Abstract
Background
Patients with metastatic breast cancer (MBC) are living longer, but the development of brain metastases often limits their survival. We conducted a systematic review and meta-analysis to determine the incidence of brain metastases in this patient population.
Methods
Articles published from January 2000 to January 2020 were compiled from four databases using search terms related to breast cancer, brain metastasis, and incidence. The overall and per patient-year incidence of brain metastases were extracted from studies including patients with human epidermal growth factor receptor-2 positive (HER2+), triple negative, and hormone receptor (HR)+/hormone receptor negative (HER2−) MBC; pooled overall estimates for incidence were calculated using random effects models.
Results
937 articles were compiled, and 25 were included in the meta-analysis. Incidence of brain metastases in patients with HER2+ MBC, triple negative MBC, and HR+/HER2− MBC was reported in 17, 6, and 4 studies, respectively. The pooled cumulative incidence of brain metastases was 31% for the HER2+ subgroup (median follow-up: 30.7 months, IQR: 24.0–34.0), 32% for the triple negative subgroup (median follow-up: 32.8 months, IQR: 18.5–40.6), and 15% among patients with HR+/HER2− MBC (median follow-up: 33.0 months, IQR: 31.9–36.2). The corresponding incidences per patient-year were 0.13 (95% CI: 0.10–0.16) for the HER2+ subgroup, 0.13 (95%CI: 0.09–0.20) for the triple negative subgroup, and only 0.05 (95%CI: 0.03–0.08) for patients with HR+/HER2− MBC.
Conclusion
There is a high incidence of brain metastases among patients with HER2+ and triple negative MBC. The utility of a brain metastases screening program warrants investigation in these populations.
Keywords: brain metastases, human epidermal growth factor receptor-2 positive (HER2+), incidence, metastatic breast cancer, triple negative
Key Points.
Brain metastases occur in almost 1 in 3 patients with human epidermal growth factor receptor-2 positive (HER2+) or triple negative metastatic breast cancer (MBC).
Incidence per patient-year is 13% for both HER2+ and triple negative MBC.
Screening for brain metastases in high-risk MBC populations warrants investigation.
Importance of the Study.
Patients with human epidermal growth factor receptor-2 positive (HER2+) or triple negative metastatic breast cancer (MBC) are living longer, but their survival is often limited by the development of brain metastases. To evaluate the possible benefit of a brain metastases screening program, we conducted a systematic review and meta-analysis to determine the incidence of brain metastases in this patient population. To account for variable follow-up durations across included studies, the incidence of brain metastases per patient-year was also calculated. We found that almost one-third of patients with HER2+ or triple negative MBC will develop brain metastases during their lifetime, with a 13% incidence per patient-year among those with HER2+ MBC and a 13% incidence per patient-year among those with triple negative MBC. To our knowledge, this is the first meta-analysis to investigate the incidence of breast cancer brain metastases among patients with MBC. Our findings support further investigation of an MRI-based screening program in high-risk MBC populations.
The incidence of brain metastases has steadily increased over time as women with metastatic breast cancer (MBC) survive longer to be at risk for developing metastasis to the central nervous system (CNS).1–10 Women with human epidermal growth factor receptor-2 positive (HER2+) or triple negative (hormone receptor negative [HER2−]) MBC, which represent approximately 30–40% of the MBC population, are at particularly high risk of brain metastases.11–18 In fact, retrospective data reveal that 30–50% of women with these high-risk breast cancer subtypes ultimately develop and succumb to CNS disease.1–5,8–10
Many studies confirm that brain metastases afford women with MBC a very poor prognosis.1,19 In a large retrospective study of 557 patients with MBC, for example, the presence of symptomatic brain metastases was independently prognostic for a shorter overall survival with a hazard ratio of 1.58 (95%CI: 1.04–2.41, P = 0.033).19 In the prospective “registHER” study, women with HER2+ MBC who developed CNS metastases also had adverse outcomes; the median survival was 26.3 months among women with brain metastases as compared to 44.6 months among those women who did not develop brain metastases.3 Although the cause of death was not reported in this specific study, approximately 50% of patients with HER2+ brain metastases are thought to die from the neurologic progression of their disease.10
Given that the overall incidence of brain metastases is fairly low in the general breast cancer population, screening procedures are not recommended in the National Comprehensive Cancer Network (NCCN) consensus or American Society of Clinical Oncology (ASCO) guidelines. Rather, patients with breast cancer undergo imaging of the brain only after symptoms suggestive of CNS involvement develop. Unfortunately, by the time that patients experience potentially debilitating symptoms of brain metastases, they often have a significant burden of disease with limited treatment options.20,21
Whether early detection of brain metastases via a magnetic resonance imaging (MRI)-based screening program may allow for early intervention and, ultimately, improved outcomes for patients with certain “high risk” populations of MBC is unknown. A very high incidence of brain metastases in certain breast cancer populations may prompt the revisiting of guidelines regarding screening practices. Hence, we conducted a systematic review and meta-analysis to determine the incidence of brain metastases among patients with a new diagnosis of MBC subtypes, which are known to portend a high risk of brain metastases.22
Methods
This systematic review was conducted and reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines23 (see Supplementary Figure 1).
Study Selection
A literature search was conducted in the MedLine, EMBASE, CENTRAL, and CDSR databases using Medical Subject Headings (MeSH) indexing terms and keywords related to breast neoplasm, breast cancer, incidence, and brain metastasis (see Supplementary Figure 2 for the full search strategy). Databases were chosen per recommendations from the Cochrane Handbook for Systematic Reviews.24 The search was conducted by a library information specialist; the search was conducted in the English language without any further publication restrictions. Experimental or observational studies that reported the first incidence of brain metastases among patients with MBC and were published between January 2000 and January 2020 were included in this systematic review. This time period was selected to capture more modern studies, which reflect the use of trastuzumab-based chemotherapy regimens that are currently administered to patients with MBC. Studies were excluded if they did not report a median follow-up of their study cohort, if they were reviews, editorials, or conference abstracts, if they investigated very specific breast cancer populations that were not representative of the MBC population in general (e.g., inflammatory breast cancer), or if they only reported on patients with symptomatic brain metastases at baseline. Furthermore, two studies that excluded patients with asymptomatic brain metastases at study entry were ineligible. References of included publications were scanned as part of the review process; however, a search of the grey literature was not conducted. In cases where studies queried the same patient database (i.e., Surveillance, Epidemiology, and End Results [SEER]) during the same time period, only the study with the largest number of patients was included, while the rest were excluded.
