Abstract
Background
High-grade meningiomas are aggressive tumors with high morbidity and mortality rates that frequently recur even after surgery and adjuvant radiotherapy. However, limited information is currently available on the biology of these tumors, and no alternative adjuvant treatment options exist. Although we previously demonstrated that high-grade meningioma cells were highly sensitive to gemcitabine in vitro and in vivo, the underlying molecular mechanisms remain unknown.
Methods
We examined the roles of hENT1 (human equilibrative nucleoside transporter 1) and dCK (deoxycytidine kinase) in the gemcitabine sensitivity and growth of meningioma cells in vitro. Tissue samples from meningiomas (26 WHO grade I and 21 WHO grade II/III meningiomas) were immunohistochemically analyzed for hENT1 and dCK as well as for Ki-67 as a marker of proliferative activity.
Results
hENT1 and dCK, which play critical roles in the intracellular transport and activation of gemcitabine, respectively, were responsible for the high gemcitabine sensitivity of high-grade meningioma cells and were strongly expressed in high-grade meningiomas. hENT1 expression was required for the proliferation and survival of high-grade meningioma cells and dCK expression. Furthermore, high hENT1 and dCK expression levels correlated with stronger tumor cell proliferative activity and shorter survival in meningioma patients.
Conclusions
The present results suggest that hENT1 is a key molecular factor influencing the growth capacity and gemcitabine sensitivity of meningioma cells and also that hENT1, together with dCK, may be a viable prognostic marker for meningioma patients as well as a predictive marker of their responses to gemcitabine.
Keywords: chemotherapy, meningioma, predictive marker, prognostic marker
Key Points.
hENT1 and dCK influence the gemcitabine sensitivity of meningioma cells.
hENT1 regulates dCK expression and cellular proliferative activity in meningioma.
hENT1 and dCK are novel prognostic markers for meningioma.
Importance of the Study.
Increasing the precision of the prognostic diagnosis of meningioma and establishing a novel therapeutic option to treat meningiomas recurring after surgery and radiotherapy are currently some of the main challenges in meningioma research. In addition to hENT1 and dCK being critical factors influencing the gemcitabine sensitivity of meningioma cells, the present results demonstrated that hENT1 is involved in the regulation of dCK and plays a critical role in the proliferation and survival of meningioma cells, which contributed to the identification of a relationship between the expression of these molecules and the survival of meningioma patients. The present results, which suggest the potential of hENT1 and dCK as excellent prognostic molecular markers of meningioma, introduce the possibility of a chemotherapeutic approach in a subset of meningioma patients with an otherwise poor prognosis and, as such, may make a significant contribution to the promotion of precision medicine for meningioma.
Meningiomas are the most common intracranial neoplasms originating from the arachnoid cap cells of the leptomeninges.1 These tumors are categorized into three histopathological grades (I-III) in the World Health Organization (WHO) classification system, with the mitotic index serving as a key criterion in grading meningiomas.2 A high Ki-67 labeling index, an indicator of cell proliferation, has been closely associated with a high risk of recurrence and a poor prognosis in patients with meningiomas.3 However, the molecular mechanisms responsible for the proliferative activity of meningioma cells remain unclear.
In contrast to benign WHO grade I meningiomas that account for ~80% of all meningioma cases and may be cured by surgical resection, high-grade (WHO grade II and III) meningiomas, which account for the remainder (~20%), pose substantial therapeutic challenges.4 While nearly half of grade II meningiomas reportedly recur even after surgical resection,5–7 the prognosis of grade III anaplastic meningioma is more dismal, with median overall survival being less than 3 years.8–10 Postoperative radiotherapy is recommended for all grade III meningiomas and incompletely resected grade II meningiomas,11 and chemotherapy is considered when no further surgical or radiotherapy options exist. However, chemotherapy for meningiomas is still experimental; a few candidate drugs have been tested in clinical trials and none have yet to demonstrate efficacy in high-grade meningiomas.12–14 Therefore, the development of novel chemotherapies for high-grade meningiomas is needed.
We recently identified gemcitabine (2′,2′-difluorodeoxycytidine [dFdC]) from a panel of chemotherapeutic agents as a highly effective drug against high-grade meningioma cells in vitro. We demonstrated that it controlled tumor growth for an extended period in vivo in a mouse xenograft model of meningioma,15 which warrants clinical trials to evaluate its efficacy in patients with high-grade meningioma. However, due to the lack of sufficient information on the molecular mechanisms underlying the high sensitivity of high-grade meningioma cells to gemcitabine, there are currently no molecular biomarkers with which to select patients most likely to benefit from gemcitabine. Therefore, we herein searched for factors that contribute to the sensitivity of meningioma cells to gemcitabine and also examined their roles in the biology of meningioma.
Materials and Methods
Cell Culture
HKBMM, a human malignant meningioma cell line, was obtained from the Riken BioResource Research Center (Tsukuba, Japan) and maintained in Ham’s F12 medium supplemented with 10% fetal bovine serum (FBS). IOMM-Lee, a human malignant meningioma cell line, and IMR90, normal human fetal lung fibroblasts, were obtained from the American Type Culture Collection (Manassas, VA, USA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS. M-20-U, M-10-S, and M-16-N were established by H.H. from the brain tumor tissues of benign meningiomas (M-20-U and M-10-S) and an atypical meningioma (M-16-N), as described in detail in the Supplementary Materials and Methods, and were maintained in RPMI-1640 medium supplemented with 20% FBS.
Meningioma Patient Cohort and Specimens
Clinical and histopathological information as well as tissue specimens were retrospectively collected from all high-grade meningiomas (9 WHO grade II meningiomas from 7 patients) that were treated surgically at the Department of Neurosurgery, Yamagata University between 2009 and 2019 and with surgical tissue specimens available for immunohistochemical analyses. Clinical and histopathological information as well as surgical specimens were collected from all high-grade meningiomas (11 WHO grade II meningiomas from 11 patients and 1 WHO grade III from 1 patient) treated surgically at the Department of Neurosurgery, Kagoshima University between 1996 and 2018. Low-grade meningiomas (26 WHO grade I meningiomas from 26 patients) treated at each institution during the same periods were used as controls. Criteria for the selection of low-grade meningioma cases are described in the Supplementary Materials and Methods. The histopathological diagnosis and grading of meningiomas were performed by the pathology departments of the treating institutions according to the WHO classification available at the time of surgery.
