Abstract
Background
Erlotinib combined with whole-brain radiotherapy (WBRT) demonstrated a favorable objective response rate in a phase II single-arm trial of non–small cell lung cancer (NSCLC) patients with brain metastases. We assessed whether concurrent erlotinib with WBRT is safe and benefits patients in a phase III, randomized trial.
Methods
NSCLC patients with two or more brain metastases were enrolled and randomly assigned (1:1) to WBRT (n = 115) or WBRT combined with erlotinib arms (n = 109). The primary endpoint was intracranial progression-free survival (iPFS) and cognitive function (CF) was assessed by the Mini-Mental State Examination (MMSE).
Results
A total of 224 patients from 10 centers across China were randomized to treatments. Median follow-up was 11.2 months. Median iPFS for WBRT concurrent erlotinib was 11.2 months vs 9.2 months for WBRT-alone (P = .601). Median PFS and overall survival (OS) of combination group were 5.3 vs 4.0 months (P = .825) and 12.9 vs 10.0 months (P = .545), respectively, compared with WBRT-alone. In EGFR-mutant patients, iPFS (14.6 vs 12.8 months; P = .164), PFS (8.8 vs 6.4 months; P = .702), and OS (17.5 vs 16.9 months; P = .221) were not significantly improved in combination group over WBRT-alone. Moreover, there were no significant differences in patients experiencing MMSE score change between the treatments.
Conclusion
Concurrent erlotinib with WBRT didn’t improve iPFS and excessive CF detriment either in the intent-to-treat (ITT) population or in EGFR-mutant patients compared with WBRT-alone, suggesting that while safe for patients already taking the drug, there is no justification for adding concurrent EGFR-TKI with WBRT for the treatment of brain metastases.
Trial registration: Clinical trials.gov identifier: NCT01887795
Keywords: brain metastases, epidermal growth factor receptor, erlotinib, non–small cell lung cancer, whole-brain radiation therapy
Key Points.
Concurrent erlotinib with whole-brain radiotherapy (WBRT) didn’t improve intracranial progression-free survival or overall survival for NSCLC patients with BM.
Concurrent erlotinib with WBRT is safe for non–small cell lung cancer patients with BM.
Combination of WBRT with concurrent erlotinib was well tolerated with no unexpected neurotoxicity.
Importance of the Study.
Brain metastasis (BM) is a leading cause of death in patients with non–small cell lung cancer (NSCLC). As the two most effective strategies available, combination of both EGFR-TKIs and whole-brain radiation therapy (WBRT) might be expected to improve the control of multiple brain metastases. This is the first prospective controlled phase III trial evaluating efficacy and safety of WBRT concurrent erlotinib for NSCLC patients with brain metastases from solid tumors. We found that concurrent erlotinib with WBRT didn’t improve intracranial progression-free survival or overall survival for NSCLC patients with BM, but the combination therapy was well tolerated with no unexpected neurotoxicity.
Brain metastasis (BM) is a leading cause of death in patients with non–small cell lung cancer (NSCLC), the most common cause of cancer death.1 Approximately, 10%–40% of untreated metastatic NSCLC patients are diagnosed with brain metastases at some point in their disease course2,3 and is associated with a poor prognosis.4
Therapeutic modalities for BM include whole-brain radiation therapy (WBRT), stereotactic radiosurgery (SRS), surgery, chemotherapy, tyrosine kinase inhibitors (TKIs), or a combination of these therapies.5 Historically, the prognosis of NSCLC patients with BMs has been poor, however, median survival time is improving at 3.0-46.8 months with new therapies.2 WBRT remains standard-of-care treatments for NSCLC patients with multiple BMs. Surgery and SRS are suitable for selected patients with a more limited number of BMs.6 However, the local control rate is still unsatisfactory when treated with WBRT-alone,7 which poses challenges for clinical practice.
EGFR is a novel therapeutic target in NSCLC, and EGFR-TKIs are the standard-of-care first-line systemic treatment for EGFR-mutant patients,8,9 and is found in 20% of Caucasian and more than 60% of Asian patients, usually in nonsmokers.10,11EGFR-mutant patients with BMs can benefit from especially newer generation EGFR-TKIs, such as gefitinib and erlotinib,12 and a randomized, open-label, multicentre, phase III clinical study showed that EGFR-TKIs were superior to WBRT and concurrent or sequential chemotherapy.13
EGFR can be induced by radiotherapy and cause radiotherapy resistance14,15 and WBRT may have a synergistic effect on EGFR-TKIs by disrupting the blood-brain barrier (BBB).16,17 Therefore, it can be hypothesized that combination of both EGFR-TKIs and WBRT will improve the control of BMs treated with WBRT.18 A previous phase II single-arm clinical trial showed a favorable objective response rate (ORR) for WBRT with concurrent erlotinib.19 Another phase II study showed that compared with WBRT monotherapy, combination of erlotinib and WBRT significantly improves intracranial ORR (54.84% vs 95.65%, P = .001), and prolonged local progression-free survival (LPFS), progression-free survival (PFS), and overall survival (OS) in patients with multiple brain metastases of lung adenocarcinoma.20 However, no randomized controlled phase III trial comparing WBRT with concurrent EGFR-TKIs with WBRT-alone in NSCLC patients with multiple BMs has been performed to date, despite increasing interest in exploring local therapeutic measures combined with EGFR-TKIs. We therefore undertook the ENTER (The Efficacy and Toxicity of Erlotinib concurrent with WBRT), a phase III trial to better assess the benefits and safety of adding concurrent erlotinib to WBRT in brain-metastatic NSCLC.
Methods
Study Design
This was a phase III, multicenter, randomized, open-label trial undertaken in 10 centers in China (ClinicalTrials.gov identifier: NCT01887795). Patients were randomly assigned in a 1:1 ratio to the WBRT-plus concurrent erlotinib or WBRT-alone arms using central permuted block randomization. Patients and investigators were not blinded to the treatment assignment. The study was approved by the institutional review boards at all sites and conformed to the Declaration of Helsinki guidelines. Written informed consent was obtained from all patients.
