Abstract
Psoriasis is a chronic inflammatory skin disease, which affects 2-3% of the U.S. population. The immune response in psoriasis includes enhanced activation of T cells and myeloid cells, platelet activation, and upregulation of interferons, tumor necrosis factor-α, and interleukin (IL)s IL-23, IL-17, and IL-6, which are linked to vascular inflammation and atherosclerosis development. Patients with psoriasis are up to 50% more likely to develop cardiovascular disease (CV) disease, and this CV risk increases with skin severity. Major society guidelines now advocate incorporating a psoriasis diagnosis into CV risk prediction and prevention strategies. While registry data suggest treatment targeting psoriasis skin disease reduces vascular inflammation, coronary plaque burden, and may reduce CV risk, randomized placebo-controlled trials are inconclusive to date. Further studies are required to define traditional CV risk factor goals, the optimal role of lipid-lowering and antiplatelet therapy, and targeted psoriasis therapies on CV risk.
Condensed Abstract:
Psoriasis is a chronic inflammatory skin disease linked to enhanced cardiovascular (CV) risk. The inflammatory milieu in psoriasis includes T-cells, myeloid cells, and cytokines such as interferons, TNF-α, and the interleukin (IL)s IL-23, IL-17, and IL-6, which are linked to atherosclerosis development. Guidelines now suggest incorporating a psoriasis diagnosis into CV risk prediction and prevention strategies. While observational data suggests treatment of psoriasis can reduce CV risk, randomized controlled trials assessing treatments to reduce CV risk are inconclusive. Further studies are required to define CV risk factor goals and degree of psoriasis skin disease control.
Keywords: Psoriasis, Inflammation, Cardiovascular Disease, Cardiovascular Risk
Case vignette:
A 50-year-old male comes to your office to establish care. He has a history of plaque psoriasis since 35 years of age, previously involving the elbows, anterior shins, and lower abdomen (covering >10% of his body), which has cleared with an interleukin (IL) 17A inhibitor. He does not smoke and has no family history of early heart disease. On presentation, his resting blood pressure is 138/84 mmHg, and body mass index of 28 kg/m2. He has a hemoglobin A1c of 5.4%, total cholesterol of 210 mg/dL, triglycerides of 145 mg/dL, high-density lipoprotein cholesterol (HDL-C) of 42 mg/dL, calculated low-density lipoprotein cholesterol (LDL-C) of 139 mg/dL, and a high sensitivity C-reactive protein (hs-CRP) of 1.5 mg/dL. He asks about his future risk of cardiovascular disease (CVD) and what can be done to lower his risk.
The clinical problem:
Despite advances, CVD remains the leading cause of mortality in the United States.(1) Identifying high cardiovascular (CV) risk patients who derive the largest benefit from prevention therapies is a key step towards reducing clinical CV events.(2) Standard CV risk calculators such as the American College of Cardiology (ACC)/American Heart Association (AHA) pooled cohort equations incorporate traditional CV risk factors, such as age, male sex, race, hypertension, hyperlipidemia, diabetes, and smoking.(2) Recognizing that CV risk scores may underestimate CV risk in certain populations,(3) guidelines now incorporate CV risk enhancers including pro-inflammatory conditions, such as psoriasis, when calculating the 10-year risk of a CV event to guide prevention strategies.(2) However, uncertainty remains in the assessment and implementation of therapies to reduce CV risk in psoriasis. Therefore, the goal of this review is to discuss the identification and management of CV risk in individuals with psoriasis, areas of uncertainty, and future directions.
Psoriasis, of which psoriasis vulgaris is the most common type, is a chronic, pro-inflammatory condition of the skin presenting primarily as thick, well-demarcated, and erythematous scaly plaques. (4) Psoriasis affects 2-3% of all Americans. (4) There is no gender predilection and a bimodal age distribution, with incidence peaking between 30 - 39 and 50 – 69 years of age.(4) Early work starting in the 1970’s described a possible connection between psoriasis and vascular disease.(5) However, in 2006, a seminal study by Gelfand et al. utilized a large United Kingdom prospective registry of ~130,000 psoriasis and ~500,000 controls with a mean follow-up of 5.4 years to describe an overall 50% elevated risk of myocardial infarction in psoriasis.(6) Since then, many, although not all, studies show a positive association between psoriasis and CVD.(7) A meta-analysis encompassing 75 studies with ~500,000 psoriasis patients reported up to a 50% increased odds of CVD in psoriasis compared to those without psoriasis.(8) Severe psoriasis confers the highest CV risk (compared to controls), including up to a 3-fold increased odds of myocardial infarction, 60% higher odds of stroke, and 40% higher odds of CV death.(7)
Proposed link between psoriasis and atherosclerosis:
The inflammatory milieu within psoriatic lesional skin includes activated T cell subsets and myeloid cells, which produce TNF-α, interferons (IFN), IL-17 isoforms, IL-23, and IL-22 in a cutaneous environment where many innate (and pro-atherosclerotic) cytokines, such as IL-1, IL-6, and IL-8 are co-expressed. (4) Collectively, these cytokines display strong synergistic interactions, further amplifying inflammation, and driving keratinocyte proliferation in the skin (Figure 1).(4) The blood vessels in psoriasis lesions are dilated and fenestrated, and upregulated pro-inflammatory cytokines (e.g. TNF-α, IL-17A, IFNƔ, IL-23, and IL-6) present in psoriasis lesional skin (Figure 1) readily exchange with blood plasma, and circulate systemically.(4)
Figure 1. Pathogenesis of Psoriasis.
