Abstract
Purpose of review:
AKI is a complex clinical syndrome with many etiologies and there is a broad range of clinical presentations, that vary according to duration, severity and context. Established consensus definitions of AKI are non-specific and limited to kidney function. This reduces treatment options to generic approaches rather than individualised, etiology-based strategies that have limited both understanding and management of AKI.
Recent findings:
The context and the temporal phase of kidney injury are critical features in the course of AKI and critical to timing relevant intervention. These features are missing in generic definitions and terms used to describe AKI. Subphenotypes of AKI can be identified from novel damage biomarkers, from functional changes including creatinine trajectories, from the duration of change and from associated clinical characteristics and comorbidities. Subphenotype parameters can be combined in risk scores, or by association strategies ranging from a simple function-damage matrix to complex methods such as machine learning. Examples of such strategies are reviewed along with tentative proposals for a revised nomenclature to facilitate description of AKI subphenotypes.
Summary:
Appropriate intervention requires refinement of the nomenclature of AKI to identify subphenotypes that facilitate correctly timed and selectively targeted intervention.
Keywords: AKI Subphenotypes, Damage Biomarkers, Subclinical AKI and CKD, Risk Prediction
Introduction
Consensus definitions of AKI, beginning with RIFLE, refined by AKIN and eventually united in the KDIGO definition have unified the previously numerous and diverse classifications of AKI. These function-based definitions reflect a path of increasing severity stage to kidney “failure” when treatment must be instituted to preserve life. The definitions have facilitated comparisons amongst studies, facilitated epidemiological analysis, highlighted the linkage between AKI severity and outcome, raised awareness of the high prevalence of AKI and allowed greater insight into the short and long term adverse outcomes of AKI including the development of CKD 1, 2. A measure of the success of these function-based definitions, is the significant growth of interest in AKI by industry as well as nephrology and intensive care specialists. However, despite this success, it could be argued that a unified functional definition implies that a syndrome with as many diverse aetiologies as AKI might have a single remedy. Unfortunately this approach is not only unhelpful but potentially dangerous. For instance, the classification of AKI into “prerenal, renal and post-renal” states tacitly encourages an automatic trial of intravenous fluids in conditions that maybe considered “pre-renal” with very different management requirements ranging from dehydration, to cardiac failure with or without preserved ejection fraction and hepatorenal syndrome. Similarly, using the same functional definition to trial treatments experimentally-based on prevention or early intervention after timed ischaemia or toxic injury have been spectacularly unsuccessful, since the functional changes are delayed in relation to the cellular damage that itself leads to the later functional decline.
These observations highlight the need for systematic approaches to improve personalized management of patients. Recent studies in ARDS and sepsis 3, 4 have demonstrated that patients can be categorized into subphenotypes (endophenotypes) based on clinical and biological features and observable patient characteristics to optimize management. Subphenotyping is useful for identifying subgroups of patients that have similar modifiable biological mechanisms, differences in treatment responses or a higher risk of mortality to permit targeted interventions4, 5. In this article, we review the current status and future prospects of subphenotyping in AKI.
Need for Subphenotyping in AKI
AKI is heterogeneous in its etiology, settings, severity, course and outcomes that are influenced by inherent disease mechanisms, the patient’s response and the process of care. As a result there is considerable inter-individual variation in the risk of AKI, possibility of outcomes and the therapeutic response to administered agents. Clinicians rely largely on clinical manifestations mainly urine output and serum creatinine trends and associated complications to manage these patients without clear knowledge of how the underlying comorbidities and genetic predisposition influence the underlying pathophysiology of injury and repair in individual patients. The current major causes of AKI are illustrated in Table 1. In addition, the associated commentary highlights how different the mechanisms of injury leading to AKI are in these conditions. Our current diagnosis and staging criteria do not differentiate the varied causes, timing of injury and the underlying stage of kidney health to determine the course and potential response to therapy. Correctly targeted treatment requires identification of the aetiology of a particular episode of AKI, regardless of whether the treatment is prevention or intervention. Otherwise the same problems besetting the functional definition of AKI, such as inappropriate volume replacement, will hinder or delay specific and appropriate therapy. Personalized management under these circumstances is challenging and often unsuccessful.
