Abstract
Aims
Periprosthetic joint infection (PJI) is a devastating complication following total knee arthroplasty (TKA). Two-stage revision has traditionally been considered the gold standard of treatment for established infection, but increasing evidence is emerging in support of one-stage exchange for selected patients. The objective of this study was to determine the outcomes of single-stage revision TKA for PJI, with mid-term follow-up.
Methods
A total of 84 patients, with a mean age of 68 years (36 to 92), underwent single-stage revision TKA for confirmed PJI at a single institution between 2006 and 2016. In all, 37 patients (44%) were treated for an infected primary TKA, while the majority presented with infected revisions: 31 had undergone one previous revision (36.9%) and 16 had multiple prior revisions (19.1%). Contraindications to single-stage exchange included systemic sepsis, extensive bone or soft-tissue loss, extensor mechanism failure, or if primary wound closure was unlikely to be achievable. Patients were not excluded for culture-negative PJI or the presence of a sinus.
Results
Overall, 76 patients (90.5%) were infection-free at a mean follow-up of seven years, with eight reinfections (9.5%). Culture-negative PJI was not associated with a higher reinfection rate (p = 0.343). However, there was a significantly higher rate of recurrence in patients with polymicrobial infections (p = 0.003). The mean Oxford Knee Score (OKS) improved from 18.7 (SD 8.7) preoperatively to 33.8 (SD 9.7) at six months postoperatively (p < 0.001). The Kaplan-Meier implant survival rate for all causes of reoperation, including reinfection and aseptic failure, was 95.2% at one year (95% confidence interval (CI) 87.7 to 98.2), 83.5% at five years (95% CI 73.2 to 90.3), and 78.9% at 12 years (95% CI 66.8 to 87.2).
Conclusion
One-stage exchange, using a strict debridement protocol and multidisciplinary input, is an effective treatment option for the infected TKA. This is the largest single-surgeon series of consecutive cases reported to date, with broad inclusion criteria.
Cite this article: Bone Jt Open 2021;2(5):305–313.
Keywords: Knee arthroplasty, Periprosthetic infection, Debridement, Single-stage, One-stage, Revision, Re-revision
Introduction
Periprosthetic joint infection (PJI) is a devastating complication following arthroplasty, and is currently the third most common indication for revision TKA in the UK.1 Registry data indicate that around 1.03% primary TKAs are revised for infection.2 However, the increasing incidence of both primary and revision TKA means that the burden of PJI also continues to rise.3
Two-stage revision TKA, originally described by Insall et al4 in 1983, has traditionally been considered the gold standard procedure for established PJI. It involves explantation, debridement, and the insertion of an antibiotic-loaded spacer in the first stage, followed by a course of systemic antibiotics—typically over several weeks—before the second stage, where further debridement is undertaken and the spacer is exchanged for a definitive prosthesis.
Single-stage revision was initially performed at the Endo-Klinik for infected hip arthroplasties,5 and subsequently described by Freeman et al6 in 1985 for the infected TKA. While the published success rates for one- versus two-stage revision TKA vary quite considerably, there is no superiority in terms of reinfection rate with respect to either method in the current literature.7-13 Furthermore, no randomized controlled trial (RCT) has been performed to date to compare these, and there is wide heterogeneity in patient demographic data, selection criteria, microbiology, and surgical techniques across existing studies. As PJI has significant physical, psychological, and economic impacts, there are inherent advantages to performing single-stage revision surgery, including reduced costs, less time in hospital, decreased morbidity, and greater patient satisfaction.14,15
The primary aim of this study was to report the outcomes of single-stage revision TKA for PJI in terms of reinfection, using a standardized debridement protocol and multidisciplinary input, with relatively broad patient selection criteria. The secondary aims were to analyze implant survival, characterize the effect of causative organisms with respect to treatment success or failure, and to assess functional status pre- and postoperatively, using patient-reported outcome measures (PROMs). This study presents the largest single-surgeon series of consecutive cases reported to date and is intended to supplement a growing evidence base for one-stage exchange.
Methods
In this study, we retrospectively reviewed data for 84 consecutive patients (53 males, 31 females) with a mean age of 68 years (36 to 92) who underwent single-stage revision TKA for PJI at Cardiff and Vale Orthopaedic Centre (UK) between December 2006 and October 2016. Overall, 37 patients (44%) presented with an infected primary TKA, while the majority of patients (56%, n = 47) were referred to our tertiary centre with infected TKA revisions. All patients had received previous courses of antibiotics, either within the community or at a different institution, prior to being referred for investigation or treatment of PJI. Preoperative demographic data are presented in Table I.
Table I.
Preoperative patient characteristics.
| Characteristic | Number |
|---|---|
| Sex, M:F | 53/31 |
| Mean age, yrs (SD; range) | 68 (11.4; 36 to 92) |
| Operated knee, L:R | 41/43 |
| Primary TKA (including four complex primary procedures), n (%) | 37 (44) |
| 1 previous revision, n (%) | 31 (36.9) |
| 2 previous revisions, n (%) | 14 (16.7) |
| 3 previous revisions, n (%) | 1 (1.2) |
| 4 previous revisions, n (%) | 1 (1.2) |
| ASA grade, n (%) | |
| 1 | 5 (6) |
| 2 | 47 (56) |
| 3 | 29 (34.5) |
| 4 | 3 (3.5) |
ASA, American Society of Anesthesiologists; SD, standard deviation; TKA, total knee arthroplasty.
