Abstract
Background
Limited population-based data exist for the brainstem gliomas for children ages ≤19 years, which includes high-grade aggressively growing tumors such as diffuse intrinsic pontine glioma (DIPG). We examined the overall incidence and survival patterns in children with brainstem high-grade glioma (HGG) by age, sex, and race and ethnicity.
Methods
We used data from Central Brain Tumor Registry of the United States (CBTRUS), obtained through data use agreements with the Centers for Disease Control (CDC) and the National Cancer Institute (NCI) from 2000 to 2017, and survival data from the CDCs National Program of Cancer Registries (NPCR), from 2001 to 2016 for malignant brainstem HGG for ages ≤19 years (per WHO ICD-O-3 codes). HGG was determined by established histologic and/or imaging criteria. Age-adjusted incidence rates and survival data were used to assess differences overall and by age, sex race, and ethnicity.
Results
The incidence of brainstem HGG was higher among the female and Non-Hispanic population. Majority (69.8%) of these tumors were diagnosed radiographically. Incidence was higher in children aged 1-9 years compared to older children. Whites had a higher incidence compared to Blacks. However, the risk of death was higher among Blacks and Other race compared to Whites. There was no difference in survival by sex.
Conclusions
We report the most comprehensive incidence and survival data on these lethal brainstem HGGs. Incidence and survival among patients with brainstem HGGs differed significantly by race, ethnicity, age-groups, and grade.
Keywords: brainstem, CBTRUS, DIPG, glioma, high-grade glioma
Key Points.
1. The incidence and survival of Brainstem high-grade gliomas (HGG) among children and adolescents in the United States were estimated using population-based data..
2. Incidence and survival among patients with Brainstem HGGs differed significantly by race, ethnicity, age groups, and grade.
Importance of the Study.
Brainstem high-grade gliomas (HGG) are much more common in children and adolescents than in adults, accounting for 10-20% of all central nervous system (CNS) tumors in children in this age group. There has been a lack of necessary epidemiological information for these HGGs. To date, no population-based analysis has been published for children ages ≤19 years in the United States diagnosed with brainstem HGG. In this study, we provide an in-depth examination of incidence, prevalence, and survival differences in the United States over a 18 year period from 2000 to 2017 for children and adolescents with brainstem HGG.
Gliomas originate from the glial cells and account for the majority of (45.7%) brain and other central nervous system (CNS) tumors both non-malignant (tumors with ICD-O-3 (International Classification of Diseases for Oncology, 3rd edition) behavior codes of /0 benign and /1 uncertain) and malignant (ICD-O-3 behavior code/3) in children and adolescents age 0-19 years.1 Gliomas located within the brainstem are considered to be more common in children and decrease in incidence with age. Historically, brainstem gliomas have not been categorized according to World Health Organization (WHO) histology classification of CNS tumors as assigned with gliomas in other locations due to difficulty and risk of biopsy and/or surgical resection in the brainstem. Rather, they are frequently grouped according to location and appearance using magnetic resonance (MRI).2 Approximately 80% of pediatric brainstem gliomas arise within the pons, while the remaining 20% arise in the medulla, midbrain, or cervicomedullary junction.3,4 Clinical features such as pontine location, rapid growth, and presence of contrast enhancement suggest high-grade pathology.
Surgical resection is considered a standard initial treatment for gliomas. However, the brainstem location represents a unique surgical challenge for safe resection and/or stereotactic biopsy, especially for infiltrative, HGG. Standard treatment for high-grade tumors involving the brainstem consists of radiation therapy with or without chemotherapy, while surgical resection is often curative for low-grade tumors in this location.5 Recent advances in molecular profiling and identification of driver mutations in gliomas have resulted in the initiation of clinical trials using targeted therapies.6 Treatment options for intrinsic brainstem glioma, especially HGG remain challenging, and no treatment has been found to be significantly effective in improving the survival of patient with this tumor.7 Specifically for brainstem gliomas, these can be characterized as either diffuse intrinsic pontine gliomas (DIPG) or non-DIPG which include either focal brainstem gliomas, tectal gliomas, or dorsally exophytic gliomas.8,9
There has been a lack of necessary epidemiological information for brainstem gliomas and specifically HGGs. To date, no population-based analysis has been published for children in the United States diagnosed with brainstem HGG. In this study, we report incidence, prevalence, and survival rates among children age 0-19 years with brainstem HGG. We provide results for by race/ethnicity, sex, and age, in order to define the factors most strongly associated with their incidence, prevalence, and survival rates of brainstem HGG in children ages ≤19 years.
