Abstract
The management of patients with glioma usually requires multimodality treatment including surgery, radiotherapy, and systemic therapy. Accurate neuroimaging plays a central role for radiotherapy planning and follow-up after radiotherapy completion. In order to maximize the radiation dose to the tumor and to minimize toxic effects on the surrounding brain parenchyma, reliable identification of tumor extent and target volume delineation is crucial. The use of positron emission tomography (PET) for radiotherapy planning and monitoring in gliomas has gained considerable interest over the last several years, but Class I data are not yet available. Furthermore, PET has been used after radiotherapy for response assessment and to distinguish tumor progression from pseudoprogression or radiation necrosis. Here, the Response Assessment in Neuro-Oncology (RANO) working group provides a summary of the literature and recommendations for the use of PET imaging for radiotherapy of patients with glioma based on published studies, constituting levels 1-3 evidence according to the Oxford Centre for Evidence-based Medicine.
Keywords: amino acid PET, FDG, glioblastoma, radiation injury, target volume
Radiotherapy is an indispensable component of glioma treatment.1,2 In recent years, substantial technological progress has improved the delivery of radiotherapy, primarily to modulate the therapeutic window in favor of reduced normal tissue complication probability. Techniques such as external beam fractionated stereotactic radiotherapy, radiosurgery, intensity-modulated radiotherapy (IMRT), image-guided radiotherapy, particle therapy, three-dimensional brachytherapy, and intraoperative radiotherapy allow the delivery of radiation with ever-increasing precision.3 Molecular (biologic) imaging, radiomics, and machine-learning approaches offer the potential to significantly influence clinical decision-making and treatment planning, which could help address whether we have reached a therapeutic ceiling or whether inadequate targeting is responsible for the perceived lack of clinical benefit from dose escalation beyond 60 Gy.3–5
Target volume delineation for radiotherapy planning is currently based on CT and MRI. The high spatial resolution of MR imaging allows for accurate anatomic definition. In general, the contrast-enhancing region on T1-weighted MRI and the signal abnormality on T2/FLAIR sequences are contoured as putative radiotherapy targets. During the last two decades, biological imaging methods demonstrated improved prognostic capability compared to standard anatomic MRI, with the potential for improving tumor delineation and treatment planning.5–7 In particular, beyond the standard, anatomically defined gross, clinical, and planning target volume (GTV, CTV, and PTV), the introduction of the biological tumor volume (BTV) based on biological imaging techniques3 could result in superior tumor coverage.
A promising method to investigate tumor biology is positron emission tomography (PET). A large variety of PET probes are able to non-invasively target various metabolic and molecular processes. Although research continues into a broad array of tracers, PET with radiolabeled amino acids has been validated as an important diagnostic tool in brain cancer.7–10 The overexpression of large neutral amino acid transporters in gliomas11 as well as in brain metastases12 compared to normal brain make these tumors a prime indication for amino acid PET imaging.
In this review, the PET/RANO (Response Assessment in Neuro-Oncology) working group summarizes the available literature and provides evidence-based recommendations for the use of PET imaging for radiotherapy of glioma patients.
Search Strategy, Selection Criteria, and Levels of Validation
A detailed description of search strategy, selection criteria, and levels of validation of the published literature is presented in the Supplemental Material.
Overview on PET Tracers
The PET tracer most commonly used in oncological diagnostics is [18F]-2-fluoro-2-deoxy-d-glucose (FDG). In the brain, the high glucose metabolism decreases the precision of tumor delineation; therefore, the value of FDG PET in radiotherapy planning is severely limited. In contrast to FDG, radiolabeled amino acids exhibit low uptake in normal brain, permitting brain tumor visualization with a high tumor-to-background signal. Commonly used amino acid tracers are [11C-methyl]-l-methionine (MET), O-(2-[18F]-fluoroethyl)-l-tyrosine (FET), 3,4-dihydroxy-6-[18F]-fluoro-l-phenylalanine (FDOPA), α-[11C]-methyl-l-tryptophan (AMT), or anti-1-amino-3-[18F]fluorocyclobutane-1-carboxylic acid (FACBC or fluciclovine).9 An important feature of these tracers is their ability to cross the intact blood-brain barrier via the transport system L for large neutral amino acids, allowing for visualization of tumor extent beyond contrast enhancement on MRI. This makes these tracers particularly suitable for radiotherapy planning. In contrast to amino acid tracers and FDG, the proliferation marker 3′-deoxy-3′-[18F]fluorothymidine (FLT) is not adequately able to pass the intact blood-brain barrier and usually accumulates only in portions of the tumor where the blood-brain barrier has already been disrupted, a region very similar to that observed on contrast enhancement on MRI.13 Similarly, [11C]choline or [18F]fluorocholine as markers of cell membrane phospholipids in brain tumors can only detect tumor in disrupted blood-brain barrier areas and are therefore less suitable for the delineation of tumor extent.9 An important approach is to investigate intratumoral hypoxia using PET tracers such as [18F]fluoromisonidazole (FMISO).14 This could be useful for volume-selective tumor dose-intensification, aiming to deliver higher radiation dose to hypoxic subvolumes in order to overcome hypoxia-induced radioresistance.15 Another promising target for brain tumor imaging is the mitochondrial translocator protein (TSPO), which is strongly expressed in gliomas.16 Accumulation of TSPO ligands might extend beyond the tumor margins on amino acid PET and indicate an infiltration zone with activated microglia showing further tumor spread.17 However, the importance of this method for radiotherapy planning has not yet been established.
Applications of PET Imaging for Radiotherapy
Target Delineation
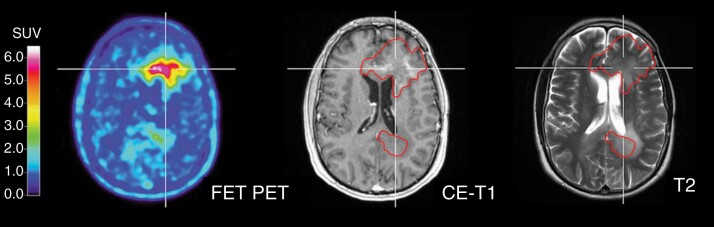
Conventional MRI sequences are limited in their ability to differentiate between edema, non-enhancing tumor and infiltrating, enhancing tumor in gliomas, and inadequately assessing tumor margins in non-enhancing gliomas. For PET, a number of studies have correlated histological findings with amino acid accumulation and provide evidence that amino acids detect the solid mass of gliomas and metabolically active tumor more reliably than conventional MRI.18–23 Therefore, amino acid PET is a highly valuable tool for target delineation. A previous PET/RANO report7 proposed that the delineation of the BTV using amino acid PET might more accurately disclose the true tumor volume beyond that visualized by conventional MRI (Figure 1). These biologically active tumor subvolumes could allow for adequate treatment and/or boosting of the high-risk tumor subregions.7 Conventional FDG PET, with its poor tumor-to-background contrast and high glucose utilization within the healthy brain parenchyma, is inadequate for these purposes.24
Fig. 1.
Patient with a multifocal IDH-wild-type glioblastoma. The extent of increased FET uptake based on a tumor-to-background threshold of >1.6 (left image; red contour transferred onto MR images) is considerably larger than the contrast enhancement (middle image) and the extent of the signal hyperintensity on the T2-weighted MR image (right image). Abbreviations: FET, O-(2-[18F]-fluoroethyl)-l-tyrosine; IDH, isocitrate dehydrogenase.
MET PET
Several studies compared MR and MET PET images for target volume delineation25–27 and reported that in the majority of glioma patients the region of MET uptake was larger than that of the contrast enhancement implying the possibility that biologically active disease might extend considerably beyond the visualized enhancement on MRI. Additionally, various studies suggested that pre-radiotherapy MET PET could identify areas at highest risk for glioma recurrence following radiotherapy.28–30 Moreover, the higher sensitivity and specificity of MET for neoplastic tissue has been demonstrated in imaging studies including histological confirmation.18,31
The actual clinical value of such information can be assessed once radiotherapy trials are performed wherein such areas are selectively dose-intensified and local relapse within them is consequentially eliminated.
