Abstract
Coronavirus disease (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2), has claimed many victims worldwide due to its high virulence and contagiousness.
The person-to-person transmission of SARS-Cov-2 when in close contact is facilitated by respiratory droplets containing the virus particles, and by skin contact with contaminated surfaces. However, the large number of COVID-19 infections cannot be explained only by droplet deposition or contact contamination. It seems very plausible that aerosols are important in transmitting SARS-Cov-2. It has been demonstrated that SARS-CoV-2 remains viable in aerosols for hours, facilitating rapid distribution of the virus over great distances. Aerosols may, therefore, also be responsible for so-called super-spreader events.
Indirect evidence points to a correlation between ventilation and the transmission and spread of SARS-Cov-2, supporting ventilation as an important factor in preventing airborne transmission. Further actions to avoid transmission of COVID-19 include social distancing, hygiene measures, and barrier measures, such as face-coverings. Professional masks offer better protection than cloth masks.
These protection measures are especially relevant to health care workers, when performing endotracheal intubation, but the risk from non-invasive ventilation and nebulizing treatment seems to be moderate.
Keywords: aerosols, COVID-19, prevention, SARS-CoV-2, transmission
The Coronavirus disease (COVID-19), first emerging in Wuhan in late 2019, and caused by the severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) has claimed many victims worldwide due to its high virulence and contagiousness. The person-to-person transmission of SARS-Cov-2 in close contact is facilitated by respiratory droplets containing the virus particles, and by skin contact with contaminated surfaces. Another route is airborne transmission by aerosols (Fig. 1). COVID-19 is transmitted by both symptomatic patients and asymptomatic carriers.2 Measures to prevent transmission of SARS-Cov-2 are absolutely necessary to curb the pandemic, and to relieve the heavy burden on society and hospitals. Knowledge of and respect for the route of transmission is pivotal to taking precautions.
FIGURE 1.
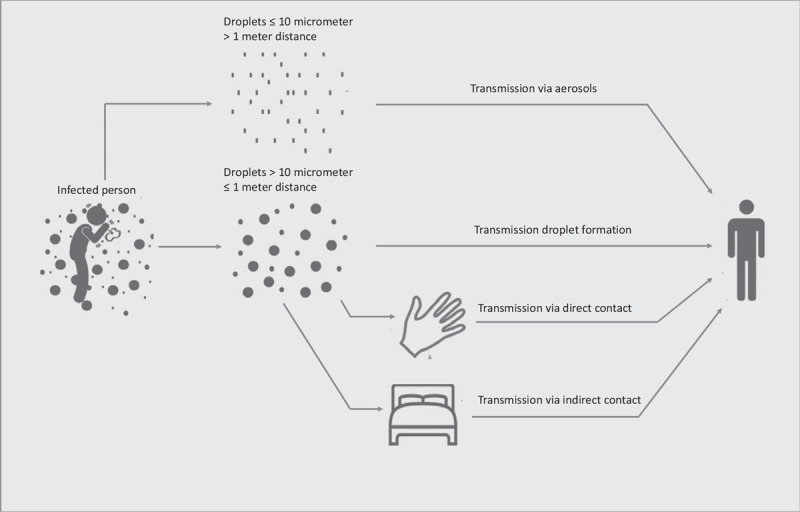
Routes of virus transmission. This figure is reproduced and modified from Otter et al (2016).1
EVIDENCE OF VIRUS TRANSMISSION
Droplet Transmission
Virus transmission of SARS-Cov-2 from person to person (direct transmission) is facilitated by respiratory droplets containing virus particles. The German bacteriologist Carl Flügge (1847–1923) demonstrated as far back as 1894 that “speaking”—patients in a study emitted droplets that showed bacterial growth on a culture medium close by. An American health scientist, William Wells (1887–1963), exposed the interplay between gravity and evaporation. Droplets larger than a humidity-determined threshold fall to the ground due to gravity and smaller droplets evaporate to become aerosols that drift in the air and remain airborne (Fig. 2). Droplets released by coughing/speaking do not spread further than approximately 3 ft due to gravity.3,4
FIGURE 2.

The Wells evaporation-falling curve. This figure is reproduced and modified from Wells and Xie.3,4
The current government social distancing measures were based on these historical data of William Wells: the 5 foot distancing in, for example, the Netherlands, the stricter 6.5 foot distancing in Switzerland and Sweden, and less strict measures of 3 foot distancing in France and Austria. The study by Chen et al5 demonstrated that droplet deposition by gravity only dominates when the droplets are larger than 100 μm and when the subjects are within 0.2 m while talking, or 0.5 m while coughing.