Table 1:
Heterogenity of AKI
| Aetiology of AKI | Comment on mechanisms of injury |
|---|---|
|
Perfusion-Dependent AKI (BM+ or BM−) Decreased Intravascular Volume Decreased Cardiac Output (LVF) Decreased Cardiac Output (RVF) Mesenteric Vasoconstriction |
Hypoxia may be the only unifying link between under perfusion of the kidneys associated with these disparate aetiologies. Right ventricular failure contributes to increased venous congestion and a reduced gradient for glomerular filtration pressure. There currently no specific urinary or plasma biomarkers of renal hypoxia. |
| Sepsis | Both globally increased renal perfusion and regional renal under perfusion occur in sepsis. The primary mechanism of kidney cellular damage may be inflammation associated with PAMPS and DAMPS but the mechanisms remain uncertain |
| Immune/Vasculitis | Inflammation and Hypoxia appear to be the likely mechanisms |
| Nephrotoxins | Direct and indirect cellular toxicity precipitate injury but inflammation may be the overwhelming mechanism leading to loss of GFR |
| Outflow Obstruction | Mechanical obstruction can raise intratubular pressure and reduce GFR, but with time, inflammation and apoptosis are both precipitated during ureteric obstruction. |
Key: BM+ damage biomarker positive; BM- damage biomarker negative; LVF left ventricular failure; RVF right ventricular failure; PAMPS pathogen-associated molecular patterns; DAMPS danger-associated molecular patterns; GFR glomerular filtration rate
Nomenclature is critical in conveying medical understanding to patients and medical staff. Perhaps for historical reasons dating to when AKI was described as acute renal ‘failure’, the present functional definition does not convey a sense of urgency in the investigation and management of AKI. Indeed, creatinine-based definitions of AKI have an inherent delay, due partly to the slow rate of increase in creatinine after a sudden loss of GFR. However, there is also the need to confirm an apparent creatinine rise, because of possible laboratory error and a wide normal range. This delays intervention and continues the tradition of ‘watchful waiting’ instead of timely intervention.
In contrast, specifying the cause of AKI within the AKI definition could immediately define an appropriate and immediate intervention. For example, ‘Hypovolaemic-AKI’ would encourage volume replacement, whereas Cardiac Failure-AKI would encourage direct management of the cardiac failure and reduce overemphasis on the increase in creatinine itself. Similarly, since we now know that there is no successful prevention possible for ‘Contrast-associated AKI’6, it is important to monitor kidney function in patients who require intraarterial contrast rather than delay potentially life-saving investigations or interventions. In contrast, the steps to diagnosis and management of obstructive AKI, rapidly progressive glomerulonephritis-associated AKI or vasculitis-associated AKI are inherent in the descriptors used. These descriptors define subphenotypes of AKI and simultaneously highlight correct and urgent management.
To address these deficiencies it is important to consider that not all AKI is created equal. Underlying susceptibilities and exposures vary across the world 7 and present in different ways. AKI secondary to snakebite in India and myocardial infarction and shock in Canada, both may result in oliguric AKI requiring dialysis however their course and outcomes will likely be different. The underlying health care environment contributes to the recognition, diagnosis and management of AKI as the process of care e.g. availability of lab tests, imaging, fluids, drugs and dialysis support is markedly different across different countries and within a country8. Additionally, the response to AKI and its treatment is highly variable and affects outcomes. Several studies have shown that fluid accumulation in AKI patients is associated with a higher risk of mortality, reduced kidney recovery and can be improved by fluid removal 9. Over recent years we have made tremendous progress in understanding the underlying mechanisms of AKI with specific biomarkers of kidney injury, repair and function and longitudinal studies of patient course and outcomes in different settings. We are thus well positioned to apply this knowledge to create subphenotypes to improve our approach to this disorder.
