the bone marrow (BM) of male mice transplanted with 5x104 hCB-CD34+ cells 4 months after transplantation. (C) Percentage of hCD34+ cells within the human population in the BM of male mice transplanted with 5x104 hCB-CD34+ cells 4 months after transplantation. (D) Percentage of hCD34+hCD38- within the human population in the BM of the male mice transplanted with 5x104 hCB-CD34+ cells. (E) Percentage of human engraftment (hCD45+) in the BM of secondary NSG mice transplanted with 1x106 sorted hCD45+ cells from the primary recipients and analyzed 4 months after transplantation. Data were obtained from four biological replicates and are presented by dots and box-plots that represent the interquartile range (p75, upper edge; p25, lower edge; p50, midline; p95, line above the box; and p5, line below the box). Statistical significance was analyzed by the Mann-Whitney U test: **P<0.01 and ****P<0.001.
E4 affects mesenchymal stromal cells within the mouse hematopoietic niche
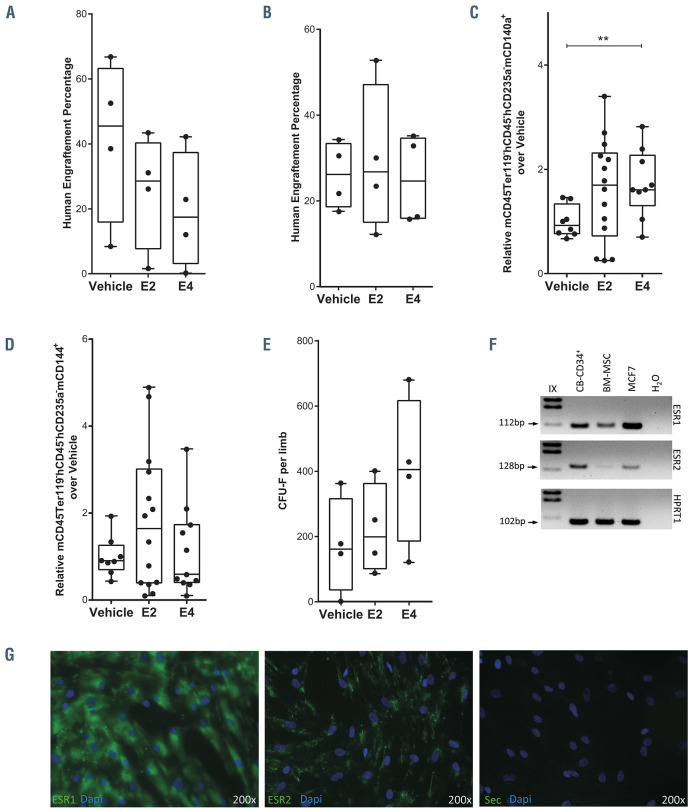
To obtain further insight into the positive impact of estrogens on promoting human hematopoietic engraftment, we assessed whether estrogens act in vivo on human HSPC to promote hematopoietic engraftment directly or indirectly through niche cells. We therefore cultured 5x104 CB-CD34+ cells with 100 nM of E2 or E4 for 4 days and transplanted the resulting cells after culture into NGS mice. As shown in Figure 6A, human engraftment of in vitro estrogen-treated HSPC was lower than that of vehicle- treated cells, which might indicate an indirect mechanism of estrogens in enhancing hematopoietic engraftment in NSG mice. Additionally, there was no difference in the percentage of lymphoid, myeloid or HSPC subpopulations among mice in the different groups (Online Supplementary Figure S6A). To gain a better understanding of the difference in engraftment between HSPC treated in vitro with estrogen and the in vivo effect of estrogens after HSC transplantation, we co-cultured 5x104 CB-CD34+ cells with human irradiated BM-MSC in the presence of 100 nM of E2 or E4 for 1 week and then transplanted the resulting cells into sublethally irradiated NSG mice. Human engraftment and lineage distribution were similar among mice in the different groups (Figure 6B; Online Supplementary Figure S6B), which indicated that the loss of engraftment ability due to in vitro estrogen-mediated expansion might be offset by the BM-MSC. Next, we examined the contribution of the hematopoietic niche to the engraftment of human HSPC after in vivo estrogentreatment. To do this, we analyzed the mesenchymal and vascular endothelial compartments of the mouse BM niche 4 months after being transplanted and treated with E2 or E4. The percentages of mouse MSC (mCD140a+, also called Pdgfra+) and vascular endothelial cells (mCD144+, also called VE-Cadherin+) in the nonhematopoietic compartment were analyzed (Online Supplementary Figure S6C). Surprisingly, mCD140a+ cells, but not mCD144+ cells, were increased in the mice treated with E4 in comparison with vehicle-treated animals (Figure 6C and D). To focus on this finding, mice were sublethally irradiated and treated with estrogens without human HSPC transplantation. Surprisingly, there were more nucleated cells in the BM of mice treated with E4 (Online Supplementary Figure S6F). These mouse BM cells were cultured to study their ability to form fibroblast colony-forming units (CFU-F). We identified a tendency to more CFU-F in the BM of estrogen-treated mice than in the BM of vehicle-treated mice (Figure 6E; Online Supplementary Figure S6G), which might indicate a beneficial role of estrogens in improving the BM niche after irradiation.
