Abstract
Opioid addiction, also referred to as opioid use disorder, continues to be a devastating problem throughout the world. Familial relation and twin studies have revealed opioid addiction, like other addictive diseases, to be profoundly influenced by genetics. Genetics studies of opioid addiction have affirmed the importance of genetics contributors in susceptibility to develop opioid addiction, and also have important implications on treatment for opioid addiction. But the complexity of the interactions of multiple genetic variants across diverse genes, as well as substantial differences in allelic frequencies across populations, thus far limits the predictive value of individual genetics variants.
The A118G variant of the μ opioid receptor (MOR) gene, OPRM1, has been robustly shown to have a significant association with opioid addiction, as well as alcoholism, in specific populations. Further, the molecular mechanism of this variant conferring substitution of an aspartate residue for an asparagine residue in the 40th amino acid position in the amino terminus of the receptor and conferring altered expression, β-endorphin binding, and stress responsivity in humans has been elucidated in a manner that other gene variants contributing to addiction have not yet been studied. Given the overall genetic variability among humans including the confound of many other genetic variants being present in any single person, which can alter the impact of the variant under study, the effect of any single variant can be difficult, but sometimes possible, to discern. In rodent models, particularly in inbred strains, the effects of such single variants can be investigated with much better control and without the confound of additional genetics variants. For example, recent studies of a mouse model of the A118G OPRM1 variant have indicated mice homozygous for the 118G allele self-administer almost double the amount of heroin as their homozygous 118A allele littermates, indicating a profound effect of this single single-nucleotide polymorphism (SNP) on opioid intake behavior. In addition to genetics contributions to the vulnerability to addiction, the contribution of genetic variants to the success of treatment, as measured by prevention of recidivism following successful detoxification of opioids by the medications approved for treatment of opioid addiction, are also of interest. Specifically, genetics association studies have been successful in identifying variants in genes encoding enzymes, which metabolize or transport methadone that modestly contributes to individual variation in the optimal dosing of methadone for treatment, as well as genetic variants in components of related neurotransmitter or hormone subsystems. Genetics studies have informed our understanding of the biological basis of opioid addiction and treatment at multiple levels.
The search for the genetic variants that contribute to the heritability of propensity to addiction to opioids such as heroin and oxycodone, which at this point is more than two decades old and has been documented in thousands of publications, has yielded valuable information but has not resulted in a comprehensive description of the genetic factors underlying opioid addiction vulnerability. Twin and family studies have shown that genetic heritability of substance addictions in general, and addiction to opioids in particular, account for ∼50% of the risk for vulnerability (Tsuang et al. 1996, 1998; Kreek et al. 2004; Brick et al. 2019; Gillespie et al. 2019). However, the contribution of the genetic variants identified to date to contribute to this overall genetic variability sums to a much lower level. This “missing heritability” problem (Manolio et al. 2009) has been described as a problem not only for addictive diseases, but for many other biological features such as height, and diseases such as type 2 diabetes.
The current review is not an attempt to be exhaustive with regard to all published studies to date on opioid addiction/opioid use disorder, which numbers in the thousands, and for which numerous reviews already exist (Kreek et al. 2004; Reed et al. 2014; Burns et al. 2019). Rather, it will document the exciting discoveries in genetics that extend our understanding of the neurobiology of opioid addiction and the treatment of opioid addiction. It will include discussion of the first gene variant discovered to have a contribution to vulnerability to opioid addiction (Bond et al. 1998) and which remains to date the most studied and most replicated genetic variant contributing to vulnerability to opioid addiction, as well as a discussion of the rationale for using animal models in the study of human genetics and recent pharmacogenetics studies of methadone maintenance treatment of opioid addiction.
A118G VARIANT OF OPRM1
Because the MOR, encoded by OPRM1 in humans, is the primary target of exogenous opiates of abuse and mediates both analgesic and reinforcing effects among other downstream effects of opiates, it was the earliest protein for which genetic variants that might underlie the known genetic vulnerability to opiate addiction were systematically studied. Following cloning of the human MOR gene (Wang et al. 1994; Mestek et al. 1995), sequencing in the U.S. and Finnish Caucasians as well as the U.S. Southwest Native Americans led to the initial discovery of four SNPs in the OPRM1 gene, two of which were rare (<0.02% of reported alleles) and two of which were common, the A118G missense variant resulting in an amino acid change in the amino-terminal region of the MOR protein as well as an intronic variant C691G (Bergen et al. 1997). In a concurrent independent study, two OPRM1 variants were found in a mixed (Caucasian and African-American) U.S. population, one that was in a noncoding promoter region of the gene and the other that was a missense mutation now referred to as C17T, resulting in the replacement of an alanine residue in the prototype MOR with a valine residue at the sixth position (Berrettini et al. 1997).
Independently, in a collaboration of our laboratory with the molecular biology laboratory of Lei Yu, we found the 118AG and 17CT polymorphisms of OPRM1, as well as performed the first mechanistic studies of the 118A prototype receptor versus the 118G version (Bond et al. 1998). The major functional consequences of the amino acid substitution, which exchanges an asparagine in the prototype MOR for an aspartate residue (N40D) in the mutant protein, are to alter selectively the binding characteristics of the endogenous ligand β-endorphin, resulting in enhanced binding by this peptide (Bond et al. 1998) as well as reducing cellular expression concordant with altered glycosylation by removing one of the four extracellular glycosylation acceptor asparagine residues (Beyer et al. 2004; Kroslak et al. 2007). Further, messenger RNA (mRNA) transcripts of the 118G allele of OPRM1 have been described as less efficiently produced and thus present at lowered overall levels in comparison with the 118A allele (Zhang et al. 2005). The decreased expression of the 118G allele compared with 118A, initially reported in heterologous cell lines, has been supported both in differentiated human stem cell lines expressing endogenous MOR (Halikere et al. 2019) as well as in MOR-directed positron-emission tomography (PET) imaging studies (Weerts et al. 2013; Peciña et al. 2015).
The allelic frequencies of the A118G variant of OPRM1, subsequently indexed as rs1799971, varies substantially across different populations with the 118G allele present in frequencies globally at 22%, as indicated by the results of the 1000 Genomes Project (see grch37.ensembl.org). This allele is present at 30%–50% in South and East Asian populations, 10%–20% in European Caucasians, 15%–25% in indigenous American populations, and <5% in populations of African descent, with this frequency being <1% in current sub-Saharan African populations (see Table 1). That there are profound differences in the allelic frequencies of this variant across diverse populations is typical of the majority of variants that have been shown to be associated with substance use disorders. For instance, the C17T variant of OPRM1, mentioned above and shown to have an association with increased substance use in female African-Americans (Crystal et al. 2012), is present in only ∼1% of Caucasians of European descent and in ∼20% of African-Americans (Crowley et al. 2003). These allelic frequency differences may ultimately impact our understanding of the underlying biology of addiction as well as its treatment (see below).
Table 1.