Approaches for Subphenotyping in AKI
Several approaches have been utilized for sub-phenotyping patients. Clinical characteristics are a first step that can differentiate patients. It is well recognized that oliguric patients have a different course and worse effects than non-oliguric patients, and the duration and number of episodes of oliguria influence the outcomes. Similarly, age, underlying CKD, heart failure, diabetes, liver disease and cancer condition the response to injury and recovery. Clinical risk scores have been developed to identify these high risk patients with good success particularly in pediatric patients 10, 11. Analysis of trajectories of serum creatinine values has also been proposed to characterize patients predicting recovery of kidney function12. The duration of AKI, number of episodes and severity are all recognized as important factors associated with outcomes and could be utilized to determine therapeutic strategies. Similarly, the furosemide stress test (FST) assessing the urinary output following a single dose of furosemide has been shown to predict stage progression in AKI and has been used a method to stratify patients for intervention studies 13
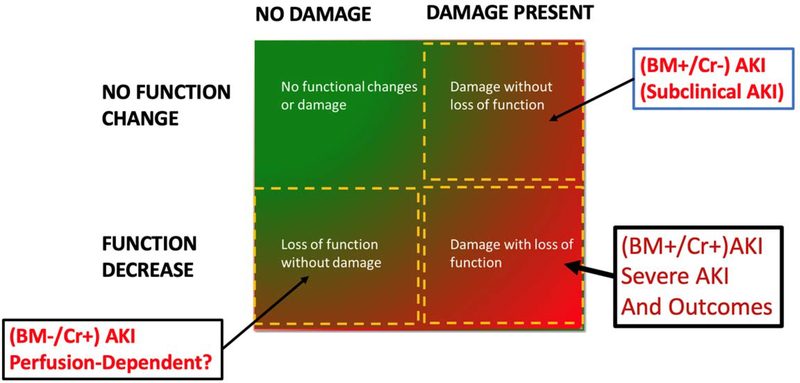
The discovery and validation of kidney damage biomarkers has allowed us to step back and recast the syndrome of AKI into a different light14. For example, a matrix definition of AKI incorporating functional and damage biomarkers (Fig 1) allows AKI to be subdivided into subclinical AKI where damage biomarkers are increased but functional change has not yet occurred and hypoperfusion AKI where creatinine has increased but damage biomarkers are absent14, 15. Interestingly, these subtypes have the same risk of dialysis or death 16. While these studies have delineated a path forward to characterize the kidneys response to injury they have not been as yet evaluated extensively with systemic disease biomarkers 17. Experience form ARDS and sepsis studies suggest that subphenotyping requires a comprehensive approach that correlates the clinical features with systemic biomarkers of the underlying mechanism and specific biomarkers of the organ response. 4, 18, 19.
Fig. 1.
Combining functional and damage biomarkers reveals two categories of AKI with similar prognosis for dialysis or death, namely BM +/Cr − (subclinical AKI) and BM−/Cr+ - (perfusion -dependent AKI). Modified from15. However, subclinical and perfusion-dependent AKI also fail to identify cause of AKI and must be further subphenotyped to allow effective management. 15
These comprehensive approaches are now possible with access to longitudinal patient data in electronic health records, coupled with advanced statistical techniques such as latent class and cluster analysis 18. Bhatraju et al 20 applied these tools to evaluate over the course and outcomes of 1800 ICU patients, enrolled in a prospective study, of whom 794 had AKI. Using latent class analysis to the clinical and biomarker data from plasma samples in the first 48 hrs after ICU admission they identified two independent populations with 29 clinical and biological variables that were distinguished by the levels of endothelial biomarkers angiopoietin1, angiopoietin II, vascular adhesion molecule and inflammatory markers TNF receptor 1, IL-6 and IL-8. The subphenotype 1 characterized by low ratio of ANG2/Ang 1 and sTNFR-1 signifying a quiescent vascular bed and low inflammatory state had lower mortality than those with Subphenotype 2 with high ANG2/Ang1 ratios and higher sTNFR-1 representing a hyperinflammtory state and increased vascular permeability. Their findings were further replicated in data from 800 patients enrolled in the VASST trial and additionally showed that patients with Subphenotype 2 had higher rates of mortality and dialysis and had a better response to nor-epinephrine and vasopressin combination with a tend to improved recovery at 7 days. More recently, the same group identified genetic variations that support the role of Angiopoietin 2 as a causal mechanism in the AKI subphenotype 2 21. A post hoc analysis of the Finnish Acute Kidney Injury (FiNNAKI) study of patients with sepsis associated AKI similarly identified two subphenotypes of AKI with Subphenotype 2 having elevated levels of inflammatory and endothelial markers that was associated with a lower short term recovery and a higher 90 day mortality22.