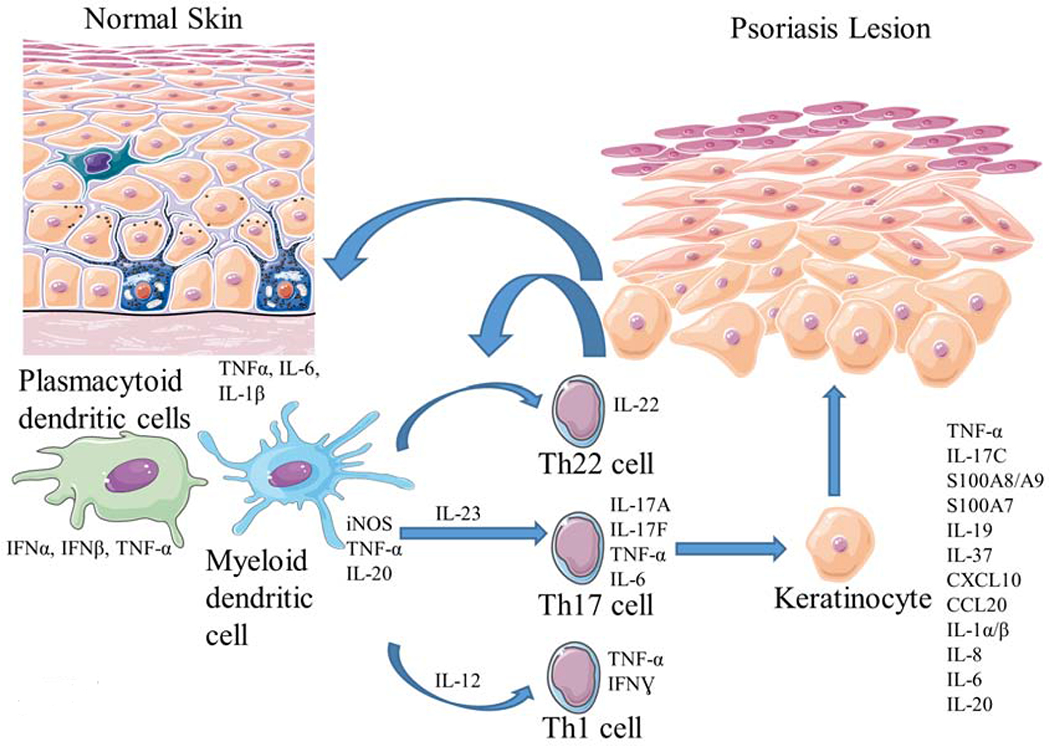
External stimuli combined with genetic pre-disposition generates an inflammatory cascade and activation of dendritic cells. Upregulated cytokines, including IL-23, lead to T cell activation and differentiation (Th17 cells). Synergistic action between IL-17 isoforms (A/F) along with TNF-α and IL-1 amplify inflammation and drive keratinocyte proliferation in the skin. IFN; Interferon, IL; Interleukin, TNF; Tumor necrosis factor.
Vascular endothelial activation and dysfunction is a first step in atherosclerosis development.(9) A transcriptomic comparison between psoriasis lesional skin and atherosclerotic plaque found a dominant overlap of IFNƔ and TNF-α driven processes that synergistically inflame the endothelium, including a >5,000-fold increase (relative to control endothelial cells) of transcripts including VCAM-1 and CXCL10.(10) In a murine model of epidermal IL-17A overexpression, endothelial dysfunction, enhanced vascular stiffness, and oxidative stress are present.(11) In analysis of directly obtained endothelial cells, patients with psoriasis (compared to controls) display a significant 2 to 8-fold upregulation of pro-inflammatory and chemotactic transcripts, including VCAM-1, IL-1β, CXCL10, and COX-2.(12). These directly obtained endothelial cells exhibit a similar expression profile to endothelial cells stimulated in vitro by combinations of TNF-α, IL-17A, and IFNƔ, highlighting the inflammatory pathogenic overlap of psoriasis and atherosclerosis (Figure 2).(12)
Figure 2. Factors Influencing Cardiovascular Disease in Psoriasis.
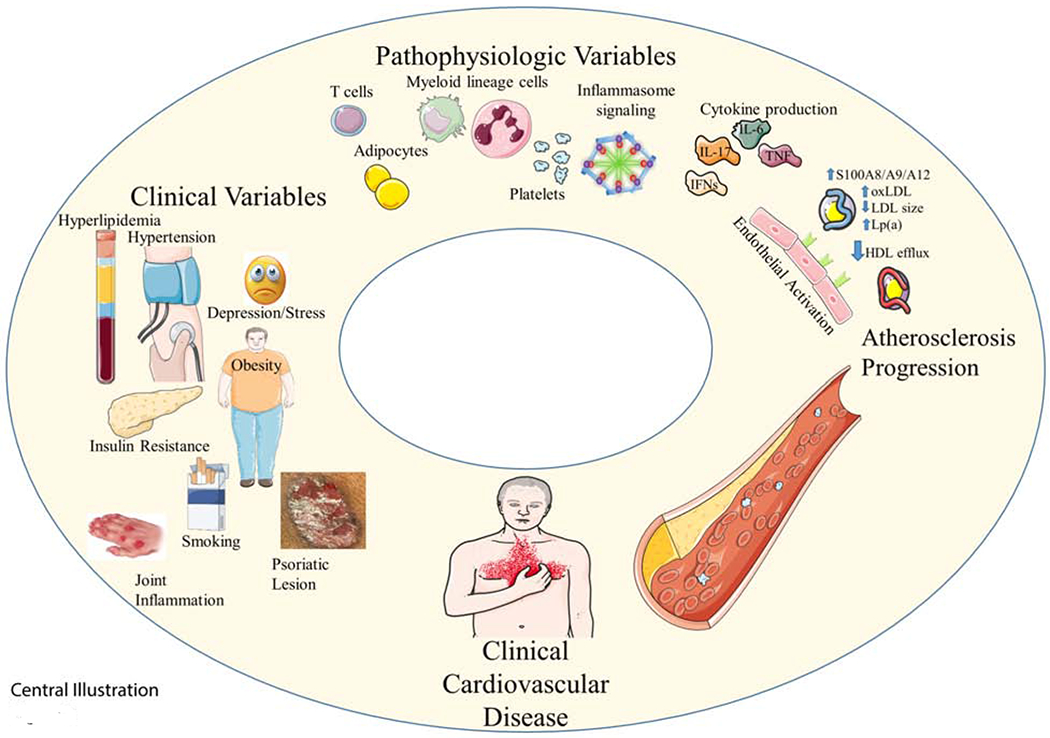
The suspected pathogenesis of cardiovascular disease in psoriasis includes a combination of cutaneous and systemic immune system activation along with the contribution of co-existing cardiometabolic conditions. HDL; High-density lipoprotein, IFN; interferon, IL; Interleukin, LDL; Low-density lipoprotein, Lp(a); Lipoprotein(a), Ox; oxidized, TNF; Tumor necrosis factor.
Extending beyond direct cytokine-induced endothelial damage, systemic inflammasome signaling (IL-1) with downstream IL-6 production is induced via TNF-α/IL-17A synergism, is the highest differentially expressed systemic pathway in psoriasis, and causal in atherosclerosis development.(12,13) Th1 cells (in psoriasis lesional skin and systemically) are present in atherosclerotic plaques, chemotactic, and promote plaque instability.(14) Macrophages in a murine model of psoriasis prone to atherosclerosis exhibit increased lipid uptake and foam cell formation.(15) Neutrophils, specifically, the neutrophil sub-type, low-density granulocytes, are 30% higher in psoriasis (compared to non-psoriasis), and correlate with coronary atherosclerosis.(16) These low-density granulocytes co-localize with platelets and induce 50% higher in vitro endothelial damage and apoptosis through neutrophil extracellular traps (termed NETosis) when compared to other neutrophil subtypes (normal density granulocytes).(16)
Platelets appear to also be an important factor in the pathogenesis of vascular dysfunction in psoriasis. Markers of platelet activation including mean platelet volume, platelet-derived P-selectin, platelet-neutrophil, and lymphocyte aggregates are elevated in psoriasis and correlate with psoriasis severity.(17,18) Psoriasis platelet RNA sequencing shows an interferon signature and elevated expression of COX-1 which correlates with psoriasis skin severity (r=0.81, p=0.01) (19,20) Finally, platelets are preferentially found in psoriasis lesional skin and induce a >20-fold increase in endothelial cell pro-atherosclerotic transcripts compared to platelets from non-psoriasis patients (19) raising the hypothesis that targeting COX-1 (via aspirin) may be beneficial in psoriasis.
