Abstract
Background
Increasing incidence of central nervous system (CNS) tumors has been noted in some populations. However, the influence of changing surgical and imaging practices has not been consistently accounted for.
Methods
We evaluated average annual percentage change (AAPC) in age- and gender-stratified incidence of CNS tumors by tumor subtypes and histological confirmation in Wales, United Kingdom (1997-2015) and the United States (2004-2015) using joinpoint regression.
Findings
In Wales, the incidence of histologically confirmed CNS tumors increased more than all CNS tumors (AAPC 3.62% vs 1.63%), indicating an increasing proportion undergoing surgery. Grade II and III glioma incidence declined significantly (AAPC −3.09% and −1.85%, respectively) but remained stable for those with histological confirmation. Grade IV glioma incidence increased overall (AAPC 3.99%), more markedly for those with histological confirmation (AAPC 5.36%), suggesting reduced glioma subtype misclassification due to increased surgery. In the United States, the incidence of CNS tumors increased overall but was stable for histologically confirmed tumors (AAPC 1.86% vs 0.09%) indicating an increase in patients diagnosed without surgery. An increase in grade IV gliomas (AAPC 0.28%) and a decline in grade II gliomas (AAPC −3.41%) were accompanied by similar changes in those with histological confirmation, indicating the overall trends in glioma subtypes were unlikely to be caused by changing diagnostic and clinical management.
Conclusions
Changes in clinical practice have influenced the incidence of CNS tumors in the United Kingdom and the United States. These should be considered when evaluating trends and in epidemiological studies of putative risk factors for CNS tumors.
Keywords: cancer registry, epidemiology, incidence, population-based, trends
Key Points.
In Wales (United Kingdom), a higher proportion of patients with a CNS tumor receiving surgery with subsequent histological confirmation reduced misclassification of gliomas.
In the United States, more CNS tumors were diagnosed without subsequent surgical management.
Importance of the Study.
Some ecological studies have observed rising trends of CNS tumors, hypothesizing environmental or lifestyle factors might explain such trends. Interpretation of incidence trends must also account for diagnostic and clinical practice, which can impact the identification and classification of tumor subtypes. We aimed to assess how changes in diagnostic and clinical management may affect the incidence of brain and CNS tumors by evaluating the trends of tumors both overall and with histological confirmation of diagnosis in two population-based cancer databases. Rising incidence of brain and other CNS tumors is driven in part by identifying incidental tumors. In Wales (United Kingdom), a shift away from clinical toward histological diagnosis likely reduced misclassification of gliomas, contributing to the previously reported controversial observation of rising grade IV glioma incidence. Evolving diagnostic and imaging practices are important factors that need to be accounted for in CNS tumor incidence reporting in the United Kingdom and other populations for international comparisons.
Reports suggest that the incidence of brain and other central nervous system (CNS) tumors has increased worldwide since 1990.1 Improvements in cancer registration and increased use of imaging may have contributed to the observed increase.2 Since CNS tumors are a heterogenous group of benign and malignant tumors, reporting of aggregate CNS tumor incidence rates masks trends of individual tumor subtypes. Several population-based studies in England, Finland, Australia, and North America have reported an increase in the incidence of glioblastoma, the most common primary brain tumor.3–6 Advances in neuroimaging technologies, increased clinical awareness, and the aging population have been suggested as contributing factors,3,4 while environmental and lifestyle factors, in particular ionizing radiation exposure, air pollution, and mobile phone use, have been suggested as putative risk factors.5 Cancer registries do not collect relevant information that enable such risk factors to be properly addressed in epidemiological studies. Furthermore, there is substantial variability in brain tumor incidence worldwide,1,7 and an increasing trend in glioblastoma incidence is not consistently reported.8,9 Evaluating incidence trends in multiple different populations by brain tumor subtypes in the context of all other brain and CNS tumors may provide additional insights into the influence of clinical and histological tumor classification.
A recent analysis of data from the English cancer registry suggested increasing incidence of glioblastomas in the United Kingdom and received much media attention.5 A limitation of this analysis was the lack of accounting for diagnostic changes that might have resulted in more accurate classification of grade IV gliomas and less misclassification of lower grade II/III gliomas. Histological confirmation is the gold standard for tumor subtype classification due to the limited specificity (78%) of classifying brain and other CNS tumors using radiological investigations only.10 Obtaining tumor samples for histological examination almost always results from an operation for brain and other CNS tumors. Hence, a recorded histological diagnosis in cancer registries provides evidence of a surgical intervention. Incidence rates of histologically diagnosed tumors represent the more accurate classification of tumor subtypes. Comparison of incidence trends in overall and histologically diagnosed tumors should therefore inform on how changes in clinical practice have influenced observed incidence trends. The extent of these changes differs between countries due to variation in national guidelines and practices. Diagnostic practice using neuroimaging has an impact on incidental findings since potentially serious findings including intracranial mass lesions occur in 1.4% of brain MRI.11 Clarifying changes in clinical and diagnostic practices can aid better interpretation of CNS tumor incidence trends and international comparison.
We aimed to describe trends in incidence of brain and other CNS tumors and their subtypes by histological diagnosis in two population-based cancer databases in the United Kingdom and the United States, and to examine changes in diagnostic and clinical practice that might affect these.
Methods
Study Design and Setting
We conducted a retrospective population-based study using routinely collected, individual-level patient records from the Secure Anonymised Information Linkage (SAIL) Databank12 (https://saildatabank.com/) and the Surveillance, Epidemiology, and End Results (SEER) database.13 The SAIL Databank is a data platform holding de-identified and linkable datasets of individuals living in Wales, United Kingdom (approximately three million people). The SEER 18 incidence database includes data on all incident tumors for about one-third of the population of the United States (approximately 114 million people). The study period for both databases was January 1, 1997 to December 31, 2015.