The diagnosis of PJI remains challenging, and there are several definitions according to the criteria established by the Musculoskeletal Infection Society (MSIS),16 the Infectious Diseases Society of America (IDSA),17 and the International Consensus Meetings (ICM) in 2013 and 2018.18,19 Although criteria have been evolving with the introduction of new tests, such as alpha-defensin and next-generation sequencing,20,21 there is no test with complete sensitivity or specificity. All cases in this series, however, retrospectively fulfilled the ICM 2013 definition of PJI.18
Patients were assessed with a detailed history, physical examination, blood tests (including full blood count (FBC), serum ESR, serum CRP, and blood cultures), and radiology (radiographs and nuclear medicine imaging, if required, with bone scans or white cell scintigraphy). Any antibiotics were withheld for a minimum of two weeks before joint aspiration was performed under sterile conditions. Arthroscopic tissue biopsy was undertaken only in cases where synovial fluid culture was negative for organisms. Cases were discussed preoperatively at multidisciplinary team (MDT) meetings, which included the input of microbiology and anaesthetics.
All procedures were performed by the senior author (RMJ) and, in every case, using a standardized debridement protocol, which incorporates principles developed by Lautenbach.22,23 The operation is a ‘true’ one-stage procedure, with explantation, debridement, and reconstruction carried out sequentially without any breaks. This approach therefore differs somewhat from the ‘two-in-one procedure’ that has been described in the literature.24,25
Patients were excluded from one-stage exchange if they were systemically septic, if the soft-tissue envelope was considered at risk and primary wound closure was not likely to be achievable, if massive bone resection was required, or if there was disruption of the extensor mechanism. Patients were not excluded for culture-negative PJI or the presence of a sinus. During the study period, 28 additional patients did not meet the inclusion criteria and required alternative procedures, representing a quarter of cases that were referred to the senior author (RMJ) for surgical management of an infected TKA. Debridement, antibiotics and implant retention (DAIR) was undertaken in six patients, two-stage revision was undertaken in 17 patients, and knee arthrodesis was performed in five patients.26
Surgical technique
Debridement is paramount in the treatment of the infected TKA. The exposure is through an extensile approach (Figure 1), which generally incorporates a previous longitudinal midline incision that can be extended proximally and distally as required. Any sinuses in the line of incision are excised (Figure 2), while isolated sinuses elsewhere are curetted with excision of the deep sinus tract. A medial parapatellar arthrotomy is undertaken and the medial and lateral gutters are developed from within the capsule. A wide osteotome is used to lift the extensor mechanism off the femoral surface, while protecting the periosteal layer.
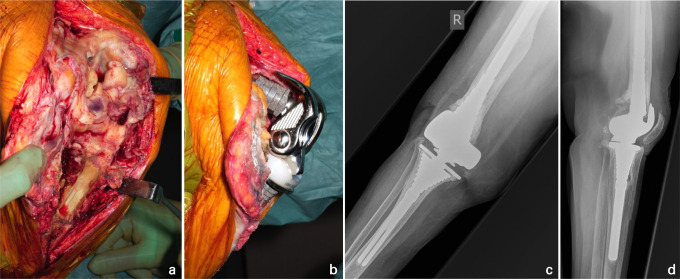
Fig. 1.
a) Intraoperative clinical photographs demonstrate an extensile approach—incorporating a tibial crest osteotomy (TCO)—to explant an infected complex primary total knee arthroplasty in a 76-year-old female, revealing extensive biofilm. b) Following debridement, cementless reimplantation was undertaken with a hinged prosthesis, as the collateral ligaments were unsalvageable. c) Anteroposterior and d) lateral right knee radiographs at four years postoperatively show the TCO has healed and the components appear well-fixed.
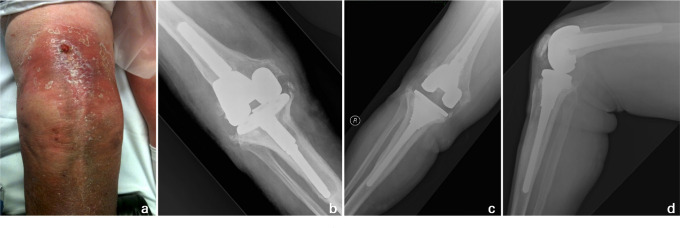
Fig. 2.
a) Preoperative clinical photograph demonstrates a sinus in the line of a previous scar with surrounding erythema. b) The corresponding anteroposterior right knee radiograph shows extensive osteolysis around the underlying revision total knee arthroplasty prosthesis. This 78-year-old female underwent single-stage re-revision. Excision of the collateral ligaments was not required during the debridement in this particular case, and increased constraint was accordingly unnecessary. Stable zonal fixation with cementless components was achieved, as shown on c) anteroposterior and d) lateral right knee radiographs at three years postoperatively.
A tibial crest osteotomy (TCO) was used in all cases to allow unimpeded access to the joint (Figures 1 and 2), protect the extensor mechanism, and encourage bone-on-bone healing.27 It is based upon Whiteside’s principles of using low-energy osteotomy, and provides a larger surface area for healing than with a conventional tibial tubercle osteotomy.28 The technique is further modified with the use of intraosseous sutures to repair the osteotomy at the end of the revision, rather than screw fixation or cerclage wiring, which avoids symptomatic hardware.27
While sharp rigid osteotomes are used for the TCO and the removal of any necrotic bone, flexible osteotomes are an option for explantation at the cement-bone interface. Surgical debridement involves a thorough synovectomy and excision of all visible infected membrane or biofilm (Figure 1) with sharp dissection. Multiple samples are obtained with separate, non-contaminated instruments, and sent urgently for laboratory processing. These comprise a joint aspirate, synovial samples, femoral joint surface tissue, tibial joint surface tissue, tibial canal membrane, and femoral canal membrane.29
Mechanical debridement is undertaken in a compartmental and cyclical fashion, with any surfaces cleared of any residual membrane, nonviable tissue, and remaining cement using curettage and rongeurs. The intramedullary canals are reamed under power successively, before sterile Normal Saline is used as powered pulse lavage to clear residual debris and, more importantly, to create oedema in any remaining membrane and biofilm. A second cycle of curettage and reaming is performed to remove the residual oedematous membrane and biofilm, with a third cycle undertaken as clinically indicated.
