Abstract
Introduction:
Renal dysfunction is a recognized risk factor for mortality after allogeneic hematopoietic cell transplantation (alloHCT). Yet, understanding of the effect of different levels of renal dysfunction at time of transplant on outcomes remains limited. This study explores the impact of different degrees of renal dysfunction on HCT outcomes and examines whether utilization of incremental degrees of renal dysfunction based on estimated glomerular filtration rate (eGFR) improve the predictability of the hematopoietic cell transplantation-comorbidity index (HCT-CI).
Methods:
Study population included two cohorts: cohort 1 with patients age 40 years and older who received alloHCT for treatment of hematologic malignancies from 2008 to 2016 (N=13,505; cohort selected given very low incidence of renal dysfunction in <40 years population) and cohort 2 with all patients on dialysis at the time of HCT (N=46). Estimated GFR was measured using Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) method. Patients in cohort 1 were assigned into four categories: eGFR ≥90 mL/min (n=7062), eGFR 60-89 mL/min (n=5264), eGFR 45-59 mL/min (n=897) and eGFR <45 mL/min (n=282) to assess the impact of degree of renal dysfunction on transplant outcomes. Transplant outcomes in patients on dialysis at the time of alloHCT were analyzed separately.
Results:
eGFR <60 mL/min was shown to be associated with increased risk for non-relapse mortality (NRM) and requirement for dialysis post-HCT. Compared to eGFR ≥90 group, HR for NRM was 1.46 (p=0.0001) for eGFR 45-59 and 1.74 (p=0.004) for eGFR <45 group. Compared to eGFR ≥90, eGFR 45-59 (HR 2.45, p<0.0001) and eGFR <45 groups (HR 3.09, p<0.0001) had higher risks of renal failure requiring dialysis after alloHCT. In addition, eGFR <45 mL/min was associated with an increased overall mortality (HR 1.63, p<0.0001). An eGFR-based revised HCT-CI was also developed and was shown to be predictive of overall survival (OS) and NRM, with predictive performance similar to the original HCT-CI. Among 46 patients on dialysis at alloHCT, one-year probability of OS and NRM was 20% and 67%, respectively.
Conclusion:
The degree of pre-transplant renal dysfunction is an independent predictor of OS, NRM, and probability of needing dialysis after alloHCT. An eGFR-based HCT-CI is a validated index in predicting outcomes in adults with hematologic malignancies undergoing alloHCT. The outcomes of alloHCT recipients on dialysis are dismal. Therefore, one should strongly weigh the significant risks of being on HD as a factor in determining alloHCT candidacy.
Keywords: Renal dysfunction, HCT-CI, allogeneic transplant, dialysis
INTRODUCTION
Allogeneic hematopoietic cell transplant (alloHCT) is a potentially curative option for a variety of hematologic malignancies and non-malignant diseases. Due to the high risk of morbidity and mortality, patients with significant renal impairment are often excluded from receiving alloHCT1,2. Much of the excess transplant-related morbidity in patients with renal dysfunction relates to the toxic effects of conditioning regimens, which have a narrow therapeutic index, even when the renal function is normal2. Myeloablative conditioning regimens are associated with significant morbidity and mortality in patients with advanced renal dysfunction owing to the effect of renal function on conditioning agent pharmacokinetics2. Advances in the field of alloHCT over the past decades such as development of reduced-intensity and non-myeloablative conditioning regimens and improved supportive care practices have allowed alloHCT in patients with comorbidities which would otherwise have caused transplant-ineligibility3. Decisions regarding conditioning intensity/regimen and post-transplant graft-versus-host disease (GVHD) prophylaxis are often determined by patient’s performance status and underlying comorbidities. Pre-transplant renal dysfunction is a recognized risk factor for mortality following alloHCT and is included in the risk scoring indices used to predict posttransplant mortality4–6, and yet data regarding the prognostic role of varying degrees of renal impairment before HCT are limited.
Hematopoietic cell transplant-comorbidity index (HCT-CI) is a measure of health status at the time of alloHCT based on the comorbidities present and has been incorporated into the pre-HCT assessment to determine the risk of non-relapse mortality (NRM). Currently, pre-transplant renal dysfunction defined by serum creatinine >2 mg/dL, requirement for dialysis or history of kidney transplant is included in the HCT-CI model and is given a score of 2, as the hazard ratio (HR) for mortality was 2.1 in these patients as described in the seminal publication5. However, patients with pre-transplant serum creatinine >2 mg/dL and/or with renal failure requiring dialysis uncommonly receive alloHCT. In addition, as the normal serum creatinine value varies depending on the patient’s age, sex and race, a single serum creatinine cutoff or the same range of values is not the most reliable method for identifying suboptimal renal function in all patients. Therefore, a measure of renal function using estimated Glomerular Filtration Rate (eGFR) that takes age, sex and race into account may provide a more accurate assessment of renal function than serum creatinine in the alloHCT patient population. In addition, performing alloHCT in a patient on dialysis poses several challenges. Several anecdotal reports and single center studies suggest that alloHCT for dialysis-dependent patients may be feasible7–10. However, these series are limited by the small sample sizes.
The primary aim of this study was to evaluate the impact of severity of renal dysfunction based on eGFR on outcomes after alloHCT. In addition, we examined whether utilization of eGFR (rather than serum creatinine) as a measure of renal dysfunction improved the discriminative capacity of an eGFR-based HCT-CI (vs. the original HCT-CI). Furthermore, this study evaluated the outcomes after alloHCT in patients with renal impairment requiring dialysis (at the time of alloHCT), with the objective of assessing feasibility and utilization of transplantation in such patients.
METHODS
Data source
The CIBMTR® (Center for International Blood and Marrow Transplant Research®) is a research collaboration between the National Marrow Donor Program®/Be The Match® and the Medical College of Wisconsin11—. It comprises a voluntary working group of approximately 420 centers worldwide that contribute detailed data on allogeneic and autologous HCT and cellular therapies. Studies conducted by the CIBMTR are performed in compliance with all applicable US federal regulations pertaining to the protection of human research participants. Protected Health Information used in the performance of such research is collected and maintained in CIBMTR’s capacity as a Public Health Authority under the HIPAA Privacy Rule. The CIBMTR collects data pre-transplant, at 100 days and six months post-transplant, annually until year 6 post-transplant and biannually thereafter until death.
Study population
Patients aged 40 years and older with hematologic malignancies who underwent first alloHCT between January 2008 and December 2016 and had serum creatinine and eGFR values reported prior to HCT were included in the cohort that was analyzed to evaluate the impact of pre-HCT eGFR on post-transplant outcomes and in the development of eGFR-based HCT-CI. Patients less than 40 years of age were excluded from this cohort due to low incidence of renal dysfunction in patients <40 years. Patients receiving ex vivo T cell-depletion or CD34+ selected grafts were excluded. A second cohort of patients on dialysis at the time of alloHCT was evaluated separately and included patients of all ages and with any indication for alloHCT.
Statistical analysis
Based on pre-transplant eGFR using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) method12,13, patients were grouped into four categories: eGFR ≥90 mL/min, eGFR 60-89 mL/min, eGFR 45–59 mL/min and eGFR <45 mL/min. Patients on dialysis at the time of HCT were examined separately. Patient-, disease-and treatment-related factors were compared using the Chi-square test for categorical and the Kruskall-Wallis test for continuous variables. Kaplan-Meier curves were used to estimate the probability of overall survival (OS). OS was defined as the time from alloHCT to death from any cause or until last follow-up. Survivors were censored at last follow-up. NRM was defined as death from any cause in continuous remission and any death within 28 days of alloHCT and was summarized by cumulative incidence estimate with relapse as competing risk. Relapse was summarized by cumulative incidence estimate with NRM as competing risk. For relapse and NRM, patients in continuous complete remission were censored at last follow-up. Acute and chronic GVHD were defined by the consensus criteria14, 15. Cumulative incidence was used to estimate the probability of acute and chronic GVHD, hepatic sinusoidal obstruction syndrome (SOS), thrombotic microangiopathy (TMA), idiopathic pneumonia syndrome (IPS) and need for dialysis post-transplant.
The overall study cohort (N=13,505) was randomly split at 60/40 into a training cohort (n=8102) and a validation cohort (n=5403). In the discovery set, multivariable analysis of overall mortality, NRM, disease relapse, GVHD, SOS, TMA, IPS and need for dialysis post-transplant were performed using Cox proportional hazards model with renal dysfunction as the main testing variable. Variables included in the multivariable analysis included patients’ age, gender, race, Karnofsky Performance Score (KPS), HCT-CI, graft source, donor type, conditioning intensity, GVHD prophylaxis, and year of transplant. All variables were tested for the affirmation of the proportional hazards assumption. Variables violating the proportional hazards assumption were adjusted through stratification. Then a stepwise forward and backward model building procedure was used to select the adjusted covariates for each outcome separately with a threshold of 0.05 for both entry and stay in the model.
