Abstract
Background:
Premenstrual dysphoric disorder (PMDD) is a new DSM-5 diagnosis characterized by the cyclical emergence of emotional and physical symptoms in the luteal phase of the menstrual cycle, with symptom remission in the follicular phase. Converging evidence highlights the possibility of distinct subtypes of PMDD with unique pathophysiologies, but temporal subgroups have yet to be explored in a systematic way.
Methods:
In the present work, we use group-based trajectory modeling (GBTM) to identify unique trajectory subgroups of core emotional and total PMDD symptoms across the perimenstrual frame (days −14 to +9, where day 0 is menstrual onset) in a sample of 74 individuals prospectively diagnosed with DSM-5 PMDD.
Results:
For the total daily symptom score, the best-fitting model was comprised of three groups: a group demonstrating moderate symptoms only in the premenstrual week (65%), a group demonstrating severe symptoms across the full two weeks of the luteal phase (17.5%), and a group demonstrating severe symptoms in the premenstrual week that were slow to resolve in the follicular phase (17.5%).
Conclusions:
These trajectory groups are discussed in the context of the latest work on the pathophysiology of PMDD. Experimental work is needed to test for the presence of possible pathophysiologic differences in trajectory groups, and whether unique treatment approaches are needed.
Keywords: menstrual cycle, premenstrual syndrome, premenstrual dysphoric disorder, group-based trajectory modeling
Introduction
Premenstrual dysphoric disorder (PMDD) is a new DSM-5 disorder characterized by the cyclical emergence of emotional and physical symptoms in the luteal phase of the menstrual cycle with remission in the week following menses (APA 2013). Although just 5.5% of females meet DSM-5 diagnostic criteria for PMDD, which requires five distinct cycling symptoms (at least one emotional (Gehlert et al. 2009)), it is estimated that an additional 7.5-13.5% show milder or subthreshold symptoms of PMDD that interfere with daily life (Halbreich et al., 2003). Some effective treatments for PMDD are available, including selective serotonin reuptake inhibitors (SSRIs; (Marjoribanks et al. 2013)), drospirenone-containing oral contraceptives on a 24-4 schedule (Lopez et al. 2012), and, in severe cases, elimination of ovarian cycling using a GnRH agonist or oopheretomy (Wyatt et al. 2004). Despite this, the majority of patients in clinical trials for PMDD do not respond adequately (Halbreich 2008; Kleinstäuber et al. 2012). A clearer understanding of the causes of PMDD is necessary for targeted treatment development.
One limiting factor for treatment development is the implicit assumption that PMDD represents a singular disorder with one pathophysiology. In fact, even samples comprised exclusively of those who have been prospectively diagnosed with PMDD (using two months of daily ratings) demonstrate heterogeneity with respect to symptom type, severity, and treatment response, raising the question of whether distinct subtypes of PMDD might exist (Pearlstein et al. 2005; Halbreich 2008). Heterogeneity of treatment response has been observed in RCTs of both SSRIs and oral contraceptives for PMDD (Halbreich, 2008; Halbreich et al., 2006; Freeman et al., 2011). Surprisingly, individuals with PMDD also differ in response to treatment with GnRH agonist (which induces low, stable hormone levels similar to menopause); only 50-60% of those with prospectively-confirmed PMDD seem to respond to this treatment (Wyatt et al., 2004), with the rest developing persistent symptoms during treatment. Given that GnRH agonists reliably suppress hormone change, which is the widely-accepted pathophysiological trigger for PMDD (Schmidt et al. 1998), this would seem to point to an alternative pathophysiology in some cases, such as sensitivity to hormone withdrawal or deprivation (e.g., see Eisenlohr-Moul et al., 2018). It is possible that unique risk processes underpin different subtypes of PMDD, which may help to inform treatment development and selection.
Previous work has focused on subtypes of PMDD with respect to content or degree of background symptoms, but no work to date has used empirical methods to derive temporal subtypes (defined by the timing of symptom onset and offset). A few studies have distinguished symptom content subgroups on the basis of physical vs. emotional complaints, or high-vs-low arousal symptoms (e.g., PMDD symptom of irritability vs. depression; Freeman et al., 2011). Still other papers make theoretical or empirical distinctions based on the presence or severity of background symptoms, such as differentiating between “pure” PMDD (those with minimal or absent postmenstrual symptoms) and those with premenstrual exacerbation (PME) of underlying psychiatric disorders, who show chronic symptoms that are exacerbated premenstrually (ISPMD, 2016; Freeman et al., 1997). However, to our knowledge, no studies to date have examined longitudinal data for evidence of subtypes of the perimenstrual timing (i.e., trajectory) of PMDD symptom onset or offset (i.e., temporal subtypes). As the time dynamics of symptoms are the defining criteria for the diagnosis of PMDD, individual differences in symptom timing may be important.
The timing of PMDD symptom onset (relative to ovulation or menses) varies substantially (Pearlstein et al., 2005; Epperson et al., 2011), with PMDD symptom onset occurring anywhere from the beginning of the luteal phase (i.e., immediately following ovulation) to the final few days before menses. This timing can substantially alter the overall burden of symptoms. For example, if symptoms appear at ovulation and remit when menses starts, the patient would suffer the full 12-14 days of a typical luteal phase; however, if symptoms appear only in the 4 days prior to menses and remit when menses starts, the patient would experience just 4 days of symptoms. These timing differences could also correspond to pathophysiological differences in severity or type. For example, PMDD symptoms that arise quickly following ovulation and remit quickly with menses onset could indicate a marked, rapidly-developing reaction to the early luteal surge in the neurosteroid metabolites of progesterone (as in (Martinez et al. 2016), or a reaction to the pre-ovulatory surge in estradiol, which is capable of triggering symptoms in some with PMDD (Schmidt et al. 1998). As another example, PMDD symptoms arising closer to menstrual onset may indicate a more slowly-developing reaction to luteal progesterone changes (Redei & Freeman 1995), or a rapid deleterious response to late luteal ovarian steroid withdrawal (Lovick et al. 2017).