Methods
Data Source and Data Collection
Incidence data for all brain and other CNS tumors were obtained from the Central Brain Tumor Registry of the United States (CBTRUS), through a data-release agreement with the Centers for Disease Control and Prevention (CDC) and through the research dataset of the National Cancer Institute (NCI). CBTRUS data are derived from central cancer registries (CCR) of which 50 were state cancer registries, the District of Columbia, and Puerto Rico. The final incidence dataset included 48 CDC National Program of Cancer Registries (NPCR) and four NCI Surveillance, Epidemiology and End Results (SEER) CCR, and provided incidence data representing 100% of the US population. Survival data for malignant brain and other CNS tumors were obtained from CDC for 45 NPCR registries for the years 2001 to 2016. This dataset provides population-based information for approximately 93% of the US population and is a subset of the data used for the incidence calculations. Survival information is derived from both active and passive follow-up.10
The CBTRUS database was queried for cases diagnosed between 2000 and 2017 of individuals age 19 years or less at time of diagnosis for incidence calculations. For this analysis, we only included histologies coded with malignant (/3) International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) behavior code. Brainstem tumors were defined as tumors occurring at ICD-O-3 site code C71.7, which includes the following sites: cerebral peduncle, choroid plexus of fourth ventricle, fourth ventricle—not otherwise specified, infratentorial brain—not otherwise specified, medulla oblongata, midbrain, olive, pons and pyramid.11 The CBTRUS Histology Grouping Scheme used in the CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2012-2016 provided the basis for the definition for Gliomas in this report.1,12 These histologies were re-organized to be more reflective of the clinical organization of brain tumors that are specific to infancy and childhood.13 The specific histologies included under HGG for this analysis were ICD-O-3 codes: 9380 (Site C71.7 only), 9401, 9440, 9441, 9442, 9451, 9460, 9400 (Site C71.7 only). All cases included were either histologically confirmed or based upon a radiographic impression consistent with a grade 3/4 tumor.2 Majority of these tumors that were diagnosed using radiological criteria and were not graded.
Data Analysis
The SEER*Stat software, version 8.3.8 (Surveillance, Epidemiology, and End Results [SEER] Program) was used to calculate frequencies, incidence rates incidence rate ratios, and R version 3.5.2 (R Core Team, 2018) was used for survival analysis and to generate figures.14 Age-adjusted incidence rates with 95% confidence intervals (CI) for children and adolescents (ages 19 years or younger) were calculated for all brainstem gliomas overall, and by selected age-groups (less than 1 year, 01-04, 05-09, 10-14 and 15-19 years or older), sex, race, ethnicity, and histology groups. Age-adjustment was based on 1-year age groupings and standardized to the 2000 US standard population. The race categories included White, Black, and Others. Due to relatively low sample size (6.3% during study period), Other race category was created to include American Indian/Alaskan Native, Chinese, and Japanese. The Joinpoint Regression Program 4.6.0.0 was used to compute annual percentage change (APC) in incidence rates from 2000 to 2017 to examine trends over time. Joinpoint software selects a minimum number of joinpoints to prohibit statistically significant improvement if one additional joinpoint is added (http://surveillance.cancer.gov/joinpoint).
Complete prevalence estimates for 2017 were calculated using methodology previously described by Zhang et al., using incidence data from the CBTRUS analytic file from 2000 to 2017 and survival data from CDCs NPCR survival data.10,15 Crude prevalence rates were calculated using all prevalent cases in those patients age 19 years and younger in 2017 using the estimated US population for 2017 obtained from SEER (estimated using the 2010 decennial census).12 Age-adjusted prevalence rates (PR) adjusted to the 2000 US standard population were calculated by histology group using the R package “asht.” 16
Survival differences were assessed among age-groups, sex, race, ethnicity, and WHO grade (grade 3, 4, and unknown). The unknown grade was further stratified in to those who were diagnosed radiologically and those who were true unknown (histologic confirmation but without grading information). There were a total of 3,665 patients with primary malignant brainstem HGG age 0-19 in NPCR databases used for survival analysis. Survival was assessed with Kaplan-Meier survival curves (generating median survival times and log rank tests) and multivariable Cox proportional hazards models (generating hazard ratios (HR) with 95% confidence intervals (95% CI)). Adjusted estimates included all covariates (age at diagnosis, sex, race, ethnicity, and grade) a priori, regardless of the individual significance level. Statistical significance was set at P < 0.05.