Published studies investigating associations of rs1799971 with diverse diseases
| Population (location) | No. sampled | MAF | Disease/phenotype studied | Association found? | References |
|---|---|---|---|---|---|
| Uyghur (Northwest China) | 210–220 | 0.30 | Obesity | Yes | Xu et al. 2009 |
| Caucasian French Canadian (Quebec City) | 749 | 0.17 | Type 2 diabetes mellitus | No | Ruchat et al. 2008 |
| Caucasian (Northeast United States) | 107 | 0.18 | Adolescent alcohol use disorder (AUD) | Yes | Miranda et al. 2010 |
| Caucasian (Czech Republic) | 582 | 0.14 | Schizophrenia | Yes | Šerý et al. 2010 |
| Caucasian (Chicago, IL, United States) | 161 | 0.12 | Amphetamine euphoria | No | Dlugos et al. 2011 |
| Caucasian (Connecticut and South Carolina, United States) | 434 | 0.11 | Suicidality | No | Arias et al. 2012 |
| Caucasian (Germany) | 2879 | 0.12 | Alcohol dependence | Yes | Koller et al. 2012 |
| Caucasian (United States) | 161 | 0.15 | HIV, viral load response to treatment | Yes | Proudnikov et al. 2012 |
| African (United States) | 691 | 0.03 | HIV, viral load response to treatment | Yes | |
| Hispanic (United States) | 179 | 0.14 | HIV, viral load response to treatment | No | |
| Caucasian (Norway) | 252 | 0.13 | Pain | Yes | Olsen et al. 2012 |
| White (Colorado, United States) | 137 | 0.15 | Exercise/exertion | Yes | Karoly et al. 2012 |
| African (Colorado, United States) | 9 | 0.00 | Exercise/exertion | ||
| Asian (Colorado, United States) | 22 | 0.14 | Exercise/exertion | ||
| Hispanic (Colorado, United States) | 22 | 0.43 | Exercise/exertion | ||
| Asian (Taiwan) | 366 | 0.36 | Nicotine/conitine concentration | Yes | Chen et al. 2013 |
| Han Chinese (Zhejiang) | 528 | 0.40 | Schizophrenia | Yes | Ding et al. 2013 |
| Caucasian (Estonia) | 102 | 0.10 | Chronic postsurgical pain | Yes | Kolesnikov et al. 2013 |
| Caucasian (Finland) | 4762 | 0.19 | Alcohol dependence | No | Rouvinen-Lagerström et al. 2013 |
| Han Chinese (Shanghai) | 112 | 0.38 | Cancer pain, opioid requirements | Yes | Gong et al. 2013 |
| Han Chinese (Beijing) | 284 | 0.29 | Tobacco smoking | No | Fang et al. 2014 |
| Caucasian (Turkey) | 208 | 0.16 | Fibromyalgia | No | Solak et al. 2014 |
| Caucasian (European-American, Northeast) | 107 | 0.10 | Alcohol/naltrexone and disulfiram response | No | Arias et al. 2014 |
| Asian (Japan) | 85 | 0.49 | Postoperative nausea | No | Sugino et al. 2014 |
| Caucasian (Norway) | 118 | 0.10 | Disc herniation and pain | Yes | Hasvik et al. 2014 |
| Caucasian (Germany) | 214 | 0.11 | Social alcohol drinking | Yes | Pfeifer et al. 2015 |
| Caucasian (Spain) | 763 | 0.17 | Alcohol, maladaptive behaviors | Yes | Francès et al. 2015 |
| Han Chinese (Wuhan) | 285 | 0.39 | Cancer pain | No | Wang et al. 2015 |
| Asian (Malaysia) | 146 | 0.40 | Cold pressor pain | No | Zahari et al. 2015 |
| African (Cameroon) | 436 | 0.01 | Sickle cell anemia | No | Wonkam et al. 2018 |
| Mixed (Tunisia) | 129 | 0.12 | Cancer pain | No | Chatti et al. 2017 |
| Caucasian (Arab, U.A.E., and Egypt) | 458 | 0.15 | Substance use disorder | No | Alblooshi et al. 2018 |
| Caucasian (Sweden) | 201 | 0.23 | Pain hypersensitivity | Yes | Heddini et al. 2014 |
| Caucasian (Poland) | 339 | 0.12 | Alcoholism | Yes | Samochowiec et al. 2019 |
| Han Chinese (Zhejiang) | 215 | 0.45 | Sufentanil analgesia | Yes | Zhao et al. 2019 |
| African-American | 241 | 0.03 | Perioperative pain | No | Li et al. 2019 |
| European Caucasian | 277 | 0.13 | No | ||
| Caucasian (France) | 496 | 0.15 | Suicide ideation | Yes | Nobile et al. 2019 |
| Mixed (Brazil) | 57 | 0.32 | Painful bladder syndrome | Yes | Cassão et al. 2019 |
| Caucasian (Hungary) | 3743 | 0.13 | Psoriasis | Yes | Szentkereszty-Kovács et al. 2019 |
| Caucasian (Spain) | 114 | 0.15 | Dental pain | No | López-Valverde et al. 2019 |
| Caucasian (Spain) | 231 | 0.21 | Low back pain | Yes | Margarit et al. 2019 |
| Asian (Korea) | 55 | 0.40 | Gambling disorder | No | Kim et al. 2019 |
The listed studies are not intended to be exhaustive, but were chosen to illustrate the diverse diseases in which the μ opioid receptor polymorphism might be implicated in vulnerability, as well as the differences in mean allelic frequencies between different populations.
The OPRM1 118G allele has been shown to be associated with heroin dependence in both European Caucasian and Southeast Asian populations (Szeto et al. 2001; Bart et al. 2004). There have been conflicting reports in the literature about association of this variant with opioid addiction, but the majority of studies indicate that there is an association in these populations as concluded by a rigorous meta-analysis (Haerian and Haerian 2013) and also an association with substance use disorders in general (Schwantes-An et al. 2016). Given the low prevalence of this variant in persons of African descent, the A118G SNP does not substantially contribute to the vulnerability to heroin addiction in African-American populations.
Similar studies of the genetics of alcoholism have shown an association of rs1799971 (e.g., Bart et al. 2005). Further studies have investigated whether this variant may influence responses of patients to alcohol to one of the few available pharmacotherapeutic treatments for alcoholism, naltrexone, which acts at least in part via antagonism of MOR and also potentially via partial antagonism of the κ opioid receptor (Butelman et al. 2020). Reported findings indicated that in patients with at least one copy of the 118G allele, naltrexone substantially extended duration of abstinence from alcohol, whereas naltrexone was ineffective in treating 118AA homozygotes (Jonas et al. 2014).
The endogenous opioid system is important to the regulation of the stress response system (Kreek and Koob 1998; Koob and Kreek 2007), as shown in part by the activation of the hypothalamic-pituitary-adrenal (HPA) axis by opioid antagonists (Schluger et al. 1998) as well as the suppression of the HPA axis by exogenous opioid agonists (Kreek et al. 1983). The rs1799971 variant has been studied to determine its effects on endogenous stress responsiveness. In persons with at least one copy of the 118G allele, cortisol response to naloxone or stress were elevated compared with persons homozygous for the 118A allele (Wand et al. 2002; Chong et al. 2006). Further, in a stress-minimized setting, subjects with at least one copy of the 118G allele had lower resting levels of cortisol compared with those with two 118A alleles (Bart et al. 2006).
Since the discovery of the A118G polymorphism and its importance in conferring a small component of the genetic variability in vulnerability to substance use disorders, a great number of studies probing a genetics association of rs1799971 have been performed both on substance use disorder as well as diverse related phenomena in which the endogenous opioid system are known or suspected to be involved. Findings of these studies have shown that this variant is pleiotropic; a subset of these studies is listed in Table 1. Although not all of these reports found a positive association of rs1799971, it is clear that this particular variant has a significant impact on the endogenous opioid system and its role in human physiology.
ANIMAL MODELS FOR THE STUDY OF SUBSTANCE USE DISORDER GENETICS
Although thousands of studies have been published on genetics of addictive diseases in humans and thousands more studies have been published on animal models of addictive diseases, the study of genetics in animal models of addiction has to date been comparatively limited. The tools and ability for control of genetics in animal models, especially in rodents for which robust models of various aspects of substance use disorder are abundant, suggest that insights could be gleaned that may impact on our understanding of the genetics of addiction in humans. Although numerous studies have been conducted using genes that have been inactivated (“knocked out”), either constitutively or in response to gene excision factors in a location and/or time and/or cell-type-specific manner, these are typically not guided by human genetics as much as findings from prior pharmacological probes. Also, given that they do not involve specific variants but rather inactivation of entire genes (or specific splice variants of gene transcripts), they have not been informative to our understanding of human genetics of drug addiction per se. Rather, the two avenues in which animal models have been informative to our understanding of human genetics include (1) genetics studies of select strains that differ in their response to specific drugs of abuse, whether such strains have been found by happenstance or have been specifically bred for differential responsiveness to drugs on one or more parameter, for example, preferring (P) ethanol versus nonpreferring (NP) ethanol-bred rats; and (2) animals genetically altered to have a specific variant that aligns with a known genetic variant in humans, followed by genetic inbreeding, with offspring of animals homozygous for the variant studied in comparison with littermates (siblings from the same parental pair born from the same pregnancy).