Case Identification and Grouping
We identified benign and malignant primary brain and other CNS tumors using the following International Classification of Diseases, 10th revision (ICD-10) codes: C70-72, C75·1-C75·3, D18·0, D32-D33, D35·2-D35·4, D42-D43, D44·3-D44·5.14 We used the International Classification of Diseases for Oncology third edition (ICD-O-3) histology codes to group tumors into three broad categories: meningiomas, gliomas, and all others, following the definitions from the Central Brain Tumour Registry of the United States (CBTRUS) (Supplementary Appendix).15 We further categorized gliomas into four grades according to ICD-O-3 (Supplementary Appendix). If there were multiple entries for an individual, we included only the earliest entry for each tumor subtype. We subdivided the basis of diagnosis into two groups: histological or clinical (without histological confirmation). Of note, in SEER, reporting of benign tumors did not start until 2004; incidence data prior to 2004 include malignant tumors only. SAIL provides data for all benign and malignant tumors from 1997.
Variables and Data Sources
Baseline characteristics included age at diagnosis, gender, year of diagnosis, and basis of diagnosis. Where information on any of these characteristics was missing within the cancer registry for individuals in the SAIL database (Wales, United Kingdom), we obtained them from linked data from the birth registry, death registry, or hospital episode data.
Diagnostic Imaging Dataset
There is no publicly available diagnostic imaging usage data in Wales or the United States. However, a collection of diagnostic imaging tests in the National Health Service (NHS) performed in England since 2012, the Diagnostic Imaging Dataset (DID), provides information on numbers of brain MRI scans performed each year and those requested by general practitioners (GP). We used these data to assess trends in the numbers of brain MRI scans performed each year in the United Kingdom from 2012 to 2018.
Statistical Analysis
For SAIL and SEER data, we calculated age- and gender-standardized incidence rates (ASR) using direct standardization to the European Standard Population 2013. We obtained gender- and year-specific population structure data from StatsWales (https://statswales.gov.wales/) for Wales, and directly from SEER for the United States. We performed all analyses using Stata 16 (StataCorp LLC, College Station, TX, USA).
To assess trends, we entered the standardized incidence rates and their corresponding standard errors into the joinpoint software version 4·7·0·0 (https://surveillance.cancer.gov/joinpoint/). Joinpoint regression was designed to identify trends in cancer incidence and uses a grid-search method to fit a regression function with unknown joinpoints.16 The annual percentage change (APC) refers to the change within a specified period; the average annual percentage change (AAPC) refers to the overall change over the whole study period. We performed trend analyses for each of the three main tumor categories and calculated the AAPC and APC with associated 95% confidence intervals to determine if they deviated significantly from 0%. To estimate trends in incidence of the subset of brain tumor patients receiving a surgical intervention, we performed analyses on tumors with a histological diagnosis. For DID data, we performed trend analysis to determine the AAPC of the number of scans per 100 000 people in England using joinpoint regression.
Ethical Statement
The Information Governance Review Panel in the SAIL Databank reviewed and approved the use of the data for this project (ref: 0918). We obtained access to SEER data using the standard procedure (https://seer.cancer.gov/data/access.html). We analyzed and reported the data in accordance with the data usage agreements.
Results
Brain and Other CNS Tumors in Wales
There were 11 770 brain and other CNS tumors diagnosed in Wales between 1997 and 2015 (Table 1). Median age at diagnosis was 62 years (interquartile range [IQR] 46-74 years). Overall, 53.5% of the incident tumors were among females and 55.4% were nonmalignant. Histological confirmation was established in 55.8% of tumors, with some variation in this proportion over time (change from 46.0% in 1997-2003 to 57.2% in 2010-2015), among tumor subgroups (ie, 60.7% in meningiomas, 66.9% in gliomas) and by glioma grade (ie, 81.9% in grade I and 69.5% in grade IV gliomas) (Supplementary Table 1). Among glioma patients, we noted almost a doubling in the proportion of patients having histological confirmation from 40% in 1997 to 80% starting around 2005 (Supplementary Figure 1). The overall ASR of brain and CNS tumors in Wales was 21.7 (95% CI 21.3-22.1) per 100 000 person-years, with similar ASR for women and men (21.5 and 21.8 per 100 000 person-years, respectively). The ASR per 100 000 person-years for tumor subtype groups were: 5.1 (95% CI 4.9-5.3) for meningioma; 8.5 (95% CI 8.2-8.7) for gliomas; and 8.8 (95% CI 8.6-9.1) for all other tumors.
Table 1.
Incident Brain and CNS Tumors Diagnosed in Wales, United Kingdom (1997-2015) and SEER, United States (2004-2015)
| SAIL, Wales, United Kingdom | SEER, United States | |||
|---|---|---|---|---|
| N | % | N | % | |
| Number of tumors | 11 770 | 243 723 | ||
| Age (y) | ||||
| Median (IQR) | 62 (46-74) | 58 (43-71) | ||
| 0-29 y | 1243 | 10.6 | 29 196 | 12.0 |
| 30-49 y | 2173 | 18.5 | 53 148 | 21.8 |
| 50-59 y | 1896 | 16.1 | 45 586 | 18.7 |
| 60-69 y | 2397 | 20.4 | 47 321 | 19.4 |
| 70-79 y | 2314 | 19.7 | 38 972 | 16.0 |
| 80+ y | 1747 | 14.8 | 29 500 | 12.1 |
| Gender | ||||
| Female | 6293 | 53.5 | 141 752 | 58.2 |
| Male | 5477 | 46.5 | 101 971 | 41.8 |
| Year of diagnosis | ||||
| 1997-2003 | 3578 | 40.3 | - | - |
| 2004-2009 | 3728 | 31.7 | 109 606 | 45.0 |
| 2010-2015 | 4464 | 37.9 | 134 117 | 55.0 |
| Malignant behavior | ||||
| Nonmalignant | 6524 | 55.4 | 169 773 | 69.7 |
| Malignant | 5246 | 44.6 | 73 953 | 30.3 |
| Tumor types | ||||
| Meningioma | 2595 | 22.0 | 91 555 | 37.6 |
| Gliomas | 4455 | 37.9 | 65 397 | 26.8 |
| Grade I gliomasa | 270 | 6.1 | 7723 | 11.9 |
| Grade II gliomasa | 643 | 14.4 | 8790 | 13.5 |
| Grade III gliomasa | 1406 | 31.6 | 14 649 | 22.5 |
| Grade IV gliomasa | 2136 | 47.9 | 33 928 | 52.1 |
| Other tumors | 4720 | 40.1 | 86 771 | 35.6 |
| Basis of diagnosis | ||||
| Histological | 6562 | 55.8 | 145 008 | 59.5 |
| Clinical (no histology) | 5208 | 44.3 | 98 715 | 40.5 |
aPercentages relate to the total number of gliomas.