Two reviewers (YG and AJD) independently reviewed the abstracts of all papers and selected papers for inclusion. Any discrepancies or conflicts were adjudicated by a third reviewer (MK).
Data Extraction
Two authors reviewed the papers and extracted the data (MK, KJJ). The reported incidence of brain metastases and follow-up duration were extracted from studies. Some studies presented two arms of data (i.e., control and treatment): where possible, we calculated median follow-up times to obtain a value for the entire cohort. Notably, the treatment arm of one randomized control trial20 that studied the efficacy of prophylactic cranial irradiation was omitted from our analysis because this practice is not standard-of-care. In cases where our patient population of interest was a subgroup of the originally reported publication, or if certain patients in the study group did not fit our inclusion criteria (i.e., patients with brain metastases at baseline), we removed those patients from the values reported in the studies. If there was no reliable way to adjust the incidence values, the studies were excluded. This was unfortunately the case with two large randomized phase III trials. While the EMILIA25 and CLEOPATRA26 trials are both important trials in the field, both studies screened for asymptomatic brain metastases prior to study entry and excluded patients with CNS involvement of their disease. Given a systematic reduction in the incidence of brain metastases, both studies were excluded from our analysis.
During the data extraction process, a level of evidence was assigned to each paper based on the hierarchy for “Prognosis” in the Australian National Health and Medical Research Council (NHMRC) guidelines.27
Statistical Analysis
The primary outcome of interest was the incidence rate of brain metastases per patient-year among patients with MBC. The secondary endpoint was the cumulative incidence of brain metastases. The incidence rate per patient-year was calculated to adjust for variable follow-up durations, with 2-sided 95% confidence intervals (95% CI) based on the measured variances. Subgroup analyses were performed for HER2+ and triple negative breast cancer subtypes; the incidence of brain metastases among studies that did not specify breast cancer subtypes were also pooled. Finally, raw data regarding the overall incidence of brain metastases for each breast cancer subtype (as opposed to incidence per unit time) were reported with median follow-up durations.
In order to compute the pooled estimate for incidence of brain metastases, a random effects model (RE Model) was carried out for the overall cohort as well as each of the three subgroups. A forest plot was generated, based on the random intercept Poisson regression model with the computed 95% CI. For each RE model, a test of heterogeneity (Q-statistic) was calculated using a restricted maximum likelihood estimator. The total heterogeneity (τ2) and the total heterogeneity relative to the variance (I2) was also calculated.
Publication bias was evaluated and presented as funnel plots, which illustrate the incidence of brain metastases against standard error with 2-sided 95% confidence intervals. To test for publication bias, a regression analysis was performed using Egger’s test to assess the symmetry of the funnel plot (α = 0.05). An Egger’s test was appropriate as there were fewer than 25 studies for each subgroup.28
A within-study assessment of bias was performed for all studies included in the analysis. The Newcastle Ottawa Scale was used to assess bias in case-control and cohort studies while the Cochrane Risk of Bias 2.0 tool was used for randomized controlled trials (RCTs).
Data Analysis
Data analysis was carried out using the methods previously described.29 All data analysis was performed using SAS (Version 9.4; SAS Institute, Cary, NC, USA) and R Statistics (Version 3.6.1; R Core Team, 2019). R Statistics Version 3.6.1 and GraphPad Prism 8.4.3 (GraphPad Software Inc, CA, USA) were used to create the figures. Subgroup analyses were carried out for HER2+, triple negative, and HR+/HER2− metastatic breast cancer subtypes.
Results
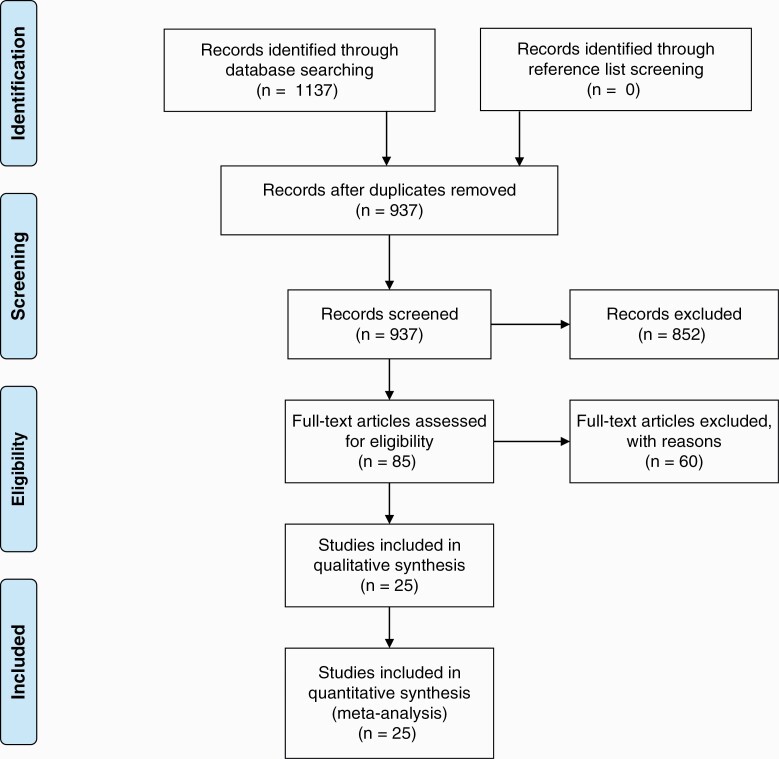
After the removal of duplicates, 937 studies were identified in our literature review. After assessment by reviewers, 85 papers were assessed for inclusion. During the full-text screen, an additional 60 papers were excluded based on specific exclusion criteria, including if they did not report a median follow-up of their study cohort. This is detailed in Supplementary Figure 3. 25 trials were included in the final analysis (Figure 1—Adapted from PRISMA 2009 Flowchart).1–4,14,15,20,30–47 Data extracted from the selected studies is summarized in Table 1 and the full data is included in Supplementary Figure 4. In total, 3 studies were randomized controlled trials, 1 study was a non-randomized controlled trial, and 21 studies were observational studies.
