ABSTRACT
Background: Posttraumatic stress disorder (PTSD) is a frequently observed stress-related disorder after acute myocardial infarction (AMI) and it is characterized by numerous symptoms, such as flashbacks, intrusions and anxiety, as well as uncontrollable thoughts and feelings related to the trauma. Biological correlates of severe stress might contribute to identifying PTSD-vulnerable patients at an early stage.
Objective: Aims of the study were (1) to determine whether blood levels of trimethylamine N-oxide (TMAO) vary immediately after AMI in patients with/without AMI-induced PTSD symptomatology, (2) to investigate whether TMAO is a potential biomarker that might be useful in the prediction of PTSD and the PTSD symptom subclusters re-experiencing, avoidance and hyperarousal, and (3) to investigate whether TMAO varies immediately after AMI in patients with/without depression 6 months after AMI.
Method: A total of 114 AMI patients were assessed with the Hamilton-Depression Scale after admission to the hospital and 6 months later. The Clinician Administered PTSD Scale for DSM-5 was used to explore PTSD-symptoms at the time of AMI and 6 months after AMI. To assess patients’ TMAO status, serum samples were collected at hospitalization and 6 months after AMI.
Results: Participants with PTSD-symptomatology had significantly higher TMAO levels immediately after AMI than patients without PTSD-symptoms (ANCOVA: TMAO(PTSD x time), F = 4.544, df = 1, p = 0.035). With the inclusion of additional clinical predictors in a hierarchical logistic regression model, TMAO became a significant predictor of PTSD-symptomatology. No significant differences in TMAO levels immediately after AMI were detected between individuals with/without depression 6 months after AMI.
Conclusions: An elevated TMAO level immediately after AMI might reflect severe stress in PTSD-vulnerable patients, which might also lead to a short-term increase in gut permeability to trimethylamine, the precursor of TMAO. Thus, an elevated TMAO level might be a biological correlate for severe stress that is associated with vulnerability to PTSD.
KEYWORDS: Trimethylamine N-oxide (TMAO), acute myocardial infarction, gut permeability, posttraumatic stress disorder (PTSD), depression, biomarker
HIGHLIGHTS
An elevated TMAO level might be a biological correlate for severe stress that is associated with vulnerability to PTSD.
Abstract
Antecedentes: El trastorno de estrés postraumático (TEPT) es un trastorno relacionado con el estrés que se observa con frecuencia después de un infarto agudo de miocardio (IAM) y se caracteriza por numerosos síntomas, como flashbacks, intrusiones y ansiedad, así como pensamientos y sentimientos incontrolables relacionados con el trauma. Los correlatos biológicos del estrés severo podrían contribuir a identificar a los pacientes vulnerables al TEPT en una etapa temprana.
Objetivo: Los objetivos del estudio fueron (1) determinar si los niveles sanguíneos de N-óxido de trimetilamina (TMAO, por sus siglas en ingles) varían inmediatamente después del IAM en pacientes con o sin sintomatología de TEPT inducida por IAM, (2) investigar si el TMAO es un biomarcador potencial que podría ser útil en la predicción de TEPT y los subgrupos de síntomas de TEPT que experimentan, evitación e hiperactivación, y (3) para investigar si el TMAO varía inmediatamente después del IAM en pacientes con o sin depresión 6 meses después del IAM.
Método: Un total de 114 pacientes con IAM fueron evaluados con la Escala de Depresión de Hamilton tras su ingreso al hospital y 6 meses después. La Escala de TEPT para el DSM-5 administrada por el médico se utilizó para explorar los síntomas de TEPT en el momento del IAM y 6 meses después del IAM. Para evaluar el estado de TMAO de los pacientes, se recolectaron muestras de suero en la hospitalización y 6 meses después del IAM.
Resultados: Los participantes con sintomatología de TEPT tenían niveles de TMAO significativamente más altos inmediatamente después del IAM que los pacientes sin síntomas de TEPT (ANCOVA: TMAO (TEPT x tiempo), F = 4.544, df = 1, p = 0.035). Con la inclusión de predictores clínicos adicionales en un modelo de regresión logística jerárquica, TMAO se convirtió en un predictor significativo de la sintomatología del TEPT. No se detectaron diferencias significativas en los niveles de TMAO inmediatamente después del IAM entre individuos con o sin depresión 6 meses después del IAM.
Conclusiones: Un nivel elevado de TMAO inmediatamente después del IAM podría reflejar un estrés severo en pacientes vulnerables al TEPT, lo que también podría conducir a un aumento a corto plazo de la permeabilidad intestinal a la trimetilamina, el precursor de TMAO. Por lo tanto, un nivel elevado de TMAO podría ser un correlato biológico del estrés severo asociado con la vulnerabilidad al TEPT.
PALABRAS CLAVE: N-óxido de trimetilamina (TMAO), Infarto agudo del miocardio, Permeabilidad intestinal, Trastorno de estrés postraumático (TEPT), Depresión, Biomarcador
Abstract
背景: 创伤后应激障碍 (PTSD) 是急性心肌梗塞 (AMI) 后常被观测的应激相关疾病, 其特点是出现例如闪回, 闯入和焦虑, 以及无法控制的创伤相关想法和感觉的多种症状。 严重应激的生物学相关因素可能有助于在早期识别PTSD易感患者。
目的: 本研究旨在 (1) 确定有/无AMI诱发PTSD症状的患者在AMI后氧化三甲胺 (TMAO) 的血液水平是否立即不同, (2) 考查TMAO是否是一种可能有助于预测PTSD和PTSD再体验, 回避和高唤起症状亚簇的潜在生物标志物, 并且 (3) 考查AMI后6个月有无抑郁的患者在AMI后TMAO是否立即不同。
方法: 在入院后及6个月后共使用汉密尔顿抑郁量表评估了114例AMI患者。使用 DSM-5的临床用PTSD量表探究AMI期间及AMI后6个月的PTSD症状。为评估患者的TMAO状态, 在住院时和AMI后6个月收集了血清样本。
结果: 有PTSD症状的参与者在AMI后的TMAO水平立即显著高于无PTSD症状患者的 (ANCOVA:TMAO (PTSD x时间), F= 4.544, df = 1, p= 0.035) 。在分层逻辑回归模型中纳入其他临床预测因素后, TMAO成为PTSD症状的一个显著预测因素。在AMI后6个月有或无抑郁的个体之间未发现AMI后即刻TMAO水平的显著差异。
结论: AMI后立即升高的TMAO水平可能反映了PTSD易感患者的严重应激, 这也可能导致肠道对TMAO前体三甲胺的通透性的短期提升。因此, 升高的TMAO水平可能是与PTSD易感性相关的严重应激的生物学相关因素。
关键词: 氧化三甲胺 (TMAO), 急性心肌梗塞, 肠胃渗透性, 创伤后应激障碍 (PTSD), 抑郁, 生物标志物
1. Introduction
1.1. Acute myocardial infarction (AMI) and psychiatric comorbidities
Acute myocardial infarction (AMI) is a major biopsychosocial health care issue and often occurs unexpectedly (Andersson, Pesonen, & Ohlin, 2007). Because of prolonged stress and impairments in health-related quality of life, many AMI patients suffer from clinically significant depression. In a recent meta-analysis, the pooled prevalence of depression among patients with AMI was 28.7% (Feng et al., 2019). In comparison, the point prevalence of major depression in the Danish general population has been reported as 3.3% (Olsen, Mortensen, & Bech, 2004). Another frequent stress-related secondary psychiatric disease following AMI is post-traumatic stress disorder (PTSD) (Jbilou et al., 2019). In scientific studies the prevalence of full PTSD after AMI has ranged from 4 to 32%, depending on the methods used to assess PTSD (Edmondson et al., 2012). In comparison, the U.S. National Comorbidity Survey Replication estimated the lifetime prevalence of PTSD in the general population of adult Americans to be 6.8% (Kessler et al., 2005).
1.2. AMI-induced post-traumatic stress disorder (PTSD)
PTSD is a common psychiatric stress disorder that is triggered by a severe traumatic event, such as AMI (Rothenhäusler, Stepan, Kreiner, Baranyi, & Kapfhammer, 2008). It is characterized by numerous symptoms, such as flashbacks, intrusions, nightmares and severe anxiety, as well as uncontrollable, intense and disturbing thoughts and feelings related to the trauma (Baranyi, Krauseneck, & Rothenhäusler, 2013). The DSM-5 lists the following criteria for the diagnosis of PTSD: Criterion A: severe or life-threatening traumatic event (defined as AMI in the present study); Criterion B: re-experiencing symptoms; intrusions and flashbacks; Criterion C: avoidance symptoms; Criterion D: negative alterations in cognition and mood; Criterion E: hyperarousal; Criterion F: significant disturbance lasting at least one month; and Criterion G: disturbance causing significant impairment (American Psychiatric Association, 2013).
