A boost from infection
During clinical trials of severe acute respiratory syndrome coronavirus 2 vaccines, no one who had survived infection with the virus was tested. A year after the pandemic was declared, vaccination of previously infected persons is a reality. Reynolds et al. address the knowledge gap in a cohort of UK health care workers given the Pfizer/BioNTech vaccine in which half of the participants had experienced natural virus infections early in the pandemic (see the Perspective by Crotty). Genotyping indicated that a genetic component underlies heterogeneity in immune responses to vaccine and to natural infection. After vaccination, naïve individuals developed antibody responses similar to those seen in naturally infected persons, but T cell responses were more limited and sometimes absent. However, antibody and memory responses in individuals vaccinated after infection were substantially boosted to the extent that a single vaccine dose is likely to protect against the more aggressive B.1.1.7 variant. It is possible that the messenger RNA vaccine has an adjuvant effect, biasing responses toward antibody generation.
Science, abh1282, this issue p. 1418; see also abj2258, p. 1392
Previous infection results in enhanced variant cross-protective T and B cell responses to a single BNT162b2 vaccine dose.
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine rollout has coincided with the spread of variants of concern. We investigated whether single-dose vaccination, with or without prior infection, confers cross-protective immunity to variants. We analyzed T and B cell responses after first-dose vaccination with the Pfizer/BioNTech messenger RNA vaccine BNT162b2 in health care workers (HCW) followed longitudinally, with or without prior Wuhan-Hu-1 SARS-CoV-2 infection. After one dose, individuals with prior infection showed enhanced T cell immunity, antibody-secreting memory B cell response to the spike protein, and neutralizing antibodies effective against variants B.1.1.7 and B.1.351. By comparison, HCW receiving one vaccine dose without prior infection showed reduced immunity against variants. B.1.1.7 and B.1.351 spike mutations resulted in increased, abrogated, or unchanged T cell responses, depending on human leukocyte antigen (HLA) polymorphisms. Single-dose vaccination with BNT162b2 in the context of prior infection with a heterologous variant substantially enhances neutralizing antibody responses against variants.
During worldwide rollout of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines, it is vital to understand how vaccination influences immune responses and protection among those who have had prior natural SARS-CoV-2 infection. This is a knowledge gap because a history of previous infection was an exclusion criterion in phase 3 vaccine trials (1). Countries have adopted diverse approaches—among them, the UK policy to maximize deployment of first doses to the largest possible number of people by extending the time interval to second dose. At the end of 2020, it became apparent that several virus variants had emerged (2, 3) and that these might affect vaccine rollout. The B.1.1.7 variant, possessing the spike Asn501→Tyr (N501Y) mutation, first emerged in the UK in December 2020 and spread rapidly (4). Additional variants of concern (VOC) include the B.1.351 variant, which emerged at about the same time in South Africa, and the P.1 variant, which emerged in January 2021 in Brazil. In addition to the N501Y mutation, both of these variants have the E484K mutation, which is implicated in escape from neutralizing antibodies (nAbs) (5, 6).
The Pfizer/BioNTech mRNA vaccine BNT162b2 encodes a prefusion-stabilized, membrane-anchored SARS-CoV-2 full-length spike protein modified by two proline substitutions (1, 7, 8). A two-dose regimen of 30 μg BNT162b2, 21 days apart, confers 95% protection against Wuhan-Hu-1 SARS-CoV-2 (1), eliciting high nAb titers as well as CD4 and CD8 cell responses (8). When given as a single 60-μg dose, BNT162b1 induced virus Ab neutralization, but T cell responses were reduced compared with the standard prime-boost regime (8). A single 30-μg dose of BNT162b1 was not reported beyond day 21. However, the cumulative incidence of COVID-19 cases among 21,676 placebo and 21,699 vaccine recipients diverged 12 days after the first dose, indicating possible early-onset first-dose protection (1). For those who were previously infected, single-dose vaccination may act as a boost after natural infection. Therefore, we aimed to test the impact of prior SARS-CoV-2 infection on T and B cell responses to first-dose vaccination.
To do this, we analyzed T and B cell immunity after the first 30-μg dose of the Pfizer/BioNTech mRNA vaccine BNT162b2 in a cohort of UK hospital health care workers (HCW) (9–12). The COVIDsortium HCW cohort has been studied longitudinally since the end of March 2020, providing accurate infection and immune history in the context of genotyping, including human leukocyte antigen (HLA) imputation (10–12). Our aim was to compare T and B cell immunity after a first dose of vaccine in December 2020 in postinfection (after natural infection), vaccinated postinfection (vaccination in the context of prior SARS-CoV-2 infection), and vaccinated naïve (single-dose vaccination) individuals. We sought to explore whether there is evidence for altered T cell recognition of the B.1.1.7 and B.1.351 variants and, in particular, of the N501Y mutation shared by several VOC.
The UK has deployed a heterodox vaccination regimen to maximize immune protection and slow spread of the B.1.1.7 lineage, giving an initial 30-μg dose of BNT162b2 followed by boosting up to 12 weeks later (13). A cross-sectional substudy (n = 51 individuals) of the existing longitudinal HCW cohort (9–12) was recruited 22 (±2) days after the first dose. After the start of the study, the majority of acute infections had already occurred among this cohort (11). At the time of receiving their first vaccine dose in December 2020, prior to the emergence of VOC, 25 individuals were ~39 weeks removed from SARS-CoV-2 infection with the Wuhan-Hu-1 strain, and 26 were confirmed uninfected, having tested negative in longitudinal serology for spike and nucleocapsid (N) proteins (table S1 and fig. S1).
We first measured SARS-CoV-2 N antibody longitudinally up to 16 to 18 weeks, then at 28 to 30 weeks, and finally at 42 weeks after recruitment, to confirm that there was no laboratory evidence of new infection at the time of drawing blood for the vaccine study at 42 weeks; none of the previously uninfected HCW had become seropositive (Fig. 1A). T cell responses to spike protein and mapped epitope peptides (MEPs) in either postinfection, vaccinated postinfection, and vaccinated naïve individuals were compared (Fig. 1B). Ninety-six percent (22/23) of vaccinated postinfection individuals mounted a T cell response to spike protein compared with 70% (16/23) of vaccinated naïve individuals, with a fourfold increase in the magnitude of the T cell response. Furthermore, while the T cell response to spike protein in vaccinated naïve individuals increased (P = 0.0440), it was lower than that of vaccinated postinfection individuals (P = 0.0557) (Fig. 1C). As expected, there was no significant change in T cell response to N (a measure of immunity to natural infection) (fig. S2A).
Fig. 1. Impact of prior natural infection with SARS-CoV-2 during the first wave on T and B cell responses to a single dose of the mRNA SARS-CoV-2 vaccine BNT162b2.