We then evaluated whether human MSC might interact with these estrogens. So, we analyzed the expression of ESR1 and ESR2 in the human BM-MSC compartment by RT-PCR (Figure 6F) and immunofluorescence (Figure 6G; Online Supplementary Figure S6H). Both estrogen receptors were present in human BM-MSC, indicating that the presence of estrogens could influence the behavior of the human stromal cells and indirectly affect the biology and/or engraftment of humans HSPC. To investigate the effect of estrogens on human BM-MSC, a limiting number of human BM-MSC were seeded and treated with estrogens and their CFU-F potential was assessed. As shown in Online Supplementary Figure S6I, the estrogens had no effect on human BM-MSC. The numbers of CFU-F dropped when the human BM-MSC had been previously irradiated. However, we observed an increase in the number of CFU-F when the BM-MSC were treated with estrogens after irradiation (Online Supplementary Figure S6I). In conclusion, estrogens, in particular E4, might facilitate and favor the hematopoietic engraftment of human progenitors through enhancing the mesenchymal compartment of the hematopoietic niche, in addition to having a direct effect on HSPC.
Discussion
The present study examines the potential use of estrogens to modify human HSPC engraftment in BM upon transplantation. On the basis of the differences in the level of human hematopoietic engraftment between female and male recipient mice (Figure 1; Online Supplementary Figure S1), and the expression of estrogen receptors in different subsets of human HSPC (Figure 2; Online Supplementary Figure S2), we explored the impact of estrogen treatment on hematopoietic cells engraftment. E2 and E4 showed a positive effect on the expansion of these cells in vitro by activating the cell cycle (Figure 3; Online Supplementary Figure S3), with E4 being better tolerated than E2 (Figure 3; Online Supplementary Figure S3). Despite the modest role of these estrogens in modulating human progenitor activity in vitro, we found that E2, and even more E4, was able to boost human hematopoietic engraftment in immunodeficient mice (Figure 5; Online Supplementary Figure S5). This better performance of human HSPC in estrogen-treated animals might reflect observed gender differences. Furthermore, an apparent expansion of the mouse mesenchymal stromal compartment was identified in animals treated with E4, which may suggest an additional indirect regulation of the estrogens, enhancing human hematopoietic engraftment through niche regulation (Figure 6; Online Supplementary Figure S6). Thus, estrogens could act directly on HSPC as well as indirectly, through the modification of the BM stroma, or BM niche, to enhance CD34+ cell engraftment. These findings might be clinically relevant, since the use of E4 could facilitate HSPC transplantation when only a limited number of cells can be infused.
We have shown that estrogens improve the engraftment of human cells in immunodeficient mice, which reinforces the role of sex hormones in HSPC regulation and might explain the superior performance of female mice as recipients of hematopoietic transplants26 (Figure 1). The importance of estrogens in regulating HSPC functions has been explored for a long time, without any clear conclusion being reached. E3 has been found to trap mouse hematopoietic progenitors in the liver.29 E2 has been described as promoting the proliferation of very primitive mouse HSC. The increase of estrogen levels during pregnancy has also been associated with greater HSC division, higher HSC frequency and an increase in erythropoiesis. 19,27 E2 has also been reported to expand human CB-CD34+ cells in vitro.28 These data contrast with those previously described by Illing et al., who found that longterm treatment of mice with E2 stimulated murine HSPC in the vascular niche but not in the endosteal niche, impairing long-term reconstitution potential.18 On the other hand, high doses of E2 suppressed hematopoiesis in mouse BM.30 This negative effect caused by estrogens has also been shown by tamoxifen treatment, which increased mouse HSC proliferation, but not self-renewal, and induced apoptosis in short-term HSC and MPP.24 Here, we found that human HSPC had different sensitivities to the four natural estrogens. E1 and, to a lesser extent, E2 and E3 were toxic for human HSPC (Figure 3; Online Supplementary Figure S3). E4 was better tolerated and was able to promote some degree of expansion of the human HSPC by activating their cell cycle and inducing less apoptosis (Figures 3 and 4; Online Supplementary Figures S3 and S4). These observations might explain the apparently divergent effects described previously for estrogens, since different doses and different estrogens were used in the above-mentioned reports.
