Abstract
There are individual differences in the rates of cognitive decline across later adulthood. Personality traits are among the factors that may account for these differences. The current project investigated whether personality traits were associated with trajectories of cognitive decline, and whether the associations were different before and after dementia diagnosis. The data was analyzed using linear mixed effects regression. Across study aims is a focus on replicability and generalizability. Each question was addressed in four independent longitudinal studies (EAS, MAP, ROS, SATSA), then meta-analyzed, providing estimates of replicability. Results indicated that low neuroticism and high openness were associated with total cognitive function. We detected evidence for cognitive decline in all four samples, and openness was associated with decline post dementia diagnosis.
Introduction
There are substantial individual differences in rates of cognitive decline in older adults. Some persons are able to maintain healthy levels of cognitive ability before experiencing substantial functional loss (Stern, 2012), while others decline more quickly. There is also variation in the timing of decline trajectories relative to dementia onset. The theory of compression of morbidity predicts later onset of dementia along with steeper decline after such onset, thus compressing the amount of time living with severe cognitive impairment (Fries, 2005). There is some empirical evidence that some individuals have slower decline before the threshold of dementia is met, and then faster decline after meeting that threshold (Contador, Bermejo-Pareja, Pablos, Villarejo, & Benito-León, 2017; Yu et al., 2012). This finding is consistent with the compression of morbidity hypothesis, in which the onset of major decline may be compressed into a shorter period before the time of death. Thus, identifying variables related to individual differences in rate of change, as well as timing of cognitive decline is a high priority. Personality traits may be related to both maintained cognitive function and compression of morbidity, given their association with dementia occurrence in later life (Terracciano et al., 2014), as well as other disease and mortality outcomes (Graham et al., 2017; Mroczek & Spiro, 2007). The present study examined associations between personality traits and cognitive decline, and tested whether personality traits are also related to faster decline after dementia diagnosis.
Cognitive Aging
Cognitive decline varies greatly among individuals in old age (Bäckman, Jones, Berger, Laukka, & Small, 2005; Lipnicki et al, 2019; Mella, Fagot, Renaud, Kliegel, & De Ribaupierre, 2018; Zammit et al., 2018). It is well established that most cognitive abilities decrease among healthy older adults, although timing, pace, and direction of change varies (Mella et al., 2018; Salthouse, 2009; Soubelet & Salthouse, 2011; Wilson et al., 2002). However, heterogeneity in cognitive trajectories suggests that some individuals still remain relatively stable or even continue to increase in their abilities throughout midlife (Aartsen, Smits, Tilburg, Knipscheer, & Deeg, 2002; Rönnlund, Nyberg, Bäckman, & Nilsson, 2005; Schaie, 1989). Accounting for this variability has been a key focus of much of the cognitive aging field over the last several decades.
A high priority for current research in this area is the identification of factors that may be related to, or account for, individual variation in cognitive decline. A recent meta-analysis of previously published work showed support for the associations among education, occupation, and maintained cognitive function (Opdebeeck, Martyr, & Clare, 2016). This active model provides a good framework to help explain why individuals with higher education or occupational achievement are able to maintain better cognitive function before showing clinical signs of decline. Recent work has found that educational attainment and cognitive participation are associated with better cognitive function prior to the onset of decline (Borland, Stomrud, Westen, Hansson, & Palmqvist, 2020; Wilson et al., 2019), and are also associated with faster cognitive decline after a change point (Yu et al., 2012). Some individuals engage in healthful activities throughout their lives and show better functioning, more efficient processing, and slower decline as they age, perhaps a demonstrative of cognitive reserve. These individuals may be more resilient to the brain pathologies that tend to develop with age, may experience slower progression to dementia, but also faster rates of decline after diagnosis, a concept known as the compression of morbidity.
Compression of Morbidity
The compression of morbidity is a concept that describes the process by which some individuals maintain functionality throughout old age, and experience steep declines in health and functioning only as they approach end of life. Some individuals retain their cognitive health for a longer period of time, and decline faster after disease onset, thus living longer in a healthy state, and spending a shorter time in a diseased state (Fries, 1980, 2005). This fits the model of compression of morbidity for cognitive decline and onset of dementia. For some, cognitive decline may be relatively slow and the onset of dementia is delayed, but the rate of decline accelerates after a diagnosis of dementia (Contador et al., 2017; Yu et al., 2012).
The causes of functional loss and death among today’s aging population are primarily in the category of chronic conditions (as opposed to infectious diseases as was the case in prior eras, until recently) (Fries, 2005). As such, the aging population has much more agency in maintaining their quality of life (via preventing chronic conditions) than ever before. Maintaining a healthy lifestyle can help individuals achieve healthy aging, and lengthen the amount of time before the onset of conditions and reduced functioning. This compression is considered preferable, because it allows individuals to have high quality of life and minimizes the amount of time they spend in an impaired state. For gerontologists, understanding how to achieve this compression of morbidity is a key goal for the increasingly large aging population, as it necessarily reduces the burden on the healthcare industry and caregivers, as well as reducing negative stigma about the experience of aging.
Personality, Cognitive Aging, and Compression of Morbidity.
There is some evidence for psychosocial factors related to slower decline before the threshold for dementia is met, and then faster decline after. Personality traits may be one of the factors related to both overall cognitive aging and the compression of morbidity. There is a breadth of evidence linking personality traits to cognitive ability and decline across the adult lifespan. Many of these studies have found that individuals higher in openness and conscientiousness, and lower in neuroticism, typically have better cognitive performance (across multiple domains), and less decline over time, see (Curtis, Windsor, & Soubelet, 2015; Luchetti, Terracciano, Stephan, & Sutin, 2016) for a review, with somewhat more mixed evidence regarding extraversion and agreeableness. In addition to their associations with cognitive function, personality traits are perhaps most well known in their associations with other aspects of behavior (Mroczek, Spiro, & Turiano, 2009; Turiano, Whiteman, Hampson, Roberts, & Mroczek, 2012), disease (Weston & Jackson, 2015; Weston, Hill, & Jackson, 2014), and mortality (Graham et al., 2017) at all stages of the adult lifespan. Personality traits are associated with factors that lead to disease or early mortality, most notably health behaviors (Turiano, Chapman, Agrigoroaei, Infurna, & Lachman, 2014) and physician adherence (Hill & Roberts, 2011).
Factors that are associated with less decline include education, occupation, and activity engagement (physical, social, intellectual), all of which are related to personality (Scarmeas & Stern, 2004). Some studies have found that individuals with higher education tend to decline more quickly once dementia is diagnosed (Contador et al., 2017; Hall et al., 2007, 2009; Scarmeas, Albert, Manly, & Stern, 2006). Given that neuroticism, conscientiousness, and openness also have been linked to health behaviors and education (Chapman, Duberstein, & Lyness, 2007; Graham et al., 2020; Hampson, Goldberg, Vogt, & Dubanoski, 2007), this suggests that personality traits may be a predictor of cognitive decline (or maintenance) and compression of morbidity. Thus, personality traits may be associated with cognitive changes both before and after a dementia diagnosis takes place. Personality traits are associated with patterns of decline (Curtis et al., 2015; Luchetti et al., 2016). Those with higher levels of particular personality traits (e.g., openness, conscientiousness, and lower neuroticism) may have slower rates of cognitive decline, meaning less impairment and better functioning for a longer length of time, but have faster rates of decline after dementia onset. Although this hypothesis has not been tested, indirect evidence from recent studies of older adults have indicated that conscientiousness predicts terminal cognitive decline (Wilson et al., 2015b), and both lower conscientiousness and higher neuroticism are associated with risk of developing Alzheimer’s disease (Duberstein et al., 2011; Terracciano et al., 2014). Neuroticism is associated with higher levels of perceived stress (Jiang et al., 2017) and perceived stress is associated with the onset of amnestic mild cognitive impairment and cognitive decline (Katz et al, 2016; Jiang et al, 2017).
Compressing morbidity into a relatively small number of years in an aging individuals’ life is a goal that envisions optimal health for as long in the life cycle as possible (Fries, Bruce, & Chakravarty, 2011). To achieve this, one strategy is to focus on modifiable lifestyle factors that can reduce or delay the onset of age-related conditions, including cognitive decline and dementia. As discussed previously, personality traits are related to many of these health behaviors, as well as directly related to many age-related health outcomes. As such, personality traits may be directly associated with the compression of morbidity as it applies to cognitive decline. The current study seeks to test this.