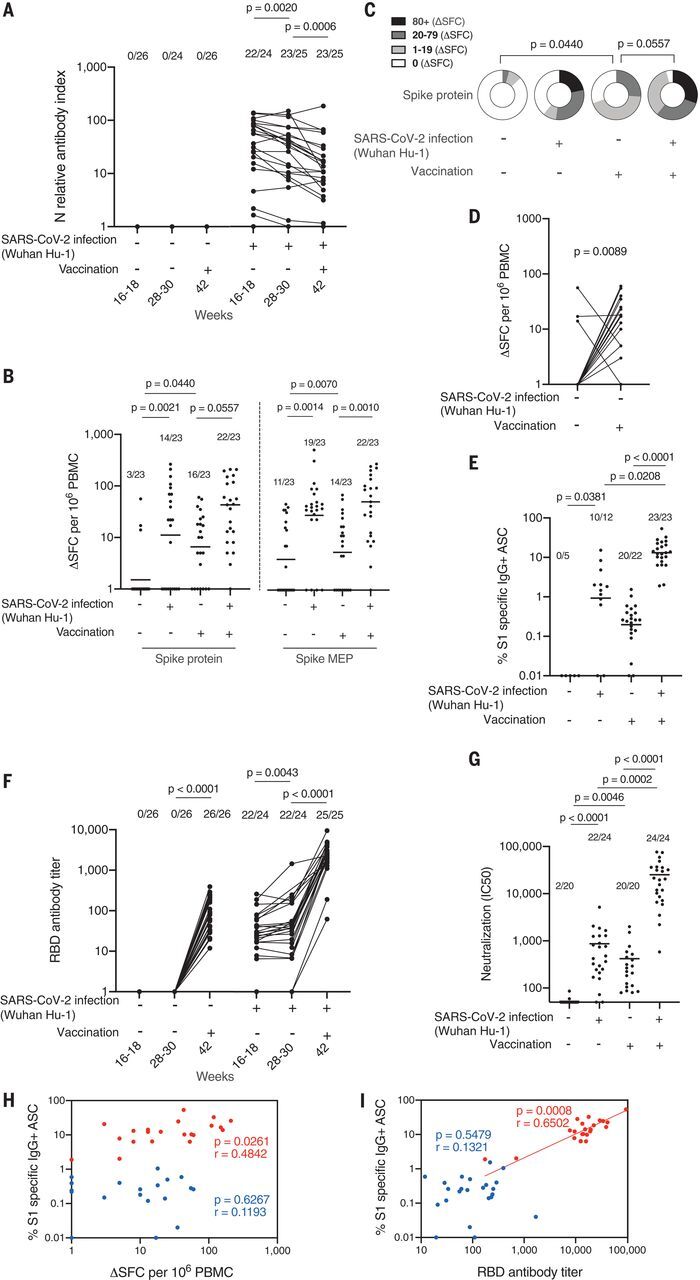
(A) Nucleocapsid Abs measured by electrochemiluminescence immunoassay analyzer (ECLIA) in serum samples from HCW with (n = 25 individuals) and without (n = 26 individuals) laboratory-confirmed SARS-CoV-2 infection (Wuhan-Hu-1, during the first wave) 3 weeks after a single dose of the mRNA SARS-CoV-2 vaccine BNT162b2. (B) Magnitude of T cell response to spike protein and spike mapped epitope peptides (MEPs) in HCW with and without laboratory-confirmed SARS-CoV-2 infection (n = 23 per group). Data are shown prevaccination (16 to 18 weeks after infection) and 3 weeks after the first-dose vaccination (week 42) with line at geometric mean. (C) Proportion of HCW with (n = 23) and without (n = 23) laboratory-confirmed SARS-CoV-2 infection (during the first wave) with a T cell response to spike protein within the range of 0, 1 to 19, 20 to 79, and >80 ΔSFC/106 PBMC before and 3 weeks after first-dose vaccination. (D) Magnitude of T cell response to spike protein in HCW without a history of SARS-CoV-2 infection, plotted pairwise at 16 to 18 weeks and 42 weeks (3 weeks after first-dose vaccination). (E) Percentage of S1-specific IgG+ antibody-secreting cells (ASCs) in vaccinated HCW with (n = 23) and without (n = 22) prior SARS-CoV-2 infection and in unvaccinated HCW with (n = 12) and without (n = 5) prior infection. Line at geometric mean. (F) RBD Ab titers measured by ECLIA in serum samples from HCW with (n = 25) and without (n = 26) laboratory-confirmed SARS-CoV-2 infection following first-dose vaccination. (G) Neutralizing antibody titer (IC50) against Wuhan-Hu-1 authentic virus in HCW with (n = 24) and without (n = 20) laboratory-confirmed SARS-CoV-2 infection. Line at arithmetic mean. (H) Correlation between percentage of S1-specific ASC and magnitude of T cell response to spike protein in vaccinated HCW with (n = 21, red) and without (n = 19, blue) a history of SARS-CoV-2 infection during the first wave. (I) Correlation between percentage of S1-specific ASC and RBD Ab titer in HCW with (n = 23, red) and without (n = 23, blue) a history of SARS-CoV-2 infection. [(A), (B), (E), and (F)] Numbers of HCW in each group with detectable responses are shown. [(F) and (G)] Data are shown prevaccination (16 to 18 weeks after infection) and 3 weeks after the first-dose vaccination (week 42). [(A), (D), and (F)] Wilcoxon matched-pairs signed rank test. [(B), (C), (E), and (G)] Kruskal Wallis multiple comparison analysis of variance (ANOVA) with Dunn’s correction. [(H) and (I)] Spearman’s rank correlation. Ab, antibody; HCW, health care workers; RBD, receptor binding domain; S1, spike subunit 1; SFC, spot forming cells.
Paired analysis of T cell immunity to spike protein in previously uninfected individuals, analyzed at the 16- to 18-week time point and 3 weeks after vaccination, showed a significantly increased response (P = 0.0089) (Fig. 1D). Three individuals who previously showed a response, despite lack of laboratory evidence for infection (therefore presumably a cross-reactive response to an endemic human coronavirus), showed an unchanged or decreased response to spike after vaccination.