Fig. 1.
Schematic illustrating study selection process. Adapted from PRISMA flow diagram 2009. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1.
Characteristics and Incidence of Brain Metastases in the Included Studies
| Type of study | Sample size | Cumulative incidence | Median follow-up (months) | Time to brain metastasis (months) | Level of evidencea | |
|---|---|---|---|---|---|---|
| HER2+ | ||||||
| Canney et al. 2015 | RCT | 26 | 0.346 | 30.7 | NR | III-2 |
| Ono et al. 2009 | Case control | 204 | 0.363 | 53.6 | 13.6 | III-3 |
| Montagna et al. 2009 | Case control | 73 | 0.425 | 35.3 | 35.8 | III-3 |
| Gori et al. 2007 | Retrospective cohort | 118 | 0.297 | 28.0 | 12.0 | III-3 |
| Brufsky et al. 2011 | Prospective cohort | 997 | 0.363 | 29.0 | 13.3 | II |
| Dawson et al. 2006 | Retrospective cohort | 28 | 0.393 | 31.0 | 11.2 | III-3 |
| Pivot et al. 2015 | RCT | 501 | 0.064 | 19.0 | 5.0 | II |
| Awada et al. 2016 | RCT | 237 | 0.173 | 23.0 | NR | III-2 |
| Bergen et al. 2014 | Retrospective cohort | 76 | 0.474 | 47.5 | NR | III-3 |
| Bian et al. 2013 | Nonrandomized controlled clinical trial | 120 | 0.075 | 5.8 | NR | II |
| Witzel et al. 2011 | Retrospective cohort | 75 | 0.387 | 24.0 | NR | III-3 |
| Bartsch et al. 2009 | Retrospective cohort | 97 | 0.412 | 24.0 | NR | III-3 |
| Clayton et al. 2004 | Retrospective cohort | 93 | 0.247 | 10.8 | 10.0 | III-3 |
| Aversa et al. 2013 | Retrospective cohort | 79 | 0.494 | 34.0 | NR | III-3 |
| Kim et al. 2018 | Retrospective cohort | 1045 | 0.138 | 31.0 | NR | III-3 |
| D’Hondt et al. 2014 | Retrospective cohort | 20 | 0.550 | 31.5 | NR | III-3 |
| Darlix et al. 2019 | Retrospective cohort | 2182 | 0.558 | 42.8 | NR | III-3 |
| Triple negative | ||||||
| Jin et al. 2018 | Retrospective cohort | 415 | 0.263 | 48.1 | 10 | III-3 |
| Lin et al. 2008 | Retrospective cohort | 100 | 0.370 | 14.2 | NR | III-3 |
| Aversa et al. 2014 | Retrospective cohort | 64 | 0.219 | 34 | NR | III-3 |
| Kim et al. 2018 | Retrospective cohort | 1441 | 0.124 | 12 | NR | III-3 |
| D’Hondt et al. 2014 | Retrospective cohort | 36 | 0.417 | 31.5 | NR | III-3 |
| Darlix et al. 2019 | Retrospective cohort | 2046 | 0.670 | 42.8 | NR | III-3 |
| MBC (irrespective of subtype) | ||||||
| Ren et al. 2019 | Case control | 13066 | 0.0718 | 19 | NR | III-3 |
| Ryberg et al. 2005 | Case control | 579 | 0.2142 | 137 | NR | III-3 |
| Azim et al. 2018 | Case control | 246 | 0.0935 | 43.2 | NR | III-3 |
| Danese et al. 2012 | Retrospective cohort | 562 | 0.2224 | 13.7096 | NR | III-3 |
| Yan et al. 2013 | Retrospective cohort | 291 | 0.1718 | 12 | 8.0 | III-3 |
| Aversa et al. 2014 | Retrospective cohort | 488 | 0.2357 | 34 | NR | III-3 |
| Kim et al. 2018 | Retrospective cohort | 10432 | 0.0851 | 29 | NR | III-3 |
| Heitz et al. 2011 | Prospective cohort | 626 | 0.1054 | 48 | NR | II |
| D’Hondt et al. 2014 | Retrospective cohort | 169 | 0.2840 | 31.5 | NR | III-3 |
| Darlix et al. 2019 | Retrospective cohort | 15499 | 0.188 | 42.8 | NR | III-3 |
NR, not reported; RCT, randomized controlled trial.
aBased on the hierarchy for “Prognosis” in the Australian National Health and Medical Research Council (NHMRC) guidelines.25
Pooled Incidence of Brain Metastases Among Patients with HER2+ MBC
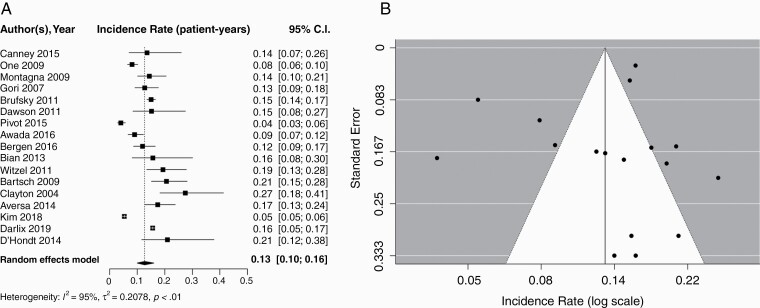
Seventeen studies including 5971 patients reported brain metastasis incidence among patients with HER2+ MBC. The pooled cumulative incidence of brain metastases was 0.31 (95% CI: 0.22–0.38) with a median follow-up of 30.7 months (IQR: 24.0–34.0). Using a random effects model, the pooled incidence of brain metastases per patient-year was 0.13 (95% CI: 0.10–0.16). Corresponding forest and funnel plots are presented in Figure 2A and B, respectively. An Egger’s test did not find asymmetry in the funnel plot and, therefore, publication bias in this subset of data was unlikely (P = 0.30).