Similarly, the offset of PMDD symptoms may vary. Although PMDD generally demonstrates symptom offset during or just after menses, several studies have noted variability in the timing and speed of this symptom offset. There appears to be a normative rapid symptom offset in the first 3 days of menses (Pearlstein et al., 2005), but some symptoms, especially those related to depression, sometimes demonstrate a slower offset in PMDD (Bancroft et al. 1994; Dawson et al. 2018) and in PME of borderline personality disorder (Eisenlohr-Moul et al. 2018b). Although the mechanisms of these offset differences remain relatively understudied, both emotion regulation strategies (Dawson et al., 2018) and differences in response to hormone withdrawal (Eisenlohr-Moul et al., 2018) may play a role.
Although some authors have mentioned these timing differences as possible reasons that standardized diagnostic systems fail to capture all individuals with cyclical mood change (Schnurr 1989; Eisenlohr-Moul et al. 2017), no studies have used longitudinal data reduction methods to extract possible temporal subtypes of PMDD using prospective daily ratings in samples with prospectively-confirmed PMDD. Therefore, the purpose of this study was to use one month of daily symptom ratings and GBTM to identify underlying subgroups of symptom timing in PMDD. Since many patients do not respond adequately to existing treatments (Halbreich 2008; Kleinstäuber et al. 2012), it is our hope that identification of subtypes may aid in targeted treatment development and personalized medicine.
GBTM was chosen as a statistical method to identify temporal subtypes, since it allows for a data-driven (i.e., bottom-up) determination of unique trajectory groups. Other analytic options (e.g., group mixture modeling, multilevel modeling) assume that indicators of subgroup membership are measured in one’s dataset (e.g., testing whether different genotypes demonstrate unique effects of time on symptoms in a multilevel growth model), whereas GBTM allows for the extraction of statistically unique symptom trajectory groups regardless of whether causes or correlates of these subgroups are available (Nagin & Odgers 2010).
Hypotheses
We used a prospective design and GBTM to test the following hypotheses:
We hypothesized the existence of a full luteal phase trajectory group for each symptom characterized by onset around ovulation and offset during menses (two weeks of symptoms), and a premenstrual week trajectory group for each symptom characterized by onset in the week before menses and remission during menses (one week of symptoms; as observed in Eisenlohr-Moul et al., 2017).
Consistent with previous work (e.g., Bancroft et al., 1994, Eisenlohr-Moul et al., 2018; Dawson et al., 2017), we also hypothesized that, especially for depressive symptoms, one trajectory group would show a late offset of symptoms, with symptoms arising at any point in the luteal phase but remitting more slowly or incompletely in the menstrual and postmenstrual weeks.
Methods
Participants
Participants of this study were recruited in the context of two studies: In one study, emotional interference, attentional processes, and emotion regulation were examined in PMDD. The other study (Kues et al. 2014) was clinical trial for an Internet-based cognitive behavioral program for PMDD (clinicaltrials.gov identifier: NCT01961479). Both studies included secondary analyses of diary data were approved by local institutional review boards. In both studies, participants were recruited via media advertisements (articles, local television, social networks) and through gynecologists and primary care physicians.
Inclusion criteria included a) 18-45 years of age, b) regular menstrual cycles, c) sufficient knowledge of German, and d) meeting DSM-5 criteria for PMDD across two months of daily ratings (American Psychiatric Association, 2013). Exclusion criteria were a) gynecological problems (e.g., hysterectomy, gynecological cancer, infertility), b) pregnancy or childbirth or lactation in past 3 months, c) psychosis, bipolar disorder, eating disorder, moderate or severe depression, somatic symptom disorder or suicidal ideation, d) intake of psychotropic or hormonal medication, or e) current psychotherapy.
Of 355 females who initially decided to participate by signing informed consent for one of the two studies, 235 (66%) were ineligible, and 46 (13%) were excluded due to insufficient symptom ratings. The final sample comprised 74 individuals with mean age of 31.57 years (SD = 6.15, range: 20 to 44 years; see Table 1 for demographics).
Table 1.
Participant characteristics (N = 74)
| Variable | Mean (SD); n (%) |
Range |
|---|---|---|
| Age | 31.57 (6.15) | 20 - 44 |
| German nationality | 67 (90.54) | |
| Education Level | ||
| Secondary school degree | 8 (10.81) | |
| Senior high school degree | 24 (32.43) | |
| Academic degree | 42 (56.75) | |
| Marital status | ||
| Married | 25 (33.78) | |
| Stable relationship | 28 (37.84) | |
| Single | 19 (25.68) | |
| Divorced/separated | 2 (2.70) | |
| Average cycle length in days | ||
| 28 +/−5 | 59 (79.73) | |
| > 33 | 15 (20.27) | |
| Age at menarche in years | 13.00 (1.60) | 9 - 17 |
| Biological children | 27 (36.49) | |
| Previously received treatment due to premenstrual complaints | 21 (28.38) |
Procedure
Initially, those interested in participating signed informed consent and completed an initial retrospective screening for PMDD (Ditzen et al. 2011) which covers all DSM-5 criteria (American Psychiatric Association, 2013) and completed demographic, menstrual cycle and medication questions, and the Brief Web-Based Screening Questionnaire for Mental disorders (Donker et al. 2009). Eligible individuals completed a telephone interview based on the International Diagnostic Checklists for ICD-10 (Janca & Hiller 1996) to assess comorbid mental disorders. Eligible individuals were then asked to complete daily records of their premenstrual symptoms over two consecutive menstrual cycles.
Measures and prospective diagnosis of PMDD
We applied a validated daily symptom report (Janda et al. 2017) with 27 items assessing all 11 DSM-5 symptoms of PMDD and 3 items measuring impairment. All items are rated on a four-point Likert scale ranging from 0=not true at all, to 3=absolutely true. In the present study, we examined the daily total score, muscle pain (i.e., cramps), and four items that capture the core emotional symptoms listed in DSM-5 PMDD: (1) sudden sadness (representing mood lability symptoms), (2) anger/irritability, (3) anxiety, and (4) sadness (representing depressive symptoms). Therefore, six outcomes were examined.
As mentioned above, to be included in the study, participants had to fulfill strict numerical criteria for DSM-5 PMDD which was operationalized as follows: a) a score of ≥ 2 (on a scale of 0-3) on at least five of the 11 DSM-5 PMDD symptoms (including at least one affective symptom) for at least 2 days during the premenstrual week (corresponding to days −7 to −1 where menstrual onset is day 0), and b) a score of ≥ 2 for at least one dimension of functional impairment for at least 2 days during the premenstrual week. The symptoms which were elevated during the luteal phase had to remit to a score ≤ 1 (on the scale of 0-3) during the mid-follicular phase. As the follicular phase (determined as days 6–10 of a menstrual cycle with a cycle length of 28 days) varies between individuals (Chiazze et al. 1968), it was adjusted for each participant related to the individual cycle length (see Janda et al. 2017, for details). The diagnosis was made during a baseline menstrual cycle (the same one used herein) using the numerical cut scores described above.