The size of the SARS-CoV-2 spike subunit 1 (S1)–specific memory B cell (MBC) pool was investigated by B cell enzyme-linked immunosorbent spot (ELISpot) assay (Fig. 1E and fig. S2B). As for T cell responses, the number of S1-specific immunoglobulin G (IgG+) antibody-secreting cells (ASCs) was far greater in vaccinated postinfection individuals than in vaccinated naïve individuals (P < 0.0001). Prior infection generated a 63-fold increase in S1-specific ASCs. There were no preexisting S1-specific ASCs in uninfected HCW before vaccination. Twenty of 22 vaccinated naïve individuals had detectable S1-specific ASCs composing 0.02 to 1.54% of the MBC pool. By comparison, all vaccinated postinfection individuals had detectable S1-specific ASCs (1.90 to 50% of the MBC pool). We previously reported (14) spike receptor binding domain (RBD) enhanced Ab responses in the vaccinated postinfection group. In this work, the vaccinated naïve group attained antibody titers similar to those of the postinfection group at 16 to 18 weeks and 28 to 30 weeks (Fig. 1F). Vaccinated naïve individuals demonstrated a lower nAb response to wild-type virus than was seen after natural infection at 16 to 18 weeks, although this did not achieve statistical significance. In line with the findings for MBC and RBD binding, there was a significantly enhanced nAb response in vaccinated postinfection individuals compared with the vaccinated naïve group (Fig. 1G), with a mean value of 25,273 compared with 420, that is, a 60-fold increase. To put this in context, these values are 43-fold higher than the values recorded after two vaccine doses in the phase 1 trial (7). There was no correlation between the magnitude of the spike protein T cell response and the percentage of S1-specific ASCs (Fig. 1H). As expected, there was a positive correlation between the percentage of S1-specific ASCs and the serum titer of RBD antibody in the vaccinated postinfection individuals [correlation coefficient (r) = 0.6502; P = 0.0008] (Fig. 1I). After vaccination, two previously infected individuals showed lower percentages of S1-specific memory B cells and reduced serum RBD-specific antibody levels than the rest of the group; prior infection involving case-definition symptoms tended to be associated with a higher specific B cell frequency than milder disease (Fig. 1F and fig. S2C). These individuals who, despite infection, had also not shown a detectable T cell response (one never seroconverted, and the other rapidly became seronegative during longitudinal follow-up) had a poor or absent response to infection that was only minimally overcome by vaccination.
The data in Fig. 1 indicate that there is a strong prime-boosting effect of prior infection on single-dose vaccination. Augmentation is seen more strongly in MBC frequency, anti-RBD, and nAb responses than for T cell response frequency. Furthermore, there was no correlation between S1 ASC frequency and T cell response frequency (Fig. 1H). There is, however, a correlation between S1 ASC and RBD antibody titers, indicating that individuals with higher numbers of MBCs mount stronger antibody responses, and individuals who had experienced infection clustered at the higher end of this response (Fig. 1I).
Shortly before the vaccination program was initiated, several VOC emerged, including B.1.1.7. This variant has nine mutations in the spike protein. Several studies have reported weaker nAb responses to B.1.1.7 relative to the previously circulating Wuhan-Hu-1 strain (2–6, 15–18). The majority of SARS-CoV-2 immune naïve individuals made no nAb response to the B.1.1.7 (18/20) and B.1.351 (17/20) variants after single-dose vaccination. In contrast, almost all vaccinated postinfection individuals made a strong nAb response to the B.1.1.7 (24/24) and B.1.351 (23/24) variants after a single-dose vaccination, with a 46-fold (B.1.1.7) and 63-fold (B.1.351) increase in mean nAb half-maximal inhibitory concentration (IC50) in vaccinated postinfection individuals compared with vaccinated naïve individuals. In a paired analysis, we observed in vitro significantly reduced nAb potency to authentic B.1.1.7 variant (mean: 35) with a 96% fall compared to that of Wuhan-Hu-1(mean: 866; P < 0.0001) in sera from individuals with a past medical history of natural infection (Fig. 2B). Worryingly, after single-dose vaccination, 90% (18/20) of vaccinated naïve individuals showed no detectable nAbs (IC50 < 50) against B.1.1.7 (mean IC50: 37; range: 0 to 184; P = 0.2090), but they did show demonstrable nAb responses to Wuhan-Hu-1 SARS-CoV-2 virus (mean IC50: 420; range: 80 to 2004; P = 0.0046). In contrast, all vaccinated postinfection individuals responded to single-dose vaccination with substantially enhanced nAb responses, neutralizing not just Wuhan-Hu-1 SARS-CoV-2 (mean IC50: 25,273; range: 581 to 76,369) but also the B.1.1.7 (mean IC50: 1717; range: 52 to 4919) and B.1.351 (mean IC50: 5451; range: 41 to 20,411) variants (Fig. 2, A and B, and fig. S3). We show a 93% reduction in neutralization (IC50) responses to the SARS-CoV-2 B.1.1.7 variant (mean: 1717) compared with the Wuhan-Hu-1 (mean: 25,273) virus in vaccinated postinfection individuals. However, despite this fall, the majority (22/24) remain within a “protective threshold.” This was not the case for vaccinated naïve individuals. There was a 91% reduction in neutralization (IC50) responses against the SARS-CoV-2 B.1.1.7 variant (mean: 37) compared with the Wuhan-Hu-1 virus (mean: 420), resulting in the majority of individuals (19/20) falling below the “protective threshold.” This result was mirrored in the SARS-CoV-2 S1-specific MBC pool, where reduced numbers of S1-specific IgG+ ASC are seen (in vaccinated naïve individuals compared with vaccinated postinfection individuals) responding to S1 antigen containing the N501Y, K417N, and E484K mutations. Prior infection substantially enhances the specific MBC pool after single-dose vaccination (Fig. 2C). We looked at correlations between RBD binding antibodies, B cell responses, T cell responses, and IC50, comparing neutralization of Wuhan-Hu-1, B.1.1.7, and B.1.351 live virus (Fig. 2D). Despite the lower neutralization of B.1.1.7 and B.1.351 variants, the pattern was retained of strong correlation between RBD antibody titer and S1-specific B cell frequency and neutralization and somewhat weaker correlation between T cell response and neutralization.
Fig. 2. Impact of vaccination and prior natural infection with SARS-CoV-2 during the first wave on T and B cell responses to the UK B.1.1.7 and South African B.1.351 variants.