Fig. 2.
A) Forest plot of pooled incidence rate (patient-years) for HER2+ MBC. Random effects model presented. Incidence per year of follow-up ranged from 0.04 to 0.27. Median follow-up times ranged from 5.8 to 53.6 months. B) Funnel Plot for publication bias according to studies reporting brain metastasis in HER2+ MBC. Egger’s test; P = 0.30. HER2+, human epidermal growth factor receptor-2 positive; MBC, metastatic breast cancer.
Six studies reported brain metastasis incidence among patients with HER2+ MBC and also provided hormone receptor (HR) status. The pooled incidence value of brain metastases per patient-year was calculated for each subgroup using a random effects model; the HR−/HER2+ group (comprising of 2092 patients) had a higher pooled incidence of 0.13 (95% CI: 0.08–0.20) than the HR+/HER2+ group (0.08 [95% CI: 0.05–0.13]), which comprised of 3480 patients. Forest and funnel plots for the HR−/HER2+ and HR+/HER2+ groups are presented in Supplementary Figures S5 and S6, respectively. Compared to patients with HR−/HER2+ MBC, those with HR+/HER2+ disease were less likely to develop brain metastases with an incidence rate ratio of 0.71 (95% CI: 0.64–0.78).
Pooled Incidence of Brain Metastases Among Patients with HR+/HER2− MBC
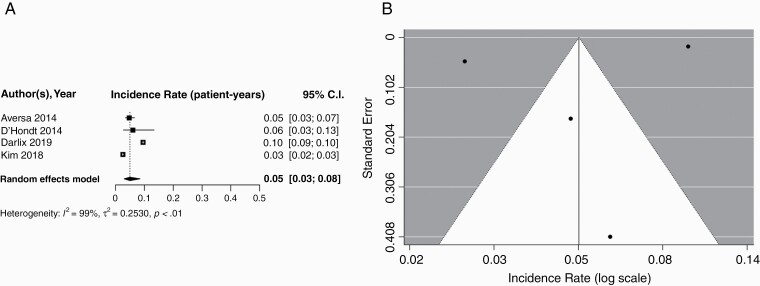
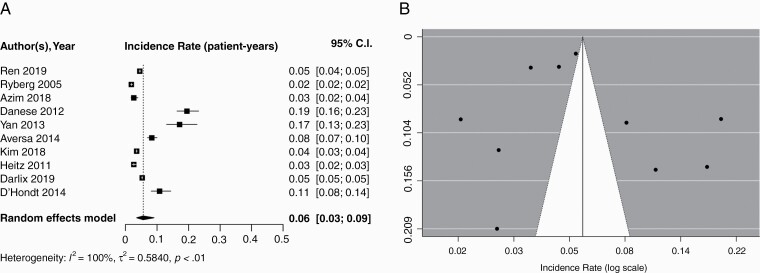
Four studies including 14656 patients reported the incidence of brain metastases specifically in patients with HR+/HER2− MBC. Pooled cumulative incidence of brain metastases among this subgroup was 0.15 (95% CI: 0.078–0.27), with a median follow-up of 33.0 months (IQR: 31.9–36.2). Using a random effects model, the pooled incidence per patient-year was found to be 0.05 (95% CI: 0.03–0.08); corresponding forest and funnel plots are displayed in Figure 3. Because only four studies were included in this subgroup, an Egger’s test was not performed.
Fig. 3.
A) Forest plot of pooled incidence rate (patient-years) for HR+/HER2− MBC. Random effects model presented. Incidence per year of follow-up ranged from 0.03–0.10. Median follow-up times ranged from 32.0–42.8 months. B) Funnel plot for publication bias according to studies reporting brain metastasis in HR+/HER2− MBC. HER2−, hormone receptor negative; HR+, hormone receptor; MBC, metastatic breast cancer.
Pooled Incidence of Brain Metastases Among Patients with Triple Negative MBC
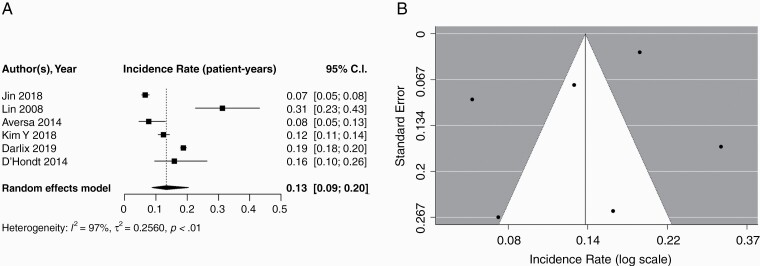
Six studies including 4102 patients reported the incidence of brain metastases among patients with triple negative MBC. The pooled cumulative incidence of brain metastases was 0.32 (95% CI: 0.19–0.49) with a median follow-up of 32.8 months (IQR: 18.5–40.6). Using a random effects model, the pooled incidence of brain metastases per patient-year was 0.13 (95% CI: 0.09–0.20). A forest plot of these data is presented in Figure 4A. The incidence of brain metastases plotted against the standard error and presented with 95% CIs is illustrated in Figure 4B. Because only 6 studies were included in this subset, an Egger’s test was not performed.
Fig. 4.
A) Forest plot of pooled incidence rate (patient-years) for triple negative MBC. Random effects model presented. Incidence per year of follow-up ranged from 0.07 to 0.31. Median follow-up times ranged from 12.0 to 48.1 months. B) Funnel plot illustrating studies reporting brain metastasis in triple negative MBC. MBC, metastatic breast cancer.
Pooled Incidence of Brain Metastases Among Patients, Irrespective of Breast Cancer Subtype
In total, 10 studies including 41,958 patients reported the incidence of brain metastases irrespective of breast cancer subtype. The pooled cumulative incidence of brain metastases in all patients with MBC was 0.15 (95% CI: 0.11–0.20) with a median follow-up of 32.7 months (IQR: 21.5–43.1). Using a random effects model, the pooled incidence of brain metastases per patient-year was 0.06 (95% CI: 0.03–0.09) (Figure 5A). In the assessment of publication bias, we plotted the incidence of brain metastases against the standard error and presented it with 95% CI in a funnel plot (Figure 5B). An Egger’s test did not find asymmetry in the funnel plot, which suggests publication bias in this subset of data is unlikely (P = 0.88).
