See the article by Adolph et al. in this issue, pp. 1012–1023.
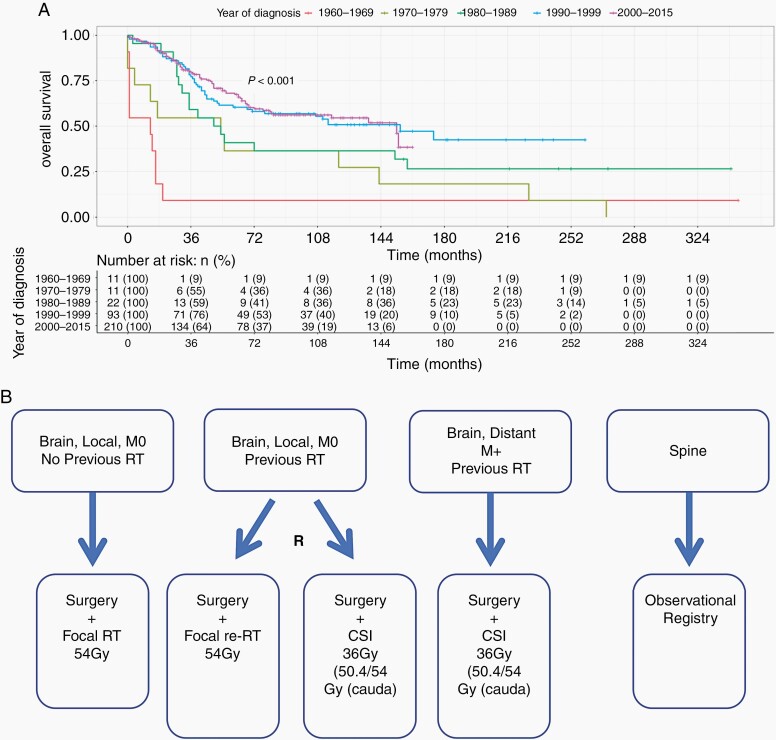
Primary therapies for ependymoma are insufficient for the prevention of tumor recurrence or progression in nearly half of cases.1,2 The majority of recurrences happen within 2 years of diagnosis, however, some occur many years later, emphasizing the importance of reporting long-term follow-up. Moreover, whilst some children die quickly following recurrence, others experience a chronically relapsing course. These multiple recurrences result in extensive morbidity accrued through increasingly debilitating, but ultimately futile, treatments.1–3 International clinical trials at recurrence are overdue and despite the known propensity to recurrence, there has been little success in reaching consensus on the best approach. Additionally, there have been no recent novel treatments resulting in improved outcomes. Whilst the UK experience indicates that survival from primary childhood ependymoma has improved over the last half-century (Figure 1A), this is most likely due to a focus on achieving complete tumor resection, improved state-of-the-art radiotherapy techniques (N. Thorp, Personal Communication) and supportive therapies. Recurrence remains associated with dismal outcomes.
Fig. 1.
(A) Improvement in survival of childhood ependymoma from 1960 to 2015 in the UK. Based on 347 retrospectively analyzed UK cases—unpublished data associated with the Ritzmann et al.’s 2020 study.2 (B) Proposed structure for a relapsed ependymoma clinical trial allowing the comparison of craniospinal and focal radiotherapy. Abbreviations: CSI, craniospinal irradiation; M0, no metastatic disease; RT, radiotherapy.
Presently, we do not have adequate strategies for identifying and treating such children. Promising innovations, such as CAR-T cell therapy, are on the horizon4 but these are as yet unproven in clinical practice, potentially costly, and only available in limited centers.
The bleak picture of therapy contrasts with the burgeoning knowledge of ependymoma biology. International, collaborative studies have identified distinct subgroups, associated with varied clinical behavior and risk profiles.5,6 Others have better delineated the tumor microenvironment in the hope of identifying new therapeutic avenues.7
There is preliminary evidence that adjuvant chemotherapy in addition to complete surgical resection (CR) and quality-assured radiotherapy confers a significant survival benefit for primary disease (A. Smith, personal communication), however, unscientific prejudgment on the role of chemotherapy prevented the COG9031 study from reaching a clear outcome. Extent of surgical resection also appears to be important in improving outcomes at recurrence; this finding being replicated in multiple studies.1–3 Additionally, there is evidence that reirradiation at relapse may be beneficial, but recent data suggest this is only short term2,8,9 whilst the likely side effects of significant doses of radiotherapy are yet to be fully quantified.
In the linked study,10 Adolph et al. provide valuable data on 53 children and adolescents with relapsed ependymoma. Participants received two courses of oral temozolomide, followed by oral etoposide and trofosfamide for further progression. This was accompanied by standard approaches targeting CR and consideration of first or repeated radiotherapy. The authors concluded that CR of relapsed disease was the only factor associated with longer-term survival. The results for oral chemotherapy with temozolomide were disappointing, with 85.7% of evaluable patients showing disease progression. Radiotherapy, either first- or reirradiation, showed some benefit in children who did not undergo, or achieved only subtotal, resection. There was no clear evidence that radiotherapy benefitted those with CR in the longer term.
