Abstract
Background:
Increasing numbers of patients undergo hematopoietic cell transplantation (HCT). However, further characterization of late kidney outcomes is needed.
Objectives:
To describe long-term kidney outcomes in HCT survivors and compare the risk of late kidney morbidity/mortality with non-HCT cancer survivors and the general population.
Study Design:
A cohort of long-term (≥2 years) allogeneic and autologous HCT survivors, treated at our institution from 1992–2009 for cancer (n=1,792) was compared with a non-HCT cancer cohort selected from the state cancer registry (n=5,455) matched on diagnosis, sex, and age at/year of cancer diagnosis/HCT (index date). Additional comparisons were made with a matched general population sample drawn from state driver’s licensing files (DOL, n=16,340). Statewide hospital discharge codes and death registry codes (ICD-9/10) were used to determine acute kidney failure (AKF) and chronic kidney disease (CKD) occurring ≥2 years after the index date. Cumulative incidence rates and hazard ratios (HR, per multivariable proportional hazard models) estimated the absolute and relative risks of AKF and CKD. Among HCT survivors, we examined the influence of additional characteristics including estimated glomerular filtration rate (eGFR) at 1-year post-HCT.
Results:
Cumulative incidence rates of late kidney complications were slightly greater in HCT vs. non-HCT cancer survivors 10-years after the index date and both groups were more likely to experience late AKF or CKD morbidity/mortality compared with the general population: AKF (HCT 9.4%, non-HCT 7.7%, DOL 1.8%); CKD (HCT 5.7%, non-HCT 5.0%, DOL 1.2%). Differences among HCT vs. non-HCT survivors were primarily seen starting 5 years after the index date, with increased hazards for late AKF (HR 1.4, 95% CI 1.1–1.9) and CKD (HR 1.9, 95% CI 1.3–2.8). Among allogeneic HCT survivors, the presence of hypertension (present <2 years post-HCT) was significantly associated with subsequent AKF (HR 2.9, 95% CI 1.7–5.0) and CKD (HR 5.2, 95% CI 2.7–10.0) 2–10 years post-HCT, with similar associations for autologous HCT survivors. Low eGFR (<60mL/min/1.73m2) at 1-year post-HCT was associated with late AKF morbidity/mortality for both allogeneic (HR 5.3, 95% CI 2.1–13.2) and autologous transplant (HR 2.7, 95% CI 1.2–6.3) compared with survivors with normal eGFR (>90mL/min/1.73m2).
Conclusions:
Overall, the risk for hospitalization or death from AKF or CKD continues to increase with time from HCT and exceeds that of non-HCT cancer survivors more than 5 years after treatment. Appropriate screening and early intervention with medication adjustments or lifestyle modifications in those with hypertension or evidence of abnormal eGFR post-HCT could potentially mitigate this risk.
Keywords: Hematopoietic cell transplant, acute kidney failure, chronic kidney disease, long-term outcomes
INTRODUCTION
Hematopoietic cell transplantation (HCT) is increasingly used as a curative therapy for patients with certain high-risk malignancies, with over 500,000 HCT survivors expected to be living in the United States by the year 2030.1 As more patients become long-term survivors, characterization of their health outcomes is critical to support their healthcare needs given the high prevalence of late complications.1–4 HCT survivors have greater rates of hospitalization and all-cause mortality compared with non-HCT cancer survivors, while both groups experience higher morbidity and mortality compared with the general population.5 Late kidney injury is a well-known complication of cancer and HCT treatment due to the use of nephrotoxic medications and therapies that can be compounded by additional patient-specific risk factors and co-morbidities.6–8 While patients with chronic kidney disease (CKD) can progress to kidney failure (previously known as end-stage renal disease (ESRD) or end-stage kidney disease (ESKD)), reduced kidney function without need for dialysis or kidney transplantation still carries greater risk for cardiovascular disease and other late morbidity and mortality.7,9 Previous studies in long-term kidney outcomes in HCT survivors have been limited by small sample sizes, lack of comparison groups, or reliance on dialysis or kidney transplant registries, which fail to capture the spectrum of kidney dysfunction.7,8,10,11 Additionally, it is unclear if HCT survivors experience a higher frequency of late kidney complications beyond those seen in non-HCT cancer survivors.
To address these current gaps in knowledge, we examined a cohort of long-term (≥2 years) allogeneic and autologous HCT survivors to characterize the general incidence and pattern of late kidney morbidity and mortality compared with a matched non-HCT cancer survivor cohort, as well as a matched general population sample.5,12 This population-based approach allowed us to compare the risk of serious kidney complications for each group. By identifying the specific risk factors and patient subsets most likely to experience acute kidney failure or chronic kidney disease, we may be able to improve targeted screening and preventative measures for this high-risk population.13
METHODS
HCT Survivor and Comparison Cohorts
HCT survivors were treated at the Fred Hutchinson Cancer Research Center (FHCRC), a National Cancer Institute-designated comprehensive cancer center in Washington state. The FHCRC HCT database was screened to identify patients who received allogeneic and autologous HCT from 1992–2009 (n=7,108), survived ≥2 years post-transplant (n=4,081), and lived in Washington state (n=1,929) at time of HCT for underlying malignancy (n=1,792). Sex, race/ethnicity, pre-transplant diagnosis, and transplant-related exposures (donor type, stem cell source, conditioning regimen, chronic graft-versus-host-disease (cGVHD) status) were obtained from the FHCRC research database. In addition, serum creatinine values from pre-HCT and 1-year post-HCT were extracted from the research database for the subset of individuals with available laboratory data.