To get more insight into transmission routes, in 1981 Breese Hall et al performed experiments with Respiratory Syncytial Virus in infected infants. Volunteers were exposed to the infected infants in three ways: (1) the cuddlers (caring for the infants in the usual manner; feeding, playing, cleaning a diaper), (2) touchers (contact with surfaces contaminated by the infants), and (3) sitters (sitting at a distance from the infants). The most, and most severely infected volunteers were seen in the cuddler group, followed by the toucher group.6
The findings of the most clinically relevant systematic review and meta-analysis by Chu et al on COVID-19, SARS, and Middle East Respiratory Syndrome (MERS) (44 comparative studies; n = 25,697 patients) suggest evidence that the current policies of at least 1 m distancing were associated with a large reduction in infections, but a distance of 2 m might be even more effective. However, the studies on droplets transmission continue to be debated, because there is not full transparency about the measurement of transmission distance and the time of remaining exposed to the virus.
Surface Transmission
The person-to-person transmission of SARS-Cov-2 is also facilitated by direct skin contact or indirect contact via contaminated surfaces. Van Doremalen et al7 demonstrated that SARS-Cov-2 may be viable for days on surfaces, a precondition for contact contamination. A Japanese video showed using a fluorescent substance, only visible under black light, how easily viruses can spread by contact transmission in restaurants from one infected person (weblink 1). However, it is noteworthy that in home measurements with COVID-19 patients, the virus was detectable on less than 5% of the tested surfaces.8 The large numbers of simultaneous COVID-19 group infections, the so-called super-spreader events (SSEs) cannot be explained solely by droplet deposition or contact contamination. It is very plausible that aerosols may be important in transmitting SARS-Cov-2.9
Weblink 1
Aerosol Transmission
Aerosols are fine solid particles or droplets dispersed in gas. Aerosols containing virus particles are released when infected persons cough, or even talk or breathe. The number of particles expelled from the airways when talking varies from person to person and can be as much as 300,000 particles at a time, with an average size of 0.7 to 10 μm, but range in size in the flume of patients. Wang et al10 showed that a single cough by a person with a high viral load in their respiratory fluid (2.35 × 109 copies per mL) may generate as many as 1.23 × 105 copies of viruses. This aerosol transmission is well demonstrated in Japanese micro-droplet spreading experiments (weblink 2).
Previously, evidence indicated that aerosols were an important mode of transmission for Influenza A virus and SARS-CoV-1.11,12 The small aerosols (less than 5 μm) with virus material can be inhaled and directly transported deep into the airways and alveolar compartment.13 For Influenza A, it has been demonstrated that transmission via aerosols was associated with more severe illness.14 Larger droplets (greater than 5 μm) are more likely to infect the upper airways.13 The potential for droplet transmission also depends on environmental conditions. The study by Xie et al investigated using numerical computations, among other things, the effects of humidity on large droplet evaporation to aerosols, with subsequent dispersion. They demonstrated that at lower humidity, more droplets suspended in the air increase the probability of subsequent inhalation.3 In contrast, sunlight rapidly inactivates SARS-CoV-2 in small-particle aerosols.15
William Wells demonstrated in his book (1955) that all laboratory animals exposed to aerosols containing influenza die quickly compared to animals who were inoculated with large droplets containing influenza.4 A study by Yu et al12 showed during the SARS-COV-1 outbreak using an airflow simulation model how the airborne spread of the SARS-COV-1 takes place in different buildings, and supported the probability of airborne spread.
Aerosols may also be important in transmitting SARS-Cov-2 and it has been demonstrated that SARS-CoV-2 remains viable in aerosols for hours, facilitating rapid distribution of the virus over great distances.7,9 Several other model studies made it likely that SARS-CoV-2 airborne transmission is even more relevant than large droplets or contact transmission.16,17 An illustrative example is contamination with SARS-CoV-2 in a Chinese restaurant: only people who were in the index patient's airflow were infected. The air flow was passing continuously through a recirculating cooling system without a filter, while the basic ventilation system for fresh air supply was switched off.18 Another example is the outbreak in a nursing home in the Netherlands that was likely the result of aerosol transmission due to inadequate ventilation. SARS-CoV-2 RNA was detected in dust present on the air conditioners and in four block filters from 3 of the 8 ventilation cabinets.19
Weblink 2
ATTENTION TO ROUTES OF TRANSMISSION
The World Health Organization and governments focus mainly on the prevention of droplet and surface transmission through hygiene measures, limiting human contact and social distancing. The third route, aerosol transmission, has been underestimated for a long time. However, this airborne route may be a plausible transmission route for SARS-CoV-2, facilitating its fast spread all over the world. Knowledge and awareness of all routes of transmission is pivotal to managing the pandemic.