Current Study
The current study expanded upon prior work by examining associations between personality traits and cognitive change in persons both with and without diagnosed dementia. This work was completed in a multi-study coordinated analysis format to assess the replicability and generalizability of the proposed models (Graham et al., in press; Weston, Graham, & Piccinin, 2019). The goal of the current project was to better understand how personality traits were related to the process of cognitive change both before and after a diagnosis of dementia has been received. First, we examined whether personality traits were related to cognitive decline. Specifically, we predicted that lower neuroticism would be associated with less decline in cognitive function. We predicted that higher openness would be associated with less cognitive decline. Lastly, we predicted that higher conscientiousness would be associated with less cognitive decline. Second, we examined whether these traits are related to a deviation in the overall rate of cognitive decline after the development of dementia in persons who were diagnosed with dementia over follow-up. Our hypotheses for these questions reflect that of compression of morbidity. We predicted that individuals lower in neuroticism would experience slower rates of decline overall, and steeper declines after a dementia diagnosis. Similarly, we predicted that individuals higher in openness would be associated with slower rates of decline overall, and steeper declines after a dementia diagnosis. Lastly, we predicted that higher conscientiousness would be associated with slower rates of decline overall, and steeper declines after a dementia diagnosis. The literature is somewhat mixed regarding extraversion and agreeableness, so we did not have a clear hypothesis for these traits. As such, analyses for extraversion and agreeableness were exploratory.
Methods
The current study used a multi-study framework to address our research questions, specifically coordinated integrative data analysis. Using the Integrative Analysis of Longitudinal Studies of Aging and Dementia (IALSA) (Hofer & Piccinin, 2009) framework, we identified four studies with appropriate data for testing the above described hypotheses: the Religious Orders Study (ROS), the Rush Memory and Aging Project (MAP), the Einstein Aging Study (EAS), and the Swedish Adoption/Twin Study of Aging (SATSA). These four studies each contain a baseline measurement of the Big Five personality traits, several clinical assessments of dementia that took place after the personality assessment, as well as a minimum of three measurement occasions of cognitive ability. Variations in studies have long been considered a constraint on the generality of findings in longitudinal research (Simons, Shoda, & Lindsay, 2017). However, using a coordinated analysis approach helps to reduce these constraints on generality. In coordinated analysis, these differences actually can be a strength: instead of regarding these study-level characteristics as sources of error, researchers have the option to test these differences systematically as sources of heterogeneity.
Studies
The Einstein Aging Study (EAS) is a study of older, ethnically diverse, community residing individuals from the Bronx, New York. Data collection began in 1993, with rolling enrollment (N=2,600 of which 733 are used in these analyses). At study entry, participant age ranged from 70-99 years, with follow up occasions approximately every 12 months (Katz et al., 2012). Included in follow-up assessments are measures of personality (IPIP), a cognitive battery including Speed (digit symbol), Episodic Memory (immediate and delayed recall), Working Memory (digit span), and Reasoning (block design), and a dementia screening. On average, participants completed 3.94 (SD = 2.67) measurement occasions of cognition overall, an average of 3.86 (SD = 2.68) measurements prior to dementia, and 1.51 (SD = .67) after dementia diagnosis. Average follow up time prior to dementia was .6 (SD = .73), and after dementia diagnosis was 2.45 (SD = 2.37) years.
The Rush Memory and Aging Project (MAP) is a longitudinal, epidemiologic clinical-pathologic cohort study of common chronic conditions of aging with emphasis on decline in cognitive and motor function and risk of Alzheimer’s Disease (AD). Participants (N=1,700) are older adults, aged 65 and older, who were recruited from retirement communities and subsidized senior housing facilities throughout Chicagoland and northeastern Illinois. Participants do not have known dementia at baseline and agree to annual clinical evaluation, cognitive testing, and brain and other tissue donation after death (Bennett et al., 2018, 2012). Enrollment began in 1997 and is ongoing. Clinical evaluations and cognitive assessments occur annually. Neuroticism was assessed starting in 2004, while extraversion and conscientiousness were assessed starting in 2008 (openness and agreeableness were not assessed), using the NEO-FFI (Costa & McCrae, 1989). The annual cognitive tests include Episodic Memory (immediate/delayed word list recall), Speed (digit symbol), Working Memory (forward/backward digits span), and Reasoning (progressive matrices). On average, participants completed 6.57 (SD = 4.48) measurement occasions of cognition overall; an average of 5.89 (SD = 4.46) measurements prior to dementia and 2. 55 (SD = 1.91) after dementia diagnosis. Average follow up time prior to dementia was 1.69 (SD = 2.24), and after dementia diagnosis was 5.36 (SD = 4.33) years.
The Religious Orders Study (ROS) is a longitudinal, epidemiologic clinical-pathologic cohort study of aging and Alzheimer’s disease (AD) that enrolls older Catholic nuns, priests, and brothers from more than 40 groups across the US. Participants (N=1,200) are aged 65 and older at enrollment, do not have known dementia at baseline and agree to annual clinical evaluation, cognitive testing, and brain and other tissue donation after death (Bennett et al., 2018; Bennett, Schneider, Arvanitakis, & Wilson, 2012). Enrollment began in 1994 and is ongoing. Clinical evaluations and cognitive assessments occur annually. The NEO-FFI (Costa & McCrae, 1989) personality assessment was collected at baseline. The annual cognitive tests used for the current study are Episodic Memory (word list recall), Speed (digit symbol), Working Memory (forward/backward digits span), and Reasoning (progressive matrices). Dementia status was evaluated at each assessment. On average, participants completed 9.83 (SD = 6.44) measurement occasions of cognition overall; an average of 7.71 (SD = 6.31) prior to dementia and 3.16 (SD = 2.29) after dementia diagnosis. Average follow up time prior to dementia was 2.56 (SD = 2.94), and after dementia diagnosis was 8.8 (SD = 6.38) years.
The Swedish Adoption/Twin Study of Aging (SATSA) is a sample of 2,280 adults aged 26-93 years that began in 1984 that examines the genetic and environmental factors associated with aging (Pedersen et al., 1991). Personality traits were assessed in 1984 using the NEO-PI and Eysenck Personality Questionnaire (EPQ), dementia was assessed at every measurement occasion using an in person mini-mental test, and the following cognitive abilities that we will focus on for this study were assessed in every measurement occasion and includes Reasoning (block design), Episodic Memory (Thurstone picture memory), Working Memory (digit span), and Speed (digit symbol). See Table 1 for study level descriptions. On average, participants completed 1.85 (SD = 2.68) measurement occasions of cognition overall; an average of 1.79 (SD = 2.63) prior to dementia and 1.74 (SD = 0.95) after dementia diagnosis. Average follow up time prior to dementia was 0.54 (SD = 0.99), and after dementia diagnosis was 2.15 (SD = 2.78) years.
Table 1:
Study Descriptions
| Study | Country | Year | Personality | MO | Interval | Episodic | Speed | Reasoning | WM | N |
|---|---|---|---|---|---|---|---|---|---|---|
| EAS | U.S. | 1993 | IPIP items | 12 | 1 | Free-Cued Selective Reminding: Total Recall score (Free + Cued) | WAIS Digit Symbol | WAIS Block Design | WAIS Digit Span | 736 |
| MAP | U.S. | 2004 | NEO-FFI | 16 | 1 | Immediate/Delayed Recall | Symbol Digits Modality Test (oral) | Progressive Matrices | Digit Span | 2175 |
| ROS | U.S. | 1994 | NEO-FFI | 27 | 1 | Immediate/Delayed Recall | Symbol Digit Modality Test (oral) | Progressive Matrices | Digit Span | 1476 |
| SATSA | Sweden | 1984 | Eysenck (N & E); NEO-PI (C-O-A) | 9 | 3 | Thurstone’s Picture Memory | Symbol Digit | Koh’s Block Design | Digit Span (forward and backward) | 641 |
Data analysis
We used R (Version 3.6.2; R Core Team, 2019) and the R-packages simr (Green et al., 2016), lmerTest (Kuznetsova et al., 2017), ggplot2 (Version 3.2.1; Wickham, 2016), lme4 (Version 1.1.21; Bates, Mächler, Bolker, & Walker, 2015), metafor (Version 2.1.0; Viechtbauer, 2010), and papaja (Version 0.1.0.9942; Aust & Barth, 2018) for all analyses and manuscript preparation.