FET PET
A prospective trial reported significant discordance in size and location between contrast enhancement on MRI and FET PET.32 In more than 30% of cases, FET uptake extended at least 20 mm beyond the margin of contrast enhancement. A subsequent study reported that FET PET-based BTVs were significantly larger than corresponding GTVs based on contrast-enhanced MRI.33 In more than half of the patients, there were major volumetric discordances. More recent studies confirmed these observations.34,35 Moreover, another study reported that the spatial congruence of MRI and FET PET for the identification of glioma GTVs was poor (mean uniformity index, 0.39).36 Alarmingly, MRI-based PTVs missed 17% of FET PET-based GTVs. Accordingly, a low spatial similarity between contrast-enhanced MRI volumes and FET PET-based BTVs has also been described.35 A more recent prospective trial in patients with WHO grade III or IV glioma reported that in approximately 90% of patients that the FET PET-positive volume would be included within a CTV based on contrast-enhanced MRI with a 20-mm margin.37
Similarly, in non-enhancing gliomas volumetric analyses showed that CTVs defined on MRI were significantly smaller than BTVs based on FET PET.38
Regarding the comparability of FET with other amino acid PET tracers such as MET in terms of GTV delineation, 29 glioma patients were prospectively evaluated.39 This study suggested that the GTV delineation can be enabled using MET and FET PET with a high likelihood of correlation, indicating that MET PET and FET PET yield comparable target volumes. Similar to MET PET, the higher sensitivity and specificity of radiolabeled amino acid FET for tumor has also been demonstrated in several studies including validation of imaging findings by histology.19,22,23,40,41
Therefore, the consistent findings in the FET PET and the MET PET imaging trials are that the BTVs are larger than the contrast-enhanced MR GTVs, and that once the MR GTVs are expanded by an approximate 20 mm CTV margin, almost 90% of BTVs are subsumed within these CTVs. Thus, BTVs could well represent both the true extent of the volume and target at greatest risk of relapse and could therefore be considered as a testable hypothesis in a randomized trial. For example, a recent study reported that a 1.5 cm margin on FET PET-based BTV and MR-based GTV yielded equivalent results according to recurrence patterns compared to classical 2 cm margins while significantly reducing dose exposure to healthy brain parenchyma.42 Secondly, a recent study demonstrated improved survival in glioblastoma when the resection was extended beyond the area of enhancement into the T2/FLAIR abnormality,43 laying the background for not restricting surgical and radiotherapy target contours to only the contrast-enhanced portion identified on MRI. On the other hand, larger lesions, especially those adjacent to eloquent cortex or critical white matter pathways, should be evaluated with caution. The potential for acute toxicity associated with radiotherapy increases substantially for larger lesions.44
FDOPA PET
Similar to MET PET and FET PET, initial studies have also suggested that in glioma patients radiotherapy target volumes delineated by FDOPA are larger than the extent of contrast enhancement on MRI.20,45 In comparison to MET and FET, FDOPA seems to be comparable in terms of delineation of tumor extent.46,47
The most frequently used radiolabeled amino acids MET, FET, and FDOPA may improve the delineation of radiotherapy target volumes beyond conventional MRI and identify additional tumor parts that should be targeted by irradiation (evidence level 2).
The Prognostic Value of PET Prior to Radiotherapy
The potential of PET as a prognostic biomarker has been evaluated in several studies. Static amino acid PET parameters such as the postoperative BTV as a measure of the active tumor burden are prognostic in patients with newly diagnosed or relapsed glioblastoma. In particular, a smaller FET PET BTV appears to be a favorable prognostic imaging biomarker for progression-free and overall survival in multivariate analyses, independent of O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation status, clinical performance status, and age.34,48 Additionally, dynamic FET PET parameters (eg, time-to-peak values) prior to re-irradiation also seem to carry prognostic value.49,50 A recent randomized phase II trial failed to demonstrate prolongation of overall survival from the addition of re-irradiation to bevacizumab.51 This trial utilized conventional contrast-enhanced MR imaging for contouring and whether BTV-driven re-irradiation could prolong overall survival remains an unanswered question.
Similar to amino acid PET, in patients with newly diagnosed WHO grade III or IV glioma, increased metabolic activity on FDG PET prior to radiotherapy has also been significantly associated with worse outcome.52,53 Increased metabolic activity on FDG PET after completion of first-line radiotherapy also portends an unfavorable outcome.54,55
Both the BTV derived from static amino acid PET and the dynamic analysis of FET uptake provide helpful prognostic information in glioblastoma patients prior to radiotherapy. Similar to amino acid PET, FDG PET provides also valuable prognostic information (evidence level 2).
PET-Based Radiotherapy in Patients With Newly Diagnosed Glioma
The concept of “dose-painting” radiotherapy, in which heterogeneous delivery of radiation to a target volume is defined by functional or molecular imaging, has been tested in newly diagnosed glioma patients. In a prospective phase II trial, 22 glioblastoma patients received postoperative temozolomide chemoradiation using integrated boost IMRT with FET PET-adapted local dose escalation.56 Overall survival of the entire cohort was 14.8 months, and the progression-free survival (PFS) was 7.8 months, neither of which provided a signal of improved local control. Acute and late toxicity were not increased indicating that dose escalation in glioblastoma patients beyond 60 Gy is feasible.56 This was confirmed by a more recent FDOPA PET study.57 A larger, single-institution study using FDOPA PET for tumor targeting with dose-escalated radiotherapy in newly diagnosed glioblastoma is ongoing.58 For MET PET, prospective studies reported that radiation dose escalation to metabolically hyperactive foci in newly diagnosed glioblastoma patients is feasible and safe, with a median overall survival of 20 months.29,59,60
PET-based dose painting in newly diagnosed glioma patients seems to be safe, but only preliminary evidence for a potential benefit has been presented (evidence level 3).
PET-Based Re-Irradiation in Patients With Relapsed Glioma
Improved understanding of the tolerance of the brain and its various substructures to irradiation,61 the availability of effective treatment options for symptomatic radiation necrosis,62 and the substantial advances in radiation technology and neuroimaging,5,9 and the modest activity of current systemic therapies63 have led to growing consideration of re-irradiation of patients with relapsed glioma.64,65 Target volume definition in these patients is an essential step for radiotherapy planning. However, there are two main problems: (i) the differentiation between relapsed tumor and treatment-related changes such as pseudoprogression or radiation necrosis and (ii) the precise delineation of tumor extent in order to minimize irradiation of healthy brain.
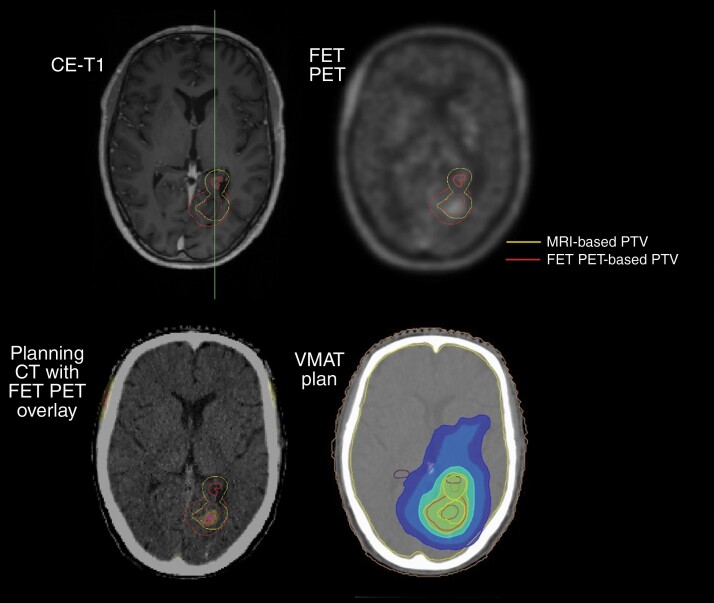
Generally, the target volume for re-irradiation of glioma relapse is based on conventional MRI. However, the higher sensitivity and specificity of the radiolabeled amino acids FET and MET for neoplastic tissue has been demonstrated in several imaging studies including histological confirmation.19,22,40,66 A small number of single-center clinical trials have utilized MET PET or FET PET for target volume delineation for the planning of stereotactic radiotherapy,67 IMRT,68 or particle radiotherapy69 in patients with recurrent glioma (Figure 2). Importantly, a small prospective trial suggested that MET PET-based re-irradiation may lead to improved survival compared with radiotherapy planning based on conventional MRI.67 Currently, a multicenter phase II trial (GLIAA, NOA-10/ARO 2013-1) is seeking to evaluate whether re-irradiation planning using FET PET improves clinical outcome in patients with recurrent glioblastoma compared to contrast-enhanced MRI.70
Fig. 2.