To verify the authenticity of tissue specimens prior to conducting immunohistochemical analyses, a hematoxylin and eosin-stained section from a specimen of each meningioma was reviewed by a board-certified pathologist (M.Y.) to confirm whether it was consistent with the histopathological diagnosis originally made at the treating institution. The following data were retrieved for clinical information: patient age at the time of surgery, sex, date of surgery, and date of tumor progression or the last follow-up. Tumor progression and progression-free survival (PFS) were defined as documented growth of the tumor after surgery on follow-up imaging by magnetic resonance imaging (MRI) or computed tomography (CT) and the time from surgery to tumor progression, respectively. Two meningiomas (2 WHO grade II from 1 patient of the Yamagata cohort) were excluded from the survival analysis because of the difficulty obtaining the exact date of tumor progression due in part to the multiplicity of tumors. The study protocol was reviewed and approved by the respective institutional review boards. Written informed consent was obtained from all participants.
Statistical Analysis
Results were expressed as the mean and standard deviation (SD), and differences were compared using the Student’s t-test, Kruskal-Wallis test, one-sample t-test, or one-way analysis of variance (ANOVA) with Tukey’s post-hoc test. Relationships between histopathological factors were analyzed using a simple linear regression analysis. PFS curves were obtained using the Kaplan-Meier method. PFS was compared between groups using the Log-rank test. A P value of <.05 was considered to be significant. All analyses were performed using GraphPad Prism version 8 for Mac (GraphPad Software, San Diego, CA, USA).
The details of materials and other methods are described in the Supplementary Material.
Results
High-Grade Meningioma Cells Are Highly Sensitive to Gemcitabine and Express High Levels of hENT1 and dCK
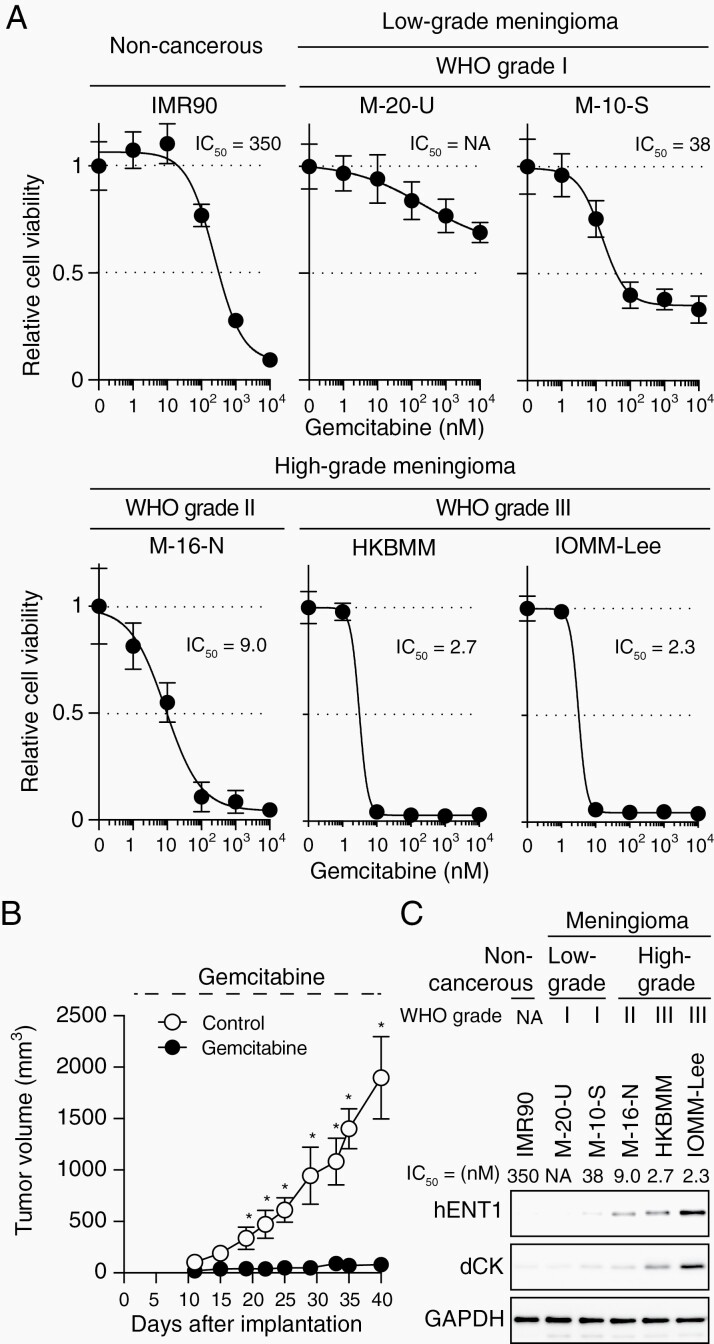
We previously reported that high-grade meningioma cell lines were as or more sensitive to gemcitabine than gemcitabine-sensitive human cancer cell lines derived from cancers for which gemcitabine is used in clinical practice.15 However, it remained unclear whether the sensitivity of meningioma cells to gemcitabine was dependent on their grade of malignancy. Therefore, we examined the growth inhibitory effects of gemcitabine on human meningioma cell lines including M-20-U and M-10-S (derived from WHO grade I meningiomas) and IOMM-Lee (derived from WHO grade III meningioma) in addition to those used in our previous study.15 The results obtained showed that high-grade meningioma cell lines including IOMM-Lee were more sensitive to gemcitabine than low-grade meningioma cell lines and IMR90 normal human fibroblasts, as indicated by their lower IC50 values (Figure 1A). Furthermore, IOMM-Lee cells were sensitive to gemcitabine-induced cell cycle arrest in in vivo subcutaneous xenograft mouse models similar to HKBMM malignant meningioma cells (Figure 1B, Supplementary Figure S1), which were previously shown to be highly sensitive to gemcitabine in vivo.15 Reflecting the high sensitivity of IOMM-Lee cells to gemcitabine observed in vivo in the subcutaneous models, systemic gemcitabine prolonged the survival of mice with intracranial meningiomas in a brain tumor treatment model (Supplementary Figure S2). Collectively, these results suggest that high-grade meningioma cells have increased sensitivity to gemcitabine, which may be of therapeutic significance.