Variable Construction and Data Transformations
Personality Traits.
Big Five personality trait measures were taken from the initial (baseline) assessment for each study. For ROS (1994) and MAP (2004) participants completed the five-factor inventory (NEO-FFI; (Costa & McCrae, 1992)). ROS participants completed the questionnaire for all five traits (Neuroticism, Extraversion, Openness, Conscientiousness, and Agreeableness). MAP participants completed the questionnaire for Neuroticism, Extraversion, and Conscientiousness only. For SATSA, personality traits were assessed at baseline (1984) using the NEO-PI (Openness, Conscientiousness, Agreeableness, (Costa & McCrae, 1985)) and the EPQ (Neuroticism, Extraversion (Eysenck, 1975)). In the EAS study, participants completed an assessment of the Big Five using IPIP adjectives (10 items per trait, 50 items total; Goldberg, 1992). Within each study, traits were z-standardized for all analyses. This allowed for interpretation of results to be in standard deviation units, and optimized cross-study comparability. All participants were free of dementia at the time of personality assessment.
Cognitive Ability
Each study assessed cognitive ability at each measurement occasion using a robust battery of tests. Across studies, the exact test varied, but ostensibly assessed similar domains of ability. For the current project, we selected similar or identical cognitive tests across the studies for optimal comparability. Working memory was assessed in all four studies using a forward and backward digit span task (Wechsler, 1997; Wilson et al., 2015a). Speed was assessed using a digit symbol task (Wechsler, 1997; Wilson et al., 2015a). Episodic Memory was assessed using an immediate and delayed recall task in ROS, MAP, and EAS (Word list recall, names/faces recall), (Grober, Lipton, Hall, & Crystal, 2000; Wilson et al., 2015a), and using the Thurstone picture memory task in SATSA. Reasoning was assessed using a block design task in SATSA and EAS (Wechsler, 1997), and a progressive matrices tasks in ROS and MAP (Wilson et al., 2015a). Within each study, each cognitive test was z-standardized. These z-scores were then averaged within each measurement occasion, then re-standardized, resulting in a single standardized “global cognition” score for each measurement occasion.
Dementia Status.
At each measurement occasion, all studies evaluated participants for potential dementia. In SATSA, the following criteria were used to identify potential dementia: a score below 24 on the MMSE, a decline on the MMSE of 3 or more points since the last measurement occasion, low cognitive test scores, suspected dementia by a research nurse, or having a proxy document cognitive problems (Gatz et al., 1997). These criteria were used at the screening stage. If any of the criteria were met, the participants was then given a full clinical workup for dementia. All diagnoses were given using the current version of the Diagnostic and Statistical Manual of Mental Disorders (DSM-III or DSM-IV). For those flagged with a dementia code in the hospital discharge registry, this triggered a full clinical workup, which included a home visit as well as medical records. In the ROS and MAP samples, dementia was evaluated using a clinical diagnosis of cognitive status, based on a three-stage process including computer score of cognitive tests, clinical judgment by neuropsychologist, and diagnostic classification by a clinician. EAS also used a clinical dementia rating and case conference review to evaluate dementia status applying DSM-IV criteria for dementia.
Time Metrics.
We used two time metrics for these analyses: Age (years), and time since dementia (0 until diagnosed; time in years since diagnosis thereafter). At each measurement occasion, each study evaluated participants for dementia and assigned participants values based on this assessment (1 = dementia, 0 = no dementia).
Covariates.
Covariates for the current project were baseline education (years), sex, and race. Education was z-standardized, and sex was binary (1=female, 0=male), as was race (1= Caucasian; 0=not Caucasian). Race was not available in SATSA, as this is a Swedish sample with high racial homogeneity.
Individual Study Analysis.
We used a series of linear mixed effects regression models to examine the associations of baseline personality traits with the overall rate of cognitive decline, and determine whether baseline personality traits are significantly associated with a change in the rate of cognitive decline after dementia diagnosis, compared to the rate of cognitive decline before diagnosis, in those who developed dementia over follow-up.
The models were built from the least to most complex, starting with the unconditional random intercept model, expressed as Yti = π0i + εti, where Y is global cognition score at a given measurement occasion t for person i. This model provided an estimate of the intraclass correlation, which is the proportion of the variation in cognitive scores due to within versus between-person differences. Next, we modelled change in cognitive function, using age as the time metric. We individually added the fixed and random effects of age to estimate cognitive change over time and account for individual differences in change over time, respectively. While these models were not the key focus of the current paper, we report and meta-analyze the estimates from these simpler models, before moving to the more complex models.
We then included fixed time since dementia to ascertain whether cognitive decline shifts post dementia diagnosis, accounting for individual-level variation in this shift. We included the covariates of sex, race, and education to adjust for the effect these factors may have on overall cognition. The covariates predicted intercepts only. We included these covariates because each has been independently associated with cognitive function, and have relatively wide representation in the proposed datasets. As such, we reasoned that our models should be tested after accounting for these factors (McCarrey, An, Kitner-Triolo, Ferrucci, & Resnick, 2016; Weuve et al., 2018a).
To estimate the effect of personality on these cognitive trajectory models, we then included, one at a time, terms for personality trait, the interaction of trait and age, and the interaction of trait and time since dementia diagnosis. These interaction terms tell us, respectively, if a personality trait is related to overall cognitive decline, and if the trait is related to the deviation in cognitive decline after a dementia diagnosis. One final model was generated per personality trait, resulting in five individual models of cognition change over time, answering our primary research question: whether personality was associated with whether individuals diagnosed with dementia have different cognitive trajectories after a dementia diagnosis.
Although we estimated models by adding additional terms individually, we focus our results on the final, covariate-adjusted models. The raw data for the individual studies are not publicly available. The output objects (Rdata) for the results of each individual study analysis are available on OSF (https://osf.io/32wgf/), and include descriptive statistics and all model outputs.
Meta-Analysis
We combined the individual study results and summarized them using random effects meta-analysis (Borenstein, Hedges, Higgins, & Rothstein, 2010). All meta-analytic estimates were weighted by study sample size, and include standard errors/confidence intervals, as well as estimates of heterogeneity (I2, Q) (Borenstein, Higgins, Hedges, & Rothstein, 2017). We estimated the average cognitive slope, the average cognitive slope post-dementia diagnosis, as well as the average estimate of each personality trait predictor of cognitive slope both overall and post dementia. All models reported in the manuscript were adjusted for sex, education, and race (see supplemental material for the results from unadjusted models). Since the MAP study did not include measures of agreeableness or openness, the meta-analytic estimates that we used to test our hypotheses will be based on three studies for agreeableness and conscientiousness. The meta-analytic summaries for neuroticism, extraversion, and openness are based on all four studies. We interpreted the magnitude and direction of the individual study estimates, but tested our hypotheses and draw conclusions based on the meta-analytic estimates, using a more conservative alpha criteria (α = .01).
All authors had some prior knowledge of these datasets. Authors James, Jackson, Boyle, Wilson, and Bennett have worked with the ROS and MAP datasets. Authors Katz and Lipton have worked with the EAS data. Authors Beam, Pedersen, and Reynolds have worked with the SATSA datasets. Authors Graham, Willroth, Luo, and Mroczek have worked with all datasets. None of the authors have worked previously with the personality trait, cognitive function, and dementia status variables in combination with one another in these datasets.
Deviations from the pre-registrations.
Our pre-registeredanalysisplan stated that each study would include a measure of episodic memory (assessed using an immediate and delayed recall task (Word list recall, names/faces recall), (Grober et al., 2000; Wilson et al., 2015a)) in the global cognition variable. Upon accessing the data, it was discovered that SATSA did not have this measure of episodic memory until their fifth measurement occasion, but did have Thurstone picture memory at all waves. We therefore switched the SATSA episodic memory task to this alternative measure. Additionally, after approval we discovered that the time metrics in our models were not aligned. We therefore adjusted our metrics so that they were expressed in years, and time since dementia is zero until diagnosed, and then time in years since diagnosis thereafter. In some instances, patients were diagnosed with dementia, but did not have that diagnosis at subsequent time points. We re-evaluated these cases and determined that once a patient was diagnosed with dementia, they should carry that diagnosis forward for all time points in which they had other visit data present. Lastly, for consistency across models, we adjusted our random effects so that all models contained a random effect of age only.