Fig. 5.
A) Forest plot of pooled incidence rate (patient-years) for MBC, irrespective of subtype. Random effects model presented. Incidence per year of follow-up ranged from 0.02 to 0.19. Median follow-up times ranged from 12.0 to 137.0 months. B) Funnel Plot for publication bias according to studies reporting brain metastasis in MBC, irrespective of subtype. Egger’s test, P = 0.88. MBC, metastatic breast cancer.
Pooled Incidence of Brain Metastases Among All Patients from 2000 to 2010 Compared to 2011 to 2020
When comparing the incidence rates of brain metastases in studies published in 2000–2010 and 2011–2020, the brain metastasis incidence did not differ between the 2 groups (P = 0.23). It is worth noting that all 3 studies with the largest patient populations were published after 2010.
Assessment of Bias
The Newcastle Ottawa Scale for estimating the risk of bias in the cohort and case-control studies is illustrated in Supplementary Figure 7. The average score (out of 9) for all studies included was 7.38, and only 3 studies ranked “Poor” in their assessment of bias. Results of The Cochrane Risk of Bias 2.0 assessment of included RCTs are presented in Supplementary Figure 8A and B, respectively.
Discussion
Our study is the first meta-analysis to investigate the incidence of brain metastases among patients with MBC. Given that patients with HER2+ or triple negative MBC are known to have the highest risk of brain metastases, our analyses focused on these subgroups. Further, to account for wide variations in median follow-up times in the literature, we report the incidence of brain metastases per patient-year, in addition to pooled cumulative incidence. For patients with HER2+ MBC, the pooled cumulative incidence of brain metastases was 31% with a median follow-up of 30.7 months (IQR: 24.0–34.0). The incidence of brain metastases per patient-year was 13%. For patients with triple negative MBC, the pooled cumulative incidence of brain metastases was 32% with a median follow-up of 32.8 months (IQR: 18.5–40.6); when accounting for follow-up duration, the incidence of brain metastases per patient-year was 13%.
This study builds upon a narrative review of the literature, which was previously published by our group.22 The present study included a systematic literature search utilizing a larger number of databases and search terms, as well as a formal meta-analysis to pool incidence rates of brain metastases. Our results indicate a high incidence of brain metastases among patients with HER2+ or triple negative MBC (13% per patient-year). The largest and highest quality study (based on our level of evidence assessment) included in our analysis was the prospective “registHER” observational study.3 RegistHER reported an incidence of brain metastases in patients with HER2+ MBC of 37%,3 with a median follow-up of 29.0 months, which is consistent with our results.
Unfortunately, only six studies reported the incidence of brain metastases in triple negative MBC, with a large range in reported cumulative incidences of 12.4%–41.7% (pooled estimate of 13% per patient-year). The largest, highest quality paper that assessed the incidence of brain metastases among patients with triple negative MBC was published by investigators at the Fudan University Shanghai Cancer Center; with a median follow-up of 48.1 months, the incidence of brain metastases was 26.3%.40 Unfortunately, clinical trials for patients with metastatic triple negative breast cancer do not typically capture brain metastases as “events,” limiting our understanding of the incidence and CNS-specific efficacy of systemic therapies in this high-risk patient population.
Importantly, most of the studies in our pooled analysis did not screen patients for asymptomatic brain metastases. Brain metastases were typically detected after the onset of neurological symptoms, which necessitated a CT scan or MRI of the brain. This suggests that while the pooled incidence estimates for brain metastases are high, they may be under-estimated. Furthermore, while not all studies specified the type of imaging that was used to detect brain metastases; at least three studies indicated that a CT scan was performed while 11 studies indicated that either a CT scan or MRI scan were used to detect brain metastases. Given that MRI is the recommended method for the detection of brain metastases,48 studies that did not use MRI to detect brain metastases have likely underestimated the incidence of brain metastases in their patient populations. Given that almost one-third of patients with triple negative or HER2+ metastatic breast cancer will experience brain metastases in their lifetime, there may be a role for an MRI-based brain metastases screening program in this patient population. It is possible that the early detection of brain metastases and early intervention may improve patient outcomes, but properly conducted randomized controlled trials that incorporate quality-of-life endpoints, as well as CNS-specific progression and ideally overall survival, are required before screening for brain metastases can be recommended in practice guidelines.
Historically, the brain was considered to be a sanctuary site for breast cancer progression given that systemic therapies were felt to have poor penetration through the blood-brain barrier.49 However, even large antibodies such as trastuzumab have been shown to penetrate the CNS in radiolabelled positron emission tomography (PET) studies.50 The clinical activity of anti-HER2 antibodies in the CNS has since been demonstrated in the CLEOPATRA study, which suggests that the addition of pertuzumab to trastuzumab plus docetaxel delays the onset of CNS metastases.26 Trastuzumab emtansine (T-DM1) has also demonstrated CNS-specific benefit in patients with HER2+ MBC and brain metastases in the KAMILLA study.51 Given the approval of these CNS-penetrating therapies over the previous decade, we compared the incidence of brain metastases in studies published in 2000–2010 to studies published in 2011–2020 and found no statistical difference. This may be due to delayed uptake and funding of pertuzumab and T-DM1 in certain jurisdictions, reducing their impact on the incidence of brain metastases in studies that were published between 2011 and 2020. It is also possible that two competing effects on brain metastases incidence may be at play. While CNS penetrating drugs may delay or foreseeably even prevent brain metastases (although data for the latter is currently lacking), improved control of extra-cranial disease and longer survival times may conversely increase the incidence of brain metastases.