Chemical debridement is the next stage, in order to create a hostile environment for pathogens, but with low toxicity to host tissues. Prior to the insertion of new implants, instruments and surgical gloves are changed; meanwhile the operative field is soaked for around ten to 20 minutes with an antimicrobial agent. In October 2013, our technique evolved from using povidone-iodine solution to 3% acetic acid.30 Other sterile antimicrobials, such as chlorhexidine or hydrogen peroxide, are widely used alternatives.
Restoration of joint line, reconstruction of bone loss, and level of constraint were judged on a case-by-case basis and according to intraoperative findings. No bone grafts were used and all patients underwent uncemented or hybrid fixation of tibial and femoral components (DePuy Synthes Knee Revision Portfolio; DePuy Orthopaedics, USA), according to the principles of zonal fixation.31 A hinged prosthesis was only required in around half our cases (Figure 1), as the collateral ligaments are not routinely excised as part of the debridement protocol (Figure 2). Primary wound closure was achieved for all patients in this series and no drains were used.
Patients were started on a short postoperative course of intravenous antibiotics—usually five to seven days of teicoplanin and meropenem—and thereafter changed to dual oral antibiotics, tailored to each patient as per culture sensitivities, to complete a six-week course. Antibiotic management was discussed at weekly MDT meetings with input from a musculoskeletal microbiologist. Patients received standardized postoperative follow-up at six weeks, six months, and then annually. The Oxford Knee Score (OKS) was recorded for patients preoperatively and at six months postoperatively.32,33 At each review, the patient was assessed clinically with an examination of the knee joint, radiographs, and blood tests. If any patient was suspected of having an infection recurrence, they underwent additional sampling with blood cultures, aspiration of the joint in theatre under aseptic conditions, and further tests (such as synovial fluid biomarkers or nuclear medicine imaging) as clinically required.
Statistical analysis
Data were analyzed using statistical software (GraphPad Prism v. 9.0.0; USA). Fisher’s exact test was performed to examine any association between binary categorical variables, such as culture-negative/-positive PJI and infection recurrence. The Cochran-Armitage test for trend was performed to compare ordinal variables, such as the preoperative American Society of Anesthesiologists (ASA) physical status classification,34 with binary outcomes, such as reoperation. A paired t-test was used to investigate the changes between the preoperative and postoperative functional scores. For all statistical tests, a p-value < 0.05 was considered significant. Kaplan-Meier curves were generated to estimate implant survival, with infection recurrence and reoperation for all causes as the endpoints, using 95% confidence intervals (CIs).
Results
The mean follow-up was seven years (one to 12), and no patients were lost to follow-up. Four patients died from unrelated causes one year postoperatively, and follow-up of at least two years was achieved in the remaining 80 patients. In total, 76 patients (90.5%) were infection-free at most recent review, while there were eight recurrences (9.5%) of PJI (Table II). Three of these patients underwent knee arthrodesis as a limb salvage procedure, two underwent repeat single-stage revision TKA, and one underwent two-stage re-revision. DAIR was successfully undertaken in one patient with an early postoperative recurrence, while another patient with a late reinfection also underwent DAIR (having declined any further re-revision procedures) and was treated with long-term suppressive antibiotic therapy. No amputations were undertaken. Seven patients (8.3%) in our cohort required aseptic re-revision TKA, which comprised six single-stage re-revisions for instability or loosening, and one implant arthrodesis due to inadequate bone stock and extensor mechanism failure.
Table II.
Details of recurrent infection cases following single-stage revision total knee arthroplasty.
| Case | Age, yrs/sex | Previous implant/number of revisions | Initial infective organism(s) cultured | Time to recurrence, mths | Infective organism(s) cultured following reinfection | Management |
|---|---|---|---|---|---|---|
| 1 | 76 F | Primary TKA | CNS | 98 | MSSA | DAIR, Long-term antibiotic suppression |
| 2 | 56 F | Revision TKA (R1) | MSSA | 87 | Polymicrobial (Pseudomonas aeruginosa, Enterococcus faecalis, Enterococcus faecium) | Repeat single-stage revision TKA |
| 3 | 77 M | Revision TKA (R1) | Polymicrobial (CNS, Escherichia coli) | 1 | Polymicrobial (E. coli, Acinetobacter baumannii) | DAIR |
| 4 | 76 M | Complex primary TKA (previous trauma) | Polymicrobial (CNS, E. faecalis, Pseudomonas aeruginosa, Corynebacterium) | 10 | Polymicrobial (CNS, P. aeruginosa, Enterobacter cloacae) | Repeat single-stage revision TKA |
| 5 | 89 F | Revision TKA (R1) | Polymicrobial (CNS, MSSA, Mixed coliforms) | 27 | Polymicrobial (CNS, MSSA) | Arthrodesis |
| 6 | 77 M | Revision TKA (R1) | Polymicrobial (CNS, MSSA, Corynebacterium) | 24 | Polymicrobial (CNS, MSSA) | Arthrodesis |
| 7 | 69 M | Multi-revised TKA (R4) | Polymicrobial (CNS, Cutibacterium acnes) | 35 | Polymicrobial (CNS, Aspergillus niger) | Two-stage revision TKA |
| 8 | 53 M | Complex primary TKA (previous extensor mechanism surgery with skin graft) | Polymicrobial (CNS, MSSA) | 58 | MRSA | Arthrodesis |
CNS, coagulase-negative Staphylococcus ; DAIR, debridement, antibiotics and implant retention; MRSA, methicillin-resistant Staphylococcus aureus ; MSSA, methicillin-sensitive Staphylococcus aureus; TKA, total knee arthroplasty.
