See the article by Patil et al. in this issue, pp. 990–998.
Brainstem high-grade gliomas (HCG) account for approximately 10-15% of childhood CNS tumors.1 However, due to their invasion to eloquent structures, histological confirmation has been routinely avoided, and clinical and radiological characteristics have been used for diagnosis. Recent molecular studies unveiled a significant biological heterogeneity among them and demonstrated the prognostic significance of different driver mutations in this population2 thus, explaining the prolonged survival reported in of some patients3 in an otherwise lethal disease.
In this issue of Neuro-Oncology, Patil et al.4 provide an overview of the epidemiological landscape of children and adolescents diagnosed with brainstem gliomas in the United States using a large population-based cohort assembled using the Central Brain Tumor Registry of the United States (CBTRUS) and NCI Surveillance, Epidemiology and End Results (SEER) cancer registry over a 17-year period.
In a laudable effort, the authors provide comprehensive epidemiological characterization powered by the large number of patients. A total of 4486 patients were included in this study, making it the largest cohort of children and adolescents with brainstem gliomas to date. The overall age-adjusted incidence rate (AAIR) was estimated to be 0.305 per 100 000 population and identified a higher incidence in white children (0.23) and children between the ages of 0-4 and 5-9 years (0.38 and 0.5 × 100 000, respectively). The incidence rates are equivalent to smaller cohorts previously reported in the United States5 although it compares slightly higher than a recent report from the Canadian Pediatric Brain Tumor Consortium (CPBTC) that only included patients with diffuse intrinsic pontine glioma (DIPG) after careful central radiological review. Suggesting the inclusion of non-DIPG in the present study may account for the slightly higher incidence rate.6
This study highlights the historical scarcity of biological samples in brainstem gliomas and its contribution to our poor understanding of the molecular underpinnings and the paucity of accurate epidemiological estimates of this entity. In this study, 70% of the study population lacked histological confirmation,4 leading to the inevitable inclusion of other pathologies and therefore encumbering the interpretation of the results. Furthermore, this study provides an impetus to establish stereotactic biopsies and tissue diagnosis as the standard of care for proper classification and treatment of brainstem gliomas.
Accounting for 80% of childhood brainstem tumors, malignant midline gliomas frequently harbor hotspot point mutations in the histone variants H3.1 and H3.32 and remain a disheartening fatal diagnosis. Recent studies demonstrated that brainstem gliomas encompass different entities with distinct oncogenic mechanisms, clinical characteristics, and disparate outcomes.7 Due to the inability to account for the biological heterogeneity of brainstem HCG in the present study, the survival outcomes may seem overestimated and require careful interpretation.
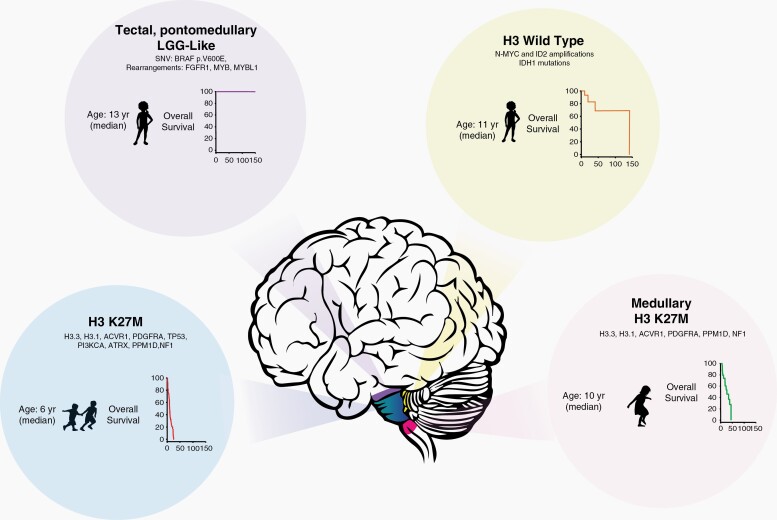
Another important confounder of this population-based analysis is the need to rely on the International Classification of Diseases for Oncology, third edition (ICD-O-3) coding, to identify the study subjects. In combination with the absence of histological confirmation, the probable inclusion of low-grade gliomas located in the brainstem may explain the unexpectedly high survival of the entire cohort and particularly in the subgroup of patients diagnosed by radiological criteria. In contrast, the survival of patients with histologically proven HGG is akin to the results as previously described in other registry studies3 and prospective clinical trials.8 Thus, the survival presented illustrates the summation of wide spectrum of diseases as depicted in Figure 1.