We also observed that treatment with E2 or E4 in vivo enhanced human hematopoietic engraftment in male mice transplanted with 5x104 human HSPC (Figure 5B; Online Supplementary Figure S5), but only to a minor degree in animals transplanted with very limited numbers of CBCD34+ cells (Online Supplementary Figure 5E-G). The apparent lack of effectiveness in female mice might be due to the presence of endogenous estrogens in these female animals. Future experiments should be done with ovariectomized mice or taking the estrous cycle of female recipients into account to identify the real effect of estrogen treatment on HSPC in female recipients. Furthermore, E4 treatment enlarged the hCD34+ cell population in already boosted human hematopoietic engraftment, but it had less impact on the hCD34+hCD38- cell population and on secondary transplant (Figure 5D and E). Nevertheless, although the percentage of hCD34+hCD38- cells was unmodified by estrogen treatment, the total cell number of this primitive population was increased since human engraftment was higher in estrogen-treated mice (Figure 5B and D).
Figure 6.
Estrogens modulate the hematopoietic niche. (A) Human engraftment in bone marrow (BM) of male mice transplanted with cells expanded from an initial dose of 5x104 hCB-CD34+ cells after 4 days in culture in the presence of 100 nM E2 or E4. Human engraftment was analyzed 2 months after transplantation. (B) Human engraftment in BM of male mice transplanted with cells expanded from an initial dose of 5x104 hCB-CD34+ cells after 1 week in co-culture with irradiated human BM-mesenchymal stromal cells (MSC) in the presence of 100 nM E2 or E4. Human engraftment was analyzed 3 months after transplantation. (C) Relative percentage of mouse MSC (mCD45-Ter119-hCD45-hCD235a-mCD140a+) in the BM of male mice transplanted with 5x104 hCB-CD34+ cells, analyzed 4 months after transplantation. (D) Relative percentage of mouse vascular endothelial cells (mCD45-Ter119-hCD45-hCD235a-mCD144+) in the BM of the male mice transplanted with 5x104 hCB-CD34+ cells, analyzed 4 months after transplantation. (E) Number of fibroblast colony-forming units (CFU-F) derived from the BM of vehicle- or estrogen- treated mice after sublethal irradiation. (F) Representative agarose gel showing the quantitative real-time polymerase chain reaction products of ESR1 (top panel), ESR2 (middle panel) and HPRT1 (bottom panel) in human BM-MSC. (G) Representative immunofluorescence image of human BM-MSC stained with anti- ESR1 (green, left panel), anti-ESR2 (green, middle panel) or secondary antibody (green, right panel) and DAPI (blue). Data were obtained from three biological replicates and are presented by dots and box-plots that represent the interquartile range (p75, upper edge; p25, lower edge; p50, midline; p95, line above the box; and p5, line below the box). Statistical significance was analyzed by the Mann-Whitney U test: **P<0.01.
We have described a positive effect of E2 or E4 treatment on the engraftment of human cells, with the impact of E4 being more significant. It is important to note that E4 is synthesized exclusively by the human liver during pregnancy. It is detected at 9 weeks of pregnancy and reaches high levels in the second trimester, with concentrations rising steadily towards the end of pregnancy.16 The fetal liver is a hematopoietic organ during the last half of gestation. During the hematopoietic stage of the fetal liver, different signaling pathways are coordinated to promote both massive expansion of HSC through the activation of the HSC cycle and massive production of erythroid cells. After birth, the HSC migrate from the liver to the adult BM, where the most primitive HSC are largely quiescent.1,31 The concurrence of E4 synthesis in the fetal liver, when it is a hematopoietic organ, may suggest an indirect link between this estrogen and the expansion of human HSPC during pregnancy. The association between estrogens and hematopoietic development has previously been described during zebrafish development,32 in mice19,27 and in the hematopoietic differentiation of human induced pluripotent stem cells.28 So estrogens have a clear impact on HSC emergence. Oguro et al. demonstrated coordination between E2 and 27-hydroxycholesterol in the regulation of hematopoiesis during pregnancy.27 Consequently, we hypothesize that E4 likely plays a role in modulating early human hematopoiesis during embryonic development.