Patients
Patients were eligible if they were ≥18 years old, had histologically confirmed stage IV or recurrent NSCLC, and multiple metastatic lesions (two or more)21 in the brain (one or more lesions of >10 mm in diameter) by contrast-enhanced magnetic resonance imaging (MRI), Karnofsky performance status score of 70 or better, baseline graded prognostic assessment (GPA) score between 0.5 and 3.5 with adequate organ function,22 and a life expectancy of at least 3 months. Patients were excluded if they had previously received any brain radiotherapy, had previous or concomitant malignancies at other sites or any other neurocognitive dysfunction diseases, a history or presence of poorly controlled systemic diseases, or if any antitumor drug or other investigational drug had been administered within 4 weeks of randomization.
Treatment
Patients received either WBRT (40 Gy in 20 fractions) plus concurrent oral erlotinib at a dose of 150 mg per day (induction erlotinib for 6 days then concurrently with WBRT) or WBRT-alone until unacceptable adverse events (AEs) occurred. Reduction or interruption of dosing of erlotinib was permitted due to AEs. Erlotinib dose could be initially reduced to 100 mg/day and then to 50 mg/day.
Subsequent Systemic Treatments and Assessments
After the protocol-specified treatment, subsequent systemic treatment was standard first-line treatment.23 In EGFR wild-type subgroup, cisplatin-based chemotherapy combined with pemetrexed or paclitaxel were subsequent treatments for adenocarcinoma and squamous carcinoma, respectively. EGFR-mutant patients received continued erlotinib. Therefore, this trial design enabled a parallel investigation of WBRT with concurrent erlotinib compared with WBRT with sequential erlotinib in an EGFR-mutant subgroup.
Patients were assessed 1 month after WBRT treatment and then every 8 weeks by contrast-enhanced MRI for intracranial evaluation and contrast-enhanced computed tomography (CT) or positron emission tomography (PET)-CT for extracranial lesions according to RECIST 1.1.24 New intracranial metastases were defined as new intracranial lesions with a diameter of more than 5 mm in the horizontal plane.
Evaluation of Cognitive Function
Once the patient entered the clinical trial, his/her cognitive function (CF) was evaluated using the Mini-Mental State Examination (MMSE)25 every 2 months, and the analyzed time points in this study included baseline and months 1, 3, 5, 7, 9, 11, and 13 after WBRT. Following previous reports, an MMSE score of 26 or lower was the cutoff for impaired function. Significant MMSE score decline was defined as a decrease of >3 points; significant gain was defined as an increase of >3 points; no change was defined as any MMSE score change ≤3 points.26,27 All MMSE score changes were a comparison of baseline MMSE score. The dynamic changes in the MMSE were collected as part of the patient clinical evaluation at each study follow-up date.
End Points
The primary end point was intracranial progression-free survival (iPFS), defined as time from randomization to either intracranial disease progression or death from any cause, whichever occurred first. Secondary end points included PFS, OS, ORR, disease control rate (DCR), safety, and AEs.
EGFR Mutation Analyses
EGFR status was tested in DNA extracted from formalin-fixed paraffin-embedded primary cancer tissue samples using a commercially available kit approved by the China Food and Drug Administration (ADx EGFR Mutations Detection kit; Amoy Diagnostics, Xiamen, China), as previously described.28
Statistical Analysis
We predicted patients randomly assigned to the WBRT-plus concurrent erlotinib arm and WBRT-alone arm to have a median iPFS of 10.0 months20 and 6.0 months,29,30 respectively, with a hazard ratio (HR) of 0.6, based on previous studies. We calculated the sample size with a two-sided significance level of 0.05 and 90% statistical power using a two-sample log-rank test. Assuming 10% of patients would be ineligible or unassessable, we calculated a total recruitment target of 206 patients; 103 patients for each arm. With the investigators blinded to the size of each block to reduce selection bias, we calculated that this current study required a total sample size of 224 patients. iPFS was estimated from randomization to either intracranial disease progression or death using the Kaplan-Meier method. PFS and OS were calculated using the same method as iPFS. The Cox proportional hazard model was used to test for differences in iPFS, PFS, and OS between the two arms and to determine HRs and confidence intervals (CIs). All testing was performed at the 0.05 significance level.
The safety population comprised all patients in the WBRT-alone and with concurrent erlotinib arms who had begun radiotherapy and had at least one post-baseline safety assessment. AEs were assessed according to National Cancer Institute Common Terminology Criteria for AEs, version 4.02. The AE rates were compared using Fisher’s exact test and incidences of adverse effects were compared by χ 2 test. With MMSE score as a continuous variable, average MMSE score over time was analyzed using a linear mixed-effects model, with intercept and coefficients of time as random effects and treatment as fixed effects. The significance threshold was a P-value <.05.
Results
Patients
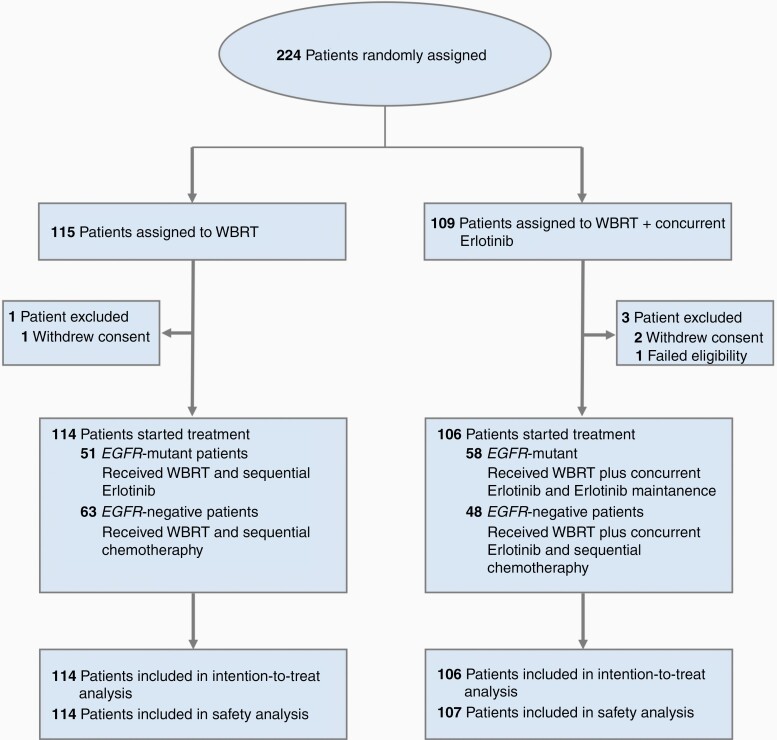
Between August 7, 2013 and November 25, 2016, 267 patients were screened from 10 centers across China, and 224 were randomly assigned to the treatment arms as the intent-to-treat (ITT) population, including 115 patients in the WBRT-alone arm and 109 patients in the WBRT with concurrent erlotinib arm (CONSORT diagram shown in Figure 1). In the WBRT with concurrent erlotinib arm, 2 patients withdrew informed consent before treatments were initiated, and another was excluded for ineligibility (a solitary BM). In the WBRT-alone arm, 1 patient withdrew informed consent before treatment. Thus, a total of 220 patients were included in the treatment and safety analyses (106 patients in the WBRT with concurrent erlotinib arm and 114 patients in the WBRT-alone arm). The study population was balanced at baseline (Table 1). Follow-up was conducted between August 7, 2013 and August 31, 2017. Median follow-up was 11.2 months (interquartile range [IQR], 4.6-18.2). The clinical data cutoff point was either at intracranial disease progression or death from any cause, whichever occurred first. The full trial protocol is available in Supplementary Material 1.