Genetics of Rodent Strains or Substrains
A number of rat strains have been developed for alcohol responsivity as a model for vulnerability to the development of alcoholism (Ciccocioppo 2013). One of the earliest and best characterized pair of lines were developed from the outbred strain of Wistar rats, selecting for low versus high ethanol drinking, resulting in rat lines named NP for ethanol-nonpreferring and P for ethanol-preferring (Li et al. 1993). Subsequent inbreeding of these lines allowed for identification of a quantitative trace locus (QTL) on rat chromosome 4, which accounts for 33% of the genetic variability (Carr et al. 1998). Several genes, including those for neuropeptide Y, located proximal to or within this QTL remain candidates to mediate the genetic difference between the inbred strains underlying the differences in drinking behavior (Carr et al. 2007). Although identification of the genes underlying differences in alcohol-preferring behavior in these rodent models may elucidate mechanisms underlying propensity for alcoholism, the particular gene variants discovered will not necessarily, and may indeed even be unlikely to, correspond with genetic variants in humans that contribute to vulnerability to alcoholism.
Similar attempts have been made to investigate mouse strains with inherent differences in opioid preference. The relatively high morphine-preferring mouse strain, C57BL/6, was crossbred with the relatively low morphine-preferring mouse strain DBA/2 (Berrettini et al. 1994). A QTL underlying the differences in morphine preference was localized to a region of chromosome 10 containing the gene for the MOR, Oprm1 (Belknap et al. 1995; Bergeson et al. 2001; Doyle et al. 2014). Identification of the particular variant(s) within a QTL can be complex and labor- and cost-intensive. The particular variants contributing to the differential morphine preference in these two mouse strains could potentially inform our understanding of mechanisms of MOR agonist reward, but they have not yet been reported.
Mouse Models of Particular Genetic Variants
For the most part, genetics studies in animal models are unlikely to yield direct parallels with SNPs to be found in human genetics studies. However, recently, rodent models have proved to be crucial for investigating the effects of isolated functional SNPs on aspects of the disease that can be modeled by animal studies. Human genetics studies are susceptible to variation in many other genes. By studying a SNP in inbred mice, we can isolate the effects of this SNP on physiology or behavior both under baseline conditions as well as in response to perturbations.
Two separate animal models have been developed to investigate the effects of the polymorphism A118G in inbred mouse models. The genetic and resulting amino acid sequence of the MOR in the mouse differs from that of human MOR. There is an analogous arginine residue in the mouse at amino acid sequence 38 (compared with amino acid 40 in the human receptor), which is potentially glycosylated. One of the mouse models induced a mutation of the 112 adenine to guanine, with concomitant amino acid substitution of the Arg38 to an aspartate residue, which parallels the human situation at amino acid 40 (Mague et al. 2009). The effects of this mutation in the mouse MOR gene largely parallel the effects of the A118G mutation in the human MOR gene with reduced expression in the 112GG homozygous mice compared with the 112AA homozygous mice and reduced glycosylation in in vitro studies as expected (Mague et al. 2009). These studies were performed on offspring of maternal and paternal mice who are heterozygous for the two alleles, Oprm1-112AG, in littermate siblings who are homozygous for the 112GG allele or 112AA allele. This is analogous to the situation of nonidentical twin siblings who are the offspring of parents who are both heterozygous (OPRM1 118AG) and who are 118GG or 118AA homozygotes (one in four chance for each child of two heterozygotes to be homozygous for each allele).
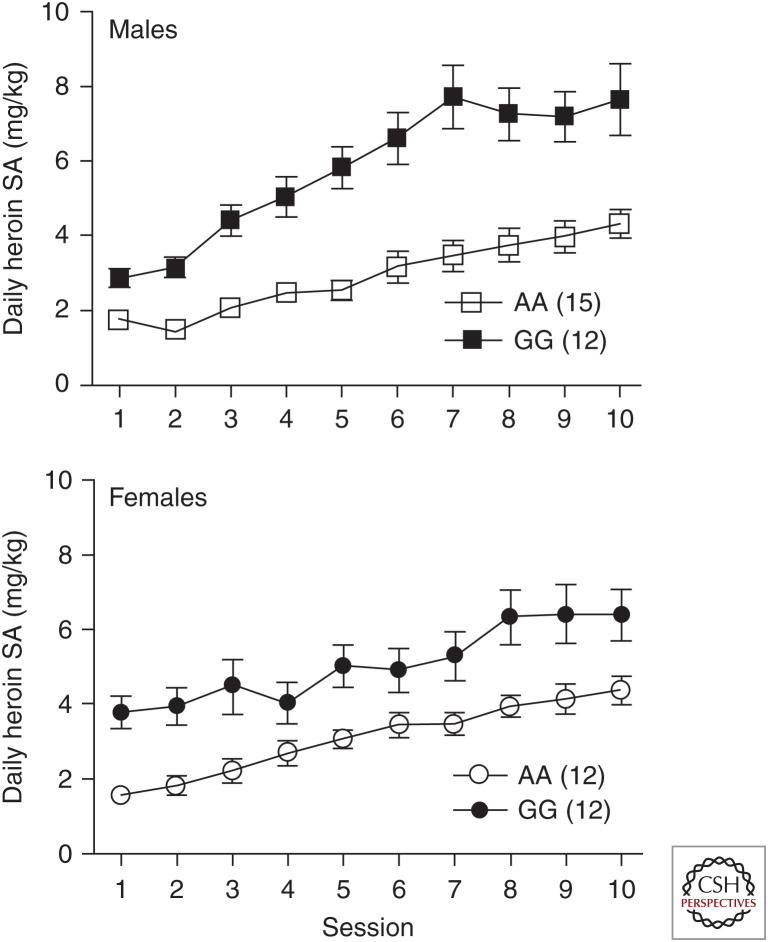
This model, developed in the laboratory of Julie Blendy of the University of Pennsylvania, has now been more extensively studied with respect to the differential responsiveness of the mice to opioids. Most extraordinarily, our laboratory, in collaboration with the Blendy laboratory, discovered that 112GG mice self-administer roughly twice the amount of heroin as 112AA mice in both male and female mice (Fig. 1; Zhang et al. 2015). Very similar findings emerged in studies of oxycodone self-administration (Collins et al. 2019). The 112GG homozygous mice also showed enhanced striatal dopamine release in response to heroin compared with their 112AA littermates, although baseline extracellular dopamine in the striatum did not differ between the two groups (Zhang et al. 2015).
Figure 1.
Male mice self-administration of heroin. Mice with the 112GG genotype of Oprm1 self-administer significantly greater amounts of heroin than mice homozygous for the prototype Oprm1 112AA genotype. SA, Self-administration. (From Zhang et al. 2015; modified, with permission, from the authors.)
We have further characterized the heterozygous mice, 112AA versus 112GG, investigating differences in baseline (opioid-naive) gene expression of several genes that interact with the endogenous opioid system in diverse brain regions of adult mice (Collins et al. 2018). We found several gene expression alterations, most strongly the expression levels of the precursors of the neuropeptides arginine vasopressin and galanin in the hypothalamus and the expression of opioid receptor-like receptor (ORL1; target of orphanin FQ/nociceptin peptide) and cannabinoid receptor type 1 (CB1) in the hippocampus. In addition to the effects of the altered MOR function and expression levels possibly being directly on the reinforcing and rewarding effects of heroin, the altered expression of these opioid-interacting genes may lead to an altered responsivity to heroin that is reflected by increased self-administration in the 112GG homozygotes.