Statistical Analyses
Creating a Standardized Timeline for Trajectory Analysis.
The purpose of our analyses was to identify, for each daily symptom examined, subgroups with unique trajectories of symptom onset and offset across the menstrual cycle (i.e., where day of the menstrual cycle represents the time variable on the X axis, and the daily symptom rating was the outcome variable on the Y axis). When using GBTM, it is important to ensure that the time variable takes on the same meaning for all participants (Nagin & Odgers 2010). In the case of the menstrual cycle, our goal was to standardize the hormonal meaning of each menstrual cycle day on the timeline. Of note, differences or changes in the length of the menstrual cycle (e.g., 28 days vs. 35 days) are known to be due almost entirely to fluctuations in the length of the follicular phase, while the length of the luteal phase is relatively fixed at 12-14 days due to the predetermined lifespan of the corpus luteum. Therefore, a “menses-to-menses” cycle was rejected as our timeline due to differences in length of the follicular phase (menses onset to ovulation), which would change the hormonal meaning of a forward-count timeline (in which day 0 = menses onset). Instead, an “ovulation to ovulation” cycle was created using a combination of backward and forward count (as in Edler et al. 2007). A backward count from menses onset was used to identify days −15 to −1 (the day before menses onset), which correspond to ovulation (days −15 to −12), the mid-luteal phase (−9 to −5), and the premenstrual phase (days −4 to 0). For the follicular phase, a forward count from day 0 (the day of menses onset) to day +9 was utilized to identify the menstrual (1 to +5) and mid-follicular ( +6 to +9) phases. Therefore, this timeline uses a combination of backward and forward count strategically to align each person’s timeline to predictable hormonal events (Schmalenberger et al. 2019). This timeline consists of 24 days centered around the perimenstrual frame (Eisenlohr-Moul et al. 2017), when PMDD symptoms generally emerge and then remit.
Group-Based Trajectory Modeling (GBTM).
We utilized GBTM to identify, for each symptom examined, groups showing different trajectories of change across the ovulation-to-ovulation timeline (days −15 to +9). This statistical approach yields discrete trajectory groups that exhibit statistically unique patterns of change over time for a particular repeated measure (Charnigo et al., 2011; Nagin & Odgers, 2010). PROC TRAJ (Jones & Nagin, 2007) was utilized in SAS statistical software (Version 9.4; SAS Institute Inc., Cary, NC). PROC TRAJ allows persons with missing data to contribute to estimation of model parameters. Because PMDD is characterized by many asymptomatic days (i.e., ratings of 0), all daily symptom outcomes (except the total) violated assumptions of normality due to zero-inflation of responses. Therefore, trajectory groups were estimated using zero-inflated Poisson distributions, except for the total score, which was estimated using a normal model. Because an appropriate number of trajectory groups was not known a priori, we used the Bayesian information criterion (BIC; lower values preferred) and Akaike information criterion (AIC; lower values preferred) to select the best-fitting number of groups, examining one, two, and three groups for each symptom (more groups were not expected and could replicability of groups given our moderate sample size; Nagin & Odgers 2010). Likewise, for each group we used the BIC and AIC to select the polynomial orders of mean-level symptom change for each group across the timeline (examining 0=flat/absent, 1=linear, 2=quadratic, 3=cubic) as well as the polynomial orders of zero inflation across the timeline for each group (0,1,2,3). For each outcome, a macro was used to test each permutation of (1) number of groups, (2) all possible combinations of group polynomials for change over time, and (3) all possible combinations of zero-inflation polynomials (representing the shape of zero-inflation over time for each group). We present fit indices for the best-fitting models (described in Table 2).
Table 2.
Results of Zero-Inflated Poisson Group-Based Trajectory Modeling of PMDD Symptom Trajectories Across the Cycle (Days −14 to +9 relative to menses onset=0; N=74)
| Symptom | Group 1 % (N) (shape of trajectory) |
Group 2 % (N) (shape of trajectory) |
Group 3 % (N) (shape of trajectory) |
Fit Indices for Selected Model |
|---|---|---|---|---|
| Total Symptom Score |
Moderate Premenstrual Week 65% (48) (quadratic) |
Severe Premenstrual Week with Late Offset 17.5% (13) (cubic) |
Severe Full Luteal Phase 17.5% (13) (cubic) |
BIC: −1501.99 AIC: −1463.66 |
|
Sadness (Depression) |
Severe Premenstrual Week with Late Offset 42.9% (32) (quadratic, quadratic ZI) |
Severe Premenstrual Week 38.2% (28) (cubic, quadratic ZI) |
Moderate Premenstrual Week 18.9% (14) (quadratic, quadratic ZI) |
BIC: −1751.55 AIC: −1775.32 |
| Anxiety |
Moderate Premenstrual Week 47.2% (35) (quadratic, quadratic ZI) |
Asymptomatic 31.2% (23) (none) |
Severe Full Luteal Phase with Late Offset 21.6% (16) (quadratic, quadratic ZI) |
BIC: −1875.89 AIC: −1834.85 |
|
Sudden Sadness (Mood Lability) |
Severe Premenstrual Week 50.7% (38) (quadratic, quadratic ZI) |
Severe Full Luteal Phase 25.8% (19) (cubic, quadratic ZI) |
Mild Premenstrual Week 23.5% (17) (quadratic, quadratic ZI) |
BIC: −1465.89 AIC: −1427.59 |
|
Anger/ Irritability |
Moderate Full Luteal Phase 55.4% (41) (quadratic, quadratic ZI) |
Moderate Premenstrual Week 23% (17) (quadratic, quadratic ZI) |
Asymptomatic 21.6% (16) (none) |
BIC: −1487.64 AIC: −1454.81 |
| Muscle Pain |
Moderate Full Luteal Phase 35% (26) (quadratic, quadratic ZI) |
Mild Premenstrual Week 30% (22) (quadratic, quadratic ZI) |
Asymptomatic 35% (26) (none) |
BIC: −1045.94 AIC: −1013.15 |
Note: Percentages refer to the proportion of the sample estimated as belonging to each of the model-identified PMDD symptom trajectories for that specific symptom. BIC = Bayesian Information Criterion; AIC = Akaike Information Criterion. ZI=zero-inflated, referring to the shape of the zero-inflation trajectory. Groups characterized by premenstrual week patterns are highlighted in pink, and groups characterized by full luteal phase patterns are highlighted in green.