Results
Of the participants across the four studies, 1,006 participants were diagnosed with dementia at some point during the study. This includes 43 (6%) from EAS, 28 (3%) from SATSA, 457 (31%) from ROS, and 478 (22%) from MAP. See Table 2 for descriptive statistics.
Table 2:
Descriptive Statistics
| Variable | N Valid | Mean | SD | Min | Max |
|---|---|---|---|---|---|
| SATSA | |||||
| Age | 641 | 65.53 | 8.39 | 39.76 | 87.98 |
| Agreeableness | 707 | 0.03 | 0.97 | −3.61 | 2.80 |
| Conscientiousness | 700 | 0.07 | 0.92 | −3.25 | 2.38 |
| Age of Dementia | 28 | 76.20 | 7.13 | 63.19 | 91.00 |
| Education | 827 | 0.04 | 1.02 | −0.66 | 2.83 |
| Extraversion | 822 | −0.01 | 1.01 | −2.10 | 1.83 |
| Neuroticism | 823 | −0.03 | 0.98 | −1.19 | 2.62 |
| Openness | 678 | 0.02 | 0.97 | −2.90 | 2.98 |
| Sex | 836 | 0.59 | 0.49 | 0.00 | 1.00 |
| ROS | |||||
| Age | 1476 | 75.98 | 7.75 | 36.50 | 102.15 |
| Agreeableness | 1466 | 0.00 | 1.00 | −3.82 | 3.37 |
| Conscientiousness | 1471 | 0.00 | 1.00 | −4.53 | 2.70 |
| Age of Dementia | 457 | 85.53 | 6.77 | 63.02 | 107.23 |
| Education | 1476 | 0.00 | 1.00 | −4.59 | 3.44 |
| Extraversion | 1470 | 0.00 | 1.00 | −3.19 | 2.90 |
| Neuroticism | 1471 | 0.00 | 1.00 | −2.83 | 3.30 |
| Openness | 1468 | 0.00 | 1.00 | −4.22 | 3.38 |
| Race | 1477 | 0.92 | 0.27 | 0.00 | 1.00 |
| Sex | 1477 | 0.71 | 0.45 | 0.00 | 1.00 |
| MAP | |||||
| Age | 2175 | 80.01 | 7.59 | 53.35 | 100.47 |
| Conscientiousness | 1233 | 0.00 | 1.00 | −4.71 | 2.41 |
| Age of Dementia | 478 | 87.40 | 6.60 | 64.06 | 104.87 |
| Education | 2175 | 0.00 | 1.00 | −4.46 | 4.53 |
| Extraversion | 2058 | 0.00 | 1.00 | −3.56 | 2.68 |
| Neuroticism | 1781 | 0.00 | 1.00 | −2.15 | 4.20 |
| Race | 2175 | 0.93 | 0.25 | 0.00 | 1.00 |
| Sex | 2175 | 0.73 | 0.44 | 0.00 | 1.00 |
| EAS | |||||
| Age | 736 | 79.00 | 5.27 | 69.00 | 94.00 |
| Agreeableness | 737 | 0.00 | 1.00 | −3.99 | 1.84 |
| Conscientiousness | 737 | 0.00 | 1.00 | −4.35 | 1.84 |
| Age of Dementia | 43 | 85.09 | 3.98 | 77.00 | 92.00 |
| Education | 737 | 0.00 | 1.00 | −3.54 | 2.88 |
| Extraversion | 737 | 0.00 | 1.00 | −3.39 | 2.45 |
| Neuroticism | 737 | 0.00 | 1.00 | −1.79 | 3.74 |
| Openness | 737 | 0.00 | 1.00 | −2.95 | 2.07 |
| Race | 737 | 0.64 | 0.48 | 0.00 | 1.00 |
| Sex | 737 | 0.61 | 0.49 | 0.00 | 1.00 |
Note:
Age of Dementia = the average age that individuals were diagnosed with dementia. This was calculated only for individuals diagnosed during the course of the study
Cognitive Change over Time and Dementia
Cognitive change over time.
At the individual study level (Table 3), there was cross-study consistency in the overall pattern of cognitive change across time. The meta-analytic summary across the four studies indicates that global cognition declines over time (B = −0.05, 95% CI = [−0.06, − 0.04], p =< .001). There was statistically significant heterogeneity in this effect (I2 = 95.03, Q = 85.11, df = 3, p =< .001). See Figure 1 for a visualization of the overall pattern of cognitive change across time. Each line represents one of the four studies, and the black line is the weighted average trajectory.
Table 3:
Cognitive Trajectories Across Age
| coef | EAS | MAP | ROS | SATSA |
|---|---|---|---|---|
| Intercept (se) | 0.50 | 0.64 | 0.40 | 0.27 |
| 0.11 | 0.1 | 0.09 | 0.05 | |
| p < .001 | p < .001 | p < .001 | p < .001 | |
| Age (se) | −0.05 | −0.06 | −0.06 | −0.04 |
| 0.01 | 0 | 0 | 0 | |
| p < .001 | p < .001 | p < .001 | p < .001 | |
| Sex (se) | 0.03 | 0.30 | 0.38 | 0.11 |
| 0.06 | 0.05 | 0.05 | 0.06 | |
| p = 0.327 | p < .001 | p < .001 | p = 0.027 | |
| Race (se) | 0.69 | 0.53 | 0.59 | NA |
| 0.06 | 0.08 | 0.08 | NA | |
| p < .001 | p < .001 | p < .001 | NA | |
| Education (se) | 0.25 | 0.32 | 0.21 | 0.34 |
| 0.03 | 0.02 | 0.02 | 0.03 | |
| p < .001 | p < .001 | p < .001 | p < .001 | |
| Intercept Variance | 1.70 | 2.87 | 1.46 | 0.64 |
| Slope Variance | 0.01 | 0.01 | 0.00 | 0.00 |
| Int-Slope Cov | −0.09 | −0.11 | −0.07 | −0.01 |
| Residual(σ2) | 0.27 | 0.14 | 0.16 | 0.15 |
| Npeople | 736 | 2,175 | 1,476 | 827 |
| Ntotalobs | 2,906 | 14,293 | 14,499 | 3,691 |
| Log Likelihood | −3061 | −11035 | −10996 | −3148 |
Figure 1:
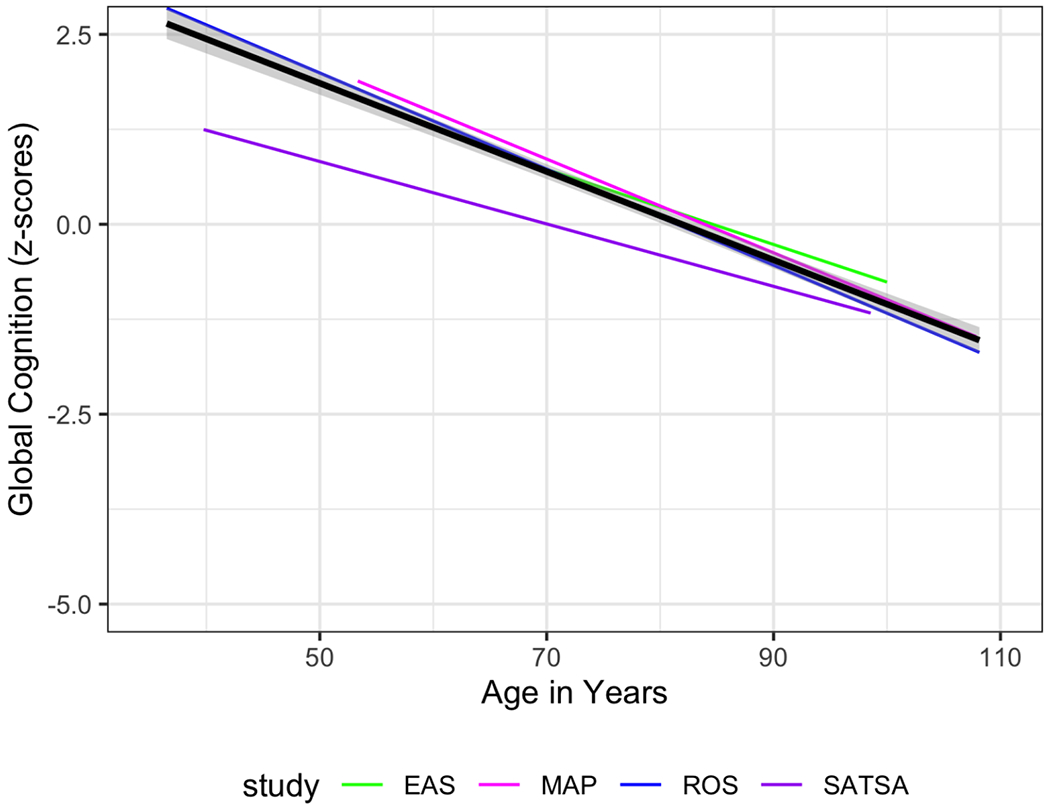
Linear Trajectories of Cognitive Performance Over Time. Black line represents the average trajectory, weighted by sample size.