Throughout the pandemic, we have seen that SARS-CoV-2 follows a cluster super-spreader pattern, generating a disproportionately large number of infections. SARS-CoV-2 spread for example at après-ski bars, choirs, concert rehearsals, naval and cruise ships, gyms, nursing homes, restaurants, slaughterhouses, festivals, and weddings. SARS-CoV-2 has an estimated basic reproduction (R)-number of 3.49 in the early stage of the epidemic, which only partly explains the high contamination rate.20 The R-number reflects the average transmission number in a population, while in COVID-19 only 10% of the population is responsible for 80% of the infections and can be defined by the co-called dispersion K factor of 0.1. This skewing phenomenon can only be explained by aerosol transmission during SSEs.21 Another observation that SSEs occur almost exclusively indoors in poorly ventilated areas is consistent with the role of aerosol transmission.22
PREVENTION OF TRANSMISSION
Ventilation
The correlation between ventilation and the transmission and spread of several infectious diseases has previously been extensively demonstrated.23 A study in the Netherlands analyzed the droplet size distribution, travel distance, and velocity from coughing and speaking, and the airborne time in relation to the level of air ventilation. They applied a laser diffraction measurement, using a spray droplet measurement system and performed the experiment in three rooms with different levels of ventilation: no ventilation, mechanical ventilation only and mechanical ventilation supported by the opening of an entrance door and a small window. The droplets halved in the best ventilated room after 30 s, whereas with no ventilation this took about 5 min and in a poorly ventilated room, the number of droplets was halved in 14 min.24 This supports the argument that ventilation is an important factor in preventing airborne transmission.
More recently, focus on improving ventilation, not only in government institutions and hospitals, but also in nursing homes, general practices, offices, restaurants, and schools has become urgent. It must be stressed that fresh air supply by using windows that can be opened, mechanical ventilation systems, avoiding central recirculation (reintroduction of return air) may be a key factor in diminishing aerosols and so the spread of the virus. The high concentration of infection droplets in aerosols around infectious individuals highlights the importance of personalized ventilation.5
It is demonstrated in an experimental setting that Mobile High-Efficiency Particulate Air (HEPA) filters have added value if there is no ventilation option.25 Ventilation systems with ultra violet light to disinfect air may be helpful, although no published studies have demonstrated efficacy, and these methods are currently not recommended in the infection prevention guidelines from the World Health Organization.26
Masks
Face coverings act as a source control measure to prevent aerosol transmission by infected patients who sneeze, cough and speak.27 The use of a face covering in public indoor areas is advised to prevent large droplet transmission. Aerosols are more difficult to block with a face covering. The added value of wearing a face mask in addition to social distancing for the prevention of SARS-CoV-2 transmission is, therefore, still under debate.
The first in vivo experimental study supported a significant benefit of surgical masks in preventing SARS-CoV-2 transmission in a hamster model.14 The use of masks by humans suggested beneficial effects on the course of the pandemic in several countries immediately at the start of the lockdown.17 The University of Kansas found a 50% reduction in the spread of COVID-19 in countries that had compulsory masks, compared to those without. Leffler et al28 demonstrated in a univariate analysis of 198 countries that the duration of mask-wearing in general by the public was significantly negatively correlated with mortality, and also international travel controls are independently associated with lower per-capita mortality from COVID-19. The findings of a systematic review and meta-analysis identified 172 observational studies across 16 countries, supporting face mask use being able to result in a large reduction in risk of infection.29 Serial cross-sectional data from approximately 7000 German participants demonstrate that implementing a mandatory policy increased actual compliance despite moderate acceptance. An obligation to wear a face mask appears to be an effective solution to curb transmissions of airborne viruses.30 Therefore, government policies in many countries make properly worn masks mandatory in public areas.28 If approximately 40% of the population wore a mask that filters out particles ranging from 0.3 to 10 mm in diameter, it would be possible to curb the pandemic.31
There are many differences in face covering methods such as the professional Filtering Face Pieces (FFP, Europe)/N-95 Mask (USA)/KN95 (China), surgical mask (simpler 3-layer nose-mouth) and (homemade) cloth masks, neck gaiters, and face shield. Protection by these masks varies considerably.32 Professional masks offer better protection than simpler surgical masks, although the latter may also offer protection.13,31 At least a professional mask should be used during airborne precaution situations.33 The filtration efficiency and air flow resistance of cloths mask vary strongly depending on the different textiles used.34,35 A variety of commonly available homemade mask types were tested in a proof-of-principle study and this study demonstrated that some mask types approach the performance of standard surgical masks.32 Clapp et al36 demonstrated also that simple modifications as nylon hosiery sleeve placed over the procedure mask can improve medical mask fit and can substantially improve filtration efficiency. However, it is not recommended that cloth masks should be used by health care workers.37
Face shields and neck gaiters are more comfortable and, therefore, may be worn more consistently. However, face shields only protect against large drops and not against aerosols.38 Several studies demonstrated that aerosols from the infected person whirl around the face shield and enter the environment.39 Neck gaiters also offer very little protection.32 The Technical University in Delft, the Netherlands, developed a detection method for objective mask leakage on a plastic head. They demonstrated that the homemade masks give the least protection, because of their poor fit and showed that mask protection is not fool proof (weblink 3).