A comparison cohort of patients with cancer (non-HCT group) was identified from the Washington State Cancer Registry, a population-based cancer incidence registry affiliated with the National Program of Cancer Registries. Relapse status was not available from the registry. After excluding records of the FHCRC HCT survivors, remaining state residents with initial cancer diagnosis from 1992–2009 and survived ≥2 years were randomly selected and matched to HCT survivors on a 3:1 basis by sex and age at/year of cancer diagnosis/HCT, as well as cancer diagnosis group (n=5,455). A separate non-cancer comparison cohort was drawn from the Washington State Department of Licensing (DOL) files for 1992–2009, excluding persons in the HCT or non-HCT groups. Subjects were randomly selected and matched to HCT survivors who were state residents and ≥16 years of age at HCT (n=1,634) on a 10:1 basis by sex and age at/year of driver’s licensing/HCT (n=16,340). State death records were screened to ensure that the comparison subjects did not die within 2 years following licensing. Study procedures were approved by the institutional review boards at FHCRC and the Washington State Department of Health.
Outcomes Ascertainment
To assess primary outcomes, we screened the Washington State Comprehensive Hospital Abstract Reporting System, containing up to 9 diagnosis codes (International Classification of Diseases, 9th revision [ICD-9]) for all hospital discharges from non-federal facilities during the years of study. Kidney-specific ICD-9 and equivalent ICD-10 diagnosis codes were divided into categories of acute kidney failure (AKF), chronic kidney disease (CKD), and an “all kidney disease” category that included AKF, CKD, unspecified kidney failure, and dialysis codes (Supplemental Table 1). We also examined the proportion of hospitalizations with kidney disease in all cohorts. Identifiers allowed for longitudinal tracking of individuals across multiple hospitalizations and linkage to outside data sources. To assess mortality, we screened the state death registry, which records the primary and up to six contributing causes of death (ICD-9 and 10th revision) and includes out-of-state deaths for Washington residents. A sequential deterministic linkage strategy was used to link survivors and comparison subjects to hospital discharge and death records from 1992–2011.5,14 Variables used for linkage included components of names, birth date, sex, zip code, county of residence, date of HCT and corresponding hospitalization, and date of death (if applicable). We then assigned linked ICD-9 and equivalent ICD-10 codes to major kidney outcome categories occurring ≥2 years after the index date (i.e., transplant for HCT group, cancer diagnosis for non-HCT group, and driver’s licensing date for DOL group).
Statistical Analysis
Follow-up of individuals without events was censored on December 31, 2011 or earlier if they died from any cause other than the specific outcome category examined. We reported the cumulative incidence rates of late kidney morbidity/mortality in the specific outcome categories per 1,000 person years at 5 and 10 years since the index date of HCT, cancer diagnosis, or driver’s licensing. We used Cox proportional hazard models to estimate the hazard ratios and associated 95% confidence intervals for each outcome between cohort groups.15 Proportional hazards assumptions were assessed using Schoenfeld residuals and log-log plots. Models were adjusted for sex and year of diagnosis/HCT. In analyses restricted to the HCT group, we stratified by allogeneic and autologous donor status and examined the contributions of sex, race/ethnicity, age at HCT, relapse of the original disease (within the first 2 years after HCT), history of cGVHD (allogeneic survivors only), and co-morbidities of hypertension and diabetes noted by hospital discharge codes within the first two years of HCT. Additionally, in the sub cohort of HCT survivors with available laboratory data, we assessed the impact of estimated glomerular filtration rate (eGFR) at pre-HCT and 1-year post-HCT on late morbidity/mortality from AKD and CKD. eGFR was calculated using the CKD-EPI creatinine equation.16–18 All analyses were performed using STATA, version 16 (StataCorp, College Station, TX).
RESULTS
The age and sex of the HCT survivor, non-HCT cancer survivor, and DOL comparison groups were similar by design (Table 1). The median age at HCT was 46 years vs. 49 years for the non-HCT group at cancer diagnosis and 48 years for the DOL group. Among individuals not deceased, the HCT group had a median follow up of 7.1 years vs. 7.2 and 7.7 years for the non-HCT and DOL groups, respectively (range 2–20 for all groups). Among HCT survivors, 925 (51.6%) received an allogeneic transplant while 867 (48.4%) received an autologous transplant.
TABLE 1.