Fig. 1.
CONSORT diagram. This flowchart demonstrates trial profile recruitment and inclusion of patients in the study.
Table 1.
Baseline Characteristics for the Intention-to-Treat Study Population
| Variable | WBRT + erlotinib (n = 106) | WBRT (n = 114) | P-value |
|---|---|---|---|
| n (%) | n (%) | ||
| Sex | |||
| Male | 63 (59.4) | 69 (60.5) | .869 |
| Female | 43 (40.6) | 45 (39.5) | |
| Age at random assignment, y | |||
| Median | 55.5 | 56.0 | .746 |
| Range | 26-70 | 27-70 | |
| Smoking status | |||
| Never | 62 (58.5) | 65 (57.0) | .717 |
| Current | 14 (13.2) | 12 (10.5) | |
| Former | 30 (28.3) | 37 (32.5) | |
| Pathological examination | |||
| Adenocarcinoma | 102 (96.2) | 103 (90.4) | .07 |
| Squamous carcinoma | 4 (3.8) | 6 (5.3) | |
| Adenosquamous carcinoma | 0 (0) | 3 (2.6) | |
| Others | 0 (0) | 2 (1.8) | |
| Neurological symptoms | |||
| Present | 63 (59.4) | 74 (64.9) | .236 |
| Absent | 43 (40.6) | 40 (35.1) | |
| EGFR status | |||
| Mutant | 58 (54.7) | 51 (44.7) | .001 |
| Negative | 48 (45.3) | 63 (55.3) | |
| Brain metastases, no. | |||
| ≤3 | 53 (50.0) | 48 (42.1) | .24 |
| >3 | 53 (50.0) | 66 (57.9) | |
| Extracranial metastases | |||
| Present | 59 (55.7) | 67 (58.8) | .641 |
| Absent | 47 (44.3) | 47 (41.2) | |
| Karnofsky performance status score | |||
| 70 | 19 (17.9) | 23 (20.2) | .409 |
| 80 | 62 (58.5) | 63 (55.3) | |
| 90 | 25 (23.6) | 26 (22.8) | |
| 100 | 0 (0) | 2 (1.7) | |
| GPA | |||
| 0.5 | 11 (10.4) | 13 (11.4) | .573 |
| 1 | 20 (18.9) | 23 (20.2) | |
| 1.5 | 24 (22.6) | 28 (24.5) | |
| 2 | 29 (27.4) | 23 (20.2) | |
| 2.5 | 13 (12.2) | 13 (11.4) | |
| 3 | 6(5.7) | 13(11.4) | |
| 3.5 | 3(2.8) | 1(0.9) |
Abbreviations: WBRT, whole-brain radiotherapy; EGFR, epithelial growth factor receptor; GPA, graded prognostic assessment.
Efficacy
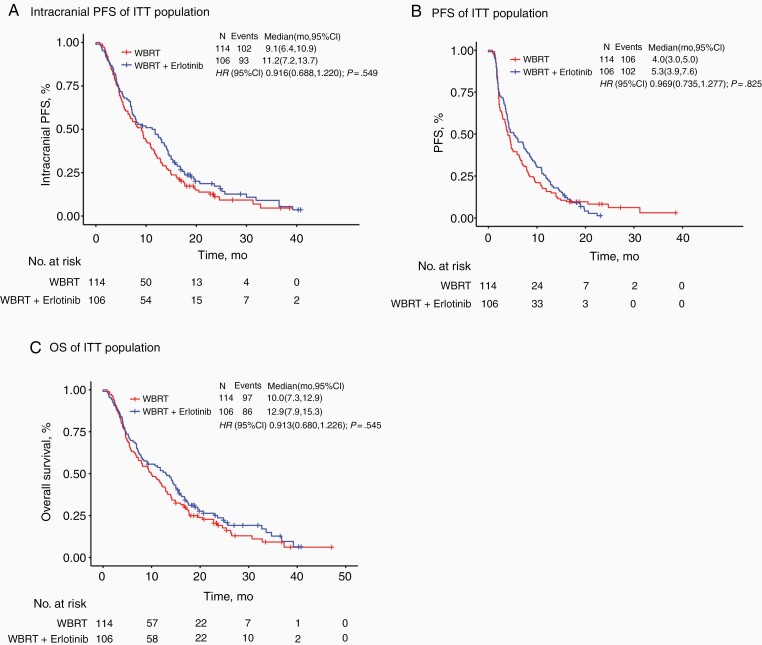
Median iPFS in the ITT population was 11.2 months (95% CI: 7.2-13.7) in the WBRT with concurrent erlotinib arm vs 9.1 months (95% CI: 6.4-10.9) for WBRT-alone. The Kaplan-Meier curve for iPFS in the ITT population by treatment arms is depicted in Figure 2A. There was no statistically significant difference between the two treatment arms (HR, 0.916; 95% CI: 0.688-1.220; P = .549). An iPFS benefit in favor of WBRT with concurrent erlotinib was observed in a subgroup of patients younger than 65 years. However, the iPFS improvement was not statistically significant across other subgroups, including gender, BM number, GPA, pathological type, smoking status, and so on (eFigure 1 in Supplementary Material 2).
Fig. 2.