Patient with a progressive IDH-wild-type glioblastoma 12 months after first-line chemoradiation with temozolomide (2.0/1.8 Gy × 30 using the radiation technique volumetric modulated arc therapy (VMAT); 60 Gy to the metabolically active occipital lesion, and 54 Gy to the non-enhancing T2 hyperintense parahippocampal lesion). After neuropathological confirmation of multifocal progression using stereotactic biopsy, FET PET was used to define the re-irradiation target volume (3.0 Gy × 13; FET PET-based PTV in red). Importantly, the re-irradiation target volume based on conventional MRI (MRI-based PTV in yellow) is considerably smaller. VMAT plan (bottom right) with 37.05 Gy (green), 31.2 Gy (light blue), 20 Gy (blue), and 15 Gy (dark blue) isodose lines. Abbreviations: FET, O-(2-[18F]-fluoroethyl)-l-tyrosine; IDH, isocitrate dehydrogenase; PET, positron emission tomography.
The majority of studies evaluating the impact of PET for re-irradiation in patients with relapsed glioma have compared conventional MRI sequences with PET images. A question remains whether advanced MRI has sufficient sensitivity and specificity for evaluating glioma extent compared to amino acid PET and if it can be also used for target volume delineation. For example, apparent diffusion coefficient values calculated from diffusion-weighted MRI overlapped only partially with FET PET and contrast-enhanced MRI.71 Nevertheless, the impact of advanced MRI techniques in comparison to amino acid PET warrants further investigation.
Up to now, there is no clear evidence for a potential benefit of PET-based radiotherapy in patients with recurrent glioma (evidence level 3). Randomized trials are required (and ongoing) to address this question.
Assessment of Response to Radiotherapy
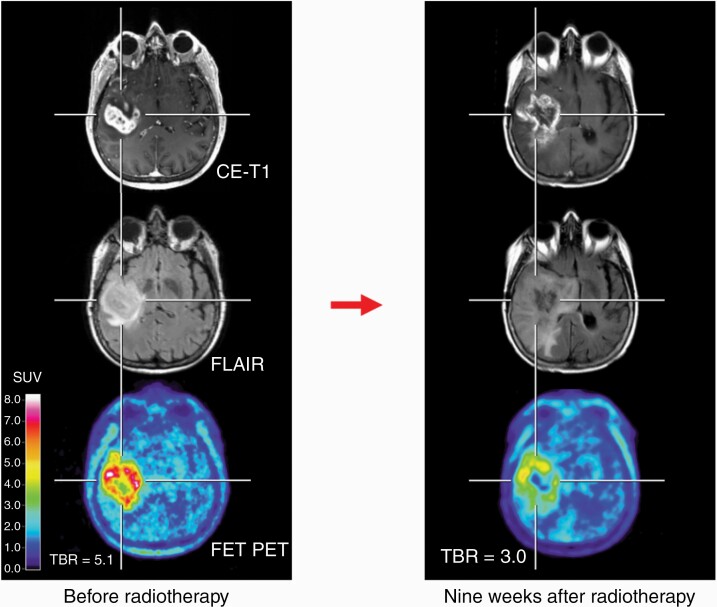
In glioma patients, changes in the size or extent of contrast enhancement on T1-weighted MRI are frequently used for response assessment.72 Clinical condition, corticosteroid use, and changes of T2- and/or FLAIR-weighted MR signal are also taken into consideration for response assessment.72,73 However, treatment-related effects, especially after radiotherapy, such as pseudoprogression or radiation necrosis, limit the reliability of conventional MRI for response assessment (Figure 3).
Fig. 3.
FET PET and conventional MR images of a 67-year-old patient with an IDH-wild-type glioblastoma with methylated MGMT promoter before radiotherapy plus of lomustine-temozolomide chemotherapy (left column). Nine weeks after radiotherapy, conventional MRI 9 suggests tumor progression (right column). In contrast, follow-up FET PET shows a substantial decrease of metabolic activity compared to the baseline scan and is consistent with pseudoprogression. The maximum tumor/brain ratios (TBR) decreased from 5.1 to 3.0 (41%). Abbreviations: FET, O-(2-[18F]-fluoroethyl)-l-tyrosine; IDH, isocitrate dehydrogenase; PET, positron emission tomography.
Several studies reported only a limited value of early post-radiotherapy quantitative FDG PET changes for the assessment of response to radiotherapy, either alone or with concomitant temozolomide.74,75 On the other hand, FMISO PET combined with FDG PET provides information on aerobic and anaerobic glycolysis that might be helpful to assess response to radiotherapy.76
Early changes of tumor-to-brain FET uptake ratios following chemoradiation with temozolomide in newly diagnosed glioblastoma patients have been shown to be a strong predictor for progression-free and overall survival.77,78 In contrast, changes in the volume of contrast enhancement on MRI were not associated with survival.
More recently, Wang and colleagues used MET PET in 18 glioblastoma patients for response assessment to standard chemoradiation with temozolomide.79 Four weeks after completing chemoradiation, MGMT promotor methylated tumors showed significantly greater reductions of static PET parameters (eg, tumor/brain ratios). However, in that study, these parameters were not correlated with survival. In contrast, a more recent study demonstrated that among 37 newly diagnosed glioblastoma patients treated in a prospective phase II dose-escalation study with correlative MET PET before and 3 months after chemoradiation that patients with complete metabolic response had superior PFS.80 On multivariate analysis, a larger metabolic tumor volume 3 months’ post-chemoradiation was significantly associated with worse PFS, whereas the contrast-enhancing tumor volume on MRI was not.
MET PET was also used in patients with WHO grade II glioma for assessing the response to radiotherapy.81 During long-term follow-up (median time, 33 months), stable or decreasing uptake of MET in the tumor area after radiotherapy compared to the MET PET scan obtained prior to radiotherapy seems to be a favorable sign for a stable clinical course.
Amino acid PET seems to provide valuable information for radiotherapy response assessment in glioma patients (evidence level 2).
Differentiation of Radiation Injury From Glioma Relapse
Following surgery, radiotherapy, or chemoradiation, neurooncologists are not infrequently confronted with findings on conventional MRI which can be either related to glioma progression or to treatment-related injury. Contrast-enhanced MRI is the cornerstone of brain imaging, but its specificity for the differentiation between blood-brain barrier disturbances related either to the treatment or to tumor progression is low, despite the excellent spatial resolution.9,82 In clinical routine, the most frequently observable imaging phenomena following radiation or chemoradiation are pseudoprogression and radiation necrosis.
The phenomenon of progressive, radiation-, or chemoradiation-induced, enhancing MRI abnormality in glioma patients, with spontaneous improvement without any treatment change, has been termed pseudoprogression.83 Pseudoprogression occurs typically within the first 12 weeks after radiotherapy completion,72,83 and this time-dependent definition has been incorporated into the criteria defined by the RANO group.72 In more detail, RANO criteria state that tumor progression should not be diagnosed radiographically earlier than 12 weeks after completion of chemoradiation with temozolomide, unless new enhancement outside the radiotherapy field occurs or tumor progression has been neuropathologically confirmed. Notwithstanding, some pseudoprogression cases occurring later than 12 weeks have been observed,84 particularly after chemoradiation using temozolomide in combination with lomustine.85
On the other end of the spectrum of radiation injury is radiation necrosis, which is the most important type of delayed toxicity after radiotherapy. In contrast to pseudoprogression, radiation necrosis typically occurs more than 6 months after radiotherapy and can even occur up to several years later.86 The rate of radiation necrosis following focal radiotherapy may vary considerably (approximately 5%-25%) and depends on the irradiated volume, radiation dose, and fractionation scheme,87,88 as well as possibly also on concurrently applied therapies such as targeted therapy89,90 or immunotherapy using checkpoint inhibitors.91,92
FDG PET provides only moderate additional diagnostic information for distinguishing between relapse and radiation injury, especially due to low specificity.93,94 In contrast, FET66,84,95–99 or FDOPA PET94,100–102 studies have consistently suggested that this differentiation can be obtained with a high diagnostic accuracy between 80% and 90%. Importantly, parameters derived from dynamic FET PET acquisition may further increase diagnostic accuracy.95,98,99 The diagnostic accuracy of MET PET regarding this clinical question is approximately 75%,93,103 which is most probably related to the higher affinity of MET for inflammation.104 Initial PET studies using AMT or FACBC suggest that these tracers may be also of value for the differentiation of radiation injury from relapsing glioma.105,106
Follow-up serial FET PET imaging after 6 months and subsequent examinations following stereotactic brachytherapy using iodine-125 may also be helpful in differentiating between radiation injury and local glioma progression.107
In early and late stages after radiotherapy, amino acid PET is useful for the differentiation between local relapse of gliomas and radiation-induced changes with high sensitivity and specificity (evidence level 2).