Demographic and treatment characteristics of ≥2-year HCT, non-HCT cancer survivors, and general population (DOL) comparison group
| Characteristic | HCT | Non-HCT | DOL | |||
|---|---|---|---|---|---|---|
| N=1,792 |
N=5,455a |
N=16,340b |
||||
| n | (%) | n | (%) | n | (%) | |
| Female | 819 | (45.7) | 2,381 | (43.6) | 7,500 | (45.9) |
| Race/ethnicity | ||||||
| White non-Hispanic | 1,504 | (83.9) | 4,543 | (83.3) | - | |
| Black | 40 | (2.2) | 202 | (3.7) | - | |
| Hispanic | 81 | (4.5) | 270 | (4.9) | - | |
| Asian | 75 | (4.2) | 241 | (4.4) | - | |
| Other/unknown | 92 | (5.1) | 199 | (3.6) | - | |
| Age at index date, yearsc | ||||||
| <20 | 277 | (15.5) | 767 | (14.1) | 570 | (3.5) |
| 20–39 | 376 | (21.0) | 934 | (17.1) | 4,100 | (25.1) |
| 40–59 | 843 | (47.0) | 2,590 | (47.5) | 8,660 | (53.0) |
| ≥60 | 296 | (16.5) | 1,164 | (21.3) | 3,010 | (18.4) |
| Year of index event | ||||||
| 1992–1999 | 520 | (29.0) | 1,417 | (26.0) | 4,480 | (27.4) |
| 2000–2009 | 1272 | (71.0) | 4,038 | (74.0) | 11,860 | (72.6) |
| Diagnosis categoryd | ||||||
| Hematologic malignancy | 1,617 | (90.2) | 4,808 | (88.1) | - | |
| Non-hematologic solid tumor | 175 | (9.8) | 647 | (11.9) | - | |
| Donor type | ||||||
| Autologous/syngeneic | 867 | (48.4) | - | - | ||
| Related allogeneic | 503 | (28.1) | - | - | ||
| Unrelated allogeneic | 422 | (23.5) | - | - | ||
| History of total body irradiation | ||||||
| None | 969 | (54.1) | - | - | ||
| <10 Gy | 221 | (12.3) | - | - | ||
| ≥10 Gy | 602 | (33.6) | - | - | ||
| History of cGVHDe | 642 | (36.1) | - | - | ||
| History of cancer relapse after index date | 434 | (24.2) | - | - | ||
| Died during the follow-up period | 388 | (21.7) | 997 | (18.3) | 581 | (3.6) |
DOL, Department of Licensing; cGVHD, chronic graft versus host disease; HCT, hematopoietic cell transplantation
Matched on 3:1 basis by sex, and age at and year of HCT/cancer diagnosis to HCT survivors. Data on relapse status are not collected by the state cancer registry
Matched on 10:1 basis by sex, and age at and year of HCT/driver’s licensing to HCT survivors age ≥16 years at time of transplant. Race/ethnicity data not available
Age at transplant (HCT survivors), initial cancer diagnosis (non-HCT cancer survivors), or driver’s licensing (general population)
A more detailed breakdown of diagnoses can be found in the Supplemental Table 1
Limited to those who required systemic immunosuppressive therapy (14 with unknown status)
When the 5- and 10-year cumulative incidences of hospitalization or death due to AKF, CKD, and all kidney disease outcomes were examined (Figure 1; Table 2), rates among HCT and non-HCT survivors were both significantly increased compared with the DOL group (p<0.001). When we examined kidney diagnoses as a proportion of all hospitalization codes, kidney outcomes remained more common in the HCT cohort (14.5%) compared with the DOL cohort (9.4%, p<0.001). Cumulative incidence rates for AKF and CKD between HCT and non-HCT survivors were not significantly different at 5-years post-HCT (5.2% vs 5.0% (p=0.81) for AKF; 3.0% vs. 3.5% (p=0.34) for CKD). Similarly, there was no significant difference in late outcomes between HCT and non-HCT survivors at 10-years post-HCT (9.4% vs. 7.7% (p=0.07) for AKF and 5.7% vs. 5.0% (p=0.31) for CKD). However, there was a suggestion of a divergence between the two groups after 5 years. For example, when the relative hazards between the HCT and non-HCT survivor groups were examined, HRs associated with kidney outcomes occurring within 5-years of the index time point were not significantly increased (Table 3). In contrast, HRs associated with outcomes occurring after five years showed a significantly increased risk associated with HCT status compared with non-HCT cancer survivors (HR 1.4 [95% CI 1.1–1.9] for AKF and HR 1.9 [95% CI 1.3–2.8] for CKD).
FIGURE 1.