Polymicrobial infections accounted for 25% of cases, while no organism was found in 19% of patients (Table III). The most commonly identified organism was coagulase-negative Staphylococcus, followed by Staphylococcus aureus. Gram-negative organisms more frequently contributed to polymicrobial infections rather than monomicrobial cases (Table IV). We compared microbiology results with outcome in terms of reinfection. Culture-negative PJI did not show any significant association with outcome (p = 0.343, Fisher's exact test). However, there was a statistically significantly increased rate of recurrence among patients with polymicrobial infections (p = 0.003, Fisher's exact test).
Table III.
Summary of microbiology results.
| Organism | Patients, n (%) |
|---|---|
| CNS | 28 (33.3) |
| MSSA | 8 (9.5) |
| MRSA | 3 (3.6) |
| Streptococcus | 3 (3.6) |
| Enterococcus | 1 (1.2) |
| Pseudomonas aeruginosa | 1 (1.2) |
| Proteus mirabilis | 1 (1.2) |
| Serratia marcescens | 1 (1.2) |
| Granulicatella adiacens | 1 (1.2) |
| Polymicrobial | 21 (25) |
| No growth | 16 (19) |
CNS, Coagulase-negative Staphylococcus; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus
Table IV.
Frequency of organisms identified among all patients with positive microbiology cultures.
| Organism | Frequency of cases, n |
|---|---|
| Gram-positive cocci | |
| CNS | 42 |
| MSSA | 15 |
| MRSA | 3 |
| Enterococcus faecalis | 7 |
| Enterococcus faecium | 2 |
| Alpha-haemolytic Streptococcus | 3 |
| Beta-haemolytic Streptococcus | 2 |
| Micrococcus luteus* | 2 |
| Granulicatella adiacens | 1 |
| Gram-positive bacilli | |
| Corynebacterium* | 2 |
| Cutibacterium acnes* | 2 |
| Gram-negative bacilli | |
| Pseudomonas aeruginosa | 3 |
| Escherichia coli* | 2 |
| Klebsiella pneumoniae* | 2 |
| Proteus mirabilis | 1 |
| Serratia marcescens | 1 |
| Serratia proteamaculans* | 1 |
| Achromobacter xylosoxidans* | 1 |
| Enterobacter cloacae* | 1 |
| Klebsiella (Enterobacter) aerogenes* | 1 |
| Fungi | |
| Aspergillus fumigatus* | 1 |
| Candida tropicalis* | 1 |
These organisms were exclusively cultured among polymicrobial infections.
CNS, Coagulase-negative Staphylococcus; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus.
Increasing numbers of previous revision TKA procedures did not demonstrate a significant association with infection recurrence alone (p = 0.451, Cochran-Armitage test), but there was a statistically significant association with reoperation due to all causes (reinfection and aseptic failure collectively, p = 0.0151; Cochran-Armitage test). The preoperative ASA score did not demonstrate a significant association with infection recurrence (p = 0.219, Cochran-Armitage test), or reoperation for all causes (p = 0.875, Cochran-Armitage test), within our cohort.
PROMs improved following single-stage revision TKA for infection. The mean OKS showed a significant difference from 18.7 (SD 8.7; 1 to 31) preoperatively to 33.8 (SD 9.7; 16 to 47) at six months postoperatively (p < 0.001, paired t-test).
The Kaplan-Meier implant survival rate for infection recurrence alone (Figure 3) was 97.6% at one year (95% CI 90.8 to 99.4), 91.1% at five years (95% CI 81.8 to 95.6), and 88.3% at 12 years (95% CI 76.6 to 94.3). The Kaplan-Meier implant survival rate for all causes of reoperation (Figure 4) was 95.2% at one year (95% CI 87.7 to 98.2), 83.5% at five years (95% CI 73.2 to 90.3), and 78.9% at 12 years (95% CI 66.8 to 87.2).
Fig. 3.
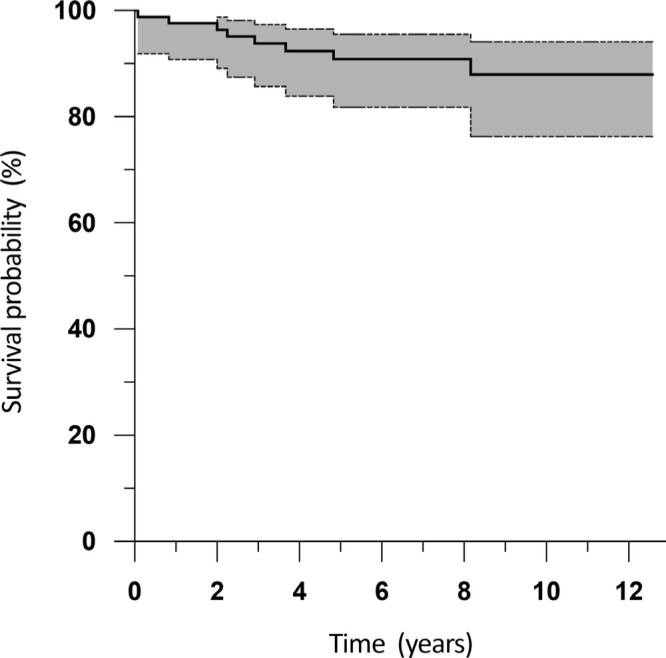
Kaplan-Meier survival analysis following single-stage revision total knee arthroplasty for infection, with infection recurrence as the endpoint. The shaded area indicates the 95% confidence interval.
Fig. 4.