The precondition for the development of PTSD is exposure to extreme stress caused by an often-life-threatening traumatic event that alters the internal balance. However, with regard to the development of PTSD, individual stress perception might also be crucial because, not every serious traumatic event leads to PTSD in every affected person. Studies indicate that a higher level of perceived threat increases PTSD symptoms (Lancaster, Cobb, Lee, & Telch, 2016). This means that the individual experience of the severity of stress during trauma might also vary substantially. To date, only a few biological correlates (e.g. cortisol, metanephrine) of such an individual perception of stress that can affect the development of PTSD in the long term have been identified (Duke, 1971; LoPilato et al., 2020). One potential biomarker that could be useful in the assessment of individual stress is trimethylamine-N-oxide (TMAO). This gut bacteria-derived metabolite is an important cardiovascular risk marker that appears to interfere with many important physiological regulatory pathways (Aliev et al., 2020; Giannoni-Pastor, Eiroa-Orosa, Fidel Kinori, Arguello, & Casas, 2016).
1.3. Trimethylamine N-oxide (TMAO)
The small organic molecule TMAO is an amine oxide generated via gut microbial metabolism from dietary phospholipids, such as choline, betaine, and L-carnitine. For example, eggs, red meat, cheese and ocean fish contain large amounts of choline, betaine, and L-carnitine. In detail, the gut microbiota transforms L-carnitine, choline and betaine into the TMAO precursor trimethylamine (TMA). After subsequent absorption into the bloodstream, TMA is further oxidized to TMAO by the hepatic flavin-containing monooxygenase (FMO) family members FMO-1 and FMO-3 (Bennett et al., 2013; Querio, Antoniotti, Levi, & Gallo, 2019; Tang et al., 2013). TMAO acts as an important protein stabilizer, preserving protein folding. In addition, TMAO is an electron acceptor that enhances vascular inflammation and oxidative stress (Li, Chen, Gua, & Li, 2017; Ufnal, Zadlo, & Ostaszewski, 2015). TMAO also expedites endothelial cell senescence, platelet hyper-reactivity and thrombosis (Zhu et al., 2016). Thus, high circulating concentrations of TMAO may lead to the progression of atherosclerosis and other severe cardiovascular diseases, such as acute coronary syndrome (Querio et al., 2019).
A previous experiment with rats suggested that heart failure might be associated with functional and structural alterations in intestinal integrity, leading to increased gut-to-blood penetration of TMA and a subsequent elevation in serum TMAO levels (Drapala et al., 2020). In contrast to heart failure, acute coronary syndrome, which includes AMI, is an acute cardiological disease and a severe medical emergency associated with a sudden reduction in blood flow to the heart. In a recent study by Alhmoud et al., human patients with acute coronary syndrome had increased intestinal tight junctional permeability (leaky gut) but no immediate increase in TMAO concentrations (Alhmoud et al., 2019). This finding of a non-immediate TMAO increase after acute coronary syndrome corresponds with the hypothesis that while TMAO has less clinical significance in acute cardiological events, high TMAO levels might enhance the risk of severe cardiac events in the future (Alhmoud et al., 2019).
1.4. Gut permeability and gut microbial metabolism
1.4.1. Impacts of acute and chronic stress and gut microbial metabolism on gut permeability and TMAO
In the last decade, the gut-brain axis theory, which involves interactions between the gut microbiome and the brain, has gained substantial attention. This complex bidirectional interaction and communication pattern leads to a plethora of physiological consequences, such as (1) alterations in intestinal motility, (2) modifications in the production of local neurotransmitters and bacterial metabolites, and (3) a significant modulation of gut-blood barrier (GBB) permeability, causing leaky gut syndrome (Carabotti, Scirocco, Maselli, & Severi, 2015; Meinitzer et al., 2020). Correspondingly, numerous previous studies have shown that severe psychological stress may cause alterations in gut permeability, causing leaky gut syndrome (Hiki et al., 2001; Rezzi et al., 2009).
eaky gut syndrome might also have an impact on the release of the TMAO precursor TMA into the bloodstream (Jaworska et al., 2020). In confirmation of this, previous animal models of surgical stress with subsequently increased TMAO levels showed a strong relationship between acute stress and elevated blood TMAO concentrations (Kinross et al., 2011).
1.4.2. Impact of acute myocardial infarction on gut microbial metabolism
Another potential cause, in addition to the stress-related increase in intestinal permeability, might be direct microbial alterations after AMI. In addition, there is a bidirectional relationship between stress and gut microbial metabolism and many stressors impact gut microbial metabolism (Karl et al., 2018). In a study by Wu et al. (2017), it was shown that the gut microbiota was altered in rats 7 days after AMI. The gut microbiota is essential for the formation of TMA, the precursor of TMAO. Thus, alterations in gut microbial community structures might also have an impact on TMAO production (Wu et al., 2017).
In summary, changes in TMAO levels in patients after AMI might be caused by stress-related changes in intestinal permeability (leaky gut) or alterations in the intestinal microbiome.
1.4.3. Impact of depression on gut microbial metabolism
In addition to PTSD, many AMI patients suffer from depression due to prolonged impairments in health-related quality of life in the period after myocardial infarction (Kala et al., 2016). Regarding TMAO, a previous study indicated that individuals with depression also have alterations in gut microbial metabolites, such as TMAO, dimethylamine and dimethylglycine (Zheng et al., 2013).
1.5. Theoretical background for TMAO analyses in AMI patients with PTSD
Exposure to severe mental stress due to a potential traumatic event, such as AMI, might affect the intestinal microbiome and gut-blood barrier permeability, thereby inducing leaky gut syndrome (Hiki et al., 2001; Rezzi et al., 2009; Wu et al., 2017). Such alterations might increase TMAO concentrations in the blood (Hiki et al., 2001; Jaworska et al., 2020).
The precondition for subsequent development of PTSD after an AMI is severe stress at the time of the AMI (Burg & Soufer, 2016). TMAO might indicate stress and a stress-induced increase in gut permeability. Thus, in PTSD vulnerable AMI patients TMAO might be a potential biomarker of severe stress and a stress-induced increase in gut permeability. Biological correlates of severe stress might contribute to identifying PTSD-vulnerable patients at an early stage.
2. Objectives
The first aim of the study was to determine whether blood levels of TMAO vary immediately after acute myocardial infarction (AMI) in patients with/without AMI-induced PTSD symptomatology (subsyndromal and full PTSD).
The second aim was to investigate whether TMAO is a potential biomarker that could be useful in the prediction of AMI-induced PTSD symptomatology and the PTSD symptom subclusters re-experiencing, avoidance and hyperarousal.
The third aim was to investigate whether TMAO varies immediately after AMI in patients with/without subsequent depression 6 months after AMI.
3. Methods
3.1. Participants and procedures
All 116 study participants were drawn from AMI inpatients hospitalized at the Division of Cardiology of the Department of Internal Medicine, Medical University of Graz, Austria. Of the 116 study participants, two patients died due to AMI shortly after hospital admission; therefore, the overall sample consisted of 114 patients (96 males, 84.2%; 18 females, 15.8%). All participants were Caucasian and the mean age was 59.9 (± 11.5) years. Exclusion criteria for enrolment in this study were pre-existing PTSD prior to AMI, delirium and dementia, as well as additional acute and severe somatic illnesses other than AMI.
After admission to the hospital and then again 6 months after AMI, patients were assessed by an experienced consulting psychiatrist (A.B.) with a standardized clinical psychiatric interview based on the Hamilton Depression Scale (HAMD-17; Hamilton, 1967). None of the participants had clinically significant depression at the time of AMI.
Baseline CAPS-5 (Clinician Administered PTSD Scale for DSM-5; Weathers et al., 2018) screenings for potential trauma A criteria prior to AMI and pre-existing PTSD symptoms were conducted to exclude pre-existing PTSD symptomatology prior to AMI. None of the participants had PTSD prior to acute myocardial infarction.
In addition, substance abuse and previous psychiatric morbidities other than PTSD and depression were recorded shortly after AMI in a baseline clinical psychiatric standard interview, based on the ICD-10 classification.
Six months after AMI the CAPS-5 was used to explore AMI-induced PTSD symptoms. These CAPS-5 assessments 6 months after AMI were conducted by investigating symptoms related to only the index trauma, that is, AMI. No symptoms related to any other trauma types were assessed.