Notwithstanding all these studies, which in summary show the benefit of using masks, this preventive measure is still under debate. A randomized study of more than 6000 people in Denmark from April to June showed that wearing a face mask did not significantly reduce the SARS-CoV-2 infection at 1 month by antibody testing.40 Their results were however heavily criticized because of the design of the study.
Weblink 3
https://innovationorigins.com/nl/kunststofhoofd-maakt-lekkage-bij-mondkapjes-zichtbaar/
Furthermore, there are some general disadvantages of face coverings, such as low compliance due to discomfort, and incorrect use of the face covering from re-use or by physical contact with the infected parts of the mask. The study MacIntyre et al warned against the use of cloth masks, because moisture retention, reuse of cloth masks and poor filtration may result in increased risk of infection. In contrast, face coverings can optically increase fear of approach and act as a reminder of infection risks, and it demonstrated that mask wearing correlated positively with other protective behaviors.40,41
In conclusion, it is plausible that appropriately wearing a surgical mask has an added value, especially when good ventilation measures are lacking or cannot be guaranteed (Fig. 3).42
FIGURE 3.
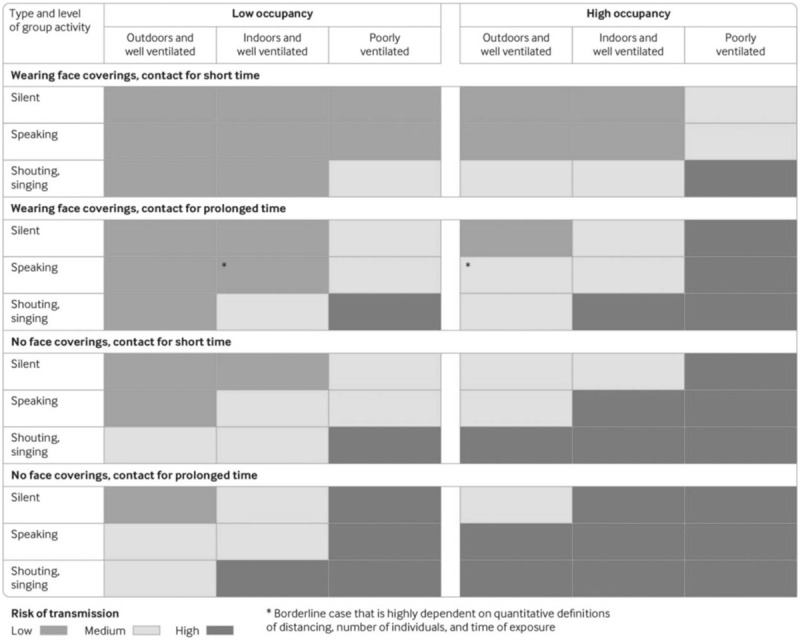
Transmission risk of SARS-CoV-2 in different settings and different occupation times, venting, and crowding levels. This figure is from Jones NR.42 SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Toilet Flushing
An underestimated source of infection may be flushing a toilet used by SARS-CoV-2 infected patients. Kang et al17 demonstrated that faecal transmission may have caused the community outbreak of COVID-19 in a high-rise building.17 The study by Döhla et al43 showed that in 21 households under quarantine conditions, surfaces in the domestic environment as well as the wastewater from washbasins, showers and toilets showed SARS-CoV-2 contamination.43 Preventive measures in the public domain are flushing the toilet with the lid closed and leaving the toilet door open between toilet visits to improve ventilation. Another option is disposable toilet seat covers to ensure a more hygienic toilet visit.