As previously reported, the observations could be attributed to an intricate regulation mediated by the estrogens.17 The complexity of estrogen signaling pathways starts with the existence of several estrogen receptors. Three of these receptors (ESR1, ESR2 and GPER33) are expressed in hematopoietic cells, but only ESR1 has been described to play a role in the regulation of HSC.18,19,24 A second level of complexity is that the expression of these estrogen receptors tends to differ among hematopoietic subpopulations24 (Figure 2). Moreover, different estrogens vary in their binding affinity for different estrogen receptors; for example, E2 has a 7-fold higher affinity for ESR1 (inhibition constant, Ki=0.21 nM) than for ESR2 (Ki=0.015 nM), and E4 has a 400-fold higher affinity for ESR1 (Ki=4.9 nM) than for ESR2 (Ki=19 nM).34 Once the estrogen and receptor are bound, specific cell responses are triggered by two different mechanisms: (i) gene expression programs, which can be initiated through estrogen nuclear signaling, and (ii) the estrogens acting through membrane-initiated steroid signaling (MISS), which is a rapid extra-nuclear cellular response to the estrogen signal.15 These two types of estrogen signaling may also explain the differences we observed between the effects of E4 and E2, from their toxicity and expansion in vitro (Figure 3; Online Supplementary Figure S3) to their in vivo effects (Figure 6). E4 uncouples nuclear activation and MISS, in contrast to E2 which does not.15 For example, E4 acts as an estrogen antagonist on breast cancer cells.16,35 Moreover, the lower affinity of E4 for estrogen receptors, in comparison with the affinity of E2, might suggest a very limited effect of E4 on HSPC; however, the E4 doses, whose effects on HSPC were observed (Figures 3 and 4), were the same doses used by Abbot et al. for which ERE transcriptional activity could be detected.15 Furthermore, given that E4 lacks MISS activity, it is possible that the impact of E2 and E4 on human HSPC is due to their nuclear signaling, with similar transcriptional output, but this point will have to be analyzed in depth. Additionally, the presence of E2 or E4 increased the levels of both ESR1 and ESR2 and modified their cellular localization. The increment of cells in S/G2/M-phase mediated by estrogens could be partially blocked by ESR1 and GPER antagonists in the case of E2, and by an ESR2 antagonist in the case of E4 (Online Supplementary Figure S3J). The implication of this is that different estrogen receptors in human HSPC might be involved in the signaling triggered by E2 or E4; however, this point will require more in-depth study. More interestingly, E2 might activate estrogen receptor-mediated MISS, since a clear polarized location of the estrogen receptors in the cytoplasm membrane was found (Online Supplementary Figure S3O and P). On the other hand, E4 might activate nuclear estrogen signaling, since E4 is unable to induce MISS15 and a clear increment of ESR2 in the cytoplasm was detected (Online Supplementary Figure S3N). The consequences of ESR1 and ESR2 upregulation and localization should be explored in future experiments. Additionally, Oguro et al.27 described two different ESR1 ligands, E2 and 27-hydroxycholesterol, which regulated HSPC differently during pregnancy. Both ESR1 ligands collaborated to induce HSC proliferation, mobilization and extramedullary hematopoiesis. In a similar way, E2 and E4 might collaborate together with a differential impact on human HSPC.