Contribution of traditional CV risk factors to CVD in psoriasis:
In addition to systemic immune activation, cardiometabolic derangements also occur. The traditional modifiable CV risk factors hypertension, diabetes, hyperlipidemia, obesity, and smoking, along with metabolic syndrome, are highly prevalent (in aggregate >50%), under-recognized, and undertreated in psoriasis (Figure 3).(21) Among 3000 psoriasis participants enrolled in clinical trials (average age ~46 years, 68% male) investigating an IL-12/23 inhibitor to improve psoriasis skin severity, 59% of participants had at least two traditional CV risk factors while 29% had three or more.(22) Approximately 20% of psoriatics diagnosed with diabetes and hypertension, and nearly 40% with hyperlipidemia were not treated. Among psoriasis patients receiving pharmacologic therapy to treat CV risk factors, ~60% were not at goal. (22) Psoriasis patients are also less physically active (23), almost 40% more likely to carry a diagnosis of depression, and have a reduced quality of life than those without psoriasis.(24,25) Smoking is also highly prevalent and exhibits a dose-response relationship with psoriasis severity.(26)
Figure 3. Association between Psoriasis and Traditional Cardiovascular Risk Factors.
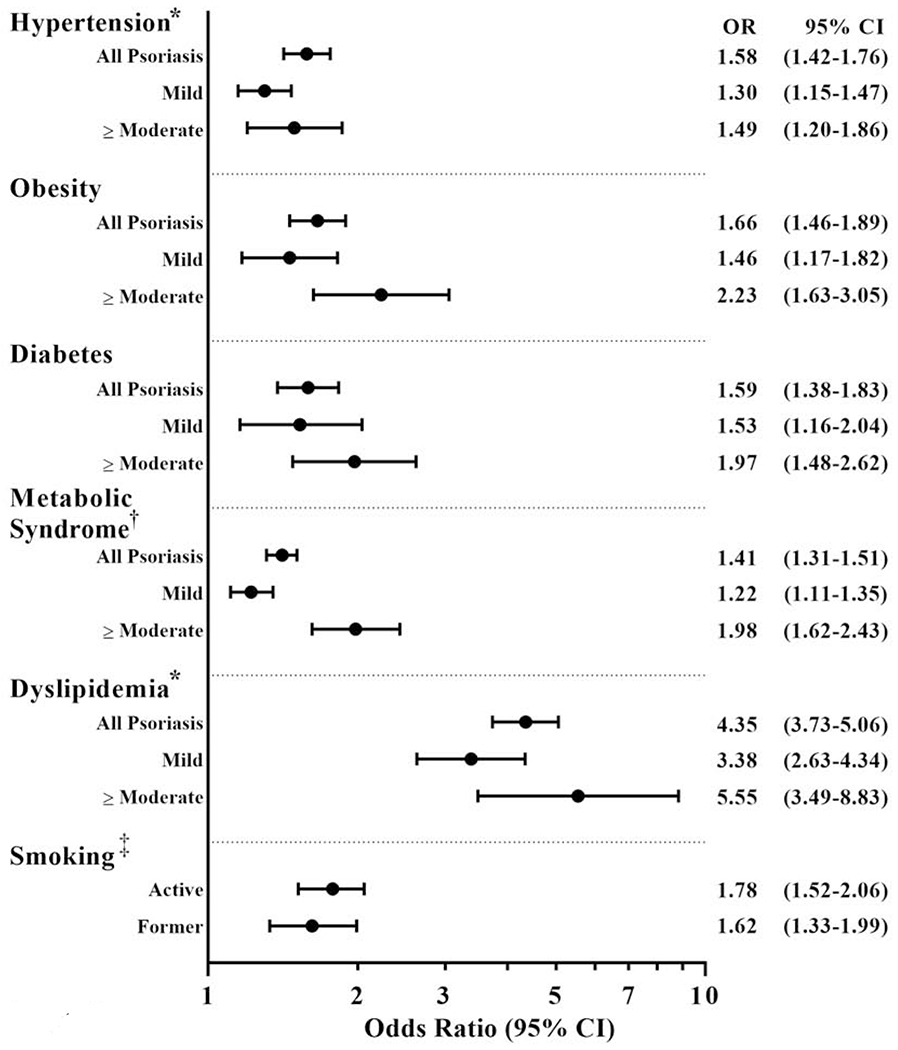
From the American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. *Some studies find less robust associations between psoriasis, hypertension, and, dyslipidemia. †In a meta-analysis encompassing 42,000 psoriasis patients, the odds ratio for metabolic syndrome was as high as 2.26 (95% CI 1.70 - 3.01). ‡Association between smoking and psoriasis. CI; Confidence interval.
Psoriasis patients have up to a ~15% lower HDL, and almost 80% reduced HDL efflux capacity.(27,28) Nuclear magnetic resonance spectroscopy shows a lipid profile similar to diabetics, including increased LDL particle concentration and decreased LDL size.(29) Compared to controls, psoriasis patients have a higher lipoprotein(a) and 15% higher oxidized HDL with the degree of oxidized LDL and oxidized HDL in psoriasis correlating with non-calcified coronary plaque. (28) Hypertension severity associates with IL-17A levels and psoriasis skin severity in both human and murine models.(30,31) Finally, obesity with metabolically active visceral adipose tissue potentiates insulin resistance and metabolic syndrome in psoriasis, associates with vascular arterial inflammation and atherosclerosis burden, and represents a further link between psoriasis and CVD (Figure 2).(32) Taken together, atherosclerotic development in psoriasis is a combination of psoriatic induced immune system activation and pan-arterial inflammation, combined with the contribution of co-existing cardiometabolic conditions (Figure 2).
CV risk screening:
Assessment of 10-year CV risk is a core component of primary CVD prevention.(2) Traditional 10-year risk estimator’s underestimate CV risk in psoriasis who present with a myocardial infarct, on average, 5-years younger than those without psoriasis.(33,34) Patients with psoriasis requiring systemic therapy exhibit an absolute 10-year risk of a coronary event or stroke 6.2%, beyond what is expected from traditional CV risk estimates.(35) Psoriasis chronicity, such as age of diagnosis and duration, tracks with vascular inflammation and up to a 1% absolute increase in CV risk per year of psoriasis.(36,37) The Joint American Academy of Dermatology — National Psoriasis Foundation guidelines, recognizing this, advocate that dermatologists inform psoriasis patients of their elevated CV risk and ensure engagement with their primary care doctor or cardiologist.(38) They further suggest that in patients with a >10% body surface area of psoriasis or candidates for systemic therapy or phototherapy apply a 1.5 multiplication factor to a 10-year CV risk score.(38) In contrast, the ACC/AHA suggests psoriasis as a CV risk-enhancing feature (when assessing CV risk) without specifying a psoriasis severity threshold (Table 1).(2)
Table 1. Considerations to identify and treat cardiovascular risk in psoriasis.