Results
Age-Adjusted Incidence Rate and Incidence Rate Ratio
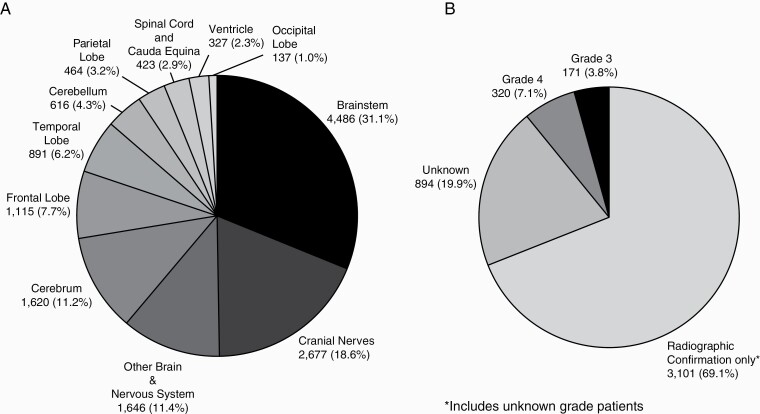
Overall, from 2000 to 2017, there were 14,402 patients with primary malignant HGG originating from brain and other CNS locations among children and adolescents ages 0-19 years, of which 4,486 (31.1%) patients had a tumor located in the brainstem (Fig. 1). Of those with brainstem HGG, 69.8% (N = 3,131) lacked a histologic diagnosis but met imaging criteria used to define HGG in the brainstem location (Fig. 1). For the purpose of this report, those lacking a histologic diagnosis, but meeting HGG imaging criteria were labeled as radiologically confirmed only. The overall age adjusted incidence rate (AAIR) of brainstem HGG was 0.305 per 100,000 population (Table 1). When stratifying by patient sex, the overall AAIR for males (0.288) and females (0.324) was significantly different (incidence rate ratio [IRR] = 1.13, 95% CI 1.06–1.19, P < 0.001) (Table 1). The AAIR was highest for Whites (0.23, 95% CI: 0.22–0.24) compared to Blacks (0.05, 95% CI: 0.05–0.06) and Other race (0.02, 95% CI: 0.02–0.02). The AAIR was 0.28 for Hispanic ethnicity and 0.32 for non-Hispanic ethnicity (IRR = 1.14, 95% CI: 1.06–1.23, P < .001). Among different age-groups, the highest incidence was found in the children ages 0–04 (AAIR = 0.38, 95% CI: 0.36–0.40) and 05–09 years (AAIR = 0.51, 95% CI: 0.48–0.53) compared to other age groups (Table 1).
Fig. 1.
Distribution of malignant primary childhood and adolescent (age ≤19 years) brain and other CNS high grade gliomas by (A) site (N = 14,402) and B) for brainstem (ICD-O-3 site code C71.7) only by grade (N = 4,486); 2000-2017, CBTRUS.
Table 1.
Age-Adjusted Incidence Rates, Incidence Rate Ratio, and Prevalence Rate with 95% Confidence Intervals (95% CI) for Primary Higher-grade Brainstem Glioma in Children and Adolescents (age ≤19 years) 2000-2017, CBTRUS (N = 4,486).
| Total Patients 2000-2017 (%) | Ratea | 95% Confidence Interval (CI) | Incidence Rate Ratio (95% CI) | P-value | Prevalence Rate (95% CI) | |
|---|---|---|---|---|---|---|
| Overall | 4,486 | 0.305 | 0.296-0.314 | – | – | 1.49 (1.40-1.57) |
| Sex | ||||||
| Males | 2,162 (48.2) | 0.288 | 0.276-0.300 | – | – | 1.50 (1.39-1.62) |
| Females | 2,324 (51.8) | 0.324 | 0.311-0.337 | 1.13 (1.06-1.19) | <.001 | 1.47 (1.36-1.59) |
| Race | ||||||
| White | 3,439 (76.7) | 0.234 | 0.226-0.242 | – | – | 1.62 (1.57-1.67) |
| Black | 750 (16.7) | 0.051 | 0.048-0.055 | 0.22 (0.20-0.24) | <.001 | 0.72 (0.66-0.78) |
| Other | 297 (6.6) | 0.020 | 0.018-0.023 | 0.08 (0.07-0.09) | <.001 | 0.99 (0.91-1.08) |
| Ethnicity | ||||||
| Hispanic | 914 (19.7) | 0.283 | 0.265-0.302 | – | – | 0.89 (0.81-0.96) |
| Non-Hispanic | 3,735 (80.3) | 0.325 | 0.315-0.336 | 1.14 (1.06-1.23) | <.001 | 1.60 (1.55-1.65) |
| Age at Diagnosis | ||||||
| <1 year | 80 (1.8) | 0.111 | 0.088-0.138 | – | – | 0.12 (0.04-0.29) |
| 01-04 years | 1,093 (24.4) | 0.381 | 0.359-0.404 | 3.43 (2.73-4.36) | <.001 | 0.58 (0.47-0.71) |
| 05-09 years | 1,843 (41.1) | 0.508 | 0.485-0.531 | 4.56 (3.65-5.78) | <.001 | 1.68 (1.51-1.87) |
| 10-14 years | 944 (21.0) | 0.250 | 0.235-0.267 | 2.25 (1.79-2.86) | <.001 | 1.76 (1.58-1.95) |
| 15-19 years | 526 (11.7) | 0.137 | 0.125-0.149 | 1.23 (0.97-1.57) | .093 | 1.97 (1.78-2.17) |
| Grade b | ||||||
| Radiographic confirmation onlyc | 3,101 (69.1) | 0.211 | 0.204-0.219 | – | – | – |
| Grade 3 | 171 (3.8) | 0.012 | 0.010-0.013 | 0.055 (0.047-0.064) | <.001 | – |
| Grade 4 | 320 (7.1) | 0.022 | 0.020-0.024 | 0.104 (0.092-0.116) | <.001 | – |
| Unknownd | 894 (19.9) | 0.061 | 0.057-0.065 | 0.288 (0.267-0.310) | <.001 | – |
aIncidence and Prevalence rates are per 100,000 and are age-adjusted to the 2000 US standard population.