Fig. 1.
hENT1 and dCK expression and gemcitabine sensitivity in cultured meningioma cells. (A) Viability of cells treated with gemcitabine. IMR90 (a noncancerous, normal human fetal fibroblast cell line), M-20-U, M-10-S (low-grade meningioma cell lines), M-16-N, HKBMM, and IOMM-Lee (high-grade meningioma cell lines) cells treated with various concentrations of gemcitabine (0, 1, 10, 102, 103, or 104 nM) for 3 days were cultured without gemcitabine for another 3 days. Cell viability was then evaluated by the WST-8 assay in 5 replicates. IC50 values were calculated by a nonlinear regression model. (B) Systemic administration of gemcitabine inhibits the growth of IOMM-Lee xenografts. On the day after the subcutaneous implantation of IOMM-Lee cells, mice were treated with gemcitabine (20 mg/kg, intraperitoneal injection, three times a week, n = 6) or vehicle alone (Control, n = 6). (C) The relationship between the IC50 for gemcitabine and the expression of hENT1 and dCK was analyzed by Western blotting. Values in (A) and (B) are shown as the mean ± SD. *P < .05. Abbreviations: dCK, deoxycytidine kinase; hENT1, human equilibrative nucleoside transporter 1, NA, not applicable.
We then investigated the molecular factors contributing to differential gemcitabine sensitivity among high- and low-grade meningioma cell lines. Since hENT1 (human equilibrative nucleoside transporter 1), a major transporter of gemcitabine,16,17 and dCK (deoxycytidine kinase), the rate-limiting enzyme for the intracellular activation of gemcitabine,18 have been shown to be critically involved in the gemcitabine sensitivity of some cancer types, such as pancreatic and biliary tract cancers, both in vitro and in clinical settings,17–23 we examined their expression levels in the meningioma cell lines. The results obtained indicated that both of these molecules were expressed at higher levels in the high-grade than in the low-grade meningioma cell lines (Figure 1C). The expression levels of hENT1 and dCK strongly correlated with the IC50 values for gemcitabine and also showed very close parallelism between themselves (Figure 1C). Therefore, parallel increases in the expression of hENT1 and dCK may be responsible for the increased sensitivity of high-grade meningioma cells to gemcitabine.
Increased Expression of hENT1 and dCK in High-Grade Meningiomas
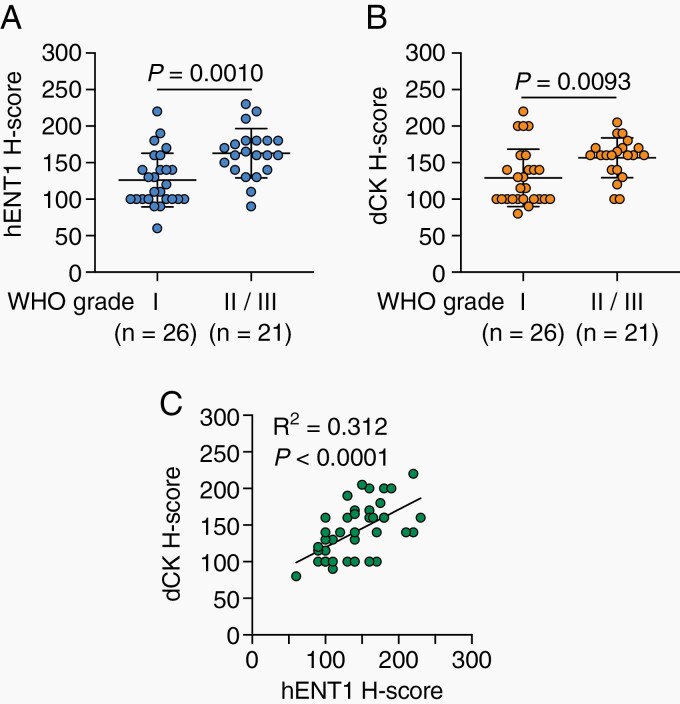
To clarify whether WHO grade-dependent parallel increases in the expression of hENT1 and dCK in meningioma cell lines in vitro reflect hENT1 and dCK expression in vivo in patient meningiomas, we conducted an immunohistochemical analysis of hENT1 and dCK expression using surgically resected meningioma tissues (26 low-grade [WHO grade I] meningiomas and 21 high-grade [WHO grade II/III] meningiomas). The expression levels of hENT1 and dCK in high-grade meningioma tissues were both significantly higher than those in low-grade meningioma tissues (Figure 2A and B). Furthermore, a correlation was observed between the expression levels of hENT1 and dCK in patient meningioma tissues (Figure 2C), which was consistent with in vitro results (Figure 1C).
Fig. 2.
Relationship among hENT1 expression, dCK expression, and WHO grades in meningiomas. The H-scores of hENT1 (A) and dCK (B) expression in surgical specimens from patients with low-grade (WHO grade I) and high-grade (WHO grade II/III) meningiomas. Horizontal bars show the mean ± SD. P values were calculated by the Student’s t-test. (C) Correlation between hENT1 and dCK expression in surgical specimens from meningioma patients. R-squared (R2) and P values were calculated by a linear regression analysis. Abbreviations: dCK, deoxycytidine kinase; hENT1, human equilibrative nucleoside transporter 1.