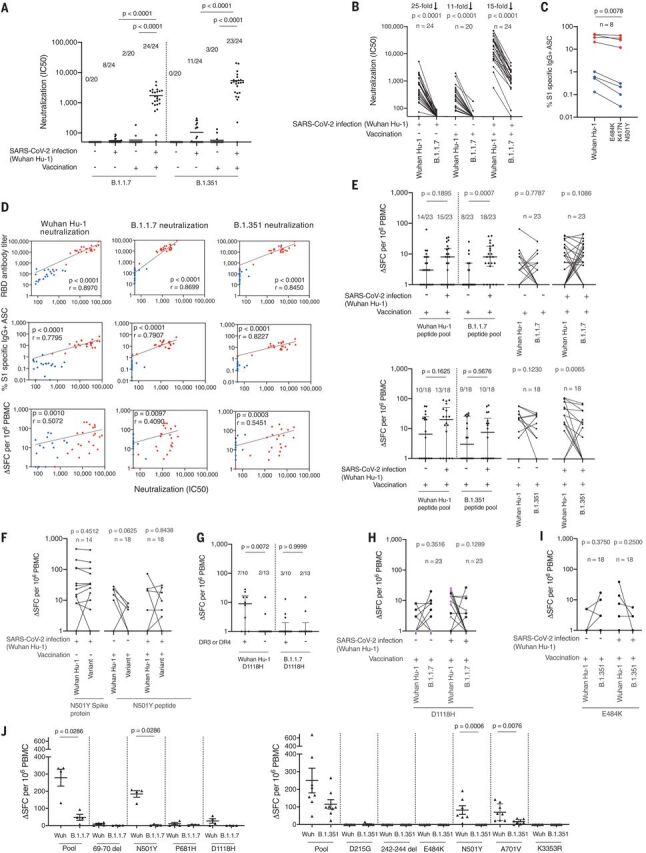
(A) Neutralizing antibody (nAb) titer (IC50) against B.1.1.7 and B.1.351 authentic virus in HCW with (n = 24) and without (n = 20) laboratory-confirmed SARS-CoV-2 infection (Wuhan-Hu-1). Lines at arithmetic mean. Data are shown prevaccination (16 to 18 weeks after infection) and 3 weeks after the first-dose vaccination (week 42). (B) nAb (IC50) titers against Wuhan-Hu-1 and B.1.1.7 authentic viruses plotted pairwise by individual. (C) Percentage of Wuhan-Hu-1 S1 and S1 containing variant mutations (E484K, K417N, and N501Y) specific IgG+ antibody-secreting cells (ASCs) in vaccinated HCW with (n = 4) and without (n = 4) prior SARS-CoV-2 infection. Single-letter abbreviations for the amino acid residues are as follows: A, Ala; C, Cys; D, Asp; E, Glu; F, Phe; G, Gly; H, His; I, Ile; K, Lys; L, Leu; M, Met; N, Asn; P, Pro; Q, Gln; R, Arg; S, Ser; T, Thr; V, Val; W, Trp; and Y, Tyr. (D) Correlations between nAb (IC50) titers of Wuhan-Hu-1, B.1.1.7, or B.1.351 authentic virus and RBD Ab titer, percentage of S1-specific ASC, and magnitude of T cell response to S1 protein in vaccinated HCW with (n = 22 to 24, red) and without (n = 18 to 20, blue) a history of SARS-CoV-2 infection. (E) Magnitude of T cell response to Wuhan-Hu-1, B.1.1.7, or B.1.351 peptide pools in vaccinated HCW with (n = 23 or 18) and without (n = 23 or 18) SARS-CoV-2 infection (Wuhan-Hu-1), plotted as grouped data (median plus interquartile range) and pairwise for each individual. (F) Magnitude of T cell response to Wuhan-Hu-1 S1 protein and N501Y variant spike RBD protein in unvaccinated HCW with laboratory-confirmed SARS-CoV-2 infection (n = 14) or to Wuhan-Hu-1 and N501Y mutated peptide in vaccinated HCW with (n = 18) and without (n = 18) a history of SARS-CoV-2 infection, plotted pairwise by individual. (G) Magnitude of T cell response to Wuhan-Hu-1 or B.1.1.7 D1118H peptide in vaccinated HCW with a history of SARS-CoV-2 infection (n = 23), plotted by DRB1*0301 or DRB1*0401 status. Lines at median plus interquartile range. (H) Magnitude of T cell response to Wuhan-Hu-1 or B.1.1.7 D1118H peptide in vaccinated HCW with (n = 23) and without (n = 23) a history of SARS-CoV-2 infection, plotted pairwise by individual and with individuals carrying DRB1*0301 or DRB1*0401 alleles marked in purple. (I) Magnitude of T cell response to Wuhan-Hu-1 or B.1.351 E484K mutated peptide in vaccinated HCW with (n = 18) and without (n = 18) a history of SARS-CoV-2 infection, plotted pairwise by individual. (J) Magnitude of T cell response to Wuhan-Hu-1 (Wuh), B.1.1.7, or B.1.351 peptide pools and individual peptides in Wuhan-Hu-1 peptide immunized HLA-DRB1*04:01 transgenic mice (left-hand panel, n = 4; right-hand panel, n = 8; lines at arithmetic mean + SEM). (A) Kruskal Wallis multiple comparison ANOVA with Dunn’s correction. [(B), (C), (E) (right-hand panels), (F), (H), and (I)] Wilcoxon matched-pairs signed rank test. (D) Spearman’s rank correlation. [(E) (left-hand panels), (G), and (J)] Mann-Whitney U test. ASC, antibody-secreting cells; HCW, health care workers; RBD, receptor binding domain; S1, spike subunit 1; SFC, spot forming cells.
A lack of Ab-mediated protection in single-dose vaccinees could be mitigated by a broader repertoire of T cell responses (18). To investigate differences in T cell recognition, we designed peptide pools covering the affected regions of Wuhan-Hu-1, B.1.1.7, and B.1.351 variant sequence (table S2). We compared T cell responses to these peptide pools in peripheral blood mononuclear cells (PBMCs) from vaccinated postinfection and vaccinated naïve individuals (Fig. 2E). Responses in postinfection vaccinees were in general higher than in the vaccinated naïve individuals (note an enhanced response to the B.1.1.7 peptide pool). T cell responses were heterogeneous; responses to variant pools could be either higher or lower than to Wuhan-Hu-1 pools. Alterations in affinity for the T cell receptor can lead to altered peptide ligand effects and differential polarization of cytokine effector programs, as we have previously observed in Zika virus infection (19). We wondered whether this was also occurring for SARS-CoV-2; however, we found no evidence for immune deviation to interleukin (IL)–4, IL-5, IL-10, IL-13, IL-17A, or IL-23 (fig. S3).
For B.1.1.7 and B.1.351, attention has centered on the N501Y mutation, as this is implicated in altered angiotensin-converting enzyme 2 (ACE2) binding and enhanced infectivity and transmission but is also a target for B and T cell recognition. We initially looked at T cell responses after natural infection and found that at 16 to 18 weeks postinfection, the N501Y mutation appeared to have no substantial differential impact on the T cell response (Fig. 2F), unlike nAb recognition (5).
The specific impact of any T cell epitope changes on the immune response against VOC depends on changes in peptide binding to the peptide-presenting HLA molecules. Because the HLA complex is the most polymorphic part of the human genome, any alteration to core HLA binding motifs will differentially affect people with certain HLA alleles over others. We performed in silico analysis (using NetMHCIIpan) to predict which of the B.1.1.7 and B.1.351 mutations were found in HLA core binding motifs and how this might affect binding to common HLAII alleles (DRB1*0101, DRB1*0301, DRB1*0401, DRB1*0701, DRB1*1101, DRB1*1301, and DRB1*1501) (tables S3 and S4). Some of the mutations did not fall in a region predicted to bind the HLAII alleles tested (D3L, T716I, T1001I, A1708D, and 3675-7 SGF del). Although several mutations were not predicted to significantly change affinity for the HLAII alleles, others did show predicted differential affinities depending on host HLAII type (tables S3 and S4). Analyzing altered responses to the D1118H mutation, we noted that individuals who carried DRB1*0301 and DRB1*0401 showed enhanced T cell responses to the Wuhan-Hu-1 peptide compared with those who did not (P = 0.0072) (Fig. 2G). T cell responses to the variant peptide appeared to be reduced in individuals carrying DRB1*0301 and DRB1*0401 (Fig. 2H). There is a basis for this in terms of differential HLAII binding as the D-to-H mutation is predicted to lose the T cell epitope for people carrying DRB1*0301 and DRB1*0401 but not, for example, in those who carry DRB1*0701 or DRB1*1501, who would be predicted to show an enhanced response (table S3). People carrying DRB1*1301 are predicted to gain a response as a consequence of this mutation. Analyzing responses to the E484K mutation seen in B.1.351 and P.1 variants, we noted that it did not fall in a region predicted to bind the HLAII alleles tested (table S4). The mutation appeared to have no substantial or differential impact on T cell responses (Fig. 2I).