For the assessment of TMAO, serum samples were collected at hospitalization and 6 months after AMI.
3.1.1. Ethics statement
This research project was approved by the Institutional Review Board of the Medical University of Graz (Approval number: 28–126 ex 15/16). Data protection measures met the standards by Austrian law. The methods were carried out in accordance with the approved guidelines. All participants in this study had to provide signed informed consent, and subjects could decide to withdraw from this research project at any time. Research participants were not compensated for their time.
3.2. Psychiatric interviews
The widely used observer rating scale for depression HAMD-17, which was designed by Hamilton (1967), was used immediately after AMI and 6 months later to ascertain the severity of AMI-induced depressive symptoms. As recommended by Zimmerman et al., the severity of depression was classified as no depression (total score of 0–7 on the HAMD-17); mild depression (total score of 8–16); moderate depression (total score of 17–23); and severe depression (total score of ≥24 on the HAMD-17) (Zimmerman, Martinez, Young, Chelminski, & Dalrymple, 2013).
After admission to the hospital and then again 6 months after AMI, the 30-item structured Clinician Administered PTSD Scale for DSM-5 (CAPS-5, National Center of PTSD, 2020; Weathers et al., 2018) was administered to assess pre-existing PTSD prior to AMI and AMI-induced PTSD symptoms (subsyndromal PTSD and full PTSD) 6 months after AMI. The 30 items on the CAPS-5 cover the following main DSM-5 PTSD criteria: A: severe or life-threatening traumatic event (defined as AMI in the present study); B: re-experiencing symptoms, intrusions and flashbacks; C: avoidance symptoms; D: negative alterations in cognition and mood; E: hyperarousal; F: significant disturbance lasting at least one month; and G: disturbance causing significant impairment. For the diagnosis of full PTSD, a patient must fulfil criteria A, F, and G and at least one criterion B symptom, at least one criterion C symptom, at least two criterion D symptoms, and at least two criterion E symptoms (American Psychiatric Association, 2013). Subsyndromal PTSD symptomatology was diagnosed when one or two of the criteria from B-E were missing (Baranyi et al., 2010).
Substance abuse and previous psychiatric morbidities other than PTSD and depression were recorded shortly after AMI in a baseline clinical psychiatric standard interview, based on the ICD-10 classification that was performed by an experienced consultation-liaison psychiatrist (A.B.).
3.3. Sociodemographic characteristics
To obtain the participants’ sociodemographic characteristics, an author-compiled sociodemographic questionnaire was used to assess age, sex, years of education and/or vocational training, and employment status at the time of psychiatric assessment. The patient’s employment status was categorized as paid work (full- or part-time) or no paid work (disability, retired, or unemployed). Marital status was categorized as married, single, divorced, or widowed.
3.4. Anthropometry, renal function, cardiac status at the time of admission to the hospital, percutaneous coronary intervention (PCI)-related parameters, in-hospital outcome and cardiac risk factors of AMI patients
The following clinical data were obtained for this study to characterize the study sample and to investigate the predictive value of TMAO, in addition to AMI-related clinical factors, for the diagnosis of PTSD symptoms 6 months after AMI:
Anthropometry: Height (cm), weight (kg).
Cardiac status at the time of inpatient admission due to AMI: Type of myocardial infarction, Killip class (=post-AMI mortality risk stratification), cardiogenic shock, abnormal level of troponin T, and AMI-related reanimation.
Percutaneous coronary intervention (PCI): coronary artery disease (number of affected vessels); thrombolysis in myocardial infarction flow (TIMI) before PCI, TIMI flow after PCI, and multivessel PCI.
Outcome: Death, stroke, major bleeding, reinfarction, and left ventricular rejection fraction (%).
Cardiac risk factors: nicotine abuse, peripheral arterial occlusive disease, insulin-dependent diabetes mellitus (IDDM), non-insulin-dependent diabetes mellitus (NIDDM), hypertension, hyperlipidaemia, relevant family history, previous myocardial infarction, and severe liver disease.
Renal function: Glomerular filtration rate (GFR), representing renal function, is well known to have an impact on TMAO levels.
Substance abuse at the time of the AMI.
Previous psychiatric morbidity other than PTSD: e.g. depression, adjustment disorder, burn-out.
3.5. Laboratory analyses
TMAO was measured using a stable-isotope dilution assay and high-performance liquid chromatography (HPLC) with electrospray ionization tandem mass spectrometry on a SCIEX QTRAP 4500 triple quadrupole instrument (Applied Biosystems, Framingham, MA, USA) equipped with an Agilent 1260 Infinity HPLC system (Agilent Technologies, Santa Clara, CA, USA). The intra- and inter-day coefficients of variation (CVs) ranged between 2.2 and 3.4% and 6.9 and 9.9%, respectively (Enko, Zelzer, et al., 2020a). The estimated GFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI)-GFR equation (Levey et al., 2009).
4. Statistical analyses
Descriptive statistics were conducted based on sociodemographic and treatment-related data. Data are presented as the means and standard deviations (SDs). To analyse continuous variables, t-tests and Pearson’s correlation coefficients were used. For categorical variables, χ2 tests were performed. In the case of multiple comparisons, an alpha adjustment (Bonferroni) was performed. TMAO levels in patients with/without PTSD symptomatology were analysed with analysis of covariance (ANCOVA), in which age, sex, body mass index (BMI), renal function (glomerular filtration rate), previous psychiatric morbidity other than PTSD and substance abuse at the time of AMI were included as covariates. The same statistical procedures were performed for the patients with/without depression 6 months after AMI. Logistic and linear regression models were used (1) to examine the impact of TMAO as a predictor of AMI-related PTSD symptomatology (subsyndromal and full PTSD) and (2) as a predictor of the PTSD-symptom subclusters of re-experiencing, avoidance and hyperarousal. Interaction analyses were performed to consider the relationship among the variables. In the first step of the logistic regression analyses we regressed TMAO at the time of hospital admission against AMI-induced PTSD-symptomatology (PTSD-symptoms present: yes/no) (Model 1). In the second step, the hierarchical logistic regression model control variables age, sex, body mass index (BMI), renal function (GFR), previous psychiatric morbidity other than PTSD and substance abuse at the time of AMI (Model 2) were introduced. In the third step, the hierarchical logistic regression model clinical predictors coronary artery disease (number of affected vessels) and anxiety at the time of AMI (HAMD-17 item psychic anxiety) (Model 3) were added to the model.
All statistical analyses were performed with SPSS 25.0 for Windows (SPSS; Chicago, IL).
5. Results
5.1. AMI-induced PTSD symptomatology (full and subsyndromal PTSD)
Six months after AMI, 49/114 (43%) patients had acute myocardial infarction-induced PTSD symptomatology [full PTSD: 14/114 (12.3%), subsyndromal PTSD (one CAPS-V criterion from B-E missing): 21/114 (18.4%), subsyndromal PTSD (two CAPS-V criteria B-E missing): 14/114 (12.3%)].
Table 1 shows the sociodemographic characteristics and pre-existing psychiatric morbidities other than PTSD in the overall sample and in the patients with or without AMI-induced PTSD-symptomatology.
Table 1.