Kaplan-Meier survival curves for (A) intracranial progression-free survival (iPFS), (B) progression-free survival (PFS), and (C) overall survival (OS) in the intent-to-treat (ITT) study population. (A) Median iPFS in the ITT population was 11.2 months (95% CI: 7.2-13.7) in the WBRT with concurrent erlotinib arm vs 9.1 months (95% CI: 6.4-10.9) for WBRT-alone. (B) Median PFS of concurrent erlotinib compared with WBRT-alone was 5.3 months vs 4.0 months, respectively. There was no statistically significant difference in PFS between the two treatment arms (HR, 0.969; 95% CI: 0.735-1.277; P = .825). (C) Median OS was 12.9 vs 10.0 months for WBRT with concurrent erlotinib and WBRT-alone, respectively. There was no statistically significant difference in OS between the two treatment arms (HR, 0.913; 95% CI: 0.680-1.226; P = .545). CI, confidence interval; WBRT, whole-brain radiotherapy; HR, hazard ratio.
The median PFS of concurrent erlotinib compared with WBRT-alone was 5.3 months vs 4.0 months, respectively. There was no statistically significant difference in PFS between the two treatment arms (HR, 0.969; 95% CI: 0.735-1.277; P = .825) (Figure 2B).
Among the 220 patients recruited to this trial, a total of 183 had died at the time of clinical data cutoff; 86 in the WBRT with concurrent erlotinib arm and 97 in the WBRT-alone arm. Median OS was 12.9 vs 10.0 months for WBRT with concurrent erlotinib and WBRT-alone, respectively. There was no statistically significant difference in OS between the two treatment arms (HR, 0.913; 95% CI: 0.680-1.226; P = .545) (Figure 2C).
In the subgroup of 109 EGFR-mutant patients, iPFS was not significantly longer in those who received WBRT with concurrent erlotinib than in those treated with WBRT-alone followed by sequential erlotinib (14.6 [95%: CI 11.8-17.7] vs 12.8 [95% CI: 7.9-14.9] months; HR, 0.743; 95% CI: 0.489-1.129; P = .164) (eFigure 3A in Supplementary Material 2). Similar results were found in both PFS (8.8 [95%: CI 5.9-11.4] vs 6.4 [95% CI: 3.6-9.5] months; HR, 0.926; 95% CI: 0.623-1.375; P = .702) and OS (17.5 [95%: CI 14.3-22.9] vs 16.9 [95% CI: 11.7-19.5] months; HR, 0.762; 95% CI: 0.493-1.178; P = .221) (eFigure 3B and C in Supplementary Material 2). The iPFS benefit of WBRT with concurrent erlotinib was observed in the subgroup with four or more BMs. However, no significance was observed in between two arms in other subgroups (eFigure 2 in Supplementary Material 2).
Safety
The safety population included all patients who received at least one dose of either erlotinib (107 patients) or WBRT-alone (114 patients). During treatment, 99.1% of patients treated with WBRT-plus concurrent erlotinib experienced at least one drug-related AE compared with 97.4% of those treated with WBRT-alone; drug-related AEs of any grade in the two arms were 73.8% and 38.6%, respectively. Significance differences were observed between the two arms for acneiform rash, dry skin, increased aspartate aminotransferase (AST)/alanine aminotransferase (ALT), increased bilirubin, paresthesia, and cough.
Drug-related acneiform rash was the most frequently reported AE and was more common in the WBRT with concurrent erlotinib arm than in the WBRT-alone arm at any grade (59.8% vs 27.2%, respectively) and at grades 3 or higher (11.2% vs 1.7%, respectively), with a statistically significant risk difference. Incidence of dry skin of any grade was 22.4% in the WBRT with concurrent erlotinib arm, and 8.8% in the WBRT-alone arm; however, there was a similar incidence of grade 3 or higher (0.9% vs 0.0%) AEs for both groups. For increased AST/ALT and increased bilirubin, incidence of any grade was higher in the WBRT-plus concurrent erlotinib arm than in the WBRT-alone arm (9.3% vs 2.6%, and 13.1% vs 5.3%, respectively), while grade 3 or higher AEs occurred at similar rates. Incidence of cough at any grade was 60.7% in the WBRT with concurrent erlotinib arm and 43.0% in the WBRT-alone arm, but there was a similar occurrence of grade 3 or higher AEs (5.6% in the WBRT with concurrent erlotinib arm vs 5.3% in the WBRT-alone arm, respectively). Grade 3 or higher AEs of any type are summarized in Table 2.
Table 2.
Selected Grade 3-5 Drug-Related Adverse Event (AE) Summary
| Drug-related AE | WBRT + erlotinib (n = 107) | WBRT (n = 114) | P any | P 3-5 | ||
|---|---|---|---|---|---|---|
| Any (%) | Grade 3-5 (%) | Any (%) | Grade 3-5 (%) | |||
| Constitutional symptoms | ||||||
| Fatigue | 59 (55.1) | 2 (1.9) | 63 (55.3) | 3 (2.6) | .9853 | 1.0000 |
| Weight loss | 14 (13.1) | 0 (0) | 15 (13.2) | 0 (0) | .9870 | - |
| Dermatology | ||||||
| Alopecia | 53 (49.5) | 2 (1.9) | 63 (55.3) | 2 (1.7) | .3939 | 1.0000 |
| Acneiform rash | 64 (59.8) | 12 (11.2) | 31 (27.2) | 2 (1.7) | <.0001 | .0039 |
| Dry skin | 24 (22.4) | 1 (0.9) | 10 (8.8) | 0 (0) | .0049 | .4842a |
| Pruritis | 33 (30.8) | 2 (1.9) | 24 (21.1) | 0 (0) | .0965 | .2333a |
| Hand-foot syndrome | 3 (2.8) | 0 (0) | 1 (0.9) | 0 (0) | .5695 | - |
| Gastrointestinal | ||||||
| Anorexia | 68 (63.6) | 1 (0.9) | 65 (57.0) | 3 (2.6) | .3214 | .6593 |
| Flatulence | 10 (9.3) | 0 (0) | 8 (7.0) | 0 (0) | .5271 | - |
| Diarrhea | 20 (18.7) | 0 (0) | 11 (9.6) | 1 (0.9) | .0531 | 1.0000a |
| Nausea | 45 (42.1) | 4 (3.7) | 45 (39.5) | 3 (2.6) | .6962 | .9321 |