HEALTH CARE
The disproportionately large number of infections among health care workers, especially in nursing homes, suggest super-spreader patterns. Health care institutions have high transmission rates due to high exposure to SARS-CoV-2, crowding, gathering in common areas and bad ventilation. Furthermore, the care homes’ supply of personal protective equipment was suboptimal at the beginning of the COVID-19 pandemic because of scarcity and reuse of materials.44 In addition, the quality of ventilation is often inadequate in hospitals and nursing homes due to recirculation. A prospective, observational cohort study in the UK and the USA noted that compared to the general community, front-line health-care workers show approximately a threefold increase in the risk of COVID-19, even after accounting for other risk factors.45 In addition, there are several specific medical procedures that generate aerosols that may increase the risk of SARS-CoV-2 transmission among health care workers.3,46
Nebulized Medication
There are contradictory study outcomes concerning the increased risk of virus transmission by nebulizer treatment.47 Nebulizers create aerosols, nonetheless it is uncertain whether these aerosols contain infectious viral particles. The nebulized medication is also relevant to generating aerosols: for example, saline has been shown to decrease aerosol formation.48
Non-Invasive Ventilation
There is no strong evidence that non-invasive ventilation (NIV) increases the risk of viral diseases.47 Therefore, NIV is not considered as an aerosol-generating procedure for virus transport.49 It has been demonstrated that NIV results in more aerosol dispersion, but the viral load appeared lower than without NIV.50 A possible explanation might be that NIV may provide a protective benefit by limiting dispersal of droplets from coughing by the patients.51 The use of specific full-face mask/helmet NIV reduces SARS-CoV-2 transmission.52,53
There is also insufficient evidence to conclude that nasal high flow (NHF) therapy should be considered an aerosol generating procedure.47 NHF therapy use does not increase the risk of dispersing aerosols over the risk from patient breathing with violent exhalation or standard oxygen mask.54,55 Vianello et al performed an NHF study in SARS-CoV-2 infected patients. None of the staff members tested positive for COVID-19 during this study, which supports the limited risk of airborne transmission by NHF.56 Previous studies also showed a low risk of airborne transmission with NHF therapy when good interface fitting is achieved.57
Intubation and Bronchoscopy
Endotracheal intubation of SARS CoV-1-infected patients has been consistently associated with viral transmission to health care workers. Four cohort studies showed that the tracheal intubation procedure is a significant risk factor for transmission of SARS CoV-1 to health care workers.46
There is little data about the risk of virus transmission by bronchoscopy. In two studies performed during the SARS CoV-1 outbreak, bronchoscopy was not associated with infection transmission.58,59 Bronchoscopy of influenza A H1N1 infected patients demonstrated an increased detection of viral aerosols.50 The extrapolation of SARS CoV-1 data is complicated by the fact that presymptomatic spread of SARS CoV-1 appears to be rare. Therefore, the aerosolizing procedures are not the greatest danger for the transmission of SARS-CoV-2, it is the daily breathing and talking to someone who is asymptomatically or pre-symptomatically infected, whilst recording the patient's history or talking to colleagues during the lunch break.
There are also several general options to reduce the risk of aerosol transmission during aerosol generating procedures. Negative-pressure rooms, high-energy particulate accumulator (HEPA) filters, and adequate personal protective equipment (PPE) are effective in protecting staff from aerosol transmission.60,61 PPE for health care workers during aerosol-generating procedures are a waterproof long-sleeved gown, double non-sterile gloves, eye protection and a respirator that ensures a level of protection equal to or greater than professional masks.62
In conclusion, clinicians should be aware of distancing, ensure correct use of equipment, use protection material and guarantee treatment of the patient in an airborne infection isolation or negative pressure room during aerosol generating procedures.57 The greatest risk of aerosol transmission is during endotracheal intubation, the risk with non-invasive ventilation seems to be moderate.
Nonetheless, it is important to realize that, in addition to aerosol generating procedures such as risk for SARS-CoV-2 transmission, the greatest likelihood of SARS-CoV-2 contamination occurs during regular contact with speaking, singing, laughing, and breathing.
CONCLUSION
The evidence for transmission via aerosols containing SARS-CoV-2 seems to be at least as strong as the evidence for transmission via deposition of droplets on surfaces. It is plausible that virus transmission via aerosols plays an important role in SSEs in the COVID-19 pandemic. Knowledge and awareness of all routes, including aerosol transmission, is pivotal for taking precautions. This is absolutely necessary to curb the pandemic and to relieve the heavy burden on society and hospitals. Important preventive measures for aerosol transmission are face protection using masks and good quality ventilation. These preventive measures are definitely needed for health care workers, especially during intubation, while the risk with non-invasive ventilation seems to be moderate.