Use of Artificial Intelligence for Radiotherapy
Over the last few years, the complexity of neuroimaging data generated in glioma patients and the resulting number of imaging parameters have substantially increased. Consequently, a timely and cost-effective evaluation of these data can be pursued by using methods from the field of artificial intelligence, especially machine-learning approaches and radiomics. Most of these methods have been applied to MRI, but PET data are also increasingly being integrated in this process.108
Tumor segmentation for radiotherapy based on artificial intelligence
For radiotherapy target volume definition, a fast and reliable tumor segmentation (ie, depicting the main tumor compartments such as the necrotic core, contrast-enhancing areas, non-enhancing tumor, and perifocal edema) is a crucial task. Currently, the best-performing segmentation tools rely on conventional MRI using artificial neural networks (especially U-Net type convolutional neural networks), to achieve considerably high similarity scores of approximately 90%.109 This automated approach has also been applied in FET PET, with slightly lower similarity scores of 82%.110
Differentiation of local relapse from radiation injury using artificial intelligence
FET PET radiomics has been used in patients with brain metastases for the differentiation of tumor relapse after radiosurgery from radiation necrosis.111 Importantly, the highest diagnostic accuracy was achieved by combining FET PET and contrast-enhanced MRI radiomics.111 Preliminary results suggest that FET PET radiomics is also of value for the detection of pseudoprogression following chemoradiation in glioblastoma patients.112
Prediction of glioma recurrence location after radiotherapy using artificial intelligence
Amino acid PET studies have reported only a small spatial overlap between initial radiotracer uptake used for radiotherapy planning and, subsequently, at recurrence.113,114 For example, a prospective FET PET study revealed that 63% of the recurrent tumor volume was located outside the initially PET-defined GTV.114 Nevertheless, these results have to be interpreted with caution because the recurrent tumor may distort the anatomy of affected brain regions. Consequently, advanced elastic registration algorithms or normalization to a standard brain template are necessary to evaluate the spatial relation to the initial target volumes.115
Furthermore, initial studies suggest that the integration of a machine-learning model for radiotherapy planning based on the combined use of MRI and FET PET can predict the location of the first recurrence with high accuracy and could therefore be helpful for personalized radiation dose escalation.116
Preliminary data suggest that valuable clinical information for tumor segmentation, the differentiation of actual tumor relapse from radiation injury, and the prediction of the glioma recurrence location can be derived from amino acid PET-based machine-learning methods and radiomics (evidence level 3).
Limitations and Conclusions
Although radiotherapy has been established as a standard of care that roughly doubles the survival of patients with WHO grade III or IV gliomas, extensive efforts at improving these results further through innovative fractionation regimens, dose escalation, or alternative radiation delivery techniques have failed to achieve this goal. What has been achieved, though, is a reduction of radiation-associated toxicity as a consequence of refined targeting of radiotherapy. It still is a matter of concern that failure in glioblastoma remains focal in more than 90% of all patients, demonstrating either an intrinsic limitation to the efficacy of conventional radiotherapy or inaccurate targeting. In this regard, dose-limiting factors and the heterogeneity of glioma subpopulations represent a major challenge. Nevertheless, adequately covering these tumor margins and securing that active tumor does not escape the radiotherapy target volume defined by MRI renders further efforts at better delineating target volumes reasonable. PET seems to be by far the most advanced technique that could hold the potential to detect tumor beyond what is achievable by conventional MRI (Table 1). The challenge of demonstrating that radiotherapy planning based on PET is superior to traditional planning either in the first-line or in the recurrent setting remains unresolved.
Table 1.
Diagnostic Value of Different Amino Acid Tracers Compared to MRI
| MET | FET | FDOPA | |
|---|---|---|---|
| Value of amino acid PET for radiotherapy target delineation | BTV larger than contrast enhancement in WHO grade III/IV gliomas, validation of imaging findings by histology18,31 | BTV larger than contrast enhancement in WHO grade III/IV gliomas, validation of imaging findings by histology19,22,23,40,41 | Preliminary studies suggest that BTV larger than contrast enhancement in WHO grade III/IV gliomas20,45 |
| FET seems to be comparable to MET39 | FDOPA seems to be comparable to MET and FET46,47 | ||
| Amino acid PET-based radiotherapy (“dose painting”) in patients with newly diagnosed glioma | Radiation dose escalation to metabolically hyperactive foci in newly diagnosed glioblastoma patients is feasible and safe, with a median OS of 20 months29,59,60 | FET PET-based radiotherapy in newly diagnosed glioblastoma is safe, but OS could not be prolonged56 | FDOPA PET-based radiotherapy in WHO grade III/IV gliomas is safe57 a larger FDOPA PET study for tumor targeting with dose-escalated radiotherapy in newly diagnosed glioblastoma is ongoing58 |
| Amino acid PET-based re-irradiation (“dose painting”) in patients with relapsed glioma | MET PET-based re-irradiation may lead to improved OS compared with radiotherapy planning based on conventional MRI67 | A prospective multicenter phase II trial is ongoing70 | n.a. |
| Use of amino acid PET for assessment of response to radiotherapy | In contrast to conventional MRI, MET PET parameter reduction post-radiotherapy was significantly associated with a longer PFS80,81 | Superior to conventional MRI; metabolic response to temozolomide chemoradiation predictive for OS77,78 | n.a. |
| Differentiation of glioma progression from radiation-induced changes | Higher accuracy than conventional MRI,93,103 but seems to be lower in comparison to FET103 | Higher accuracy than conventional MRI66,84,95–99 dynamic FET PET acquisition may further increase diagnostic accuracy95,98,99 | Higher accuracy than conventional MRI94,100–102 |
Abbreviations: BTV, biological tumor volume; FDOPA, 3,4-dihydroxy-6-[18F]-fluoro-l-phenylalanine; FET, O-(2-[18F]-fluoroethyl)-l-tyrosine; MET, [11C-methyl]-l-methionine; OS, overall survival; PFS, progression-free survival.
While molecular signatures of gliomas are increasingly utilized to select systemic therapies, radiotherapy has changed minimally over the last few decades. Radiation doses and treatment volumes are still largely independent of the increasingly complex biology and heterogeneity of individual gliomas. To date, hardly any predictive biomarker is available that can be used to predict the response of patients to radiotherapy. The increasing availability of advanced functional and molecular imaging such as PET and the potential to use artificial intelligence to better understand the data may help to spatially resolve the biological characteristics of gliomas, which could permit functionally guided dose painting (Table 2). Additionally, such imaging could provide predictive information for treatment response, which could allow for individually tailored therapies. The authors are aware that PET imaging is not available everywhere, mostly due to restrictions concerning reimbursement. However, the added knowledge and understanding of glioma biology provided within the framework of PET imaging in conjunction with radiotherapy might also help to define and evaluate surrogate parameters provided by refined MRI methods.
Table 2.
Summary of Recommendations
| Amino Acid PET (MET, FET, FDOPA) | FDG PET | Other PET Tracers | Oxford Level of Evidence | |
|---|---|---|---|---|
| Target delineation for radiotherapy planning | ++ | − | n.a. | 2 |
| Prognostic value of PET prior to radiotherapy | ++ | ++ | n.a. | 2 |
| PET-based radiotherapy in patients with newly diagnosed gliomas | (++) | n.a. | n.a. | 3 |
| PET-based re-irradiation in patients with glioma relapse | (++) | n.a. | n.a. | 3 |
| Assessment of response to radiotherapy | ++ | + | (++) | 2 |
| Differentiation of radiation injury from glioma relapse | ++ a | + | n.a. | 2 |
| Use of artificial intelligence for radiotherapy | (++) | n.a. | n.a. | 3 |
Abbreviations: FDG, [18F]-2-fluoro-2-deoxy-d-glucose; FDOPA, 3,4-dihydroxy-6-[18F]-fluoro-l-phenylalanine; FET, O-(2-[18F]-fluoroethyl)-l-tyrosine; MET, [11C-methyl]-l-methionine.