Access to large datasets in electronic health records has contributed to recent publications describing machine learning approaches for identifying phenotypes of patients at risk for AKI. In an assessment of the MIMIC 3 dataset Xu et al 23 identified 3 phenotypes of patients who had a high risk of AKI and correlated with the subsequent development and severity stage of AKI. Koola et al24 similarly developed a clinical risk prediction model for detecting hepatorenal syndrome at admission in cirrhotic patients and demonstrated that 10 predictor variables had an AUC of 0.87 in identifying development of HRS in over 33000 admissions. Tomasev et al25 applied deep learning techniques in machine learning to a large US Veterans Affairs (VA) longitudinal dataset of more than 700,000 patients across diverse clinical settings—more than 170 inpatient and 1000 outpatient sites to develop a predictive model for AKI. With a lead time of up to 48 hours, their model predicted 55.8% of all inpatient episodes of AKI and 90.2% of all dialysis-requiring AKI episodes within 90 days of the initial onset. However, the algorithm generated two false positives for every true alert. More recently Churpek et al26 described a predictive model for AKI development with internal and external validation from 3 hospital systems and almost 500 000 patients. The results showed an area under the curve as high as 0.92 for AKI and as high as 0.97 for dialysis. However given the low prevalence of these events (3.4% Stage 2 AKI) the positive predictive value is very low.
What’s the future?
These data suggest that we may be able to define patient groups with different courses for personalized management. However, we need to consider several aspects. Firstly the current KDIGO definitions based on serum creatinine and urine output alone may not be sufficient to categorize patients. A recent study showed that various approaches for the KDGIO criteria resulted in significant variations in the incidence of AKI from 28–75%27. We therefore need consistent application of the criteria. Secondly, we need to combine clinical characteristics with the kidney biomarker profiles for the site and extent of damage and functional changes other than GFR to detect subphenotypes 28. Third, we need to include information on the health of other organ systems and the underlying disease states with systemic biomarkers. This is important to establish both predictive 10, 11, 17, 29 and prognostic subphenotyping to refine the heterogeneity in AKI 20,22. Since subclinical AKI and subclinical CKD modify the course of clinically overt AKI30, we need to consider both intitial kidney ‘status’ as well as the underlying pathophysiology contributing to AKI in the assessments. A potential grouping is show in Table 2. Fourthly, we should utilize clinical tests to probe the kidney to refine the course and determine interventions13. Finally, as we learn more from big data analysis and machine learning algorithms, it should be feasible to establish profiles of patients for risk prediction, defining optimum time points for targeted interventions and prognosis 31, 32.