AAD/NPF guidelines utilize the Strength of Recommendation Taxonomy (SORT).
| Quantifying Cardiovascular Risk: | Evidence and Strength of Recommendation | |
|---|---|---|
| Screening for known variables contributing to CVD | Hypertension, obesity, diabetes mellitus, dyslipidemia, metabolic syndrome, smoking, physical inactivity, depression, stress | AAD/NPF SOR B, LOE II – III |
| CV risk score calculation | ∙ 2019 AAD/NPF guidelines - multiply cardiovascular risk score by 1.5* ∙ 2019 ACC/AHA guidelines – use psoriasis as a CV risk enhancer ∙ 2019 ESC guidelines - use of CIID to solely guide lipid lowering interventions is not recommended. |
AAD/NPF SOR C, LOE II - III ACC/AHA Class IIa, LOE B-NR ESC Class III, LOE C |
| Potential ancillary measures | ∙ Psoriasis skin disease severity assessment >10% body surface area involvement Candidates for systemic or phototherapy treatment ∙ Coronary artery calcium score** ∙ Lipoprotein (a) ∙ High sensitivity C-reactive protein |
In psoriasis, criteria to identify those that deserve more aggressive traditional CV risk factor screening (SOR B, LOE II – III) or CV risk score multiplied by 1.5 (SOR C, LOE II – III) from AAD/NPF Other ancillary measures Class and LOE per 2019 ACC/aHa guidelines on the primary prevention of CVD in the general population |
| Guideline Directed Risk Factor Reduction: | ||
| ∙ Lifestyle counseling (e.g. dietary counseling, smoking cessation, exercise) ∙ Weight management, BMI < 25 kg/m2 ∙ Blood pressure, < 130/80 mmHg ∙ Hemoglobin A1c, ≤ 7 % ∙ Statin (lipid lowering) therapy ∙ Low-dose aspirin therapy |
In psoriasis, lifestyle counseling AAD/NPF SOR B, LOE II – III In psoriasis, CV risk management, blood pressure, and lipid targets per “national guideline recommendations” AAD/NPF - SOR C, LOE III Class and LOE per 2019 ACC/AHA guidelines on the primary prevention of CVD in the general population |
|
| Reducing Inflammation: | ||
| ∙ Biologic therapy (e.g. IL-17A, IL-12/23, TNF-α inhibitors) ∙ Phototherapy ∙ Oral therapy (e.g. methotrexate, cyclosporine, acitretin, apremilast) |
Suggested benefit primarily from observational non-randomized studies Further randomized controlled trials are needed | |
>10% body surface area of psoriasis involvement or candidates for systemic or phototherapy treatment
Per ACC/AHA, in inflammatory conditions, a calcium score of 0 does not necessarily rule out enhanced cardiovascular risk. AAD/NPF; American Academy of Dermatology (AAD)/National Psoriasis Foundation. ACC/AHA; American College of Cardiology (ACC)/American Heart Association (AHA). CVD; cardiovascular disease. CIID; chronic immune-mediated inflammatory diseases. ESC; European Society of Cardiology. IL; interleukin. LOE; level of evidence. NPF; National Psoriasis Foundation. NR; non-randomized. SOR; Strength of recommendation. TNF; Tumor necrosis factor.
Biomarkers of CV risk:
Supporting the connection between psoriatic activity and atherosclerosis development, [18F]-fluorodeoxyglucose positron emission tomography evaluation displays a correlation between psoriasis skin severity and pan arterial vascular inflammation (β=0.41, p<0.01).(39) In coronary analyses, psoriasis, compared to matched non-psoriasis patients have a 2-fold greater odds of coronary artery calcium (any amount > 0) and comparable to a diabetic without psoriasis.(40) Psoriasis patients also have a 15% higher non-calcified coronary plaque burden than matched controls with the degree of high-risk non-calcified coronary plaque correlating with psoriasis skin severity.(41) Extending beyond the skin, hs-CRP tracks with psoriasis skin severity, vascular inflammation, and coronary atherosclerosis.(42–44) However, newer modalities are emerging such as circulating glycoprotein acetylation (a pro-inflammatory measure of N-glycan side chains attached to acute phase reactants), which is shown to improve the prediction of coronary atherosclerosis in psoriasis beyond traditional CV risk factors and hs-CRP.(42,43) In summary, these clinical-translational studies suggest that coronary atherosclerosis is not only prevalent in psoriasis but highlight the promise and need for future clinical trials to prospectively evaluate biomarkers for CV risk stratification.(45)
Psoriatic arthritis
Up to 25% of patients with psoriasis have psoriatic arthritis, which is also linked to CV comorbidities, including up to a >70% risk of diabetes, 90% higher prevalence of hypertension, and a 40% higher prevalence of obesity when compared to non-psoriasis controls.(46) Compared to patients with mild psoriasis and a similar traditional CV risk factor profile, psoriatic arthritis associates with a 2-fold higher hs-CRP and 30% higher carotid plaque. (47) In a Danish study evaluating 4 million controls, ~2,000 severe psoriasis, and ~670 psoriatic arthritis patients, compared to controls, those with psoriatic arthritis (relative risk 1.79, 95% CI [1.31 – 2.45]) and severe psoriasis without joint involvement (relative risk 1.58, 95% CI [1.32 – 1.84]) displayed a similar risk of myocardial infarction, stroke, or CV death.(48) Finally, in psoriatic arthritis, the number of dactylic digits increases CV risk (myocardial infarction, stroke, revascularization, or CV death) by ~20%.(46) These data highlight even with minimal skin activity, joint involvement also predisposes to CV risk in psoriasis.