Because we did not wish to assume that the trajectories for each PMDD symptom would be the same, we applied PROC TRAJ separately for each of the six outcomes (total score, sadness, anxiety, sudden sadness, anger/irritability, and muscle pain). Once the best-fitting model was identified, we labeled the derived trajectories according to timing of symptom onset and offset. Symptom trajectory groups characterized by elevated symptoms across the entire luteal phase (ovulation through menses onset) were termed “full luteal phase”. Symptom trajectory groups characterized by symptoms restricted to the week before menses were termed “premenstrual week”. In some cases, symptom trajectory groups differed in severity, and were labeled accordingly (e.g., mild, moderate, severe). For each trajectory, we also considered whether the timing of symptom offset differentiated it from other groups. It was sometimes a distinguishing characteristic that a group showed slower or less complete offset of symptoms than other groups (“late offset”).
The total score analyses represent subgroups of people showing different global symptom trajectories. However, for individual symptom analyses, a single participant could be in different trajectory groups for two different individual symptoms. While our sample size was consistent with recommendations for single-symptom trajectories (Nagin & Odgers 2010), it was inadequate for confidently modeling dual trajectory groups (Charnigo at al. 2011). Also, although each participant met prospective criteria for PMDD, not every woman met the criteria of luteal phase confinement on every PMDD symptom included in these analyses.
Results
Table 2 presents the GBTM results for each daily symptom, including the percentage of the sample represented in each group. Figures 1–3 depict group means for the three symptom trajectory groups identified for the total score and each of the emotional symptoms. For each symptom, a three-group model provided the best model fit to the data and provided sufficient additional information to be defensible relative to a two-group model (Nagin & Odgers 2010). Each retained group followed an inverted-U-shaped quadratic or cubic trajectory across the cyclical timeline, where symptoms started low around ovulation, increased perimenstrually, and then decreased in the follicular phase (occasionally increasing slightly again in the late follicular phase). Each group also retained a U-shaped quadratic zero-inflation trajectory, where likelihood of zero-inflation started high around ovulation, decreased perimenstrually, and increased in the follicular phase. As is recommended (Nagin & Odgers 2010), the average posterior probabilities of group membership were high for each trajectory group identified (ranging from 73% to 99%).
Figure 1.
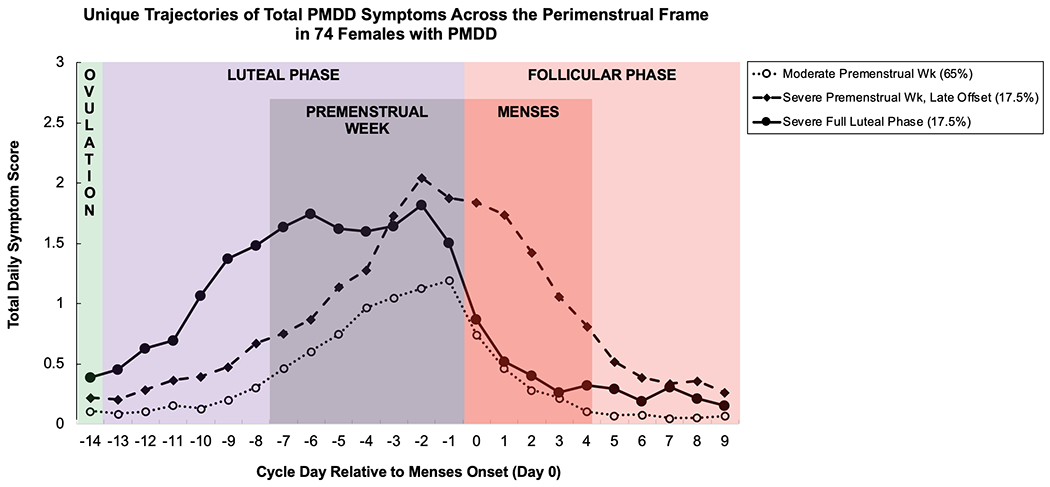
Mean daily symptom total for three PMDD symptom trajectory groups in females with PMDD, derived using group-based trajectory modeling.
Figure 3.