Whilst providing valuable data, the work by Adolph and colleagues highlights the challenges faced by the neuro-oncology community in interpreting mixed datasets to develop evidence-based prospective approaches for the treatment of recurrent disease. Most studies of ependymoma relapse have been heterogeneous with respect to patient composition due to the inclusion of retrospective cohorts of children treated on and off clinical trials with mixed tumor locations and molecular subgroups.2,3,8 Additionally, by the time of recurrence, children will have received multiple non-standardized interventions. Approaches may then vary further based on local treatment decisions and patient factors. This has made understanding the true impact of approaches to treatment at recurrence challenging. Radiotherapy seems to provide benefit in some cases and CR seems to be an important factor for overall survival in some cohorts10 but not others.2 However, this study highlights that CR at recurrence (nor at primary presentation) is not a panacea, and many children with CR still go on to experience further relapse.
Further data are needed on radiotherapy outcomes at recurrence. The linked study asks important questions, but in view of the trial design, the radiotherapy results were difficult to interpret. Participants received a mixture of focal and craniospinal irradiation (CSI) at recurrence, in addition to a mixture of first- and reirradiation, with small numbers in each cohort. This was as a result of an open trial design in which local centers primarily determined the radiotherapy approach. Ideally, randomized trials of radiotherapy at recurrence are needed to understand and quantify the relative benefits of focal radiotherapy and CSI alongside first or reirradiation, in greater depth.
Additionally, the identification of multiple ependymoma subgroups adds complexity. Almost all ependymoma recurrences are of the same subtype as the primary tumor,2,5 however, it is not clear whether the different subtypes need different approaches at recurrence to improve outcomes. In the linked study, all the patients with RELA ependymoma died. The authors suggest that whilst PFA is associated with poorer outcomes based on primary disease, RELA tumors may be associated with worse outcomes at recurrence. This is an intriguing question based on limited data and large prospective, molecularly stratified studies of ependymoma relapse are imperative to explore this further.
Although not yet proven at the biological level, we hypothesize that some ependymomas are destined to recur and argue on the basis of the linked study, and others, that attention should now focus on three factors to move toward better understanding of the underlying biology and thereby outcomes for relapsed ependymoma. A promising line of enquiry is defining whether patients are in remission based on biomarkers mirroring the definition of minimal residual disease (MRD) assessment as in Acute Lymphoblastic Leukaemia. For those not in molecular remission the role of ‘continuation therapy’ could be considered.
(1) The initiation of standardized international clinical trials to increase reproducibility and reduce the heterogeneity of studies of relapsed ependymoma; here we provide a proposal for the structure of such a trial, interrogating the efficacy of focal radiotherapy vs CSI in both radiotherapy naïve and previously irradiated patients (Figure 1B);
(2) Routine collection of matched tumor samples at primary and recurrence to better understand how ependymomas evolve over time, highlighting much needed novel therapeutic strategies at recurrence;
(3) Application of molecular stratification to recurrent ependymomas to understand differences in the behavior of subgroups at recurrence.
Only through focussed, international efforts will we improve the morbidity and survival for children with this devastating disease.
Acknowledgments
The text is the sole product of the authors and no third party had input or gave support to its writing.
Conflict of interest statement. The authors have no conflicts of interest to declare.
References
- 1. Marinoff AE, Ma C, Guo D, et al. Rethinking childhood ependymoma: a retrospective, multi-center analysis reveals poor long-term overall survival. J Neurooncol. 2017;135(1):201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ritzmann TA, Rogers HA, Paine SML, et al. A retrospective analysis of recurrent pediatric ependymoma reveals extremely poor survival and ineffectiveness of current treatments across central nervous system locations and molecular subgroups. Pediatr Blood Cancer. 2020;67(9):e28426. [DOI] [PubMed] [Google Scholar]
- 3. Zacharoulis S, Ashley S, Moreno L, Gentet JC, Massimino M, Frappaz D. Treatment and outcome of children with relapsed ependymoma: a multi-institutional retrospective analysis. Childs Nerv Syst. 2010;26(7):905–911. [DOI] [PubMed] [Google Scholar]
- 4. Donovan LK, Delaidelli A, Joseph SK, et al. Locoregional delivery of CAR T cells to the cerebrospinal fluid for treatment of metastatic medulloblastoma and ependymoma. Nat Med. 2020;26(5):720–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pajtler KW, Witt H, Sill M, et al. Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell. 2015;27(5):728–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pajtler KW, Wen J, Sill M, et al. Molecular heterogeneity and CXorf67 alterations in posterior fossa group A (PFA) ependymomas. Acta Neuropathol. 2018;136(2):211–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gillen AE, Riemondy KA, Amani V, et al. Single-cell RNA sequencing of childhood ependymoma reveals neoplastic cell subpopulations that impact molecular classification and etiology. Cell Rep. 2020;32(6):108023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Merchant TE, Boop FA, Kun LE, Sanford RA. A retrospective study of surgery and reirradiation for recurrent ependymoma. Int J Radiat Oncol Biol Phys. 2008;71(1):87–97. [DOI] [PubMed] [Google Scholar]
- 9. Tsang DS, Burghen E, Klimo P Jr, Boop FA, Ellison DW, Merchant TE. Outcomes after reirradiation for recurrent pediatric intracranial ependymoma. Int J Radiat Oncol Biol Phys. 2018;100(2):507–515. [DOI] [PubMed] [Google Scholar]
- 10. Adolph JE, Fleischhack G, Mikasch R, et al. Local and systemic therapy of recurrent ependymoma in children and adolescents: short- and long-term results of the E-HIT-REZ 2005 study [published online ahead of print December 17, 2020]. Neuro Oncol. doi: 10.1093/neuonc/noaa276. [DOI] [PMC free article] [PubMed] [Google Scholar]