bPrevalence could not be reliably estimated due to the low sample size.
cIncludes unknown grade patients.
dIncludes histologically confirmed, but unknown WHO grade patients.
Differences in Prevalence and Time Trends
As of 2017, the overall age-adjusted prevalence rate of all brainstem HGG was1.49 per 100,000 (95% CI: 1.40–1.57). The prevalence rate among male (AAPR = 1.50 per 100,000, 95% CI: 1.39–1.62) and female (AAPR = 1.47, 95% CI: 1.36–1.59) was similar. In the period from 2000 to 2017, the overall annual percentage change (APC) for brainstem HGG was 1.43% (95% CI: 0.70%–2.17%, P ≤ .001). The APC from 2000 to 2017 was significant for both male and females, both Whites and Blacks, and non-Hispanics. When stratifying by age group, the APC was significant for 10–14 years (APC: 2.84%, 95% CI: 1.41–4.30%) and 15–19 years (APC: 2.78%, 95% CI: 0.87–4.73), but not for other age groups.
Overall Survival for Brainstem HGG by Age, Race, Ethnicity, and Sex
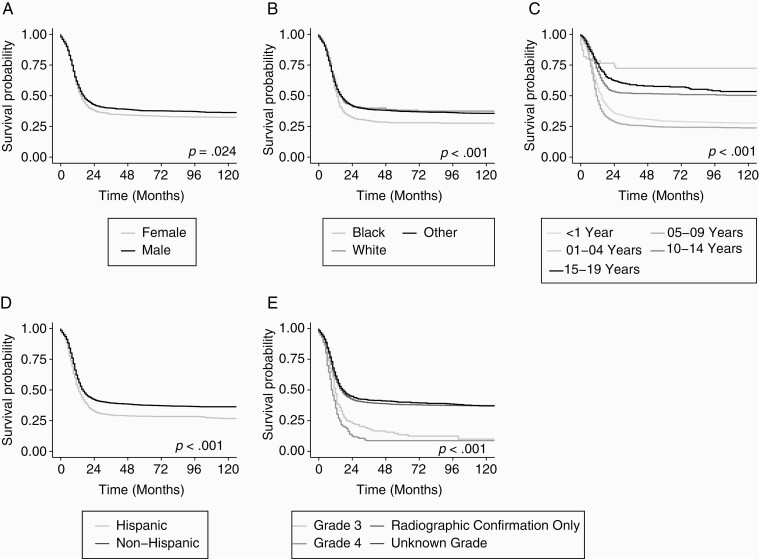
The Kaplan–Meier estimated median overall survival for brainstem HGG was 15 months (95% CI: 15, 16). Kaplan–Meier analysis showed significant differences in overall survival by sex (log-rank P < .001), race (log-rank P < .001), age-groups (log-rank P < .001), ethnicity (log-rank P < .001) and WHO grade (log-rank P < .001) (Fig. 2). The median survival times in months by sex, race, age groups, ethnicity, and WHO grade are presented in Table 2. Multivariable Cox proportional hazard regression models were used to further examine the association of patient characteristics and overall survival among brainstem HGG overall, for histologically confirmed only, and for HGG diagnosed using radiologic criteria only (Table 2). Among the total study population, the risk of death differed significantly by race, ethnicity, age at diagnosis and grade (P < .05). The risk of death was not significantly different by sex (HR for females: 1.08; 95% CI: 1.00–1.18; P = .061). Blacks had a higher risk of death compared to Whites (HR: 1.19; 95% CI: 1.07–1.33; P = .002). A separate multivariable Cox model analysis for histologically confirmed cases showed that the risk of death was not significantly different by race as seen with the total study population (Table 2). The multivariable analysis of brainstem HGG with radiographic diagnosis only showed similar findings to the total study population.