When we primed transgenic mice expressing human HLA-DRB1*0401 with the Wuhan-Hu-1 peptide pool, T cell responses to the B.1.1.7 variant peptide pool were significantly reduced (P = 0.0286) (Fig. 2J). Furthermore, the T cell response to the spike N501Y mutation common to all three of the current VOC was ablated.
In this HCW cohort, vaccinated naïve individuals made an anti-S1 RBD Ab response with a mean titer of ~100 U/ml at 22 (±2) days after vaccination, roughly equivalent to the mean peak Ab response after natural infection (14). However, the spike T cell response after one dose was lower than after natural infection, and for 30% of vaccinees, no response could be measured. However, T cell responses are enhanced fourfold in those vaccinated postinfection. This T cell enhancement is small relative to the 63-fold change in ASCs and the corresponding 140-fold change in Roche anti-S (RBD) Ab levels we observed after one vaccine dose in HCW vaccinated postinfection (14). While much has been written about the impact of rapidly waning serum antibodies, our findings confirm that MBCs are nevertheless primed and able to contribute a rapid, large response to repeat exposure. The rather large effect on B cell priming and restimulation, relative to T cells, in previously infected single dose–vaccinated individuals may reflect the fact that, among the nuanced differences between the licensed SARS-CoV-2 vaccines, aspects of the mRNA adjuvant effect appear to skew immunity to high nAb titers, which may underpin its high efficacy. Our evidence for enhanced vaccine responses after infection supports the case that only one vaccine dose is necessary to maximize immune protection for SARS-CoV-2–experienced individuals (14, 20).
It is notable that the high IC50 titers in those vaccinated after infection provide such a large protective margin that responses to authentic B.1.1.7 and B.1.351 variants are also high. In contrast, nAb responses in individuals several months on from mild infection show much lower IC50 titers against B.1.1.7 and B.1.351, often less than 100. Similarly, the majority of responses in naïve individuals after one dose show weak recognition of B.1.1.7 and B.1.351. This finding indicates potentially poor protection against B.1.1.7 and B.1.351 in individuals who have experienced natural infection or who have only had one vaccine dose.
It is important to map the effect of VOC mutations on any evasion of T cell immunity. The case has been made that reductions in antibody neutralization of mutant spike may be mitigated by protective T cells (8). A case has been made for the role of T cells as correlates of protection (21). Our evidence from this analysis 22 (±2) days after one dose is that T cell immunity is mostly variably low but also relatively unperturbed by the N501Y mutation. The other mutations we considered that overlay CD4 epitopes were, as might be predicted, distributed across the range of HLAII polymorphisms. Those alleles associated with loss of CD4 response to the variant pool tended to be those with a lysine in pocket 4 of the groove (HLA-DR residue 71β), whereas those with an increased response to the variant pool tended to be those with a smaller amino acid, alanine. In HLA-DRB1*0401 transgenics, we confirmed that in the context of a given HLAII heterodimer, the N501Y mutation can result in ablation of this part of the T cell response, demonstrating that HLA polymorphisms are likely to be significant determinants of responder and nonresponder status with respect to vaccine escape.
SARS-CoV-2 immunity now encompasses postinfection plus either zero, one, or two vaccine doses and first and second dose naïve vaccinated. Single-dose vaccination after infection achieves similar levels of S1 RBD binding antibodies to two doses in naïve vaccinated individuals and second-dose vaccination in one-dose vaccinated postinfection individuals offers no additional enhancement (22). Moving forward, it will be important to resolve the quantitative and qualitative differences between these groups in terms of neutralizing antibody repertoire as well as phenotype and durability of memory B and T cell responses. Durability of immunity to natural infection and after vaccination as well as sustained vaccine efficacy and vaccine escape need to be monitored over time.
Acknowledgments
The authors thank all the HCW participants for donating their samples and data for these analyses as well as the research teams involved in consenting, recruitment, and sampling of the HCW participants. The COVIDsortium Healthcare Workers bioresource was approved by the ethical committee of UK National Research Ethics Service (20/SC/0149) and registered on clinicaltrials.gov (NCT04318314). The study conformed to the principles of the Helsinki Declaration, and all subjects gave written informed consent. The authors thank S. Astbury for help imputing HLA genotypes from GWAS data; S. Murray, F. Pieper, and K.-M. Lin for help processing HCW PBMC and serum samples; and the James Wigg Practice, London, UK, for support. Funding: The COVIDsortium is supported by funding donated by individuals, charitable trusts, and corporations, including Goldman Sachs, Citadel, and Citadel Securities, the Guy Foundation, GW Pharmaceuticals, Kusuma Trust, and Jagclif Charitable Trust, and enabled by Barts Charity with support from UCLH Charity. Wider support is acknowledged on the COVIDsortium website (https://covid-consortium.com/). Institutional support from Barts Health NHS Trust and Royal Free NHS Foundation Trust facilitated study processes, in partnership with University College London and Queen Mary University of London. R.B. and D.M.A. are supported by MRC (MR/S019553/1, MR/R02622X/1, and MR/V036939/1), NIHR Imperial Biomedical Research Centre (BRC):ITMAT, Cystic Fibrosis Trust SRC (2019SRC015), and Horizon 2020 Marie Skłodowska-Curie Innovative Training Network (ITN) European Training Network (860325). Á.M. is supported by Rosetrees Trust, the John Black Charitable Foundation, and Medical College of St Bartholomew’s Hospital Trust. J.C.M., C.M., and T.A.T. are directly and indirectly supported by the University College London Hospitals (UCLH) and Barts NIHR Biomedical Research Centres and through a British Heart Foundation (BHF) Accelerator Award (AA/18/6/34223). T.A.T. is funded by a BHF Intermediate Research Fellowship (FS/19/35/34374). M.N. is supported by the Wellcome Trust (207511/Z/17/Z) and by NIHR Biomedical Research Funding to UCL and UCLH. M.K.M. is supported by UKRI/NIHR UK-CIC, a Wellcome Trust Investigator Award (214191/Z/18/Z), and a CRUK Immunology grant (26603). A.M.V., Á.M., C.M., and J.C.M. were supported by UKRI/MRC COVID-19 Rapid response grant COV0331 MR/V027883/1. The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report. Author contributions: R.B. conceptualized the study. C.M., T.A.T., J.C.M., M.N., Á.M., D.M.A., and R.B. designed the study. R.B. and D.M.A. designed and supervised the T cell and B cell experiments. A.M.V. supervised HLA analysis. T.B. and A.Se. supervised S1 IgG and N IgG/IgM studies. Á.M. designed and supervised the nAb experiments. C.J.R. and D.K.B. developed, performed, and analyzed the T cell and B cell experiments. J.M.G. and C.P. developed, performed, and analyzed the nAb experiments. A.D.O. performed and A.Se. analyzed the RBD and N antibody assays. T.B., C.M., Á.M., T.A.T., J.C.M., and M.N. conceptualized and established the HCW cohort. R.B., T.A.T., J.C.M., and C.M. designed the vaccine substudy recruitment. K.M., M.F., A.Sm., J.E.S.-W., C.M., T.A.T., and J.C.M. collected HCW samples. C.J.R. and D.K.B. processed HCW samples. R.B., C.J.R., D.K.B., J.M.G., C.P., Á.M., and D.M.A. analyzed the data. D.M.A., C.J.R., M.K.M., Á.M., B.C., C.M., T.A.T., J.C.M., A.Se., T.B., M.N., A.M.V., and R.B. interpreted the data. R.B. and D.M.A. wrote the manuscript with input from all the authors. All the authors reviewed and edited the manuscript and figures. Competing interests: R.B. and D.M.A. are members of the Global T cell Expert Consortium and have consulted for Oxford Immunotec outside the submitted work. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper or the supplementary materials. SARS-CoV-2 nucleoprotein (100982) and SARS-CoV-2 spike (100979) are available from P. Cherepanov, Francis Crick Institute, UK, under a material transfer agreement with Centre for AIDS Reagents (CFAR), National Institute for Biological Standards and Control (NIBSC), UK. The SARS-CoV-2 B.1.1.7 isolate was obtained from NIBSC, thanks to the contribution of PHE Porton Down and S. Funnell. The nCoV19 isolate/UK ex South African/2021 lineage B.1.351 EVA catalog code 04V-04071 was obtained from European Virus Archive Global, PHE Porton Down. The SARS-CoV-2 Wuhan-Hu-1 Human 2019-nCoV Isolate EVA catalog code 026V-03883 was obtained from European Virus Archive Global, Charité – Universitätsmedizin Berlin. This work is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/. This license does not apply to figures/photos/artwork or other content included in the article that is credited to a third party; obtain authorization from the rights holder before using such material.