Cognitive change after dementia diagnosis.
Adding time since dementia diagnosis to the model provided an estimate of the deviation from the overall cognitive slope after dementia diagnosis among individuals who were diagnosed with dementia during the study. See Table 4 for the individual study model summaries. The individual studies all showed a significant negative slope of cognitive for individuals with a dementia diagnosis, although the magnitude of these estimates varies, specifically the effect was much larger in the EAS than in the other three studies. The meta-analytic summary indicates that individuals who were diagnosed with dementia had steeper cognitive decline after diagnosis than individuals who remained healthy during the study, but this estimate was not significant (B = −0.38, 95% CI = [−0.92, − 0.04], p = .164). There was significant heterogeneity in this effect (I2 = 99.95, Q = 123.72, df = 3, p =< .001). As shown in the meta-analytic estimates, and the individual study estimates, this deviation from the overall slope was modest for most studies, suggesting that on average, the cognitive trajectory for individuals eventually diagnosed with dementia steepens in its decline after the diagnosis. See Figure 2 for a visualization of the cognitive trajectories across studies and the unique slope for individuals with dementia, and the average trajectories weighted by sample size. Solid lines indicate the trajectory before dementia diagnosis, and the dashed lines show the deviation from the non-dementia trajectory; vertical lines show the average age at dementia diagnosis (that is, the point in time in which the cognitive trajectory deviates from the original [non-dementia] trajectory).
Table 4:
Cognitive Trajectories Across Age for Individuals both With and Without Dementia
| coef | EAS | MAP | ROS | SATSA |
|---|---|---|---|---|
| Intercept (se) | 0.41 | 0.55 | 0.29 | 0.27 |
| 0.1 | 0.09 | 0.09 | 0.05 | |
| p < .001 | p < .001 | p < .001 | p < .001 | |
| Age (se) | −0.04 | −0.05 | −0.05 | −0.04 |
| 0 | 0 | 0 | 0 | |
| p < .001 | p < .001 | p < .001 | p < .001 | |
| Dementia Dx (se) | −1.22 | −0.13 | −0.12 | −0.08 |
| 0.1 | 0.01 | 0.01 | 0.03 | |
| p < .001 | p < .001 | p < .001 | p = 0.002 | |
| Sex (se) | 0.02 | 0.29 | 0.36 | 0.11 |
| 0.06 | 0.04 | 0.05 | 0.06 | |
| p = 0.366 | p < .001 | p < .001 | p = 0.027 | |
| Race (se) | 0.68 | 0.46 | 0.52 | NA |
| 0.06 | 0.08 | 0.08 | NA | |
| p < .001 | p < .001 | p < .001 | NA | |
| Education (se) | 0.25 | 0.32 | 0.22 | 0.34 |
| 0.03 | 0.02 | 0.02 | 0.03 | |
| p < .001 | p < .001 | p < .001 | p < .001 | |
| Intercept Variance | 1.24 | 2.89 | 1.40 | 0.64 |
| Slope Variance | 0.00 | 0.01 | 0.00 | 0.00 |
| Int-Slope Cov | −0.06 | −0.11 | −0.06 | −0.01 |
| Residual(σ2) | 0.26 | 0.14 | 0.16 | 0.15 |
| Npeople | 736 | 2,175 | 1,476 | 827 |
| Ntotalobs | 2,906 | 14,293 | 14,499 | 3,691 |
| Log Likelihood | −2990 | −10918 | −10820 | −3144 |
Figure 2:
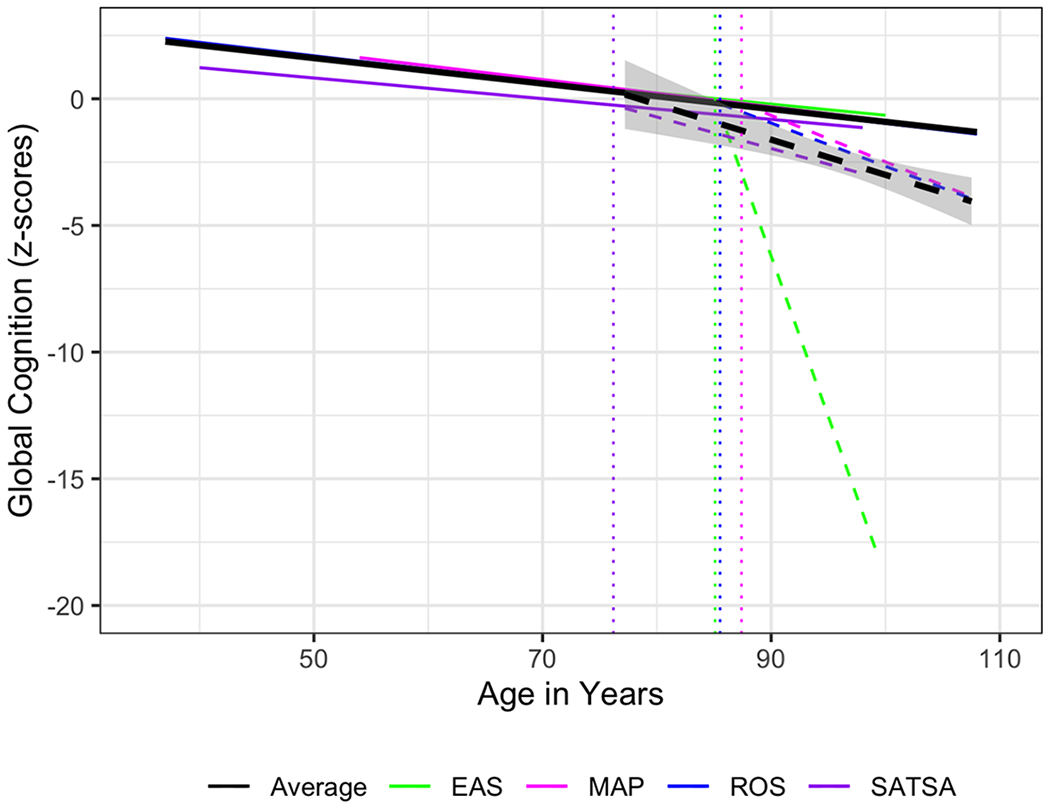
Cognitive Trajectories over time, plus the difference in trajectory among individuals with dementia. Dashed line represents trajectory post dementia diagnosis. Dotted vertical lines represent the average age of dementia diagnosis within each study. Black line represents the average trajectory, weighted by sample size.
Neuroticism
The following section reports the estimates for the associations of neuroticism with overall cognitive performance, cognitive change, and with change after dementia diagnosis. See Table 5 for the individual study model summaries. Overall, individuals higher in neuroticism tended to have worse cognitive performance, and this was supported by the meta-analytic summary (B = −0.09, 95% CI = [−0.14, − 0.05], p =< .001). At the individual study level, not all neuroticism estimates were significant, but they were fairly consistent in their direction and approximate magnitude. There was not significant heterogeneity in this effect (I2 = 6.38, Q = 2.42, df = 3, p = .490).