The Endogenous Expression of hENT1 and dCK in High-Grade Meningioma Cells Contributes to Their Increased Sensitivity to Gemcitabine
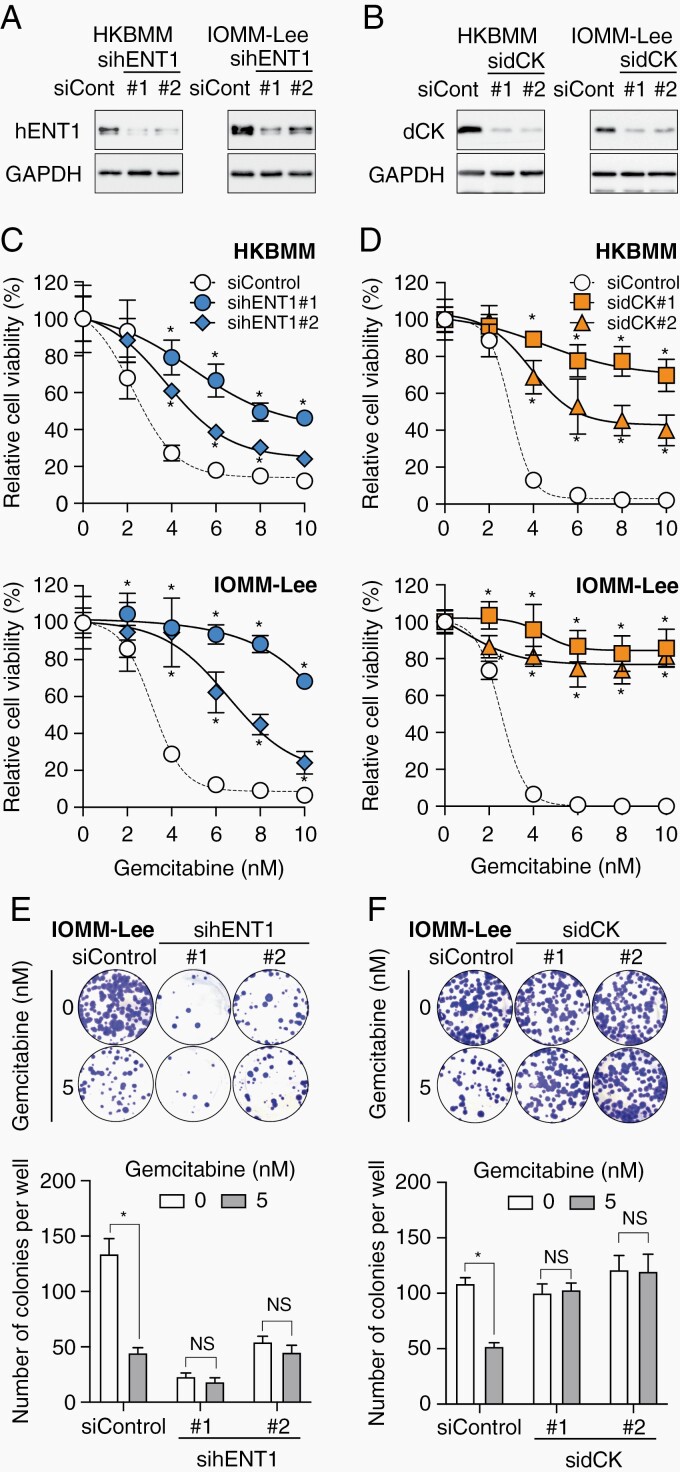
We investigated whether the increased expression of hENT1 and dCK in high-grade meningioma cells plays a critical role in their sensitivity to gemcitabine. We initially examined the effects of S-(4-nitrobenzyl)-6-thioinosine (NBTI), a specific inhibitor of the transporter activity of hENT1,24 on the gemcitabine sensitivity of high-grade meningioma cells. The pharmacological inhibition of hENT1 by the NBTI treatment impaired the growth inhibitory effects of gemcitabine on these cells (Supplementary Figure S3). This result was corroborated by the genetic knockdown of hENT1 (Figure 3A), which reduced the sensitivity of cells to gemcitabine in both short- (Figure 3C) and long-term (Figure 3E) assays. Similarly, the genetic knockdown of dCK resulted in high-grade meningioma cells that were less sensitive to gemcitabine in both short- and long-term assays (Figure 3B, D, and F). These results indicated that the endogenous expression of hENT1 and dCK plays a crucial role in the increased sensitivity of high-grade meningioma cells to gemcitabine.
Fig. 3.
Essential role for hENT1 and dCK expression in the sensitivity of high-grade meningioma cells to gemcitabine. (A-F) HKBMM and/or IOMM-Lee cells were transfected twice with siControl, sihENT1 (#1 or #2), or sidCK (#1 or #2). (A and B) Transfected cells were harvested 3 days after the second transfection and subjected to Western blotting. (C and D) Alternatively, 2 days after the second transfection, transfected cells were cultured in the presence of gemcitabine for 3 days and then in the absence of gemcitabine for another 3 days and subjected to the WST-8 assay. (E and F) Transfected IOMM-Lee cells were seeded at a colony-forming density (500 cells/well in 6-well plates), cultured in the presence of gemcitabine for 3 days, and then in the absence of gemcitabine for another 6-9 days, to perform the colony formation assay. In (C) through (F), values were shown as the mean ± SD from samples in 6 replicates of a representative experiment repeated with similar results. *P < .05, NS: P ≥ .05. Abbreviations: dCK, deoxycytidine kinase; hENT1, human equilibrative nucleoside transporter 1; NS, nonsignificant.
hENT1-Dependent Expression of dCK in Meningioma Cells
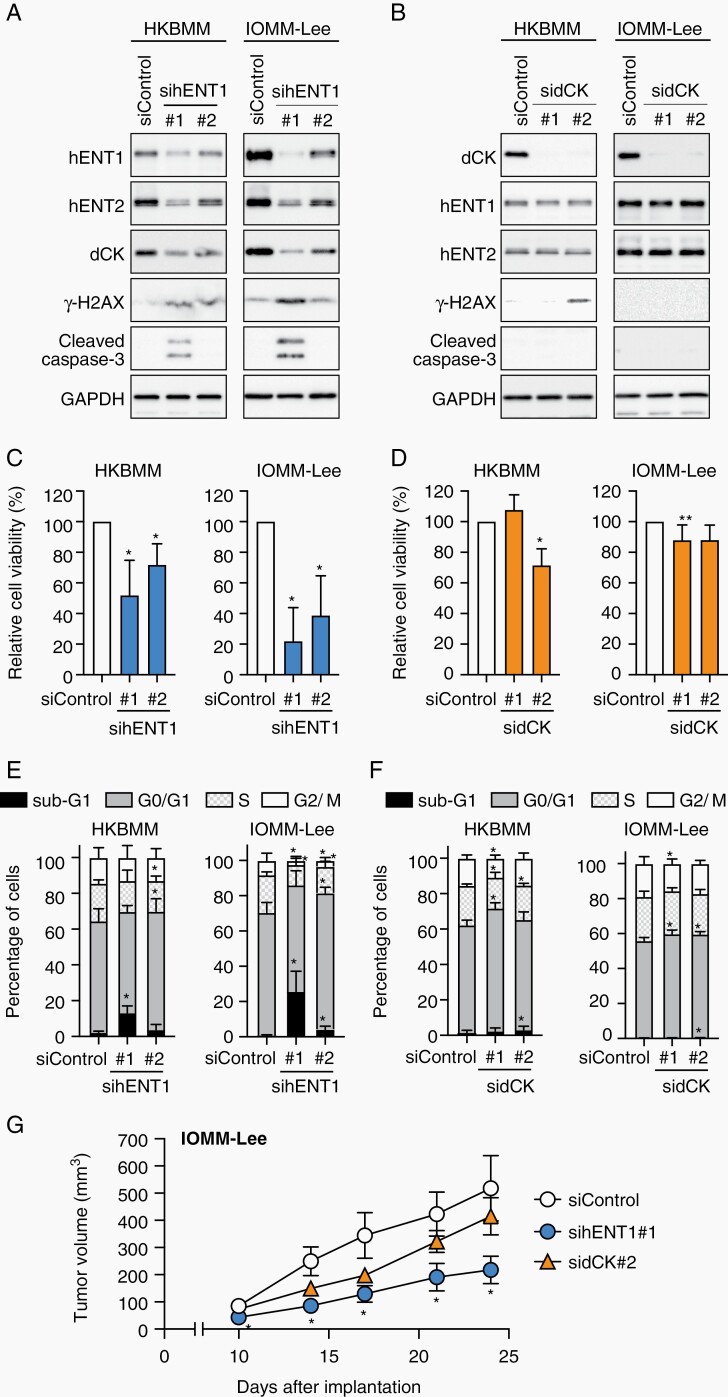
During knockdown experiments, we noted that the hENT1 knockdown reproducibly resulted in reduced dCK protein expression in the high-grade meningioma cell lines (Figure 4A), but not vice versa (Figure 4B). The hENT1 knockdown also downregulated the mRNA expression of dCK (Supplementary Figure S4), suggesting that hENT1 regulates dCK expression at the transcriptional level. These results suggested an unexpected molecular mechanism for the close relationship between the expression of hENT1 and dCK, which was observed earlier both in vitro (Figure 1C) and in vivo (Figure 2C). Since the hENT1 inhibitor NBTI failed to affect the protein (Supplementary Figure S5A) or mRNA (Supplementary Figure S5B) level of dCK, the transporter activity of hENT1 may not necessarily be required for the hENT1-mediated regulation of dCK expression. Collectively, these results implied that hENT1 acts upstream of dCK in meningioma cells and, thus, influences their sensitivity to gemcitabine.