Supplementary Materials
science.sciencemag.org/content/372/6549/1418/suppl/DC1
Materials and Methods
Figs. S1 to S4
Tables S1 to S5
UK COVIDsortium Investigators Collaborator List
UK COVIDsortium Immune Correlates Network Collaborator List
MDAR Reproducibility Checklist
Contributor Information
Collaborators: Hakam Abbass, Aderonke Abiodun, Mashael Alfarih, Zoe Alldis, Daniel M. Altmann, Oliver E. Amin, Mervyn Andiapen, Jessica Artico, João B. Augusto, Georgina L. Baca, Sasha N. L. Bailey, Anish N. Bhuva, Alex Boulter, Ruth Bowles, Rosemary J. Boyton, Olivia V. Bracken, Ben O’Brien, Tim Brooks, Natalie Bullock, David K. Butler, Gabriella Captur, Nicola Champion, Carmen Chan, Aneesh Chandran, David Collier, Jorge Couto de Sousa, Xose Couto-Parada, Teresa Cutino-Moguel, Rhodri H. Davies, Brooke Douglas, Cecilia Di Genova, Keenan Dieobi-Anene, Mariana O. Diniz, Anaya Ellis, Karen Feehan, Malcolm Finlay, Marianna Fontana, Nasim Forooghi, Celia Gaier, Joseph M. Gibbons, Derek Gilroy, Matt Hamblin, Gabrielle Harker, Jacqueline Hewson, Wendy Heywood, Lauren M. Hickling, Aroon D. Hingorani, Lee Howes, Alun Hughes, Gemma Hughes, Rebecca Hughes, Ivie Itua, Victor Jardim, Wing-Yiu Jason Lee, Melaniepetra Jensen, Jessica Jones, Meleri Jones, George Joy, Vikas Kapil, Hibba Kurdi, Jonathan Lambourne, Kai-Min Lin, Sarah Louth, Mala K. Maini, Vineela Mandadapu, Charlotte Manisty, Áine McKnight, Katia Menacho, Celina Mfuko, Kevin Mills, Oliver Mitchelmore, Christopher Moon, James C. Moon, Diana Munoz-Sandoval, Sam M. Murray, Mahdad Noursadeghi, Ashley Otter, Corinna Pade, Susana Palma, Ruth Parker, Kush Patel, Babita Pawarova, Steffen E. Petersen, Brian Piniera, Franziska P. Pieper, Daniel Pope, Mary Prossora, Lisa Rannigan, Alicja Rapala, Catherine J. Reynolds, Amy Richards, Matthew Robathan, Joshua Rosenheim, Genine Sambile, Nathalie M. Schmidt, Amanda Semper, Andreas Seraphim, Mihaela Simion, Angelique Smit, Michelle Sugimoto, Leo Swadling, Stephen Taylor, Nigel Temperton, Stephen Thomas, George D. Thornton, Thomas A. Treibel, Art Tucker, Jessry Veerapen, Mohit Vijayakumar, Sophie Welch, Theresa Wodehouse, Lucinda Wynne, Dan Zahedi, and Benjamin Chain
References and Notes
- 1.Polack F. P., Thomas S. J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J. L., Pérez Marc G., Moreira E. D., Zerbini C., Bailey R., Swanson K. A., Roychoudhury S., Koury K., Li P., Kalina W. V., Cooper D., Frenck R. W. Jr.., Hammitt L. L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D. B., Mather S., Dormitzer P. R., Şahin U., Jansen K. U., Gruber W. C.; C4591001 Clinical Trial Group , Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020). 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mascola J. R., Graham B. S., Fauci A. S., SARS-CoV-2 viral variants—tackling a moving target. JAMA 325, 1261–1262 (2021). 10.1001/jama.2021.2088 [DOI] [PubMed] [Google Scholar]
- 3.Altmann D. M., Boyton R. J., Beale R., Immunity to SARS-CoV-2 variants of concern. Science 371, 1103–1104 (2021). 10.1126/science.abg7404 [DOI] [PubMed] [Google Scholar]
- 4.A. Rambaut, N. Loman, O. Pybus, W. Barclay, J. Barrett, A. Carabelli, T. Connor, T. Peacock, D. L. Robertson, E. Volz, on behalf of COVID-19 Genomics Consortium UK (CoG-UK), Preliminary genomic characterisation of an emergent SARS-CoV-2 lineage in the UK defined by a novel set of spike mutations, virological.org (2020); https://virological.org/t/preliminary-genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-the-uk-defined-by-a-novel-set-of-spike-mutations/563.