Table 5:
Cognitive Trajectories by Neuroticism
| coef | EAS | MAP | ROS | SATSA |
|---|---|---|---|---|
| Intercept (se) | 0.37 | 0.63 | 0.29 | 0.25 |
| 0.1 | 0.1 | 0.09 | 0.05 | |
| p < .001 | p < .001 | p < .001 | p < .001 | |
| Age (se) | −0.04 | −0.05 | −0.05 | −0.04 |
| 0 | 0 | 0 | 0 | |
| p < .001 | p < .001 | p < .001 | p < .001 | |
| Dementia Dx (se) | −1.34 | −0.16 | −0.13 | −0.07 |
| 0.11 | 0.01 | 0.01 | 0.04 | |
| p < .001 | p < .001 | p < .001 | p = 0.032 | |
| Neuroticism (se) | −0.14 | −0.07 | −0.05 | −0.12 |
| 0.09 | 0.05 | 0.04 | 0.03 | |
| p = 0.058 | p = 0.078 | p = 0.121 | p < .001 | |
| Sex (se) | 0.05 | 0.27 | 0.36 | 0.14 |
| 0.06 | 0.04 | 0.05 | 0.06 | |
| p = 0.209 | p < .001 | p < .001 | p = 0.006 | |
| Race (se) | 0.70 | 0.43 | 0.53 | NA |
| 0.06 | 0.08 | 0.08 | NA | |
| p < .001 | p < .001 | p < .001 | NA | |
| Education (se) | 0.23 | 0.26 | 0.19 | 0.33 |
| 0.03 | 0.02 | 0.02 | 0.03 | |
| p < .001 | p < .001 | p < .001 | p < .001 | |
| Age x Neuroticism (se) | 0.00 | 0.00 | −0.01 | 0.00 |
| 0 | 0 | 0 | 0 | |
| p = 0.404 | p = 0.04 | p = 0.003 | p = 0.146 | |
| Dementia x Neuroticism (se) | 0.40 | −0.01 | 0.02 | −0.03 |
| 0.12 | 0.01 | 0.01 | 0.04 | |
| p < .001 | p = 0.084 | p < .001 | p = 0.222 | |
| Intercept Variance | 1.05 | 2.39 | 1.42 | 0.62 |
| Slope Variance | 0.00 | 0.00 | 0.00 | 0.00 |
| Int-Slope Cov | −0.05 | −0.09 | −0.06 | −0.01 |
| Residual(σ2) | 0.26 | 0.13 | 0.16 | 0.15 |
| Npeople | 736 | 1,781 | 1,470 | 816 |
| Ntotalobs | 2,906 | 12,712 | 14,474 | 3,653 |
| Log Likelihood | −2976 | −9031 | −10744 | −3102 |
Although not pre-registered, we decided it was prudent to report the age by trait interactions from the models that did not contain the interaction with time since dementia. This interaction term provides an estimate of the association between personality traits and overall pattern of cognitive decline. Neuroticism was not related to cognitive trajectories overall according to the meta-analytic summary (B = 0.00, 95% CI = [−0.01,0.00], p = .400). Individual study results from these models can be found in Supplemental table S12.
When both the interactions are included in the model (trait by age, trait by dementia), the terms provide estimates for the associations with cognitive change prior to dementia diagnosis and after diagnosis. Neuroticism was not related to cognitive trajectories prior to dementia diagnosis according to the meta-analytic summary (B = 0.00, 95% CI = [−0.01,0.00], p = .353). At the individual study level, we observed a significant effect in ROS, suggesting the higher neuroticism was associated with steeper declines prior to diagnosis, but this was not replicated in the other three studies, nor was it supported by the meta-analytic summary. Lastly, the interaction with dementia diagnosis suggests that neuroticism was not associated with cognitive decline after diagnosis (B = 0.07, 95% CI = [−0.09,0.23], p = .422). All told, these findings do not support our hypotheses for neuroticism.
Extraversion
The following section reports the estimates for the associations of extraversion with overall cognitive function, cognition change, and with change after dementia diagnosis. See Table 6 for the individual study model summaries. Overall, extraversion was not associated with cognitive performance, and this was supported by the meta-analytic summary (B = −0.04, 95% CI = [−0.08,0.01], p = .084). There was not heterogeneity in this effect (I2 = 0.00, Q = 0.43, df = 3, p = .933).
Table 6:
Cognitive Trajectories by Extraversion
| coef | EAS | MAP | ROS | SATSA |
|---|---|---|---|---|
| Intercept (se) | 0.40 | 0.54 | 0.31 | 0.27 |
| 0.1 | 0.09 | 0.09 | 0.05 | |
| p < .001 | p < .001 | p < .001 | p < .001 | |
| Age (se) | −0.04 | −0.05 | −0.05 | −0.04 |
| 0 | 0 | 0 | 0 | |
| p < .001 | p < .001 | p < .001 | p < .001 | |
| Dementia (se) | −0.94 | −0.15 | −0.12 | −0.09 |
| 0.1 | 0.01 | 0.01 | 0.03 | |
| p < .001 | p < .001 | p < .001 | p < .001 | |
| Extraversion (se) | −0.02 | −0.02 | −0.03 | −0.05 |
| 0.09 | 0.05 | 0.04 | 0.03 | |
| p = 0.41 | p = 0.331 | p = 0.258 | p = 0.048 | |
| Sex (se) | 0.03 | 0.24 | 0.34 | 0.11 |
| 0.06 | 0.04 | 0.05 | 0.06 | |
| p = 0.338 | p < .001 | p < .001 | p = 0.028 | |
| Race (se) | 0.68 | 0.49 | 0.51 | NA |
| 0.06 | 0.08 | 0.08 | NA | |
| p < .001 | p < .001 | p < .001 | NA | |
| Education (se) | 0.25 | 0.31 | 0.21 | 0.34 |
| 0.03 | 0.02 | 0.02 | 0.03 | |
| p < .001 | p < .001 | p < .001 | p < .001 | |
| Age x Extraversion (se) | 0.00 | 0.00 | 0.00 | 0.00 |
| 0 | 0 | 0 | 0 | |
| p = 0.293 | p = 0.043 | p = 0.05 | p = 0.129 | |
| Dementia x Extraversion (se) | 0.77 | −0.03 | −0.03 | 0.04 |
| 0.1 | 0.01 | 0.01 | 0.04 | |
| p < .001 | p < .001 | p < .001 | p = 0.158 | |
| Intercept Variance | 1.16 | 2.57 | 1.42 | 0.63 |
| Slope Variance | 0.00 | 0.00 | 0.00 | 0.00 |
| Int-Slope Cov | −0.05 | −0.10 | −0.06 | −0.01 |
| Residual(σ2) | 0.26 | 0.14 | 0.16 | 0.15 |
| Npeople | 736 | 2,058 | 1,469 | 815 |
| Ntotalobs | 2,906 | 13,793 | 14,471 | 3,656 |
| Log Likelihood | −2958 | −10239 | −10757 | −3105 |
Extraversion was not related to cognitive trajectories overall according to the meta-analytic summary, using the above stated more conservative alpha criteria (α = .01), (B = 0.00, 95% CI = [0.00,0.00], p = .054). Individual study results from these models can be found in Supplemental table S19.
Extraversion was not associated with cognitive trajectories prior to dementia across all four studies, and this was supported by the meta-analytic summary (B = 0.00, 95% CI = [0.00,0.00], p = .010). There was not heterogeneity in this effect (I2 = 0.00, Q = 0.57, df = 3, p = .904). For individuals who were diagnosed with dementia, individuals higher in extraversion did not demonstrate a deviation from the overall cognitive trajectory (B = 0.18, 95% CI = [−0.19,0.55], p = .343).
Openness
The following section reports the estimates for the associations of openness with overall cognitive change and with change after dementia diagnosis. See Table 7 for the individual study model summaries. The individual study estimates indicated that individuals higher in openness tended to have better cognitive performance, and this was supported by the meta-analytic summary (B = 0.18, 95% CI = [0.07,0.29], p = .002). There was not significant heterogeneity in this effect (I2 = 75.48, Q = 7.92, df = 2, p = .019).