Table 2:
Potential grouping for AKI categories
| AKI Precipitants = Types of AKI | Suggested Abbreviations |
|---|---|
| Reduced Perfusion | AKI-Low Perfusion (?Perfusion-Dependent-AKI) |
| - Hypovolaemia | Cardiac Surgery-AKI (CS-AKI, CTS-AKI) |
| Critical illness, Trauma including Surgery, Shock, Burns | Traumatic-AKI, Burns-AKI... |
| - CardioRenal (Tl): Right Heart Failure, Left Heart Failure | |
| - Renal Vasconstriction (HepatoRenal) | Cardiac-Failure-AKI (CF-AKI, RHF-AKI, LVF-AKL.) Hepato-Renal AKI |
|
Sepsis (incl High Output Failure (Sepsis, SIRS) - infection-associated AKI (eg PN, lepto...) |
Septic-AKI (incl ?High Flow-AKI) - Infective-AKI |
|
Immune Factors - Autoimmune diseases, vasculitis... |
Autoimmune-AKI, Vasculitic-AKI... |
| Nephrotoxins | Toxic-AKI (Tox-AKI) |
| - Direct: NSAIDS, gentamicin, paraquat... | |
| - Indirect: Allergic Reactions... | |
| - Radiocontrast agents | Contrast-induced-AKI (CI-AKI) |
| - Poisonous plants, snakes, spiders... | Venom-AKI |
| Outflow Obstruction (Ureteric or urethral) | |
| - stones, cancer, prostatic | Obstructive-AKI |
Key: “incl“, including; SIRS, systemic inflammatory response syndrome; NSAIDS non-steroidal anti-inflammatory drugs; CS-AKI Cardiac Surgery AKI; CTS-AKI Cardio-thoracic surgery AKI; CF-AKI Cardiac failure AKI; RHF-AKI Right heart failure AKI; LVF-AKI Left ventricular failure AKI; Ci-AKI Contrast induced AKI; Tox-AKI Nephrotoxin induced AKI
Conclusion
The era of individualised treatments that target specific kidney cells and pathways is beginning 33. Etiology, specific molecular mechanisms and the temporal phase in the evolution of AKI are critical to phase and mechanism-specific intervention. These features identify subphenotypes of AKI, but are missing from current generic definitions and from the terms used to describe AKI. Subphenotypes of AKI can be identified from novel damage biomarkers, from endothelial and inflammatory biomarkers, from functional changes including creatinine trajectories, from the duration of change and from associated clinical parameters and comorbidities. Subphenotype parameters can be combined in risk scores, or by association strategies ranging from a simple function-damage matrix to more complex aggregative methods such as neural network strategies used in machine learning. Tentative examples are described to shown how the current nomenclature can be modified to facilitate description of AKI subphenotypes. Consensus strategies are needed to refine the current nomenclature to allow identification of AKI subphenotypes that will facilitate correctly timed and selectively targeted intervention. These strategies are critical to both understanding clinical AKI and to facilitate targeted management.
Key Points.
Simplification of the complex syndrome of AKI into treateable categories requires identification of AKI subphenotypes.
AKI subphenotypes require identification of etiology, specific molecular mechanisms and temporal phase.
AKI nomenclature needs to incorporate AKI subphenotypes to facilitate research and clinical management.
Acknowledgments
Financial Support
This work was supported by NIH NIDDK Grant DK079337 for the UAB-UCSD O’Brien Centre for AKI Research in Birmingham, Alabama and San Diego, California, USA and by funding to the Australian Kidney Biomarker Reference Laboratory from the Prince of Wales Hospital Foundation, Sydney, NSW, Australia.
Footnotes
Conflicts of Interest
Zoltan H Endre; None
Ravindra L Mehta; None
References
- 1.Mehta RL, Cerda J, Burdmann EA, et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385(9987): 2616–2643. [DOI] [PubMed] [Google Scholar]
- 2.Ostermann M, Bellomo R, Burdmann EA, et al. Controversies in acute kidney injury: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Conference. Kidney Int 2020;98(2): 294–309.** This article provides an update on the KDIGO guidelines for AKI proposing contemporrary approaches for detection and management of AKI.