Fig. 2.
Kaplan-Meier survival curve for primary brainstem high-grade glioma in children and adolescents (age ≤19 years) by (A) sex (B) race (White, Blacks vs Others) (C) age group (<1 year, 01-04 year, 05-09 years, 10-14 years and 15-19 years) (D) ethnicity (Hispanic vs Non-Hispanic) and (E) grade; NPCR 2001-2016 (N = 3,665).
Table 2.
Multivariable Cox Proportional Hazard Ratios (With 95% CI and P-Values) For Overall Survival for Primary High-Grade Brainstem Glioma in Childhood and Adolescent (age ≤19 years); NPCR 2001-2016 (N = 3,665)
| Overall N = 3,665 | Histologically confirmed N = 1,118 | Radiographically confirmed only N = 2,547 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median survival time in months (95% CIa) | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Sex | ||||||||||
| Male | 16 (15-18) | – | – | – | – | – | – | |||
| Female | 14 (14-15) | 1.08 | 1.00, 1.18 | .060 | 1.06 | 0.91, 1.22 | .457 | 1.08 | 0.98, 1.20 | .137 |
| Race | ||||||||||
| White | 16 (15-17) | – | – | – | – | – | – | |||
| Black | 13 (12-14) | 1.19 | 1.07, 1.33 | .002 | 1.15 | 0.94, 1.40 | .177 | 1.22 | 1.07, 1.39 | .004 |
| Other | 16 (13-22) | 1.00 | 0.84, 1.19 | .977 | 1.11 | 0.81, 1.53 | .500 | 0.97 | 0.79, 1.20 | .789 |
| Ethnicity | ||||||||||
| Non-Hispanic | 16 (15-17) | – | – | – | – | – | – | |||
| Hispanic | 13 (12-15) | 1.22 | 1.10, 1.36 | <.001 | 1.17 | 0.98, 1.40 | .086 | 1.28 | 1.13, 1.46 | <.001 |
| Age at diagnosis | ||||||||||
| <1 years | ** | – | – | – | – | – | – | – | – | – |
| 01-04 years | 15 (13-16) | 2.94 | 1.79, 4.83 | <.001 | 2.51 | 1.11, 5.67 | .027 | 3.08 | 1.64, 5.76 | <.001 |
| 05-09 years | 11 (11-12) | 3.47 | 2.11, 5.68 | <.001 | 3.99 | 1.78, 8.97 | <.001 | 3.17 | 1.70, 5.92 | <.001 |
| 10-14 years | ** | 1.74 | 1.06, 2.88 | .030 | 2.55 | 1.12, 5.80 | .025 | 1.41 | 0.75, 2.66 | 0.289 |
| 15-19 years | b | 1.49 | 0.89, 2.48 | .128 | 2.61 | 1.14, 6.00 | .023 | 1.03 | 0.53, 1.97 | 0.937 |
| Grade | ||||||||||
| Radiographic confirmation only | 16 (15-18) | – | – | – | – | – | – | – | – | – |
| Grade 3 | 12 (11-13) | 1.67 | 1.39, 2.00 | <.001 | 1.83 | 1.49, 2.24 | <.001 | – | – | – |
| Grade 4 | 10 (9-11) | 1.98 | 1.70, 2.31 | <.001 | 2.10 | 1.74, 2.52 | <.001 | — | — | — |
| Unknowna | 17 (15-21) | 0.94 | 0.84, 1.05 | .258 | – | – | – | – | – | – |
Abbreviations: HR, Hazard Ratio; CI, Confidence Interval.
aUnknown is the reference category for histologically confirmed cases model.
bMedian survival times could not be estimated.