Table 7:
Cognitive Trajectories by Openness
| coef | EAS | ROS | SATSA |
|---|---|---|---|
| Intercept (se) | 0.39 | 0.34 | 0.34 |
| 0.1 | 0.09 | 0.05 | |
| p < .001 | p < .001 | p < .001 | |
| Age (se) | −0.04 | −0.05 | −0.04 |
| 0 | 0 | 0 | |
| p < .001 | p < .001 | p < .001 | |
| Dementia Dx (se) | −1.34 | −0.13 | −0.09 |
| 0.13 | 0.01 | 0.03 | |
| p < .001 | p < .001 | p = 0.002 | |
| Openness (se) | 0.29 | 0.08 | 0.21 |
| 0.09 | 0.04 | 0.03 | |
| p < .001 | p = 0.027 | p < .001 | |
| Sex (se) | 0.03 | 0.31 | 0.08 |
| 0.06 | 0.05 | 0.06 | |
| p = 0.32 | p < .001 | p = 0.076 | |
| Race (se) | 0.69 | 0.50 | NA |
| 0.06 | 0.08 | NA | |
| p < .001 | p < .001 | NA | |
| Education (se) | 0.18 | 0.17 | 0.28 |
| 0.03 | 0.02 | 0.03 | |
| p < .001 | p < .001 | p < .001 | |
| Age x Openness (se) | −0.01 | 0.00 | 0.00 |
| 0 | 0 | 0 | |
| p = 0.04 | p = 0.086 | p = 0.146 | |
| Dementia x Openness (se) | −0.12 | −0.02 | −0.05 |
| 0.1 | 0.01 | 0.02 | |
| p = 0.117 | p < .001 | p = 0.008 | |
| Intercept Variance | 1.06 | 1.38 | 0.53 |
| Slope Variance | 0.00 | 0.00 | 0.00 |
| Int-Slope Cov | −0.05 | −0.06 | −0.01 |
| Residual(σ2) | 0.26 | 0.16 | 0.15 |
| Npeople | 736 | 1,467 | 672 |
| Ntotalobs | 2,906 | 14,438 | 3,089 |
| Log Likelihood | −2978 | −10718 | −2557 |
Openness was not related to cognitive trajectories overall according to the meta-analytic summary (B = 0.00, 95% CI = [−0.01,0.00], p = .423). Individual study results from these models can be found in Supplemental table S26.
Openness was not associated with cognitive trajectories prior to dementia, and the meta-analytic summary indicates a null effect (B = 0.00, 95% CI = [−0.01,0.00], p = .631). There was not significant heterogeneity in this effect (I2 = 71.96, Q = 5.88, df = 2, p = .053). For individuals who were diagnosed with dementia, individuals higher in openness did demonstrate a deviation from the overall cognitive trajectory (B = −0.03, 95% CI = [−0.05, −0.01], p = .003). There was not heterogeneity in this effect (I2 = 17.71, Q = 2.12, df = 2, p = .346). These findings partially support our hypotheses. See Figure 3 for a visualization of the overall cognitive trajectories across studies and the unique slope for individuals with dementia, among individuals high and low in openness, and the average trajectories weighted by sample size. It appears that individuals higher in openness had somewhat flatter cognitive slopes prior to diagnosis, and steeper slopes after dementia was diagnosed.
Figure 3:
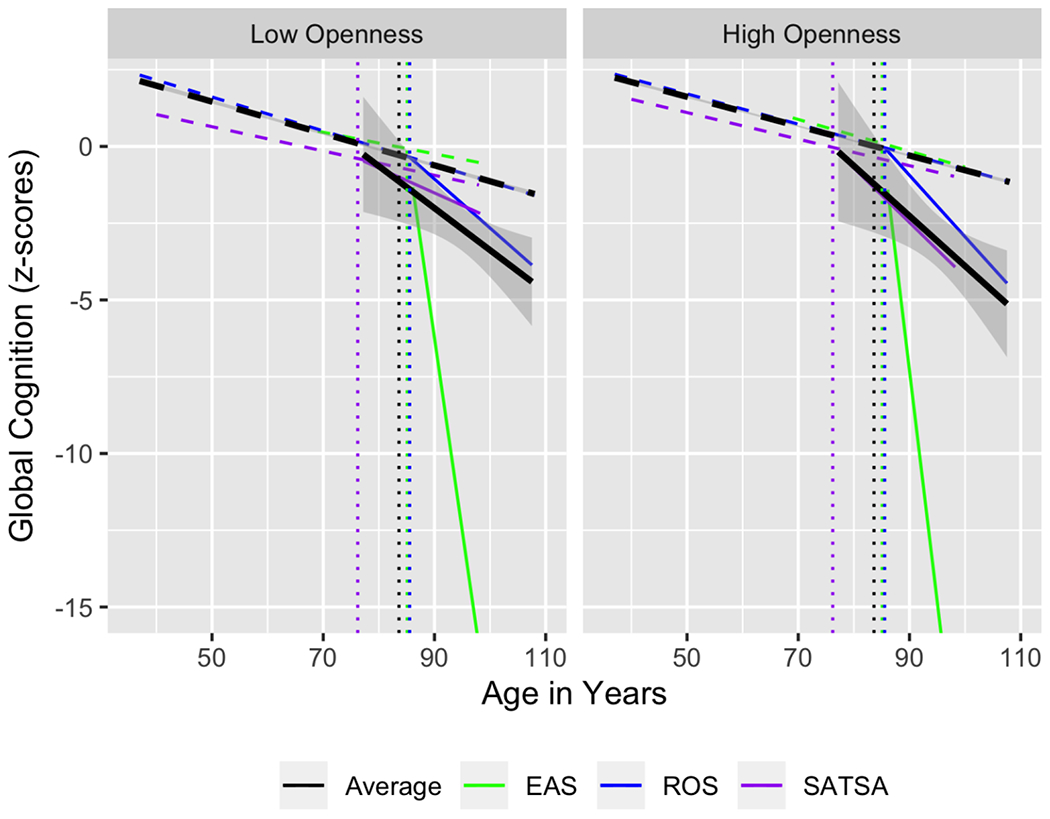
Overall cognitive trajectories across studies and the unique slope for individuals with dementia, among individuals high and low in openness, and the average trajectories weighted by sample size.
Conscientiousness.
The following section reports the estimates for the associations of conscientiousness with overall cognitive function, with cognitive change, and with change after dementia diagnosis. See Table 8 for the individual study model summaries. The individual study estimates suggest that conscientiousness was not associated with cognitive performance, and this was supported by the meta-analytic summary (B = −0.06, 95% CI = [−0.10, −0.01], p = .017). There was not heterogeneity in this effect (I2 = 0.09, Q = 4.34, df = 3, p = .227).
Table 8:
Cognitive Trajectories by Conscientiousness
| coef | EAS | MAP | ROS | SATSA |
|---|---|---|---|---|
| Intercept (se) | 0.41 | 0.76 | 0.35 | 0.34 |
| 0.1 | 0.11 | 0.09 | 0.05 | |
| p < .001 | p < .001 | p < .001 | p < .001 | |
| Age (se) | −0.04 | −0.04 | −0.05 | −0.04 |
| 0 | 0 | 0 | 0 | |
| p < .001 | p < .001 | p < .001 | p < .001 | |
| Dementia Dx (se) | −1.27 | −0.15 | −0.13 | −0.09 |
| 0.12 | 0.01 | 0.01 | 0.03 | |
| p < .001 | p < .001 | p < .001 | p = 0.002 | |
| Conscientiousness (se) | 0.08 | −0.13 | −0.06 | −0.04 |
| 0.09 | 0.05 | 0.04 | 0.04 | |
| p = 0.196 | p = 0.007 | p = 0.091 | p = 0.124 | |
| Sex (se) | 0.02 | 0.15 | 0.31 | 0.08 |
| 0.06 | 0.05 | 0.05 | 0.06 | |
| p = 0.374 | p < .001 | p < .001 | p = 0.096 | |
| Race (se) | 0.68 | 0.41 | 0.50 | NA |
| 0.06 | 0.09 | 0.08 | NA | |
| p < .001 | p < .001 | p < .001 | NA | |
| Education (se) | 0.24 | 0.23 | 0.20 | 0.34 |
| 0.03 | 0.02 | 0.02 | 0.03 | |
| p < .001 | p < .001 | p < .001 | p < .001 | |
| Age x Conscientiousness (se) | 0.00 | 0.01 | 0.01 | 0.00 |
| 0 | 0 | 0 | 0 | |
| p = 0.33 | p < .001 | p < .001 | p = 0.315 | |
| Dementia x Conscientiousness (se) | −0.06 | −0.01 | −0.03 | −0.07 |
| 0.1 | 0.01 | 0.01 | 0.04 | |
| p = 0.267 | p = 0.304 | p < .001 | p = 0.065 | |
| Intercept Variance | 1.20 | 2.03 | 1.42 | 0.58 |
| Slope Variance | 0.00 | 0.00 | 0.00 | 0.00 |
| Int-Slope Cov | −0.06 | −0.08 | −0.06 | −0.01 |
| Residual(σ2) | 0.26 | 0.13 | 0.16 | 0.15 |
| Npeople | 736 | 1,233 | 1,470 | 694 |
| Ntotalobs | 2,906 | 10,216 | 14,474 | 3,199 |
| Log Likelihood | −2989 | −6597 | −10747 | −2669 |
Conscientiousness was not related to cognitive trajectories overall according to the meta-analytic summary (B = 0.00, 95% CI = [0.00,0.01], p = .188). Individual study results from these models can be found in Supplemental table S33.