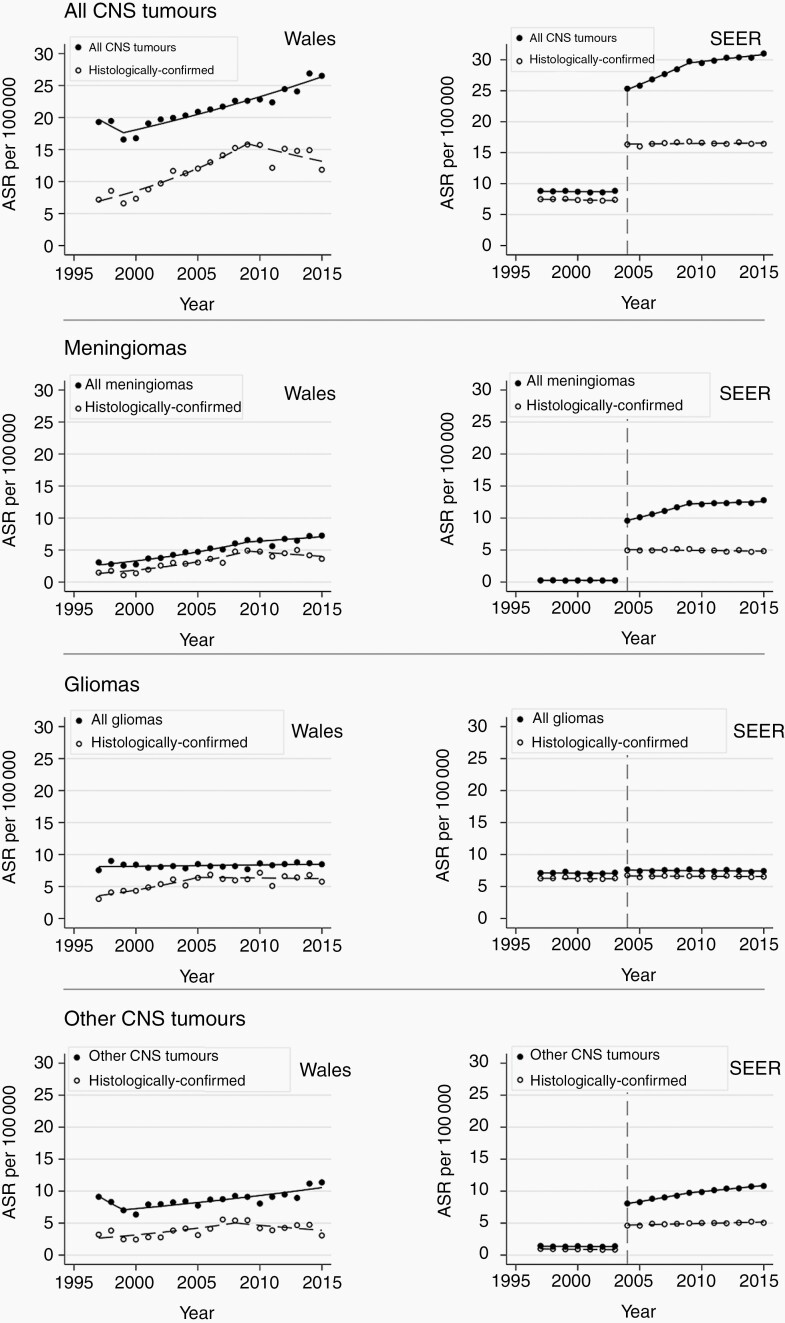
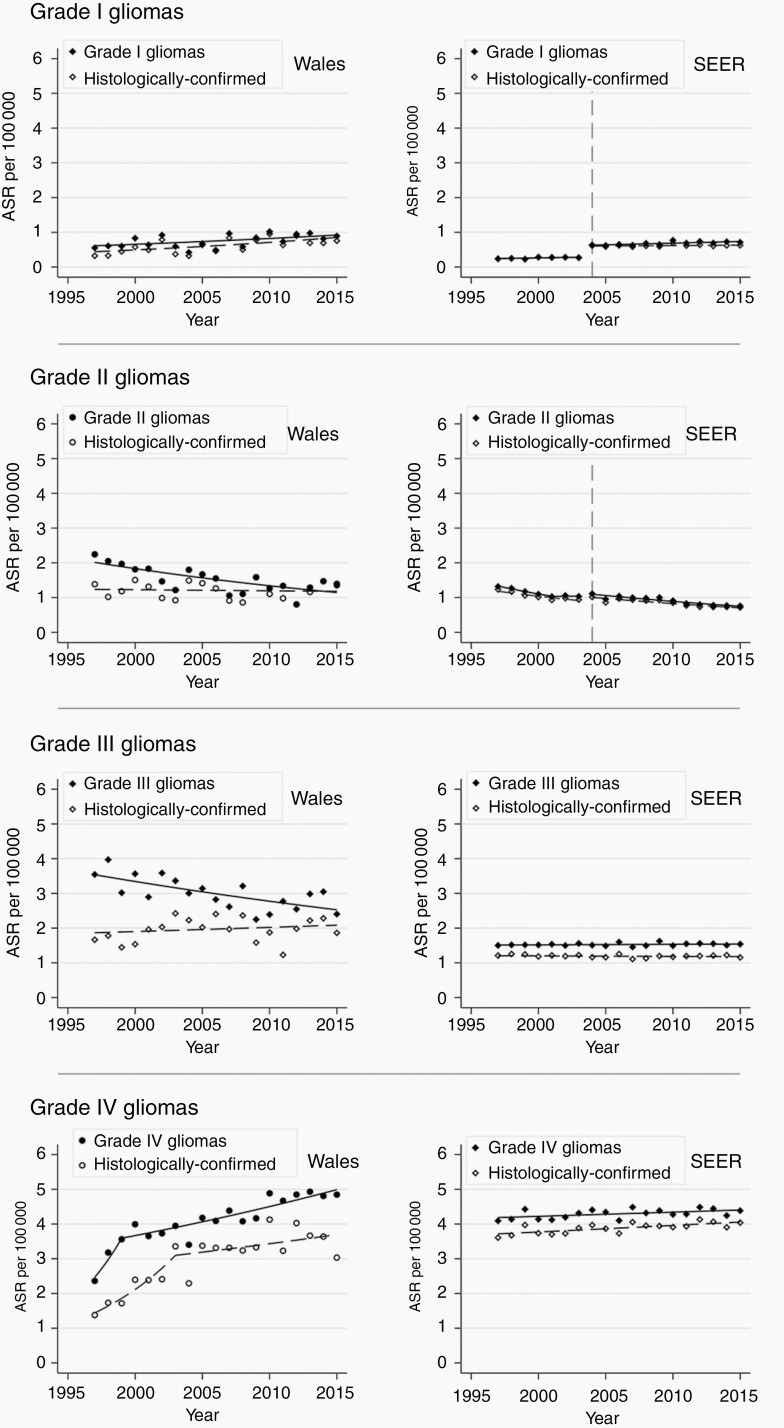
Overall, the incidence of brain and other CNS tumors in Wales increased between 1997 and 2015 from 19.3 to 26.5 per 100 000 person-years (AAPC 1.63%; 95% CI 0.40-2.87; P = .01) (Figure 1). Analysis by tumor subtypes showed that the incidence of gliomas overall did not change during the study period. In contrast, the incidence of meningiomas increased, albeit less steeply after 2009, and there was no significant change in the incidence of other CNS tumors (Figure 1, Table 2). Amongst gliomas, the incidence of grade IV gliomas increased while that of grade II and III gliomas declined (Figure 2, Table 2).
Fig. 1.
Trends in age- and gender-standardized incidence rate of brain and other CNS tumors in Wales and SEER by tumor types 1997-2015. Data from the Secure Anonymised Information Linkage (SAIL) Databank in Wales and from the Surveillance, Epidemiology, and End Results (SEER) in the United States contributed to this analysis. Incidence rates were standardized to the European Standard Population 2013. Vertical dotted line denotes the year at which SEER began to include benign tumors.
Table 2.
Average Annual Percentage Change (AAPC) of Brain and Other CNS Tumor Incidence by Tumor Types
| Brain and Other CNS Tumors | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| SAIL, Wales, United Kingdom (1997-2015) | SEER, United States (2004-2015)a | ||||||||
| ASR in 1997 | ASR in 2015 | AAPC | 95% CI | P Value | ASR in 2004 | ASR in 2015 | AAPC | 95% CI | P Value |