With an evolving landscape of CNS-penetrating systemic therapies for patients with MBC, particularly those with HER2+ disease, the outcomes of patients with breast cancer brain metastases are likely to improve over time. In fact, the addition of tucatinib (small molecule tyrosine kinase inhibitor of HER2) to trastuzumab plus capecitabine has recently demonstrated significant intracranial response, reduced risk of intracranial progression or death, and even improved survival in a randomized controlled trial.52 In addition, the poly(adenosine diphosphate-ribose) inhibitor talazoparib has shown efficacy among patients with HER2 negative MBC and a germline BRCA mutation, irrespective of the presence of CNS metastases.53 Unfortunately, despite rationale for intracranial efficacy of immunotherapy,54 particularly among patients with triple negative, programmed death-ligand 1 (PD-L1) positive metastatic breast cancer, patients with brain metastases were not well represented in relevant phase III randomized controlled trials.55–57 Evidence for CNS-specific activity of CDK4/6 inhibitors58,59 and PI3K inhibitors60,61 in combination with endocrine therapy among patients with HR+/HER2− MBC is emerging but still limited.
Study Limitations
Overall, we included 25 studies in our analysis. This number represents only a portion of all the studies that assessed the incidence of brain metastases in patients with MBC. This is largely due to inconsistencies in the way that data were reported across studies, limiting our ability to pool results. Many studies made no mention of median follow-up time, and others varied in how follow-up times were measured (e.g., from initial diagnosis of early breast cancer, from diagnosis of MBC, from initiation of treatment).
It is noted that few studies reported data for HER2+ and triple negative MBC subgroups, and brain metastasis incidence was rarely broken down by hormone receptor status. Even fewer studies differentiated between parenchymal brain metastases and leptomeningeal disease. Future studies should acknowledge these types of CNS involvement, as their prognoses and treatment options vary significantly. Quality-of-life data among patients with brain metastases were also lacking. Importantly, one limitation of the incidence rate ratio analysis to compare HR-/HER2+ and HR+/HER2+ subgroups is that the same median follow-up time between the two groups had to be assumed, as separate follow-up times were not reported. Finally, it is noted that small sample sizes lead to wide confidence intervals and reduced precision around estimates in some cases. As the calculation of true confidence intervals require at least 12 samples within a group,62 assessment of publication bias was omitted for the triple negative and HR+/HER2− subgroups.
Conclusion
Patients and clinicians and should be aware of the high risk of brain metastases among patients with HER2+ and triple negative MBC. Vigilant monitoring for neurologic symptoms may help to identify CNS spread of disease early and enable appropriate and timely treatment. Although clinical practice guidelines do not currently recommend screening for brain metastases, this is largely due to a lack of available data to demonstrate the merits of an MRI-based screening program for early detection. Clinical trials, such as the “MRI Screening Versus SYMptom-directed Surveillance for Brain Metastases Among Patients With Triple Negative or HER2+ MBC” (SYMPToM trial; NCT03881605), are ongoing to investigate the potential risks and benefits of early detection of brain metastases and subsequent early intervention.
Supplementary Material
Acknowledgments
We would like to thank Ekaterina Petkova, Reena Besa, and Christina DeLonghi at Sunnybrook Library for their assistance with the database search.
Funding
No specific funding sources were used for this work.
Conflict of interest statement. To the best of our knowledge, none of the companies mentioned below have an interest in the results or materials of this study. KJJ is a speaker/advisor board/consultant for Amgen, Apo Biologix, Eli Lilly, Esai, Genomic Health, Pfizer, Roche, Novartis, Purdue Pharma. KJJ received research funding: Eli Lilly, Astra Zeneca. SD is an advisor/consultant for AbbVie, Xpan Medical, Synaptive, Omniscient. SD is also a board member in Subcortical Surgery Group and provided past educational seminars: AbbVie, Subcortical Surgery Group, Congress of Neurological Surgeons, American Association of Neurological Surgeons, Society for NeuroOncology. SD received research grants from Alkerme, Medicenna and received travel accommodations/expenses from Subcortical Surgery Group, Congress of Neurological Surgeons, American Association of Neurological Surgeons, Society for NeuroOncology, Integra. AS is an advisor/consultant for AbbVie, Merck, Roche, Varian (Medical Advisory Group), Elekta (Gamma Knife Icon), BrainLAB, and VieCure (Medical Advisory Board). AS is a board member in International Stereotactic Radiosurgery Society (ISRS) and is a co-chair in AO Spine Knowledge Forum Tumor. AS also provided educational seminars in Elekta AB, Accuray Inc., Varian (CNS Teaching Faculty), BrainLAB, Medtronic Kyphon; received research grant from Elekta AB; received travel accommodations/expenses: Elekta, Varian, BrainLAB. AS also belongs to the Elekta MR Linac Research Consortium, Elekta Spine, Oligometastases and Linac Based SRS Consortia.
Authorship statement. Generation and Drafting of Manuscript: M.K., K.J.J. Conception and Design of Experiment: M.K., K.J.J. Data Collection: M.K., Y.G., A.J.D., K.J.J. Data Analysis and Interpretation: M.K., W.T., A.K., A.S.K., C.H., A.S., S.D., K.K.C., K.J.J. Manuscript Revision and Final Approval: M.K., Y.G., W.T., C.H., A.K., A.S.K., A.J.D., A.S., S.D., K.K.C., K.J.J.