The main testing variables in this study include eGFR and a revised HCT-CI score. Based on pre-transplant eGFR using the Chronic Kidney Disease Epidemiology Collaboration [(CKD-EPI) method, eGFR = 141 × min(serum creatinine/k, 1)α × max(serum creatinine /K, 1)−1.209 × 0.993Age × 1.018 [if female] × 1.159 [if black], where k is 0.7 for females and 0.9 for males, alpha is −0.329 for females and −0.411 for males and serum creatinine is measured in mg/dL12, 13], patients were grouped into four categories; eGFR ≥90 mL/min (eGFR ≥90, reference), eGFR 60-89 mL/min (eGFR 60-89), eGFR 45-59 mL/min (eGFR 45-59) and eGFR <45 mL/min (eGFR <45). A revised HCT-CI score was developed based on eGFR and the score of the original HCT-CI minus the renal component (see details in the Results section). The association of eGFR and the revised HCT-CI with transplant outcomes were tested. This revised eGFR-based HCT-CI was also compared with the original HCT-CI for prediction of NRM and OS using C-statistics. Patients on dialysis at time of HCT (n=46) were not included in the above analysis.
Interactions between the main testing variables and the adjusted covariates were tested at the significance level of 0.01. No significant interactions between the main testing variable and the adjusted covariates were detected in any of the models. All p-values were two-sided. To adjust for multiple testing, the significance level of 0.01 was used for association of a main testing variable with an endpoint. The statistical software SAS version 9.4 (SAS Institute, Cary, NC) was used for all the analyses.
RESULTS
Baseline characteristics
Baseline characteristics of the main study cohort (n=13,505; excluding 46 patients on dialysis at HCT, which formed a separate second cohort) are summarized in Table 1. Patients in the main cohort were put into four groups based on pre-transplant eGFR: eGFR ≥90 mL/min (n=7062; reference group), eGFR 60-89 mL/min (n=5264), eGFR 45-59 mL/min (n=897) and eGFR <45 mL/min (n=282). Fifty-two percent of patients had eGFR ≥90. The patients with eGFR 60-89 comprised the second largest group at 39%, followed by eGFR 45-59 at 7%, and eGFR <45 at 2%. Patients with eGFR ≥90 were younger (median age, 56 years) compared to patients with eGFR 45-59 (median age, 64 years) and eGFR <45 (median age, 63 years). A higher proportion of the patients with eGFR ≥90 received myeloablative conditioning (54%) compared to patients with eGFR <90 (38%, 33% and 31% for GFR 60-89, 45-59 and <45 groups, respectively). As expected, a higher proportion of patients with eGFR <60 had HCT-CI scores ≥3 (57% and 55% for eGFR <45 and eGFR 45-60 groups, respectively) compared to the groups with eGFR ≥60 (47% and 42% for eGFR 60-89 and eGFR ≥90, respectively). A higher proportion of patients with eGFR <45 mL/min also had history of cardiovascular disease, prior malignancy, and concomitant diabetes. Calcineurin-inhibitor based GVHD prophylaxis was used more frequently in GFR ≥60 groups (90% and 89% in GFR ≥90 and 60-89 groups, respectively) than in GFR <60 groups (81% and 84% in GFR 45–89 and <45 groups, respectively). Posttransplant cyclophosphamide-based prophylaxis was used more commonly in eGFR <45 group (11 % vs. 7–8% in GFR ≥45 groups). There were no major differences in gender, race, KPS, refined disease risk index (R-DRI), graft source, donor type, among the groups. Median follow up of survivors was at least 4 years since HCT (50, 49, 48 and 60 months for eGFR >90, 60–89, 45–59 and <45 groups, respectively).
Table 1.
Characteristics of adults age 40 or older with hematologic malignancies who underwent first allogeneic HCT between 2008 and 2016
| Variable | Level of renal function N (%) |
|||
|---|---|---|---|---|
| (eGFR <45) | (eGFR 45-59) | (eGFR 60-89) | (eGFR ≥90) | |
| Number of patients | 282 | 897 | 5264 | 7062 |
| Number of centers | 88 | 127 | 165 | 172 |
| Patient-related | ||||
| Recipient age, median (range) | 63 (40-82) | 64 (40-78) | 62 (40-83) | 56 (40-78) |
| Male | 146 (51.8) | 509 (56.7) | 3269 (62.1) | 4092 (57.9) |
| Karnofsky performance score | ||||
| 90-100 | 136 (48.2) | 478 (53.3) | 3063 (58.2) | 4055 (57.4) |
| < 90 | 139 (49.3) | 396 (44.1) | 2079 (39.5) | 2874 (40.7) |
| Missing | 7 (2.5) | 23 (2.6) | 122 (2.3) | 133 (1.9) |
| Race | ||||
| White | 253 (89.7) | 811 (90.4) | 4794 (91.1) | 6007 (85.1) |
| Black or African American | 19 (6.7) | 44 (4.9) | 246 (4.7) | 535 (7.6) |
| Asian | 8 (2.8) | 28 (3.1) | 174 (3.3) | 429 (6.1) |
| Others | 2 (0.8) | 14 (1.6) | 50 (0.9) | 91 (1.3) |
| Ethnicity | ||||
| Hispanic or Latino | 12 (4.3) | 26 (2.9) | 198 (3.8) | 492 (7) |
| Not Hispanic or Latino | 256 (90.8) | 829 (92.4) | 4834 (91.8) | 6176 (87.5) |
| Missing | 14 (5.0) | 42 (4.6) | 232 (4.4) | 394 (5.6) |
| HCT-CI | ||||
| 0 | 55 (19.5) | 163 (18.2) | 1227 (23.3) | 1886 (26.7) |
| 1 | 25 (8.9) | 109 (12.2) | 686 (13) | 1015 (14.4) |
| 2 | 19 (6.7) | 104 (11.6) | 729 (13.8) | 949 (13.4) |
| ≥3 | 160 (56.9) | 494 (55.0) | 2466 (47.0) | 2995 (42.4) |
| Missing | 23 (8.2) | 27 (3) | 156 (3) | 217 (3.1) |
| Serum creatinine | ||||
| ≤2 mg/dL | 237 (84) | 897 | 5264 | 7062 |
| >2 mg/dL | 45 (16) | 0 | 0 | 0 |
| Cardiac comorbidity | ||||
| No | 217 (77) | 744 (83) | 4391 (83) | 6073 (86) |
| Yes | 49 (17) | 125 (14) | 718 (14) | 765 (11) |