- 5.Wang Z., Schmidt F., Weisblum Y., Muecksch F., Barnes C. O., Finkin S., Schaefer-Babajew D., Cipolla M., Gaebler C., Lieberman J. A., Oliveira T. Y., Yang Z., Abernathy M. E., Huey-Tubman K. E., Hurley A., Turroja M., West K. A., Gordon K., Millard K. G., Ramos V., Da Silva J., Xu J., Colbert R. A., Patel R., Dizon J., Unson-O’Brien C., Shimeliovich I., Gazumyan A., Caskey M., Bjorkman P. J., Casellas R., Hatziioannou T., Bieniasz P. D., Nussenzweig M. C., mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature 592, 616–622 (2021). 10.1038/s41586-021-03324-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collier D. A., De Marco A., Ferreira I. A. T. M., Meng B., Datir R. P., Walls A. C., Kemp S. A., Bassi J., Pinto D., Silacci-Fregni C., Bianchi S., Tortorici M. A., Bowen J., Culap K., Jaconi S., Cameroni E., Snell G., Pizzuto M. S., Pellanda A. F., Garzoni C., Riva A., Elmer A., Kingston N., Graves B., McCoy L. E., Smith K. G. C., Bradley J. R., Temperton N., Ceron-Gutierrez L., Barcenas-Morales G., Harvey W., Virgin H. W., Lanzavecchia A., Piccoli L., Doffinger R., Wills M., Veesler D., Corti D., Gupta R. K.; CITIID-NIHR BioResource COVID-19 Collaboration; COVID-19 Genomics UK (COG-UK) Consortium , Sensitivity of SARS-CoV-2 B.1.1.7 to mRNA vaccine-elicited antibodies. Nature 593, 136–141 (2021). 10.1038/s41586-021-03412-7 [DOI] [PubMed] [Google Scholar]
- 7.Walsh E. E., Frenck R. W. Jr.., Falsey A. R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M. J., Bailey R., Swanson K. A., Li P., Koury K., Kalina W., Cooper D., Fontes-Garfias C., Shi P. Y., Türeci Ö., Tompkins K. R., Lyke K. E., Raabe V., Dormitzer P. R., Jansen K. U., Şahin U., Gruber W. C., Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N. Engl. J. Med. 383, 2439–2450 (2020). 10.1056/NEJMoa2027906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L. M., Vormehr M., Baum A., Pascal K., Quandt J., Maurus D., Brachtendorf S., Lörks V., Sikorski J., Hilker R., Becker D., Eller A. K., Grützner J., Boesler C., Rosenbaum C., Kühnle M. C., Luxemburger U., Kemmer-Brück A., Langer D., Bexon M., Bolte S., Karikó K., Palanche T., Fischer B., Schultz A., Shi P. Y., Fontes-Garfias C., Perez J. L., Swanson K. A., Loschko J., Scully I. L., Cutler M., Kalina W., Kyratsous C. A., Cooper D., Dormitzer P. R., Jansen K. U., Türeci Ö., COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 586, 594–599 (2020). 10.1038/s41586-020-2814-7 [DOI] [PubMed] [Google Scholar]
- 9.Treibel T. A., Manisty C., Burton M., McKnight Á., Lambourne J., Augusto J. B., Couto-Parada X., Cutino-Moguel T., Noursadeghi M., Moon J. C., COVID-19: PCR screening of asymptomatic health-care workers at London hospital. Lancet 395, 1608–1610 (2020). 10.1016/S0140-6736(20)31100-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Augusto J. B., Menacho K., Andiapen M., Bowles R., Burton M., Welch S., Bhuva A. N., Seraphim A., Pade C., Joy G., Jensen M., Davies R. H., Captur G., Fontana M., Montgomery H., O’Brien B., Hingorani A. D., Cutino-Moguel T., McKnight Á., Abbass H., Alfarih M., Alldis Z., Baca G. L., Boulter A., Bracken O. V., Bullock N., Champion N., Chan C., Couto-Parada X., Dieobi-Anene K., Feehan K., Figtree G., Figtree M. C., Finlay M., Forooghi N., Gibbons J. M., Griffiths P., Hamblin M., Howes L., Itua I., Jones M., Jardim V., Kapil V., Jason Lee W.-Y., Mandadapu V., Mfuko C., Mitchelmore O., Palma S., Patel K., Petersen S. E., Piniera B., Raine R., Rapala A., Richards A., Sambile G., Couto de Sousa J., Sugimoto M., Thornton G. D., Artico J., Zahedi D., Parker R., Robathan M., Hickling L. M., Ntusi N., Semper A., Brooks T., Jones J., Tucker A., Veerapen J., Vijayakumar M., Wodehouse T., Wynne L., Treibel T. A., Noursadeghi M., Manisty C., Moon J. C., Healthcare Workers Bioresource: Study outline and baseline characteristics of a prospective healthcare worker cohort to study immune protection and pathogenesis in COVID-19. Wellcome Open Res. 5, 179 (2020). 10.12688/wellcomeopenres.16051.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reynolds C. J., Swadling L., Gibbons J. M., Pade C., Jensen M. P., Diniz M. O., Schmidt N. M., Butler D. K., Amin O. E., Bailey S. N. L., Murray S. M., Pieper F. P., Taylor S., Jones J., Jones M., Lee W. J., Rosenheim J., Chandran A., Joy G., Di Genova C., Temperton N., Lambourne J., Cutino-Moguel T., Andiapen M., Fontana M., Smit A., Semper A., O’Brien B., Chain B., Brooks T., Manisty C., Treibel T., Moon J. C., Noursadeghi M., Altmann D. M., Maini M. K., McKnight Á., Boyton R. J.; COVIDsortium investigators; COVIDsortium immune correlates network , Discordant neutralizing antibody and T cell responses in asymptomatic and mild SARS-CoV-2 infection. Sci. Immunol. 5, eabf3698 (2020). 10.1126/sciimmunol.abf3698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manisty C., Treibel T. A., Jensen M., Semper A., Joy G., Gupta R. K., Cutino-Moguel T., Andiapen M., Jones J., Taylor S., Otter A., Pade C., Gibbons J., Lee J., Bacon J., Thomas S., Moon C., Jones M., Williams D., Lambourne J., Fontana M., Altmann D. M., Boyton R., Maini M., McKnight A., Chain B., Noursadeghi M., Moon J. C., Time series analysis and mechanistic modelling of heterogeneity and sero-reversion in antibody responses to mild SARS‑CoV-2 infection. EBioMedicine 65, 103259 (2021). 10.1016/j.ebiom.2021.103259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. COVID-19 vaccines: Acting on the evidence. Nat. Med. 27, 183 (2021). 10.1038/s41591-021-01261-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manisty C., Otter A. D., Treibel T. A., McKnight Á., Altmann D. M., Brooks T., Noursadeghi M., Boyton R. J., Semper A., Moon J. C., Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet 397, 1057–1058 (2021). 10.1016/S0140-6736(21)00501-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen X., Tang H., McDanal C., Wagh K., Fischer W., Theiler J., Yoon H., Li D., Haynes B. F., Sanders K. O., Gnanakaran S., Hengartner N., Pajon R., Smith G., Glenn G. M., Korber B., Montefiori D. C., SARS-CoV-2 variant B.1.1.7 is susceptible to neutralizing antibodies elicited by ancestral spike vaccines. Cell Host Microbe 29, 529–539.e3 (2021). 10.1016/j.chom.2021.