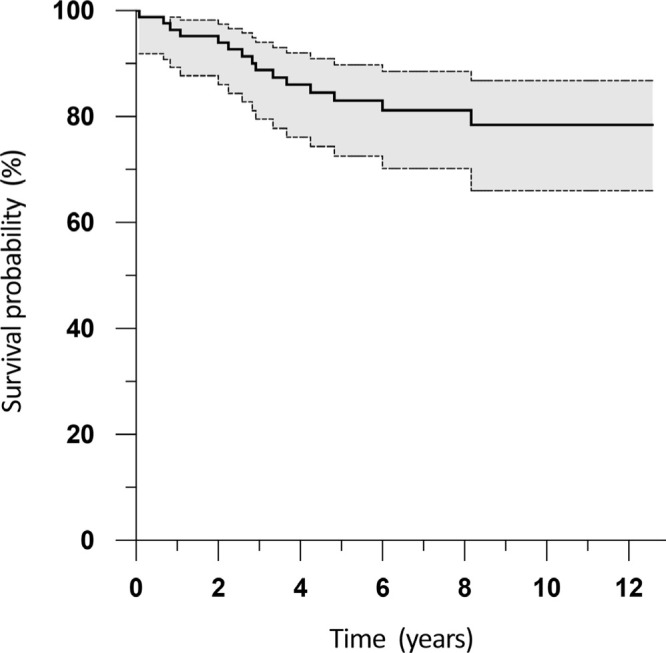
Kaplan-Meier survival analysis following single-stage revision total knee arthroplasty for infection, with reoperation for any indication (including aseptic failure or reinfection) as the endpoint. The shaded area indicates the 95% confidence interval.
Discussion
The management of PJI is complex and reflects the multifactorial nature of the problem. While this study advocates single-stage revision, we recognize the role of two-stage procedures in many cases according to surgical, microbiological, or patient factors. Reported success rates following single-stage revision for the infected TKA represent diverse selection criteria and techniques among different centres and different surgeons, in addition to the learning curve involved in managing PJI with a one-stage exchange.
An early study on the outcomes of single-stage revision TKA for infection from the Endo-Klinik reported an eradication rate of 73% in 104 patients.35 The following decade, Buechel36 reported a success rate of 90.9% in 22 patients. More recently, Parkinson et al24 reported no cases of recurrent infection in 12 patients treated with a ‘two-in-one’ revision at mean two years follow-up, while a study from NHS Fife reported success in 96.2% of patients following the ‘two-in-one’ technique, with statistically significant improvements in functional outcomes.25 Zahar et al37 reported a success rate of 93% in 70 patients treated at the Endo-Klinik with one-stage exchange arthroplasty and a rotating hinge implant, with minimum nine years’ follow-up. In contrast to our study, however, they excluded patients with culture-negative PJI, routinely performed a subvastus approach (as opposed to TCO) for exposure, aggressively debrided the collateral ligaments (requiring a hinged prosthesis), and used antibiotic-loaded polymethylmethacrylate (PMMA) cement in each case. The high number of re-revisions in our series also differentiates from previous studies.
Several reviews have been performed in recent years to assimilate the evidence on single-stage revision TKA for infection. A systematic review of five cohort studies by Nagra et al8 found no significant differences in reinfection rates between one- and two-stage procedures. A meta-analysis by Kunutsor et al9 compared ten single-stage studies with 108 two-stage studies for generally unselected patients, and found similar reinfection rates of 7.6% and 8.8% respectively, although they mention an inherent limitation of heterogeneity. A recent PRISMA systematic review of 16 single-stage articles reported a mean reinfection rate of 15.42%.10 While acknowledging that studies with careful patient selection criteria have demonstrated good results, Yaghmour et al10 also highlighted the practical benefits of a single procedure in higher-risk patients (such as avoiding two exposures to anaesthesia and less morbidity associated with a temporary spacer). Pangaud et al11 recently performed a systematic review of 14 single-stage articles involving 687 patients and 18 two-stage articles involving 1,086 patients. The mean reinfection rate was 12.9% for single-stage revision and 15.2% for two-stage revision, with similar function across both groups.
The selection criteria for single-stage revision in PJI have traditionally been very strict, reflecting the complexity of cases and the need for a favourable environment in which to implant a new prosthesis. A number of the cases included in this study would not have been considered suitable for a one-stage exchange at many other institutions, especially patients with culture-negative PJI.13,37 There are several potential reasons that an infective organism might not be identified preoperatively; these may include successive courses of antibiotics, an insufficient period without antibiotics before sampling, inadequate culture times, low-virulence organisms, limitations of sampling techniques, or the lack of diagnostic facilities for rare organisms. In our experience, none of these reasons were sufficient to prohibit the use of a single-stage revision, as the debridement was considered imperative in every case, whether the organism was identified or not. Culture-negative PJI therefore did not influence our surgical technique. Furthermore, a short course of broad-spectrum intravenous antibiotics was generally administered to all patients after surgery—even if a pathogen had been identified preoperatively—to account for the possibility of other (perhaps less virulent) organisms being cultured from intraoperative tissue samples.