We identified that the underlying mechanism mediated by estrogens is activation of the cell cycle in vitro, as previously described, which promotes the expansion of hematopoietic progenitors.19,24 However, estrogens might also have other effects, including the activation of telomerase activity to facilitate the expansion of HSPC,20-22 or an increase of the unfolded protein response to promote hematopoietic regeneration after a proteotoxic stress, such as irradiation.23,36 In our in vivo model, E2 or E4 might activate the gene signaling involved in the cell cycle,19,24 telomerase activity20-22 or unfolded protein response,23 but these estrogens could also activate apoptosis24,27 when high doses are used (Figure 3; Online Supplementary Figure S3G and H). Surprisingly, the estrogen-mediated expansion observed in vitro was not enough to explain the improvement in human hematopoietic engraftment. Indeed, the in vitro proliferation of human HSPC induced by the estrogens was counterproductive to the enhancement of hematopoietic engraftment (Figure 6A). This might be due to the reduction of long-term engraftment ability of cycling HSPC, and the decoupling of HSPC expansion and stem cell properties in vitro.37 As HSC quiescence, selfrenewal and differentiation are controlled through intrinsic HSC signaling and extrinsic niche signaling and we observed that the co-culture of HSPC with human BMMSC was able to expand hematopoietic cells (Figure 4) and maintain engraftment potential (Figure 6B), in vitro expansion of HSPC might be compensated by niche signaling. In accordance with this, estrogens could also modulate hematopoiesis by affecting the capacity of MSC to promote osteogenesis.30,38 Furthermore, osteogenic differentiation might favor the proliferation of HSPC.25 The beneficial effect of E2 on the expansion of both HSPC and MSC was noted previously by Kitajima et al.39 As shown in Figure 6, an increase in MSC was also detected in our in vivo model after E4 treatment. Besides, the presence of estrogen might favor the recovery of MSC after irradiation (Figure 6E; Online Supplementary Figure S6E-G), as previously described for HSPC.23,36 Consequently, the impact of estrogens on promoting human hematopoietic engraftment in immunodeficient mice might be mediated through regeneration of the MSC compartment of the BM niche after irradiation, or a combined effect on human HSPC (Figures 2 and 3) and niche cells (Figure 6). We can, therefore, hypothesize that estrogens might coordinate HSPC proliferation and recovery of the BM niche in the context of HSC transplantation.
We suggest that the results reported could have some significant clinical implications. E4 has a safer therapeutic window than E2, which facilitates its clinical use.16 Additionally, E4 has been tested in several clinical trials and its safety and efficacy have been determined in different conditions, such as contraception,40 menopause,41 osteoporosis42 and breast cancer.43 The clinical application of E4 to modulate HSPC could, therefore, be considered for improving HSPC transplantation in the near future. The clinical use of E4 might potentially facilitate the transplantation of single cord blood units, the autologous transplantation of genetically modified HSPC to treat inherited hematopoietic diseases or in any situation in which a limited number of HSPC has to be infused. The administration of a clinically approved estrogen, such as E4, after HSPC transplantation could lead to an improvement in overall hematopoietic engraftment in the recipient.
Supplementary Material
Acknowledgments
The authors would like to thank Miguel A. Martin for the careful maintenance of NSG mice, and Norman Feltz for reviewing the manuscript.
Funding Statement
Funding This work was supported by grants from “Ministerio de Economía, Comercio y Competitividad y Fondo Europeo de Desarrollo Regional (FEDER)” (SAF2017-84248-P), “Fondo de Investigaciones Sanitarias, Instituto de Salud Carlos III” (RD16/0011/0011), TERCEL (“Red de Terapia Celular” of the “Instituto de Salud Carlos III”) and AvanCell consortium of “Comunidad de Madrid”. The authors also thank Fundación Botín for promoting translational research at the Hematopoietic Innovative Therapies Division of the CIEMAT. CIBERER is an initiative of the “Instituto de Salud Carlos III” and “Fondo Europeo de Desarrollo Regional (FEDER)”.