Treatment of modifiable CV risk factors in psoriasis (Table 1):
Optimal lifestyle modification in psoriasis is a cornerstone of strategies to reduce CVD and improve skin severity. In the obese or overweight psoriatic, the National Psoriasis Foundation recommends a hypocaloric diet. (49) A meta-analysis of 7 randomized controlled trials involving ~900 overweight or obese psoriasis patients found that weight loss via caloric restriction improves psoriasis skin severity, including a 3-fold higher skin clearance rate with diet plus psoriasis treatment as opposed to psoriasis treatment alone.(50) There is a weak (strength of recommendation 2B) recommendation from the National Psoriasis Foundation emphasizing a Mediterranean diet as those psoriasis patients who are more adherent show psoriasis skin improvement, lower fat mass, and lower hs-CRP.(49) Finally, smoking cessation should be encouraged as it reduces CVD, and may also improve psoriasis skin severity. (26)
Clinical trials evaluating traditional CV risk factor treatment thresholds and goals in the psoriasis population are lacking. However, given the pattern of dyslipidemia, early vascular dysfunction, and higher prevalence of coronary plaque, lipid-lowering should play a key role in CV risk reduction strategies in psoriasis. In a retrospective study of ~9000 psoriasis patients followed a median of 4.3 years, statin therapy associated with a reduction (hazard ratio 0.31 [0.22 −0.43]) in incident myocardial infarction.(51) A post-hoc analysis of two secondary prevention lipid-lowering (high intensity vs. low intensity statin) trials identified ~500 (out of ~19,000) patients with psoriasis.(52) Psoriasis patients on high-intensity statins displayed a similar reduction in lipids and CV events when compared to non-psoriasis patients, highlighting the efficacy of statins in this high-risk population. Whether statin therapy in psoriasis confers additional anti-inflammatory benefit beyond lipid-lowering is not yet known. Finally, circulating proprotein convertase subtilisin/kexin type 9 is elevated in psoriasis (compared to non-psoriasis), preferentially expressed in psoriatic lesional skin, and associated with vascular inflammation, further emphasizing a potential benefit of lipid-lowering in psoriasis.(53)
Aspirin in the primary prevention of CVD is controversial even in the non–psoriatic patient.(2) While older studies suggest aspirin may reduce myocardial infarction and stroke in those with higher circulating inflammatory biomarkers, (54) CV outcomes data is lacking in the psoriasis population. In a small randomized controlled trial of 30 patients with psoriasis randomized to COX-1 inhibition (81mg of aspirin) or no-treatment, vascular endothelial inflammation was reduced over 70% in the aspirin group. The degree of endothelial inflammation improvement significantly correlated with degree of platelet inhibition highlighting the potential utility of aspirin in the psoriatic population.(19) Lastly, while it is reasonable to promote aggressive blood pressure and hemoglobin A1c goals, in-line with ACC/AHA recommendations in those at elevated risk of CVD (Table 1), clinical studies evaluating this approach in psoriasis are needed.(38)
Targeting inflammation in psoriasis to reduce CV risk:
In an observational analysis of almost 9000 psoriasis patients, in those treated with a biologic (e.g., TNF-α inhibitor) when compared to topical therapy, myocardial infarction was reduced by 50%. (51) Consistently, surrogates of CV risk, including hs-CRP, IL-6, glycoprotein acetylation, and platelet-lymphocyte aggregates, are all reduced in psoriasis patients undergoing treatment of their skin disease across a variety of psoriasis therapies.(18,45,55) In prospective studies, improvement in lipid parameters after treatment of psoriasis is also seen, including a small (3%), but statistically significant increase in HDL-C(44) and 75% decrease in oxidized HDL.(28) In moderate disease psoriasis patients with low CV risk, at 1-year follow-up, biologic treatment corresponded to a 6% reduction in non-calcified plaques and a 6% reduction in vascular aortic inflammation.(45,55) A non-randomized clinical trial evaluated the impact of an IL-17A inhibitor, cyclosporine, or methotrexate on myocardial and vascular function.(56) At 12 months, while psoriasis skin improvement was noted across all groups, the authors observed a 14% improvement in global longitudinal strain and 11% reduction in vascular stiffness (measured by pulse wave velocity) in the IL-17A inhibitor group as opposed to minimal changes with other treatments.(56)
Despite these observational data, randomized placebo-controlled trials targeting psoriasis skin disease to reduce vascular inflammation remain inconclusive (Table 2). Compared to placebo, three months of an IL-17A inhibitor did not improve brachial artery flow-mediated dilatation.(57) In randomized placebo controlled clinical trials of TNF-α inhibitor (43), phototherapy, (43), and IL-17A inhibitor therapy (58), the majority of treated patients displayed an adequate skin response to treatment and a reduction in circulating pro-inflammatory biomarkers; yet, vascular arterial inflammation, the primary outcome was not reduced after 3 months (Table 2). This contrasts to a smaller clinical trial of IL-12/23 inhibition, which found a significant reduction (compared to placebo) in vascular arterial inflammation at three months; however, this effect was not sustained at 1-year (Table 2).(59).
Table 2. Randomized placebo controlled clinical trials assessing the impact of biologics on vascular health in psoriasis.
In all listed studies, a crossover to active treatment occurred at the end of the randomized portion.
| Clinical Trial | Year | N | Inclusion Criteria | Treatment (N) | Duration | Primary Outcome | Main Findings | Misc |
|---|---|---|---|---|---|---|---|---|
| Adalimumab | ||||||||
| NCT01722214 TNF-α Antagonist and Vascular Inflammation in Psoriasis Vulgaris | 2017 | 107 | -≥ 5% BSA - Elevated TBR |
Adalimumab (54) Placebo (53) |
16 weeks | Change from baseline in TBR of ascending aorta | −0.002 (−0.048 to 0.053) −0.002 (−0.053 to 0.049d) |
−30% hs-CRP reduction in adalimumab group |
| NCT01553058 Vascular Inflammation in Psoriasis (VIP) | 2018 | 97 | -≥ 10% BSA | Adalimumab (33) Placebo (31) UVB (33) |
12 weeks | Percent change from baseline in maximum aortic TBR | −1.84% (−7.17% to 3.47%) −2.49% (−6.29% to 1.31%) −4.09% (−7.78 to −0.39%) |
-GlycA reduced in adalimumab group -HDL-p increased in phototherapy group |
| Ustekinumab | ||||||||
| NCT02187172 Vascular Inflammation in Psoriasis (VIP-U) | 2020 | 43 | - ≥ 10% BSA | Ustekinumab (22) Placebo (21) |
12 weeks | Change from baseline of TBR in 5 aortic segments | −6.58% (−13.64% to 0.47%) 12.07% (3.26% to 20.88%) |
-Difference between groups significant at 12 weeks (p<0.01) -TBR reductions not maintain at 52 weeks |
| Secukinumab | ||||||||
| NCT02690701 Vascular Inflammation in Psoriasis (VIP-S) | 2020 | 91 | -≥ 10% BSA | Secukinumab (46) Placebo (45) |
12 weeks | Change from baseline in maximum aortic TBR | 2.6% (−2.5% to 7.6%) 3.3% (−0.8 to 7.5) |
|
|
NCT02559622 Evaluation of Cardiovascular Risk Markers in Psoriasis Patients treated with Secukinumab (CARIMA) |
2020 | 151 | - ≥ 10 Psoriasis area and severity index score | Secukinumab 300mg (48) Secukinumab 150mg (54) Placebo (26)* Placebo (23)* |
12 weeks | Change in brachial artery flow mediated dilatation | 4.6% ± 3.5% → 5.1% ± 5.2% 4.6% ± 4.6% → 4.8% ± 3.9% 3.9% ± 3.9% → 3.6% ± 3.7% 3.7% ± 3.6%→ 3.6% ± 4.6% |
- No difference between groups at 12 weeks. |
Crossover to Secukinumab 300mg and 150mg doses at week 12 through 52.
BSA; body surface area (of active psoriasis). CAD; Coronary artery disease. HDL-p; high-density lipoprotein particle number. Hs-CRP; high sensitivity C-reactive protein. TBR; Target:background ratio (of 18F-fludeoxyglucose [FDG] uptake assessed by positron emission tomography/computed tomography [PET/CT]). UVB; Ultraviolet B (phototherapy).