The most commonly identified organisms in our series (Table IV) were consistent with many other studies on PJI.12,13,38-40 Interestingly, we had no reinfections in patients with culture-negative PJI. A recent meta-analysis of eight articles indicated that the outcomes of culture-negative PJI are not worse than culture-positive infections, although two-stage exchange was the selected technique in all included studies.41 In an earlier series of 60 consecutive patients undergoing two-stage revision TKA for infection at our own institution, 43% chose not to undergo a second-stage procedure as the initial debridement and interval prosthesis had eradicated the infection, resolved pain, and achieved good functional outcome.42 In all five patients (8%) who developed recurrent infection in that series, the organism was known, while there were no reinfections among six patients with culture-negative PJI (10%). A retrospective cohort study from France comparing the outcomes of one-stage revision TKA between selected and unselected patients with PJI reported no significant differences between the two groups in terms of recurrences.40
In the present study, polymicrobial infections were associated with a statistically significantly worse outcome. This finding is supported by the Second ICM in 2018, where a strong consensus (97%) was reached that polymicrobial PJIs generally demonstrate inferior treatment results when compared to single organism infections.43 A more recent study by Kavolus et al44 likewise found that polymicrobial infections in total hip arthroplasties were associated with a significantly lower treatment success rate. It may be that a two-stage procedure is more favourable in these cases. Two patients in our series had polymicrobial infections involving fungal pathogens (Table IV). Although both of these infections were successfully cleared, the evidence regarding fungal PJI indicates that they are often more difficult to treat than bacterial cases, and can be associated with systemic compromising factors in the host.45 While Klatte et al46 have suggested that single-stage exchange is feasible in such a scenario, the majority of existing studies advocate two-stage revision for fungal PJI.38,45,47
Zmistowski et al48 investigated whether recurrent PJI is a result of persistent infection with the same organism or new infections, by comparing microbiology cultures at different stages of treatment in 92 patients who had failed two-stage exchange. They found that in the majority of cases (68.5%), the ‘new’ profile of organism(s) was different to the initial PJI, but that failures following staphylococcal infections were more likely to be persistent. This largely reflects our experience (Table II), although it is unclear whether some of the ‘new’ organisms may have been present, but not cultured, during the initial treatment. Similarly, Bongers et al49 found new organisms in 14 out of 23 recurrent PJI cases (61%) after revision TKA. Although an earlier study at our institution found that infection is a more predominant cause of failure in revision TKA compared with primary TKA,50 the reinfections in the present study were insufficient to determine any statistically significant correlation with numbers of previous procedures. It is interesting to note, however, that higher numbers of previous procedures were associated with a significantly increased rate of reoperation, when analyzing reinfection and aseptic failure collectively. This is consistent with the available evidence on patients who undergo knee arthrodesis as limb salvage.26
The use of 3% acetic acid has been shown to be safe in revision TKA for infection, whether or not a tourniquet is used.30 It is effective in lowering environmental pH and demonstrates activity against both Gram-positive and Gram-negative organisms. Some surgeons advocate the use of hydrogen peroxide as part of the debridement process due to the effervescence it provides via oxygen release in the tissues, and the fact that it is synergistic with both chlorhexidine and povidone-iodine solution.51 Controversy remains as to whether there is a risk of air embolism using hydrogen peroxide, but this risk is theoretically mitigated by the use of a tourniquet. Surgihoney, which provides a local osmolar effect and sustained release of hydrogen peroxide, has been developed as a potential antimicrobial agent in a variety of infections, including PJI.52 The importance of developing alternative strategies in treating orthopaedic infections cannot be overstated, with the increasing prevalence of antibiotic-resistant organisms.53
This study has certain limitations. Its observational design reflects the majority of existing evidence on PJI, and we recognize the need for a prospective multicentre RCT to provide more robust evidence on the differences between one- and two-stage revision procedures.7,11 We also acknowledge that our cohort represents a heterogeneous group of organisms and host factors, but all patients were treated with a specific debridement protocol and received MDT input at the same institution. The decision to perform a single-stage exchange in patients with culture-negative PJI is controversial, but there were no reinfections in these patients. Among numerous available PROMs, we selected the OKS as a validated instrument at two specific timepoints. We recognize that the contemporary literature varies in terms of scoring systems used, and that repeat OKS measurements at longer-term follow-up would have been useful to determine any changes in postoperative function over time. Finally, it is important to note that the diagnosis of PJI can vary depending on the diagnostic criteria selected, although we have generally found the ICM 2013 definition to be most relevant in our everyday practice.18
In conclusion, single-stage revision is an effective option for the infected TKA and, where appropriate, provides significant potential benefits for both for the patient and healthcare system. Our results indicate that culture-negative PJI should not be considered an absolute contraindication, while polymicrobial infections might be better served with a two-stage procedure, although more research is required in this area.43 In our experience, the success of any revision surgery for the infected TKA is contingent upon the debridement (which should not be compromised in favour of reconstruction), alongside a multidisciplinary approach.
Take home message
- Single-stage revision is an effective treatment option for the infected total knee arthroplasty (TKA) and can be performed with uncemented or hybrid component fixation.
- A strict debridement protocol is paramount for patients undergoing single-stage revision TKA for periprosthetic joint infection (PJI) and each case should receive multidisciplinary input.
- Culture-negative PJI was not associated with increased recurrence of infection following one-stage exchange, but polymicrobial infections demonstrated significantly worse outcomes.
Acknowledgements
The authors would like to thank S. S. Chitnis and P. J. Jenkins for their assistance with the database. We also gratefully acknowledge H. C. Hughes, A. Mehta, and our colleagues at Cardiff and Vale Orthopaedic Centre for contributing to the care of patients in this study.
Footnotes
Author contributions: N. Razii: Designed the study, Collected and analyzed the data, Prepared and approved the manuscript.
J. M. Clutton: Collected and analyzed the data, Prepared and approved the manuscript.
R. Kakar: Collected and analyzed the data, Prepared and approved the manuscript.
R. Morgan-Jones: Designed the study, Performed the operations, Collected and analyzed the data, Prepared and approved the manuscript.
Funding statement: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement: R. Morgan-Jones reports payment for lectures, including service on speakers' bureaus, from DePuy Synthes, Biocomposites, and LINK, unrelated to this study.
Ethical review statement: This study did not require ethical approval as it was deemed a service evaluation by the institution’s research and development department.
Twitter: Follow Cardiff and Vale University Health Board @CV_UHB
References
- 1. No authors listed . 16th Annual Report. National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. 2019. https://reports.njrcentre.org.uk/Portals/0/PDFdownloads/NJR%2016th%20Annual%20Report%202019.pdf (date last accessed 25 January 2020).