++ high diagnostic value; (++) high diagnostic value, but limited data available; + limited diagnostic accuracy; − not helpful; n.a. = only preliminary or no data available.
aIncreased accuracy when using dynamic FET PET.
A limitation for the widespread clinical use especially of amino acid PET for radiotherapy planning and monitoring in glioma patients remains the lack of general approval and reimbursement issues by national insurances. Nevertheless, considerable progress has been made in recent years. For example, the radiolabeled amino acid FET has been approved for brain tumor diagnostics in Switzerland and France. Additionally, the amino acid PET tracer FDOPA is also approved and available in several other European countries. In the United States, the amino acid FACBC has recently been granted orphan drug status for glioma imaging. Currently, the number of clinical studies with this tracer is still low, but further efforts are ongoing. Furthermore, in the United States, FDOPA is FDA-approved for Parkinson’s syndromes and offers the opportunity for off-label use of this tracer for brain tumor diagnostics. In order to convince health insurance to reimburse the costs, our recently published guideline may be of value.117 This guideline was developed in close collaboration between Society for Neuro-Oncology (SNO)/European Society of Neuro-Oncology (EANO) and both the American Society of Nuclear Medicine and Molecular Imaging (SNMMI) and the European Association of Nuclear Medicine (EANM).
In conclusion, in order to improve existing treatment paradigms and to develop novel approaches for the personalization of radiotherapy for gliomas, biological information regarding inter- and intra-individual glioma heterogeneity available through metabolic imaging could prove to be crucial. The biological and imaging characterization of individual gliomas may potentially enable personalized parametrization of mathematical models for tumor control and normal tissue complication probabilities, thereby evolving, evaluating, and benchmarking these models with the aim of implementation into the treatment planning process for biology-dependent dose painting.
Future Perspective
Recent literature suggests that newer PET ligands targeting the TSPO might help to distinguish glioma from activated microglia.118,119 This might be of importance in distinguishing radiation-induced changes as well as to identify prognostically relevant patterns of biological tumor heterogeneity.
The concept of theranostics is currently being evaluated in prostate cancer and meningioma.120 By substituting the radionuclide used for diagnostic PET such as [18F] with a therapeutic radioisotope, typically β-emitters like [177Lu] or [90Y], the same tracer can be used for delivery of radiotherapy. Appropriate combinations of highly tumor-specific ligands with either diagnostic or therapeutic isotopes could pave new avenues for both highly selective imaging and radiotherapy.121
Funding
None declared.
Conflict of interest statement. M.P.M. reports consulting with Mevion, Karyopharm, Tocagen, Blue Earth Diagnostics and is on the Board of Directors of Oncoceutics, with stock options. The other authors report no conflicts of interest related to the present work.
Supplementary Material
References
- 1. Weller M, van den Bent M, Tonn JC, et al. ; European Association for Neuro-Oncology (EANO) Task Force on Gliomas . European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18(6):e315–e329. [DOI] [PubMed] [Google Scholar]
- 2. Wen PY, Weller M, Lee EQ, et al. . Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22(8):1073–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Niyazi M, Brada M, Chalmers AJ, et al. . ESTRO-ACROP guideline “target delineation of glioblastomas”. Radiother Oncol. 2016;118(1): 35–42. [DOI] [PubMed] [Google Scholar]
- 4. Sahiner B, Pezeshk A, Hadjiiski LM, et al. . Deep learning in medical imaging and radiation therapy. Med Phys. 2019;46(1):e1–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kocher M, Ruge MI, Galldiks N, Lohmann P. Applications of radiomics and machine learning for radiotherapy of malignant brain tumors. Strahlenther Onkol. 2020;196(10):856–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Unterrainer M, Eze C, Ilhan H, et al. . Recent advances of PET imaging in clinical radiation oncology. Radiat Oncol. 2020;15(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Albert NL, Weller M, Suchorska B, et al. . Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016;18(9):1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Galldiks N, Albert NL, Sommerauer M, et al. . PET imaging in patients with meningioma - report of the RANO/PET Group. Neuro Oncol. 2017;19(12):1576–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Langen KJ, Galldiks N, Hattingen E, Shah NJ. Advances in neuro-oncology imaging. Nat Rev Neurol. 2017;13(5):279–289. [DOI] [PubMed] [Google Scholar]
- 10. Galldiks N, Langen KJ, Albert NL, et al. . PET imaging in patients with brain metastasis - report of the RANO/PET group. Neuro Oncol. 2019;21(5):585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wiriyasermkul P, Nagamori S, Tominaga H, et al. . Transport of 3-fluoro-l-α-methyl-tyrosine by tumor-upregulated L-type amino acid transporter 1: a cause of the tumor uptake in PET. J Nucl Med. 2012;53(8):1253–1261. [DOI] [PubMed] [Google Scholar]
- 12. Papin-Michault C, Bonnetaud C, Dufour M, et al. . Study of LAT1 expression in brain metastases: towards a better understanding of the results of positron emission tomography using amino acid tracers. PLoS One. 2016;11(6):e0157139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herholz K. Brain tumors: an update on clinical pet research in gliomas. Semin Nucl Med. 2017;47(1):5–17. [DOI] [PubMed] [Google Scholar]
- 14. Langen KJ, Eschmann SM. Correlative imaging of hypoxia and angiogenesis in oncology. J Nucl Med. 2008;49(4):515–516. [DOI] [PubMed] [Google Scholar]
- 15. Bekaert L, Valable S, Lechapt-Zalcman E, et al. . [18F]-FMISO PET study of hypoxia in gliomas before surgery: correlation with molecular markers of hypoxia and angiogenesis. Eur J Nucl Med Mol Imaging. 2017;44(8):1383–1392. [DOI] [PubMed] [Google Scholar]
- 16. Albert NL, Unterrainer M, Fleischmann DF, et al. . TSPO PET for glioma imaging using the novel ligand 18F-GE-180: first results in patients with glioblastoma. Eur J Nucl Med Mol Imaging. 2017;44(13):2230–2238. [DOI] [PubMed] [Google Scholar]
- 17. Unterrainer M, Fleischmann DF, Diekmann C, et al. . Comparison of 18F-GE-180 and dynamic 18F-FET PET in high grade glioma: a double-tracer pilot study. Eur J Nucl Med Mol Imaging. 2019;46(3):580–590. [DOI] [PubMed] [Google Scholar]
- 18. Kracht LW, Miletic H, Busch S, et al. . Delineation of brain tumor extent with [11C]l-methionine positron emission tomography: local comparison with stereotactic histopathology. Clin Cancer Res. 2004;10(21):7163–7170. [DOI] [PubMed] [Google Scholar]
- 19. Pauleit D, Floeth F, Hamacher K, et al. . O-(2-[18F]fluoroethyl)-l-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain. 2005;128(Pt 3):678–687. [DOI] [PubMed] [Google Scholar]
- 20. Pafundi DH, Laack NN, Youland RS, et al. . Biopsy validation of 18F-DOPA PET and biodistribution in gliomas for neurosurgical planning and radiotherapy target delineation: results of a prospective pilot study. Neuro Oncol. 2013;15(8):1058–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roodakker KR, Alhuseinalkhudhur A, Al-Jaff M, et al. . Region-by-region analysis of PET, MRI, and histology in en bloc-resected oligodendrogliomas reveals intra-tumoral heterogeneity. Eur J Nucl Med Mol Imaging. 2019;46(3):569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Verburg N, Koopman T, Yaqub MM, et al. . Improved detection of diffuse glioma infiltration with imaging combinations: a diagnostic accuracy study. Neuro Oncol. 2020;22(3):412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schön S, Cabello J, Liesche-Starnecker F, et al. . Imaging glioma biology: spatial comparison of amino acid PET, amide proton transfer, and perfusion-weighted MRI in newly diagnosed gliomas. Eur J Nucl Med Mol Imaging. 2020;47(6):1468–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Douglas JG, Stelzer KJ, Mankoff DA, et al. . [F-18]-fluorodeoxyglucose positron emission tomography for targeting radiation dose escalation for patients with glioblastoma multiforme: clinical outcomes and patterns of failure. Int J Radiat Oncol Biol Phys. 2006;64(3):886–891. [DOI] [PubMed] [Google Scholar]