A second model involved the exchange of exon 1 of the mouse Oprm1 gene, encoding the amino terminus and transmembrane region 1, exon 1 of the human gene (Mahmoud et al. 2011). This yields a receptor that has the substituted amino acid residue in the same precise location as the human variant in the 40th position of the extracellularly localized amino terminus. It should be noted that other amino acids encoded by this human cassette are also altered as they are not all conserved between the two species. There have been no published reports of opioid self-administration in this “humanized” mouse model of the A118G variant, but in studies of morphine conditioned place preference, there was no difference between the 118AA and 118G homozygous mice (Henderson-Redmond et al. 2016), although both mouse models observed a decrease in analgesic response to exogenous opioids (Mague et al. 2009; Mahmoud et al. 2011).
As mentioned above, and indicated by the results in Table 1, the effects of the A118G variation of OPRM1 are pleiotropric with effects on multiple disease states and aspects of physiology. The two mouse constructs described could be helpful in elucidating the mechanism of the genetic contribution of this variant to the development of these disease states. Head-to-head comparison of these mouse constructs would also be helpful to further understand the role of mouse versus human amino acids surrounding the variant asparagine/aspartate residue in MOR binding/signaling/physiology.
GENETICS OF METHADONE MAINTENANCE THERAPY
Pharmacogenetics refers to the interaction of pharmaceutical therapeutic effectiveness with genes and genetic variants. The two main successful treatments for opioid addiction are methadone and buprenorphine/naloxone. Although the bulk of efforts into the genetics of opioid addiction have focused on vulnerabilities toward the development of an addiction, several recent studies from our own laboratory and others have investigated genetic associations with the success of maintenance treatment with rather few studies investigating buprenorphine pharmacogenetics published to date. Although methadone maintenance therapy for opioid addiction is by far the most successful treatment for any addictive disease to date, 10%–30% of patients are refractory. In addition, methadone is usually administered at a dose between 80 and 150 mg/d, but some patients have been shown to require higher doses such as >150 mg/d. Genetics studies have investigated associations of particular variants in genes involved in the neurochemistry and metabolism of methadone.
The primary pharmacotherapeutic target of methadone is MOR (Neil 1984) with a lower potency binding to N-methyl-D-aspartate (NMDA) receptors (Gorman et al. 1997). Although certain noncoding or synonymous variants of the OPRM1 have been associated with methadone dosing, the A118G and C17T variants were not found to be associated with this parameter (Levran et al. 2013b; Smith et al. 2017; Crist et al. 2018). A genome-wide association study (GWAS) of methadone dosing revealed that in an African-American population, but not European-American, a SNP localized near the OPRM1 locus is associated with methadone dosing requirements in treatment of opioid addiction with the minor allele contributing to a requirement for higher methadone doses (Smith et al. 2017). Variants in the genes encoding for NMDA receptor subcomponents have not been reported to be assessed with respect to methadone dosing and treatment response in opioid addiction.
In addition to the binding sites, several studies have probed genetic variants in enzymes known to be involved in the metabolism of methadone, namely, the cytochrome P450 (CYP) family members (Eap et al. 2002). Variants of methadone metabolizing enzymes in this family have been shown to alter methadone bioavailability (Wang et al. 2011). Further, variants in multiple CYP genes have been shown to be associated with required methadone dosing with rather modest effects (Levran et al. 2013a).
Methadone is a substrate of the P-glycoprotein (P-gp) 170 transporter encoded in humans by the ABCB1 gene, which serves in part to transport drugs out of the brain, limiting blood–brain barrier penetration (Levran et al. 2008). Genetics association studies of nine SNPs of this gene with doses required for methadone indicate a significant, albeit modest, association of a single, synonymous polymorphism in the coding region of this gene, rs1128503, comparing formerly heroin-addicted persons in treatment requiring high dose methadone (>150 mg/d) and those requiring lower doses of methadone (≤150 mg/d). Further, a haplotype of this SNP and two others, the nonsynonymous SNP rs2032582 as well as the synonymous SNP rs1045642, both also in the coding region, was significantly associated with requirements for a higher methadone dose (Levran et al. 2008). A similar earlier study also indicated that haplotypes of the ABCB1 variants were associated with methadone dose requirements (Coller et al. 2006).
An association study of methadone treatment success of heroin-addicted patients and the dopamine D2 receptor DRD2 Taq1A variant rs1800497, which causes a carboxy-terminal amino acid substitution, is associated with poor treatment outcome with higher relapse (Lawford et al. 2000), but this was not replicated in a similar population (Barratt et al. 2006). Given known effects of brain-derived neurotrophic factor on methadone response, we investigated polymorphisms in the gene encoding for nerve growth factor (β polypeptide) NGFB. Of the 14 polymorphisms of this gene investigated, a single intronic variant, rs2239622, showed significant association with methadone dose requirements (Levran et al. 2012).
Although methadone maintenance is effective in normalizing opioid-exposure-induced dysfunction and can lead to long-term abstinence from illicit opioid use, it has also been shown to be effective in reducing cocaine use. However, some patients continue to abuse cocaine while in methadone treatment. A recent genetics study revealed that an association of a gene variant, rs1500, a noncoding SNP downstream from the corticotropin-releasing hormone-binding protein (CRHBP), is associated with a propensity to abstain from cocaine use while in methadone maintenance (Peles et al. 2019).
The cumulative genetics effect on methadone dosing is relatively low, and other factors such as prior illicit opioid exposure in terms of duration of use and daily dosing level at time of recovery initiation likely have much more important effects on methadone dosing requirements. Additionally, other medications can interact with methadone, altering metabolism, which can impact the required dosing, including phenytoin and rifampin, which can accelerate methadone metabolism. Thus, patients on these medications would require higher dosing (Kreek et al. 1976; Tong et al. 1981).
CONCLUSIONS
Although it has long been known and appreciated that there are genetic risk factors underlying propensity to the development of addictive diseases, a full accounting of the genetic components of this risk has thus far proven elusive, as has been the case for other polygenic risk factors contributing to many other complex diseases. Future studies may help further our understanding, perhaps via studies of larger populations, a closer accounting of addictive disease phenotypes, inclusion of considerations of comorbid disorders, and/or a clearer understanding of gene–gene and gene–environment interactions. Genetics studies to date have certainly enhanced our understanding of the biology of addiction and addiction treatment. For the vast majority of the variants that have been identified to date in replicated studies of having an association with opioid addiction, little to no work has been performed to examine the functional mechanisms, in vitro and in vivo, which might relate the consequences of the variant to the responses to opioids and alterations of the endogenous opioid system. In the case of the A118G OPRM1 variant, which has been discussed here, the early finding of functional consequences in vitro helped to spur the numerous subsequent studies that have since elucidated our understanding of the effects of this variant in vivo. However, as noted above, this variant is present only in very low levels or not at all in sub-Saharan African populations in which heroin use is a large and growing problem (Acuda et al. 2011; Lancaster et al. 2018). The effect size of the G allele is high in mouse model studies of the A118G OPRM1 variant, but overall the contribution of this allele to addiction propensity is low with persons homozygous for 118A allele also developing addictions. It will be critical to further elucidate the genetic risk factors, both in terms of the discovery of new variants and their interactions with known contributing variants, in furthering our understanding of the mechanisms of those variants that have already been replicated.
ACKNOWLEDGMENTS
Support has been provided by the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (M.J.K.).