| All brain and CNS tumors | |||||||||
| 19.3 | 26.5 | 1.63 | 0.40 to 2.87 | .01 | 25.3 | 31.0 | 1.86 | 1.60 to 2.12 | <.01 |
| Meningiomas | |||||||||
| 3.1 | 7.3 | 5.56 | 4.11 to 7.03 | <.01 | 9.6 | 12.8 | 2.47 | 2.09 to 2.85 | <.01 |
| Others | |||||||||
| 9.1 | 11.4 | 0.74 | −1.82 to 3.37 | .57 | 8.1 | 10.8 | 2.77 | 2.40 to 3.14 | <.01 |
| Gliomas | |||||||||
| 7.6 | 8.5 | 0.26 | −0.12 0.64 | .17 | 7.7 | 7.4 | −0.18 | −0.47 to 0.11 | .19 |
| Grade I gliomas | |||||||||
| 0.6 | 0.9 | 2.31 | 0.38 to 4.27 | .02 | 0.6 | 0.7 | 1.42 | 0.50 to 2.35 | .01 |
| Grade II gliomas | |||||||||
| 2.2 | 1.4 | −3.09 | −4.48 to −1.67 | <.01 | 1.1 | 0.8 | −3.41 | −4.30 to −2.52 | <.01 |
| Grade III gliomas | |||||||||
| 3.5 | 2.4 | −1.85 | −2.80 to −0.88 | <.01 | 1.5a | 1.5a | 0.11a | −0.15 to 0.37 | .40 |
| Grade IV gliomas | |||||||||
| 2.4 | 4.8 | 3.99 | 1.46 to 6.59 | <.01 | 4.1a | 4.4a | 0.28a | 0.04 to 0.53 | .03 |
aAAPC for grade III and IV gliomas calculated from 1997 to 2015.
Fig. 2.
Trends in age- and gender-standardized incidence rate of gliomas in Wales and SEER by glioma grades 1997-2015.Data from the Secure Anonymised Information Linkage (SAIL) Databank in Wales and from the Surveillance, Epidemiology, and End Results (SEER) in the United States contributed to this analysis. Incidence rates were standardized to the European Standard Population 2013. Vertical dotted line denotes the year at which SEER began to include benign tumors.