Footnotes
Consent for publication: The authors agree with the submission of the manuscript.
Dr in ‘t Veen has some financial relationships.
The authors report no conflict of interest.
Authors’ contributions: J.P.M. van der Valk, MD, PhD Main author.
J.C.C.M. in ’t Veen, MD, PhD Main reviewer.
The authors read and approved the manuscript.
Clinical significance: Aerosol transmission is one of the dominant ways of spreading of SARS-Cov-2. SARS-CoV-2 remains viable in aerosols for hours, facilitating rapid virulent distribution. Adequate ventilation, with avoidance of air and thus virus recirculation, is pivotal in preventing virus transmission next to barrier measurements in the public domain as well in health care setting.
Contributor Information
Johanna P.M. van der Valk, Department of Pulmonary Medicine Franciscus Gasthuis and Vlietland Rotterdam, The Netherlands.
Johannes C.C.M. in ’t Veen, Department of Pulmonary Medicine Franciscus Gasthuis and Vlietland Rotterdam, The Netherlands; Department of Pulmonary Medicine ErasmusMC Rotterdam, The Netherlands..
REFERENCES
- 1.Otter JA, Donskey C, Yezli S, Douthwaite S, Goldenberg SD, Weber DJ. Transmission of SARS and MERS coronaviruses and influenza virus in health care settings: the possible role of dry surface contamination. J Hosp Infect 2016; 92:235–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA 2020; 323:1406–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie X, Li Y, Chwang AT, Ho PL, Seto WH. How far droplets can move in indoor environments—revisiting the Wells evaporation-falling curve. Indoor Air 2007; 17:211–225. [DOI] [PubMed] [Google Scholar]
- 4. Wells WF. Airborne Contagion and Air Hygiene. Cambridge, MA: Harvard University Press; 1955; 25:235–260. [Google Scholar]
- 5.Chen W, Zhang N, Wei J, Yen H, Li Y. Short-range airborne route dominates exposure of respiratory infection during close contact. medRxiv 2020; doi: 10.1101/2020.03.16.20037291. [Google Scholar]
- 6.Hall CB, Douglas RG. Modes of transmission of respiratory syncytial virus. J Pediatr 1981; 99:100–103. [DOI] [PubMed] [Google Scholar]
- 7.van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of HCoV-19 (SARS-CoV-2) compared to SARS-CoV-1. medRxiv 2020; doi: 10.1101/2020.03.09.20033217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dohla M, Boesecke C, Schulte B, et al. Rapid point-of-care testing for SARS-CoV-2 in a community screening setting shows low sensitivity. Public Health 2020; 182:170–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morawska L, Milton DK. It is time to address airborne transmission of COVID-19. Clin Infect Dis 2020; 71:2311–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Xu G, Huang YW. Modeling the load of SARS-CoV-2 virus in human expelled particles during coughing and speaking. PLoS One 2020; 15:e0241539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tellier R. Review of aerosol transmission of influenza A virus. Emerg Infect Dis 2006; 12:1657–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu ITS, Li Y, Wai Wong T, et al. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med 2004; 350:1731–1739. [DOI] [PubMed] [Google Scholar]
- 13.Fennelly KP. Particle sizes of infectious aerosols: implications for infection control. Lancet Respir Med 2020; 8:914–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tellier R, Li Y, Cowling BJ, Tang JW. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect Dis 2019; 19:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuit M, Ratnesar-Shumate S, Yolitz J, et al. Airborne SARS-CoV-2 is rapidly inactivated by simulated Sunlight. J Infect Dis 2020; 222:564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan JF, Yuan S, Zhang AJ, et al. Surgical mask partition reduces the risk of non-contact transmission in a golden Syrian hamster model for Coronavirus Disease 2019 (COVID-19). Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang R, Li Y, Zhang AL, Wang Y, Molina MJ. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci U S A 2020; 117:14857–14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Qian H, Hang J, et al. Evidence for probable aerosol transmission of SARS-CoV-2 in a poorly ventilated restaurant. MedRxiv 2020; doi: 10.1101/2020.04.16.20067728. [Google Scholar]
- 19. De Man P, Paltansing S, Ong DSY, Vaessen N, van Nielen G, Koeleman JGM. Outbreak of COVID-19 in a nursing home associated with aerosol transmission as a result of inadequate ventilation. Clin Infect Dis. 28 augustus 2020 (epub). Medline. doi: 10.1093/cid/ciaa1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, You XY, Wang YJ, et al. Estimating the basic reproduction number of COVID-19 in Wuhan, China. Chin J Epidemiol 2020; 41:476–479. [DOI] [PubMed] [Google Scholar]