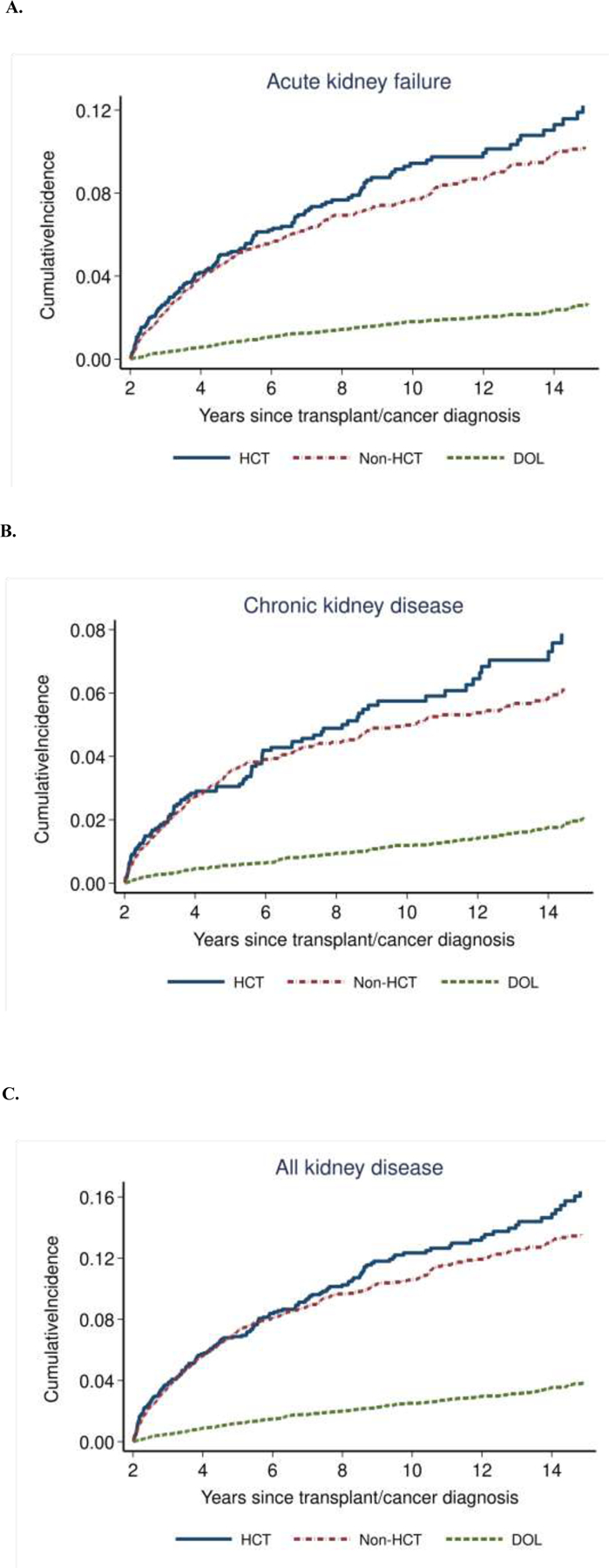
Cumulative incidence of late morbidity/mortality for A) acute kidney failure, B) chronic kidney disease, and C) all kidney disease among HCT survivors (blue solid line), non-HCT matched cancer survivors (red dashed line), and DOL general population group (green dashed line)
TABLE 2.
Five- and ten-year cumulative incidence rates with 95% confidence intervals (CI) of kidney outcomes among HCT vs. comparison groups (non-HCT cancer survivors and the general population [DOL])
| HCT |
Non-HCT |
DOL |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | No. events | 5y (95% CI) | 10y (95% CI) | No. events | 5y (95% CI) | 10y (95% CI) | No. events | 5y (95% CI) | 10y (95% CI) |
| Acute kidney failure | 142 | 5.2 (4.2–6.3) | 9.4 (7.9–11.2) | 364 | 5.0 (4.4–5.7) | 7.7 (6.9–8.6) | 258 | 0.8 (0.7–1.0) | 1.8 (1.6–2.1) |
| Chronic kidney disease | 94 | 3.0 (2.3–4.0) | 5.7 (4.5–7.1) | 234 | 3.5 (3.0–4.1) | 5.0 (4.3–5.7) | 176 | 0.6 (0.4–0.7) | 1.2 (1.0–1.4) |
| All kidney disease | 190 | 6.9 (5.7–8.2) | 12.4 (10.6–14.3) | 510 | 7.3 (6.6–8.1) | 10.7 (9.8–11.7) | 367 | 1.2 (1.0–1.4) | 2.5 (2.2–2.8) |
DOL, Department of Licensing; HCT, hematopoietic cell transplantation
TABLE 3.
Relative hazards (HR) with 95% confidence intervals (CI) of kidney outcomes occurring between 2–5 and ≥5-years among HCT vs. non-HCT survivors (referent group)*
| Outcome | 2–5 y | ≥5y |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Acute kidney failure | 1.1 (0.8–1.4) | 1.4 (1.1–1.9) |
| Chronic kidney disease | 0.9 (0.7–1.3) | 1.9 (1.3–2.8) |
| All kidney disease | 1.0 (0.8–1.2) | 1.5 (1.2–1.9) |
Models adjusted for sex and diagnosis year.