Sociodemographic characteristics and pre-existing psychiatric morbidities other than PTSD in the patients with and without AMI-induced PTSD symptomatology
| Category | Total Sample (n = 114) |
p | PTSD-Symptomatology (n = 49/114, 43%) |
No PTSD (n = 65/114, 57%) |
p | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | n (%) | 96 (84.2%) | χ2 = 53.368, df = 1 p < .001a |
39 (79,6%) | 57 (87.7%) | χ2 = 1.38, df = 1 p = 0.24a |
| Female | n (%) | 18 (15.8%) | 10 (20.4%) | 8 (12.3) | ||
| Age | mean (SD) | 59.9 (±11.48) | - | 59.2 (±11.52) | 60.6 (±11.51) | t = 0.650, df = 112 p = 0.52b |
| Marital Status | ||||||
| Single | n (%) | 14 (12.3%) | χ2 = 117.643, df = 3 p < .001a |
8 (17%) | 6 (78.5%) | χ2 = 7.17, df = 3 p = 0.07a |
| Married | n (%) | 77 (67.5%) | 26 (55.3%) | 51 (9.2%) | ||
| Widowed | n (%) | 4 (3.5%) | 2 (4.3%) | 2 (3.1%) | ||
| Divorced | n (%) | 17 (14.9%) | 11 (23.4%) | 6 (9.2%) | ||
| Maximum Educational Level | ||||||
| Secondary School without Graduation | n (%) | 7 (6.1%) | χ2 = 116.642, df = 7 p < .001a |
4 (8.7%) | 3 (5%) | χ2 = 6.49, df = 7 p = 0.48a |
| Secondary School with Graduation | n (%) | 49 (43.0%) | 23 (50%) | 26 (43.3%) | ||
| Business School without Diploma | n (%) | 6 (5.3%) | 2 (4.3%) | 4 (6.7%) | ||
| Business School with Diploma | n (%) | 11 (9.6%) | 5 (10.9%) | 6 (10%) | ||
| Grammar School without Graduation | n (%) | 7 (6.1%) | 2 (4.3%) | 5 (8.3%) | ||
| Grammar School with Graduation | n (%) | 11 (9.6%) | 5 (10.9%) | 6 (10%) | ||
| A-level without University degree | n (%) | 2 (1.8%) | 2 (4.3%) | 0 (0%) | ||
| A-level with University Degree | n (%) | 13 (11.4%) | 3 (6.5%) | 10 (16.7%) | ||
| Employment Status | ||||||
| Paid Work (full- or part-time) | n (%) | 53 (46.5%) | χ2 = 87.754, df = 3 p < .001a |
21 (42.9%) | 32 (49.2%) | χ2 = 7.85, df = 3 p = 0.05a |
| Homemaker | n (%) | 3 (2.6%) | 0 (0%) | 3 (4.6%) | ||
| Retired | n (%) | 54 (47.4%) | 24 (49%) | 30 (46.2%) | ||
| Unemployed | n (%) | 4 (3.5%) | 4 (8.1%) | 0 (0%)5 | ||
|
Previous Mental Illness other than PTSD (Depression, Adjustment Disorder, Burn-out) |
n (%) | 14 (12.3%) | - | 8 (16.3%) | 6 (9.2%) | χ2 = 1.306, df = 1 p = 0.25a |
| Previous Psychopharma-cological Medication | n (%) | 11 (9.6%) | - | 5 (10.2%) | 6 (9.2%) | χ2 = 0.03, df = 1 p = 0.86a |
| Substance Abuse | ||||||
| Alcohol | n (%) | 1 (0.87%) | - | 1 (2.0%) | 0 (0%) | p = 0.429c |
| Illicit Drugs | n (%) | 0 (0%) | - | 0 (0%) | 0 (0%) | - |
Legend: a χ2 – test; b t-test; c Fisher exact
Table 2 presents the anthropometry, renal function, cardiac status at the time of admission to the hospital, percutaneous coronary intervention (PCI)-related parameters, in-hospital outcome and cardiac risk factors in the overall sample and in the patients with or without AMI-induced PTSD-symptomatology.
Table 2.
Anthropometry, renal function, cardiac status at the time of admission to the hospital, percutaneous coronary intervention (pci)-related parameters, in-hospital outcome and cardiac risk factors in the patients with and without ami-induced ptsd symptomatology
| Category | Total Sample (n = 114) |
p | PTSD-Symptomatology (n = 49/114; 43%) | No PTSD (n = 65/114; 57%) |
||
|---|---|---|---|---|---|---|
| Anthropometry | ||||||
| Height (cm) | Mean (SD) | 174.79 (±8.15) | - | 173.5 (±8.27) | 176.0 (±7.97) | t = 1.435, df = 109 p = 0.154a |
| Weight (kg) | Mean (SD) | 86.64 (±13.89) | - | 87.7 (±16.82) | 85.81 (±11.20) | t = −0.710, df = 108 p = 0.479a |
| BMI | Mean (SD) | 28.31 (±-4.0) | - | 29.01 (±4.89) | 27.76 (±3.07) | t = −1.643, df = 108 p = 0.103a |
| Renal Function | ||||||
| Glomerular Filtration Rate | Mean (SD) | 84.69 (±17.87) | - | 83.6 (±18.89) | 85.53 (±17.17) | t = 0.578, df = 112 p = 0.56a |
| Cardiac Situation at the Time of Admission to the Hospital | ||||||
| NSTEMI | n (%) | 43 (37.7%) | - | 20 (40.8%) | 23 (35.4%) | χ2 = 0.351, df = 1 p = 0.55b |
| STEMI | n (%) | 71 (62.3%) | 29 (59.2%) | 42 (64.6%) | ||
| Killip Mortality Risk Stratification: | ||||||
| Killip Class I | n (%) | 88 (77.2%) | χ2 = 197.62, df = 3 p < .001a |
40 (85.1%) | 48 (84.2%) | χ2 = 0.807, df = 3 p = 0.85b |
| Killip Class II | n (%) | 8 (7%) | 3 (6.4%) | 5 (8.8%) | ||
| Killip Class III | n (%) | 5 (4.4%) | 2 (4.3%) | 3 (5.3%) | ||
| Killip Class IV | n (%) | 3 (2.6) | 2 (4.3%) | 1 (1.8%) | ||
| Abnormal Level of Troponin T |
n (%) | 114 (100%) | - | 49 (100%) | 65 (100%) | - |
| Cardiogenic Shock | n (%) | 0 (%) | - | 0 (0%) | 0 (0%) | - |
| AMI-related Reanimation | n (%) | 3 (2.6%) | - | 2 (4.1%) | 1 (1.5%) | p = 0.576c |
| PCI-related Parameters | ||||||
| TIMI Flow before PCI: | ||||||
| 0-I | n (%) | 85 (74.6%) | χ2 = 108.571.62, df = 2 p < .001a |
35 (76.1%) | 50 (84.7%) | χ2 = 1.325, df = 2 p = 0.52b |
| II | n (%) | 15 (13.2%) | 8 (17.4%) | 7 (11.9%) | ||
| III | n (%) | 5 (4.4%) | 3 (6.5%) | 2 (3.4% | ||
| TIMI flow after PCI: | ||||||
| 0-I | n (%) | 4 (3.5%) | χ2 = 156.054.62, df = 2 p < .001a |
0 (0%) | 4 (6.3%) | χ2 = 4.269, df = 2 p = 0.12b |
| II | n (%) | 8 (7%) | 5 (10.6%) | 3 (4.7%) | ||
| III | n (%) | 99 (86.8%) | 42 (89.4%) | 57 (89.0%) | ||
| Multivessel PCI | n (%) | 23 (20.2%) | - | 13 (27.7%) | 10 (16.7%) | χ2 = 1.887, df = 1 p = 0.17b |
| Coronary Artery Disease (number of affected vessels) | Mean (SD) | 1.82 (±0.79) | 1.84 (±0.83) | 1.82 (±0.76) | t-test = −0.074, df = 112 p = 0.89a |
|
| In-hospital Outcome | ||||||
| Major Bleeding | n (%) | 0 (0%) | - | 0 (0%) | 0 (0%) | - |
| Reinfarction | n (%) | 2 (1.8%) | - | 1 (2.0%) | 1 (1.5%) | χ2 = 0.042, df = 1 p = 0.84b |
| Left-Ventricular Ejection Fraction (%) | Mean (SD) | 53.15 (±10.559) | - | 53.24 (±11.73) | 53.07 (±9.60) | t-test = −0.074, df = 79 p = 0.94a |
| Cardiac Risk Factors | ||||||
| Nicotine Abuse | n (%) | 39 (34.2%) | - | 23 (46.9%) | 30 (46.2%) | χ2 = 0.007, df = 1 p = 0.93b |
| Peripheral Arterial Occlusive Disease | n (%) | 3 (2.6%) | - | 2 (4.1%) | 1 (1.5%) | χ2 = 0.681, df = 1 p = 0.41b |
| IDDM | n (%) | 2 (1.8%) | - | 1 (2.0%) | 1 (1.5%) | χ2 = 0.041, df = 1 p = 0.84b |
| NIDDM | n (%) | 17 (14.9%) | - | 11 (22.4%) | 6 (9.2%) | χ2 = 3.847, df = 1 p = 0.05b |
| Hypertension | n (%) | 102 (89.5%) | - | 42 (85.7%) | 60 (92.3%) | χ2 = 1.290, df = 1 p = 0.26b |
| Hyperlipidaemia | n (%) | 59 (51.8%) | - | 28 (56.0%) | 31 (47.7) | χ2 = 1.077, df = 1 p = 0.30b |
| Relevant Family History | n (%) | 23 (20.2%) | - | 10 (23.3%) | 13 (25.5%) | χ2 = 0.063, df = 1 p = 0.80b |
| Previous Myocardial Infarction | n (%) | 12 (10.5%) | - | 8 (16.3%) | 4 (6.5%) | χ2 = 2.768, df = 1 p = 0.096 |
| Severe Liver Disease | n (%) | 2 (1.8%) | - | 0 (0%) | 2 (3.0%) | χ2 = 1.54, df = 1 p = 0.22b |
Legend: a t-test; b χ2 – test, c Fisher exact; Abbreviations: AMI = Acute Myocardial Infarction; IDDM = Insulin-dependent Diabetes Mellitus; NIDDM = Non-Insulin-dependent Diabetes Mellitus; PCI = Percutaneous Coronary Intervention; TIMI flow = Thrombolysis in Myocardial Infarction Flow.