| Missing | 16 (6) | 28 (3) | 155 (3) | 224 (3) |
| Cerebrovascular disease | ||||
| No | 258 (91) | 839 (94) | 4958 (94) | 6717 (95) |
| Yes | 8 (3) | 27 (3) | 148 (3) | 122 (2) |
| Missing | 16 (6) | 31 (3) | 158 (3) | 223 (3) |
| Diabetes | ||||
| No | 215 (76) | 734 (82) | 4492 (85) | 6074 (86) |
| Yes | 51 (18) | 135 (15) | 618 (12) | 768 (11) |
| Missing | 16 (6) | 28 (3) | 154 (3) | 220 (3) |
| History of prior malignancy (solid tumor) | ||||
| No | 192 (68) | 634 (71) | 3955 (75) | 5653 (80) |
| Yes | 79 (28) | 247 (28) | 1186 (23) | 1221 (17) |
| Missing | 11 (4) | 16 (2) | 123 (2) | 188 (3) |
| Disease-related | ||||
| Disease | ||||
| AML | 98 (34.8) | 284 (31.7) | 1849 (35.1) | 3058 (43.3) |
| ALL | 5 (1.8) | 38 (4.2) | 269 (5.1) | 649 (9.2) |
| MDS | 76 (27) | 319 (35.6) | 1718 (32.6) | 1593 (22.6) |
| MPN/CML | 31 (11) | 108 (12) | 485 (9.2) | 579 (8.2) |
| NHL/HL | 44 (15.6) | 85 (9.5) | 518 (9.8) | 708 (10) |
| Others | 28 (10.0) | 63 (7.0) | 428 (8.1) | 475 (6.8) |
| Refined disease risk index | ||||
| Low | 21 (7.4) | 57 (6.4) | 478 (9.1) | 705 (10) |
| Intermediate | 150 (53.2) | 467 (52.1) | 2738 (52) | 3828 (54.2) |
| High | 84 (29.8) | 318 (35.5) | 1796 (34.1) | 2148 (30.4) |
| Very high | 17 (6) | 32 (3.6) | 144 (2.7) | 251 (3.6) |
| Missing | 10 (3.5) | 23 (2.6) | 108 (2.1) | 130 (1.8) |
| Previous autologous transplant | ||||
| No | 242 (85.8) | 803 (89.5) | 4938 (93.8) | 6623 (93.8) |
| Yes | 36 (12.8) | 78 (8.7) | 294 (5.6) | 394 (5.6) |
| Missing | 4 (1.4) | 16 (1.8) | 32 (0.6) | 45 (0.6) |
| Transplant-related | ||||
| Conditioning regimen intensity | ||||
| Myeloablative | 87 (30.9) | 295 (32.9) | 2024 (38.4) | 3796 (53.8) |
| RIC | 130 (46.1) | 422 (47) | 2183 (41.5) | 2232 (31.6) |
| NMA | 65 (23) | 179 (20) | 1041 (19.8) | 1015 (14.4) |
| Missing | 0 | 1 (0.1) | 16 (0.3) | 19 (0.3) |
| Graft source | ||||
| Bone marrow | 26 (9.2) | 90 (10) | 569 (10.8) | 813 (11.5) |
| Peripheral blood | 229 (81.2) | 713 (79.5) | 4166 (79.1) | 5381 (76.2) |
| Umbilical cord blood | 27 (9.6) | 94 (10.5) | 529 (10) | 867 (12.3) |
| Donor type | ||||
| HLA-identical sibling | 66 (23.4) | 239 (26.6) | 1285 (24.4) | 2010 (28.5) |
| Other related (≥7/8 match) | 17 (6) | 75 (8.4) | 402 (7.6) | 569 (8.1) |
| Other related (≤6/8 match) | 10 (3.5) | 13 (1.4) | 92 (1.7) | 114 (1.6) |
| Well-matched unrelated (8/8) | 127 (45) | 387 (43.1) | 2426 (46.1) | 2706 (38.3) |
| Partially-matched unrelated (7/8) | 22 (7.8) | 72 (8) | 430 (8.2) | 612 (8.7) |
| Mis-matched unrelated (≤6/8) | 2 (0.7) | 3 (0.3) | 16 (0.3) | 35 (0.5) |
| Cord blood | 27 (9.6) | 94 (10.5) | 529 (10) | 867 (12.3) |
| Missing | 11 (3.9) | 14 (1.6) | 84 (1.6) | 149 (2.1) |
| GVHD prophylaxis | ||||
| Post-CY +/− other(s) | 30 (10.6) | 70 (7.8) | 381 (7.3) | 461 (6.5) |
| Calcineurin inhibitor-based | 236 (83.7) | 797 (80.7) | 4704 (89.3) | 6377 (90.3) |
| Other(s)2 | 7 (2.5) | 19 (2.1) | 88 (1.7) | 112 (1.6) |
| Missing | 9 (3.2) | 11 (1.2) | 91 (1.7) | 112 (1.6) |
| ATG/alemtuzumab | ||||
| ATG and/or alemtuzumab | 78 (27.7) | 234 (26.1) | 1507(28.6) | 1886 (26.7) |
| No ATG or alemtuzumab | 204 (72.3) | 662 (73.8) | 3753 (71.3) | 5170 (73.2) |
| Missing | 0 | 1 (0.1) | 4 (0.1) | 6 (0.1) |
| Year of transplant | ||||
| 2008-2011 | 110 (39) | 292 (32.6) | 1952 (37.1) | 2873 (40.7) |
| 2012-2016 | 172 (61) | 605 (67.4) | 3312 (62.9) | 4189 (59.3) |
| Median follow-up of survivors (range), months | 60 (3-124) | 48 (3-126) | 49 (3-125) | 50 (3-126) |
Other conditioning regimens: Flu + TBI: n=787, TBI ± other: n=288, Flu + Cy: n=307, TLI ± other: n=141, Bu ± other: n=139, Flu ± other: n=82, BEAM: n=79, Mel ± other: n=75, Cy ± other: n=21, BuMel: n=10, Cytarabine ± other: n=3, Pentostatin alone: n=2, Emiddleoside alone: n=2
Other GVHD prophylaxis: MMF + sirolimus ± other: n=83; sirolimus ± other: n=21; other: n=122
Hypothesis testing: a Kruskal-Wallis test b Pearson chi-square test
Effect of pre-transplant renal dysfunction on transplant outcomes
Multivariable analysis to assess the impact of renal dysfunction (as measured by eGFR) on NRM and OS after adjusting for patient-and transplant-related variables based on the training cohort is shown in Figure 1 and Tables 2–3. Decreased eGFR was independently associated with an increase in NRM as illustrated by an increase in hazards of mortality with lower eGFR groups (eGFR <60): compared to eGFR ≥90 group, HR for NRM was 1.12 (95% CI 0.99-1.26, p=0.07) for eGFR 60–89 group, 1.46 (95% CI 1.21-1.76, p=0.0001) for eGFR 45–59 group, and 1.74 (95% CI 1.19-2.54, p=0.004) for eGFR <45 group. Similarly, eGFR <45 mL/min was associated with significantly decreased OS. Compared to the eGFR ≥90 group, the HR for mortality was 1.0 (95% CI 0.95-1.06, p=0.98) for eGFR 60-89 group, 1.17 (95% CI 1.02-1.34, p=0.03) for eGFR 45-59, and 1.63 (95% CI 1.33-2.01, p<0.0001) for eGFR <45 group. Renal function at alloHCT was not significant risk factor for post-transplant relapse (Supplemental Table 1).
Figure 1:
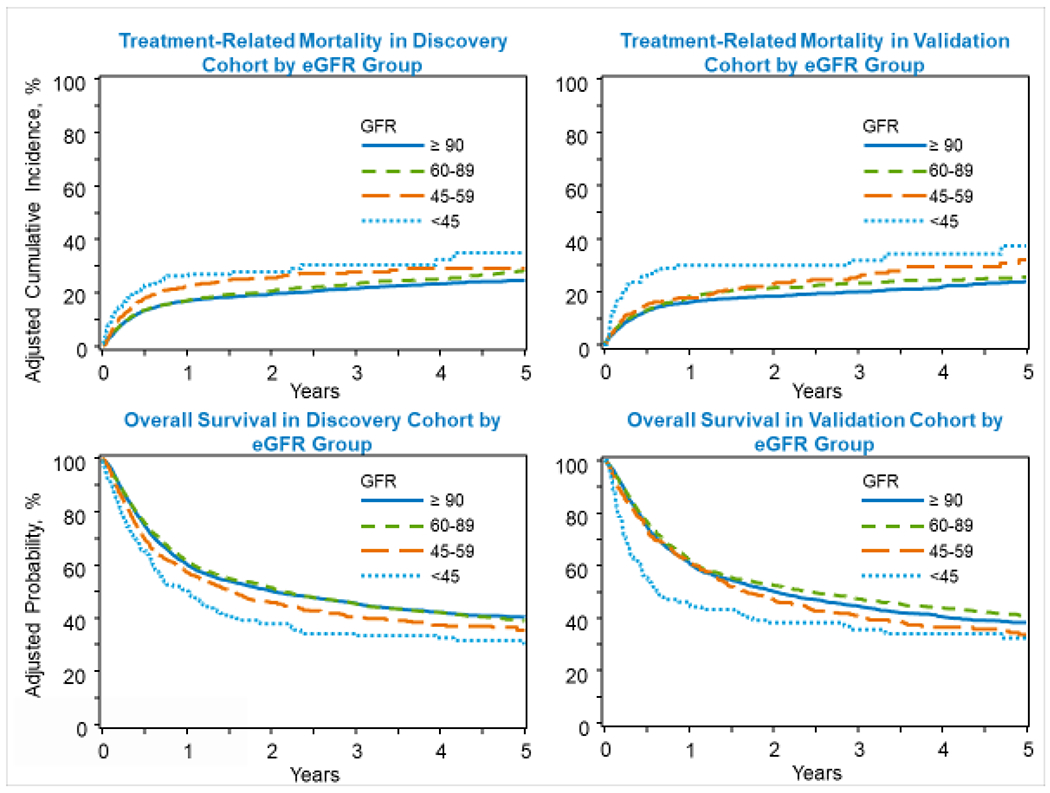
Impact of degree of renal dysfunction measured by estimated glomerular filtration rate on non-relapse mortality and overall survival after allogeneic transplant in adults age 40 and older with hematologic malignancy (GFR, estimated glomerular filtration rate).
Table 2.