References
- 1. Gori S, Rimondini S, De Angelis V, et al. Central nervous system metastases in HER-2 positive metastatic breast cancer patients treated with trastuzumab: incidence, survival, and risk factors. Oncologist. 2007;12(7):766–773. [DOI] [PubMed] [Google Scholar]
- 2. Clayton AJ, Danson S, Jolly S, et al. Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer. 2004;91(4):639–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brufsky AM, Mayer M, Rugo HS, et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17(14):4834–4843. [DOI] [PubMed] [Google Scholar]
- 4. Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer. 2008;113(10):2638–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shmueli E, Wigler N, Inbar M. Central nervous system progression among patients with metastatic breast cancer responding to trastuzumab treatment. Eur J Cancer. 2004;40(3):379–382. [DOI] [PubMed] [Google Scholar]
- 6. Sanna G, Franceschelli L, Rotmensz N, et al. Brain metastases in patients with advanced breast cancer. Anticancer Res. 2007;27(4C):2865–2869. [PubMed] [Google Scholar]
- 7. Yau T, Swanton C, Chua S, et al. Incidence, pattern and timing of brain metastases among patients with advanced breast cancer treated with trastuzumab. Acta Oncol. 2006;45(2):196–201. [DOI] [PubMed] [Google Scholar]
- 8. Lower EE, Drosick DR, Blau R, Brennan L, Danneman W, Hawley DK. Increased rate of brain metastasis with trastuzumab therapy not associated with impaired survival. Clin Breast Cancer. 2003;4(2):114–119. [DOI] [PubMed] [Google Scholar]
- 9. Lai R, Dang CT, Malkin MG, Abrey LE. The risk of central nervous system metastases after trastuzumab therapy in patients with breast carcinoma. Cancer. 2004;101(4):810–816. [DOI] [PubMed] [Google Scholar]
- 10. Bendell JC, Domchek SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97(12):2972–2977. [DOI] [PubMed] [Google Scholar]
- 11. Arvold ND, Oh KS, Niemierko A, et al. Brain metastases after breast-conserving therapy and systemic therapy: incidence and characteristics by biologic subtype. Breast Cancer Res Treat. 2012;136(1):153–160. [DOI] [PubMed] [Google Scholar]
- 12. Gabos Z, Sinha R, Hanson J, et al. Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J Clin Oncol. 2006;24(36):5658–5663. [DOI] [PubMed] [Google Scholar]
- 13. Pestalozzi BC, Zahrieh D, Price KN, et al. ; International Breast Cancer Study Group (IBCSG) . Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG). Ann Oncol. 2006;17(6):935–944. [DOI] [PubMed] [Google Scholar]
- 14. Ryberg M, Nielsen D, Osterlind K, Andersen PK, Skovsgaard T, Dombernowsky P. Predictors of central nervous system metastasis in patients with metastatic breast cancer. A competing risk analysis of 579 patients treated with epirubicin-based chemotherapy. Breast Cancer Res Treat. 2005;91(3):217–225. [DOI] [PubMed] [Google Scholar]
- 15. Yan M, Lü HM, Liu ZZ, et al. High risk factors of brain metastases in 295 patients with advanced breast cancer. Chin Med J (Engl). 2013;126(7):1269–1275. [PubMed] [Google Scholar]
- 16. Hung MH, Liu CY, Shiau CY, et al. Effect of age and biological subtype on the risk and timing of brain metastasis in breast cancer patients. PLoS One. 2014;9(2):e89389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Niwińska A, Murawska M. New breast cancer recursive partitioning analysis prognostic index in patients with newly diagnosed brain metastases. Int J Radiat Oncol Biol Phys. 2012;82(5):2065–2071. [DOI] [PubMed] [Google Scholar]
- 18. Altaha R, Crowell E, Hobbs G, Higa G, Abraham J. Increased risk of brain metastases in patients with HER-2/neu-positive breast carcinoma. Cancer. 2005;103(3):442–443. [DOI] [PubMed] [Google Scholar]
- 19. Jung SY, Rosenzweig M, Sereika SM, Linkov F, Brufsky A, Weissfeld JL. Factors associated with mortality after breast cancer metastasis. Cancer Causes Control. 2012;23(1):103–112. [DOI] [PubMed] [Google Scholar]
- 20. Canney P, Dixon-Hughes J, Lewsley LA, Paul J, Murra E. A prospective randomised Phase III clinical trial testing the role of prophylactic cranial radiotherapy in patients treated with trastuzumab for metastatic breast cancer - Anglo Celtic VII. Clin Oncol (R Coll Radiol). 2015;27(8):460. [DOI] [PubMed] [Google Scholar]
- 21. Cagney DN, Martin AM, Catalano PJ, et al. Implications of screening for brain metastases in patients with breast cancer and non-small cell lung cancer. JAMA Oncol. 2018;4(7):1001–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Komorowski AS, Warner E, MacKay HJ, Sahgal A, Pritchard KI, Jerzak KJ. Incidence of brain metastases in nonmetastatic and metastatic breast cancer: is there a role for screening? Clin Breast Cancer. 2019. doi: 10.1016/j.clbc.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 23. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lefebvre C, Glanville J, Briscoe S, et al. Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from: www.training.cochrane.org/handbook. [Google Scholar]
- 25. Krop IE, Lin NU, Blackwell K, et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol. 2015;26(1):113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Swain SM, Baselga J, Miles D, et al. Incidence of central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: results from the randomized phase III study CLEOPATRA. Ann Oncol. 2014;25(6):1116–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. National Health and Medical Research Council. NHMRC additional levels of evidence and grades for recommendations for developers of guidelines. Canberra; 2009. Available from: www.mja.com.au/sites/default/files/NHMRC.levels.of.evidence.2008-09.pdf.