5.2. Trimethylamine N-oxide (TMAO)
5.2.1. Overall sample
In the overall sample (regardless of secondary psychiatric diseases), there was a slight trend for an increase in TMAO 6 months after acute myocardial infarction [t-test for paired samples; mean TMAO concentration at time of hospital admission: 3.63 µmol/l (SD = ±3.38); mean TMAO concentration after 6 months: 4.23 µmol/l (SD = ±2.39), t = −1.68, df = 113, p = 0.09].
Baseline TMAO levels immediately after myocardial infarction were not significantly correlated with cardiological parameters of the acute cardiac event (Killip class – post-AMI mortality risk stratification: Pearson’s r = −0.77, p = 0.438; LVEF: Pearson’s r = 0.109, p = 0.332).
5.2.2. TMAO in patients with AMI-induced PTSD symptomatology (full and subsyndromal PTSD) six months after AMI
Patients with AMI-induced full or subsyndromal PTSD symptomatology 6 months after AMI had significantly higher TMAO values immediately after AMI than patients without PTSD symptoms. Six months after AMI, TMAO levels remained high in patients with full or subsyndromal AMI induced PTSD symptomatology. In comparison to the levels at the time of AMI, patients without PTSD symptoms had higher TMAO levels 6 months after AMI [ANCOVA: TMAO (time) F = 4.824, df = 1, p = 0.03; TMAO (PTSD) F = 0.243, df = 1, p = 0.122; TMAO (PTSD × time) F = 4.544, df = 1, p = 0.035; covariate age: F = 0.021, df = 1, p = 0.886; covariate sex: F = 13.936, df = 1, p < 0.01; covariate BMI: F = 2.129, df = 1, p = 0.148; covariate renal function (GFR): F = 0.053, df = 1, p = 0.819; covariate previous psychiatric morbidity: F = 3.914, df = 1, p = 0.051; substance abuse: F = 0.102, df = 1, p = 0.750]. Figure 1 shows the levels of TMAO in patients with and without PTSD symptomatology.
Figure 1.
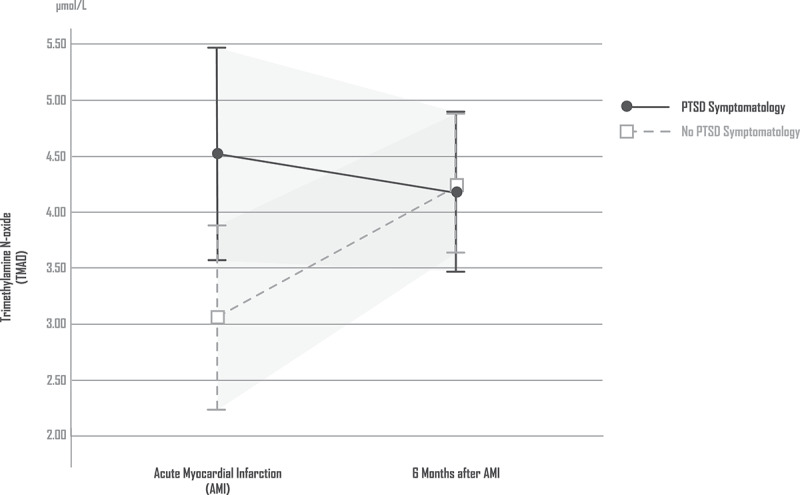
TMAO in patients with and without PTSD symptomatology
5.2.3. Multivariate analyses: TMAO as a predictor of AMI-related PTSD-symptoms (full and subsyndromal PTSD)
A three-step hierarchical logistic regression model was used to examine potential correlates of AMI-induced PTSD symptomatology. In the first step, we regressed TMAO at the time of hospital admission against AMI-induced PTSD-symptomatology (PTSD-symptoms present: yes/no) (Model 1). In the second step, we introduced the hierarchical logistic regression model control variables age, sex, body mass index (BMI), renal function (glomerular filtration rate), previous psychiatric morbidity other than PTSD and substance abuse at the time of AMI (Model 2). In the third step, we introduced the hierarchical logistic regression model clinical predictors coronary artery disease (number of affected vessels) and anxiety at the time of AMI (HAMD-17 item psychic anxiety) (Model 3).
Regarding Model 1, baseline TMAO alone tended to be predictive of AMI-induced PTSD symptomatology (TMAO: p = 0.079). When the control variables (Model 2) and the clinical predictors (Model 3) were included in the hierarchical logistic regression analysis, TMAO became a significant predictor of AMI-induced PTSD symptomatology (TMAO: p = 0.048).
Table 3 shows the three-step hierarchical logistic regression model of AMI-induced PTSD symptomatology.
Table 3.
Three-step hierarchical logistic regression model of AMI-induced PTSD symptomatology
| B | S.E. | WALD | df | Exp (B) | p | |
|---|---|---|---|---|---|---|
|
Step 1 (Model 1) DV = PTSD Symptomatology (Yes/No) |
||||||
| Constant | −0.741 | 0.320 | 5.357 | 1 | 0.477 | 0.021 |
| TMAO at time of hospital admission | 0.128 | 0.073 | 3.080 | 1 | 1.136 | 0.079 |
| R2 COX & Snell: 0.036 (Omnibus: χ2 = 4.046, df = 1, p = 0.044; Hosmer-Lemeshow-Test: χ2 = 7.151, df = 8, p = 0.520) |
||||||
|
Step 2 (Model 2) DV = PTSD Symptomatology (Yes/No) |
||||||
| Constant | 19.673 | 40,193.16 | 0.00 | 1 | 349,734,226.1 | 1.00 |
| TMAO at time of hospital admission | 0.163 | 0.082 | 3.929 | 1 | 1.177 | 0.047 |
| Sex | 0.434 | 0.638 | 0.463 | 1 | 1.544 | 0.496 |
| Age | −0.023 | 0.21 | 1.210 | 1 | 0.978 | 0.271 |
| BMI | 0.106 | 0.055 | 3.681 | 1 | 1.111 | 0.055 |
| GFR | −0.007 | 0.013 | 0.247 | 1 | 0.993 | 0.619 |
| Previous psychiatric morbidity other than PTSD | −0.492 | 0.690 | 0.508 | 1 | 0.611 | 0.476 |
| Substance abuse at the time of AMI | −21.284 | 40,193.16 | 0.00 | 1 | 0.000 | 1.00 |
| R2 COX & Snell: 0.116 (Omnibus: χ2 = 13.381, df = 7, p = 0.063; Hosmer-Lemeshow-Test: χ2 = 8.872, df = 8, p = 0.353) |
||||||
|
Step 3 (Model 3) DV = PTSD Symptomatology (Yes/No) |
||||||
| Constant | 18.441 | 40,192.93 | .000 | 1 | 102,068,407.1 | 1.00 |
| TMAO at time of hospital admission | 0.167 | 0.085 | 3.908 | 1 | 1.182 | 0.048 |
| Sex | 0.332 | 0.689 | 0.232 | 1 | 1.394 | 0.630 |
| Age | −0.016 | 0.022 | 0.568 | 1 | 0.984 | 0.451 |
| BMI | 0.111 | 0.058 | 3.669 | 1 | 1.118 | 0.055 |
| GFR | −0.006 | 0.014 | 0.209 | 1 | 0.994 | 0.647 |
| Previous psychiatric morbidity other than PTSD | −0.437 | 0.718 | 0.370 | 1 | 0.646 | 0.543 |
| Substance abuse at the time of AMI | −21.312 | 40,192.936 | 0.000 | 1 | 0.000 | 1.00 |
| PCI – coronary artery disease (number of affected vessels) | 0.100 | 0.291 | 0.119 | 1 | 0.625 | 0.730 |
| Anxiety at the time of AMI (HAMD-17 item psychic anxiety). | 0.428 | 0.178 | 5.775 | 1 | 1.082 | 0.016 |
| R2 COX & Snell: 0.165 (Omnibus: χ2 = 19.716, df = 9, p = 0.020; Hosmer-Lemeshow-Test: χ2 = 5.573, df = 8, p = 0.695) |
||||||
5.2.4. TMAO as a predictor of the AMI-induced PTSD symptom subcluster re-experiencing
In a linear regression model, we regressed TMAO at the time of hospital admission against the PTSD symptom subcluster re-experiencing. In this linear regression analysis, TMAO showed a minor trend (p = 0.079) for the prediction of the PTSD symptom subcluster re-experiencing.