As opposed to potential benefit, there was early concern of adverse CV effects of biologics in psoriasis (specifically IL-12/23 blockade).(60) However, in a meta-analysis of 38 randomized controlled trials assessing biologics to improve psoriasis skin severity and encompassing almost 18,000 patients, no statistically significant difference in adverse CV events were found across mainstays of psoriasis treatment including TNF-α, IL-12/23, and IL-17 inhibitors.(60) Despite these studies, TNF-α inhibitor use in the heart failure population is associated with adverse outcomes and not recommended in patients with psoriasis who have a history of heart failure.(38) Oral medications such as cyclosporine exhibit substantial drug-drug interactions and are associated with hypertension, while acitretin can worsen lipid profiles.(38) At higher doses than typically used in psoriatic disease, janus kinase inhibition (high dose Tofacitinib in rheumatoid arthritis pts with > 1 CV risk factor), was linked to venous thromboembolism and now carries a black box warning.(61) In summary, whether findings from observational studies or ones evaluating surrogates of CV risk translate into reductions in CV events are unknown and highlight the need for larger clinical trials with clinically meaningful endpoints to investigate targeting inflammation to reduce CV risk in psoriasis.
Residual CV risk in psoriasis:
Despite the ability of biologics to reduce visible skin and joint manifestations of psoriasis, once psoriatic pathology occurs, not all is reversible, even in the skin.(62) Memory T-cells are retained in healed skin lesions while whole transcriptome data reveal a residual disease signature containing ~25% of affected genes.(62) Translating this concept to CV risk, in a clinical study evaluating the impact of various psoriasis therapies on 157 inflammatory or CV risk associated proteins, many proteins but not all, were decreased in psoriasis treatment skin responders with variable protein reductions noted across different psoriasis therapies.(63) These data highlight the need to define the residual inflammatory burden in psoriasis and the differential impact of psoriasis medications on CV risk reduction.
Summary and conclusions:
The clinical approach to identifying and treating CV risk in patients with psoriasis are represented in Table 1 and Figure 4. Using the ACC/AHA pooled cohort equation, the patient in the opening clinical vignette has a 10-year risk of CV death, nonfatal stroke, or nonfatal myocardial infarction of 5.1%. While his psoriasis is minimally active, his use of a biologic, psoriasis duration, and prior severe psoriasis suggests a significant risk of CVD. Consistent with new guidelines, we advocate that prevalent psoriasis is a risk enhancer, and should be used in evaluating CV risk. This patient was recommended lifestyle modification and a moderate-high intensity statin, given his >5% CV risk and psoriasis.
Figure 4. Approach to Primary Prevention in Psoriasis.
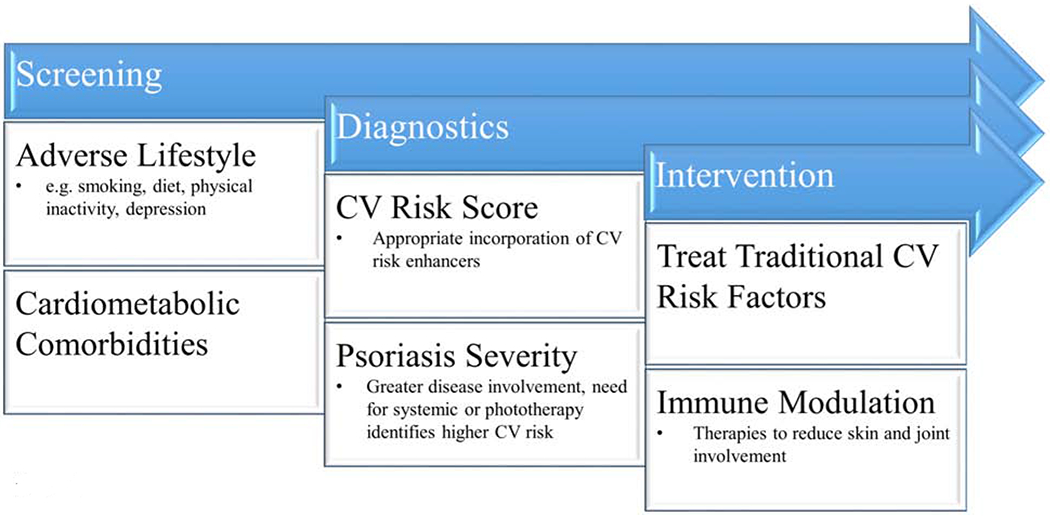
A suggested approach to cardiovascular (CV) risk management in psoriasis including early screening, the appropriate use of diagnostic tests, and therapeutic interventions.
In conclusion, the bulk of epidemiologic and clinical-translational data suggest a strong contribution of psoriasis to CVD. CV risk assessment in psoriasis requires incorporating traditional CV risk factors (Table 1, Figure 4) and other guideline-directed CV risk enhancers as appropriate. A history of moderate-to-severe psoriasis qualifies a patient with psoriasis as having a significant elevation in CV risk. In those that do not meet this criteria, a large burden of psoriatic disease (either extended duration or prolonged history of untreated disease) and psoriatic arthritis, also exhibit elevated CV risk and should be taken into consideration.
Given the heightened CV risk in psoriasis, we advocate for an aggressive approach to both lifestyle and medication therapy, recognizing that clinical trials and observational data on antiplatelet and statin therapy, blood pressure, lipid, and hemoglobin A1c goals are limited in this population and require further study (Table 1, Figure 4). Increased patient and provider awareness of the connection between atherosclerosis and psoriasis is also required to facilitate and initiate conversations on CV preventive measures. Despite compelling observational data, adequately powered randomized trials with hard clinical endpoints are necessary to investigate the benefit of CV prevention strategies including targeting inflammation to reduce CV risk in psoriasis.
Highlight bullet points.
Patients with psoriasis are at increased risk of cardiovascular disease
Cutaneous and systemic inflammation coupled with a background of traditional cardiovascular risk factors are thought to increase cardiovascular risk in psoriasis.
Randomized controlled trials are needed to determine if treatment of psoriasis reduces the risk of developing cardiovascular disease.
Acknowledgments:
We thank Dr. Tessa J. Barrett for key contributions to figure and table conceptualization.
Sources of Funding: Financial support was provided by, in part, an American Heart Association Career Development Grant (Dallas, TX) 18CDA34080540 and National Psoriasis Foundation Bridge Grant (Portland, OR), awarded to Michael S. Garshick. Nicole L. Ward was supported, in part, by NIH (Bethesda, MD) grants P50AR070590, R01AR063437, R01AR073196. Jeffrey S. Berger was supported, in part, by NIH (Bethesda, MD) grants R01HL139909, R01HL114978 and R35HL144993.