- 25. Grosu AL, Weber WA, Riedel E, et al. . l-(Methyl-11C) methionine positron emission tomography for target delineation in resected high-grade gliomas before radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63(1):64–74. [DOI] [PubMed] [Google Scholar]
- 26. Matsuo M, Miwa K, Tanaka O, et al. . Impact of [11C]methionine positron emission tomography for target definition of glioblastoma multiforme in radiation therapy planning. Int J Radiat Oncol Biol Phys. 2012;82(1):83–89. [DOI] [PubMed] [Google Scholar]
- 27. Mahasittiwat P, Mizoe JE, Hasegawa A, et al. . l-[Methyl-11C] methionine positron emission tomography for target delineation in malignant gliomas: impact on results of carbon ion radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70(2):515–522. [DOI] [PubMed] [Google Scholar]
- 28. Lee IH, Piert M, Gomez-Hassan D, et al. . Association of 11C-methionine PET uptake with site of failure after concurrent temozolomide and radiation for primary glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2009;73(2):479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tsien CI, Brown D, Normolle D, et al. . Concurrent temozolomide and dose-escalated intensity-modulated radiation therapy in newly diagnosed glioblastoma. Clin Cancer Res. 2012;18(1):273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hirata T, Kinoshita M, Tamari K, et al. . 11C-methionine-18F-FDG dual-PET-tracer-based target delineation of malignant glioma: evaluation of its geometrical and clinical features for planning radiation therapy. J Neurosurg. 2019;131(3):676–686. [DOI] [PubMed] [Google Scholar]
- 31. Mosskin M, Ericson K, Hindmarsh T, et al. . Positron emission tomography compared with magnetic resonance imaging and computed tomography in supratentorial gliomas using multiple stereotactic biopsies as reference. Acta Radiol. 1989;30(3):225–232. [PubMed] [Google Scholar]
- 32. Weber DC, Zilli T, Buchegger F, et al. . [(18)F]Fluoroethyltyrosine- positron emission tomography-guided radiotherapy for high-grade glioma. Radiat Oncol. 2008; 3:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Niyazi M, Geisler J, Siefert A, et al. . FET-PET for malignant glioma treatment planning. Radiother Oncol. 2011;99(1):44–48. [DOI] [PubMed] [Google Scholar]
- 34. Suchorska B, Jansen NL, Linn J, et al. . Biological tumor volume in 18FET-PET before radiochemotherapy correlates with survival in GBM. Neurology. 2015; 84(7):710–719. [DOI] [PubMed] [Google Scholar]
- 35. Lohmann P, Stavrinou P, Lipke K, et al. . FET PET reveals considerable spatial differences in tumour burden compared to conventional MRI in newly diagnosed glioblastoma. Eur J Nucl Med Mol Imaging. 2019;46(3):591–602. [DOI] [PubMed] [Google Scholar]
- 36. Rieken S, Habermehl D, Giesel FL, et al. . Analysis of FET-PET imaging for target volume definition in patients with gliomas treated with conformal radiotherapy. Radiother Oncol. 2013;109(3):487–492. [DOI] [PubMed] [Google Scholar]
- 37. Munck Af Rosenschold P, Costa J, Engelholm SA, et al. . Impact of [18F]-fluoro-ethyl-tyrosine PET imaging on target definition for radiation therapy of high-grade glioma. Neuro Oncol. 2015; 17(5):757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hayes AR, Jayamanne D, Hsiao E, et al. . Utilizing 18F-fluoroethyltyrosine (FET) positron emission tomography (PET) to define suspected nonenhancing tumor for radiation therapy planning of glioblastoma. Pract Radiat Oncol. 2018;8(4):230–238. [DOI] [PubMed] [Google Scholar]
- 39. Grosu AL, Astner ST, Riedel E, et al. . An interindividual comparison of O-(2-[18F]fluoroethyl)-l-tyrosine (FET)- and l-[methyl-11C]methionine (MET)-PET in patients with brain gliomas and metastases. Int J Radiat Oncol Biol Phys. 2011;81(4):1049–1058. [DOI] [PubMed] [Google Scholar]
- 40. Kunz M, Thon N, Eigenbrod S, et al. . Hot spots in dynamic 18FET-PET delineate malignant tumor parts within suspected WHO grade II gliomas. Neuro Oncol. 2011;13(3):307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Song S, Cheng Y, Ma J, et al. . Simultaneous FET-PET and contrast-enhanced MRI based on hybrid PET/MR improves delineation of tumor spatial biodistribution in gliomas: a biopsy validation study. Eur J Nucl Med Mol Imaging. 2020;47(6):1458–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fleischmann DF, Unterrainer M, Schön R, et al. . Margin reduction in radiotherapy for glioblastoma through 18F-fluoroethyltyrosine PET?—a recurrence pattern analysis. Radiother Oncol. 2020;145:49–55. [DOI] [PubMed] [Google Scholar]
- 43. Molinaro AM, Hervey-Jumper S, Morshed RA, et al. . Association of maximal extent of resection of contrast-enhanced and non-contrast-enhanced tumor with survival within molecular subgroups of patients with newly diagnosed glioblastoma. JAMA Oncol. 2020;6(4):495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Møller S, Munck Af Rosenschöld P, Costa J, et al. . Toxicity and efficacy of re-irradiation of high-grade glioma in a phase I dose- and volume escalation trial. Radiother Oncol. 2017;125(2):223–227. [DOI] [PubMed] [Google Scholar]
- 45. Kosztyla R, Chan EK, Hsu F, et al. . High-grade glioma radiation therapy target volumes and patterns of failure obtained from magnetic resonance imaging and 18F-FDOPA positron emission tomography delineations from multiple observers. Int J Radiat Oncol Biol Phys. 2013;87(5):1100–1106. [DOI] [PubMed] [Google Scholar]
- 46. Lapa C, Linsenmann T, Monoranu CM, et al. . Comparison of the amino acid tracers 18F-FET and 18F-DOPA in high-grade glioma patients. J Nucl Med. 2014;55(10):1611–1616. [DOI] [PubMed] [Google Scholar]
- 47. Becherer A, Karanikas G, Szabó M, et al. . Brain tumour imaging with PET: a comparison between [18F]fluorodopa and [11C]methionine. Eur J Nucl Med Mol Imaging. 2003;30(11):1561–1567. [DOI] [PubMed] [Google Scholar]
- 48. Poulsen SH, Urup T, Grunnet K, et al. . The prognostic value of FET PET at radiotherapy planning in newly diagnosed glioblastoma. Eur J Nucl Med Mol Imaging. 2017; 44(3):373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fleischmann DF, Unterrainer M, Bartenstein P, Belka C, Albert NL, Niyazi M. 18F-FET PET prior to recurrent high-grade glioma re-irradiation-additional prognostic value of dynamic time-to-peak analysis and early static summation images? J Neurooncol. 2017;132(2):277–286. [DOI] [PubMed] [Google Scholar]
- 50. Niyazi M, Jansen N, Ganswindt U, et al. . Re-irradiation in recurrent malignant glioma: prognostic value of [18F]FET-PET. J Neurooncol. 2012;110(3):389–395. [DOI] [PubMed] [Google Scholar]
- 51. Tsien C, Pugh S, Dicker AP, et al. . Randomized phase II trial of re-irradiation and concurrent bevacizumab versus bevacizumab alone as treatment for recurrent glioblastoma (NRG Oncology/RTOG 1205): initial outcomes and RT plan quality report. Int J Radiat Oncol Biol Phys. 2019; 105(1):S78. [Google Scholar]
- 52. Tralins KS, Douglas JG, Stelzer KJ, et al. . Volumetric analysis of 18F-FDG PET in glioblastoma multiforme: prognostic information and possible role in definition of target volumes in radiation dose escalation. J Nucl Med. 2002;43(12):1667–1673. [PubMed] [Google Scholar]
- 53. Colavolpe C, Metellus P, Mancini J, et al. . Independent prognostic value of pre-treatment 18-FDG-PET in high-grade gliomas. J Neurooncol. 2012;107(3):527–535. [DOI] [PubMed] [Google Scholar]