Table 4 shows the linear regression model for the PTSD symptom subcluster re-experiencing.
Table 4.
Linear regression model – PTSD-symptom subcluster re-experiencing
| DV = PTSD Subcluster Re-Experiencing | B | Beta | S.E. | p |
|---|---|---|---|---|
| Constant | 2.572 | 0.441 | 0.000 | |
| TMAO at time of hospital admission | 0.154 | 0.161 | 0.089 | 0.087 |
5.2.5. TMAO as a predictor of the AMI-induced PTSD symptom subcluster avoidance
In a linear regression model, we regressed TMAO at the time of hospital admission against the PTSD symptom subcluster avoidance. In this linear regression analysis, TMAO was not a predictor (p = 0.594) of the PTSD-symptom subcluster avoidance.
5.2.6. TMAO as a predictor of the AMI-induced PTSD symptom subcluster hyperarousal
In a linear regression model, we regressed TMAO at time of hospital admission against the PTSD symptom subcluster arousal. In this linear regression analysis, TMAO was not a predictor (p = 0.910) of the PTSD symptom subcluster hyperarousal.
5.3. TMAO in patients with depression 6 months after acute myocardial infarction
Six months after AMI, 60/114 (52.6%) patients had depressive symptomatology. In the group of depressed patients, the HAMD-17 score increased significantly after 6 months [mean HAMD-17 score at the time of hospital admission: 5.30 (SD = ±2.52); mean HAMD-17 score after 6 months: 13.73 (SD = ±4.79)]. In the group of non-depressed patients, the HAMD-17 score decreased after 6 months [mean HAMD-17 score at admission: 3.98 (SD = ±2.0); mean HAMD-17 score at 6 months: 1.77 (SD = ±1.87); ANOVA (depression × time): F = 201,483; df = 1, p < .001].
In comparison with the non-depressed patients, patients with depression 6 months after AMI did not have significantly different TMAO levels [ANCOVA: TMAO (time) F = 4.511, df = 1, p = 0.036; TMAO (Depression) F = 3.184, df = 1, p = 0.569; TMAO (Depression × time) F = 0.083, df = 1, p = 0.774; covariate age: F = 0.0, df = 1, p = 0.987; covariate sex: F = 14.712, df = 1, p < 0.01; covariate BMI: F = 1.246, df = 1, p = 0.267; covariate renal function (GFR): F = 0.021, df = 1, p = 0.885; covariate previous psychiatric morbidity: F = 3.459, df = 1, p = 0.066; substance abuse: F = 0.019, df = 1, p = 0.892]. Figure 2 shows the levels of TMAO in patients with and without depression.
Figure 2.
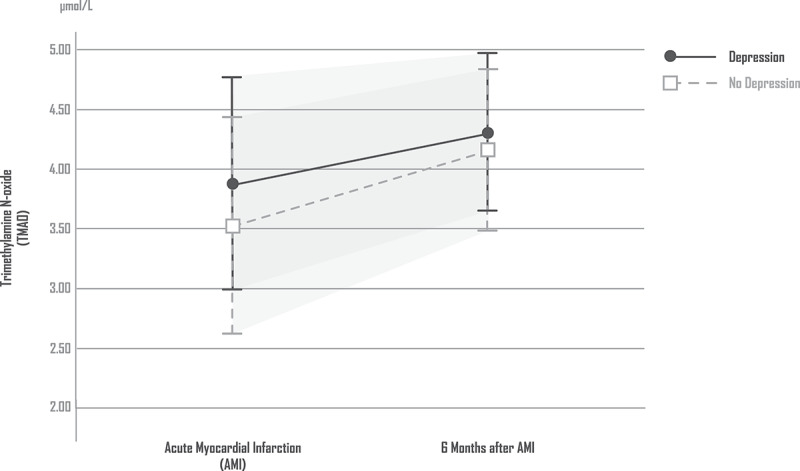
TMAO in patients with and without depression
6. Discussion
6.1. TMAO levels after AMI
In the overall group, regardless of psychiatric symptoms, we found a trend for a slight increase in serum TMAO levels 6 months after AMI. This observed TMAO increase might be caused by AMI-induced changes in microbial community structures (Wu et al., 2017) and by chronic long-term stress after myocardial infarction. The results of our study are also partly consistent with the results of the study by Alhmoud et al. In this study, there was no immediate increase observed in TMAO after acute coronary syndrome (Alhmoud et al., 2019).
However, when secondary psychiatric diseases were included in our data analysis, a more differentiated pattern emerged. Thus, we found no significant difference in TMAO levels immediately after AMI between individuals with or without depression 6 months after AMI. In contrast, our study participants with AMI-induced PTSD symptoms had significantly higher TMAO levels immediately after AMI than patients without PTSD symptoms. This might be explained by the fact that the emergence of PTSD requires an individual perception of severe acute stress at the time of myocardial infarction. Several studies have demonstrated that such severe mental stress has a distinct impact on the microbiota-gut-brain axis, which becomes obvious based on the consequences of increased gut permeability (Obrenovich, Siddiqui, McCloskey, & Reddy, 2020; Rezzi et al., 2009). A leaky gut-brain axis is a serious consideration given the potential for the transmission of bacteria and gut bacteria-derived metabolites, which may also have impacts on the pathophysiology of cardiovascular and psychiatric diseases (Carabotti et al., 2015; Meinitzer et al., 2020). Therefore, in our study, the observed increased TMAO levels immediately after AMI may reflect such an increase in gut permeability with regard to the TMAO precursor TMA. These considerations are also in line with the findings of a previous study by Kinross et al. (2011), which described elevated TMAO levels after stressful surgical interventions in Wistar rats (Kinross et al., 2011). In a recent study, we further demonstrated that the small molecule TMAO can also cross the blood-CSF barrier via passive diffusion (Enko, Zelzer, et al., 2020b). Considering this fact, TMAO could also play a direct role in the central nervous system. However, the impact of TMAO on the pathophysiology of the central nervous system requires further studies (Del Rio et al., 2017).
While increased TMAO levels immediately after AMI may reflect an increase in gut permeability to the TMAO precursor TMA due to an acute overwhelming stress perception in PTSD-vulnerable patients, the observed high TMAO levels 6 months after AMI in the entire group might more likely be explained by AMI-induced prolonged alterations of gut microbial community structures. These long-term changes in gut microbial community structures are likely to emerge independent of PTSD symptoms due to acute myocardial infarction itself (Wu et al., 2017) and chronic long-term stress after myocardial infarction (familial, employment, health problems and other stressors), which do not reach a sufficiently high enough levels to trigger PTSD. Exposure to severe psychological stressors can modify microbial community structures, turning a normally stable microenvironment into a volatile dysbiotic profile (Galley, Mackos, Varaljay, & Bailey, 2017; Knowles, Nelson, & Palombo, 2008). These alterations in gut microbial community structures are also expected to be present in our study group of patients without PTSD. This might explain why in our study, even patients without PTSD had elevated TMAO levels 6 months after myocardial infarction.
With the exception of sex, none of the covariates (age, body mass index (BMI), renal function (glomerular filtration rate), previous psychiatric morbidity other than PTSD and substance abuse at the time of AMI significantly impacted the study results.
In our study, TMAO immediately after myocardial infarction was not correlated with cardiological markers, such as Killip class (=post-AMI mortality risk stratification) and LVE. This means that while TMAO is thought to have less clinical significance in acute cardiological events, it might enhance the risk of severe cardiac events in the future (Alhmoud et al., 2019; Querio et al., 2019).
In addition, our study results (see Tables 1 and 2) showed that the differences in TMAO concentrations found between patients with and without new-onset AMI-induced PTSD were unlikely to be explained by anthropometry, renal function, cardiac status at the time of admission to the hospital, percutaneous coronary intervention (PCI)-related parameters, in-hospital outcomes or cardiac risk factors.