References
- 1.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132(4):631-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson A, Laurenti E, Oser G, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135(6): 1118-1129. [DOI] [PubMed] [Google Scholar]
- 3.Broxmeyer HE, Farag S. Background and future considerations for human cord blood hematopoietic cell transplantation, including economic concerns. Stem Cells Dev. 2013;22(Suppl 1):103-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lund TC, Boitano AE, Delaney CS, Shpall EJ, Wagner JE. Advances in umbilical cord blood manipulation-from niche to bedside. Nat Rev Clin Oncol. 2015;12(3):163-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith AR, Wagner JE. Alternative haematopoietic stem cell sources for transplantation: place of umbilical cord blood. Br J Haematol. 2009;147(2):246-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zonari E, Desantis G, Petrillo C, et al. Efficient ex vivo engineering and expansion of highly purified human hematopoietic stem and progenitor cell populations for gene therapy. Stem Cell Reports. 2017;8(4):977-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner JEJ, Brunstein CG, Boitano AE, et al. Phase I/II trial of stemregenin-1 expanded umbilical cord blood hematopoietic stem cells supports testing as a stand-alone graft. Cell Stem Cell. 2016;18(1):144-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gori JL, Chandrasekaran D, Kowalski JP, et al. Efficient generation, purification, and expansion of CD34(+) hematopoietic progenitor cells from nonhuman primateinduced pluripotent stem cells. Blood. 2012;120(13):e35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boitano AE, Wang J, Romeo R, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329(5997):1345-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fares I, Chagraoui J, Gareau Y, et al. Cord blood expansion. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science. 2014;345 (6203):1509-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cutler C, Multani P, Robbins D, et al. Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood. 2013;122(17):3074-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goessling W, Allen RS, Guan X, et al. Prostaglandin E2 enhances human cord blood stem cell xenotransplants and shows long-term safety in preclinical nonhuman primate transplant models. Cell Stem Cell. 2011;8(4):445-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernández-García M, Yañez RM, Sánchez-Domínguez R, et al. Mesenchymal stromal cells enhance the engraftment of hematopoietic stem cells in an autologous mouse transplantation model. Stem Cell Res Ther. 2015;6(1):165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perlin JR, Robertson AL, Zon LI. Efforts to enhance blood stem cell engraftment: Recent insights from zebrafish hematopoiesis. J Exp Med. 2017;214(10): 2817-2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abot A, Fontaine C, Buscato M, et al. The uterine and vascular actions of estetrol delineate a distinctive profile of estrogen receptor modulation, uncoupling nuclear and membrane activation. EMBO Mol Med. 2014;6(10):1328-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coelingh Bennink HJT, Holinka CF, Diczfalusy E. Estetrol review: profile and potential clinical applications. Climacteric. 2008;11(Suppl)147-158. [DOI] [PubMed] [Google Scholar]
- 17.Heo H-R, Chen L, An B, Kim K-S, Ji J, Hong S-H. Hormonal regulation of hematopoietic stem cells and their niche: a focus on estrogen. Int J Stem Cells. 2015;8(1):18-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Illing A, Liu P, Ostermay S, et al. Estradiol increases hematopoietic stem and progenitor cells independent of its actions on bone. Haematologica. 2012;97(8):1131-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakada D, Oguro H, Levi BP, et al. Oestrogen increases haematopoietic stemcell self-renewal in females and during pregnancy. Nature. 2014;505(7484):555-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cha Y, Kwon SJ, Seol W, Park K-S. Estrogen receptor-alpha mediates the effects of estradiol on telomerase activity in human mesenchymal stem cells. Mol Cells. 2008;26(5):454-458. [PubMed] [Google Scholar]
- 21.Calado RT, Yewdell WT, Wilkerson KL, et al. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood. 2009;114(11):2236-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeap BB, Hui J, Knuiman MW, et al. Crosssectional associations of sex hormones with leucocyte telomere length, a marker of biological age, in a community-based cohort of older men. Clin Endocrinol (Oxf) 2019;90(4): 562-569. [DOI] [PubMed] [Google Scholar]