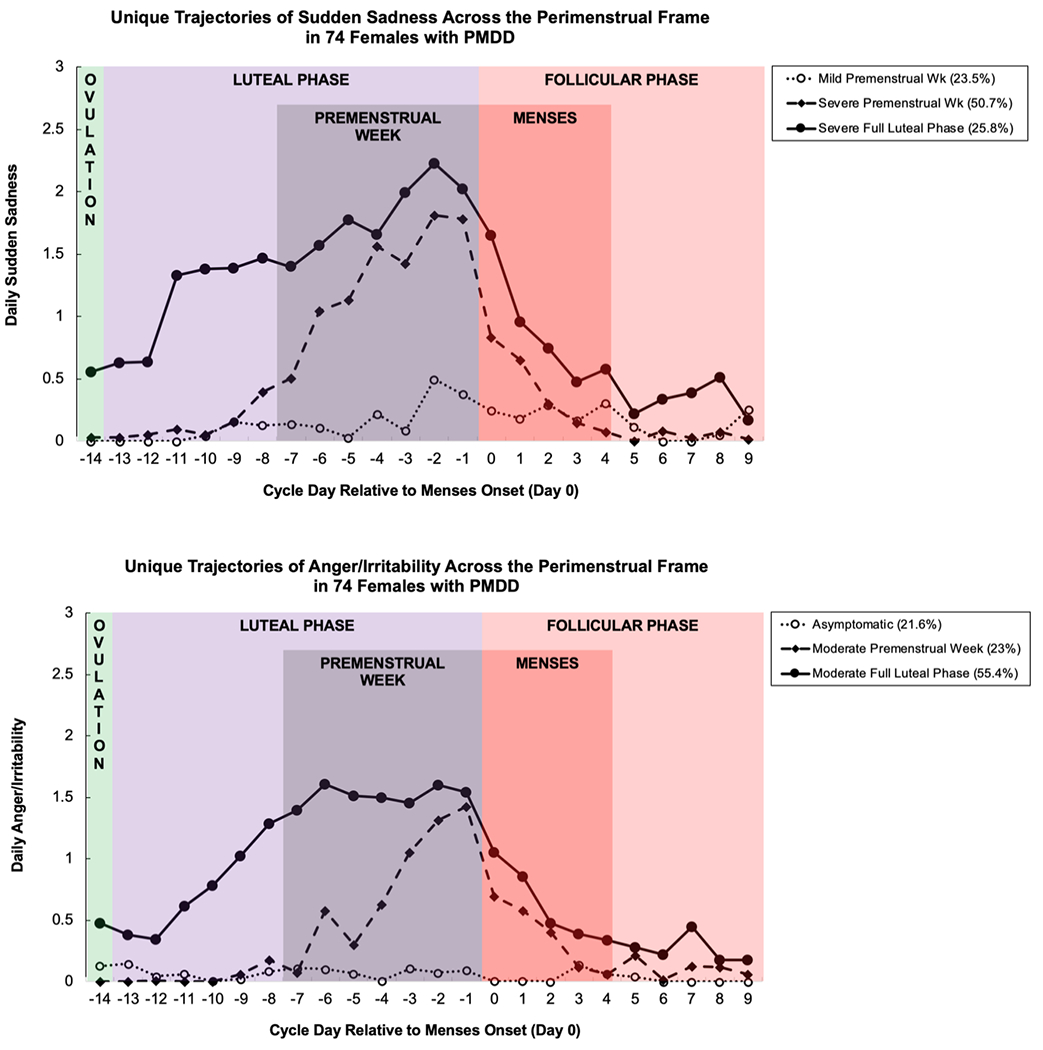
Mean daily sudden sadness (TOP) and anger/irritability (BOTTOM) for three PMDD symptom trajectory groups in females with PMDD, derived using group-based trajectory modeling.
Hypothesis 1: Full luteal phase vs. premenstrual week trajectory groups
We hypothesized that, for each symptom, we would observe at least one full luteal phase trajectory group (two weeks of symptoms from ovulation to menses) and at least one premenstrual week trajectory group (one premenstrual week of symptoms). This hypothesis was generally supported, with at least one full luteal and one premenstrual week group identified for the total score and most outcomes (Table 2). The exception was sadness, for which each of the trajectory groups showed primarily premenstrual week symptoms.
Hypothesis 2: Normal vs. late symptom offset trajectory groups
Hypothesis 2 was that sadness would demonstrate at least one group characterized by late offset of symptoms. Consistent with our hypothesis, sadness demonstrated one late-offset group. However, we also observed one late-offset trajectory group for anxiety and the total score. Symptom severity in both of these late-clearing groups appeared somewhat higher than other groups.
Discussion
Derived trajectory groups of PMDD symptoms generally included both a full luteal phase group, with symptoms occurring in the full two weeks between ovulation to menses, and a premenstrual week group, with symptoms occurring only in the final premenstrual week. Further, analyses for the depressive symptom of sadness demonstrated a subtype consistent with relatively later offset of PMDD. Unexpectedly, anxiety as well as the total score also demonstrated late offset trajectory groups.
Early Luteal Onset vs. Late Luteal Onset of PMDD
The majority of the symptom trajectory groups identified had symptoms confined to the premenstrual week. Summary evidence suggests that these premenstrual week groups were often split further into two groups, including a briefer or milder premenstrual week group and a longer and more severe premenstrual week group. Given their greater prevalence and milder subtypes, these premenstrual week groups may represent the upper range of an underlying, normally distributed risk for premenstrual symptoms in the general population (Hartlage et al. 2004). These delayed-onset symptoms could reflect a slow-developing form of post-ovulatory hormone sensitivity (i.e., relative to the full luteal group), in which symptoms are caused by an abnormal sensitivity to normal neurosteroid changes (Martinez et al. 2016) and altered cellular processing of steroids (Dubey et al. 2017), and are not related to late luteal hormone withdrawal (Schmidt et al. 2010). Alternatively, these late-occurring symptoms could be caused by the precipitous withdrawal of ovarian steroids in the late luteal phase (Lovick et al. 2017; Eisenlohr-Moul et al. 2018a). If a hormone-withdrawal-sensitive subtype of PMDD does exist, it could also signal increased risk during other periods of life when ovarian steroid withdrawal or deprivation also occurs, especially postpartum and the late menopause transition (Schiller et al. 2013; Schmidt et al. 2015). Experimental work should examine whether prevention of cyclical hormone withdrawal might uniquely benefit these patients (Eisenlohr-Moul et al. 2018a).
There was also evidence for a smaller number of trajectory groups in which symptoms arise quickly following ovulation and persist through the full luteal phase. In general, these subgroups were characterized by high severity. We suspect that this early-onset PMDD trajectory group is overrepresented in many of the most rigorous experimental studies of PMDD (e.g., Schmidt et al. 1998; Dubey et al. 2017). In these studies, clinical samples are strictly selected on the basis of positive clinical response to GNRH agonist (injectable medications that cause a reversible menopausal state)—and such a positive response to GnRH agonist are more common in those PMDD patients with a slower, smoother change rather than erratic change across the cycle. (Pincus et al. 2011). The full luteal phase symptom pattern, which creates a roughly 14-day symptomatic luteal phase (followed by an asymptomatic follicular phase for around 14 days, and so on), might be more consistent with the predicable, smooth changes that are seen among those who benefit from GnRH agonist-induced menopause (Pincus et al. 2011)). Therefore, since the seminal studies on the pathophysiology of PMDD have selected the subgroup of patients with PMDD who show a positive response to GnRH agonist, the findings in these studies may be most likely to reflect the pathophysiology of a full luteal phase trajectory of PMDD symptoms (e.g., (Schmidt et al. 1998, 2010, 2017)), and it is possible that they may not accurately reflect the pathophysiology of all patients with PMDD.
Given that the full luteal phase trajectory groups show emergence of symptoms during the post-ovulatory steroid surge, they may also be vulnerable during other events characterized by steroid surges, such as during puberty, pregnancy (Putnam et al. 2017)), and the early peri-menopause (Gordon et al. 2015). If it is true that these individuals respond better to medical menopause using GnRH agonist (Freeman et al. 1997; Pincus et al. 2011), they may also show greater benefit from oophorectomy (Cronje 2004). Similarly, if it is true that this group demonstrates post-ovulatory onset of symptoms due to neurosteroid change sensitivity (Martinez et al. 2016), they may show a more beneficial response to 5-alpha reductase blockers such as dutasteride or finasteride, which prevent the typical luteal conversion of progesterone to neurosteroids (Martinez et al. 2016).