- 54. Omuro A, Beal K, Gutin P, et al. . Phase II study of bevacizumab, temozolomide, and hypofractionated stereotactic radiotherapy for newly diagnosed glioblastoma. Clin Cancer Res. 2014;20(19):5023–5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Barker FG 2nd, Chang SM, Valk PE, Pounds TR, Prados MD. 18-Fluorodeoxyglucose uptake and survival of patients with suspected recurrent malignant glioma. Cancer. 1997;79(1):115–126. [PubMed] [Google Scholar]
- 56. Piroth MD, Pinkawa M, Holy R, et al. . Integrated boost IMRT with FET-PET-adapted local dose escalation in glioblastomas. Results of a prospective phase II study. Strahlenther Onkol. 2012;188(4):334–339. [DOI] [PubMed] [Google Scholar]
- 57. Kosztyla R, Raman S, Moiseenko V, Reinsberg SA, Toyota B, Nichol A. Dose-painted volumetric modulated arc therapy of high-grade glioma using 3,4-dihydroxy-6-[18F]fluoro-l-phenylalanine positron emission tomography. Br J Radiol. 2019;92(1099):20180901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Laack N, Pafundi D, Anderson S, et al. . Preliminary safety and efficacy of a phase II trial of 18F-FDOPA PET-guided, dose-escalated radiotherapy in the treatment of glioblastoma. Neuro Oncol. 2018; 20(suppl_6):vi13. [Google Scholar]
- 59. Miwa K, Matsuo M, Ogawa S, et al. . Hypofractionated high-dose irradiation with positron emission tomography data for the treatment of glioblastoma multiforme. Biomed Res Int. 2014; 2014:407026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fitzek MM, Thornton AF, Rabinov JD, et al. . Accelerated fractionated proton/photon irradiation to 90 cobalt gray equivalent for glioblastoma multiforme: results of a phase II prospective trial. J Neurosurg. 1999;91(2):251–260. [DOI] [PubMed] [Google Scholar]
- 61. Nieder C, Andratschke NH, Grosu AL. Re-irradiation for recurrent primary brain tumors. Anticancer Res. 2016;36(10):4985–4995. [DOI] [PubMed] [Google Scholar]
- 62. Levin VA, Bidaut L, Hou P, et al. . Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79(5):1487–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wick W, Gorlia T, Bendszus M, et al. . Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954–1963. [DOI] [PubMed] [Google Scholar]
- 64. Shi W, Scannell Bryan M, Gilbert MR, et al. . Investigating the effect of reirradiation or systemic therapy in patients with glioblastoma after tumor progression: a secondary analysis of NRG oncology/radiation therapy oncology group trial 0525. Int J Radiat Oncol Biol Phys. 2018;100(1):38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Niyazi M, Adeberg S, Kaul D, et al. . Independent validation of a new reirradiation risk score (RRRS) for glioma patients predicting post-recurrence survival: a multicenter DKTK/ROG analysis. Radiother Oncol. 2018;127(1):121–127. [DOI] [PubMed] [Google Scholar]
- 66. Bashir A, Mathilde Jacobsen S, Mølby Henriksen O, et al. . Recurrent glioblastoma versus late posttreatment changes: diagnostic accuracy of O-(2-[18F]fluoroethyl)-l-tyrosine positron emission tomography (18F-FET PET). Neuro Oncol. 2019;21(12):1595–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Grosu AL, Weber WA, Franz M, et al. . Reirradiation of recurrent high-grade gliomas using amino acid PET (SPECT)/CT/MRI image fusion to determine gross tumor volume for stereotactic fractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63(2):511–519. [DOI] [PubMed] [Google Scholar]
- 68. Miwa K, Matsuo M, Ogawa S, et al. . Re-irradiation of recurrent glioblastoma multiforme using 11C-methionine PET/CT/MRI image fusion for hypofractionated stereotactic radiotherapy by intensity modulated radiation therapy. Radiat Oncol. 2014;9:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Debus C, Waltenberger M, Floca R, et al. . Impact of 18F-FET PET on target volume definition and tumor progression of recurrent high grade glioma treated with carbon-ion radiotherapy. Sci Rep. 2018;8(1):7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Oehlke O, Mix M, Graf E, et al. . Amino-acid PET versus MRI guided re-irradiation in patients with recurrent glioblastoma multiforme (GLIAA) - protocol of a randomized phase II trial (NOA 10/ARO 2013-1). BMC Cancer. 2016;16(1):769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Popp I, Bott S, Mix M, et al. . Diffusion-weighted MRI and ADC versus FET-PET and GdT1w-MRI for gross tumor volume (GTV) delineation in re-irradiation of recurrent glioblastoma. Radiother Oncol. 2019;130:121–131. [DOI] [PubMed] [Google Scholar]
- 72. Wen PY, Macdonald DR, Reardon DA, et al. . Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-Oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 73. Nayak L, DeAngelis LM, Brandes AA, et al. . The neurologic assessment in neuro-oncology (NANO) scale: a tool to assess neurologic function for integration into the Response Assessment in Neuro-Oncology (RANO) criteria. Neuro Oncol. 2017;19(5):625–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Spence AM, Muzi M, Graham MM, et al. . 2-[(18)F]Fluoro-2-deoxyglucose and glucose uptake in malignant gliomas before and after radiotherapy: correlation with outcome. Clin Cancer Res. 2002;8(4):971–979. [PubMed] [Google Scholar]
- 75. Charnley N, West CM, Barnett CM, et al. . Early change in glucose metabolic rate measured using FDG-PET in patients with high-grade glioma predicts response to temozolomide but not temozolomide plus radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66(2):331–338. [DOI] [PubMed] [Google Scholar]
- 76. Leimgruber A, Hickson K, Lee ST, et al. . Spatial and quantitative mapping of glycolysis and hypoxia in glioblastoma as a predictor of radiotherapy response and sites of relapse. Eur J Nucl Med Mol Imaging. 2020;47(6):1476–1485. [DOI] [PubMed] [Google Scholar]
- 77. Galldiks N, Langen KJ, Holy R, et al. . Assessment of treatment response in patients with glioblastoma using O-(2-18F-fluoroethyl)-l-tyrosine PET in comparison to MRI. J Nucl Med. 2012;53(7):1048–1057. [DOI] [PubMed] [Google Scholar]
- 78. Piroth MD, Pinkawa M, Holy R, et al. . Prognostic value of early [18F]fluoroethyltyrosine positron emission tomography after radiochemotherapy in glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2011;80(1):176–184. [DOI] [PubMed] [Google Scholar]
- 79. Wang Y, Rapalino O, Heidari P, et al. . C11 methionine PET (MET-PET) imaging of glioblastoma for detecting postoperative residual disease and response to chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2018;102(4):1024–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Miller S, Li P, Schipper M, et al. . Metabolic tumor volume response assessment using (11)C-methionine positron emission tomography identifies glioblastoma tumor subregions that predict progression better than baseline or anatomic magnetic resonance imaging alone. Adv Radiat Oncol. 2020;5(1):53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nuutinen J, Sonninen P, Lehikoinen P, et al. . Radiotherapy treatment planning and long-term follow-up with [(11)C]methionine PET in patients with low-grade astrocytoma. Int J Radiat Oncol Biol Phys. 2000;48(1):43–52. [DOI] [PubMed] [Google Scholar]
- 82. Kumar AJ, Leeds NE, Fuller GN, et al. . Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology. 2000;217(2):377–384. [DOI] [PubMed] [Google Scholar]
- 83. Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9(5):453–461. [DOI] [PubMed] [Google Scholar]
- 84. Kebir S, Fimmers R, Galldiks N, et al. . Late pseudoprogression in glioblastoma: diagnostic value of dynamic O-(2-[18F]fluoroethyl)-l-tyrosine PET. Clin Cancer Res. 2016;22(9):2190–2196. [DOI] [PubMed] [Google Scholar]
- 85. Stuplich M, Hadizadeh DR, Kuchelmeister K, et al. . Late and prolonged pseudoprogression in glioblastoma after treatment with lomustine and temozolomide. J Clin Oncol. 2012;30(21):e180–e183. [DOI] [PubMed] [Google Scholar]
- 86. Strauss SB, Meng A, Ebani EJ, Chiang GC. Imaging glioblastoma posttreatment: progression, pseudoprogression, pseudoresponse, radiation necrosis. Radiol Clin North Am. 2019;57(6):1199–1216. [DOI] [PubMed] [Google Scholar]
- 87. Marks JE, Baglan RJ, Prassad SC, Blank WF. Cerebral radionecrosis: incidence and risk in relation to dose, time, fractionation and volume. Int J Radiat Oncol Biol Phys. 1981;7(2):243–252. [DOI] [PubMed] [Google Scholar]