Disclosures: Dr. Krueger has received grants from: Novartis, Pfizer, Amgen, Lilly, Boehringer, Innovaderm, BMS, Janssen, Abbvie, Paraxel, Leo Pharma, Vitae, Akros, Regeneron, Allergan, Novan, Biogen MA, Sienna, UCB, Celgene, Botanix, Incyte, Avillion, Exicure. He has received personal fees from: Novartis, Pfizer, Amgen, Lilly, Boehringer, BiogenIdec, Abbvie, Leo Pharma, Escalier, Valeant, Aurigne, Allergan, Asana, UCB, Sienna, Celgene, Nimbus, Menlo, Aristea, Sanofi, Sun Pharma, Almirall, Arena, BMS. Drs Garshick, Ward, and Berger have no relevant financial relationships with industry.
Abbreviations:
- Hs-CRP
High sensitivity C-reactive protein
- ACC
American College of Cardiology
- AHA
American Heart Association
- CV
Cardiovascular
- IL
Interleukin
- CVD
Cardiovascular disease
- TNF
Tumor necrosis factor
- IFN
interferon
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benjamin EJ, Muntner P, Alonso A et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 2.Arnett DK, Blumenthal RS, Albert MA et al. 2019. ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary. J Am Coll Cardiol. 2019 September 10;74(10):1376–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stern R Discordance of individual risk estimates. J Am Coll Cardiol 2010;56:743; author reply 743–4. [DOI] [PubMed] [Google Scholar]
- 4.Greb JE, Goldminz AM, Elder JT et al. Psoriasis. Nat Rev Dis Primers 2016;2:16082. [DOI] [PubMed] [Google Scholar]
- 5.McDonald CJ, Calabresi P. Psoriasis and occlusive vascular disease. Br J Dermatol 1978;99:469–75. [DOI] [PubMed] [Google Scholar]
- 6.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA 2006;296:1735–41. [DOI] [PubMed] [Google Scholar]
- 7.Samarasekera EJ, Neilson JM, Warren RB, Parnham J, Smith CH. Incidence of cardiovascular disease in individuals with psoriasis: a systematic review and meta-analysis. J Invest Dermatol 2013;133:2340–2346. [DOI] [PubMed] [Google Scholar]
- 8.Miller IM, Ellervik C, Yazdanyar S, Jemec GB. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J Am Acad Dermatol 2013;69:1014–24. [DOI] [PubMed] [Google Scholar]
- 9.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004;109:III27–32. [DOI] [PubMed] [Google Scholar]
- 10.Mehta NN, Teague HL, Swindell WR et al. IFN-gamma and TNF-αlpha synergism may provide a link between psoriasis and inflammatory atherogenesis. Sci Rep 2017;7:13831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karbach S, Croxford AL, Oelze M et al. Interleukin 17 drives vascular inflammation, endothelial dysfunction, and arterial hypertension in psoriasis-like skin disease. Arterioscler Thromb Vasc Biol 2014;34:2658–68. [DOI] [PubMed] [Google Scholar]
- 12.Garshick MS, Barrett T, Wechter T et al. Inflammasome Signaling and Impaired Vascular Health in Psoriasis. Arterioscler Thromb Vasc Biol 2019:ATVBAHA118312246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verma D, Fekri SZ, Sigurdardottir G, Eding CB, Sandin C, Enerback C. Enhanced inflammasome activity in patients with psoriasis promotes systemic inflammation. J Invest Dermatol 2020. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong AW, Voyles SV, Armstrong EJ, Fuller EN, Rutledge JC. A tale of two plaques: convergent mechanisms of T-cell-mediated inflammation in psoriasis and atherosclerosis. Exp Dermatol 2011;20:544–9. [DOI] [PubMed] [Google Scholar]
- 15.Baumer Y, Ng Q, Sanda GE et al. Chronic skin inflammation accelerates macrophage cholesterol crystal formation and atherosclerosis. JCI Insight 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teague HL, Varghese NJ, Tsoi LC et al. Neutrophil Subsets, Platelets, and Vascular Disease in Psoriasis. JACC Basic Transl Sci 2019;4:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan Z, Wang L, Jiang H, Lin Y, Wang Z. Platelet Dysfunction and Its Role in the Pathogenesis of Psoriasis. Dermatology 2020:1–10. [DOI] [PubMed] [Google Scholar]
- 18.Sanz-Martinez MT, Moga E, Sanchez Martinez MA et al. High Levels of Platelet-Lymphocyte Complexes in Patients with Psoriasis Are Associated with a Better Response to Anti-TNF-alpha Therapy. J Invest Dermatol 2020;140:1176–1183. [DOI] [PubMed] [Google Scholar]
- 19.Garshick MS, Tawil M, Barrett TJ et al. Activated Platelets Induce Endothelial Cell Inflammatory Response in Psoriasis via COX-1. Arterioscler Thromb Vasc Biol 2020;40:1340–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vila L, Cullare C, Sola J, Puig L, de Castellarnau C, de Moragas JM. Cyclooxygenase activity is increased in platelets from psoriatic patients. J Invest Dermatol 1991;97:922–6. [DOI] [PubMed] [Google Scholar]
- 21.Cea-Calvo L, Vanaclocha F, Belinchon I, Rincon O, Julia B, Puig L. Underdiagnosis of Cardiovascular Risk Factors in Outpatients with Psoriasis Followed at Hospital Dermatology Offices: The PSO-RISK Study. Acta Derm Venereol 2016;96:972–973. [DOI] [PubMed] [Google Scholar]
- 22.Kimball AB, Szapary P, Mrowietz U et al. Underdiagnosis and undertreatment of cardiovascular risk factors in patients with moderate to severe psoriasis. J Am Acad Dermatol 2012;67:76–85. [DOI] [PubMed] [Google Scholar]
- 23.Frankel HC, Han J, Li T, Qureshi AA. The association between physical activity and the risk of incident psoriasis. Arch Dermatol 2012;148:918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol 2010;146:891–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puig L, Strohal R, Husni ME et al. Cardiometabolic profile, clinical features, quality of life and treatment outcomes in patients with moderate-to-severe psoriasis and psoriatic arthritis. J Dermatolog Treat 2015;26:7–15. [DOI] [PubMed] [Google Scholar]
- 26.Li W, Han J, Choi HK, Qureshi AA. Smoking and risk of incident psoriasis among women and men in the United States: a combined analysis. Am J Epidemiol 2012;175:402–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holzer M, Wolf P, Curcic S et al. Psoriasis alters HDL composition and cholesterol efflux capacity. J Lipid Res 2012;53:1618–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorokin AV, Kotani K, Elnabawi YA et al. Association Between Oxidation-Modified Lipoproteins and Coronary Plaque in Psoriasis. Circ Res 2018;123:1244–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehta NN, Li R, Krishnamoorthy P et al. Abnormal lipoprotein particles and cholesterol efflux capacity in patients with psoriasis. Atherosclerosis 2012;224:218–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeshita J, Wang S, Shin DB et al. Effect of psoriasis severity on hypertension control: a population-based study in the United Kingdom. JAMA Dermatol 2015;151:161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orejudo M, Rodrigues-Diez RR, Rodrigues-Diez R et al. Interleukin 17A Participates in Renal Inflammation Associated to Experimental and Human Hypertension. Front Pharmacol 2019;10:1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong Y, Nakamizo S, Tan KJ, Kabashima K. An Update on the Role of Adipose Tissues in Psoriasis. Front Immunol 2019;10:1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eder L, Chandran V, Gladman DD. The Framingham Risk Score underestimates the extent of subclinical atherosclerosis in patients with psoriatic disease. Ann Rheum Dis 2014;73:1990–6. [DOI] [PubMed] [Google Scholar]