Fig. 1.
Schema illustrating some of the different entities among brainstem gliomas, clinical characteristics, most frequent alterations, and overall survival outcomes.
Notably, the identification of age as a prognostic factor is presumably a surrogate for biology as the majority of patients with histone mutated tumors present between 1-9 years of age. Furthermore, the superb survival reported in children younger than 1 year could represent the outcome of low-grade gliomas in which higher mitotic figures are frequently observed and often histologically “upgraded” 9 or the unique biological behavior of infantile gliomas.10
The true epidemiology of brainstem gliomas remains to be elucidated and will require the centralized selection of cases and the incorporation of biological markers for accurate estimation. Disease-specific registries such as the International Diffuse Intrinsic Glioma Registry (IDPGR) (https://dipgregistry.org/) and the European Society for Pediatric Oncology (SIOPE) DIPG network registry (https://www.dipgregistry.eu/) have modeled the acquisition and curation of clinical, radiological, and biological data, and aim to improving the veracity of the information collected and providing reliable estimations. Notwithstanding, the retrospective nature of these registries has its own set of limitations.
Patil et al.4 lay a foundation with this remarkable study and highlight the overwhelming importance of incorporating biological data into population-based dataset for proper disease characterization as we evolve into a molecularly driven classification of maladies.
Acknowledgments
The text is the sole product of the authors and no third party had input or gave support to its writing.
Funding
AF is supported by the WE LOVE YOU CONNIE FOUNDATION.
References
- 1. Ostrom QT, Cioffi G, Gittleman H, et al. CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 2019;21(Supplement_5):v1–v100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schwartzentruber J, Korshunov A, Liu XY, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012; 482(7384):226–231. [DOI] [PubMed] [Google Scholar]
- 3. Hoffman LM, Veldhuijzen van Zanten SEM, Colditz N, et al. Clinical, radiologic, pathologic, and molecular characteristics of long-term survivors of diffuse intrinsic pontine glioma (DIPG): a collaborative report from the International and European Society for Pediatric Oncology DIPG registries. J Clin Oncol. 2018;36(19):1963–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patil N, Kelly ME, Yeboa DN, et al. Epidemiology of brainstem high-grade gliomas in children and adolescents in the United States, 2000–2017 [published online ahead of print December 21, 2020]. Neuro Oncol. doi: 10.1093/neuonc/noaa295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang AS, Ostrom QT, Kruchko C, Rogers L, Peereboom DM, Barnholtz-Sloan JS. Complete prevalence of malignant primary brain tumors registry data in the United States compared with other common cancers, 2010. Neuro Oncol. 2017;19(5):726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fonseca A, Afzal S, Bowes L, et al. Pontine gliomas a 10-year population-based study: a report from The Canadian Paediatric Brain Tumour Consortium (CPBTC). J Neurooncol. 2020;149(1):45–54. [DOI] [PubMed] [Google Scholar]
- 7. Chen LH, Pan C, Diplas BH, et al. The integrated genomic and epigenomic landscape of brainstem glioma. Nat Commun. 2020;11(1):3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hargrave D, Bartels U, Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol. 2006;7(3):241–248. [DOI] [PubMed] [Google Scholar]
- 9. Fouladi M, Hunt DL, Pollack IF, et al. Outcome of children with centrally reviewed low-grade gliomas treated with chemotherapy with or without radiotherapy on Children’s Cancer Group high-grade glioma study CCG-945. Cancer. 2003;98(6):1243–1252. [DOI] [PubMed] [Google Scholar]
- 10. Ryall S, Zapotocky M, Fukuoka K, et al. Integrated molecular and clinical analysis of 1,000 pediatric low-grade gliomas. Cancer Cell. 2020;37(4):569–583.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]