Footnotes
Editors: R. Christopher Pierce, Ellen M. Unterwald, and Paul J. Kenny
Additional Perspectives on Addiction available at www.perspectivesinmedicine.org
REFERENCES
- Acuda W, Othieno CJ, Obondo A, Crome IB. 2011. The epidemiology of addiction in sub-Saharan Africa: a synthesis of reports, reviews, and original articles. Am J Addict 20: 87–99. [DOI] [PubMed] [Google Scholar]
- Alblooshi H, Hulse G, Osman W, El Kashef A, Shawky M, Al Ghaferi H, Al Safar H, Tay GK. 2018. The frequency of DRD2 rs1076560 and OPRM1 rs1799971 in substance use disorder patients from the United Arab Emirates. Ann Gen Psychiatry 17: 22. 10.1186/s12991-018-0192-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias AJ, Chan G, Gelernter J, Farrer L, Kranzler HR. 2012. Variation in OPRM1 and risk of suicidal behavior in drug-dependent individuals. Am J Addict 21: 5–10. 10.1111/j.1521-0391.2011.00195.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias AJ, Gelernter J, Gueorguieva R, Ralevski E, Petrakis IL. 2014. Pharmacogenetics of naltrexone and disulfiram in alcohol dependent, dually diagnosed veterans. Am J Addict 23: 288–293. 10.1111/j.1521-0391.2014.12102.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt DT, Coller JK, Somogyi AA. 2006. Association between the DRD2 A1 allele and response to methadone and buprenorphine maintenance treatments. Am J Med Genet B Neuropsychiatr Genet 141B: 323–331. 10.1002/ajmg.b.30319 [DOI] [PubMed] [Google Scholar]
- Bart G, Heilig M, LaForge KS, Pollak L, Leal SM, Ott J, Kreek MJ. 2004. Substantial attributable risk related to a functional µ-opioid receptor gene polymorphism in association with heroin addiction in central Sweden. Mol Psychiatry 9: 547–549. 10.1038/sj.mp.4001504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bart G, Kreek MJ, Ott J, LaForge KS, Proudnikov D, Pollak L, Heilig M. 2005. Increased attributable risk related to a functional µ-opioid receptor gene polymorphism in association with alcohol dependence in central Sweden. Neuropsychopharmacolgy 30: 417–422. 10.1038/sj.npp.1300598 [DOI] [PubMed] [Google Scholar]
- Bart G, LaForge KS, Borg L, Lilly C, Ho A, Kreek MJ. 2006. Altered levels of basal cortisol in healthy subjects with a 118G allele in exon 1 of the µ opioid receptor gene. Neuropsychopharmacolgy 31: 2313–2317. 10.1038/sj.npp.1301128 [DOI] [PubMed] [Google Scholar]
- Belknap JK, Mogil JS, Helms ML, Richards SP, O'Toole LA, Bergeson SE, Buck KJ. 1995. Localization to chromosome 10 of a locus influencing morphine analgesia in crosses derived from C57BL/6 and DBA/2 strains. Life Sci 57: PL117–PL124. 10.1016/0024-3205(95)02040-P [DOI] [PubMed] [Google Scholar]
- Bergen AW, Kokoszka J, Peterson R, Long JC, Virkkunen M, Linnoila M, Goldman D. 1997. µ Opioid receptor gene variants: lack of association with alcohol dependence. Mol Psychiatry 2: 490–494. 10.1038/sj.mp.4000331 [DOI] [PubMed] [Google Scholar]
- Bergeson SE, Helms ML, O'Toole LA, Jarvis MW, Hain HS, Mogil JS, Belknap JK. 2001. Quantitative trait loci influencing morphine antinociception in four mapping populations. Mamm Genome 12: 546–553. 10.1007/s003350020022 [DOI] [PubMed] [Google Scholar]
- Berrettini WH, Ferraro TN, Alexander RC, Buchberg AM, Vogel WH. 1994. Quantitative trait loci mapping of three loci controlling morphine preference using inbred mouse strains. Nat Genet 7: 54–58. 10.1038/ng0594-54 [DOI] [PubMed] [Google Scholar]
- Berrettini WH, Hoehe MR, Ferraro TN, Demaria PA, Gottheil E. 1997. Human µ opioid receptor gene polymorphisms and vulnerability to substance abuse. Addict Biol 2: 303–308. 10.1080/13556219772598 [DOI] [PubMed] [Google Scholar]
- Beyer A, Koch T, Schroder H, Schulz S, Hollt V. 2004. Effect of the A118G polymorphism on binding affinity, potency and agonist-mediated endocytosis, desensitization, and resensitization of the human µ-opioid receptor. J Neurochem 89: 553–560. 10.1111/j.1471-4159.2004.02340.x [DOI] [PubMed] [Google Scholar]
- Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM, et al. 1998. Single-nucleotide polymorphism in the human µ opioid receptor gene alters β-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci 95: 9608–9613. 10.1073/pnas.95.16.9608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brick LA, Micalizzi L, Knopik VS, Palmer RHC. 2019. Characterization of DSM-IV opioid dependence among individuals of European ancestry. J Stud Alcohol Drugs 80: 319–330. 10.15288/jsad.2019.80.319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JA, Kroll DS, Feldman DE, Kure Liu C, Manza P, Wiers CE, Volkow ND, Wang GJ. 2019. Molecular imaging of opioid and dopamine systems: insights into the pharmacogenetics of opioid use disorders. Front Psychiatry 10: 626. 10.3389/fpsyt.2019.00626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butelman ER, Fry RS, Kimani R, Reed B, Kreek MJ. 2020. Neuroendocrine effects of naltrexone and nalmefene in humans. Hum Psychopharmacol 10.1002/hup.2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr LG, Foroud T, Bice P, Gobbett T, Ivashina J, Edenberg H, Lumeng L, Li TK. 1998. A quantitative trait locus for alcohol consumption in selectively bred rat lines. Alcohol Clin Exp Res 22: 884–887. 10.1111/j.1530-0277.1998.tb03883.x [DOI] [PubMed] [Google Scholar]
- Carr LG, Kimpel MW, Liang T, McClintick JN, McCall K, Morse M, Edenberg HJ. 2007. Identification of candidate genes for alcohol preference by expression profiling of congenic rat strains. Alcohol Clin Exp Res 31: 1089–1098. 10.1111/j.1530-0277.2007.00397.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassão VD, Reis ST, Pimenta R, Lucon M, Leite KRM, Srougi M, Bruschini H. 2019. Single nucleotide polymorphism analysis in interstitial cystitis/painful bladder syndrome. PLoS ONE 14: e0215201. 10.1371/journal.pone.0215201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatti I, Woillard JB, Mili A, Creveaux I, Ben Charfeddine I, Feki J, Langlais S, Ben Fatma L, Saad A, Gribaa M, et al. 2017. Genetic analysis of μ and κ opioid receptor and COMT enzyme in cancer pain Tunisian patients under opioid treatment. Iran J Public Health 46: 1704–1711. [PMC free article] [PubMed] [Google Scholar]
- Chen YT, Tsou HH, Kuo HW, Fang CP, Wang SC, Ho IK, Chang YS, Chen CH, Hsiao CF, Wu HY, et al. 2013. OPRM1 genetic polymorphisms are associated with the plasma nicotine metabolite cotinine concentration in methadone maintenance patients: a cross sectional study. J Hum Genet 58: 84–90. 10.1038/jhg.2012.139 [DOI] [PubMed] [Google Scholar]
- Chong RY, Oswald L, Yang X, Uhart M, Lin PI, Wand GS. 2006. The μ-opioid receptor polymorphism A118G predicts cortisol responses to naloxone and stress. Neuropsychopharmacolgy 31: 204–211. 10.1038/sj.npp.1300856 [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R. 2013. Genetically selected alcohol preferring rats to model human alcoholism. Curr Top Behav Neurosci 13: 251–269. 10.1007/978-3-642-28720-6_199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller JK, Barratt DT, Dahlen K, Loennechen MH, Somogyi AA. 2006. ABCB1 genetic variability and methadone dosage requirements in opioid-dependent individuals. Clin Pharmacol Ther 80: 682–690. 10.1016/j.clpt.2006.09.011 [DOI] [PubMed] [Google Scholar]