Fig. 4.
hENT1-dependent growth of high-grade meningioma cells. HKBMM and IOMM-Lee cells transfected twice with siControl, sihENT1 (#1 or #2), or sidCK (#1 or #2) were harvested 3 days after the second transfection and subjected to Western blotting (A and B) and cell cycle analyses (n = 3-5) (E and F). Alternatively, transfected cells were seeded on 96-well plates (1000 cells/well for HKBMM and 500 cells/well for IOMM-Lee) on the day after the second transfection and, after 7 days, cell viability was evaluated by the WST-8 assay (n = 5-7) (C and D). (G) IOMM-Lee cells transfected twice with siControl, sihENT1#1, or sidCK#2 siRNAs were subcutaneously implanted into nude mice (1 × 106 viable cells/injection, n = 8, each group) on the day following the second transfection, and the tumor volume was measured. In (C) through (G), values in the graphs were the mean and SD. In (C) and (D), the average absorbance for siControl was set to 100% for each experiment, and P values were calculated by a one-sample t-test. In (E) and (F), P values from comparisons with the values of siControl were calculated by a one-way ANOVA with Tukey’s post-hoc test. In (G), P values from comparisons with the values of siControl group were calculated by the Kruskal-Wallis test. *P < .05. Abbreviations: ANOVA, analysis of variance; dCK, deoxycytidine kinase; hENT1, human equilibrative nucleoside transporter 1.
hENT1-Dependent Growth and Survival of High-Grade Meningioma Cells
During knockdown experiments, we also noted that the hENT1 knockdown reproducibly resulted in reduced viability in both HKBMM and IOMM-Lee cells (Figure 4C), whereas the effects of the dCK knockdown on cell viability were negligible (Figure 4D). A cell cycle analysis revealed that modest reductions in hENT1 expression (by use of sihENT1 #2) led to a significant increase in the G0/G1 fraction accompanied by a slight increase in the sub-G1 fraction (Figure 4A and E). The increase in the sub-G1 fraction, which was most likely due to apoptosis based on elevated expression levels of cleaved caspase 3 (Figure 4A), became more pronounced when hENT1 expression was more potently reduced by sihENT1 #1 (Figure 4A and E). These results suggested that the inhibition of hENT1 activity resulted in cellular phenotypes ranging from a proliferation block to apoptotic cell death depending on the severity of hENT1 inhibition. In contrast, the impact of the dCK knockdown on the cell cycle status was less pronounced (Figure 4F). Decreased cell viability as a result of the hENT1 knockdown was accompanied by the increased expression of γH2AX, an indicator of DNA double-strand breaks (Figure 4A). These results are consistent with previous findings showing that an insufficient supply of nucleosides and the resultant imbalance in the dNTP pool may aggravate “replication stress,” thereby inducing DNA double-strand breaks and, consequently, apoptotic cell death.25,26 In an attempt to validate these results pharmacologically, we also examined the effects of different NBTI concentrations on the viability of high-grade meningioma cells. NBTI only decreased cell viability at 100 µM (Supplementary Figure S3A), a concentration at which it inhibits not only hENT1, but also hENT2, which is another human equilibrative nucleoside transporter that has an overlapping, but somewhat different, substrate specificity and is 7000-fold less sensitive to NBTI than hENT1.24,27,28 Prompted by this result, we examined the expression of hENT2 in high-grade meningioma cells. The hENT1 knockdown consistently decreased the protein (Figure 4A), but not mRNA (Supplementary Figure S4) expression of hENT2, suggesting the hENT1-dependent protein expression of hENT2 in vitro. We also found that the hENT2 knockdown decreased the expression of hENT1 and dCK and the growth and gemcitabine sensitivity of high-grade meningioma cells (Supplementary Figure S6). Since hENT2 was recently shown to be transported to the plasma membrane to form hetero-oligomers with hENT1,29 the present results imply that the hENT2 protein recruited to the plasma membrane is stabilized and helps stabilize hENT1 when complexed with hENT1. Therefore, hENT1 may cooperate with hENT2 to support the growth of meningioma cells in vitro. To confirm whether hENT1 expression is a critical factor influencing meningioma cell growth in vivo, we implanted high-grade meningioma cells into nude mice after the knockdown of hENT1 or dCK. Although the growth of tumors formed by dCK knockdown cells was similar to or slightly less than that of control tumors, the growth of tumors from hENT1 knockdown cells was significantly inhibited and accompanied by increases and decreases in γH2AX- and Ki-67-positive tumor cell numbers, respectively (Figure 4G, Supplementary Figure S7). These results suggested that hENT1 expression is required to prevent DNA damage and sustain the growth of meningioma cells in vivo and in vitro.