- 21.Godri Pollitt KJ, Peccia J, Ko AI, et al. COVID-19 vulnerability: the potential impact of genetic susceptibility and airborne transmission. Hum Genomics 2020; 14:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian H, Miao T, Liu L, Zheng X, Luo D, Li Y. Indoor transmission of SARS-CoV-2. Indoor Air 2020; doi: 10.1111/ina.12766. 2020-10-31. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Leung GM, Tang JW, et al. Role of ventilation in airborne transmission of infectious agents in the built environment—a multidisciplinary systematic review. Indoor Air 2007; 17:2–18. [DOI] [PubMed] [Google Scholar]
- 24.Somsen GA, van Rijn C, Kooij S, Bem RA, Bonn D. Small droplet aerosols in poorly ventilated spaces and SARS-CoV-2 transmission. Lancet Respir Med 2020; 8:658–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bluyssen PM, Ortiz M, Zhang D. The effect of a mobile HEPA filter system on ‘infectious’ aerosols, sound and air velocity in the SenseLab. Build Environ 2020; doi: 10.1016/j.buildenv.2020.107475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nardell EA, Nathavitharana RR. Airborne spread of SARS-CoV-2 and a potential role for air disinfection. JAMA 2020; 324:141–142. [DOI] [PubMed] [Google Scholar]
- 27.Lindsley WG, Blachere FM, Law BF, Beezhold DH, Noti JD. Efficacy of face masks, neck gaiters and face shields for reducing the expulsion of simulated cough-generated aerosols. medRxiv 2020; doi: 10.1101/2020.10.05.20207241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leffler CT, Ing E, Lykins JD, Hogan MC, McKeown CA, Grzybowski A. Association of country-wide coronavirus mortality with demographics, testing, lockdowns, and public wearing of masks. Am J Trop Med Hyg 2020; 103:2400–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chu DK, Akl EA, Duda S, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet 2020; 395:1973–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Betsch C, Korn L, Sprengholz P, et al. Social and behavioral consequences of mask policies during the COVID-19 pandemic. Proc Natl Acad Sci U S A 2020; 117:21851–21853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teesing GR, van Straten B, de Man P, Horeman-Franse T. Is there an adequate alternative to commercially manufactured face masks? A comparison of various materials and forms. J Hosp Infect 2020; 106:246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischer EP, Fischer MC, Grass D, Henrion I, Warren WS, Westman E. Low-cost measurement of face mask efficacy for filtering expelled droplets during speech. Sci Adv 2020; 6: doi: 10.1126/sciadv.abd3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howard BE. High-risk aerosol-generating procedures in COVID-19: respiratory protective equipment considerations. Otolaryngol Head Neck Surg 2020; 163:98–103. [DOI] [PubMed] [Google Scholar]
- 34.Davies A, Thompson KA, Giri K, Kafatos G, Walker J, Bennett A. Testing the efficacy of homemade masks: would they protect in an influenza pandemic? Disaster Med Public Health Prep 2013; 7:413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konda A, Prakash A, Moss GA, Schmoldt M, Grant GD, Guha S. Aerosol filtration efficiency of common fabrics used in respiratory cloth masks. ACS Nano 2020; 14:6339–6347. [DOI] [PubMed] [Google Scholar]
- 36.Clapp PW, Sickbert-Bennett EE, Samet JM, et al. Evaluation of cloth masks and modified procedure masks as personal protective equipment for the public during the COVID-19 pandemic. JAMA Intern Med 2020; doi: 10.1001/jamainternmed.2020.8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacIntyre CR, Seale H, Dung TC, et al. A cluster randomised trial of cloth masks compared with medical masks in healthcare workers. BMJ Open 2015; 5:e006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindsley WG, Noti JD, Blachere FM, Szalajda JV, Beezhold DH. Efficacy of face shields against cough aerosol droplets from a cough simulator. J Occup Environ Hyg 2014; 11:509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verma S, Dhanak M, Frankenfield J. Visualizing droplet dispersal for face shields and masks with exhalation valves. Phys Fluids 2020; 32:091701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bundgaard H, Bundgaard JS, Raaschou-Pedersen DET, et al. Effectiveness of adding a mask recommendation to other public health measures to prevent SARS-CoV-2 infection in Danish mask wearers: a randomized controlled trial. Ann Intern Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacIntyre C, Cauchemez S, Dwyer DE, et al. Face mask use and control of respiratory virus transmission in households. Emerg Infect Dis 2009; 15:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones NR, Qureshi ZU, Temple RJ, Larwood JPJ, Greenhalgh T, Bourouiba L. Two metres or one: what is the evidence for physical distancing in covid-19? BMJ 2020; 370:m3223. [DOI] [PubMed] [Google Scholar]