- 3.Seymour CW, Kennedy JN, Wang S, et al. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA. 2019;321(20): 2003–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shankar-Hari M, Fan E, Ferguson ND. Acute respiratory distress syndrome (ARDS) phenotyping. Intensive Care Med 2019;45(4): 516–519. [DOI] [PubMed] [Google Scholar]
- 5.Hasegawa D, Nishida O. Patient selection in sepsis: precision medicine using phenotypes and its implications for future clinical trial design. J Thorac Dis 2019;11(9): 3672–3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weisbord SD, Gallagher M, Jneid H, et al. Outcomes after Angiography with Sodium Bicarbonate and Acetylcysteine. N Engl J Med 2018;378(7): 603–614. [DOI] [PubMed] [Google Scholar]
- 7.Mehta RL, Burdmann EA, Cerda J, et al. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: a multinational cross-sectional study. Lancet. 2016;387(10032): 2017–2025. [DOI] [PubMed] [Google Scholar]
- 8.Mehta RBA, Patibandla R, Chakravarthi R. Detection and Management of AKI in the Developing World: The 18th Acute Disease Quality Initiative (ADQI) International Consensus Conference. Kidney Int Rep 2017;2(4): 515–518. [Google Scholar]
- 9.Gist KM, Selewski DT, Brinton J, Menon S, Goldstein SL, Basu RK. Assessment of the Independent and Synergistic Effects of Fluid Overload and Acute Kidney Injury on Outcomes of Critically Ill Children. Pediatr Crit Care Med 2020;21(2): 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basu RK, Kaddourah A, Goldstein SL, Investigators AS. Assessment of a renal angina index for prediction of severe acute kidney injury in critically ill children: a multicentre, multinational, prospective observational study. Lancet Child Adolesc Health. 2018;2(2): 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCoy IE, Chertow GM. The ongoing search for a robust clinical prediction model of ICU AKI. Clin Nephrol 2020;93(3): 160–162. [DOI] [PubMed] [Google Scholar]
- 12.Bhatraju PK, Mukherjee P, Robinson-Cohen C, et al. Acute kidney injury subphenotypes based on creatinine trajectory identifies patients at increased risk of death. Crit Care. 2016;20(1): 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lumlertgul N, Peerapornratana S, Trakarnvanich T, et al. Early versus standard initiation of renal replacement therapy in furosemide stress test non-responsive acute kidney injury patients (the FST trial). Crit Care. 2018;22(1): 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Endre ZH, Kellum JA, Di Somma S, et al. Differential diagnosis of AKI in clinical practice by functional and damage biomarkers: workgroup statements from the tenth Acute Dialysis Quality Initiative Consensus Conference. Contrib Nephrol 2013;182: 30–44. [DOI] [PubMed] [Google Scholar]
- 15.Murray PT, Mehta RL, Shaw A, et al. Potential use of biomarkers in acute kidney injury: report and summary of recommendations from the 10th Acute Dialysis Quality Initiative consensus conference. Kidney Int 2014;85(3): 513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanski N, Menon S, Goldstein SL, Basu RK. Integration of urinary neutrophil gelatinase-associated lipocalin with serum creatinine delineates acute kidney injury phenotypes in critically ill children. J Crit Care. 2019;53: 1–7. [DOI] [PubMed] [Google Scholar]
- 17.Bhatraju PK, Zelnick LR, Katz R, et al. A Prediction Model for Severe AKI in Critically Ill Adults That Incorporates Clinical and Biomarker Data. Clin J Am Soc Nephrol 2019;14(4): 506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calfee CS, Janz DR, Bernard GR, et al. Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest. 2015;147(6): 1539–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin B, Bennett TD. Sepsis Computable Phenotypes in the Service of Observational Research. Crit Care Med 2019;47(2): 303–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhatraju PK, Zelnick LR, Herting J, et al. Identification of Acute Kidney Injury Subphenotypes with Differing Molecular Signatures and Responses to Vasopressin Therapy. Am J Respir Crit Care Med 2019;199(7): 863–872.** This study describes the characteristsics of ICU patients ina single center using latent class analysis to identify two sub-phenotypes of AKI differentiated by the presence of an inflammatory response and evidence of endothelial dysfunction associated with increased vascular permeability. The two phenotypes are shown to correlate with the response to vasopressors in a previous trial of vasopressin compared to noradrenaline. These data suggest that sub-phenotypes may be useful in identifying individuals likely to benefit from specific targeted therapies, howevre these findings ned to be confirmed in other studies.