Clinical Impact of hENT1 and dCK Expression in Meningioma
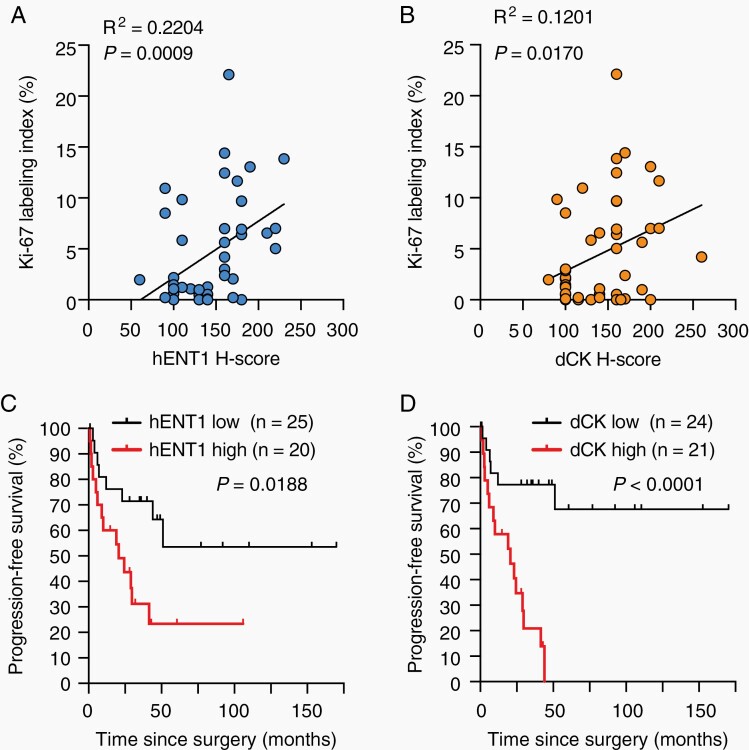
The present results suggested that hENT1 expression, which is involved in the control of dCK and hENT2 expression in vitro, is one of the key factors affecting the growth capacity of meningioma cells in culture. Therefore, we investigated the clinical relevance of the results obtained from cultured cells through an immunohistochemical analysis of surgically resected meningioma tissues. A correlation was observed between hENT1 expression and tumor cell proliferative activity represented by the Ki-67 labeling index (Figure 5A). As expected from the strong correlation between hENT1 and dCK expression described earlier (Figure 2C), a modest correlation was observed between dCK expression and the Ki-67 labeling index (Figure 5B). Based on the relationship between hENT1 expression and tumor cell proliferative activity in meningioma tissues, we investigated the impact of hENT1 expression on the PFS of meningioma patients. Consistent with its relationship with tumor cell proliferative activity, high expression levels of hENT1 were associated with shorter PFS (Figure 5C). Similarly, high expression levels of dCK were associated with shorter PFS; however, this relationship was more pronounced than that of hENT1 (Figure 5D). hENT2 was predominantly expressed in the nucleus of meningioma tissues in contrast to hENT1, which showed a diffuse pattern of expression (Supplementary Figure S8), and no correlations were observed between the expression levels of hENT2 and hENT1 (Supplementary Figure S9A), suggesting that the functional interaction between hENT1 and hENT2 in vitro is unique to in vitro culture conditions and may not be operative in vivo. In accordance with its lack of a relationship with hENT1 expression, hENT2 expression did not correlate with the Ki-67 labeling index (Supplementary Figure S9B), WHO grade (Supplementary Figure S9C), or PFS in meningioma patients (Supplementary Figure S9D); however, low rather than high expression levels of hENT2 were associated with shorter PFS (Supplementary Figure S9D). Collectively, the results of the immunohistochemical analysis of clinical meningioma samples were consistent with high hENT1 expression levels contributing to the increased malignancy of meningiomas, both clinically (as represented by shorter PFS) and histopathologically (as represented by an increased Ki-67 labeling index), by facilitating tumor cell growth. The present results also demonstrated the potential of hENT1 and dCK as prognostic biomarkers for meningiomas.
Fig. 5.
Impact of hENT1 and dCK expression in meningiomas on tumor proliferative activity and patient survival. (A and B) Correlation between the Ki-67 labeling index and the expression of hENT1 (A) and dCK (B) in surgical specimens from meningioma patients. (C and D) Kaplan-Meier plot of the relationship between progression-free survival in meningioma patients and the expression of hENT1 (C) and dCK (D). In (A) and (B), P values were calculated by a linear regression analysis. In (C) and (D), P values were calculated by the Log-rank test. Abbreviations: dCK, deoxycytidine kinase; hENT1, human equilibrative nucleoside transporter 1.
Discussion
We herein demonstrated that high-grade meningiomas expressed higher levels of hENT1 and dCK than low-grade meningiomas and that their expression critically contributed to the sensitivity of high-grade meningioma cells to gemcitabine. To exert cytotoxic effects, gemcitabine needs to be incorporated into cancer cells, mainly by hENT1,16,30,31 and is then activated through phosphorylation by dCK.32 In line with the essential roles of hENT1 and dCK in gemcitabine metabolism, the hENT1 knockdown in biliary tract cancer cells20 and dCK knockdown in pancreatic cancer cells19 attenuated cell sensitivity to gemcitabine, which is consistent with the present results. High expression levels of hENT1 or dCK were associated with better survival in gemcitabine-treated cholangiocarcinoma20,21 and pancreatic cancer,17,18,22,23 indicating that the functional roles of hENT1 and dCK in vitro reflect their roles in gemcitabine sensitivity in clinical settings. Therefore, the present results demonstrated that patients with meningiomas expressing high levels of hENT1 and dCK may benefit from gemcitabine-based chemotherapy and, thus, clinical trials to test the efficacy of gemcitabine in high-grade meningiomas are warranted.
We also identified an unexpected role for hENT1 in the proliferation and survival of meningioma cells, demonstrating that hENT1 expression is required to prevent DNA damage and sustain the growth of meningioma cells both in vitro and in vivo. However, a recent study reported that the knockdown of hENT1 in a cholangiocarcinoma cell line resulted in G1/S arrest accompanied by an increase in the subG1 fraction. Furthermore, high hENT1 expression levels were associated with a high Ki-67 labeling index and shorter overall survival in intrahepatic cholangiocarcinomas treated without gemcitabine.33 The present results are consistent with these findings and collectively suggest that the growth regulatory role of hENT1 is a feature shared by some types of cancer cells instead of being unique to meningioma cells. Although the mechanisms underlying the growth regulatory function of hENT1 remain speculative and hypothetical, hENT1 may promote the proliferative activity of tumor cells through an adequate supply of precursors for DNA synthesis based on the primary function of hENT1 as a transporter of nucleosides and nucleobases, which are precursors of nucleotides that are essential for DNA synthesis.27 In support of this concept, previous studies demonstrated that an insufficient supply of nucleotides resulted in dysfunctional proliferation, DNA damage, and, ultimately, apoptosis.25,26,34 In contrast to hENT1, our in vitro and in vivo results suggest a minor role for dCK in meningioma cell growth, implying that the reaction catalyzed by this enzyme is not rate-limiting for the proliferative activity of meningioma cells. The present results also revealed that the reduced expression of hENT1 decreased dCK expression; however, the mechanistic link between hENT1 and dCK remains elusive. We hypothesized that hENT1 may affect the availability of nucleosides, including the endogenous dCK substrate deoxycytidine, in cooperation with hENT2 in vitro, which may influence the expression of dCK.