In analysis of risk factors among HCT survivors alone, allogeneic and autologous survivors who were older at the time of HCT had an increased hazard of late morbidity/mortality for both AKF and CKD (Table 4). A history of hypertension was also independently associated with an increased risk of kidney outcomes for both groups, with a 5-fold increased hazard of late morbidity/mortality from CKD. A history of cGVHD in allogeneic survivors was only borderline associated with late CKD complications (HR 2.5 [95% CI 0.9–7.2]; p=0.09). Of note, underlying diagnosis (hematologic malignancy vs. solid tumor), donor type (allogeneic vs. autologous), and receipt of total body irradiation were not associated with kidney outcomes.
TABLE 4.
Relative hazards (HR) with 95% confidence intervals (CI) of kidney outcomes occurring 2–10 years post-HCT among allogeneic and autologous survivors*
| Outcome / risk factor | Allogeneic | Autologous |
|---|---|---|
| HCT | HCT | |
| HR (95% CI) | HR (95% CI) | |
| Acute kidney failure | ||
| Age at HCT‡ | 2.4 (1.5–3.7) | 2.0 (1.3–2.9) |
| History of post-HCT relapse | 2.8 (1.5–5.1) | 2.1 (1.1–4.0) |
| History of hypertension | 2.9 (1.7–5.0) | 2.5 (1.3–4.7) |
| History of diabetes | 1.0 (0.4–2.4) | 3.8 (1.5–9.2) |
| History of cGVHD | 1.4 (0.7–2.6) | n/a |
| Chronic kidney disease | ||
| Age at HCT‡ | 2.4 (1.3–4.3 | 1.8 (1.1–2.8) |
| History of post-HCT relapse | 0.9 (0.3–2.9) | 1.3 (0.5–3.5) |
| History of hypertension | 5.2 (2.7–10.0) | 4.8 (2.4–9.6) |
| History of diabetes | 0.7 (0.2–2.3) | 1.3 (0.3–5.6) |
| History of cGVHD | 2.5 (0.9–7.2) | n/a |
cGVHD, chronic graft vs. host disease; n/a, not applicable
Models adjusted for all risk factors listed plus sex, race/ethnicity, and year of HCT.
Calculated in 20-year increments
When the subset of HCT survivors with laboratory data was examined, the proportion of allogeneic survivors with eGFR data at pre-transplant and 1-year was 98.2% and 84.8%, respectively. eGFR data were less complete for autologous HCT survivors at 1-year, with only 50.6% available. Overall, 67.4% of all HCT survivors with laboratory data had normal eGFR (≥90 mL/min/1.73m2) pre-transplant, while 26.9% had mildly decreased eGFR (60–89 mL/min/1.73m2), and only 5.7% had CKD (eGFR <60 mL/min/1.73 m2; Supplemental Table 2). The distribution of kidney function in survivors changed from baseline to 1-year post-HCT (p<0.001), with the proportion with normal eGFR decreasing to 45.4% by 1-year post-HCT. Correspondingly, the proportion of survivors with eGFR <60 mL/min/1.73m2 increased from 5.7% at baseline to 20.3% by 1-year post-HCT.
For HCT survivors with laboratory data available at 1-year post-HCT, decreased eGFR was associated with late morbidity/mortality from AKF (Supplemental Table 3). This was especially pronounced in patients who had received allogeneic transplants. The relative hazard associated with late AKF ≥2 years after allogeneic HCT was 5.3 (95% CI 2.1–13.2) in patients with 1-year post-HCT eGFR <60 mL/min/1.73m2 compared with patients with normal eGFR. In survivors of autologous HCT, the corresponding HR was 2.7 (95% CI 1.2–6.3). In examination of late morbidity/mortality from CKD, we excluded all patients who already had eGFR <60 mL/min/1.73m2 at 1-year post-HCT. For survivors of allogeneic transplant, decreased eGFR (60–89 mL/min/1.73m2) at 1-year post-HCT was suggestive of an increased risk of late CKD complications ≥2 years from transplant compared with survivors with normal eGFR (HR 4.0 [95% CI 0.8–20.5]).
DISCUSSION
Overall, we found that the risk for hospitalization or death from AKF or CKD continued to increase with time from HCT and exceeded that of non-HCT cancer survivors more than 5 years after treatment. Specifically, HCT status was associated with 40% greater risk of late AKF complications and 90% greater risk of late CKD complications compared with non-HCT survivors. This may reflect increased cumulative toxicity from HCT-related therapies contributing to greater kidney-related morbidity or mortality compared with conventional therapy. The relative differences between HCT and non-HCT survivors only became evident several years post-treatment, possibly related to slow progression in kidney dysfunction that does not appear to be fully explained by co-morbidities such as cGVHD. While few other studies have directly compared HCT survivors with non-HCT cancer survivors, one study in pediatric patients found that HCT survivors were nearly 4-times more likely to report serious or life-threatening chronic health conditions compared with non-HCT hematologic cancer survivors.19 The cumulative incidence of late CKD morbidity/mortality as defined in our study was similar to a long-term study estimating a cumulative incidence of CKD of 5.7% (95% CI 4.2–7.2) at 10-years post-HCT using a definition of eGFR <60mL/min/1.73m2.8 Although acute kidney injury (AKI) commonly occurs in patients undergoing HCT, studies typically focus on AKI within the first 100 days after transplantation6,20–22; in contrast, our study found that HCT survivors remained at risk for acute kidney complications years after treatment. It is worth noting that various definitions of AKI/AKF and CKD have been used in the HCT literature, making comparisons between studies challenging.