Multivariate analysis of impact of renal dysfunction measured by eGFR on overall survival in discovery cohort.
| Factor | Count | Event | HR | 95% CI | P-value |
|---|---|---|---|---|---|
| Renal function groups | <.0001 | ||||
| GFR ≥90 | 4234 | 2390 | 1 | ||
| GFR 60-89 | 3157 | 1858 | 1 | (0.95, 1.06) | 0.98 |
| GFR 45-59 | 537 | 336 | 1.17 | (1.02, 1.34) | 0.03 |
| GFR <45 | 169 | 124 | 1.63 | (1.33, 2.01) | <.0001 |
| HCT-CI (excluding renal function) | <.0001 | ||||
| 0 | 1995 | 1061 | 1 | ||
| 1 | 1086 | 594 | 1.08 | (0.97, 1.21) | 0.16 |
| 2 | 1118 | 601 | 1.11 | (0.97, 1.28) | 0.13 |
| 3 | 1445 | 842 | 1.18 | (1.07, 1.29) | 0.0006 |
| 4 | 908 | 564 | 1.26 | (1.12, 1.42) | 0.0001 |
| 5 | 557 | 358 | 1.5 | (1.33, 1.7) | <.0001 |
| 6 | 371 | 247 | 1.5 | (1.24, 1.82) | <.0001 |
| 7 | 200 | 148 | 1.81 | (1.46, 2.24) | <.0001 |
| 8 | 98 | 85 | 3 | (2.32, 3.88) | <.0001 |
| 9+ | 77 | 57 | 1.8 | (1.31, 2.49) | 0.0003 |
| Patient age | <.0001 | ||||
| 40-49 | 1637 | 871 | 1 | ||
| 50-59 | 2782 | 1589 | 1.18 | (1.07, 1.31) | 0.001 |
| 60-69 | 3084 | 1876 | 1.33 | (1.18, 1.49) | <.0001 |
| ≥70 | 594 | 372 | 1.48 | (1.27, 1.73) | <.0001 |
| Donor age (HLA-identical sibling or other relative; years) | 0.0083 | ||||
| <18 | 18 | 6 | 1 | ||
| 18-29 | 2294 | 1242 | 1.86 | (0.83, 4.17) | 0.13 |
| 30-39 | 1265 | 731 | 2.05 | (0.91, 4.6) | 0.08 |
| 40-49 | 1221 | 708 | 2.16 | (0.96, 4.87) | 0.06 |
| ≥50 | 2090 | 1215 | 2.29 | (0.99, 5.27) | 0.05 |
| Donor type | <.0001 | ||||
| HLA-identical sibling | 2176 | 1206 | 1 | ||
| Haploidentical | 638 | 343 | 1.2 | (1.01, 1.44) | 0.04 |
| Other related | 147 | 94 | 1.33 | (1, 1.78) | 0.05 |
| Well-matched unrelated | 3355 | 1884 | 1.19 | (1.05, 1.34) | 0.007 |
| Partially-matched unrelated | 671 | 441 | 1.44 | (1.24, 1.69) | <.0001 |
| Mis-matched unrelated | 33 | 26 | 1.85 | (1.28, 2.67) | 0.001 |
| Unrelated (matching unknown) | 95 | 48 | 1.48 | (1.06, 2.06) | 0.02 |
| Cord blood | 919 | 625 | NA | ||
| DRI-R groupings | <.0001 | ||||
| Low | 763 | 363 | 1 | ||
| Intermediate | 4350 | 2300 | 1.19 | (1.04, 1.38) | 0.01 |
| High | 2560 | 1744 | 1.9 | (1.62, 2.23) | <.0001 |
| Very high | 252 | 186 | 2.69 | (2.08, 3.47) | <.0001 |
| Karnofsky performance score | <.0001 | ||||
| 90-100 | 4671 | 2552 | 1 | ||
| < 90 | 3254 | 2053 | 1.26 | (1.16, 1.37) | <.0001 |
| Missing | 172 | 103 | 1.12 | (0.87, 1.44) | 0.37 |
| Previous autologous transplant | 0.06 | ||||
| No | 7567 | 4364 | 1 | ||
| Yes | 465 | 295 | 1.14 | (0.98, 1.32) | 0.09 |
| Missing | 65 | 49 | 1.45 | (0.98, 2.14) | 0.06 |
| Year of transplant | <.0001 | ||||
| 2008-2011 | 3141 | 2111 | 1 | ||
| 2012-2016 | 4956 | 2597 | 0.81 | (0.75, 0.87) | <.0001 |
Note: stratified variables: graft type, conditioning regimen, conditioning regimen intensity, disease group, ATG/Campath, GVHD prophylaxis. NA, not applicable.
Table 3.
Multivariate analysis of impact of renal dysfunction measured by eGFR on non-relapse mortality in discovery cohort.
| Factor | Count | Event | HR | 95% CI | P-value |
|---|---|---|---|---|---|
| Renal function groups | <.0001 | ||||
| GFR ≥90 | 3188 | 693 | 1 | ||
| GFR 60-89 | 2532 | 617 | 1.12 | (0.99, 1.26) | 0.07 |
| GFR 45-59 | 436 | 126 | 1.46 | (1.21, 1.76) | 0.0001 |
| GFR < 45 | 138 | 49 | 1.74 | (1.19, 2.54) | 0.004 |
| HCT-CI (excluding renal function) | <.0001 | ||||
| 0 | 1560 | 330 | 1 | ||
| 1 | 825 | 179 | 1.21 | (1.01, 1.45) | 0.04 |
| 2 | 848 | 185 | 1.13 | (0.89, 1.45) | 0.31 |
| 3 | 1126 | 258 | 1.18 | (0.96, 1.45) | 0.12 |
| 4 | 686 | 176 | 1.41 | (1.1, 1.82) | 0.008 |
| 5 | 426 | 98 | 1.38 | (1.07, 1.79) | 0.01 |
| 6 | 305 | 88 | 1.87 | (1.36, 2.56) | 0.0001 |
| 7 | 164 | 53 | 2.02 | (1.4, 2.92) | 0.0002 |
| 8 | 86 | 38 | 4.66 | (3.22, 6.76) | <.0001 |
| 9+ | 59 | 18 | 2.32 | (1.39, 3.88) | 0.001 |
| Patient age | 0.01 | ||||
| 40-49 | 1204 | 255 | 1 | ||
| 50-59 | 2137 | 525 | 1.27 | (1.06, 1.52) | 0.008 |
| 60-69 | 2475 | 581 | 1.43 | (1.15, 1.77) | 0.001 |
| ≥70 | 478 | 124 | 1.67 | (1.2, 2.32) | 0.002 |
| Conditioning intensity | <.0001 | ||||
| Myeloablative | 2683 | 714 | 1 | ||
| RIC | 2427 | 538 | 0.64 | (0.51, 0.8) | 0.0001 |
| NMA | 1167 | 230 | 0.51 | (0.38, 0.69) | <.0001 |
| Donor age (HLA-identical sibling or other relative; years) | 0.003 | ||||
| <18 | 14 | 2 | 1 | ||
| 18-29 | 1833 | 422 | 1.07 | (0.15, 7.83) | 0.95 |
| 30-39 | 983 | 239 | 1.11 | (0.15, 8.2) | 0.92 |
| 40-49 | 928 | 208 | 1.3 | (0.18, 9.55) | 0.80 |
| ≥50 | 1665 | 339 | 1.57 | (0.21, 11.88) | 0.66 |
| Donor type | <.0001 | ||||
| HLA-identical sibling | 1718 | 301 | 1 | ||
| Haploidentical | 473 | 99 | 1.8 | (1.37, 2.38) | <.0001 |
| Other related | 126 | 26 | 2.11 | (1.25, 3.54) | 0.005 |
| Well-matched unrelated | 2648 | 651 | 2.01 | (1.66, 2.43) | <.0001 |
| Partially-matched unrelated | 502 | 152 | 2.56 | (2.02, 3.25) | <.0001 |
| Mis-matched unrelated | 29 | 10 | 2.14 | (1.17, 3.92) | 0.01 |
| Unrelated (matching unknown) | 71 | 14 | 2.25 | (1.15, 4.4) | 0.02 |
| Cord blood | 664 | 214 | 3.3 | (0.44, 24.92) | 0.25 |
| Patient ethnicity | 0.003 | ||||
| Hispanic or Latino | 364 | 86 | 1 | ||
| Not Hispanic or Latino | 5651 | 1330 | 0.94 | (0.71, 1.23) | 0.64 |
| Non-resident of the U.S. | 216 | 57 | 1.96 | (1.19, 3.24) | 0.009 |
| Karnofsky performance score | 0.0001 | ||||
| 90-100 | 3586 | 791 | 1 | ||
| < 90 | 2570 | 653 | 1.36 | (1.19, 1.55) | <.0001 |
| Year of transplant | <.0001 | ||||
| 2008-2011 | 2429 | 687 | 1 | ||
| 2012-2016 | 3865 | 798 | 0.7 | (0.6, 0.81) | <.0001 |
Note: stratified variables: DRI-R groupings, disease group, conditioning regimen, GVHD prophylaxis.
Pre-transplant renal dysfunction and risk of GVHD and regimen-related toxicities
In multivariable analysis, the degree of renal dysfunction as measured by eGFR was not a significant risk factor for the development of grade II-IV acute or chronic GVHD (Supplemental Table 2–3). Compared to eGFR ≥90 group, eGFR 45-59 (HR 2.45, 95% CI 1.74-3.46, p<0.0001) and eGFR <45 groups (HR 3.09, 95% CI 2.0-4.78, p<0.0001) had higher risks of renal failure requiring dialysis after alloHCT (overall p<0.0001; Supplemental Table 4). Patients with HCT-CI score (excluding the renal component) of ≥5 also had an increased risk of dialysis-dependent renal failure after alloHCT than those with HCT-CI score 0. Renal function prior to alloHCT was not found to be a significant predictor for IPS, TMA and SOS (Supplemental Tables 5–7). Patients with eGFR <45 mL/min had an HR of 2.6 for development of SOS (vs. GFR ≥90, p=0.03; overall p=0.02), with only 7 events in the group (n=167).