The incidence of histologically confirmed tumors increased from 7.2 to 11.8 per 100 000 person-years between 1997 and 2015 (AAPC 3.62%; 95% CI 1.78-5.49; P < .01; Table 3). Most of this increase occurred from 1997 to 2009, flattening thereafter (Figure 2; Supplementary Table 2). For meningiomas, the incidence of histologically confirmed cases paralleled the increasing overall trend from 1997 to 2009 but flattened (and so diverged from the overall trend) from 2009 (Figure 1). The incidence of histologically confirmed gliomas increased from 1997 to 2005 but flattened from 2005 (Figure 2, Supplementary Table 2). Similarly, for all other tumors, there was an increase (1997-2008) followed by stabilization of incidence rates from 2008 to 2015. The trends for histologically confirmed grade I and grade IV glioma cases were broadly similar and parallel to the overall trends for these glioma grades (Figure 2). However, the incidence of histologically confirmed grade II and III gliomas did not change significantly, in contrast with the reduction in overall incidence rates for these glioma subtypes (Figure 2, Table 3).
Table 3.
Average Annual Percentage Change (AAPC) of Histologically Confirmed Brain and Other CNS Tumor Incidence by Tumor Types
| Histologically Confirmed Brain and Other CNS Tumors | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| SAIL, Wales, United Kingdom (1997-2015) | SEER, United States (2004-2015)a | ||||||||
| ASR in 1997 | ASR in 2015 | AAPC | 95% CI | P Value | ASR in 2004 | ASR in 2015 | AAPC | 95% CI | P Value |
| All brain and CNS tumors | |||||||||
| 7.2 | 11.8 | 3.62 | 1.78 to 5.49 | <.01 | 16.3 | 16.4 | 0.09 | −0.13 to 0.32 | .39 |
| Meningiomas | |||||||||
| 1.5 | 3.6 | 6.20 | 3.51 to 8.96 | <.01 | 5.0 | 4.8 | −0.40 | −0.90 to 0.09 | .10 |
| Others | |||||||||
| 3.2 | 3.1 | 2.13 | −1.21 to 5.58 | .21 | 4.6 | 5.0 | 0.87 | 0.53 to 1.23 | <.01 |
| Gliomas | |||||||||
| 3.1 | 5.8 | 3.14 | 1.13 to 5.19 | <.01 | 6.8 | 6.5 | −0.12 | −0.37 to 0.13 | .33 |
| Grade I gliomas | |||||||||
| 0.3 | 0.8 | 3.72 | 1.20 to 6.30 | .01 | 0.6 | 0.6 | 0.42 | −0.46 to 1.30 | .32 |
| Grade II gliomas | |||||||||
| 1.4 | 1.4 | −0.26 | −2.10 to 1.61 | .77 | 1.0 | 0.7 | −3.19 | −4.19 to −2.19 | <.01 |
| Grade III gliomas | |||||||||
| 1.7 | 1.9 | 0.62 | −1.00 to 2.26 | .43 | 1.2a | 1.2a | −0.11a | −0.42 to 0.19 | .45 |
| Grade IV gliomas | |||||||||
| 1.4 | 3.0 | 5.36 | 2.06 to 8.77 | <.01 | 3.6a | 4.0a | 0.49a | 0.25 to 0.73 | <.01 |
aAAPC for grade III and IV gliomas calculated from 1997 to 2015.
Brain and Other CNS Tumors in the United States (SEER)
In the SEER database, there were 273 543 brain and other CNS tumors diagnosed in the United States between 1997 and 2015, of which 243 723 were diagnosed from 2004 onwards, when benign tumor reporting started (Table 1). The median age at diagnosis was 58 years (IQR 43-71 years). Overall, 58.2% of tumors were among females and 69.7% were nonmalignant. The proportion of tumors with histological confirmation was 59.5% with variation among tumor types (eg, 45.3% in meningiomas, 88.5% in gliomas) and by glioma grades (eg, 77.2% in grade III and 92.1% in grade IV gliomas) (Supplementary Table 1). The majority (90.0%) of non-histologically confirmed tumors were diagnosed radiographically and this increased between 2004 and 2015 for meningiomas and other CNS tumors (Supplementary Figure 2).
The overall ASR in SEER from 2004 to 2015 was 28.9 (95% CI 28.8-29.0) per 100 000 person-years and was higher in females than males (ASR 31.4; 95% CI 31.3-31.6 vs 26.0; 95% CI 25.9-26.2 per 100 000 person-years). The ASRs per 100 000 person-years for tumor groups were: 11.7 (95% CI 11.6-11.8) for meningiomas; 7.5 (95% CI 7.4-7.6) for gliomas; and 9.7 (95% CI 9.6-9.7) for all other tumors.
Overall incidence of brain and other CNS tumors in the United States increased between 2004 and 2015 from 25.3 to 31.0 per 100 000 person-years (AAPC 1.86; 95% 1.60-2.12; P < .01) (Figure 1). The incidence of meningiomas and other (non-meningioma, non-glioma) CNS tumors increased, but not gliomas (Table 2, Figure 1). With respect to the incidence of glioma subtypes, grade I and IV gliomas showed a small increase (AAPC 1.42% and 0.28%, respectively). Grade II gliomas decreased, and grade III gliomas did not change significantly (Table 2, Figure 2).
The incidence of histologically confirmed tumors did not change overall (Table 3), although the incidence of non-meningioma, non-glioma CNS tumors increased slightly (AAPC 0.87; 95% CI 0.53-1.23; P < .01) (Table 3). There was no change in histologically confirmed meningioma and glioma incidence in 2004-2015 (Figure 1). The grade-specific incidence trends of histologically confirmed gliomas were similar to the overall trends for these gliomas (Figure 2).