- 88. Ruben JD, Dally M, Bailey M, Smith R, McLean CA, Fedele P. Cerebral radiation necrosis: incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys. 2006;65(2):499–508. [DOI] [PubMed] [Google Scholar]
- 89. Colaco RJ, Martin P, Kluger HM, Yu JB, Chiang VL. Does immunotherapy increase the rate of radiation necrosis after radiosurgical treatment of brain metastases? J Neurosurg. 2016;125(1):17–23. [DOI] [PubMed] [Google Scholar]
- 90. Zhuang H, Tao L, Wang X, et al. . Tyrosine kinase inhibitor resistance increased the risk of cerebral radiation necrosis after stereotactic radiosurgery in brain metastases of non-small-cell lung cancer: a multi-institutional retrospective case-control study. Front Oncol. 2020;10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Galldiks N, Kocher M, Ceccon G, et al. . Imaging challenges of immunotherapy and targeted therapy in patients with brain metastases: response, progression, and pseudoprogression. Neuro Oncol. 2020;22(1):17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Martin AM, Cagney DN, Catalano PJ, et al. . Immunotherapy and symptomatic radiation necrosis in patients with brain metastases treated with stereotactic radiation. JAMA Oncol. 2018;4(8):1123–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Nihashi T, Dahabreh IJ, Terasawa T. Diagnostic accuracy of PET for recurrent glioma diagnosis: a meta-analysis. AJNR Am J Neuroradiol. 2013;34(5):944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Karunanithi S, Sharma P, Kumar A, et al. . 18F-FDOPA PET/CT for detection of recurrence in patients with glioma: prospective comparison with 18F-FDG PET/CT. Eur J Nucl Med Mol Imaging. 2013;40(7):1025–1035. [DOI] [PubMed] [Google Scholar]
- 95. Werner JM, Stoffels G, Lichtenstein T, et al. . Differentiation of treatment-related changes from tumour progression: a direct comparison between dynamic FET PET and ADC values obtained from DWI MRI. Eur J Nucl Med Mol Imaging. 2019;46(9):1889–1901. [DOI] [PubMed] [Google Scholar]
- 96. Galldiks N, Dunkl V, Stoffels G, et al. . Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[18F]fluoroethyl)-l-tyrosine PET. Eur J Nucl Med Mol Imaging. 2015;42(5):685–695. [DOI] [PubMed] [Google Scholar]
- 97. Mihovilovic MI, Kertels O, Hänscheid H, et al. . O-(2-(18F)fluoroethyl)-l-tyrosine PET for the differentiation of tumour recurrence from late pseudoprogression in glioblastoma. J Neurol Neurosurg Psychiatry. 2019;90(2):238–239. [DOI] [PubMed] [Google Scholar]
- 98. Pyka T, Hiob D, Preibisch C, et al. . Diagnosis of glioma recurrence using multiparametric dynamic 18F-fluoroethyl-tyrosine PET-MRI. Eur J Radiol. 2018;103:32–37. [DOI] [PubMed] [Google Scholar]
- 99. Galldiks N, Stoffels G, Filss C, et al. . The use of dynamic O-(2-18F-fluoroethyl)-l-tyrosine PET in the diagnosis of patients with progressive and recurrent glioma. Neuro Oncol. 2015;17(9):1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Herrmann K, Czernin J, Cloughesy T, et al. . Comparison of visual and semiquantitative analysis of 18F-FDOPA-PET/CT for recurrence detection in glioblastoma patients. Neuro Oncol. 2014;16(4):603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Karunanithi S, Sharma P, Kumar A, et al. . Comparative diagnostic accuracy of contrast-enhanced MRI and (18)F-FDOPA PET-CT in recurrent glioma. Eur Radiol. 2013;23(9):2628–2635. [DOI] [PubMed] [Google Scholar]
- 102. Chen W, Silverman DH, Delaloye S, et al. . 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med. 2006;47(6):904–911. [PubMed] [Google Scholar]
- 103. Terakawa Y, Tsuyuguchi N, Iwai Y, et al. . Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med. 2008;49(5):694–699. [DOI] [PubMed] [Google Scholar]
- 104. Salber D, Stoffels G, Pauleit D, et al. . Differential uptake of O-(2-18F-fluoroethyl)-l-tyrosine, l-3H-methionine, and 3H-deoxyglucose in brain abscesses. J Nucl Med. 2007;48(12):2056–2062. [DOI] [PubMed] [Google Scholar]
- 105. Alkonyi B, Barger GR, Mittal S, et al. . Accurate differentiation of recurrent gliomas from radiation injury by kinetic analysis of α-11C-methyl-l-tryptophan PET. J Nucl Med. 2012;53(7):1058–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Henderson F Jr, Brem S, O’Rourke DM, et al. . 18F-Fluciclovine PET to distinguish treatment-related effects from disease progression in recurrent glioblastoma: PET fusion with MRI guides neurosurgical sampling. Neurooncol Pract. 2020;7(2):152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Jansen NL, Suchorska B, Schwarz SB, et al. . [18F]fluoroethyltyrosine-positron emission tomography-based therapy monitoring after stereotactic iodine-125 brachytherapy in patients with recurrent high-grade glioma. Mol Imaging. 2013;12(3):137–147. [PubMed] [Google Scholar]
- 108. Lohmann P, Galldiks N, Kocher M, et al. . Radiomics in neuro-oncology: basics, workflow, and applications. Methods. 2020:S1046–2023(19)30317–2. [DOI] [PubMed] [Google Scholar]
- 109. Kickingereder P, Isensee F, Tursunova I, et al. . Automated quantitative tumour response assessment of MRI in neuro-oncology with artificial neural networks: a multicentre, retrospective study. Lancet Oncol. 2019;20(5):728–740. [DOI] [PubMed] [Google Scholar]
- 110. Blanc-Durand P, Van Der Gucht A, Schaefer N, Itti E, Prior JO. Automatic lesion detection and segmentation of 18F-FET PET in gliomas: a full 3D U-Net convolutional neural network study. PLoS One. 2018;13(4):e0195798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lohmann P, Kocher M, Ceccon G, et al. . Combined FET PET/MRI radiomics differentiates radiation injury from recurrent brain metastasis. Neuroimage Clin. 2018;20:537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lohmann P, Elahmadawy MA, Werner J, et al. . OS9.6 diagnosis of pseudoprogression using FET PET radiomics. Neuro-Oncology. 2019;21(Supplement_3):iii19. [Google Scholar]
- 113. Piroth MD, Galldiks N, Pinkawa M, et al. . Relapse patterns after radiochemotherapy of glioblastoma with FET PET-guided boost irradiation and simulation to optimize radiation target volume. Radiat Oncol. 2016;11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Weber DC, Casanova N, Zilli T, et al. . Recurrence pattern after [(18)F]fluoroethyltyrosine-positron emission tomography-guided radiotherapy for high-grade glioma: a prospective study. Radiother Oncol. 2009;93(3):586–592. [DOI] [PubMed] [Google Scholar]
- 115. Crinion J, Ashburner J, Leff A, Brett M, Price C, Friston K. Spatial normalization of lesioned brains: performance evaluation and impact on fMRI analyses. Neuroimage. 2007;37(3):866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lipkova J, Angelikopoulos P, Wu S, et al. . Personalized radiotherapy design for glioblastoma: integrating mathematical tumor models, multimodal scans, and bayesian inference. IEEE Trans Med Imaging. 2019;38(8):1875–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Law I, Albert NL, Arbizu J, et al. . Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: version 1.0. Eur J Nucl Med Mol Imaging. 2019;46(3):540–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Unterrainer M, Fleischmann DF, Vettermann F, et al. . TSPO PET, tumour grading and molecular genetics in histologically verified glioma: a correlative 18F-GE-180 PET study. Eur J Nucl Med Mol Imaging. 2020;47(6):1368–1380. [DOI] [PubMed] [Google Scholar]
- 119. Zinnhardt B, Müther M, Roll W, et al. . TSPO imaging-guided characterization of the immunosuppressive myeloid tumor microenvironment in patients with malignant glioma. Neuro Oncol. 2020;22(7):1030–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Seystahl K, Stoecklein V, Schüller U, et al. . Somatostatin receptor-targeted radionuclide therapy for progressive meningioma: benefit linked to 68Ga-DOTATATE/-TOC uptake. Neuro Oncol. 2016;18(11):1538–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bailly C, Vidal A, Bonnemaire C, et al. . Potential for nuclear medicine therapy for glioblastoma treatment. Front Pharmacol. 2019;10:772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.