Normal vs. Late-Offset Symptom Trajectories in PMDD
Although we predicted that a late-offset trajectory would be found only for depressive symptoms, we also observed one trajectory characterized by late offset for anxiety, as well as for the total score. The late-offset phenomenon may be due in part to individual differences in emotion regulation strategies. We have previously shown that those with high trait levels of rumination have slower offset of premenstrual depressive symptoms (Dawson et al. 2018) and some work has indicated that those with PMDD with a past history of depressive episodes (i.e., compromised emotion regulation) show slower offset of their PMDD depressive symptoms (Bancroft et al. 1994). Similar psychological processes may mediate late offset of other symptoms. There are several psychosocial theories of PMDD development, maintenance, or exacerbation that should be considered, especially in relation to these late-offset trajectories. Factors such as symptom appraisal (Blake 1995), cultural expectations and intrapsychic mode of evaluating changes of the body (Ussher 1997), or symptom-related expectations and associated emotions (Sigmon et al. 2000) may be informative.
Strengths and Limitations
We used a new data-driven approach (GBTM) to identify unique PMDD symptom trajectory groups across the cycle; prior studies had primarily focused on content subtypes of PMDD. However, the generalizability of the study is tempered by the moderate sample size and single-cycle design. Since GBTM is not intended to define literal groups (Nagin & Odgers 2010), further multimethod exploration of individual differences in trajectories of PMDD symptoms is important. Additionally, although many studies have found relative stability of PMDD symptom patterns, some within-person variance in symptom onset and offset appears to be common (Pearlstein et al. 2005); therefore, further investigation of these trajectory groups should also examine their stability within a given woman. Another limitation is that it is very possible that differences in timing of onset or offset are caused by individual differences in endometrial hormone sensitivity that drive variability in the timing of menses relative to the timing of symptoms. It should also be understood that this sample is representative of those with PMDD only; a substantial number of those who did not meet strict PMDD criteria were excluded. Some were also excluded because they either did not fulfill other eligibility criteria (e.g., significant comorbidities) or did not provide sufficient ratings. Since the diagnostic daily ratings began prior to the initial visit, no information is available regarding how individuals with insufficient daily ratings might have differed from those with sufficient daily ratings; this is a limitation of the present study.
Of note, since we did not wish to assume that the groups would be the same when examining different daily symptoms, the grouping analyses were repeated independently for the total symptom score and each of five individual symptoms. For illustration, this means that it is possible for a single individual to be in the “full luteal phase” group for one symptom (e.g., anger), but in the “premenstrual week with late offset” group for another symptom (e.g., depression). Outside of the total score, this complexity could not be captured using our sample size; however, examination of these dual trajectory subtypes (Charnigo et al. 2011) may be useful.
Kiesner and others (e.g., (Kiesner 2011; Kiesner et al. 2016) have found that in addition to those with no menstrual cycle-related symptoms and those with perimenstrual increases in symptoms (e.g., PMS/PMDD pattern), some individuals demonstrate a mid-cycle increase in symptoms. Thus, there is evidence of further heterogeneity of temporal patterns of symptom change across the menstrual cycle, among females without PMDD. Although not clearly relevant to research on PMDD, understanding this additional heterogeneity may provide clues for better understanding individual differences in response to reproductive steroids and the menstrual cycle that may provide insights for understanding PMDD and its symptom trajectories.
Conclusion
Given the observed heterogeneity of symptom timing and severity, it seems possible that patients with prospectively-confirmed PMDD, typically treated as a single group, are actually heterogeneous with respect to symptom patterns and perhaps etiology. While there may be pathophysiologic differences between these “premenstrual week” and “full luteal phase” PMDD subtypes, both trajectory groups meet DSM-5 criteria for PMDD diagnosis, on average, with symptoms present in the week before menses and absent the week following menses (APA 2013). Efforts to standardize the diagnosis of PMDD (e.g., (Eisenlohr-Moul et al. 2017)) and to identify homogeneous PMDD subgroups may improve the clarity of research findings. More work is needed to establish the replicability and stability of these trajectory groups, and to determine whether they have unique biological and/or psychosocial etiologies that could inform more personalized approaches to treatment of PMDD.
Figure 2.
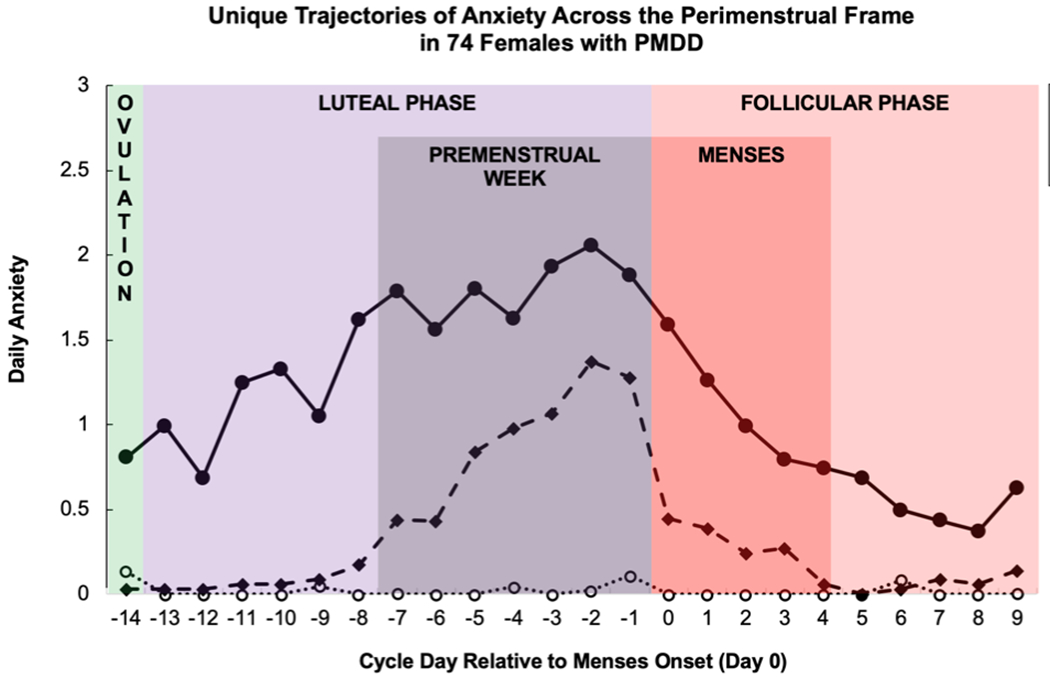
Mean daily sadness (TOP) and anxiety (BOTTOM) for three PMDD symptom trajectory groups in females with PMDD, derived using group-based trajectory modeling.