Imaging Trend in England 2012-2018
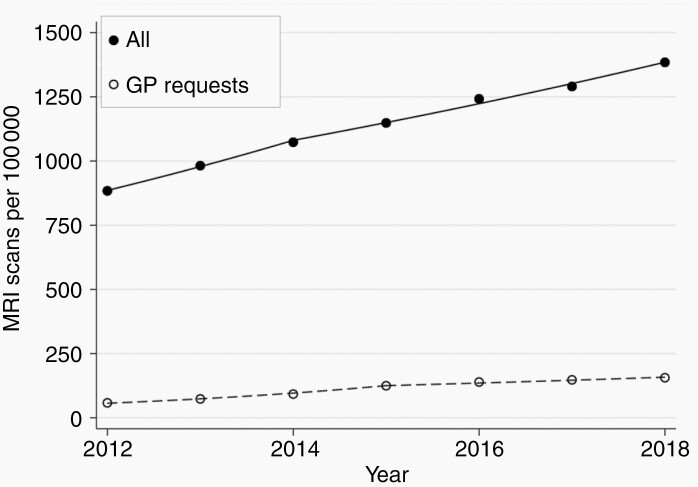
A total of 4 392 470 brain MRI scans was performed in England between 2012 and 2018, of which 435 350 were requested by GP, giving a mean of 1143 scans per 100 000 person-years overall and 113 per 100 000 person-years for GP requests. The mean number of scans performed per 100 000 increased by an average of 7.7% (n = 49 525) per year (95% CI 6.2-9.3; P < .01) and by 18.7% (n = 10 010) (95% CI 18.4-19.0; P < .01) for GP requests (Figure 3).
Fig. 3.
Number of MRI brain scans performed per 100 000 people in England 2012-2018. Data from the Diagnostic Imaging Dataset (DID) published by the National Health Service (NHS) England. Solid points denote MRI brain scans performed; unfilled points denote scans requested by the general practitioner (GP).
Discussion
We used two population-based cancer registry datasets to describe trends in the incidence of brain and other CNS tumors from 1997 to 2015 in Wales, United Kingdom and from 2004 to 2015 in the United States. We found evidence of an overall increase in incidence in both populations that was driven by increased incidence of meningiomas and other non-glioma, non-meningioma CNS tumors. In Wales, the combination of a decline in the incidence of grade II and III glioma with no change for those with histological confirmation suggests a shift from solely clinical diagnoses to histological diagnoses, likely resulting in the observed increased incidence of grade IV gliomas. In the United States, the increase in the incidence of meningiomas and other (non-glioma) CNS tumors without a corresponding increase in those with a histological diagnosis suggests an increase in clinical and radiological diagnosis of these tumors. For all glioma subtypes, overall and histologically confirmed incidence trends were parallel, suggesting no substantial change in diagnostic and clinical practice for gliomas in the United States during the time period of the study.
The increasing incidence of brain and other CNS tumors in Wales is consistent with the rest of the United Kingdom17,18 and internationally.1 The National Public Health Service for Wales reported a 80% and 100% increase in all CT and MRI, respectively, in Wales 2000-2007.19 While these findings are not specific for brain imaging, it is likely that the increased use of brain MRI observed in the English DID also applies to Wales. As well as identifying brain tumors in patients with symptoms, brain MRI also reveals incidental tumors in 1.4% of scans performed for other reasons.11 Hence, regional variation in provision and utilization of neuroimaging facilities is likely to contribute to the variation in incidence trends of brain tumors observed internationally.7 In the United States, brain imaging for medical purposes increased between 2000 and 2016, with a lower AAPC (1.2%) than in England (7.7%).20 Data from the Health Care Systems Research Network in the United States showed that MRI use increased by 11.4% each year from 2000 to 2004, and then by 2.2% each year from 2007 to 2016.21 These findings are consistent with the increasing incidence of brain and CNS tumors in SEER, which increased less steeply from around 2009, supporting our interpretation of imaging use contributing to the observed incidence trends. These data are also consistent with overall trends in increasing MRI use from 1997 to 2014 available from annual imaging usage data from NHS.22
Evaluating trends of histologically confirmed tumors aids the interpretation of overall trends. Conventional MRI techniques have >85% sensitivity in diagnosing meningiomas; surgery is not required simply for histological confirmation of likely diagnosis.23 Surgical resection is reserved as the primary treatment for symptomatic patients, although occasionally other treatment modalities such as radiotherapy and stereotactic radiosurgery are used for meningiomas in difficult or surgically inaccessible tumor locations.24 The difference between the incidence trends of overall and histologically confirmed tumors (Figures 1 and 2); therefore, represents an estimate of the proportion of patients receiving alternative treatment or no treatment at all. Since there has been no major change to clinical management of meningiomas; the proportion of patients receiving surgical and alternative treatment should have remained constant. The observation in Wales of increasing incidence of meningiomas overall with no change in the incidence of histologically confirmed meningiomas from 2009 would therefore suggest increased identification of incidental meningiomas that did not require surgery through increased use of imaging.25,26 This divergent trend was also seen in the United States, but earlier, between 2004 and 2009. Subsequent parallel trends of overall and histologically confirmed meningiomas suggest that imaging practice reached a steady state in the United States by 2009. By contrast, a further increase in the use of neuroimaging in Wales could drive a further apparent increase in meningioma incidence through diagnosing more incidental meningiomas. Furthermore, the imaging rate per 1000 person-years in 2016 was 1.4 for all MRI in England (Figure 3) but was 8 for MRI head imaging alone in the United States.20 This undoubtedly contributed to the much higher incidence rate of meningioma in the United States since incidental findings are not uncommon.11
Gliomas are a heterogeneous group of tumors. While individual patient and tumor factors have important implications for prognosis, management of gliomas is largely dependent on the tumor grade.27,28 The sensitivity of conventional MRI techniques alone to diagnose glioma grade varies with these glioma subtypes,23 which poses a challenge for clinical management. Even with contemporary imaging techniques, sensitivity (80%) and specificity (78%) are suboptimal.10 Histological confirmation, therefore, remains the diagnostic method of choice to guide clinical management. In the United Kingdom, the Royal College of Physicians published clinical guidelines in 1997 stated that tumor samples should be obtained for accurate diagnosis.29 A change in clinical practice subsequent to these guidelines could have contributed to the rise in the incidence of histologically confirmed gliomas observed in Wales, which we observed in our data where we saw a doubling in the histological confirmation of gliomas from 1997 to 2005. The reduction in the incidence of grade II and III gliomas coupled with a rise in grade IV gliomas suggests increased precision in the classification of grade IV gliomas through histological classification (Figure 1). In the United States, higher proportions of gliomas had a histological diagnosis (Supplementary Table 1) and the incidence trends of overall and histologically confirmed gliomas across all grades were parallel. Molecular markers are increasingly important in the classification and treatment of gliomas. The 2016 update of the World Health Organization classification of CNS tumors undoubtedly impacted on tumor diagnosis, and more routine incorporation of molecular markers should improve diagnostic accuracy beyond just histological confirmation from tumor samples.