| Vomiting | 35 (32.7) | 5 (4.7) | 37 (32.5) | 3 (2.6) | .9679 | .6515 |
| Altered taste | 3 (2.8) | 0 (0) | 1 (0.9) | 0 (0) | .5695 | - |
| Dehydration | 1 (0.9) | 0 (0) | 0 (0.0) | 0 (0) | .4842a | - |
| Hepatobiliary | ||||||
| Increased AST/ALT | 10 (9.3) | 1 (0.9) | 3 (2.6) | 0 (0) | .0340 | .4842a |
| Increased bilirubin | 14 (13.1) | 0 (0) | 6 (5.3) | 0 (0) | .0428 | - |
| Neurologic | ||||||
| Headache | 56 (52.3) | 5 (4.6) | 61 (53.5) | 7 (6.1) | .8615 | .6304 |
| Dizziness | 70 (65.4) | 4 (3.7) | 75 (65.8) | 9 (7.9) | .9540 | .1894 |
| Epileptic seizure | 2 (1.9) | 0 (0) | 0 (0.0) | 0 (0) | .2333a | - |
| Paresthesia | 10 (9.3) | 0 (0) | 0 (0.0) | 0 (0) | .0026 | - |
| Pulmonary | ||||||
| Cough | 65 (60.7) | 6 (5.6) | 49 (43.0) | 6 (5.3) | .0083 | .9101 |
| Dyspnea | 15 (14.0) | 2 (1.9) | 17 (14.9) | 3 (2.6) | .8504 | 1.0000 |
| Pleural effusion | 20 (18.7) | 7 (6.5) | 21 (18.4) | 3 (2.6) | .9588 | .2828 |
| WBRT-related | ||||||
| Radiation dermatitis | 2 (1.9) | 0 (0) | 4 (3.5) | 0 (0) | .7373 | - |
| Epileptic seizure | 4 (3.7) | 0 (0) | 3 (2.6) | 1 (0.9) | .9321 | 1.0000a |
| Paresthesia | 17 (15.9) | 1 (0.1) | 17 (14.9) | 2 (1.7) | .8408 | 1.0000 |
| Hydrocephalus | 6 (5.6) | 1 (0.9) | 11 (9.6) | 0 (0) | .2598 | .4842a |
| Others | 3 (2.8) | 0 (0) | 1 (0.9) | 1 (0.9) | .5695 | 1.0000a |
aAnalyzed using Fisher’s exact test; others were analyzed using chi-square test or continuity-adjusted chi-square test.
Abbreviations: WBRT, whole-brain radiotherapy; AST, aspartate transaminase; ALT, alanine transaminase.
In the WBRT with concurrent erlotinib arm, the erlotinib dose had to be reduced in 3 patients (2.8%) because of drug-related AEs, while 4 patients discontinued erlotinib (one due to a drug-related AE). Two patients experienced erlotinib delay, of which one was because of drug-related AEs. WBRT delay occurred in 1 patient but was not due to WBRT-related AEs, while 3 patients discontinued WBRT, of which 1 was due to WBRT-related AEs. In the WBRT-alone arm, 4 patients discontinued WBRT but only 1 was due to WBRT-related AEs. In summary, 9 patients (8.5%) in the WBRT with concurrent erlotinib arm experienced dose modifications of erlotinib or WBRT; 7 patients (6.6%) were due to treatment-related AEs. Four patients (3.5%) in the WBRT-alone arm experienced modification of WBRT, but only 1 was due to WBRT-related AEs. The interventions in both arms were safe and well tolerated (eTable 1 in Supplementary Material 2).
Two patients in the WBRT with concurrent erlotinib arm died from cerebral hernia, while 1 patient in the WBRT-alone arm received subsequent thoracic radiotherapy and died from radiation pneumonitis.
Cognitive Function
In brief, 220 patients were randomly assigned, 114 to the WBRT-alone arm and 106 to the WBRT with concurrent erlotinib arm. Baseline MMSE score was collected for 219 (99.5%) of 220 randomly assigned patients, and these patients made up the population for the primary analysis. There were no significant differences in compliance rates between arms at any evaluation time point (eTables 2 and 3 in Supplementary Material 2). Categorical change in MMSE score by treatment arm was summarized in Table 3 and eTable 4 in Supplementary Material 2. MMSE decline was a rare event in either of the randomized arms as well, with the overwhelming majority of patients experiencing no change in MMSE score over time. There was no significant difference in the ITT proportion or in EGFR-mutant patients experiencing MMSE score gain, no change, or decline at any of the key evaluation time points between treatment arms. To assess differences between the two treatment groups over time, multivariable linear mixed-effects model was used and the results were listed in Table 4. There was no difference in MMSE score change over time for both randomly assigned arms (P = .861). The interventions in both arms were well tolerated without excessive CF detriment.
Table 3.
Categorical Change in MMSE Score by Treatment Arm in the Intent-to-Treat (ITT) Study Population
| MMSE score changea | WBRT + erlotinib | WBRT | P-value | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| 1 mo | n = 90 | n = 99 | .579 | ||
| Decline | 13 | 14.4 | 20 | 20.2 | |
| No change | 74 | 82.2 | 76 | 76.8 | |
| Gain | 3 | 3.3 | 3 | 3.0 | |
| 3 mo | n = 66 | n = 68 | .691 | ||
| Decline | 9 | 13.6 | 13 | 19.1 | |
| No change | 54 | 81.8 | 52 | 76.5 | |
| Gain | 3 | 4.5 | 3 | 4.4 | |
| 5 mo | n = 51 | n = 53 | .495 | ||
| Decline | 7 | 13.7 | 12 | 22.6 | |
| No change | 41 | 80.4 | 38 | 71.7 | |
| Gain | 3 | 5.9 | 3 | 5.7 | |
| 7 mo | n = 47 | n = 41 | .246 | ||
| Decline | 8 | 17.0 | 13 | 31.7 | |
| No change | 35 | 74.5 | 26 | 63.4 | |
| Gain | 4 | 8.5 | 2 | 4.9 | |
| 9 mo | n = 34 | n = 25 | .626 | ||
| Decline | 6 | 17.6 | 7 | 28.0 | |
| No change | 26 | 76.5 | 17 | 68.0 | |
| Gain | 2 | 5.9 | 1 | 4.0 | |
| 11 mo | n = 28 | n = 20 | .872 | ||
| Decline | 8 | 28.6 | 7 | 35.0 | |
| No change | 18 | 64.3 | 12 | 60.0 | |
| Gain | 2 | 7.1 | 1 | 5.0 | |
| 13 mo | n = 23 | n = 13 | .913 | ||
| Decline | 4 | 17.4 | 2 | 15.4 | |
| No change | 18 | 78.3 | 10 | 76.9 | |
| Gain | 1 | 4.3 | 1 | 7.7 | |
Note. Percentages may not sum to 100% because of rounding.