- Collins D, Randesi M, da Rosa JC, Zhang Y, Kreek MJ. 2018. Oprm1 A112G, a single nucleotide polymorphism, alters expression of stress-responsive genes in multiple brain regions in male and female mice. Psychopharmacology (Berl) 235: 2703–2711. 10.1007/s00213-018-4965-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D, Zhang Y, Blendy J, Kreek MJ. 2019. Murine model of OPRM1 A118G alters oxycodone self-administration and locomotor activation, but not conditioned place preference. Neuropharmacology 167: 107864. 10.1016/j.neuropharm.2019.107864 [DOI] [PubMed] [Google Scholar]
- Crist RC, Doyle GA, Nelson EC, Degenhardt L, Martin NG, Montgomery GW, Saxon AJ, Ling W, Berrettini WH. 2018. A polymorphism in the OPRM1 3′-untranslated region is associated with methadone efficacy in treating opioid dependence. Pharmacogenomics J 18: 173–179. 10.1038/tpj.2016.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley JJ, Oslin DW, Patkar AA, Gottheil E, DeMaria PA Jr, O'Brien CP, Berrettini WH, Grice DE. 2003. A genetic association study of the μ opioid receptor and severe opioid dependence. Psychiatr Genet 13: 169–173. 10.1097/00041444-200309000-00006 [DOI] [PubMed] [Google Scholar]
- Crystal HA, Hamon S, Randesi M, Cook J, Anastos K, Lazar J, Liu C, Pearce L, Golub E, Valcour V, et al. 2012. A C17T polymorphism in the μ opiate receptor is associated with quantitative measures of drug use in African American women. Addict Biol 17: 181–191. 10.1111/j.1369-1600.2010.00265.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Chen B, Zheng Y, Lu Q, Liu L, Zhuge QC. 2013. Association study of OPRM1 polymorphisms with schizophrenia in Han Chinese population. BMC Psychiatry 13: 107. 10.1186/1471-244X-13-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugos AM, Hamidovic A, Hodgkinson C, Shen PH, Goldman D, Palmer AA, de Wit H. 2011. OPRM1 gene variants modulate amphetamine-induced euphoria in humans. Genes Brain Behav 10: 199–209. 10.1111/j.1601-183X.2010.00655.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle GA, Schwebel CL, Ruiz SE, Chou AD, Lai AT, Wang MJ, Smith GG, Buono RJ, Berrettini WH, Ferraro TN. 2014. Analysis of candidate genes for morphine preference quantitative trait locus Mop2. Neuroscience 277: 403–416. 10.1016/j.neuroscience.2014.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eap CB, Buclin T, Baumann P. 2002. Interindividual variability of the clinical pharmacokinetics of methadone: implications for the treatment of opioid dependence. Clin Pharmacokinet 41: 1153–1193. 10.2165/00003088-200241140-00003 [DOI] [PubMed] [Google Scholar]
- Fang J, Wang X, He B. 2014. Association between common genetic variants in the opioid pathway and smoking behaviors in Chinese men. Behav Brain Funct 10: 2. 10.1186/1744-9081-10-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francès F, Portolès O, Castelló A, Costa JA, Verdú F. 2015. Association between opioid receptor μ1 (OPRM1) gene polymorphisms and tobacco and alcohol consumption in a Spanish population. Bosn J Basic Med Sci 15: 31–36. 10.17305/bjbms.2015.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie NA, Bates TC, Hickie IB, Medland SE, Verhulst B, Kirkpatrick RM, Kendler KS, Martin NG, Benotsch EG. 2019. Genetic and environmental risk factors in the non-medical use of over-the-counter or prescribed analgesics, and their relationship to major classes of licit and illicit substance use and misuse in a population-based sample of young adult twins. Addiction 114: 2229–2240. 10.1111/add.14750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong XD, Wang JY, Liu F, Yuan HH, Zhang WY, Guo YH, Jiang B. 2013. Gene polymorphisms of OPRM1 A118G and ABCB1 C3435T may influence opioid requirements in Chinese patients with cancer pain. Asian Pac J Cancer Prev 14: 2937–2943. 10.7314/APJCP.2013.14.5.2937 [DOI] [PubMed] [Google Scholar]
- Gorman AL, Elliott KJ, Inturrisi CE. 1997. The D- and L-isomers of methadone bind to the non-competitive site on the N-methyl-D-aspartate (NMDA) receptor in rat forebrain and spinal cord. Neurosci Lett 223: 5–8. 10.1016/S0304-3940(97)13391-2 [DOI] [PubMed] [Google Scholar]
- Haerian BS, Haerian MS. 2013. OPRM1 rs1799971 polymorphism and opioid dependence: evidence from a meta-analysis. Pharmacogenomics 14: 813–824. 10.2217/pgs.13.57 [DOI] [PubMed] [Google Scholar]
- Halikere A, Popova D, Scarnati MS, Hamod A, Swerdel MR, Moore JC, Tischfield JA, Hart RP, Pang ZP. 2019. Addiction associated N40D μ-opioid receptor variant modulates synaptic function in human neurons. Mol Psychiatry 10.1038/s41380-019-0507-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasvik E, Iordanova Schistad E, Grøvle L, Julsrud Haugen A, Roe C, Gjerstad J. 2014. Subjective health complaints in patients with lumbar radicular pain and disc herniation are associated with a sex–OPRM1 A118G polymorphism interaction: a prospective 1-year observational study. BMC Musculoskelet Disord 15: 161. 10.1186/1471-2474-15-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heddini U, Johannesson U, Grönbladh A, Nyberg F, Nilsson KW, Bohm-Starke N. 2014. A118G polymorphism in the µ-opioid receptor gene and levels of β-endorphin are associated with provoked vestibulodynia and pressure pain sensitivity. Scand J Pain 5: 10–16. 10.1016/j.sjpain.2013.10.004 [DOI] [PubMed] [Google Scholar]
- Henderson-Redmond AN, Yuill MB, Lowe TE, Kline AM, Zee ML, Guindon J, Morgan DJ. 2016. Morphine-induced antinociception and reward in “humanized” mice expressing the µ opioid receptor A118G polymorphism. Brain Res Bull 123: 5–12. 10.1016/j.brainresbull.2015.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas DE, Amick HR, Feltner C, Wines R, Shanahan E, Rowe CJ, Garbutt JC. 2014. Genetic polymorphisms and response to medications for alcohol use disorders: a systematic review and meta-analysis. Pharmacogenomics 15: 1687–1700. 10.2217/pgs.14.121 [DOI] [PubMed] [Google Scholar]
- Karoly HC, Stevens CJ, Magnan RE, Harlaar N, Hutchison KE, Bryan AD. 2012. Genetic influences on physiological and subjective responses to an aerobic exercise session among sedentary adults. J Cancer Epidemiol 2012: 540563. 10.1155/2012/540563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KM, Choi SW, Kim D, Lee J, Kim JW. 2019. Associations among the opioid receptor gene (OPRM1) A118G polymorphism, psychiatric symptoms, and quantitative EEG in Korean males with gambling disorder: a pilot study. J Behav Addict 8: 463–470. 10.1556/2006.8.2019.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikov Y, Gabovits B, Levin A, Veske A, Qin L, Dai F, Belfer I. 2013. Chronic pain after lower abdominal surgery: do catechol-O-methyl transferase/opioid receptor µ1 polymorphisms contribute? Mol Pain 9: 19. 10.1186/1744-8069-9-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller G, Zill P, Rujescu D, Ridinger M, Pogarell O, Fehr C, Wodarz N, Bondy B, Soyka M, Preuss UW. 2012. Possible association between OPRM1 genetic variance at the 118 locus and alcohol dependence in a large treatment sample: relationship to alcohol dependence symptoms. Alcohol Clin Exp Res 36: 1230–1236. 10.1111/j.1530-0277.2011.01714.x [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. 2007. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry 164: 1149–1159. 10.1176/appi.ajp.2007.05030503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. 1998. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend 51: 23–47. 10.1016/S0376-8716(98)00064-7 [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Garfield JW, Gutjahr CL, Giusti LM. 1976. Rifampin-induced methadone withdrawal. New Engl J Med 294: 1104–1106. 10.1056/NEJM197605132942008 [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Wardlaw SL, Hartman N, Raghunath J, Friedman J, Schneider B, Frantz AG. 1983. Circadian rhythms and levels of β-endorphin, ACTH, and cortisol during chronic methadone maintenance treatment in humans. Life Sci 33(Suppl 1): 409–411. 10.1016/0024-3205(83)90529-5 [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, LaForge KS. 2004. Genes associated with addiction: alcoholism, opiate, and cocaine addiction. Neuromolecular Med 5: 85–108. 10.1385/NMM:5:1:085 [DOI] [PubMed] [Google Scholar]