- 21.Bhatraju PK, Cohen M, Nagao RJ, et al. Genetic variation implicates plasma angiopoietin-2 in the development of acute kidney injury sub-phenotypes. BMC Nephrol 2020;21(1): 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiersema R, Jukarainen S, Vaara ST, et al. Two subphenotypes of septic acute kidney injury are associated with different 90-day mortality and renal recovery. Crit Care. 2020;24(1): 150.* As shown by Bhatraju et al in refrence 19, this study similarly used latent class analysis in patients with sepsis and AKI in the prospective Finnish Acute Kidney Injury (FINNAKI) study and identified two subphenotypes with a worst prognosis in those with an increased inflamamtory response and endothelial dysfunction.
- 23.Xu Z, Chou J, Zhang XS, et al. Identifying sub-phenotypes of acute kidney injury using structured and unstructured electronic health record data with memory networks. J Biomed Inform 2020;102: 103361.*This article applied a machine learning approach on the MIMIC 3 cohort and descriebs the features of patients who develop different severity stages of AKI. The findings corroborate what has been known for some time that underlying chronic kidney disease is a risk factor for AKI.
- 24.Koola JD, Chen G, Malin BA, et al. A clinical risk prediction model to identify patients with hepatorenal syndrome at hospital admission. Int J Clin Pract 2019;73(11): e13393. [DOI] [PubMed] [Google Scholar]
- 25.Tomasev N, Glorot X, Rae JW, et al. A clinically applicable approach to continuous prediction of future acute kidney injury. Nature. 2019;572(7767): 116–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Churpek MM, Carey KA, Edelson DP, et al. Internal and External Validation of a Machine Learning Risk Score for Acute Kidney Injury. JAMA Netw Open 2020;3(8): e2012892.** This article describes the devlopment and validation of a predictive score for Stage 2 AKI in over 500,000 patients in 3 centers in the USA. Although the model works well, with AUC ranging from 0.86 for AKI and 0.95 for dialysis its utility in clinical practice is still to be determined given the low positive predictive value.
- 27.Wiersema R, Jukarainen S, Eck RJ, et al. Different applications of the KDIGO criteria for AKI lead to different incidences in critically ill patients: a post hoc analysis from the prospective observational SICS-II study. Crit Care. 2020;24(1): 164.**The ADQI consensus statement provides a comprehensive discussion of the current status of kidney function and damage biomarkers and their utilization for diagnosis and maangement of AKI. This includes a proposal to expand the diagnostic criteria of AKI to include a subclincial state based on the presence of damage biomarkers in the absence of changes in creatinine.
- 28.Ostremann M, Zarbock A, Goldstein S, Kashani k, Macedo e, Murugan R, Bell M, Forni l, Guzi L, Joannidis M, Kane-Gil S, Legrand M, Mehta R, Murray P, Pickkers P, Plebani M, Prowle J, Ricci Z, Rimmele T, Rosner M, Shaw A, Kellum JA, Ronco C. Recommendations on Acute Kidney Injury Biomarkers From the Acute Disease Quality Initiative Consensus Conference. JAMA Open. 2020. [DOI] [PubMed] [Google Scholar]
- 29.Matsuura R, Srisawat N, Claure-Del Granado R, et al. Use of the Renal Angina Index in Determining Acute Kidney Injury. Kidney Int Rep 2018;3(3): 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Succar L, Pianta TJ, Davidson T, Pickering JW, Endre ZH. Subclinical chronic kidney disease modifies the diagnosis of experimental acute kidney injury. Kidney Int 2017;92(3): 680–692. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein BA, Bedoya AD. Guiding Clinical Decisions Through Predictive Risk Rules. JAMA Netw Open. 2020;3(8): e2013101.** This editorial accompanies the paper by Churpek et al ref 25 and discusses the practical limitations of applying the predictive model for clinical decision support.
- 32.Knaus WA, Marks RD. New Phenotypes for Sepsis: The Promise and Problem of Applying Machine Learning and Artificial Intelligence in Clinical Research. JAMA. 2019;321(20): 1981–1982. [DOI] [PubMed] [Google Scholar]
- 33.Endre ZH, Erlich JH. Targeted protection of proximal tubular cells by nanoparticle-enhanced delivery of a TLR9-antagonist. Kidney Int 2020;98(1): 48–50. [DOI] [PubMed] [Google Scholar]