Abbreviations: MMSE, Folstein Mini-Mental State Examination; WBRT, whole-brain radiotherapy.
aMMSE decline, >3-point decline; MMSE gain, >3-point gain; MMSE no change, ≤3-point change.
Table 4.
Multivariable Linear Mixed-Effects Model for MMSE Score Change Over Time in both Intent-to-Treat (ITT) Study Population and EGFR-Mutant Subgroup
| Parameter (reference level) | Estimate | SE | P-value | 95% CI |
|---|---|---|---|---|
| ITT population | ||||
| Intercept | −0.67 | 0.83 | .421 | −2.30 to 0.96 |
| WBRT + erlotinib | 0 | 0 | - | - |
| WBRT | −0.56 | 0.32 | .080 | −1.20 to 0.07 |
| Time, mo | −0.02 | 0.09 | .861 | −0.19 to 0.16 |
| EGFR-mutant subgroup | ||||
| Intercept | 1.00 | 0.97 | .305 | −0.91 to 2.91 |
| WBRT + erlotinib | 0 | 0 | - | - |
| WBRT | −0.36 | 0.38 | .343 | −1.10 to 0.38 |
| Time, mo | −0.16 | 0.10 | .121 | −0.35 to 0.04 |
Abbreviations: MMSE, Folstein Mini-Mental State Examination; WBRT, whole-brain radiotherapy; ITT, Intent-to-Treat; EGFR, epithelial growth factor receptor.
Discussion
To our knowledge, this is the largest reported phase III randomized clinical trial in which WBRT with concurrent erlotinib was compared with WBRT-alone in NSCLC patients with multiple BMs. The previous phase II trial reported a favorable ORR and survival time with WBRT-plus concurrent erlotinib compared with historical controls of WBRT-alone in NSCLC patients with BMs.19 However, that study was a single-arm non-randomized study with a small patient population, and it was probably subject to a negative selection bias. Another randomized phase II study showed no statistically significant improvement in neurological PFS or OS in unselected patients treated with concurrent erlotinib and WBRT followed by maintenance erlotinib compared to placebo.31 An analysis of these studies revealed that the major reasons for this discrepancy were the variegated EGFR mutation rate and selection of patients for treatment. The ENTER study was the first phase III randomized clinical trial in which WBRT with concurrent erlotinib was compared with WBRT-alone in NSCLC patients with multiple BMs. In this trial, we optimized our maintenance strategies, cisplatin-based chemotherapy combined with pemetrexed or paclitaxel were used for EGFR wild-type subgroup. Our iPFS analysis showed no significant superiority between the WBRT with concurrent erlotinib group and the WBRT-alone group in both the ITT population and the EGFR-mutant subgroup. There were also no statistically significant differences between the two treatment arms with respect to PFS or OS in the ITT population. The efficacy was basically consistent with the consequence of Lee et al.,31 but our results preferably overcome the participants’ bias caused by extremely low frequency of EGFR mutations.
The trial design enabled a parallel investigation of WBRT with concurrent erlotinib compared with WBRT with sequential erlotinib in an EGFR-mutant subgroup. Actually, optimal management of an EGFR-mutated NSCLC with BMs is an evolving paradigm.32,33 EGFR-TKIs are a powerful and broadly effective therapeutic strategy for BMs, even more so than WBRT13; therefore, recent studies have focused on the multimodal approach of WBRT and EGFR-TKIs in EGFR-mutant NSCLC patients. A pooled retrospective clinical research demonstrated that radiation followed by administration of an EGFR-TKI resulted in longer OS than upfront EGFR-TKI with deferral of radiotherapy in patients with EGFR-mutant NSCLC,34 but this study was not randomized and had failed to use intracranial progression as the optimal primary endpoint. Additionally, we further explored the outcomes and toxicity of WBRT-plus concurrent and vs sequential erlotinib. There was no significance of iPFS, PFS, or OS was observed between the two arms in the subgroup of EGFR-mutant patients, which indicated WBRT with concurrent erlotinib may not superior to sequential erlotinib. The cause of treatment failure in patients with mutant EGFR tumors may due to the dose-related effect of WBRT on BBB for penetration of EGFR-TKIs. Currently, studies have reported that the cerebrospinal fluid penetration of gefitinib is enhanced after 30-40 Gy of radiation.35 Thus, the doses used for WBRT may be inadequate or the effects of TKIs on BMs may not be significant until WBRT is almost complete. Furthermore, erlotinib has the best cerebrospinal fluid penetration of the first-generation TKIs,36 and clinical central nervous system (CNS) responses to erlotinib alone are routinely observed, which demonstrates that erlotinib can penetrate across BBB even without the help of radiation. Thus, this may explain why additional benefit was not observed for the addition of concurrent erlotinib to WBRT in this study compared to that of WBRT with sequential erlotinib.
Drug-related AEs in the WBRT-plus erlotinib arm were similar to those in the WBRT-alone arm, except for a slightly higher occurrence of acneiform rash and dry skin in the WBRT-plus concurrent erlotinib arm. No patient was withdrawn or received reduced erlotinib dose due to adverse reactions. RTOG 0320, another phase III study involved the evaluation of efficacy and toxicity about erlotinib associated with WBRT + SRS was closed because of accrual limitations. Possible explanations for the relatively large difference in AEs between two trials may be related to the combination of SRS used in this trial or nonstandard subsequent treatments for patient with wild-type EGFRs.37 For neurocognitive function, although debate continues as to whether neurocognitive function is accurately assessed with the MMSE, compared with the Hopkins Verbal Learning Test-Revised for example, we chose to use the MMSE on the basis of findings from Aoyama and colleagues.38 According to the MMSE scores, no significant difference was recorded between the arms for the change during the 13 months of follow-up, but the average MMSE scores showed differences that were in favor of the combination group. Patients who had a baseline MMSE score <27 were numerically more likely to experience significant MMSE score gain than decline, suggesting that at least some of the baseline deficits in CF caused by the tumor itself can be reversed with effective therapy. Although patient numbers and MMSE compliance did fall over time, there was no indication that more intensive therapy was associated with higher rates of CF decline at any key evaluation time point. Thus, it appears to be safe to continue the erlotinib in EGFR-mutant patients receiving brain radiation during their course of therapy, and the combination therapy did not rouse excessive CF detriment. Similar to other study, the combination of WBRT with concurrent erlotinib was well tolerated with no unexpected neurotoxicity.