Financial Support:
This research was supported by a grant from the National Institute of Mental Health (R00MH109667) and a Brain and Behavior Research Foundation Young Investigator Grant.
Footnotes
Conflicts of Interest: None.
Ethics: The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Contributor Information
Tory A. Eisenlohr-Moul, University of Illinois at Chicago, USA
Gudrun Kaiser, University of Marburg, Germany.
Cornelia Weise, University of Marburg, Germany.
Katja M. Schmalenberger, Heidelberg University, Germany
Jeff Kiesner, Università degli Studi di Padova, Italy.
Beate Ditzen, Heidelberg University, Germany.
Maria Kleinstäuber, University of Otago, New Zealand.
References
- Association AP (2013). Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Pub. [Google Scholar]
- Bancroft J, Rennie D, Warner P (1994). Vulnerability to perimenstrual mood change: The relevance of a past history of depressive disorder. Psychosomatic Medicine 56, 225–231. [DOI] [PubMed] [Google Scholar]
- Blake F (1995). Cognitive therapy for Premenstrual Syndrome. Cognitive and Behavioral Practice 2, 167–185. [Google Scholar]
- Charnigo R, Kryscio R, Bardo MT, Lynam D, Zimmerman RS (2011). Joint Modeling of Longitudinal Data in Multiple Behavioral Change. Evaluation & the health professions 34, 181–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiazze L, Brayer FT, Macisco JJ, Parker MP, Duffy BJ (1968). The Length and Variability of the Human Menstrual Cycle. JAMA 203, 377–380. [PubMed] [Google Scholar]
- Cronje WH (2004). Hysterectomy and bilateral oophorectomy for severe premenstrual syndrome. Human Reproduction 19, 2152–2155. [DOI] [PubMed] [Google Scholar]
- Dawson DN, Eisenlohr-Moul TA, Paulson JL, Peters JR, Rubinow DR, Girdler SS (2018). Emotion-related impulsivity and rumination predict the perimenstrual severity and trajectory of symptoms in women with a menstrually related mood disorder. Journal of Clinical Psychology 74, 579–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditzen B, Nussbeck F, Drobnjak S, Spörri C, Wüest D, Ehlert U (2011). Validierung eines deutschsprachigen DSM-IV-TR basierten Fragebogens zum prämenstruellen Syndrom. Zeitschrift für Klinische Psychologie und Psychotherapie 40, 149–159. [Google Scholar]
- Donker T, van Straten A, Marks I, Cuijpers P (2009). A Brief Web-Based Screening Questionnaire for Common Mental Disorders: Development and Validation. Journal of Medical Internet Research 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey N, Hoffman JF, Schuebel K, Yuan Q, Martinez PE, Nieman LK, Rubinow DR, Schmidt PJ, Goldman D (2017). The ESC/E(Z) complex, an effector of response to ovarian steroids, manifests an intrinsic difference in cells from women with Premenstrual Dysphoric Disorder. Molecular psychiatry 22, 1172–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edler C, Lipson SF, Keel PK (2007). Ovarian hormones and binge eating in bulimia nervosa. Psychological Medicine 37, 131–141. [DOI] [PubMed] [Google Scholar]
- Eisenlohr-Moul T, Prinstein M, Rubinow D, Young S, Walsh E, Bowers S, Girdler S (2018a). S104. Ovarian Steroid Withdrawal Underlies Perimenstrual Worsening of Suicidality: Evidence From a Crossover Steroid Stabilization Trial. Biological Psychiatry 83, S387. [Google Scholar]
- Eisenlohr-Moul TA, Girdler SS, Schmalenberger KM, Dawson DN, Surana P, Johnson JL, Rubinow DR (2017). Toward the Reliable Diagnosis of DSM-5 Premenstrual Dysphoric Disorder: The Carolina Premenstrual Assessment Scoring System (C-PASS). The American journal of psychiatry 174, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenlohr-Moul TA, Schmalenberger KM, Owens SA, Peters JR, Dawson DN, Girdler SS (2018b). Perimenstrual exacerbation of symptoms in borderline personality disorder: evidence from multilevel models and the Carolina Premenstrual Assessment Scoring System. Psychological Medicine, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman EW, Sondheimer SJ, Rickels K (1997). Gonadotropin-releasing hormone agonist in the treatment of premenstrual symptoms with and without ongoing dysphoria: a controlled study. Psychopharmacology Bulletin 33, 303–309. [PubMed] [Google Scholar]
- Gehlert S, Song IH, Chang C-H, Hartlage SA (2009). The prevalence of premenstrual dysphoric disorder in a randomly selected group of urban and rural women. Psychological Medicine 39, 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JL, Girdler SS, Meltzer-Brody SE, Stika CS, Thurston RC, Clark CT, Prairie BA, Moses-Kolko E, Joffe H, Wisner KL (2015). Ovarian Hormone Fluctuation, Neurosteroids, and HPA Axis Dysregulation in Perimenopausal Depression: A Novel Heuristic Model. American Journal of Psychiatry 172, 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbreich U (2008). Selective Serotonin Reuptake Inhibitors and Initial Oral Contraceptives for the Treatment of PMDD: Effective But Not Enough. CNS Spectrums 13, 566–572. [DOI] [PubMed] [Google Scholar]
- Hartlage SA, Brandenburg DL, Kravitz HM (2004). Premenstrual exacerbation of depressive disorders in a community-based sample in the United States. Psychosomatic Medicine 66, 698–706. [DOI] [PubMed] [Google Scholar]
- Janca A, Hiller W (1996). ICD-10 checklists--a tool for clinicians’ use of the ICD-10 classification of mental and behavioral disorders. Comprehensive Psychiatry 37, 180–187. [DOI] [PubMed] [Google Scholar]
- Janda C, Kues JN, Andersson G, Kleinstäuber M, Weise C (2017). A symptom diary to assess severe premenstrual syndrome and premenstrual dysphoric disorder. Women & Health 57, 837–854. [DOI] [PubMed] [Google Scholar]