The WHO grading system classifies meningiomas based on histopathological findings, including cell proliferation evaluated by the number of mitoses per 10 high power fields.2,35 Although the criteria for grading is based on clinical relevance to predict outcomes, the current WHO grading system may not always accurately predict clinical courses, underscoring the need for novel biomarkers and/or grading systems of prognostic value. Two recent studies addressed this need and indicated that a classification based on genome-wide methylation profiling36 and unsupervised hierarchical clustering according to RNA sequencing (RNA-seq) and whole-exome sequencing data37 more accurately predicted clinical outcomes than the WHO classification. However, since genome-wide analyses are not always available in a standard clinical laboratory, it may still be difficult to apply these methods to a routine clinical diagnosis. We demonstrated using an immunohistochemical analysis that high expression levels of hENT1 or dCK correlated with shorter PFS in meningiomas. Moreover, high dCK expression levels were associated with shorter PFS even when WHO grade I or II/III tumors were selected (Supplementary Figure S10C). Therefore, the present results suggest that hENT1 and dCK function not only as feasible, but also useful prognostic markers in the routine diagnosis of meningiomas when combined with the WHO grading system. Consistent with the present results, the significance of hENT1 as a prognostic marker has already been demonstrated in intrahepatic cholangiocarcinoma,33 ampullary carcinoma,38 and gastric cancer.39 Similarly, a relationship between high dCK expression levels and a poor prognosis has been reported in esophageal cancer,40 breast cancer,41 and adenocarcinoma of the esophagus, esophagogastric junction, and gastric cardia.42 Although the underlying mechanisms for the relationship between dCK expression and a poor prognosis in meningioma remain elusive, clinical studies are warranted to evaluate the prognostic significance of hENT1 and dCK.
In conclusion, we have revealed in this study, novel roles of hENT1 and dCK in the biology of meningioma cells and thereby discovered them as potential biomarkers with which to identify meningioma patients who have poor prognosis but nevertheless are most likely to benefit from gemcitabine-based chemotherapy. The present study thus makes a significant step toward establishing precision medicine for the management of meningioma patients.
Funding
This work was supported by Grants-in-Aid for Young Scientists 18K16550 and 20K17949 from the Ministry of Education, Culture, Sports, Science and Technology of Japan (T.S.) and by a grant from the Japan Brain Foundation (M.Y.).
Conflict of interest statement. The authors declare no competing interests.
Authorship statement. Conceptualization: C.K. and M.Y. Project administration: C.K. Supervision: C.K. Data analysis and interpretation: C.K., M.Y., K.T., Y.S., H.H., and K.Y. Investigation: M.Y., T.S., S.H.S., A.S., S.Z.S., and M.O. Validation: M.Y. Resources: H.U., H.Y., N.H., T.T., Y.Y., Y.S., H.H., and K.Y. Writing—original draft: C.K. and M.Y. Writing—review and editing: C.K., M.Y., T.S., S.H.S., H.U., H.Y., N.H., T.T., Y.Y., A.S., K.T., S.Z.S., M.O., Y.S., H.H., and K.Y.
Supplementary Material
References
- 1. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol. 2018;20(suppl_4):iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 3. Perry A, Stafford SL, Scheithauer BW, Suman VJ, Lohse CM. The prognostic significance of MIB-1, p53, and DNA flow cytometry in completely resected primary meningiomas. Cancer. 1998;82(11):2262–2269. [PubMed] [Google Scholar]
- 4. Paldor I, Awad M, Sufaro YZ, Kaye AH, Shoshan Y. Review of controversies in management of non-benign meningioma. J Clin Neurosci. 2016;31:37–46. [DOI] [PubMed] [Google Scholar]
- 5. Nanda A, Bir SC, Konar S, et al. Outcome of resection of WHO Grade II meningioma and correlation of pathological and radiological predictive factors for recurrence. J Clin Neurosci. 2016;31:112–121. [DOI] [PubMed] [Google Scholar]
- 6. Klinger DR, Flores BC, Lewis JJ, et al. Atypical meningiomas: recurrence, reoperation, and radiotherapy. World Neurosurg. 2015;84(3):839–845. [DOI] [PubMed] [Google Scholar]
- 7. Aghi MK, Carter BS, Cosgrove GR, et al. Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery. 2009;64(1):56–60; discussion 60. [DOI] [PubMed] [Google Scholar]
- 8. Moliterno J, Cope WP, Vartanian ED, et al. Survival in patients treated for anaplastic meningioma. J Neurosurg. 2015;123(1):23–30. [DOI] [PubMed] [Google Scholar]
- 9. Sughrue ME, Sanai N, Shangari G, Parsa AT, Berger MS, McDermott MW. Outcome and survival following primary and repeat surgery for World Health Organization Grade III meningiomas. J Neurosurg. 2010;113(2):202–209. [DOI] [PubMed] [Google Scholar]
- 10. Peyre M, Gauchotte G, Giry M, et al. De novo and secondary anaplastic meningiomas: a study of clinical and histomolecular prognostic factors. Neuro Oncol. 2018;20(8):1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun SQ, Hawasli AH, Huang J, Chicoine MR, Kim AH. An evidence-based treatment algorithm for the management of WHO Grade II and III meningiomas. Neurosurg Focus. 2015;38(3):E3. [DOI] [PubMed] [Google Scholar]
- 12. Karsy M, Hoang N, Barth T, et al. Combined hydroxyurea and verapamil in the clinical treatment of refractory meningioma: human and orthotopic xenograft studies. World Neurosurg. 2016;86:210–219. [DOI] [PubMed] [Google Scholar]
- 13. Graillon T, Sanson M, Campello C, et al. Everolimus and octreotide for patients with recurrent meningioma: results from the phase II CEVOREM trial. Clin Cancer Res. 2020;26(3):552–557. [DOI] [PubMed] [Google Scholar]
- 14. Suppiah S, Nassiri F, Bi WL, et al. ; International Consortium on Meningiomas . Molecular and translational advances in meningiomas. Neuro Oncol. 2019;21(Suppl 1):i4–i17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Takeda H, Okada M, Kuramoto K, et al. Antitumor activity of gemcitabine against high-grade meningioma in vitro and in vivo. Oncotarget. 2017;8(53):90996–91008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Farrell JJ, Elsaleh H, Garcia M, et al. Human equilibrative nucleoside transporter 1 levels predict response to gemcitabine in patients with pancreatic cancer. Gastroenterology. 2009;136(1):187–195. [DOI] [PubMed] [Google Scholar]