- Kroslak T, Laforge KS, Gianotti RJ, Ho A, Nielsen DA, Kreek MJ. 2007. The single nucleotide polymorphism A118G alters functional properties of the human µ opioid receptor. J Neurochem 103: 77–87. [DOI] [PubMed] [Google Scholar]
- Lancaster KE, Hetrick A, Jaquet A, Adedimeji A, Atwoli L, Colby DJ, Mayor AM, Parcesepe A, Syvertsen J. 2018. Substance use and universal access to HIV testing and treatment in sub-Saharan Africa: implications and research priorities. J Virus Erad 4: 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawford BR, Young RM, Noble EP, Sargent J, Rowell J, Shadforth S, Zhang X, Ritchie T. 2000. The D2 dopamine receptor A1 allele and opioid dependence: association with heroin use and response to methadone treatment. Am J Med Genet 96: 592–598. [DOI] [PubMed] [Google Scholar]
- Levran O, O'Hara K, Peles E, Li D, Barral S, Ray B, Borg L, Ott J, Adelson M, Kreek MJ. 2008. ABCB1 (MDR1) genetic variants are associated with methadone doses required for effective treatment of heroin dependence. Hum Mol Genet 17: 2219–2227. 10.1093/hmg/ddn122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O, Peles E, Hamon S, Randesi M, Zhao C, Zhang B, Adelson M, Kreek MJ. 2012. Nerve growth factor β polypeptide (NGFB) genetic variability: association with the methadone dose required for effective maintenance treatment. Pharmacogenomics J 12: 319–327. 10.1038/tpj.2011.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O, Peles E, Hamon S, Randesi M, Adelson M, Kreek MJ. 2013a. CYP2B6 SNPs are associated with methadone dose required for effective treatment of opioid addiction. Addict Biol 18: 709–716. 10.1111/j.1369-1600.2011.00349.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O, Peles E, Randesi M, Shu X, Ott J, Shen PH, Adelson M, Kreek MJ. 2013b. Association of genetic variation in pharmacodynamic factors with methadone dose required for effective treatment of opioid addiction. Pharmacogenomics 14: 755–768. 10.2217/pgs.13.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TK, Lumeng L, Doolittle DP. 1993. Selective breeding for alcohol preference and associated responses. Behav Genet 23: 163–170. 10.1007/BF01067421 [DOI] [PubMed] [Google Scholar]
- Li J, Wei Z, Zhang J, Hakonarson H, Cook-Sather SD. 2019. Candidate gene analyses for acute pain and morphine analgesia after pediatric day surgery: African American versus European Caucasian ancestry and dose prediction limits. Pharmacogenomics J 19: 570–581. 10.1038/s41397-019-0074-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Valverde N, López-Valverde A, Gómez de Diego R, Cieza-Borrella C, Ramírez JM, González-Sarmiento R. 2019. Genetic study in patients operated dentally and anesthetized with articaine-epinephrine. J Pain Res 12: 1371–1384. 10.2147/JPR.S193745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Isiegas C, Huang P, Liu-Chen LY, Lerman C, Blendy JA. 2009. Mouse model of OPRM1 (A118G) polymorphism has sex-specific effects on drug-mediated behavior. Proc Natl Acad Sci 106: 10847–10852. 10.1073/pnas.0901800106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud S, Thorsell A, Sommer WH, Heilig M, Holgate JK, Bartlett SE, Ruiz-Velasco V. 2011. Pharmacological consequence of the A118G µ opioid receptor polymorphism on morphine- and fentanyl-mediated modulation of Ca2+ channels in humanized mouse sensory neurons. Anesthesiology 115: 1054–1062. 10.1097/ALN.0b013e318231fc11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravarti A, et al. 2009. Finding the missing heritability of complex diseases. Nature 461: 747–753. 10.1038/nature08494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margarit C, Roca R, Inda MD, Muriel J, Ballester P, Moreu R, Conte AL, Nunez A, Morales D, Peiro AM. 2019. Genetic contribution in low back pain: a prospective genetic association study. Pain Pract 19: 836–847. 10.1111/papr.12816 [DOI] [PubMed] [Google Scholar]
- Mestek A, Hurley JH, Bye LS, Campbell AD, Chen Y, Tian M, Liu J, Schulman H, Yu L. 1995. The human µ opioid receptor: modulation of functional desensitization by calcium/calmodulin-dependent protein kinase and protein kinase C. J Neurosci 15: 2396–2406. 10.1523/jneurosci.15-03-02396.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R, Ray L, Justus A, Meyerson LA, Knopik VS, McGeary J, Monti PM. 2010. Initial evidence of an association between OPRM1 and adolescent alcohol misuse. Alcohol Clin Exp Res 34: 112–122. 10.1111/j.1530-0277.2009.01073.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil A. 1984. Affinities of some common opioid analgesics towards four binding sites in mouse brain. Naunyn Schmiedebergs Arch Pharmacol 328: 24–29. 10.1007/BF00496100 [DOI] [PubMed] [Google Scholar]
- Nobile B, Ramoz N, Jaussent I, Gorwood P, Olié E, Castroman JL, Guillaume S, Courtet P. 2019. Polymorphism A118G of opioid receptor µ1 (OPRM1) is associated with emergence of suicidal ideation at antidepressant onset in a large naturalistic cohort of depressed outpatients. Sci Rep 9: 2569. 10.1038/s41598-019-39622-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen MB, Jacobsen LM, Schistad EI, Pedersen LM, Rygh LJ, Roe C, Gjerstad J. 2012. Pain intensity the first year after lumbar disc herniation is associated with the A118G polymorphism in the opioid receptor µ1 gene: evidence of a sex and genotype interaction. J Neurosci 32: 9831–9834. 10.1523/jneurosci.1742-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peciña M, Love T, Stohler CS, Goldman D, Zubieta JK. 2015. Effects of the µ opioid receptor polymorphism (OPRM1 A118G) on pain regulation, placebo effects and associated personality trait measures. Neuropsychopharmacolgy 40: 957–965. 10.1038/npp.2014.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peles E, Levran O, Randesi M, Ott J, Kreek MJ, Adelson M. 2019. Genetic variant in the CRH-binding protein gene (CRHBP) is associated with cessation of cocaine use in methadone maintenance patients with opioid addiction. J Addict Med 13: 430–435. [DOI] [PubMed] [Google Scholar]
- Pfeifer P, Sariyar M, Eggermann T, Zerres K, Vernaleken I, Tuscher O, Fehr C. 2015. Alcohol consumption in healthy OPRM1 G allele carriers and its association with impulsive behavior. Alcohol Alcohol 50: 379–384. 10.1093/alcalc/agv019 [DOI] [PubMed] [Google Scholar]
- Proudnikov D, Randesi M, Levran O, Crystal H, Dorn M, Ott J, Ho A, Kreek MJ. 2012. Association of polymorphisms of the µ opioid receptor gene with the severity of HIV infection and response to HIV treatment. J Infect Dis 205: 1745–1756. 10.1093/infdis/jis264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed B, Butelman ER, Yuferov V, Randesi M, Kreek MJ. 2014. Genetics of opiate addiction. Curr Psychiatry Rep 16: 504. 10.1007/s11920-014-0504-6 [DOI] [PubMed] [Google Scholar]