Discussion
Brainstem gliomas are much more common in children than in adults, accounting for 10–20% of all CNS tumors in children.17–19 In this report, we provide an in-depth examination of incidence, prevalence, and survival differences in the United States over a 18-year period from 2000 to 2017 (2001–2016 for survival) for children and adolescents with brainstem HGG. We found that the incidence of brainstem HGG was highest in age groups 01–04 and 05–09 years compared to other pediatric age groups. The preponderance of gliomas in young children is consistent with prior reports where median age was reported as 6.5 years3,18–20 and is consistent with the suggested developmental origin of these midline tumors.21 Similar to other malignant midline tumors in children, gliomas involving the brainstem show a specific age and mutation distribution.22,23 HGG involving the pons are largely characterized by lysine-to-methionine substitution at position 27 in the H3F3A or HIST13B genes (H3.3 and H3.1 K27M) that are thought to occur in glial precursors resulting in these cells retaining a self-renewing, progenitor-like phenotype.22,23
Changes in tumor incidence and outcomes in children and adolescents with brainstem HGG observed in this study likely have multifactorial origins including intrinsic tumor behavior and environmental contributions.24 Whites, regardless of Hispanic status, had the highest incidence of brainstem HGG, which is similar to incidence patterns of glial tumors in general. While these differences likely reflect true predominance in these population, incidence rates may be biased towards higher reporting in non-Hispanic Whites given previous reports of their greater access to care and earlier diagnosis.25 These incidence differences may also be due to variation in frequency of genetic variants affecting risk for these tumors by genetic ancestry. Analyses of global incidence of glial tumors have also identified significantly higher incidence of these tumors in predominately European ancestry populations, such as found in the United States and northern European countries.26,27
Overall survival was examined for all brainstem HGG and then for histologically confirmed and radiographically confirmed, separately. Not surprisingly, the median overall survival for all brainstem HGG was very short (15 months, 95% CI: 15, 16). Most (~75%) of these high-grade glioma are believed to be DIPG, which are known to have poor survival.1,3,17,28 Also, given the site code in our analysis for brainstem could include disease just near brainstem, such as cerebral peduncle or fourth ventricle and non-DIPG histologies, these could explain some of the variation in outcomes from traditional diffuse gliomas of the brainstem reported in the literature.29 Next, although previous registry studies from the United States and Britain show overall survival of adults and children diagnosed with CNS glioma was related to sex,24,27 we did not find differences in risk of death for brainstem glioma based upon sex when analyzing the whole group or when limiting analysis to histologically confirmed or radiologically confirmed patients only.9
Histological confirmation of HGG in this study was performed in only 30.2% of patients. Rather, the majority of patients were diagnosed using radiographic criteria, which may result in the potential for over diagnosis. We observed that histologically confirmed brainstem HGG had poor survival compared to radiographically confirmed patients (Fig. 2). This may again indicate the potential for over-diagnosis in radiographically confirmed patients as patients with true DIPGs is known to have a shorter survival time.30 Imaging of a brainstem mass with high grade features are considered to be an acceptable diagnostic option in the pediatric population, in particular in cases where biopsy or surgical resection would be unsafe or unfeasible.2 When we looked at a risk of death for histologically confirmed cases only, we found similar results (Table 2), except there was no difference in survival by race. Indeed, an explanation for the lack of advancement in treatment or improvement in the abysmal survival for this group of aggressive tumors over the past two decades, can be related in part to the paucity of available tissue for molecular and genetic profiling necessary to provide biological insights and potential new therapeutic targets. The pediatric neurosurgical paradigm for stereotactic biopsy of diffuse brainstem lesion has shifted over this period, to having to risk profile considered by many to be acceptable to obtain histopathological diagnosis.31 Through the more routine application of biopsy to brain tumors, molecular diagnostics are now central to the definition diffuse midline glioma, H3-K27M mutant, WHO grade 4, for example, following the detection of recurrent H3F3A gene mutation resulting in a substitution K27M in the vast majority of what was formerly described as higher-grade glioma or DIPG tumors. New techniques, such as cell-free DNA testing of cerebrospinal fluid (CSF) or serum circulating tumor DNA testing may also offer safer noninvasive diagnosis for molecularly defined tumors, obviating the need for biopsy altogether. In this vein, histological or molecular confirmation would allow for improved diagnostic characterization of brainstem gliomas and detection of molecular features that may allow for targeted treatment options.32,33
Strength and Limitations
This analysis represents the first comprehensive population-based analysis of incidence and survival patterns for brainstem HGG in children and adolescents in the United States. There are also several inherent limitations to the type of data used for this project. Due to their deep location in the brain, most brainstem HGGs are rarely examined pathologically, and therefore the assigned histologic classification often has not been confirmed. Also, ICD-O-3 site code C71.7 for brainstem is not specific and includes many regions as noted under Data Source and data collection section, and accurate location cannot be determined. However, we believe that focus on specific high-grade histologies is reflective of the clinical organization of childhood brain tumors and will minimize this limitation.
The most common type of brainstem glioma as diagnosed by MRI is DIPG or diffuse midline glioma and it remains one of the leading causes of brain tumor-related deaths in children.3,17,28 However, specific data on these tumors cannot be accurately obtained from cancer registry data as there is no specific WHO assigned ICD-O-312 code for these tumors. Therefore, HGGs in this report includes both DIPG and non-DIPG tumors.
Though CBTRUS, in collaboration with CDC and NCI, represents the largest and most up-to-date population-based registry focused exclusively on CNS tumors from the entire US population, there is no central review of pathology or imaging review of cases within the US cancer registry system. Thus, histology code assignment at case registration is based on histology or radiographic information contained in the patient’s medical record and may contain inaccuracies. Histologies reflect the prevailing criteria for a histology designation at the time of registration and not the most recent classifications. As a result, despite the WHO Classification of Tumors of the Central Nervous System most recent revision in 2016,34 tumors reported beforehand may have been diagnosed using any of the prior classifications schemas due to the variation in the adoption timeline of new standards among participating institutions.