Development of an eGFR-based HCT-CI score
An eGFR-based HCT-CI score was developed based on eGFR using the training cohort (Table 4). Based on the Cox model for NRM, integer scores of 0-9 were first assigned to the 0-9 levels of HCT-CI minus R (with renal component excluded). Then, by treating HCT-CI minus R as a continuous variable, we compared the regression coefficients of eGFR with the slope (as 1-unit increment) of the HCT-CI minus R and assigned scores of 0, 1,3 and 5 to eGFR ≥90, eGFR 60-89, eGFR 45-59 and eGFR <45, respectively. For example, since the slope of HCT-CI minus R was 0.1048 and the regression coefficient of eGFR 60–89 was 0.1127, a score of 1 was assigned to eGFR 60-89. For eGFR 45-59 with the regression coefficient of 0.3769, a score of 3 was assigned (approximately 3 times that of the 0.1048 slope of HCT-CI minus R) to eGFR 45-59. The eGFR-based HCT-CI score was defined as the sum of the assigned eGFR score and the score for the HCT-CI minus R. This newly defined eGFR-based HCT-CI was then applied to the validation cohort to test its ability to predict NRM and OS. The eGFR-based HCT-CI was also compared with the original HCT-CI for prediction of NRM and OS using C-statistics.
Table 4.
Estimates used to build eGFR-based HCT-CI in discovery cohort
| Factor | Count | Event | β | HR: exp(β) |
95% CI | P-value | Score |
|---|---|---|---|---|---|---|---|
| Renal function groups | 0.0001 | ||||||
| eGFR ≥90 | 3188 | 693 | 0.0000 | 1.0 | 0 | ||
| eGFR 60-89 | 2532 | 617 | 0.1127 | 1.15 | (0.99, 1.26) | 0.07 | 1 |
| eGFR 45-59 | 436 | 126 | 0.3769 | 1.43 | (1.21, 1.76) | 0.0001 | 3 |
| eGFR <45 | 138 | 49 | 0.5544 | 1.70 | (1.19, 2.54) | 0.004 | 5 |
| HCT-CI minus renal function (continuous) | 6085 | 1423 | 0.1048 | 1.11 | (1.08, 1.14) | <0.0001 | 1 unit |
NOTE:
HCT-CI groups in the above models is calculated without accounting for the renal function question currently asked for the original HCT-CI. No significant interaction between eGFR and HCT-CI was found for non-relapse mortality (NRM). Patients missing HCT-CI were excluded.
By treating HCT CI (minus renal function) as a continuous variable, it has a slope of 0.105. If we treat 0.105 as one unit increment, then 0.1127, 0.3769 and 0.5544 correspond to roughly 1-, 3- or 5-units increment, respectively.
In the training data set, eGFR-based HCT-CI was shown to be significantly predictive for both OS (p<0.0001) and NRM (p<0.0001) (Supplemental Tables 8–9). In multivariable analysis of training data set, while patients with lower eGFR-based HCT-CI (1-2 vs. 0 for OS, 1-3 vs. 0 for NRM) did not show significant differences in OS or NRM, patients with eGFR HCT-CI ≥3 was associated with significantly decreased OS probability than those with HCT-CI 0 and eGFR-based HCT-CI ≥4 was associated with significantly increased NRM risk than those with HCT-CI 0. Similarly, in the validation set, eGFR-based HCT-CI was shown to be predictive for OS (p=0.001) and NRM (p<0.0001). The eGFR-based HCT-CI scores of 1-5 (vs. score of 0) did not result in statistically significant increased HRs for OS and NRM. However, eGFR-based HCT-CI score of ≥6 was independently associated with an increase in NRM and decreased OS as illustrated by increase in HR for death with higher scores (Supplemental Tables 10–11). Pairwise comparisons for eGFR-based HCT-CI scores in multivariable models of OS and NRM using the validation data set showed that a revised HCT-CI score of ≥2 (vs. 1) and ≥4 (vs. 1) increased the OS probability and NRM risk, respectively (Supplemental Table 12–13).
Comparison of the eGFR-based HCT-CI and the original HCT-CI for prediction of outcomes Statistical comparison between the two HCT-CI risk scores are summarized in Table 5. In the training set excluding patients with normal renal function, the eGFR-based HCT-CI index had similar c-statistic estimates for NRM (0.676) and OS (0.624) compared to the original HCI-CI (0.673 and 0.623, respectively). Similarly, in the validation set excluding the patients with normal renal function, the eGFR-based HCT-CI index had similar c-statistic estimates for NRM (0.686) and OS (0.638) than the original HCT-CI for TRM (0.681 and 0.634, respectively). Comparison of outcomes stratified by the eGFR-based HCT-CI and original HCT-CI are shown in Figure 2.
Table 5.
Comparisons between original HCT-CI and eGFR-based HCT-CI for prediction of non-relapse mortality (NRM) and overall survival (OS) in the training and validation set
| Outcomes | Original HCT-CI | eGFR-based HCT-CI |
|---|---|---|
| Non-relapse mortality | ||
| c-statistics training set (entire cohort) N=8102 | 0.6774 | 0.6801 |
| c-statistics validation set (entire cohort) N= 5403 | 0.6791 | 0.6823 |
| c-statistics training set (subset with renal dysfunction*) N=4237 | 0.6735 | 0.6761 |
| c-statistics validation set (subset with renal dysfunction*) N=2825 | 0.6805 | 0.6855 |
| Overall survival | ||
| c-statistics training set (entire cohort) N=8102 | 0.6251 | 0.6252 |
| c-statistics validation set (entire cohort) N= 5403 | 0.6277 | 0.6289 |
| c-statistics training set (subset with renal dysfunction*) N=4237 | 0.6225 | 0.6242 |
| c-statistics validation set (subset with renal dysfunction*) N=2825 | 0.6339 | 0.6379 |
Renal dysfunction is defined as estimated glomerular filtration rate (eGFR) ≤ 89.
Note: There was no statistically significant differences between original HCT-CI and eGFR-based eGFR.
Figure 2:
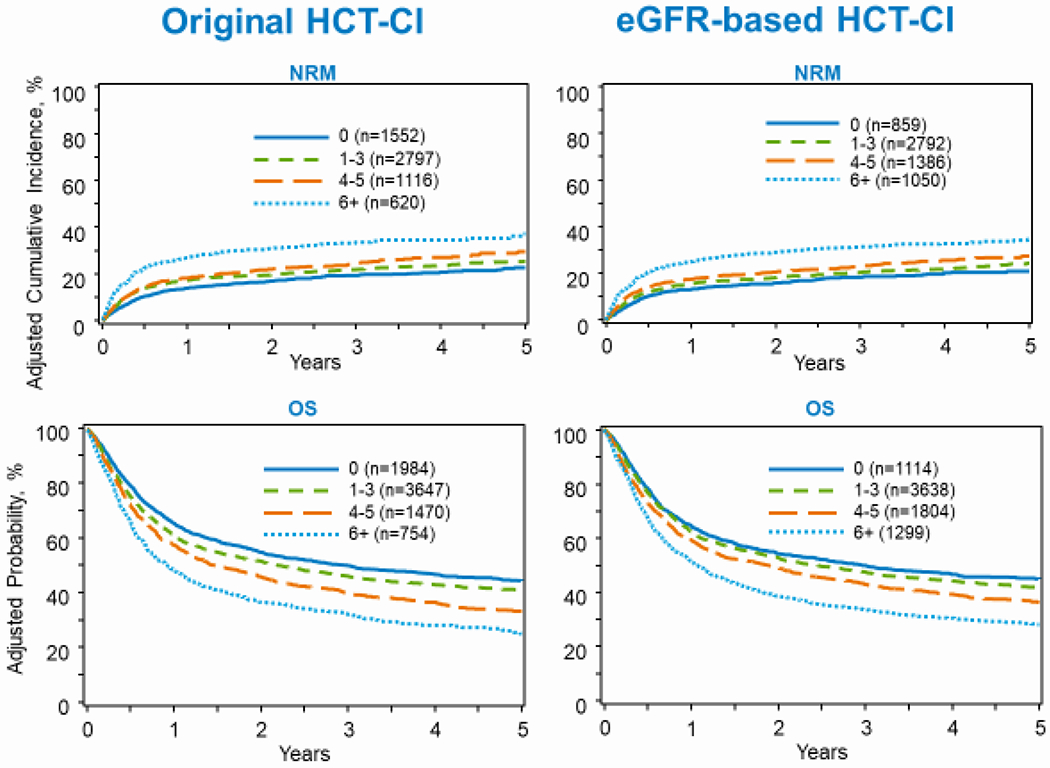
Comparison between Original HCT-CI and eGFR-based HCT-CI (HCT-CI, hematopoietic cell transplantation-comorbidity index; eGFR, estimated glomerular filtration rate; NRM, non-relapse mortality; OS, overall survival).