- 17. Greenhalf W, Ghaneh P, Neoptolemos JP, et al. Pancreatic cancer hENT1 expression and survival from gemcitabine in patients from the ESPAC-3 trial. J Natl Cancer Inst. 2014;106(1):djt347. [DOI] [PubMed] [Google Scholar]
- 18. Marechal R, Bachet JB, Mackey JR, et al. Levels of gemcitabine transport and metabolism proteins predict survival times of patients treated with gemcitabine for pancreatic adenocarcinoma. Gastroenterology. 2012;143(3):664–674.e6. [DOI] [PubMed] [Google Scholar]
- 19. Ohhashi S, Ohuchida K, Mizumoto K, et al. Down-regulation of deoxycytidine kinase enhances acquired resistance to gemcitabine in pancreatic cancer. Anticancer Res. 2008;28(4B):2205–2212. [PubMed] [Google Scholar]
- 20. Kim J, Kim H, Lee JC, et al. Human equilibrative nucleoside transporter 1 (hENT1) expression as a predictive biomarker for gemcitabine chemotherapy in biliary tract cancer. PLoS One. 2018;13(12):e0209104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Woo SM, Yoon KA, Hong EK, et al. DCK expression, a potential predictive biomarker in the adjuvant gemcitabine chemotherapy for biliary tract cancer after surgical resection: results from a phase II study. Oncotarget. 2017;8(46):81394–81404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maréchal R, Mackey JR, Lai R, et al. Deoxycitidine kinase is associated with prolonged survival after adjuvant gemcitabine for resected pancreatic adenocarcinoma. Cancer. 2010;116(22):5200–5206. [DOI] [PubMed] [Google Scholar]
- 23. Xiong J, Altaf K, Ke N, et al. dCK expression and gene polymorphism with gemcitabine chemosensitivity in patients with pancreatic ductal adenocarcinoma: a strobe-compliant observational study. Medicine (Baltimore). 2016;95(10):e2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ward JL, Sherali A, Mo ZP, Tse CM. Kinetic and pharmacological properties of cloned human equilibrative nucleoside transporters, ENT1 and ENT2, stably expressed in nucleoside transporter-deficient PK15 cells. Ent2 exhibits a low affinity for guanosine and cytidine but a high affinity for inosine. J Biol Chem. 2000;275(12):8375–8381. [DOI] [PubMed] [Google Scholar]
- 25. Oliver FJ, Collins MK, López-Rivas A. dNTP pools imbalance as a signal to initiate apoptosis. Experientia. 1996;52(10–11):995–1000. [DOI] [PubMed] [Google Scholar]
- 26. Bester AC, Roniger M, Oren YS, et al. Nucleotide deficiency promotes genomic instability in early stages of cancer development. Cell. 2011;145(3):435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boswell-Casteel RC, Hays FA. Equilibrative nucleoside transporters - a review. Nucleosides Nucleotides Nucleic Acids. 2017;36(1):7–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Narumi K, Ohata T, Horiuchi Y, et al. Mutual role of ecto-5′-nucleotidase/CD73 and concentrative nucleoside transporter 3 in the intestinal uptake of dAMP. PLoS One. 2019;14(10):e0223892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grañe-Boladeras N, Williams D, Tarmakova Z, et al. Oligomerization of equilibrative nucleoside transporters: a novel regulatory and functional mechanism involving PKC and PP1. FASEB J. 2019;33(3):3841–3850. [DOI] [PubMed] [Google Scholar]
- 30. Mackey JR, Mani RS, Selner M, et al. Functional nucleoside transporters are required for gemcitabine influx and manifestation of toxicity in cancer cell lines. Cancer Res. 1998;58(19):4349–4357. [PubMed] [Google Scholar]
- 31. Mackey JR, Yao SY, Smith KM, et al. Gemcitabine transport in Xenopus oocytes expressing recombinant plasma membrane mammalian nucleoside transporters. J Natl Cancer Inst. 1999;91(21):1876–1881. [DOI] [PubMed] [Google Scholar]
- 32. Huang P, Plunkett W. Induction of apoptosis by gemcitabine. Semin Oncol. 1995;22(4 Suppl 11):19–25. [PubMed] [Google Scholar]
- 33. Tavolari S, Deserti M, Vasuri F, et al. Membrane human equilibrative nucleoside transporter 1 is associated with a high proliferation rate and worse survival in resected intrahepatic cholangiocarcinoma patients not receiving adjuvant treatments. Eur J Cancer. 2019;106:160–170. [DOI] [PubMed] [Google Scholar]
- 34. Kohnken R, Kodigepalli KM, Wu L. Regulation of deoxynucleotide metabolism in cancer: novel mechanisms and therapeutic implications. Mol Cancer. 2015;14:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Banan R, Hartmann C. The new WHO 2016 classification of brain tumors - what neurosurgeons need to know. Acta Neurochir (Wien). 2017;159(3):403–418. [DOI] [PubMed] [Google Scholar]
- 36. Sahm F, Schrimpf D, Stichel D, et al. DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol. 2017;18(5):682–694. [DOI] [PubMed] [Google Scholar]
- 37. Patel AJ, Wan YW, Al-Ouran R, et al. Molecular profiling predicts meningioma recurrence and reveals loss of DREAM complex repression in aggressive tumors. Proc Natl Acad Sci U S A. 2019;116(43):21715–21726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Santini D, Perrone G, Vincenzi B, et al. Human equilibrative nucleoside transporter 1 (hENT1) protein is associated with short survival in resected ampullary cancer. Ann Oncol. 2008;19(4):724–728. [DOI] [PubMed] [Google Scholar]
- 39. Santini D, Vincenzi B, Fratto ME, et al. Prognostic role of human equilibrative transporter 1 (hENT1) in patients with resected gastric cancer. J Cell Physiol. 2010;223(2):384–388. [DOI] [PubMed] [Google Scholar]
- 40. Shimada Y, Okumura T, Sekine S, et al. Clinicopathological significance of deoxycytidine kinase expression in esophageal squamous cell carcinoma. Mol Clin Oncol. 2013;1(4):716–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Geutjes EJ, Tian S, Roepman P, Bernards R. Deoxycytidine kinase is overexpressed in poor outcome breast cancer and determines responsiveness to nucleoside analogs. Breast Cancer Res Treat. 2012;131(3):809–818. [DOI] [PubMed] [Google Scholar]
- 42. Peters CJ, Rees JR, Hardwick RH, et al. A 4-gene signature predicts survival of patients with resected adenocarcinoma of the esophagus, junction, and gastric cardia. Gastroenterology. 2010;139(6):1995–2004.e15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.