There remains a possibility of potential miscoding that can affect race distribution, treatment patterns etc. The actual WHO grade was not available for the majority of these higher-grade tumors. Furthermore, we excluded any patients with WHO grade 1 and 2 to limit the analysis to designated HGG in the analysis. Incidence trends may also be impacted by increased case ascertainment and identification, particularly among primary brain and other CNS tumors, over time. However, the incidence database used for this study has a catchment area that covers approximately 100% of the US population, and therefore represents the most accurate and complete estimate of the incidence of childhood and adolescent brainstem glioma in the United States.
Conclusions
During the study period (2000–2017), the incidence of primary malignant brainstem HGG in children age 0–19 was increasing. Incidence and survival varied by different patient characteristics. Most notably, the incidence was higher among whites compared to blacks, but risk of death was higher among blacks. This study represents the largest population-based descriptive epidemiology study of brainstem gliomas in the United States.
Funding
Funding for CBTRUS was provided by the Centers for Disease Control and Prevention (CDC) under Contract No. 75D30119C06056, the American Brain Tumor Association, The Sontag Foundation, Novocure, the Musella Foundation, National Brain Tumor Society, the Uncle Kory Foundation, the Zelda Dorin Tetenbaum Memorial Fund, as well as private and in-kind donations. QTO is supported by a Research Training Grant from the Cancer Prevention and Research Institute of Texas (CPRIT; RP160097T). Contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC.
Conflict of interest statement. There are no conflicts of interest or disclosures to report.
Authorship statement. Conception and design of study: N.P. and J.S.B.-S.; Acquisition of data: N.P., G.C., Q.T.O., C.K., and J.S.B.-S.; Analysis and/or interpretation of data: N.P., G.C., S.B., and J.S.B.-S.; Drafting and revising the manuscript critically for important intellectual content: N.P., M.K., D.N.Y., R.B., and J.S.B.-S.; Approval of the version of the manuscript to be published: N.P., M.K., D.N.Y., R.B., G.C., S.B., Q.T.O., C.K., and J.S.B.-S.
References
- 1. Ostrom QT, Cioffi G, Gittleman H, et al. . CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro-Oncol. 2019;21(Suppl 5):v1–v100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barkovich AJ, Krischer J, Kun LE, et al. . Brain stem gliomas: a classification system based on magnetic resonance imaging. Pediatr Neurosurg. 1990;16(2):73–83. [DOI] [PubMed] [Google Scholar]
- 3. Freeman CR, Farmer JP. Pediatric brain stem gliomas: a review. Int J Radiat Oncol Biol Phys. 1998;40(2):265–271. [DOI] [PubMed] [Google Scholar]
- 4. Epstein FJ, Farmer JP. Brain-stem glioma growth patterns. J Neurosurg. 1993;78(3):408–412. [DOI] [PubMed] [Google Scholar]
- 5. Pierre-Kahn A, Hirsch JF, Vinchon M, et al. . Surgical management of brain-stem tumors in children: results and statistical analysis of 75 cases. J Neurosurg. 1993;79(6):845–852. [DOI] [PubMed] [Google Scholar]
- 6. Buczkowicz P, Hawkins C. Pathology, molecular genetics, and epigenetics of diffuse intrinsic pontine glioma. Front Oncol. 2015;5:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vanan MI, Eisenstat DD. DIPG in children – what can we learn from the past? Front Oncol. 2015;5:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reyes-Botero G, Mokhtari K, Martin-Duverneuil N, Delattre J-Y, Laigle-Donadey F. Adult brainstem gliomas. The Oncologist. 2012;17(3):388–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. PDQ® Pediatric Treatment Editorial Board. PDQ Childhood Brain Stem Glioma Treatment. Bethesda, MD: National Cancer Institute, 2020. Available at: https://www.cancer.gov/types/brain/hp/child-glioma-treatment-pdq. Accessed November 24, 2020. PMID: 26389253. [Google Scholar]
- 10. National Program of Cancer Registries SEER*Stat Database: NPCR Survival Analytic file - 2001-2016. 2020. United States Department of Health and Human Services, Centers for Disease Control and Prevention.