Outcomes of patients on dialysis at the time of transplant
Baseline characteristics of 46 patients on dialysis at the time of alloHCT are shown in Table 6. A majority of patients was younger than 30 years (52%), male (78%) and had KPS <90 (61%). The most common transplant indication was acute leukemia (46%) followed by non-malignant hematologic disorders (22%). Twenty-eight percent patients received myeloablative conditioning. Peripheral blood was the most common stem cell graft source (65%). The most frequently used GVHD prophylaxis was calcineurin inhibitor-based (59%). Median follow up of survivors was 36 months. Based on the univariate analysis (Table 7), the probability of OS (n=46) at 1 year was 20% (95% CI, 10-32%) and 17% (95% CI, 8-29%) at 3 years. In patients with malignancy as transplant indication (n=36), the cumulative incidence of NRM at 1 year was 67% (95% CI, 50-81%). The probability of disease-free survival at 1 and 3 years was 11% (95% CI, 3-23%) and 7% (95% CI, 1-19%), respectively. The most common causes of death in this cohort were organ failure (33%), followed by primary disease (30%) and infection (25%).
Table 6.
Characteristics of patients on dialysis at time of allogeneic HCT from 2008-2016
| Variable | N (%) |
|---|---|
| Number of patients | 46 |
| Number of centers | 35 |
| Patient related | |
| Recipient age, median (range) | 27 (<1-70) |
| Male | 36 (78) |
| Karnofsky performance score | |
| 90-100 | 13 (28) |
| <90 | 28 (61) |
| Missing | 5 (11) |
| Race | |
| White | 28 (61) |
| Black or African American | 12 (26) |
| Asian | 3 (7) |
| Native American | 1 (2) |
| Missing | 2 (4) |
| HCT-CI | |
| 2 | 3 (7) |
| 3 | 8 (17) |
| 4 | 5 (11) |
| 5+ | 21 (46) |
| Missing | 9 (20) |
| Disease related | |
| Disease | |
| AML | 13 (28) |
| ALL | 8 (17) |
| MDS/MPN | 9 (20) |
| NHL/HL | 3 (7) |
| MM/PCD | 2 (4) |
| CML | 1 (2) |
| Non-malignant hematologic disease2 | 10 (22) |
| Transplant related | |
| Number of alloHCT | |
| 1st allo | 30 (65) |
| 2nd or greater allo | 16 (35) |
| Conditioning regimen intensity | |
| Myeloablative | 13 (28) |
| RIC | 13 (28) |
| NMA | 12 (26) |
| No conditioning | 7 (15) |
| Missing | 1 (2) |
| Graft source | |
| Bone marrow | 7 (15) |
| Peripheral blood | 30 (65) |
| Umbilical cord blood | 9 (20) |
| Donor type | |
| HLA-identical sibling | 5 (11) |
| Twin | 2 (4) |
| Other related (≥7/8 match) | 15 (33) |
| Other related (≤6/8 match) | 1 (2) |
| Well-matched unrelated (8/8) | 6 (13) |
| Partially-matched unrelated (7/8) | 4 (9) |
| Unrelated (matching indeterminable) | 3 (7) |
| Cord blood | 9 (20) |
| Missing | 1 (2) |
| GVHD prophylaxis | |
| ex-vivo T-cell depletion | 6 (13) |
| CD34 selection | 2 (4) |
| Post-CY + other(s) | 4 (9) |
| TAC +− others | 17 (37) |
| CSA +− others | 10 (22) |
| Other(s)3 | 2 (4) |
| Missing | 5 (11) |
| ATG/alemtuzumab | |
| ATG alone | 15 (33) |
| Alemtuzumab alone | 2 (4) |
| No ATG or Alemtuzumab | 24 (52) |
| Missing | 5 (11) |
| Year of transplant | |
| 2008-2011 | 15 (33) |
| 2012-2016 | 31 (67) |
| Median follow-up of survivors (range), months | 36 (13-120) |
Missing pre-TED: These cases did not have pre-TED triggered as they had a second allogeneic transplant where the pre-TED was not required a second time.
Non-malignant indications: Severe aplastic anemia: n=4; Schwachmann-Diamond: n=1, Diamond-Blackfan anemia: n=1, Omenn syndrome: n=1; Wiskott Aldrich syndrome: n=1, Leukocyte adhesion deficiency: n=1; SCID: n=1
Other GVHD prophylaxis: MMF + sirolimus: n=1; MMF +− other: n=1
Abbreviation: HCT, hematopoietic cell transplantation; CI, comorbidity index; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; CML, chronic myeloid leukemia; NHL, non-Hodgkin lymphoma; HL, Hodgkin lymphoma, RIC, reduced-intensity conditioning; NMA, nonmyeloablative regimen; MM, multiple myeloma; PCD, plasma cell disorder; Cy, cyclophosphamide; TBI, total body irradiation; Bu, busulfan; Flu, fludarabine; Mel, melphalan; CB, cord blood; TAC, tacrolimus; MMF, mycophenolate mofetil; MTX, methotrexate; CSA, cyclosporine; ATG, anti-thymocyte globulin
Table 7.
Univariate analysis: Transplant outcomes for patients on hemodialysis (HD) at time of allogeneic HCT (N=46)
| Outcomes | N Eval | Prob (95% CI) |
|---|---|---|
| Overall survival | 46 | |
| 1-year | 20 (10-32)% | |
| 3-year | 17 (8-29)% | |
| Relapse (malignant disease only) | 36 | |
| 1-year | 22 (10-38)% | |
| 3-year | 26 (12-43)% | |
| NRM (malignant disease only) | 36 | |
| 1-year | 67 (50-81)% | |
| 3-year | 67 (50-81)% | |
| Disease-free survival (malignant disease only) | 36 | |
| 1-year | 11 (3-23)% | |
| 3-year | 7 (1-19)% | |
| Acute GVHD grade II-IV | 46 | |
| 1-year | 20 (10-33)% | |
| Acute GVHD grade III-IV | 46 | |
| 1-year | 14 (5-25)% | |
| Chronic GVHD | 46 | |
| 1-year | 12 (4-25)% | |
| TMA | 46 | |
| 1-year | 4 (0-12)% | |
| VOD | 44 | |
| 1-year | 7 (1-16)% | |
| IPS | 46 | |
| 1-year | 4 (0-12)% |
DISCUSSION
This large observational study evaluated the association between the severity of pre-transplant renal dysfunction as measured by eGFR and outcomes after alloHCT for hematologic malignancies. Approximately nine percent patients in the main study cohort had pre-transplant renal dysfunction as defined by eGFR <60 mL/min, including 0.33% with pre-transplant serum creatinine >2 mg/dL. The study results demonstrated that decreased eGFR before transplant is a determinant of post-transplant outcomes. More specifically, eGFR <60 mL/min was independently associated with increased risks of NRM and overall mortality after alloHCT and in addition, progressive increase in HR for death was observed with decreased eGFR levels. The degree of renal dysfunction did not significantly affect the rates of relapse, acute and chronic GVHD, IPS, TMA and SOS. However, an increased risk of ESRD requiring dialysis was observed in the groups with eGFR <60 mL/min before alloHCT. With the small number of events in the analysis for SOS risk, eGFR <45 group had a 2.6-fold risk (compared to eGFR ≥90) but did not meet statistical significance. It is plausible that an analysis with a larger sample size (of patients with eGFR <45) with adequate number of events may have the power to detect significant differences in SOS risk with eGFR groups.
The study results add to the data supporting the role of alloHCT in patients with hematologic malignancies and baseline renal dysfunction. Prior studies have evaluated the impact of pretransplant renal impairment on alloHCT outcomes and have been mainly limited by smaller sample sizes16–19. In a small study (n=13), Kersting et al. reported that patients with eGFR <60 mL/min could be safely offered non-myeloablative transplant (with fludarabine and total body irradiation as conditioning)17. In a single center study of 141 patients with acute myeloid leukemia and myelodysplastic syndrome undergoing alloHCT with fludarabine and melphalan (Flu/Mel)-based reduced intensity conditioning (RIC), mild-to-moderate renal dysfunction (eGFR <60 mL/min) was not associated with worse OS and NRM16. On account of a homogenous study population and use of Flu/Mel, which is not markedly nephrotoxic, these results may not apply to other conditioning regimens. A study by Shouval et al. evaluating the ability of physiological biomarkers to predict transplant-related risks in 1217 alloHCT recipients showed that eGFR <60 mL/min was predictive of NRM, consistent with our results19.