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greaney A. J., Loes A. N., Crawford K. H. D., Starr T. N., Malone K. D., Chu H. Y., Bloom J. D., Comprehensive mapping of mutations in the SARS-CoV-2 receptor-binding domain that affect recognition by polyclonal human plasma antibodies. Cell Host Microbe 29, 463–476.e6 (2021). 10.1016/j.chom.2021.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Supasa P., Zhou D., Dejnirattisai W., Liu C., Mentzer A. J., Ginn H. M., Zhao Y., Duyvesteyn H. M. E., Nutalai R., Tuekprakhon A., Wang B., Paesen G. C., Slon-Campos J., López-Camacho C., Hallis B., Coombes N., Bewley K. R., Charlton S., Walter T. S., Barnes E., Dunachie S. J., Skelly D., Lumley S. F., Baker N., Shaik I., Humphries H. E., Godwin K., Gent N., Sienkiewicz A., Dold C., Levin R., Dong T., Pollard A. J., Knight J. C., Klenerman P., Crook D., Lambe T., Clutterbuck E., Bibi S., Flaxman A., Bittaye M., Belij-Rammerstorfer S., Gilbert S., Hall D. R., Williams M. A., Paterson N. G., James W., Carroll M. W., Fry E. E., Mongkolsapaya J., Ren J., Stuart D. I., Screaton G. R., Reduced neutralization of SARS-CoV-2 B.1.1.7 variant by convalescent and vaccine sera. Cell 184, 2201–2211.e7 (2021). 10.1016/j.cell.2021.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muik A., Wallisch A. K., Sänger B., Swanson K. A., Mühl J., Chen W., Cai H., Maurus D., Sarkar R., Türeci Ö., Dormitzer P. R., Şahin U., Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera. Science 371, 1152–1153 (2021). 10.1126/science.abg6105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reynolds C. J., Suleyman O. M., Ortega-Prieto A. M., Skelton J. K., Bonnesoeur P., Blohm A., Carregaro V., Silva J. S., James E. A., Maillère B., Dorner M., Boyton R. J., Altmann D. M., T cell immunity to Zika virus targets immunodominant epitopes that show cross-reactivity with other Flaviviruses. Sci. Rep. 8, 672 (2018). 10.1038/s41598-017-18781-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krammer F., Srivastava K., Alshammary H., Amoako A. A., Awawda M. H., Beach K. F., Bermúdez-González M. C., Bielak D. A., Carreño J. M., Chernet R. L., Eaker L. Q., Ferreri E. D., Floda D. L., Gleason C. R., Hamburger J. Z., Jiang K., Kleiner G., Jurczyszak D., Matthews J. C., Mendez W. A., Nabeel I., Mulder L. C. F., Raskin A. J., Russo K. T., Salimbangon A. T., Saksena M., Shin A. S., Singh G., Sominsky L. A., Stadlbauer D., Wajnberg A., Simon V., Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N. Engl. J. Med. 384, 1372–1374 (2021). 10.1056/NEJMc2101667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertoletti A., Tan A. T., Le Bert N., The T-cell response to SARS-CoV-2: Kinetic and quantitative aspects and the case for their protective role. Oxford Open Immunology 2, iqab006 (2021). 10.1093/oxfimm/iqab006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goel R. R., Apostolidis S. A., Painter M. M., Mathew D., Pattekar A., Kuthuru O., Gouma S., Hicks P., Meng W., Rosenfeld A. M., Dysinger S., Lundgreen K. A., Kuri-Cervantes L., Adamski S., Hicks A., Korte S., Oldridge D. A., Baxter A. E., Giles J. R., Weirick M. E., McAllister C. M., Dougherty J., Long S., D’Andrea K., Hamilton J. T., Betts M. R., Luning Prak E. T., Bates P., Hensley S. E., Greenplate A. R., Wherry E. J., Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci. Immunol. 6, eabi6950 (2021). 10.1126/sciimmunol.abi6950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Public Health England, COVID-19: laboratory evaluations of serological assays, gov.uk, 16 March 2021; www.gov.uk/government/publications/covid-19-laboratory-evaluations-of-serological-assays.
- 24.Peng Y., Mentzer A. J., Liu G., Yao X., Yin Z., Dong D., Dejnirattisai W., Rostron T., Supasa P., Liu C., López-Camacho C., Slon-Campos J., Zhao Y., Stuart D. I., Paesen G. C., Grimes J. M., Antson A. A., Bayfield O. W., Hawkins D. E. D. P., Ker D. S., Wang B., Turtle L., Subramaniam K., Thomson P., Zhang P., Dold C., Ratcliff J., Simmonds P., de Silva T., Sopp P., Wellington D., Rajapaksa U., Chen Y.-L., Salio M., Napolitani G., Paes W., Borrow P., Kessler B. M., Fry J. W., Schwabe N. F., Semple M. G., Baillie J. K., Moore S. C., Openshaw P. J. M., Ansari M. A., Dunachie S., Barnes E., Frater J., Kerr G., Goulder P., Lockett T., Levin R., Zhang Y., Jing R., Ho L.-P., Cornall R. J., Conlon C. P., Klenerman P., Screaton G. R., Mongkolsapaya J., McMichael A., Knight J. C., Ogg G., Dong T.; Oxford Immunology Network Covid-19 Response T cell Consortium; ISARIC4C Investigators , Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol. 21, 1336–1345 (2020). 10.1038/s41590-020-0782-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.GISAID, Tracking of variants; www.gisaid.org/hcov19-variants/.
- 26.Reynisson B., Alvarez B., Paul S., Peters B., Nielsen M., NetMHCpan-4.1 and NetMHCIIpan-4.0: Improved predictions of MHC antigen presentation by concurrent motif deconvolution and integration of MS MHC eluted ligand data. Nucleic Acids Res. 48, W449–W454 (2020). 10.1093/nar/gkaa379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quigley K. J., Reynolds C. J., Goudet A., Raynsford E. J., Sergeant R., Quigley A., Worgall S., Bilton D., Wilson R., Loebinger M. R., Maillere B., Altmann D. M., Boyton R. J., Chronic infection by mucoid Pseudomonas aeruginosa associated with dysregulation in T-cell immunity to outer membrane porin F. Am. J. Respir. Crit. Care Med. 191, 1250–1264 (2015). 10.1164/rccm.201411-1995OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynolds C., Goudet A., Jenjaroen K., Sumonwiriya M., Rinchai D., Musson J., Overbeek S., Makinde J., Quigley K., Manji J., Spink N., Yos P., Wuthiekanun V., Bancroft G., Robinson J., Lertmemongkolchai G., Dunachie S., Maillere B., Holden M., Altmann D., Boyton R., T cell immunity to the alkyl hydroperoxide reductase of Burkholderia pseudomallei: A correlate of disease outcome in acute melioidosis. J. Immunol. 194, 4814–4824 (2015). 10.4049/jimmunol.1402862 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
science.sciencemag.org/content/372/6549/1418/suppl/DC1
Materials and Methods
Figs. S1 to S4
Tables S1 to S5
UK COVIDsortium Investigators Collaborator List
UK COVIDsortium Immune Correlates Network Collaborator List
MDAR Reproducibility Checklist