6.2. TMAO as a predictor of AMI-related PTSD-symptoms (full and subsyndromal PTSD)
Baseline TMAO at the time of AMI alone tended to be predictive of PTSD symptomatology. When the control variables and the clinical predictors were included in the hierarchical logistic regression model, TMAO became a significant predictor of PTSD symptomatology. In addition, TMAO showed a minor trend for the prediction of the PTSD symptom subcluster re-experiencing. The observation that the re-experiencing subcluster is particularly strongly reflected in biomarkers is also supported by two studies: Lima et al. (2020) investigated the association of AMI-related PTSD with mental stress-induced myocardial ischaemia among patients who survived AMI. In the study by Lima et al. (2020), the PTSD symptom-subcluster re-experiencing was most strongly associated with ischaemia during mental stress after AMI (Lima et al., 2020). Lima et al. further suggested that AMI survivors with comorbid PTSD might also have a more pronounced inflammatory response to subsequent acute psychological stress situations than AMI survivors without comorbid PTSD. In a recent study, they showed, that acute severe mental stress is associated with an increase in the inflammatory marker IL-6 in AMI survivors with comorbid PTSD. Re-Experiencing was again the PTSD-symptom-subcluster most strongly associated with an enhanced inflammatory response to subsequent acute severe mental stress (Lima et al., 2019).
6.3. Limitations
Follow-up studies should investigate the correlations of TMAO with well-known biomarkers of stress (e.g. cortisol, metanephrine). In addition, serum TMA level measurements would be of interest. Further studies should also assess stress perception with additional psychometric questionnaires. It should also be mentioned that a heart attack no longer constitutes a Criterion A trauma, according to the DSM-5 (American Psychiatric Association, 2013).
7. Conclusions
In summary, TMAO elevations immediately after AMI might reflect severe stress in PTSD-vulnerable patients, which might also lead to a short-term increase in gut permeability to TMA, the precursor of TMAO. Thus, an elevated TMAO level might be a biological correlate of AMI-related severe stress, which affects vulnerability to the development of PTSD.
Funding Statement
This work was supported by the ZARG, Zentrum für ambulante Rehabilitation GmbH, Graz, Austria under Grant [4325/Medical University of Graz].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data available Statement
To protect the confidentiality of patient information, the Medical University of Graz IRB will not allow the authors to make data publicly available. Data are available upon request from Andreas Baranyi at the Department of Psychiatry and Psychotherapeutic Medicine, Medical University of Graz, Graz, Austria, for researchers who meet the criteria for access to confidential data.
References
- Alhmoud, T., Kumar, A., Lo, C. C., Al-Sadi, R., Clegg, S., Alomari, I., … Ma, T. (2019). Investigating intestinal permeability and gut microbiota roles in acute coronary syndrome patients. Human Microbiome Journal, 13, 100059. doi: 10.1016/j.humic.2019.100059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliev, G., Beeraka, N. M., Nikolenko, V. N., Svistunov, A. A., Rozhnova, T., Kostyuk, S., … Kirkland, C. E. (2020). Neurophysiology and psychopathology underlying PTSD and recent insights into the PTSD therapies-A comprehensive review. Journal of Clinical Medicine, 9(9), 2951. doi: 10.3390/jcm9092951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders. (5th ed.). American Psychiatric Publishing. doi: 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- Andersson, S. I., Pesonen, E., & Ohlin, H. (2007). Perspectives that lay persons with and without health problems show toward coronary heart disease: An integrated biopsychosocial approach. Heart & Lung: The Journal of Critical Care, 36(5), 330–14. doi: 10.1016/j.hrtlng.2007.02.004 [DOI] [PubMed] [Google Scholar]
- Baranyi, A., Krauseneck, T., & Rothenhäusler, H. B. (2013). Posttraumatic stress symptoms after solid-organ transplantation: Preoperative risk factors and the impact on health-related quality of life and life satisfaction. Health and Quality of Life Outcomes, 11(1), 111. doi: 10.1186/1477-7525-11-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranyi, A., Leithgöb, O., Kreiner, B., Tanzer, K., Ehrlich, G., Hofer, H. P., & Rothenhäusler, H. B. (2010). Relationship between posttraumatic stress disorder, quality of life, social support, and affective and dissociative status in severely injured accident victims 12 months after trauma. Psychosomatics, 51(3), 237–247. doi: 10.1016/S0033-3182(10)70691-5 [DOI] [PubMed] [Google Scholar]
- Bennett, B. J., De Aguiar Vallim, T. Q., Wang, Z., Shih, D. M., Meng, Y., Gregory, J., … Lusis, A. J. (2013). Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metabolism, 17(1), 49–60. doi: 10.1016/j.cmet.2012.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg, M. M., & Soufer, R. (2016). Post-traumatic Stress Disorder and Cardiovascular Disease. Current Cardiology Reports, 18(10), 94. doi: 10.1007/s11886-016-0770-5 [DOI] [PubMed] [Google Scholar]
- Carabotti, M., Scirocco, A., Maselli, M. A., & Severi, C. (2015). The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Annals of Gastroenterology, 28(2), 203–209. [PMC free article] [PubMed] [Google Scholar]
- Del Rio, D., Zimetti, F., Caffarra, P., Tassotti, M., Bernini, F., Brighenti, F., … Zanotti, I. (2017). The gut microbial metabolite trimethylamine-N-oxide is present in human cerebrospinal fluid. Nutrients, 9(10), 1053. doi: 10.3390/nu9101053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapala, A., Szudzik, M., Chabowski, D., Mogilnicka, I., Jaworska, K., Kraszewska, K., … Ufnal, M. (2020). Heart failure disturbs gut-blood barrier and increases plasma trimethylamine, a toxic bacterial metabolite. International Journal of Molecular Sciences, 21(17), 6161. doi: 10.3390/ijms21176161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke, M. P. (1971). Reaction time and normetanephrine-metanephrine excretion under intense stimulation in chronic schizophrenics, non-psychotics, and normals. Perceptual and Motor Skills, 32(2), 579–586. doi: 10.2466/pms.1971.32.2.579 [DOI] [PubMed] [Google Scholar]
- Edmondson, D., Richardson, S., Falzon, L., Davidson, K. W., Mills, M. A., & Neria, Y. (2012). Posttraumatic stress disorder prevalence and risk of recurrence in acute coronary syndrome patients: A meta-analytic review. PloS One, 7(6), e38915. doi: 10.1371/journal.pone.0038915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enko, D., Zelzer, S., Baranyi, A., Herrmann, M., & Meinitzer, A. (2020a). Determination of trimethylamine-N-oxide by a simple isocratic high-throughput liquid-chromatography tandem mass-spectrometry method. Clinical Laboratory, 66(9). doi: 10.7754/Clin.Lab.2020.200122 [DOI] [PubMed] [Google Scholar]
- Enko, D., Zelzer, S., Niedrist, T., Holasek, S., Baranyi, A., Schnedl, W. J., … Meinitzer, A. (2020b). Assessment of trimethylamine-N-oxide at the blood-cerebrospinal fluid barrier: Results from 290 lumbar punctures. EXCLI Journal, 19, 1275–1281. doi: 10.17179/excli2020-2763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, L., Li, L., Liu, W., Yang, J., Wang, Q., Shi, L., & Luo, M. (2019). Prevalence of depression in myocardial infarction: A PRISMA-compliant meta-analysis. Medicine, 98(8), e14596. doi: 10.1097/MD.0000000000014596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galley, J. D., Mackos, A. R., Varaljay, V. A., & Bailey, M. T. (2017). Stressor exposure has prolonged effects on colonic microbial community structure in Citrobacter rodentium-challenged mice. Scientific Reports, 7(1), 45012. doi: 10.1038/srep45012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannoni-Pastor, A., Eiroa-Orosa, F. J., Fidel Kinori, S. G., Arguello, J. M., & Casas, M. (2016). Prevalence and predictors of posttraumatic stress symptomatology among burn survivors: A systematic review and meta-analysis. Journal of Burn Care & Research: Official Publication of the American Burn Association, 37(1), e79–e89. doi: 10.1097/BCR.0000000000000226 [DOI] [PubMed] [Google Scholar]
- Hamilton, M. (1967). Development of a rating scale for primary depressive illness. The British Journal of Social and Clinical Psychology, 6(4), 278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x [DOI] [PubMed] [Google Scholar]