- Rouvinen-Lagerström N, Lahti J, Alho H, Kovanen L, Aalto M, Partonen T, Silander K, Sinclair D, Raikkonen K, Eriksson JG, et al. 2013. µ-Opioid receptor gene (OPRM1) polymorphism A118G: lack of association in Finnish populations with alcohol dependence or alcohol consumption. Alcohol Alcohol 48: 519–525. 10.1093/alcalc/agt050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchat SM, Girard M, Weisnagel SJ, Bouchard C, Vohl MC, Pérusse L. 2008. Association between µ-opioid receptor-1 102T > C polymorphism and intermediate type 2 diabetes phenotypes: results from the Quebec family study (QFS). Clin Exp Pharmacol Physiol 35: 1018–1022. 10.1111/j.1440-1681.2008.04972.x [DOI] [PubMed] [Google Scholar]
- Samochowiec A, Samochowiec J, Pelka-Wysiecka J, Kucharska-Mazur J, Grochans E, Jablonski M, Bienkowski P, Murawiec S, Malecka I, Mak M, et al. 2019. The role of OPRM1 polymorphism in the etiology of alcoholism. Adv Clin Exp Med 28: 199–202. 10.17219/acem/78592 [DOI] [PubMed] [Google Scholar]
- Schluger JH, Ho A, Borg L, Porter M, Maniar S, Gunduz M, Perret G, King A, Kreek MJ. 1998. Nalmefene causes greater hypothalamic-pituitary-adrenal axis activation than naloxone in normal volunteers: implications for the treatment of alcoholism. Alcohol Clin Exp Res 22: 1430–1436. 10.1111/j.1530-0277.1998.tb03931.x [DOI] [PubMed] [Google Scholar]
- Schwantes-An TH, Zhang J, Chen LS, Hartz SM, Culverhouse RC, Chen X, Coon H, Frank J, Kamens HM, Konte B, et al. 2016. Association of the OPRM1 variant rs1799971 (A118G) with non-specific liability to substance dependence in a collaborative de novo meta-analysis of European-ancestry cohorts. Behav Genet 46: 151–169. 10.1007/s10519-015-9737-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šerý O, Přikryl R, Častulík L, St'astný F. 2010. A118G polymorphism of OPRM1 gene is associated with schizophrenia. J Mol Neurosci 41: 219–222. 10.1007/s12031-010-9327-z [DOI] [PubMed] [Google Scholar]
- Smith AH, Jensen KP, Li J, Nunez Y, Farrer LA, Hakonarson H, Cook-Sather SD, Kranzler HR, Gelernter J. 2017. Genome-wide association study of therapeutic opioid dosing identifies a novel locus upstream of OPRM1. Mol Psychiatry 22: 346–352. 10.1038/mp.2016.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solak O, Erdoğan MO, Yildiz H, Ulaşli AM, Yaman F, Terzi ES, Ulu S, Dundar U, Solak M. 2014. Assessment of opioid receptor µ1 gene A118G polymorphism and its association with pain intensity in patients with fibromyalgia. Rheumatol Int 34: 1257–1261. 10.1007/s00296-014-2995-1 [DOI] [PubMed] [Google Scholar]
- Sugino S, Hayase T, Higuchi M, Saito K, Moriya H, Kumeta Y, Kurosawa N, Namiki A, Janicki PK. 2014. Association of µ-opioid receptor gene (OPRM1) haplotypes with postoperative nausea and vomiting. Exp Brain Res 232: 2627–2635. 10.1007/s00221-014-3987-9 [DOI] [PubMed] [Google Scholar]
- Szentkereszty-Kovács Z, Fiatal S, Szegedi A, Kovács D, Janka E, Herszényi K, Holló P, Nikamo P, Ståhle M, Remenyik E, et al. 2019. The prevalence of ADH1B and OPRM1 alleles predisposing for alcohol consumption are increased in the Hungarian psoriasis population. Arch Dermatol Res 311: 435–442. 10.1007/s00403-019-01915-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto CY, Tang NL, Lee DT, Stadlin A. 2001. Association between µ opioid receptor gene polymorphisms and Chinese heroin addicts. Neuroreport 12: 1103–1106. 10.1097/00001756-200105080-00011 [DOI] [PubMed] [Google Scholar]
- Tong TG, Pond SM, Kreek MJ, Jaffery NF, Benowitz NL. 1981. Phenytoin-induced methadone withdrawal. Ann Intern Med 94: 349–351. 10.7326/0003-4819-94-3-349 [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, Meyer JM, Toomey R, Faraone SV, Eaves L. 1996. Genetic influences on DSM-III-R drug abuse and dependence: a study of 3,372 twin pairs. Am J Med Genet 67: 473–477. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, True W, Lin N, Toomey R, Eaves L. 1998. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch Gen Psychiatry 55: 967–972. 10.1001/archpsyc.55.11.967 [DOI] [PubMed] [Google Scholar]
- Wand GS, McCaul M, Yang X, Reynolds J, Gotjen D, Lee S, Ali A. 2002. The µ-opioid receptor gene polymorphism (A118G) alters HPA axis activation induced by opioid receptor blockade. Neuropsychopharmacolgy 26: 106–114. 10.1016/S0893-133X(01)00294-9 [DOI] [PubMed] [Google Scholar]
- Wang JB, Johnson PS, Persico AM, Hawkins AL, Griffin CA, Uhl GR. 1994. Human µ opiate receptor. cDNA and genomic clones, pharmacologic characterization and chromosomal assignment. FEBS Lett 338: 217–222. 10.1016/0014-5793(94)80368-4 [DOI] [PubMed] [Google Scholar]
- Wang SC, Ho IK, Tsou HH, Tian JN, Hsiao CF, Chen CH, Tan HK, Lin L, Wu CS, Su LW, et al. 2011. CYP2B6 polymorphisms influence the plasma concentration and clearance of the methadone S-enantiomer. J Clin Psychopharmacol 31: 463–469. 10.1097/JCP.0b013e318222b5dd [DOI] [PubMed] [Google Scholar]
- Wang XS, Song HB, Chen S, Zhang W, Liu JQ, Huang C, Wang HR, Chen Y, Chu Q. 2015. Association of single nucleotide polymorphisms of ABCB1, OPRM1 and COMT with pain perception in cancer patients. J Huazhong Univ Sci Technol Med Sci 35: 752–758. 10.1007/s11596-015-1502-6 [DOI] [PubMed] [Google Scholar]
- Weerts EM, McCaul ME, Kuwabara H, Yang X, Xu X, Dannals RF, Frost JJ, Wong DF, Wand GS. 2013. Influence of OPRM1 Asn40Asp variant (A118G) on [11C]carfentanil binding potential: preliminary findings in human subjects. Int J Neuropsychopharmacol 16: 47–53. 10.1017/S146114571200017X [DOI] [PubMed] [Google Scholar]
- Wonkam A, Mnika K, Ngo Bitoungui VJ, Chetcha Chemegni B, Chimusa ER, Dandara C, Kengne AP. 2018. Clinical and genetic factors are associated with pain and hospitalisation rates in sickle cell anaemia in Cameroon. Br J Haematol 180: 134–146. 10.1111/bjh.15011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Zhang F, Zhang DD, Chen XD, Lu M, Lin RY, Wen H, Jin L, Wang XF. 2009. OPRM1 gene is associated with BMI in Uyghur population. Obesity (Silver Spring) 17: 121–125. 10.1038/oby.2008.504 [DOI] [PubMed] [Google Scholar]
- Zahari Z, Lee CS, Ibrahim MA, Musa N, Yasin MAM, Lee YY, Tan SC, Mohamad N, Ismail R. 2015. The opposing roles of IVS2 + 691 CC genotype and AC/AG diplotype of 118A > G and IVS2 + 691G > C of OPRM1 polymorphisms in cold pain tolerance among opioid-dependent Malay males on methadone therapy. Pain Ther 4: 179–196. 10.1007/s40122-015-0041-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang D, Johnson AD, Papp AC, Sadee W. 2005. Allelic expression imbalance of human µ opioid receptor (OPRM1) caused by variant A118G. J Biol Chem 280: 32618–32624. 10.1074/jbc.M504942200 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Picetti R, Butelman ER, Ho A, Blendy JA, Kreek MJ. 2015. Mouse model of the OPRM1 (A118G) polymorphism: differential heroin self-administration behavior compared with wild-type mice. Neuropsychopharmacology 40: 1091–1100. 10.1038/npp.2014.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Lv B, Zhao X, Zhang Y. 2019. Effects of OPRM1 and ABCB1 gene polymorphisms on the analgesic effect and dose of sufentanil after thoracoscopic-assisted radical resection of lung cancer. Biosci Rep 39: BSR20181211. 10.1042/BSR20181211 [DOI] [PMC free article] [PubMed] [Google Scholar]