In terms of risk factors, we found that older age at time of transplant and hypertension were associated with late kidney complications among HCT survivors, which have been noted in previous studies.13 Older patients, in addition to advanced age, often have co-morbid conditions that increase their risk for kidney injury.6,11,23 In a study of 1190 HCT survivors, Choi et al. similarly found that older age at transplant was associated with late development of CKD (RR per 5-year increment 1.33, 95% CI 1.2–1.5) at a median of 7 years post-HCT.8 In our study, history of hypertension was an important risk factor for late AKF and particularly CKD complications (HR ~5) for both allogeneic and autologous HCT survivors. This is notable as hypertension represents one of the few modifiable risk factors in the development of CKD and related CV complications.6,7,24,25 In contrast to hypertension, history of diabetes was not associated with late kidney complications. Although we did not find an association between cGVHD status and late AKF complications, cGVHD was suggestive of an increased risk of late CKD complications. This association between cGVHD and CKD has been previously described,23,26 hypothesized to result from chronic systemic inflammation, antibody-mediated injury, or direct T-cell-mediated injury resulting in renal endothelial and tubular injury.6
Our study corroborated previous studies demonstrating that decreased eGFR occurs commonly following HCT and that survivors with lower eGFR experience a higher burden of late morbidity and mortality.7,23 This may be related to underlying kidney insufficiency or decreased kidney reserve following treatment.21 In our study, the majority (67.4%) of patients had normal kidney function pre-HCT but 20.3% developed eGFR <60mL/min/m2 by 1-year post-HCT. Similarly, another cohort study found that 23% of HCT survivors developed GFR <60mL/min/m2 during follow-up (median 191 days post-transplant, range 131–516).23 While other studies have shown that kidney function generally stabilizes within the first few years following HCT,8,26,27 our study found that the risk for hospitalization or death with AKF, CKD, and other kidney issues continued to increase with time from HCT.
A previous prospective study conducted at our institution of 294 1-year HCT survivors concluded that the largest decrease in eGFR occurred with the first year post-HCT.28 This suggests that HCT survivors should be monitored closely for kidney dysfunction in the first year post-HCT. Unfortunately, prospective or randomized studies specific to HCT survivors are minimal, so most screening guidelines and recommendations are drawn from expert panels. Current screening guidelines for CKD in HCT survivors represent a consensus from the Center for International Blood and Marrow Transplant Research (CIBMTR), European Group for Blood and Marrow Transplantation (EBMT), and American Society for Bone Marrow Transplantation (ASBMT)/American Society for Transplantation and Cellular Therapy (ASTCT) and include at minimum yearly blood pressure screening and lab monitoring with urine protein and serum BUN/creatinine.13,29 For patients who develop albuminuria and elevated blood pressure, interventions such as angiotensin-converting-enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs)6,7 could be considered, particularly given the importance of hypertension as a risk factor for late kidney complications. In a small randomized controlled trial of HCT survivors treated with the ACE inhibitor captopril versus placebo, there was a trend towards higher eGFR at 1-year post-HCT in the captopril group, although the study was limited by small number of enrollees and less than optimal adherence with the study drug.30 Other recommendations for management of progression and complications of CKD are generally drawn from experience with CKD in the general population.31 For high-risk groups such as HCT survivors, early referral to a nephrologist for evaluation and co-management can help prevent and treat complications of kidney dysfunction.13,29 Avoidance of additional nephrotoxic insults is also critical to avoid progressive kidney disease. Other interventional strategies for hypertension and other modifiable cardiovascular risk factors, such as dietary salt restriction, weight reduction, and regular exercise, may also mitigate long-term morbidity among survivors.29,32,33
Our population-based study benefited from a relatively large number of HCT survivors and the presence of a matched non-HCT cancer comparison group. Additionally, the use of population-based administrative data eliminated potential response biases that may affect long-term studies relying on self-reported data.34,35 However, hospital discharge and death records may be incomplete or incorrectly coded, leading to misclassification, and patients must be hospitalized for their outcomes to be counted. Previous studies examining the accuracy of kidney disease ICD codes compared with diagnoses made from chart review have found that specificity is typically high (93–99%) but sensitivity can be variable (3–88%), although ICD codes generally underestimate the true prevalence of kidney disease.36,37 However, this inconsistency should apply to all three cohorts in our study, and any misclassification of our outcome measures should be non-differential, which should bias results towards the null. While we did not have access to ambulatory encounter data, we were unlikely to miss patients with significant AKF or CKD, including those patients requiring dialysis, as these problems would have likely been listed as diagnosis codes with hospitalizations. Furthermore, our observation that kidney disease codes represented a greater proportion of all hospitalization codes among HCT survivors compared with the general population lends support to our overall finding of an increased risk of serious late kidney outcomes among HCT survivors. An additional limitation is that this study only included patients transplanted through 2009 and followed through 2011. Therefore, we do not have information on more recent changes in both cancer therapies and HCT conditioning regimens. Lastly, for the HCT survivor cohort, we lacked information on pre-HCT exposures that may have influenced late kidney disease, considering that a third of the patients with laboratory data already had abnormal eGFR pre-HCT.