- 11. Fritz AG, Percy C, Jack A, et al. , eds. International Classification of Diseases for Oncology: ICD-O. Third edition, First revision. Geneva, Switzerland: World Health Press, World Health Organization, 2013. [Google Scholar]
- 12. Ostrom QT, de Blank PM, Kruchko C, et al. . Alex’s Lemonade Stand Foundation infant and childhood primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro-Oncol. 2015;16(suppl_10):x1–x36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Surveillance, Epidemiology, and End Results (SEER) Program. (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2018 Sub (2000–2016) <Katrina/Rita Population Adjustment> – Linked To County Attributes – Total U.S., 1969–2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2019, based on the November 2018 submission. URL: https://seer.cancer.gov/data/treatment.html. [Google Scholar]
- 14. Surveillance Research Program. National Cancer Institute SEER*Stat Software (Seer.Cancer.Gov/Seerstat) Version 8.3.6.
- 15. Zhang AS, Ostrom QT, Kruchko C, Rogers L, Peereboom DM, Barnholtz-Sloan JS. Complete prevalence of malignant primary brain tumors registry data in the United States compared with other common cancers, 2010. Neuro-Oncol. 2017;19(5):726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fay MP. Applied Statistical Hypothesis Tests. R package version 0.9.3. asht: Applied Statistical Hypothesis Tests. https://cran.r-project.org/web/packages/asht/index.html
- 17. Johnson KJ, Cullen J, Barnholtz-Sloan JS, et al. . Childhood brain tumor epidemiology: a brain tumor epidemiology consortium review. Cancer Epidemiol Biomarkers Prev. 2014;23(12):2716–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaplan AM, Albright AL, Zimmerman RA, et al. . Brainstem gliomas in children. A Children’s Cancer Group review of 119 cases. Pediatr Neurosurg. 1996;24(4):185–192. [DOI] [PubMed] [Google Scholar]
- 19. Littman P, Jarrett P, Bilaniuk LT, et al. . Pediatric brain stem gliomas. Cancer. 1980;45(11):2787–2792. [DOI] [PubMed] [Google Scholar]
- 20. Berger MS, Edwards MS, LaMasters D, Davis RL, Wilson CB. Pediatric brain stem tumors: radiographic, pathological, and clinical correlations. Neurosurgery. 1983;12(3):298–302. [DOI] [PubMed] [Google Scholar]
- 21. Jessa S, Blanchet-Cohen A, Krug B, et al. . Stalled developmental programs at the root of pediatric brain tumors. Nat Genet. 2019;51(12):1702–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khuong-Quang DA, Buczkowicz P, Rakopoulos P, et al. . K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 2012;124(3):439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu G, Broniscer A, McEachron TA, et al. ; St. Jude Children’s Research Hospital–Washington University Pediatric Cancer Genome Project . Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44(3):251–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arora RS, Alston RD, Eden TOB, Estlin EJ, Moran A, Birch JM. Age–incidence patterns of primary CNS tumors in children, adolescents, and adults in England. Neuro-Oncol. 2009;11(4):403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ostrom QT, Cote DJ, Ascha M, Kruchko C, Barnholtz-Sloan JS. Adult glioma incidence and survival by race or ethnicity in the United States From 2000 to 2014. JAMA Oncol. 2018;4(9):1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chow EJ, Puumala SE, Mueller BA, et al. . Childhood cancer in relation to parental race and ethnicity: a 5-state pooled analysis. Cancer. 2010;116(12):3045–3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Erdmann F, Kielkowski D, Schonfeld SJ, et al. . Childhood cancer incidence patterns by race, sex and age for 2000–2006: a report from the South African National Cancer Registry. Int J Cancer. 2015;136(11):2628–2639. [DOI] [PubMed] [Google Scholar]
- 28. Hargrave D, Bartels U, Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol. 2006;7(3):241–248. [DOI] [PubMed] [Google Scholar]
- 29. Hassan H, Pinches A, Picton SV, Phillips RS. Survival rates and prognostic predictors of high grade brain stem gliomas in childhood: a systematic review and meta-analysis. J Neurooncol. 2017;135(1):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fonseca A, Afzal S, Bowes L, et al. . Pontine gliomas a 10-year population-based study: a report from The Canadian Paediatric Brain Tumour Consortium (CPBTC). J Neurooncol. 2020;149(1):45–54. [DOI] [PubMed] [Google Scholar]
- 31. Thomas R, Guilherme M, Cathrine M, et al. . Stereotactic biopsy of diffuse pontine lesions in children. J Neurosurg. 2007;107(1 Suppl):1–4. [DOI] [PubMed] [Google Scholar]
- 32. Leach JL, Roebker J, Schafer A, et al. . MR imaging features of diffuse intrinsic pontine glioma and relationship to overall survival: report from the International DIPG Registry. Neuro Oncol. 2020;22(11):1647–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chiang J, Diaz AK, Makepeace L, et al. . Clinical, imaging, and molecular analysis of pediatric pontine tumors lacking characteristic imaging features of DIPG. Acta Neuropathol Commun. 2020;8(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Louis DN, Perry A, Reifenberger G, et al. . The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]