- 23.Chapple RH, Hu T, Tseng Y-J, et al. ERpromotes murine hematopoietic regeneration through the Ire1-mediated unfolded protein response. Elife. 2018;7:e31159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sánchez-Aguilera A, Arranz L, Martín-Pérez D, et al. Estrogen signaling selectively induces apoptosis of hematopoietic progenitors and myeloid neoplasms without harming steady-state hematopoiesis. Cell Stem Cell. 2014;15(6):791-804. [DOI] [PubMed] [Google Scholar]
- 25.Qiu X, Jin X, Shao Z, Zhao X. 17-estradiol induces the proliferation of hematopoietic stem cells by promoting the osteogenic differentiation of mesenchymal stem cells. Tohoku J Exp Med. 2014;233(2):141-148. [DOI] [PubMed] [Google Scholar]
- 26.Notta F, Doulatov S, Dick JE. Engraftment of human hematopoietic stem cells is more efficient in female NOD/SCID/IL-2Rgc-null recipients. Blood. 2010;115(18):3704-3707. [DOI] [PubMed] [Google Scholar]
- 27.Oguro H, McDonald JG, Zhao Z, Umetani M, Shaul PW, Morrison SJ. 27- Hydroxycholesterol induces hematopoietic stem cell mobilization and extramedullary hematopoiesis during pregnancy. J Clin Invest. 2017;127(9):3392-3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim H-R, Lee J-H, Heo H-R, et al. Improved hematopoietic differentiation of human pluripotent stem cells via estrogen receptor signaling pathway. Cell Biosci. 2016;6(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayama T, Nawa Y, Ezaki T, Kotani M. Effects of estrogen on hepatic hemopoiesis in the adult mouse. Exp Hematol. 1983;11(7):611-617. [PubMed] [Google Scholar]
- 30.Perry MJ, Samuels A, Bird D, Tobias JH. Effects of high-dose estrogen on murine hematopoietic bone marrow precede those on osteogenesis. Am J Physiol Endocrinol Metab 2000;279(5):E1159-65. [DOI] [PubMed] [Google Scholar]
- 31.Laird DJ, von Andrian UH, Wagers AJ. Stem cell trafficking in tissue development, growth, and disease. Cell. 2008;132(4):612-630. [DOI] [PubMed] [Google Scholar]
- 32.Carroll KJ, Esain V, Garnaas MK, et al. Estrogen defines the dorsal-ventral limit of VEGF regulation to specify the location of the hemogenic endothelial niche. Dev Cell. 2014;29(4):437-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Vito C, Bergante S, Balduini A, et al. The oestrogen receptor GPER is expressed in human haematopoietic stem cells but not in mature megakaryocytes. Br J Haematol. 2010;149(1):150-152. [DOI] [PubMed] [Google Scholar]
- 34.Coelingh Bennik, HJT; Bunschoten E. Pharmaceutical composition comprising estetrol derivatives for use in cancer therapy. United States Patent. 2015; US 9034854B2. [Google Scholar]
- 35.Gérard C, Blacher S, Communal L, et al. Estetrol is a weak estrogen antagonizing estradiol-dependent mammary gland proliferation. J Endocrinol. 2015;224(1):85-95. [DOI] [PubMed] [Google Scholar]
- 36.Jin J, Wang Y, Wang J, et al. Impaired hematopoiesis and delayed thrombopoietic recovery following sublethal irradiation in SRC3 knockout mice. Mol Med Rep. 2014;9(5):1629-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tajer P, Pike-Overzet K, Arias S, Havenga M, Staal FJT. Ex vivo expansion of hematopoietic stem cells for therapeutic purposes: lessons from development and the niche. Cells. 2019;8(2):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Girasole G, Jilka RL, Passeri G, et al. 17 betaestradiol inhibits interleukin-6 production by bone marrow-derived stromal cells and osteoblasts in vitro: a potential mechanism for the antiosteoporotic effect of estrogens. J Clin Invest. 1992;89(3):883-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitajima Y, Doi H, Ono Y, et al. Estrogen deficiency heterogeneously affects tissue specific stem cells in mice. Sci Rep. 2015;5:12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kluft C, Zimmerman Y, Mawet M, et al. Reduced hemostatic effects with drospirenone-based oral contraceptives containing estetrol vs. ethinyl estradiol. Contraception. 2017;95(2):140-147. [DOI] [PubMed] [Google Scholar]
- 41.Coelingh Bennink HJT, Verhoeven C, Zimmerman Y, Visser M, Foidart J-M, Gemzell-Danielsson K. Pharmacokinetics of the fetal estrogen estetrol in a multiple-rising- dose study in postmenopausal women. Climacteric. 2017;20(3):285-289. [DOI] [PubMed] [Google Scholar]
- 42.Coelingh Bennink HJT, Verhoeven C, Zimmerman Y, Visser M, Foidart J-M, Gemzell-Danielsson K. Pharmacodynamic effects of the fetal estrogen estetrol in postmenopausal women: results from a multiple- rising-dose study. Menopause. 2017;24(6):677-685. [DOI] [PubMed] [Google Scholar]
- 43.Singer CF, Bennink HJTC, Natter C, et al. Antiestrogenic effects of the fetal estrogen estetrol in women with estrogen-receptor positive early breast cancer. Carcinogenesis. 2014;35(11):2447-2451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.