- 2. Springer BD, Cahue S, Etkin CD, Lewallen DG, McGrory BJ. Infection burden in total hip and knee arthroplasties: An international registry-based perspective. Arthroplast Today. 2017;3(2):137–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lenguerrand E, Whitehouse MR, Beswick AD, et al. Description of the rates, trends and surgical burden associated with revision for prosthetic joint infection following primary and revision knee replacements in England and Wales: an analysis of the National joint Registry for England, Wales, Northern Ireland and the Isle of man. BMJ Open. 2017;7(7):e014056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Insall JN, Thompson FM, Brause BD. Two-stage reimplantation for the salvage of infected total knee arthroplasty. J Bone Joint Surg Am. 1983;65-A(8):1087–1098. [PubMed] [Google Scholar]
- 5. Buchholz HW, Elson RA, Engelbrecht E, Lodenkämper H, Röttger J, Siegel A. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981;63-B(3):342–353. [DOI] [PubMed] [Google Scholar]
- 6. Freeman MA, Sudlow RA, Casewell MW, Radcliff SS. The management of infected total knee replacements. J Bone Joint Surg Br. 1985;67-B(5):764–768. [DOI] [PubMed] [Google Scholar]
- 7. Masters JPM, Smith NA, Foguet P, Reed M, Parsons H, Sprowson AP. A systematic review of the evidence for single stage and two stage revision of infected knee replacement. BMC Musculoskelet Disord. 2013;14(1):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nagra NS, Hamilton TW, Ganatra S, Murray DW, Pandit H. One-Stage versus two-stage exchange arthroplasty for infected total knee arthroplasty: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2016;24(10):3106–3114. [DOI] [PubMed] [Google Scholar]
- 9. Kunutsor SK, Whitehouse MR, Lenguerrand E, Blom AW, Beswick AD, Team I, INFORM Team . Re-Infection outcomes following one- and two-stage surgical revision of infected knee prosthesis: a systematic review and meta-analysis. PLoS One. 2016;11(3):e0151537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yaghmour KM, Chisari E, Khan WS. Single-Stage revision surgery in infected total knee arthroplasty: a PRISMA systematic review. J Clin Med. 2019;8(2):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pangaud C, Ollivier M, Argenson J-N. Outcome of single-stage versus two-stage exchange for revision knee arthroplasty for chronic periprosthetic infection. EFORT Open Rev. 2019;4(8):495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ribes C, Masquefa T, Dutronc H, et al. One-Stage versus two-stage prosthesis replacement for prosthetic knee infections. Med Mal Infect. 2019;49(7):519–526. [DOI] [PubMed] [Google Scholar]
- 13. Haddad FS, Sukeik M, Alazzawi S. Is single-stage revision according to a strict protocol effective in treatment of chronic knee arthroplasty infections? Clin Orthop Relat Res. 2015;473(1):8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Negus JJ, Gifford PB, Haddad FS. Single-Stage revision arthroplasty for Infection-An underutilized treatment strategy. J Arthroplasty. 2017;32(7):2051–2055. [DOI] [PubMed] [Google Scholar]
- 15. Moore AJ, Blom AW, Whitehouse MR, Gooberman-Hill R. Deep prosthetic joint infection: a qualitative study of the impact on patients and their experiences of revision surgery. BMJ Open. 2015;5(12):e009495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: from the Workgroup of the musculoskeletal infection Society. Clin Orthop Relat Res. 2011;469(11):2992–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Osmon DR, Berbari EF, Berendt AR, et al. Executive summary: diagnosis and management of prosthetic joint infection: clinical practice guidelines by the infectious diseases Society of America. Clin Infect Dis. 2013;56(1):1–10. [DOI] [PubMed] [Google Scholar]
- 18. Parvizi J, Gehrke T, Chen AF. Proceedings of the International consensus on periprosthetic joint infection. Bone Joint J. 2013;95-B(11):1450–1452. [DOI] [PubMed] [Google Scholar]
- 19. Parvizi J, Tan TL, Goswami K, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. 2018;33(5):1309–1314. [DOI] [PubMed] [Google Scholar]
- 20. Marson BA, Deshmukh SR, Grindlay DJC, Scammell BE. Alpha-Defensin and the Synovasure lateral flow device for the diagnosis of prosthetic joint infection: a systematic review and meta-analysis. Bone Joint J. 2018;100-B(6):703–711. [DOI] [PubMed] [Google Scholar]
- 21. Tarabichi M, Shohat N, Goswami K. Diagnosis of periprosthetic joint infection: The potential of next-generation sequencing. J Bone Joint Surg Am. 2018;100-A(2):147–154. [DOI] [PubMed] [Google Scholar]
- 22. Weber FA, Lautenbach EE. Revision of infected total hip arthroplasty. Clin Orthop Relat Res. 1986;211:108–115. [PubMed] [Google Scholar]
- 23. Hashmi MA, Norman P, Saleh M. The management of chronic osteomyelitis using the Lautenbach method. J Bone Joint Surg Br. 2004;86-B(2):269–275. [DOI] [PubMed] [Google Scholar]
- 24. Parkinson RW, Kay PR, Rawal A. A case for one-stage revision in infected total knee arthroplasty? Knee. 2011;18(1):1–4. [DOI] [PubMed] [Google Scholar]
- 25. Holland G, Brown G, Goudie S, Brenkel I, Walmsley PJ. Results of Using a “2-in-1” Single-Stage Revision Total Knee Arthroplasty for Infection with Associated Bone Loss: Prospective 2-Year Follow-Up. J Knee Surg. 2021;34(5):526–532. [DOI] [PubMed] [Google Scholar]
- 26. Razii N, Kakar R, Morgan-Jones R. Knee arthrodesis in the infected total knee arthroplasty : Rodríguez-Merchán E, Oussedik S. The Infected Total Knee Arthroplasty: Prevention, Diagnosis, and Treatment. Springer. 2018: 165–180. [Google Scholar]