CONCLUSIONS
We found that HCT survivors were at greater risk of being hospitalized with or dying from both acute and chronic kidney disease compared with non-HCT cancer survivors >5 years after HCT/cancer diagnosis. Overall, the risk for poor kidney outcomes continued to increase with time from HCT. Providers caring for long-term HCT survivors should be aware of these risks and consider early aggressive interventions for patients with high blood pressure, decreased eGFR, or other signs of kidney dysfunction, in order to mitigate late kidney morbidity and mortality.
Supplementary Material
Highlights.
Risk of poor kidney outcomes increases with time from hematopoietic cell transplant
Transplant survivors matched with cancer survivors and general population cohort
Transplant status associated with higher rates of acute and chronic kidney disease
Early management of hypertension or kidney dysfunction can help mitigate risk
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health (NIH), National Cancer Institute, grants CA151775, CA167451, and CA018029. A large portion of the data provided by the Washington State Cancer Registry were collected under NIH Surveillance, Epidemiology, and End Results (SEER) program contracts, National Cancer Institute grants N01-CN-67009 and N01-PC-35142, and Department of Health and Human Services/NIH grant HHSN26120130012I. This investigation was additionally supported by the NIH under the Ruth L. Kirschstein National Research Service Award T32CA009351.
Footnotes
Financial disclosures: none.
Declarations of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Majhail NS, Tao L, Bredeson C, et al. Prevalence of hematopoietic cell transplant survivors in the United States. Biology of Blood and Marrow Transplantation. 2013;19(10):1498–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun C-L, Francisco L, Kawashima T, et al. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study. Blood, The Journal of the American Society of Hematology. 2010;116(17):3129–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun C-L, Kersey JH, Francisco L, et al. Burden of morbidity in 10+ year survivors of hematopoietic cell transplantation: report from the bone marrow transplantation survivor study. Biology of blood and marrow transplantation. 2013;19(7):1073–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. Journal of clinical oncology. 2011;29(16):2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow EJ, Cushing-Haugen KL, Cheng G-S, et al. Morbidity and mortality differences between hematopoietic cell transplantation survivors and other cancer survivors. Journal of Clinical Oncology. 2017;35(3):306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hingorani S Renal complications of hematopoietic-cell transplantation. New England Journal of Medicine. 2016;374(23):2256–2267. [DOI] [PubMed] [Google Scholar]
- 7.Ando M, Ohashi K, Akiyama H, et al. Chronic kidney disease in long-term survivors of myeloablative allogeneic haematopoietic cell transplantation: prevalence and risk factors. Nephrology Dialysis Transplantation. 2010;25(1):278–282. [DOI] [PubMed] [Google Scholar]
- 8.Choi M, Sun CL, Kurian S, et al. Incidence and predictors of delayed chronic kidney disease in long-term survivors of hematopoietic cell transplantation. Cancer: Interdisciplinary International Journal of the American Cancer Society. 2008;113(7):1580–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C-y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. New England Journal of Medicine. 2004;351(13):1296–1305. [DOI] [PubMed] [Google Scholar]
- 10.Béchade C, Dejardin O, Bara S, et al. Survival of patients with cancer starting chronic dialysis: Data from kidney and cancer registries in lower Normandy. Nephrology. 2018;23(12):1125–1130. [DOI] [PubMed] [Google Scholar]
- 11.Touzot M, Elie C, van Massenhove J, Maillard N, Buzyn A, Fakhouri F. Long-term renal function after allogenic haematopoietic stem cell transplantation in adult patients: a single-centre study. Nephrology Dialysis Transplantation. 2010;25(2):624–627. [DOI] [PubMed] [Google Scholar]
- 12.Foord AM, Cushing-Haugen KL, Boeckh MJ, et al. Late infectious complications in hematopoietic cell transplantation survivors: a population-based study. Blood advances. 2020;4(7):1232–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Majhail NS, Rizzo JD, Lee SJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Hematology/oncology and stem cell therapy. 2012;5(1):1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow EJ, Mueller BA, Baker KS, et al. Cardiovascular hospitalizations and mortality among recipients of hematopoietic stem cell transplantation. Annals of internal medicine. 2011;155(1):21–32. [DOI] [PubMed] [Google Scholar]
- 15.Therneau TM, Grambsch PM. The Cox model. In: Modeling survival data: extending the Cox model. Springer; 2000:39–77. [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA. Estimating GFR using the CKD epidemiology collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. American Journal of Kidney Diseases. 2010;55(4):622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Eckardt K-U, Dorman NM, et al. Nomenclature for kidney function and disease: report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney International. 2020;97(6):1117–1129. [DOI] [PubMed] [Google Scholar]