Although the results of this multi-institutional study are potentially practice changing, there are several limitations in this study. First, previous literature to predict the sample size was limited, and we were forced to use two previous single-arm studies to power this trial. Second, the median iPFS from the previous literature were quite different from that found in this trial, which influenced the accuracy of sample size calculations inevitably and this trial may have been underpowered to detect a true difference. Third, the presence of two or more metastatic lesions in the brain was set as an eligibility criterion in our study.21 However, brain radiation technology has improved since then and SRS is often preferred for patients with up to four or more BMs.39 Although the subgroup analysis in our study suggested that WBRT-plus erlotinib was superior to WBRT-alone for EGFR-mutant patients with more than four BMs, the enrollment of patients with less than four metastatic lesions may bias the results. Fourth, the comparison between WBRT with concurrent erlotinib and sequential erlotinib was not pre-specified, the subset analysis of EGFR-mutant patients in this study was not a pre-planned question in a prospective randomized controlled trial. Fifth, the use of EGFR-TKI has changed significantly since this trial was begun, currently, osimertinib is approved for use as initial treatment (first-line therapy) for patients with a new diagnosis of EGFR-mutated lung cancer with BM.40,41 This usage of newly EGFR-TKI would presumably lead to outcomes that are different from those for the patients in this trial. Furthermore, patients and investigators were not blinded to the treatment group, thus introducing the potential for bias. In addition, the study involved only Chinese patients and thus the representativeness of the study may be limited in Western populations.
Conclusions
This multi-institutional trial failed to find an effect of concurrent erlotinib with WBRT in either the ITT population or in EGFR-mutant NSCLC patients compared with WBRT-alone. WBRT with concurrent erlotinib did not significantly improve the iPFS over the WBRT-alone arm in the EGFR-mutant subgroup, nor was the PFS or OS improved. Both interventions were safe and well tolerated.
Supplementary Material
Acknowledgments
We are indebted to all the patients who have participated in our clinical trial and to the investigators and study teams for their participation. We would like to thank the continuous academic support from Medical Department of Shanghai Roche Pharmaceuticals Ltd., in particular, Emma Li. We also thank Nikki March, PhD, of Edanz Medical Writing for providing medical writing services.
Role of the funder/sponsor. The funders/sponsors had no role in the design and conduct of the study, including collection, management, analysis, or interpretation of the data, preparation, review, or approval of the manuscript, or the decision to submit the manuscript for publication.
Previous presentation. This study was presented as a mini oral presentation at the 19th World Conference on Lung Cancer (WCLC 2018) of the International Association for the Study of Lung Cancer (IASLC) on September 23-26, 2018 in Toronto, Canada.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81972851, 81472803, Z.Y.) and the Foundation and Frontier Research Project of Chongqing (Grant No. cstc2015jcyjBX0084, Z.Y.).
Conflict of interest statement. The authors who have taken part in this study declared that they have nothing to disclose regarding funding or conflict of interest with respect to this manuscript.
Authorship statement. Drs Z.Y. and Y.Z. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs Z.Y. and Y.Z. contributed equally to this work. Specific author contributions are as follows:
(1) Study concept and design: Z.Y.
(2) Data acquisition, analysis, and interpretation: Z.Y., Y.Z., R.L., A.Y., B.R., J.S., J.L., L.C., R.Z., J.Z., X.X., Z.L., and D.P.C.
(3) Drafting of the manuscript: Y.Z.
(4) Critical revision of the manuscript for important intellectual content: Z.Y., Z.L., D.P.C.
(5) Statistical analysis: J.Z.
(6) Obtained funding: Z.Y.
(7) Administrative, technical, or material support: Z.Y., R.L., A.Y., B.R., J.S., J.L., L.C., and R.Z.
(8) Study supervision: Z.Y. and J.Z.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Sperduto PW, Yang TJ, Beal K, et al. Estimating survival in patients with lung cancer and brain metastases: an update of the graded prognostic assessment for lung cancer using molecular markers (Lung-molGPA). JAMA Oncol. 2017;3(6):827–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. El Rassy E, Botticella A, Kattan J, Le Péchoux C, Besse B, Hendriks L. Non-small cell lung cancer brain metastases and the immune system: from brain metastases development to treatment. Cancer Treat Rev. 2018;68:69–79. [DOI] [PubMed] [Google Scholar]
- 4. Owen S, Souhami L. The management of brain metastases in non-small cell lung cancer. Front Oncol. 2014;4:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suh JH, Kotecha R, Chao ST, Ahluwalia MS, Sahgal A, Chang EL. Current approaches to the management of brain metastases. Nat Rev Clin Oncol. 2020;17(5):279–299. [DOI] [PubMed] [Google Scholar]
- 6. Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15(4):387–395. [DOI] [PubMed] [Google Scholar]
- 7. Brown PD, Ahluwalia MS, Khan OH, Asher AL, Wefel JS, Gondi V. Whole-brain radiotherapy for brain metastases: evolution or revolution? J Clin Oncol. 2018;36(5):483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. [DOI] [PubMed] [Google Scholar]
- 9. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. [DOI] [PubMed] [Google Scholar]
- 10. Couraud S, Zalcman G, Milleron B, Morin F, Souquet PJ. Lung cancer in never smokers–a review. Eur J Cancer. 2012;48(9):1299–1311. [DOI] [PubMed] [Google Scholar]
- 11. Chen YJ, Roumeliotis TI, Chang YH, et al. Proteogenomics of non-smoking lung cancer in east asia delineates molecular signatures of pathogenesis and progression. Cell. 2020;182(1):226–244.e17. [DOI] [PubMed] [Google Scholar]