Our findings demonstrate that incidence trends should not be interpreted in isolation. A population-based study from England reported an increase in grade IV glioma incidence from 1995 to 2015 and suggested that there are environmental and lifestyle factors driving the rise in grade IV gliomas.5 However, data from that study also showed a decline in lower-grade gliomas, raising the question of whether the observed increase in grade IV gliomas is real or due to previous misclassification of grade IV gliomas as grade II and III gliomas. Limitations of this previous analysis were: (1) collapsing of grade III and IV tumors as one group and grade I and II as another and (2) not accounting for diagnostic practice that might reduce misclassification of grade IV tumors over time. Our findings from Welsh data are consistent with those based on previous analyses of English data but our presentation of glioma incidence rates stratified by histological confirmation add clarity to the interpretation of the observed trends. Two other studies from Finland4 and Australia3 also showed increased incidence but again there was a lack of accounting for diagnostic practice. We suggest that future reports of CNS tumor incidence trends should account for this in their analysis to avoid misinterpretation of the data whenever possible.
Strengths and Limitations
Using population-based data, we were able to assess the incidence of brain and CNS tumors in the Welsh and US populations over similar periods of time, enabling comparison between tumor types and a more detailed examination of the incidence trends. Our approach of analyzing all diagnosed tumors and histologically confirmed tumors demonstrates the potential impact clinical practice may have on routinely collected data in cancer registries. We did not have access to individual-level imaging data from the same populations as those from which the incidence data were derived to directly support our suggestion that increased use of imaging contributed to the rising incidence observed in both populations. However, although not available prior to 2012, the observed increase in brain MRI in the NHS in England is likely to be reflective of United Kingdom practice more broadly (including in Wales), given that the NHS provides the vast majority of health care UK-wide. The data from England are not stratified by age or by indication, which precludes examination of age-specific imaging rates. A study using imaging data from the United States and Canada in 2000-2016 showed a greater increase of MRI head imaging in those aged ≥65 years than those aged 18-65 years.20 It like likely that this also occurred in the United Kingdom. While treatment data are available from both databases used in this study, the Welsh database only includes the initial treatment received, leading to an underestimation of the number and proportion of patients receiving surgery. Using histological confirmation not only had the benefit of providing information on diagnostic practice and accuracy but also allowed comparable estimates of surgical intervention rates between the two populations. Lack of data on clinical variables such as tumor volume, functional status, and quality of life measures preclude investigations into the direct impact of increased diagnostic imaging on tumor management and outcomes.
Conclusions
The overall incidence of brain and other CNS tumors increased in both Wales and the United States and was attributable to tumors other than gliomas. The incidence of tumors not requiring surgical intervention increased from 2009, probably as a result of increased access to and use of neuroimaging, with increasing rates of incidental tumors. Better diagnostic accuracy from histological confirmation is likely to contribute to the rising incidence of grade IV gliomas and decline in grade II and III gliomas in Wales and should be considered when interpreting incidence rates. Efforts to integrate imaging usage, incidence, treatment, and outcome data will aid understanding of how clinical practice affects observed trends in brain and CNS tumors. The influence of changes in diagnostic and clinical practice in the last 20 years on the observed incidence of CNS tumors and their subtypes should be taken into account when considering the possible effects of lifestyle and environmental exposures. Further, as imaging and diagnostic practices vary worldwide, international comparisons of incidence trends will need to consider these factors when comparing rates between populations in order to accurately interpret associations and further our understanding of the natural history of CNS tumors.
Supplementary Material
Acknowledgment
This study makes use of anonymized data held in the Secure Anonymised Information Linkage (SAIL) Databank. We would like to acknowledge all the data providers who make anonymized data available for research.
Funding
M.T.C.P. and K.J. are funded by Cancer Research UK Brain Cancer Centre of Excellence Award (C157/A27589). Cancer Research UK did not play a role in study design, analysis, interpretation, or submission of this article. J.D.F. acknowledges personal funding from Wellcome Trust grant 207800/Z/17/Z on temporal trends in cancer incidence and mortality.
Conflict of interest statement. All authors have no conflict of interest to declare.