- Hiki, N., Mimura, Y., Ogawa, T., Kojima, J., Hatao, F., & Kaminishi, M. (2001). Pathophysiological relevance of the CD14 receptor in surgical patients: Biological activity of endotoxin is regulated by the CD14 receptor. Journal of Endotoxin Research, 7(6), 461–466. doi: 10.1177/09680519010070060101 [DOI] [PubMed] [Google Scholar]
- Jaworska, K., Konop, M., Hutsch, T., Perlejewski, K., Radkowski, M., Grochowska, M., … Ufnal, M. (2020). Trimethylamine but not Trimethylamine Oxide increases with age in rat plasma and affects smooth muscle cells viability. The Journals of Gerontology: Series A, 75(7), 1276–1283. doi: 10.1093/gerona/glz181 [DOI] [PubMed] [Google Scholar]
- Jbilou, J., Grenier, J., Chomienne, M. H., Talbot, F., Tulloch, H., D’Antono, B., Greenman, P., & MindTheHeart Project Team . (2019). Understanding men’s psychological reactions and experience following a cardiac event: A qualitative study from the MindTheHeart project. BMJ Open, 9(9), e029560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kala, P., Hudakova, N., Jurajda, M., Kasparek, T., Ustohal, L., Parenica, J., … Kanovsky, J. (2016). Depression and anxiety after acute myocardial infarction treated by primary PCI. PloS One, 11(4), e0152367. doi: 10.1371/journal.pone.0152367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl, J. P., Hatch, A. M., Arcidiacono, S. M., Pearce, S. C., Pantoja-Feliciano, I. G., Doherty, L. A., & Soares, J. W. (2018). Effects of psychological, environmental and physical stressors on the gut microbiota. Frontiers in Microbiology, 9, 2013. doi: 10.3389/fmicb.2018.02013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler, R. C., Berglund, P., Demler, O., Jin, R., Merikangas, K. R., & Walters, E. E. (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 593–602. doi: 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- Kinross, J. M., Alkhamesi, N., Barton, R. H., Silk, D. B., Yap, I. K., Darzi, A. W., … Nicholson, J. K. (2011). Global metabolic phenotyping in an experimental laparotomy model of surgical trauma. Journal of Proteome Research, 10(1), 277–287. doi: 10.1021/pr1003278 [DOI] [PubMed] [Google Scholar]
- Knowles, S. R., Nelson, E. A., & Palombo, E. A. (2008). Investigating the role of perceived stress on bacterial flora activity and salivary cortisol secretion: A possible mechanism underlying susceptibility to illness. Biological Psychology, 77(2), 132–137. doi: 10.1016/j.biopsycho.2007.09.010 [DOI] [PubMed] [Google Scholar]
- Lancaster, C. L., Cobb, A. R., Lee, H. J., & Telch, M. J. (2016). The role of perceived threat in the emergence of PTSD and depression symptoms during warzone deployment. Psychological Trauma: Theory, Research, Practice and Policy, 8(4), 528–534. doi: 10.1037/tra0000129 [DOI] [PubMed] [Google Scholar]
- Levey, A. S., Stevens, L. A., Schmid, C. H., Zhang, Y. L., Castro, A. F., 3rd, Feldman, H. I., … Coresh, J.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . (2009). A new equation to estimate glomerular filtration rate. Annals of Internal Medicine, 150(9), 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, T., Chen, Y., Gua, C., & Li, X. (2017). Elevated circulating trimethylamine N-oxide levels contribute to endothelial dysfunction in aged rats through vascular inflammation and oxidative stress. Frontiers in Physiology, 8, 350. doi: 10.3389/fphys.2017.00350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima, B. B., Hammadah, M., Pearce, B. D., Shah, A., Moazzami, K., Kim, J. H., … Vaccarino, V. (2020). Association of posttraumatic stress disorder with mental stress-induced myocardial ischemia in adults after myocardial infarction. JAMA Network Open, 3(4), e202734. doi: 10.1001/jamanetworkopen.2020.2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima, B. B., Hammadah, M., Wilmot, K., Pearce, B. D., Shah, A., Levantsevych, O., … Vaccarino, V. (2019). Posttraumatic stress disorder is associated with enhanced interleukin-6 response to mental stress in subjects with a recent myocardial infarction. Brain, Behavior, and Immunity, 75, 26–33. doi: 10.1016/j.bbi.2018.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoPilato, A. M., Addington, J., Bearden, C. E., Cadenhead, K. S., Cannon, T. D., Cornblatt, B. A., … Walker, E. F. (2020). Stress perception following childhood adversity: Unique associations with adversity type and sex. Development and Psychopathology, 32(1), 343–356. doi: 10.1017/S0954579419000130 [DOI] [PubMed] [Google Scholar]
- Meinitzer, S., Baranyi, A., Holasek, S., Schnedl, W. J., Zelzer, S., Mangge, H., … Enko, D. (2020). Sex-specific associations of trimethylamine-N-oxide and Zonulin with signs of depression in carbohydrate malabsorbers and nonmalabsorbers. Disease Markers, 2020, 7897240. doi: 10.1155/2020/7897240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center of PTSD . (2020). Clinician-administered PTSD scale for DSM-5 (CAPS-5) – PTSD. Retrieved from https://www.ptsd.va.gov/professional/assessment/adult-int/caps.asp
- Obrenovich, M., Siddiqui, B., McCloskey, B., & Reddy, V. P. (2020). The microbiota-gut-brain axis heart shunt part I: The French paradox, heart disease and the microbiota. Microorganisms, 8(4), 490. doi: 10.3390/microorganisms8040490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen, L. R., Mortensen, E. L., & Bech, P. (2004). Prevalence of major depression and stress indicators in the Danish general population. Acta psychiatrica Scandinavica, 109(2), 96–103. doi: 10.1046/j.0001-690x.2003.00231.x [DOI] [PubMed] [Google Scholar]
- Querio, G., Antoniotti, S., Levi, R., & Gallo, M. P. (2019). Trimethylamine N-oxide does not impact viability, ROS production, and mitochondrial membrane potential of adult rat cardiomyocytes. International Journal of Molecular Sciences, 20(12), 3045. doi: 10.3390/ijms20123045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezzi, S., Martin, F. P., Alonso, C., Guilarte, M., Vicario, M., Ramos, L., … Kochhar, S. (2009). Metabotyping of biofluids reveals stress-based differences in gut permeability in healthy individuals. Journal of Proteome Research, 8(10), 4799–4809. doi: 10.1021/pr900525w [DOI] [PubMed] [Google Scholar]
- Rothenhäusler, H. B., Stepan, A., Kreiner, B., Baranyi, A., & Kapfhammer, H. P. (2008). Patterns of psychiatric consultation in an Austrian tertiary care center - results of a systematic analysis of 3,307 referrals over 2 years. Psychiatria Danubina, 20(3), 301–309. [PubMed] [Google Scholar]
- Tang, W. H., Wang, Z., Levison, B. S., Koeth, R. A., Britt, E. B., Fu, X., … Hazen, S. L. (2013). Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. The New England Journal of Medicine, 368(17), 1575–1584. doi: 10.1056/NEJMoa1109400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ufnal, M., Zadlo, A., & Ostaszewski, R. (2015). TMAO: A small molecule of great expectations. Nutrition, 31(11–12), 1317–1323. doi: 10.1016/j.nut.2015.05.006 [DOI] [PubMed] [Google Scholar]
- Weathers, F. W., Bovin, M. J., Lee, D. J., Sloan, D. M., Schnurr, P. P., Kaloupek, D. G., … Marx, B. P. (2018). The clinician-administered PTSD scale for DSM-5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychological Assessment, 30(3), 383–395. doi: 10.1037/pas0000486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Z. X., Li, S. F., Chen, H., Song, J. X., Gao, Y. F., Zhang, F., & Cao, C. F. (2017). The changes of gut microbiota after acute myocardial infarction in rats. PloS One, 12(7), e0180717. doi: 10.1371/journal.pone.0180717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, P., Wang, Y., Chen, L., Yang, D., Meng, H., Zhou, D., … Xie, P. (2013). Identification and validation of urinary metabolite biomarkers for major depressive disorder. Molecular & Cellular Proteomics: MCP, 12(1), 207–214. doi: 10.1074/mcp.M112.021816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, W., Gregory, J. C., Org, E., Buffa, J. A., Gupta, N., Wang, Z., … Hazen, S. L. (2016). Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell, 165(1), 111–124. doi: 10.1016/j.cell.2016.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman, M., Martinez, J. H., Young, D., Chelminski, I., & Dalrymple, K. (2013). Severity classification on the Hamilton depression rating scale. Journal of Affective Disorders, 150(2), 384–388. doi: 10.1016/j.jad.2013.04.028 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
To protect the confidentiality of patient information, the Medical University of Graz IRB will not allow the authors to make data publicly available. Data are available upon request from Andreas Baranyi at the Department of Psychiatry and Psychotherapeutic Medicine, Medical University of Graz, Graz, Austria, for researchers who meet the criteria for access to confidential data.