Since the degree of renal dysfunction independently predicted NRM and overall mortality risks, we sought to modify the HCT-CI as a risk assessment tool by using different levels of renal impairment (as reflected by different eGFR cutoffs) to examine the utility of eGFR-based HCT-CI in alloHCT recipients. The analysis demonstrated that eGFR-based HCT-CI was a strong predictor of NRM and OS among recipients of alloHCT, even though eGFR-based HCT-CI did not improve upon the predicative ability of the original HCT-CI. In an effort to improve the predictive performance of the HCT-CI, several other studies have proposed adding other pretransplant physiological biomarkers including albumin and eGFR to the original HCT-CI. Although both eGFR and albumin level were shown in these studies to be independently predictive of NRM and OS20, 21, similar to our study, addition of these biomarkers individually to the HCT-CI have failed to show significant improvement in HCT-CI performance.
Renal injury remains a frequent post-transplant complication with progression to end-stage renal disease (ESRD) observed in up to 4% of patients with chronic kidney disease22–24. In the current study, pre-transplant renal dysfunction (eGFR <60 mL/min) was found to be an independent risk factor for ESRD requiring dialysis after alloHCT. Prior reports have shown a dramatic decline in eGFR, typically during the first year of HCT25. The ability of the pre-transplant eGFR to predict the risk of ESRD requiring dialysis after alloHCT is an important finding, and it underscore the importance of identifying patients at risk for ESRD and developing a preventative strategy in patients with eGFR <45 mL/min.
The study results also provide a benchmark for outcomes in patients on dialysis at the time of HCT as shown in the heterogeneous and highly selected second cohort, with a one-year survival of 20%. More than half the patients in this cohort were less than 30 years (median age, 27 years). In a subset of 36 patients with hematologic malignancies receiving alloHCT, 3-year probability of disease-free survival of 7% (indicating relapse or NRM in the remaining 93%) highlights the remarkably dismal prognosis associated with ESRD requiring dialysis before alloHCT. Our results are in line with the previous single center case series of 6 alloHCT recipients on either hemodialysis or peritoneal dialysis at the time of transplant which revealed an exceedingly high mortality rate (one-year mortality of 83%)9. Mortality in this cohort was largely related to regimen-related toxicity and infections, rather than primary disease.
Given its retrospective nature, there are limitations to this study, including lack of detailed granular information like the dosing of conditioning regimens and GVHD prophylaxis and if they were adjusted for pre-transplant serum creatinine or eGFR. The data on etiology, timing (with regards to HCT) and reversibility of renal dysfunction were not captured in the registry data set. While it is impressive that 32.7% patients with eGFR <60 mL/min received myeloablative conditioning, only 6.2% of myeloablative regimen recipients had pre-transplant eGFR <60 mL/min (including 1.4% for eGFR <45 mL/min). In contrast, the proportion of patients receiving RIC plus non-myeloablative conditioning and with eGFR <60 mL/min was 10.9% (including 2.7% for eGFR <45 mL/min). These low proportions are likely reflective of the reluctance of the transplant physicians in offering allogeneic HCT to patients with renal dysfunction. Given that baseline data are not captured in the registry for patients not receiving alloHCT, the proportion of patients with renal impairment not proceeding to alloHCT and of those deemed transplant-ineligible owing to renal dysfunction could not be estimated. In the small heterogeneous cohort of patients on dialysis with several disease indications for transplant and lack of information on chronology and indication for dialysis, we could not determine the risk factors associated with significantly worse outcomes. The large data set from large number of centers, however, adds to the generalizability of the study results.
Posttransplant complications such as GVHD are more challenging to prevent and manage in alloHCT patients with renal impairment: pharmacokinetics and pharmacodynamics of various drugs including GVHD prophylaxis agents may vary significantly in the setting of renal dysfunction1. The renal vasoconstricting and profibrotic effects of calcineurin inhibitors (cyclosporine and tacrolimus) are less well-tolerated in the setting of chronic kidney disease and may result in worsening renal function2. Approximately 89% of this study cohort (79% of patients with eGFR <60) received calcineurin inhibitor-based GVHD prophylaxis. While the results do not show an interaction between eGFR and GVHD prophylaxis for transplant outcomes, the impact of renal dysfunction on transplant outcomes in the setting of calcineurin inhibitor-free GVHD prophylaxis would need further evaluation26, 27. Post-transplant cyclophosphamide (ptCy)-based calcineurin inhibitor-free GVHD prophylaxis has been evaluated in prospective clinical trials of HLA-identical donor and mismatched donor transplants28–31. However, it remains to be seen if it has a renal-sparing effect in patients with eGFR <60 mL/min. Furthermore, T-cell depletion with CD34+ selected donor graft (ex vivo) or anti-thymocyte globulin or alemtuzumab (in vivo) also represent GVHD prevention approaches uninfluenced by renal function1, 2 Efficacy and safety of ptCy (calcineurin inhibitor-free prophylaxis, with bone marrow graft) and ex vivo CD34+ selected graft from HLA-identical donors are being investigated in a large prospective randomized controlled trial29. Further studies are needed to refine the dosing of conditioning regimens and optimize calcineurin inhibitor-free GVHD prophylaxis in order improve the outcomes of alloHCT recipients with baseline renal insufficiency.
In conclusion, the study results establish the role of pre-transplant renal dysfunction, as reflected by eGFR of less than 60 mL/min, in predicting the risk of NRM and overall mortality in alloHCT recipients aged 40 years and older with hematologic malignancies. These data afford benchmarking for future studies in alloHCT recipients with baseline renal dysfunction and provide a measure of transplant outcomes in these patients against which non-transplant options could potentially be weighed. While the results demonstrate significantly increased NRM and overall mortality in patients with eGFR <60 mL/min, the results should inform clinical practice in considering and undertaking alloHCT for these patients, if the alternative treatment is expected to produce worse survival outcomes. In addition, this analysis demonstrated the validity of eGFR-based HCT-CI in predicting outcomes in alloHCT patients. The outcomes in patients on dialysis at the time of transplant are, however, dismal: in patients with hematologic malignancies, alternative approaches should be considered in lieu of alloHCT given the probability of long-term disease-free survival being <10%. With rare exceptions (such as combined marrow-kidney transplant32, 33 and possibly non-malignant disease indications in young patients), alloHCT should be considered a contraindication in ESRD patients requiring dialysis and it should ideally be conducted in the setting of a clinical trial with the objective of improving safety of HCT in this group of patients.
Supplementary Material
Acknowledgment:
Allistair Abraham, Ibrahim Ahmed, Vaibhav Agrawal, Mahmoud Aljurf, Jeffrey J Auletta, Nelli Bejanyan, Christopher Dandoy, Marcos de Lima, Miguel Angel Diaz, Natalie Callander, Jan Cerny, Gregory Guilcher, William Hogan, Benjamin Laskin, Parinda Mehta, Kasiani Myers, Sunita Nathan, Roomi Nusrat, Tracy O’Brien, Seth Rotz, Mitchell Sabloff, Insara Jaffer Sathick, Amir Steinberg, Sachiko Seo, John L. Wagner, Basem M. William
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bodge MN, Reddy S, Thompson MS, Savani BN. Preparative Regimen Dosing for Hematopoietic Stem Cell Transplantation in Patients with Chronic Kidney Disease: Analysis of the Literature and Recommendations. Biology of Blood and Marrow Transplantation 2014; 20(7): 908–919. doi: 10.1016/j.bbmt.2014.02.013 [DOI] [PubMed] [Google Scholar]
- 2.Heher EC, Spitzer TR. Hematopoietic Stem Cell Transplantation in Patients With Chronic Kidney Disease. Semin Nephrol 2010; 30(6): 602–614. doi: 10.1016/j.semnephrol.2010.09.008 [DOI] [PubMed] [Google Scholar]
- 3.D’Souza A, Lee S, Zhu X, Pasquini M. Current Use and Trends in Hematopoietic Cell Transplantation in the United States. Biol Blood Marrow Transplant 2017; 23(9): 1417–1421. e-pub ahead of print 2017/06/14; doi: 10.1016/j.bbmt.2017.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parimon T, Au DH, Martin PJ, Chien JW. A risk score for mortality after allogeneic hematopoietic cell transplantation. Ann Intern Med 2006; 144(6): 407–414. e-pub ahead of print 2006/03/22; doi: 10.7326/0003-4819-144-6-200603210-00007 [DOI] [PubMed] [Google Scholar]
- 5.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessmentent before allogeneic HCT. Blood 2005; 106(8): 2912–2919. e-pub ahead of print 2005/07/05; doi: 10.1182/blood-2005-05-2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luft T, Benner A, Terzer T, Jodele S, Dandoy CE, Storb R et al. EASIX and mortality after allogeneic stem cell transplantation. Bone Marrow Transplant 2020; 55(3): 553–561. e-pub ahead of print 2019/09/29; doi: 10.1038/s41409-019-0703-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi HS, Kim SY, Lee JH, Yoon SY, Cho YH, Lee HG. Successful allogeneic stem-cell transplantation in a patient with myelodysplastic syndrome with hemodialysis-dependent end-stage renal disease. Transplantation 2011; 92(6): e28–29. e-pub ahead of print 2011/09/13; doi: 10.1097/TP.0b013e31822a79f1 [DOI] [PubMed] [Google Scholar]
- 8.Hamaki T, Katori H, Kami M, Yamato T, Yamakado H, Itoh T et al. Successful allogeneic blood stem cell transplantation for aplastic anemia in a patient with renal insufficiency requiring dialysis. Bone Marrow Transplant 2002; 30: 195. [DOI] [PubMed] [Google Scholar]
- 9.Shadman M, Hingorani S, Lanum SA, Pagel JM, Storb R, Maloney DG et al. Allogeneic hematopoietic cell transplant for patients with end stage renal disease requiring dialysis - a single institution experience. Leuk Lymphoma 2017; 58(3): 740–742. e-pub ahead of print 07/25; doi: 10.1080/10428194.2016.1211280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Besien K, Schouten V, Parsad S, Smith S, Odenike O, Artz AS. Allogeneic stem cell transplant in renal failure: engraftment and prolonged survival, but high incidence of neurologic toxicity. Leuk Lymphoma 2012; 53(1): 158–159. e-pub ahead of print 2011/07/14; doi: 10.3109/10428194.2011.604756 [DOI] [PubMed] [Google Scholar]
- 11.Report. CAP. https://www.cibmtr.org/About/AdminReports/Documents/2019CIBMTRAnnualReport.pdf. Accessed June 9, 2020. 2019.