- 43.Döhla M, Wilbringa G, Schulte B, Kümmerer BM, Diegmann C, Siba E. SARS-CoV-2 in environmental samples of quarantined households. medRxiv 2020; doi: 10.1101/2020.05.28.20114041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gordon AL, Goodman C, Achterberg W, et al. Commentary: COVID in care homes-challenges and dilemmas in health care delivery. Age Ageing 2020; 49:701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen LH, Drew DA, Joshi AD, et al. Risk of COVID-19 among frontline healthcare workers and the general community: a prospective cohort study. medRxiv 2020; doi: 10.1101/2020.04.29.20084111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tran K, Cimon K, Severn M, Pessoa-Silva C, Conly J. Aerosol-generating procedures and risk of transmission of acute respiratory infections: a systematic review. CADTH Technol Overv 2013; 3:e3101. [PMC free article] [PubMed] [Google Scholar]
- 47.Harding H, Broom A, Broom J. Aerosol-generating procedures and infective risk to healthcare workers from SARS-CoV-2: the limits of the evidence. J Hosp Infect 2020; 105:717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fiegel J, Clarke R, Edwards DA. Airborne infectious disease and the suppression of pulmonary bioaerosols. Drug Discov Today 2006; 11:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simonds AK, Hanak A, Chatwin M, et al. Evaluation of droplet dispersion during non-invasive ventilation, oxygen therapy, nebuliser treatment and chest physiotherapy in clinical practice: implications for management of pandemic influenza and other airborne infections. Health Technol Assess 2010; 14:131–172. [DOI] [PubMed] [Google Scholar]
- 50.Thompson KA, Pappachan JV, Bennett AM, et al. Influenza aerosols in UK hospitals during the H1N1 (2019) pandemic—the risk of aerosol generation during medical procedures. PLoS One 2013; 8:e56278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCracken J. Should noninvasive ventilation be considered a high-risk procedure during an epidemic? CMAJ 2009; 181:663–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rali AS, Howard C, Miller R, et al. Helmet CPAP revisited in COVID-19 pneumonia: a case series. Can J Respir Ther 2020; 56:32–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pirzada AR, Aleissi SA, Almeneessier AS, BaHammam AS. Management of aerosol during noninvasive ventilation for patients with sleep-disordered breathing: important messages during the COVID-19 pandemic. Sleep Vigil 2020; 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roberts CT, Owen LS, Manley BJ, et al. Nasal high-flow therapy for primary respiratory support in preterm infants. N Engl J Med 2016; 375:1142–1151. [DOI] [PubMed] [Google Scholar]
- 55.Li J, Fink JB, Ehrmann S. High-flow nasal cannula for COVID-19 patients: low risk of bio-aerosol dispersion. Eur Respir J 2020; 55: doi: 10.1183/13993003.00892-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vianello A, Arcaro G, Molena B, et al. High-flow nasal cannula oxygen therapy to treat patients with hypoxemic acute respiratory failure consequent to SARS-CoV-2 infection. Thorax 2020; 75:998–1000. [DOI] [PubMed] [Google Scholar]
- 57.Ari A. Practical strategies for a safe and effective delivery of aerosolized medications to patients with COVID-19. Respir Med 2020; 167:105987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raboud J, Shigayeva A, McGeer A, et al. Risk factors for SARS transmission from patients requiring intubation: a multicentre investigation in Toronto, Canada. PLoS One 2010; 5:e10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loeb M, McGeer A, Henry B, et al. SARS among critical care nurses, Toronto. Emerg Infect Dis 2004; 10:251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pfeifer M, Ewig S, Voshaar T, et al. Position paper for the state-of-the-art application of respiratory support in patients with COVID-19. Respiration 2020; 99:521–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raoof S, Nava S, Carpati C, Hill NS. High-flow, noninvasive ventilation and awake (nonintubation) proning in patients with coronavirus disease 2019 with respiratory failure. Chest 2020; 158:1992–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferioli M, Cisternino C, Leo V, Pisani L, Palange P, Nava S. Protecting healthcare workers from SARS-CoV-2 infection: practical indications. Eur Respir Rev 2020; 29: doi: 10.1183/16000617.0068-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