- 19.Armenian SH, Sun C-L, Kawashima T, et al. Long-term health-related outcomes in survivors of childhood cancer treated with HSCT versus conventional therapy: a report from the Bone Marrow Transplant Survivor Study (BMTSS) and Childhood Cancer Survivor Study (CCSS). Blood, The Journal of the American Society of Hematology. 2011;118(5):1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Didsbury MS, Mackie FE, Kennedy SE. A systematic review of acute kidney injury in pediatric allogeneic hematopoietic stem cell recipients. Pediatric transplantation. 2015;19(5):460–470. [DOI] [PubMed] [Google Scholar]
- 21.Lopes J, Jorge S. Acute kidney injury following HCT: incidence, risk factors and outcome. Bone marrow transplantation. 2011;46(11):1399–1408. [DOI] [PubMed] [Google Scholar]
- 22.Kogon A, Hingorani S. Acute kidney injury in hematopoietic cell transplantation. Seminars in nephrology. 2010;30(6):615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hingorani S Chronic kidney disease in long-term survivors of hematopoietic cell transplantation: epidemiology, pathogenesis, and treatment. Journal of the American Society of Nephrology. 2006;17(7):1995–2005. [DOI] [PubMed] [Google Scholar]
- 24.Chow EJ, Baker KS, Lee SJ, et al. Influence of conventional cardiovascular risk factors and lifestyle characteristics on cardiovascular disease after hematopoietic cell transplantation. Journal of clinical oncology. 2014;32(3):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armenian SH, Sun C-L, Vase T, et al. Cardiovascular risk factors in hematopoietic cell transplantation survivors: role in development of subsequent cardiovascular disease. Blood. 2012;120(23):4505–4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abboud I, Porcher R, Robin M, et al. Chronic kidney dysfunction in patients alive without relapse 2 years after allogeneic hematopoietic stem cell transplantation. Biology of Blood and Marrow Transplantation. 2009;15(10):1251–1257. [DOI] [PubMed] [Google Scholar]
- 27.Grönroos M, Bolme P, Winiarski J, Berg U. Long-term renal function following bone marrow transplantation. Bone marrow transplantation. 2007;39(11):717–723. [DOI] [PubMed] [Google Scholar]
- 28.Hingorani S, Pao E, Stevenson P, et al. Changes in glomerular filtration rate and impact on long-term survival among adults after hematopoietic cell transplantation: A prospective cohort study. Clinical Journal of the American Society of Nephrology. 2018;13(6):866–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abboud I, Peraldi M-N, Hingorani S. Chronic kidney diseases in long-term survivors after allogeneic hematopoietic stem cell transplantation: monitoring and management guidelines. Paper presented at: Seminars in hematology2012. [DOI] [PubMed] [Google Scholar]
- 30.Cohen EP, Irving AA, Drobyski WR, et al. Captopril to mitigate chronic renal failure after hematopoietic stem cell transplantation: a randomized controlled trial. International journal of radiation oncology, biology, physics. 2008;70(5):1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levin A, Stevens PE, Bilous RW, et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney international supplements. 2013;3(1):1–150. [Google Scholar]
- 32.Chang E, Iukuridze A, Echevarria M, et al. Feasibility and Acceptability of Using a Telehealth Platform to Monitor Cardiovascular Risk Factors in Hematopoietic Cell Transplantation Survivors at Risk for Cardiovascular Disease. Biology of Blood and Marrow Transplantation. 2020;26(6):1233–1237. [DOI] [PubMed] [Google Scholar]
- 33.Rizzo JD, Wingard JR, Tichelli A, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: joint recommendations of the European Group for Blood and Marrow Transplantation, the Center for International Blood and Marrow Transplant Research, and the American Society of Blood and Marrow Transplantation. Biology of Blood and Marrow Transplantation. 2006;12(2):138–151. [DOI] [PubMed] [Google Scholar]
- 34.Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. Journal of Clinical Oncology. 2013;31(29):3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gibson TM, Mostoufi-Moab S, Stratton KL, et al. Temporal patterns in the risk of chronic health conditions in survivors of childhood cancer diagnosed 1970–99: a report from the Childhood Cancer Survivor Study cohort. The Lancet Oncology. 2018;19(12):1590–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vlasschaert ME, Bejaimal SA, Hackam DG, et al. Validity of administrative database coding for kidney disease: a systematic review. American journal of kidney diseases. 2011;57(1):29–43. [DOI] [PubMed] [Google Scholar]
- 37.Waikar SS, Wald R, Chertow GM, et al. Validity of international classification of diseases, ninth revision, clinical modification codes for acute renal failure. Journal of the American Society of Nephrology. 2006;17(6):1688–1694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