- 27. Abbas AMI, Williams RLL, Khan WS, Ghandour A, Morgan-Jones RL. Tibial crest osteotomy in Extensile knee Exposure-A modified, low-energy, suture technique. J Arthroplasty. 2016;31(2):383–388. [DOI] [PubMed] [Google Scholar]
- 28. Whiteside LA. Exposure in difficult total knee arthroplasty using tibial tubercle osteotomy. Clin Orthop Relat Res. 1995;321:32–35. [PubMed] [Google Scholar]
- 29. Khan W, Morgan-Jones R. Debridement: defining something we all do. J Trauma Orthop. 2016;4(1):48–50. [Google Scholar]
- 30. Williams RL, Ayre WN, Khan WS, Mehta A, Morgan-Jones R. Acetic acid as part of a debridement protocol during revision total knee arthroplasty. J Arthroplasty. 2017;32(3):953–957. [DOI] [PubMed] [Google Scholar]
- 31. Morgan-Jones R, Oussedik SIS, Graichen H, Haddad FS. Zonal fixation in revision total knee arthroplasty. Bone Joint J. 2015;97-B(2):147–149. [DOI] [PubMed] [Google Scholar]
- 32. Dawson J, Fitzpatrick R, Murray D, Carr A. Questionnaire on the perceptions of patients about total knee replacement. J Bone Joint Surg Br. 1998;80(1):63–69. [DOI] [PubMed] [Google Scholar]
- 33. Murray DW, Fitzpatrick R, Rogers K, et al. The use of the Oxford hip and knee scores. J Bone Joint Surg Br. 2007;89(8):1010–1014. [DOI] [PubMed] [Google Scholar]
- 34. Dripps RD, Lamont A, Eckenhoff JE. The role of anesthesia in surgical mortality. JAMA. 1961;178(3):261–266. [DOI] [PubMed] [Google Scholar]
- 35. von Foerster G, Klüber D, Käbler U. [Mid- to long-term results after treatment of 118 cases of periprosthetic infections after knee joint replacement using one-stage exchange surgery]. Orthopade. 1991;20(3):244–252. [Article in German] [PubMed] [Google Scholar]
- 36. Buechel FF. The infected total knee arthroplasty: just when you thought it was over. J Arthroplasty. 2004;19(4 Suppl 1):51–55. [DOI] [PubMed] [Google Scholar]
- 37. Zahar A, Kendoff DO, Klatte TO, Gehrke TA. Can good infection control be obtained in one-stage exchange of the infected TKA to a rotating hinge design? 10-year results. Clin Orthop Relat Res. 2016;474(1):81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thakrar RR, Horriat S, Kayani B, Haddad FS. Indications for a single-stage exchange arthroplasty for chronic prosthetic joint infection. Bone Joint J. 2019;101-B(1_Supple_A):19–24. [DOI] [PubMed] [Google Scholar]
- 39. Nickinson RSJ, Board TN, Gambhir AK, Porter ML, Kay PR. The microbiology of the infected knee arthroplasty. Int Orthop. 2010;34(4):505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jenny JY, Barbe B, Cazenave A, Roche O, Massin P. French Society for hip and knee surgery (SFHG). patient selection does not improve the success rate of infected TKA one stage exchange. Knee. 2016;23(6):1012–1015. [DOI] [PubMed] [Google Scholar]
- 41. Reisener M, Perka C. Do culture-negative periprosthetic joint infections have a worse outcome than culture-positive periprosthetic joint infections? A systematic review and meta-analysis. Biomed Res Int. 2018;2018:6278012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Prasad N, Paringe V, Kotwal R, Ghandour A, Jones RM, Morgan-Jones R. Two-stage revision for infected total knee arthroplasty: our experience with interval prosthesis. Eur J Orthop Surg Traumatol. 2014;24(7):1279–1283. [DOI] [PubMed] [Google Scholar]
- 43. Carijo JH, Courtney PM, Goswami K, et al. Hip and knee section, pathogen factors: proceedings of international consensus on orthopedic infections. J Arthroplasty. 2019;34(2S):S381–S386. [DOI] [PubMed] [Google Scholar]
- 44. Kavolus JJ, Cunningham DJ, Rao SR, Wellman SS, Seyler TM. Polymicrobial infections in hip arthroplasty: lower treatment success rate, increased surgery, and longer hospitalization. J Arthroplasty. 2019;34(4):710–716. [DOI] [PubMed] [Google Scholar]
- 45. Gebauer M, Frommelt L, Achan P, et al. Management of fungal or atypical periprosthetic joint infections. J Arthroplasty. 2014;29(2):112–114. [DOI] [PubMed] [Google Scholar]
- 46. Klatte TO, Kendoff D, Kamath AF, et al. Single-Stage revision for fungal peri-prosthetic joint infection: a single-centre experience. Bone Joint J. 2014;96-B(4):492–496. [DOI] [PubMed] [Google Scholar]
- 47. Azzam K, Parvizi J, Jungkind D, et al. Microbiological, clinical, and surgical features of fungal prosthetic joint infections: a multi-institutional experience. J Bone Joint Surg Am. 2009;91 Suppl 6-A(Suppl 6):142–149. [DOI] [PubMed] [Google Scholar]
- 48. Zmistowski B, Tetreault MW, Alijanipour P, Chen AF, Della Valle CJ, Parvizi J. Recurrent periprosthetic joint infection: persistent or new infection? J Arthroplasty. 2013;28(9):1486–1489. [DOI] [PubMed] [Google Scholar]
- 49. Bongers J, Jacobs AME, Smulders K, van Hellemondt GG, Goosen JHM. Reinfection and re-revision rates of 113 two-stage revisions in infected TKA. J Bone Jt Infect. 2020;5(3):137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Agarwal S, Kabariti R, Kakar R, Lopez D’Jon, Morgan-Jones R. Why are revision knee replacements failing? Knee. 2019;26(3):774–778. [DOI] [PubMed] [Google Scholar]
- 51. Lu M, Hansen EN. Hydrogen peroxide wound irrigation in orthopaedic surgery. J Bone Jt Infect. 2017;2(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dryden M. Reactive oxygen species: a novel antimicrobial. Int J Antimicrob Agents. 2018;51(3):299–303. [DOI] [PubMed] [Google Scholar]
- 53. Li B, Webster TJ. Bacteria antibiotic resistance: new challenges and opportunities for implant-associated orthopedic infections. J Orthop Res. 2018;36(1):22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]