- 28. Quintana DS. From pre-registration to publication: A non-technical primer for conducting a meta-analysis to synthesize correlational data. Front Psychol. 2015; 6(OCT):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14(6):500–515. [DOI] [PubMed] [Google Scholar]
- 30. Pivot X, Manikhas A, Żurawski B, et al. CEREBEL (EGF111438): a phase III, randomized, open-label study of lapatinib plus capecitabine versus trastuzumab plus capecitabine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2015;33(14):1564–1573. [DOI] [PubMed] [Google Scholar]
- 31. Awada A, Colomer R, Inoue K, et al. Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in previously untreated metastatic ERBB2-positive breast cancer: the NEfERT-T randomized clinical trial. JAMA Oncol. 2016;2(12):1557–1564. [DOI] [PubMed] [Google Scholar]
- 32. Bergen E, Berghoff AS, Rudas M, et al. Taxanes plus trastuzumab compared to oral vinorelbine plus trastuzumab in HER2-overexpressing metastatic breast cancer. Breast Care (Basel). 2014;9(5):344–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bian L, Wang T, Zhang S, Jiang Z. Trastuzumab plus capecitabine vs. lapatinib plus capecitabine in patients with trastuzumab resistance and taxane-pretreated metastatic breast cancer. Tumour Biol. 2013;34(5):3153–3158. [DOI] [PubMed] [Google Scholar]
- 34. Witzel I, Kantelhardt EJ, Milde-Langosch K, et al. Management of patients with brain metastases receiving trastuzumab treatment for metastatic breast cancer. Onkologie. 2011;34(6):304–308. [DOI] [PubMed] [Google Scholar]
- 35. Bartsch R, De Vries C, Pluschnig U, et al. Predicting for activity of second-line trastuzumab-based therapy in her2-positive advanced breast cancer. BMC Cancer. 2009;9:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aversa C, Rossi V, Geuna E, et al. Metastatic breast cancer subtypes and central nervous system metastases. Breast. 2014;23(5): 623–628. [DOI] [PubMed] [Google Scholar]
- 37. Kim YJ, Kim JS, Kim IA. Molecular subtype predicts incidence and prognosis of brain metastasis from breast cancer in SEER database. J Cancer Res Clin Oncol. 2018;144(9):1803–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. D’hondt R, Spoormans I, Neyens N, Mortier N, Van Aelst F. Survival of patients with metastatic breast cancer: a single-centre experience. Acta Clin Belg. 2014;69(3):194–199. [DOI] [PubMed] [Google Scholar]
- 39. Darlix A, Louvel G, Fraisse J, et al. Impact of breast cancer molecular subtypes on the incidence, kinetics and prognosis of central nervous system metastases in a large multicentre real-life cohort. Br J Cancer. 2019;121(12):991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jin J, Gao Y, Zhang J, et al. Incidence, pattern and prognosis of brain metastases in patients with metastatic triple negative breast cancer. BMC Cancer. 2018;18(1):446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ren JX, Gong Y, Ling H, Hu X, Shao ZM. Racial/ethnic differences in the outcomes of patients with metastatic breast cancer: contributions of demographic, socioeconomic, tumor and metastatic characteristics. Breast Cancer Res Treat. 2019;173(1):225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Danese MD, Lindquist K, Doan J, Lalla D, Brammer M, Griffiths RI. Effect of central nervous system metastases on treatment discontinuation and survival in older women receiving trastuzumab for metastatic breast cancer. J Cancer Epidemiol. 2012;2012:819210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heitz F, Rochon J, Harter P, et al. Cerebral metastases in metastatic breast cancer: disease-specific risk factors and survival. Ann Oncol. 2011;22(7):1571–1581. [DOI] [PubMed] [Google Scholar]
- 44. Azim HA, Abdel-Malek R, Kassem L. Predicting brain metastasis in breast cancer patients: stage versus biology. Clin Breast Cancer. 2018;18(2):e187–e195. [DOI] [PubMed] [Google Scholar]
- 45. Ono M, Ando M, Yunokawa M, et al. Brain metastases in patients who receive trastuzumab-containing chemotherapy for HER2-overexpressing metastatic breast cancer. Int J Clin Oncol. 2009;14(1):48–52. [DOI] [PubMed] [Google Scholar]
- 46. Montagna E, Cancello G, D’Agostino D, et al. Central nervous system metastases in a cohort of metastatic breast cancer patients treated with trastuzumab. Cancer Chemother Pharmacol. 2009;63(2):275–280. [DOI] [PubMed] [Google Scholar]
- 47. Dawson SJ, Ranieri NF, Snyder RD, et al. Central nervous system metastases in women with HER-2 positive metastatic breast cancer after treatment with trastuzumab. Asia Pac J Clin Oncol. 2006;2(1):50–56. [Google Scholar]
- 48. Martin AM, Cagney DN, Catalano PJ, et al. Brain metastases in newly diagnosed breast cancer: a population-based study. JAMA Oncol. 2017;3(8):1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pestalozzi BC, Brignoli S. Trastuzumab in CSF. J Clin Oncol. 2000;18(11):2349–2351. [DOI] [PubMed] [Google Scholar]
- 50. Dijkers EC, Oude Munnink TH, Kosterink JG, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther. 2010;87(5):586–592. [DOI] [PubMed] [Google Scholar]
- 51. Montemurro F, Delaloge S, Barrios CH, et al. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial. Ann Oncol. 2020;31(10):1350–1358. [DOI] [PubMed] [Google Scholar]
- 52. Lin NU, Borges V, Anders C, et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol. 2020;38(23):2610–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379(8):753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brastianos PK, Lee EQ, Cohen JV, et al. Single-arm, open-label phase 2 trial of pembrolizumab in patients with leptomeningeal carcinomatosis. Nat Med. 2020;26(8):1280–1284. [DOI] [PubMed] [Google Scholar]
- 55. Schmid P, Adams S, Rugo HS, et al. ; IMpassion130 Trial Investigators . Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–2121. [DOI] [PubMed] [Google Scholar]
- 56. Cortes J, Cescon DW, Rugo HS, et al. KEYNOTE-355: Randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. J Clin Oncol. 2020;38(15_suppl):1000. [DOI] [PubMed] [Google Scholar]
- 57. Miles DW, Gligorov J, André F, et al. LBA15 Primary results from IMpassion131, a double-blind placebo-controlled randomised phase III trial of first-line paclitaxel (PAC) ± atezolizumab (atezo) for unresectable locally advanced/metastatic triple-negative breast cancer (mTNBC). Ann Oncol. 2020;31:S1147–S1148. [DOI] [PubMed] [Google Scholar]
- 58. Nguyen LV, Searle K, Jerzak KJ. Central nervous system-specific efficacy of CDK4/6 inhibitors in randomized controlled trials for metastatic breast cancer. Oncotarget. 2019;10(59):6317–6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Anders CK, Le Rhun E, Bachelot TD, et al. A phase II study of abemaciclib in patients (pts) with brain metastases (BM) secondary to HR+, HER2- metastatic breast cancer (MBC). J Clin Oncol. 2019;37(15_suppl):1017. [Google Scholar]
- 60. de Gooijer MC, Zhang P, Buil LCM, et al. Buparlisib is a brain penetrable pan-PI3K inhibitor. Sci Rep. 2018;8(1):10784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Batalini F, Moulder SL, Winer EP, Rugo HS, Lin NU, Wulf GM. Response of brain metastases from PIK3CA-mutant breast cancer to Alpelisib. JCO Precis Oncol. 2020;4:PO.19.00403. Published May 27, 2020. doi: 10.1200/PO.19.00403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. van Belle G. Statistical Rules of Thumb. 2nd ed. Hoboken, New Jersey: John Wiley & Sons, Inc.; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.