- 34.Karbach S, Hobohm L, Wild J et al. Impact of Psoriasis on Mortality Rate and Outcome in Myocardial Infarction. J Am Heart Assoc 2020;9:e016956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta NN, Yu Y, Pinnelas R et al. Attributable risk estimate of severe psoriasis on major cardiovascular events. Am J Med 2011;124:775 e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Egeberg A, Skov L, Joshi AA et al. The relationship between duration of psoriasis, vascular inflammation, and cardiovascular events. J Am Acad Dermatol 2017;77:650–656 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li WQ, Han JL, Manson JE et al. Psoriasis and risk of nonfatal cardiovascular disease in U.S. women: a cohort study. Br J Dermatol 2012;166:811–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elmets CA, Leonardi CL, Davis DMR et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol 2019. [DOI] [PubMed] [Google Scholar]
- 39.Naik HB, Natarajan B, Stansky E et al. Severity of Psoriasis Associates With Aortic Vascular Inflammation Detected by FDG PET/CT and Neutrophil Activation in a Prospective Observational Study. Arterioscler Thromb Vasc Biol 2015;35:2667–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mansouri B, Kivelevitch D, Natarajan B et al. Comparison of Coronary Artery Calcium Scores Between Patients With Psoriasis and Type 2 Diabetes. JAMA Dermatol 2016;152:1244–1253. [DOI] [PubMed] [Google Scholar]
- 41.Lerman JB, Joshi AA, Chaturvedi A et al. Coronary Plaque Characterization in Psoriasis Reveals High-Risk Features That Improve After Treatment in a Prospective Observational Study. Circulation 2017;136:263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joshi AA, Lerman JB, Aberra TM et al. GlycA Is a Novel Biomarker of Inflammation and Subclinical Cardiovascular Disease in Psoriasis. Circ Res 2016;119:1242–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mehta NN, Shin DB, Joshi AA et al. Effect of 2 Psoriasis Treatments on Vascular Inflammation and Novel Inflammatory Cardiovascular Biomarkers: A Randomized Placebo-Controlled Trial. Circ Cardiovasc Imaging 2018;11:e007394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puig L, Strohal R, Fuiman J et al. Cardiometabolic biomarkers in chronic plaque psoriasis before and after etanercept treatment. J Dermatolog Treat 2014;25:470–81. [DOI] [PubMed] [Google Scholar]
- 45.Elnabawi YA, Dey AK, Goyal A et al. Coronary artery plaque characteristics and treatment with biologic therapy in severe psoriasis: results from a prospective observational study. Cardiovasc Res 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eder L, Gladman DD. Atherosclerosis in psoriatic disease: latest evidence and clinical implications. Ther Adv Musculoskelet Dis 2015;7:187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eder L, Jayakar J, Shanmugarajah S et al. The burden of carotid artery plaques is higher in patients with psoriatic arthritis compared with those with psoriasis alone. Ann Rheum Dis 2013;72:715–20. [DOI] [PubMed] [Google Scholar]
- 48.Ahlehoff O, Gislason GH, Charlot M et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med 2011;270:147–57. [DOI] [PubMed] [Google Scholar]
- 49.Ford AR, Siegel M, Bagel J et al. Dietary Recommendations for Adults With Psoriasis or Psoriatic Arthritis From the Medical Board of the National Psoriasis Foundation: A Systematic Review. JAMA Dermatol 2018;154:934–950. [DOI] [PubMed] [Google Scholar]
- 50.Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond) 2015;39:1197–202. [DOI] [PubMed] [Google Scholar]
- 51.Wu JJ, Poon KY, Channual JC, Shen AY. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol 2012;148:1244–50. [DOI] [PubMed] [Google Scholar]
- 52.Ports WC, Fayyad R, DeMicco DA, Laskey R, Wolk R. Effectiveness of Lipid-Lowering Statin Therapy in Patients With and Without Psoriasis. Clin Drug Investig 2017;37:775–785. [DOI] [PubMed] [Google Scholar]
- 53.Garshick MS, Baumer Y, Dey AK et al. Characterization of PCSK9 in the Blood and Skin of Psoriasis. J Invest Dermatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997;336:973–9. [DOI] [PubMed] [Google Scholar]
- 55.Dey AK, Joshi AA, Chaturvedi A et al. Association Between Skin and Aortic Vascular Inflammation in Patients With Psoriasis: A Case-Cohort Study Using Positron Emission Tomography/Computed Tomography. JAMA Cardiol 2017;2:1013–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Makavos G, Ikonomidis I, Andreadou I et al. Effects of Interleukin 17A Inhibition on Myocardial Deformation and Vascular Function in Psoriasis. Can J Cardiol 2020;36:100–111. [DOI] [PubMed] [Google Scholar]
- 57.von Stebut E, Reich K, Thaci D et al. Impact of Secukinumab on Endothelial Dysfunction and Other Cardiovascular Disease Parameters in Psoriasis Patients over 52 Weeks. J Invest Dermatol 2019;139:1054–1062. [DOI] [PubMed] [Google Scholar]
- 58.Gelfand JM, Shin DB, Duffin KC et al. A Randomized Placebo-Controlled Trial of Secukinumab on Aortic Vascular Inflammation in Moderate-to-Severe Plaque Psoriasis (VIP-S). J Invest Dermatol 2020;140:1784–1793 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gelfand JM, Shin DB, Alavi A et al. A Phase IV, Randomized, Double-Blind, Placebo-Controlled Crossover Study of the Effects of Ustekinumab on Vascular Inflammation in Psoriasis (the VIP-U Trial). J Invest Dermatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rungapiromnan W, Yiu ZZN, Warren RB, Griffiths CEM, Ashcroft DM. Impact of biologic therapies on risk of major adverse cardiovascular events in patients with psoriasis: systematic review and meta-analysis of randomized controlled trials. Br J Dermatol 2017;176:890–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Voelker R Higher Dose of Tofacitinib for Rheumatoid Arthritis Poses Risks. JAMA 2019;321:1245. [DOI] [PubMed] [Google Scholar]
- 62.Benezeder T, Wolf P. Resolution of plaque-type psoriasis: what is left behind (and reinitiates the disease). Semin Immunopathol 2019;41:633–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim J, Tomalin L, Lee J et al. Reduction of Inflammatory and Cardiovascular Proteins in the Blood of Patients with Psoriasis: Differential Responses between Tofacitinib and Etanercept after 4 Weeks of Treatment. J Invest Dermatol 2018;138:273–281. [DOI] [PubMed] [Google Scholar]