- Kiesner J (2011). One woman’s low is another woman’s high: Paradoxical effects of the menstrual cycle. Psychoneuroendocrinology 36, 68–76. [DOI] [PubMed] [Google Scholar]
- Kiesner J, Mendle J, Eisenlohr-Moul TA, Pastore M (2016). Cyclical Symptom Change Across the Menstrual Cycle: Attributional, Affective, and Physical Symptoms. Clinical Psychological Science [Google Scholar]
- Kleinstäuber M, Witthöft M, Hiller W (2012). Cognitive-behavioral and pharmacological interventions for premenstrual syndrome or premenstrual dysphoric disorder: a meta-analysis. Journal of Clinical Psychology in Medical Settings 19, 308–319. [DOI] [PubMed] [Google Scholar]
- Kues JN, Janda C, Kleinstäuber M, Weise C (2014). Internet-based cognitive behavioural self-help for premenstrual syndrome: study protocol for a randomised controlled trial. Trials 15, 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez LM, Kaptein AA, Helmerhorst FM (2012). Oral contraceptives containing drospirenone for premenstrual syndrome. The Cochrane Database of Systematic Reviews, CD006586. [DOI] [PubMed] [Google Scholar]
- Lovick TA, Guapo VG, Anselmo-Franci JA, Loureiro CM, Faleiros MCM, Del Ben CM, Brandão ML (2017). A specific profile of luteal phase progesterone is associated with the development of premenstrual symptoms. Psychoneuroendocrinology 75, 83–90. [DOI] [PubMed] [Google Scholar]
- Marjoribanks J, Brown J, O’Brien PMS, Wyatt K (2013). Selective serotonin reuptake inhibitors for premenstrual syndrome. The Cochrane Database of Systematic Reviews, CD001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez PE, Rubinow DR, Nieman LK, Koziol DE, Morrow AL, Schiller CE, Cintron D, Thompson KD, Khine KK, Schmidt PJ (2016). 5α-Reductase Inhibition Prevents the Luteal Phase Increase in Plasma Allopregnanolone Levels and Mitigates Symptoms in Women with Premenstrual Dysphoric Disorder. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology 41, 1093–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagin DS, Odgers CL (2010). Group-based trajectory modeling in clinical research. Annual Review of Clinical Psychology 6, 109–138. [DOI] [PubMed] [Google Scholar]
- Pearlstein T, Yonkers KA, Fayyad R, Gillespie JA (2005). Pretreatment pattern of symptom expression in premenstrual dysphoric disorder. Journal of Affective Disorders 85, 275–282. [DOI] [PubMed] [Google Scholar]
- Pincus SM, Alam S, Rubinow DR, Bhuvaneswar CG, Schmidt PJ (2011). Predicting response to leuprolide of women with premenstrual dysphoric disorder by daily mood rating dynamics. Journal of Psychiatric Research 45, 386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam KT, Wilcox M, Robertson-Blackmore E, Sharkey K, Bergink V, Munk-Olsen T, Deligiannidis KM, Payne J, Altemus M, Newport J, Apter G, Devouche E, Viktorin A, Magnusson P, Penninx B, Buist A, Bilszta J, O’Hara M, Stuart S, Brock R, Roza S, Tiemeier H, Guille C, Epperson CN, Kim D, Schmidt P, Martinez P, Di Florio A, Wisner KL, Stowe Z, Jones I, Sullivan PF, Rubinow D, Wildenhaus K, Meltzer-Brody S (2017). Clinical phenotypes of perinatal depression and time of symptom onset: analysis of data from an international consortium. The lancet. Psychiatry 4, 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redei E, Freeman EW (1995). Daily plasma estradiol and progesterone levels over the menstrual cycle and their relation to premenstrual symptoms. Psychoneuroendocrinology 20, 259–267. [DOI] [PubMed] [Google Scholar]
- Schiller CE, O’Hara MW, Rubinow DR, Johnson AK (2013). Estradiol modulates anhedonia and behavioral despair in rats and negative affect in a subgroup of women at high risk for postpartum depression. Physiology & Behavior 119, 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Ben Dor R, Martinez PE, Guerrieri GM, Harsh VL, Thompson K, Koziol DE, Nieman LK, Rubinow DR (2015). Effects of Estradiol Withdrawal on Mood in Women With Past Perimenopausal Depression: A Randomized Clinical Trial. JAMA psychiatry 72, 714–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Martinez PE, Nieman LK, Koziol DE, Thompson KD, Schenkel L, Wakim PG, Rubinow DR (2017). Premenstrual Dysphoric Disorder Symptoms Following Ovarian Suppression: Triggered by Change in Ovarian Steroid Levels But Not Continuous Stable Levels. The American Journal of Psychiatry 174, 980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR (1998). Differential Behavioral Effects of Gonadal Steroids in Women with and in Those without Premenstrual Syndrome. New England Journal of Medicine 338, 209–216. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman LK, Grover GN, Muller KL, Merriam GR, Rubinow DR (2010). Lack of Effect of Induced Menses on Symptoms in Women with Premenstrual Syndrome. research-article 10.1056/NEJM199104253241705 [DOI] [PubMed] [Google Scholar]
- Schnurr PP (1989). Measuring amount of symptom change in the diagnosis of premenstrual syndrome. Psychological Assessment: A Journal of Consulting and Clinical Psychology 1, 277–283. [Google Scholar]
- Sigmon ST, Rohan KJ, Boulard NE, Dorhofer DM, Whitcomb SR (2000). Menstrual Reactivity: The Role of Gender-Specificity, Anxiety Sensitivity, and Somatic Concerns in Self-Reported Menstrual Distress. Sex Roles 43, 143–161. [Google Scholar]
- Wyatt KM, Dimmock PW, Ismail KMK, Jones PW, O’Brien PMS (2004). The effectiveness of GnRHa with and without ‘add-back’ therapy in treating premenstrual syndrome: a meta analysis. BJOG: an international journal of obstetrics and gynaecology 111, 585–593. [DOI] [PubMed] [Google Scholar]