Authors’ contribution. M.T.C.P., P.M.B., C.L.M.S., and J.D.F. conceptualized the study. M.T.C.P., C.L.M.S., and J.D.F. designed the study. M.T.C.P. performed data preparation and statistical analyses. M.T.C.P., P.M.B., C.L.M.S., and J.D.F. interpreted the results. M.T.C.P. drafted the manuscript. P.M.B., K.J., C.L.M.S., and J.D.F. critically revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
References
- 1. GBD 2016 Brain and Other CNS Cancer Collaborators. Global, regional, and national burden of brain and other CNS cancer, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(4):376–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barnholtz-Sloan JS. Brain and central nervous system tumor statistics: access to accurate data for all countries is critical! Neuro Oncol. 2019;21(3):291–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dobes M, Khurana VG, Shadbolt B, et al. . Increasing incidence of glioblastoma multiforme and meningioma, and decreasing incidence of Schwannoma (2000–2008): findings of a multicenter Australian study. Surg Neurol Int. 2011;2:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Korja M, Raj R, Seppa K, et al. . Glioblastoma survival is improving despite increasing incidence rates: a nationwide study between 2000 and 2013 in Finland. Neuro Oncol. 2019;21(3):370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Philips A, Henshaw DL, Lamburn G, O’Carroll MJ. Brain tumours: rise in glioblastoma multiforme incidence in England 1995-2015 suggests an adverse environmental or lifestyle factor. J Environ Public Health. 2018;2018:7910754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davis FG, Smith TR, Gittleman HR, Ostrom QT, Kruchko C, Barnholtz-Sloan JS. Glioblastoma incidence rate trends in Canada and the United States compared with England, 1995–2015. Neuro Oncol. 2020;22(2):301–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miranda-Filho A, Piñeros M, Soerjomataram I, Deltour I, Bray F. Cancers of the brain and CNS: global patterns and trends in incidence. Neuro Oncol. 2017;19(2):270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deltour I, Johansen C, Auvinen A, Feychting M, Klaeboe L, Schuz J. Time trends in brain tumor incidence rates in Denmark, Finland, Norway, and Sweden, 1974–2003. JNCI J Natl Cancer Inst. 2009;101(24):1721–1724. [DOI] [PubMed] [Google Scholar]
- 9. Natukka T, Raitanen J, Haapasalo H, Auvinen A. Incidence trends of adult malignant brain tumors in Finland, 1990–2016. Acta Oncol. 2019;58(7):990–996. [DOI] [PubMed] [Google Scholar]
- 10. Wang W, Hu Y, Lu P, et al. . Evaluation of the diagnostic performance of magnetic resonance spectroscopy in brain tumors: a systematic review and meta-analysis. PLoS One. 2014;9(11):e112577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gibson LM, Paul L, Chappell FM, et al. . Potentially serious incidental findings on brain and body magnetic resonance imaging of apparently asymptomatic adults: systematic review and meta-analysis. BMJ. 2018;363:k4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lyons RA, Hutchings H, Rodgers SE, et al. . Development and use of a privacy-protecting total population record linkage system to support observational, interventional, and policy relevant research. Lancet. 2012;380:S6. [Google Scholar]
- 13. Park HS, Lloyd S, Decker RH, Wilson LD, Yu JB. Limitations and biases of the Surveillance, Epidemiology, and End Results database. Curr Probl Cancer. 2012;36(4):216–224. [DOI] [PubMed] [Google Scholar]
- 14. ISD Scotland. Cancer | Cancer Statistics | Brain and Central Nervous System Cancer | Health Topics | ISD Scotland. Cancer Statistics. Published 2019. https://www.isdscotland.org/Health-Topics/Cancer/Cancer-Statistics/Brain-and-Central-Nervous-System/. Accessed December 19, 2019.
- 15. Ostrom QT, Cioffi G, Gittleman H, et al. . CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 2019;21(Supplement_5):v1–v100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. [DOI] [PubMed] [Google Scholar]
- 17. Cancer Research UK. Brain, other CNS and intracranial tumours statistics. Cancer Research UK. Published May 14, 2015. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/brain-other-cns-and-intracranial-tumours. Accessed November 14, 2019.
- 18. Arora RS, Alston RD, Eden TOB, et al. . Are reported increases in incidence of primary CNS tumours real? An analysis of longitudinal trends in England, 1979–2003. Eur J Cancer. 2010;46(9):1607–1616. [DOI] [PubMed] [Google Scholar]
- 19. Monaghan N, Peju F, Mary W. An horizon scan of demand for computerised tomography and magnetic resonance imaging in Wales. Published online March 4, 2009. http://www2.nphs.wales.nhs.uk:8080/HealthServiceQDTDocs.nsf/Main%20Frameset?OpenFrameSet&Frame=Right&Src=%2FHealthServiceQDTDocs.nsf%2Fcategorypublicpage%3FOpenPage%26ExpandView%26RestrictToCategory%3DService%2520planning%26AutoFramed. Accessed April 25, 2020.
- 20. Smith-Bindman R, Kwan ML, Marlow EC, et al. . Trends in use of medical imaging in US health care systems and in Ontario, Canada, 2000–2016. JAMA. 2019;322(9):843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ross TR, Ng D, Brown JS, et al. . The HMO research network virtual data warehouse: a public data model to support collaboration. EGEMS (Wash DC). 2014;2(1):1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. NHS England. NHS imaging and radiodiagnostic activity. Published online August 6, 2014. https://www.england.nhs.uk/statistics/wp-content/uploads/sites/2/2013/04/KH12-release-2013-14.pdf. Accessed February 23, 2021. [Google Scholar]
- 23. Yan PF, Yan L, Zhang Z, et al. . Accuracy of conventional MRI for preoperative diagnosis of intracranial tumors: a retrospective cohort study of 762 cases. Int J Surg. 2016;36(Pt A):109–117. [DOI] [PubMed] [Google Scholar]
- 24. Bloch O, Kaur G, Jian BJ, Parsa AT, Barani IJ. Stereotactic radiosurgery for benign meningiomas. J Neurooncol. 2012;107(1):13–20. [DOI] [PubMed] [Google Scholar]
- 25. Pouchieu C, Gruber A, Berteaud E, et al. . Increasing incidence of central nervous system (CNS) tumors (2000–2012): findings from a population based registry in Gironde (France). BMC Cancer. 2018;18(1):653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin D, Lin J, Deng X, et al. . Trends in intracranial meningioma incidence in the United States, 2004-2015. Cancer Med. 2019;8(14):6458–6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brown TJ, Bota DA, van Den Bent MJ, et al. . Management of low-grade glioma: a systematic review and meta-analysis. Neurooncol Pract. 2019;6(4):249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sim HW, Morgan ER, Mason WP. Contemporary management of high-grade gliomas. CNS Oncol. 2018;7(1):51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Davies E, Hopkins A. Good practice in the management of adults with malignant cerebral glioma: clinical guidelines. Working Group, Royal College of Physicians. Br J Neurosurg. 1997;11(4):318–330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.