- 12.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150(9): 604–612. e-pub ahead of print 2009/05/06; doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158(11): 825–830. e-pub ahead of print 2013/06/05; doi: 10.7326/0003-4819-158-11-201306040-00007 [DOI] [PubMed] [Google Scholar]
- 14.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995; 15(6): 825–828. e-pub ahead of print 1995/06/01; [PubMed] [Google Scholar]
- 15.Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med 1980; 69(2): 204–217. e-pub ahead of print 1980/08/01; doi: 10.1016/0002-9343(80)90380-0 [DOI] [PubMed] [Google Scholar]
- 16.de Souza JA, Saliba RM, Patah P, Rondon G, Ribeiro R, de Padua Silva L et al. Moderate renal function impairment does not affect outcomes of reduced-intensity conditioning with fludarabine and melphalan for allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2009; 15(9): 1094–1099. e-pub ahead of print 2009/08/08; doi: 10.1016/j.bbmt.2009.05.006 [DOI] [PubMed] [Google Scholar]
- 17.Kersting S, Verdonck LF. Successful outcome after nonmyeloablative allogeneic hematopoietic stem cell transplantation in patients with renal dysfunction. Biol Blood Marrow Transplant 2008; 14(11): 1312–1316. e-pub ahead of print 2008/10/23; doi: 10.1016/j.bbmt.2008.08.015 [DOI] [PubMed] [Google Scholar]
- 18.Oshima K, Kanda Y, Nanya Y, Tanaka M, Nakaseko C, Yano S et al. Allogeneic hematopoietic stem cell transplantation for patients with mildly reduced renal function as defined based on creatinine clearance before transplantation. Ann Hematol 2013; 92(2): 255–260. e-pub ahead of print 2012/10/12; doi: 10.1007/s00277-012-1584-1 [DOI] [PubMed] [Google Scholar]
- 19.Shouval R, de Jong CN, Fein J, Broers AEC, Danylesko I, Shimoni A et al. Baseline Renal Function and Albumin are Powerful Predictors for Allogeneic Transplantation-Related Mortality. Biol Blood Marrow Transplant 2018; 24(8): 1685–1691. e-pub ahead of print 2018/05/13; doi: 10.1016/j.bbmt.2018.05.005 [DOI] [PubMed] [Google Scholar]
- 20.Artz AS, Logan B, Zhu X, Akpek G, Bufarull RM, Gupta V et al. The prognostic value of serum C-reactive protein, ferritin, and albumin prior to allogeneic transplantation for acute myeloid leukemia and myelodysplastic syndromes. Haematologica 2016; 101(11): 1426–1433. e-pub ahead of print 2016/11/02; doi: 10.3324/haematol.2016.145847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaughn JE, Storer BE, Armand P, Raimondi R, Gibson C, Rambaldi A et al. Design and Validation of an Augmented Hematopoietic Cell Transplantation-Comorbidity Index Comprising Pretransplant Ferritin, Albumin, and Platelet Count for Prediction of Outcomes after Allogeneic Transplantation. Biol Blood Marrow Transplant 2015; 21(8): 1418–1424. e-pub ahead of print 2015/04/12; doi: 10.1016/j.bbmt.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ando M, Ohashi K, Akiyama H, Sakamaki H, Morito T, Tsuchiya K et al. Chronic kidney disease in long-term survivors of myeloablative allogeneic haematopoietic cell transplantation: prevalence and risk factors. Nephrol Dial Transplant 2010; 25(1): 278–282. e-pub ahead of print 2009/09/19; doi: 10.1093/ndt/gfp485 [DOI] [PubMed] [Google Scholar]
- 23.Hingorani S Renal Complications of Hematopoietic-Cell Transplantation. New England Journal of Medicine 2016; 374(23): 2256–2267. doi: 10.1056/NEJMra1404711 [DOI] [PubMed] [Google Scholar]
- 24.Kersting S, Koomans HA, Hené RJ, Verdonck LF. Acute renal failure after allogeneic myeloablative stem cell transplantation: retrospective analysis of incidence, risk factors and survival. Bone Marrow Transplant 2007; 39(6): 359–365. e-pub ahead of print 2007/03/08; doi: 10.1038/sj.bmt.1705599 [DOI] [PubMed] [Google Scholar]
- 25.Hingorani S, Pao E, Stevenson P, Schoch G, Laskin BL, Gooley T et al. Changes in Glomerular Filtration Rate and Impact on Long-Term Survival among Adults after Hematopoietic Cell Transplantation: A Prospective Cohort Study. Clin J Am Soc Nephrol 2018; 13(6): 866–873. e-pub ahead of print 2018/04/20; doi: 10.2215/cjn.10630917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Homsi AS, Cole K, Muilenburg M, Goodyke A, Abidi M, Duffner U et al. Calcineurin and mTOR Inhibitor-Free Post-Transplantation Cyclophosphamide and Bortezomib Combination for Graft-versus-Host Disease Prevention after Peripheral Blood Allogeneic Hematopoietic Stem Cell Transplantation: A Phase I/II Study. Biol Blood Marrow Transplant 2017; 23(10): 1651–1657. e-pub ahead of print 2017/05/28; doi: 10.1016/j.bbmt.2017.05.024 [DOI] [PubMed] [Google Scholar]
- 27.Solomon SR, Sanacore M, Zhang X, Brown S, Holland K, Morris LE et al. Calcineurin inhibitor-free graft-versus-host disease prophylaxis with post-transplantation cyclophosphamide and brief-course sirolimus following reduced-intensity peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 2014; 20(11): 1828–1834. e-pub ahead of print 2014/07/30; doi: 10.1016/j.bbmt.2014.07.020 [DOI] [PubMed] [Google Scholar]
- 28.HLA-Mismatched Unrelated Donor Bone Marrow Transplantation With Post-Transplantation Cyclophosphamide. https://clinicaltrials.gov/ct2/show/NCT02793544; ClinicalTrials.gov #NCT02793544. (Accessed 12/15/2020.).
- 29.CTN/NHLBI/NCI. B. Calcineurin Inhibitor-Free Interventions for Prevention of Graft-versus-Host Disease (BMT CTN 1301). . https://clinicaltrials.gov/ct2/show/NCT02345850.; ClinicalTrials.gov #NCT02345850. Accessed 12/15/2020.;
- 30.Kasamon YL, Ambinder RF, Fuchs EJ, Zahurak M, Rosner GL, Bolanos-Meade J et al. Prospective study of nonmyeloablative, HLA-mismatched unrelated BMT with high-dose posttransplantation cyclophosphamide. Blood Adv 2017; 1(4): 288–292. doi: 10.1182/bloodadvances.2016002766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw BE, Burns LJ, Logan B, Jimenez-Jimenez AM, Khimani F, Shaffer BC et al. Transplantation Using Bone Marrow from a (very) HLA Mismatched Unrelated Donor in the Setting of Post-Transplant Cyclophosphamide Is Feasible and Expands Access to Underserved Minorities. Biology of Blood and Marrow Transplantation 2020; 26(3): S283–S284. doi: 10.1016/j.bbmt.2019.12.553 [DOI] [Google Scholar]
- 32.Lowsky R, Strober S. Combined kidney and hematopoeitic cell transplantation to induce mixed chimerism and tolerance. Bone Marrow Transplant 2019; 54(Suppl 2): 793–797. e-pub ahead of print 2019/08/23; doi: 10.1038/s41409-019-0603-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strober S Use of hematopoietic cell transplants to achieve tolerance in patients with solid organ transplants. Blood 2016; 127(12): 1539–1543. doi: 10.1182/blood-2015-12-685107 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