- 12. Wu YL, Zhou C, Cheng Y, et al. Erlotinib as second-line treatment in patients with advanced non-small-cell lung cancer and asymptomatic brain metastases: a phase II study (CTONG-0803). Ann Oncol. 2013;24(4):993–999. [DOI] [PubMed] [Google Scholar]
- 13. Yang JJ, Zhou C, Huang Y, et al. Icotinib versus whole-brain irradiation in patients with EGFR-mutant non-small-cell lung cancer and multiple brain metastases (BRAIN): a multicentre, phase 3, open-label, parallel, randomised controlled trial. Lancet Respir Med. 2017;5(9):707–716. [DOI] [PubMed] [Google Scholar]
- 14. Chen DJ, Nirodi CS. The epidermal growth factor receptor: a role in repair of radiation-induced DNA damage. Clin Cancer Res. 2007;13(22 Pt 1):6555–6560. [DOI] [PubMed] [Google Scholar]
- 15. Chinnaiyan P, Huang S, Vallabhaneni G, et al. Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva). Cancer Res. 2005;65(8):3328–3335. [DOI] [PubMed] [Google Scholar]
- 16. Zimmermann S, Dziadziuszko R, Peters S. Indications and limitations of chemotherapy and targeted agents in non-small cell lung cancer brain metastases. Cancer Treat Rev. 2014;40(6):716–722. [DOI] [PubMed] [Google Scholar]
- 17. Proto C, Imbimbo M, Gallucci R, et al. Epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of central nervous system metastases from non-small cell lung cancer: the present and the future. Transl Lung Cancer Res. 2016;5(6):563–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Soon YY, Leong CN, Koh WY, Tham IW. EGFR tyrosine kinase inhibitors versus cranial radiation therapy for EGFR mutant non-small cell lung cancer with brain metastases: a systematic review and meta-analysis. Radiother Oncol. 2015;114(2):167–172. [DOI] [PubMed] [Google Scholar]
- 19. Welsh JW, Komaki R, Amini A, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol. 2013;31(7):895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhuang H, Yuan Z, Wang J, Zhao L, Pang Q, Wang P. Phase II study of whole brain radiotherapy with or without erlotinib in patients with multiple brain metastases from lung adenocarcinoma. Drug Des Devel Ther. 2013;7:1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665–1672. [DOI] [PubMed] [Google Scholar]
- 22. Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non-small cell lung cancer: a review. JAMA. 2019;322(8):764–774. [DOI] [PubMed] [Google Scholar]
- 24. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 25. Mowla A, Zandi T. Mini-mental status examination: a screening instrument for cognitive and mood disorders of elderly. Alzheimer Dis Assoc Disord. 2006;20(2):124. [DOI] [PubMed] [Google Scholar]
- 26. Prabhu RS, Won M, Shaw EG, et al. Effect of the addition of chemotherapy to radiotherapy on cognitive function in patients with low-grade glioma: secondary analysis of RTOG 98-02. J Clin Oncol. 2014;32(6):535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weller J, Tzaridis T, Mack F, et al. Health-related quality of life and neurocognitive functioning with lomustine-temozolomide versus temozolomide in patients with newly diagnosed, MGMT-methylated glioblastoma (CeTeG/NOA-09): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2019;20(10):1444–1453. [DOI] [PubMed] [Google Scholar]
- 28. Zhang Y, Li XY, Tang Y, et al. Rapid increase of serum neuron specific enolase level and tachyphylaxis of EGFR-tyrosine kinase inhibitor indicate small cell lung cancer transformation from EGFR positive lung adenocarcinoma? Lung Cancer. 2013;81(2):302–305. [DOI] [PubMed] [Google Scholar]
- 29. Zabel A, Debus J. Treatment of brain metastases from non-small-cell lung cancer (NSCLC): radiotherapy. Lung Cancer. 2004;45(Suppl 2):S247–S252. [DOI] [PubMed] [Google Scholar]
- 30. Mehta MP, Khuntia D. Current strategies in whole-brain radiation therapy for brain metastases. Neurosurgery. 2005;57(5 Suppl):S33–S44; discusssion S1. [DOI] [PubMed] [Google Scholar]
- 31. Lee SM, Lewanski CR, Counsell N, et al. Randomized trial of erlotinib plus whole-brain radiotherapy for NSCLC patients with multiple brain metastases. J Natl Cancer Inst. 2014;106(7):dju151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Khalifa J, Amini A, Popat S, Gaspar LE, Faivre-Finn C; International Association for the Study of Lung Cancer Advanced Radiation Technology Committee . Brain metastases from NSCLC: radiation therapy in the era of targeted therapies. J Thorac Oncol. 2016;11(10):1627–1643. [DOI] [PubMed] [Google Scholar]
- 33. Zhou L, Deng L, Lu Y. Epidermal growth factor receptor mutations in non-small-cell lung cancer with brain metastasis: can up-front radiation therapy be deferred or withheld? J Clin Oncol. 2017;35(10):1033–1035. [DOI] [PubMed] [Google Scholar]
- 34. Magnuson WJ, Lester-Coll NH, Wu AJ, et al. Management of brain metastases in tyrosine kinase inhibitor-naive epidermal growth factor receptor-mutant non-small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol. 2017;35(10):1070–1077. [DOI] [PubMed] [Google Scholar]
- 35. Zeng YD, Liao H, Qin T, et al. Blood-brain barrier permeability of gefitinib in patients with brain metastases from non-small-cell lung cancer before and during whole brain radiation therapy. Oncotarget. 2015;6(10):8366–8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Togashi Y, Masago K, Masuda S, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol. 2012;70(3):399–405. [DOI] [PubMed] [Google Scholar]
- 37. Sperduto PW, Wang M, Robins HI, et al. A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus WBRT and SRS with temozolomide or erlotinib for non-small cell lung cancer and 1 to 3 brain metastases: Radiation Therapy Oncology Group 0320. Int J Radiat Oncol Biol Phys. 2013;85(5):1312–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483–2491. [DOI] [PubMed] [Google Scholar]
- 39. Chao ST, De Salles A, Hayashi M, et al. Stereotactic radiosurgery in the management of limited (1-4) brain metasteses: systematic review and international stereotactic radiosurgery society practice guideline. Neurosurgery. 2018;83(3):345–353. [DOI] [PubMed] [Google Scholar]
- 40. Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFRmutated advanced non-small-cell lung cancer. J Clin Oncol. 2018;36(33):3290–3297. [DOI] [PubMed] [Google Scholar]
- 41. Soria JC, Ohe Y, Vansteenkiste J, et al. ; FLAURA Investigators . Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.