According to the meta-analytic summary, conscientiousness was not associated with cognitive trajectories prior to dementia, (B = 0.00, 95% CI = [0.00,0.01], p = .154). There was heterogeneity in this effect (I2 = 85.24, Q = 21.51, df = 3, p =< .001). For individuals who were diagnosed with dementia, individuals higher in conscientiousness did not demonstrate a deviation from the overall cognitive trajectory (B = −0.03, 95% CI = [−0.05,0.00], p = .053). We did observe an effect in the ROS sample suggesting that individuals with higher conscientiousness experience a steeper decline post diagnosis, but this was not replicated across studies. There was not heterogeneity in this effect (I2 = 60.05, Q = 6.77, df = 3, p = .080). All told, these findings do not support our hypotheses for conscientiousness.
Agreeableness.
The following section reports the estimates for the associations of agreeableness with overall cognitive change and with change after dementia diagnosis. See Table 9 for the individual study model summaries. The individual studies all indicated that agreeableness was not associated with cognitive performance, and this was supported by the meta-analytic summary (B = 0.02, 95% CI = [−0.03,0.07], p = .374). There was not heterogeneity in this effect (I2 = 0.00, Q = 0.10, df = 2, p = .952).
Table 9:
Cognitive Trajectories by Agreeableness
| coef | EAS | ROS | SATSA |
|---|---|---|---|
| Intercept (se) | 0.44 | 0.35 | 0.33 |
| 0.1 | 0.09 | 0.05 | |
| p < .001 | p < .001 | p < .001 | |
| Age (se) | −0.04 | −0.05 | −0.04 |
| 0 | 0 | 0 | |
| p < .001 | p < .001 | p < .001 | |
| Dementia Dx (se) | −1.26 | −0.13 | −0.10 |
| 0.11 | 0.01 | 0.03 | |
| p < .001 | p < .001 | p = 0.002 | |
| Agreeableness (se) | 0.05 | 0.02 | 0.02 |
| 0.09 | 0.04 | 0.04 | |
| p = 0.295 | p = 0.326 | p = 0.265 | |
| Sex (se) | 0.00 | 0.32 | 0.10 |
| 0.06 | 0.05 | 0.06 | |
| p = 0.484 | p < .001 | p = 0.053 | |
| Race (se) | 0.67 | 0.47 | NA |
| 0.06 | 0.08 | NA | |
| p < .001 | p < .001 | NA | |
| Education (se) | 0.25 | 0.20 | 0.34 |
| 0.03 | 0.02 | 0.03 | |
| p < .001 | p < .001 | p < .001 | |
| Age x Agreeableness (se) | 0.00 | 0.00 | 0.00 |
| 0 | 0 | 0 | |
| p = 0.404 | p = 0.038 | p = 0.008 | |
| Dementia x Agreeableness (se) | −0.07 | −0.04 | 0.07 |
| 0.09 | 0.01 | 0.06 | |
| p = 0.205 | p < .001 | p = 0.117 | |
| Intercept Variance | 1.25 | 1.42 | 0.58 |
| Slope Variance | 0.00 | 0.00 | 0.00 |
| Int-Slope Cov | −0.06 | −0.06 | −0.01 |
| Residual(σ2) | 0.26 | 0.16 | 0.15 |
| Npeople | 736 | 1,465 | 701 |
| Ntotalobs | 2,906 | 14,418 | 3,206 |
| Log Likelihood | −2987 | −10699 | −2674 |
Agreeableness was not related to cognitive trajectories overall according to the meta-analytic summary (B = 0.00, 95% CI = [−0.01,0.00], p = .581). Individual study results from these models can be found in Supplemental table S40.
Agreeableness was not associated with cognitive trajectories prior to dementia according to both the individual study estimates and meta-analytic summary (B = 0.00, 95% CI = [−0.01,0.01], p = .972). There was not heterogeneity in this effect (I2 = 71.07, Q = 8.65, df = 2, p = .013). For individuals who were diagnosed with dementia, individuals higher in agreeableness did not demonstrate a deviation from the overall cognitive trajectory (B = −0.02, 95% CI = [−0.08,0.05], p = .624). There was not significant heterogeneity in this effect (I2 = 42.06, Q = 3.45, df = 2, p = .178).
Discussion
The current study examined associations between the Big Five personality traits and cognitive ability/decline among individuals both with and without dementia. Our hypotheses and analysis plan incorporated the compression of morbidity theoretical framework, which posits that some individuals maintain a high level of function throughout most of later life followed by a rapid decline only near the end of life. Using this framework as a guide, we predicted that certain individuals would only experience a sharp downturn in cognitive function (or steep decline) after they were diagnosed with dementia, and that personality traits would be associated with this post-diagnosis decline. The analyses were completed using the methodological approach of coordinated data analysis, in which harmonized scripts were used to wrangle, clean, and model data from four independent datasets. The results from each dataset were then synthesized using random effects meta-analysis. This paper has achieved three goals, one substantive and two methodological. First, we estimated the extent of associations among personality traits and cognitive aging outcomes. Second, we provided evidence for the replicability of these estimates. And third, we demonstrated the utility of the registered report format for secondary longitudinal data analysis.
Across these four studies, results are based on almost 3 decades of cognitive assessments among over 5,000 participants, nearly a fifth of whom developed dementia over the course of the study. We found that neuroticism and openness to experience were associated with overall cognitive function. Specifically, we found that higher neuroticism was associated with worse cognitive function and openness was associated with better cognitive function, which is consistent with prior work (Curtis et al., 2015; Luchetti et al., 2016). Conscientiousness, agreeableness, and extraversion were not associated with cognitive function. We predicted that neuroticism, openness, and conscientiousness would be associated, and also indicated that our analyses for extraversion and agreeableness were exploratory. We did find a significant effect of conscientiousness on cognition for one study (MAP) but this effect was not replicated across studies.
Consistent with the rich cognitive aging literature (e.g., (Bäckman et al., 2005; Mella et al., 2018), we found that global cognition declines significantly over time, and this was replicated consistently across all four studies. Individuals who were diagnosed with dementia deviated from the overall cognitive trajectory within each sample after dementia diagnosis, demonstrating a steepening of cognitive among participants with dementia. Curiously, while the individual study estimates for post dementia cognitive slope were all statistically significant, the meta-analytic summary was not. We suspect that this was due, in part, to the fact that the slope estimate for EAS is inflated due to lower power. In EAS, only 6% of the sample developed dementia, and participants were dropped from the study one year after diagnosis unless they signed an autopsy consent, resulting in fewer measurement occasions with which to model change than desired. The overall pattern is consistent with the other three studies, and overall we conclude that individuals who were diagnosed with dementia do indeed experience steeper cognitive decline.
The test of whether personality traits account for individual differences in cognitive decline (consistent with lifespan developmental theory (Baltes, 1987; Baltes, Lindenberger, & Staudinger, 2006)) yielded mostly null results. This was the key substantive goal in the current study: to ascertain the extent of associations among personality traits and cognitive decline, both before and after dementia diagnosis. Our results show evidence that openness to experience is associated with steeper slopes after dementia diagnosis, which supported our hypotheses. This is consistent with the compression of morbidity theory, such that individuals higher in openness appear to retain better function leading up to dementia, but then decline more rapidly after diagnosis. According to the theoretical framework of the compression of morbidity, some individuals are expected to experience a steep decline post diagnosis after a period of fairly high function. These individuals, in theory, are able to optimize their high-functioning years, and only experience significant loss of function toward the end of life (Fries, 1980, 2005). Individual difference factors have been previously linked to maintained function (Opdebeeck et al., 2016), and with better function before the onset of decline (Wilson et al., 2019). Openness to experience, which is highly correlated with education, was associated with post dementia decline, even after accounting for the effect of education on cognition.
These findings can also be interpreted in light of the health behaviors models of personality (Friedman; 2000; Smith, 2006; Graham et al., 2020). According to this framework, the associations among personality and health outcomes in later adulthood can be partially attributed to the salubrious (or deleterious) behaviors that individuals with high levels of certain traits are likely to engage in, which then influences their health. In the case of the current study, individuals who were higher in openness are typically more educated, and may have been engaging in cognitive stimulating behaviors throughout middle and later adulthood which helped them maintain a higher level of cognitive function and slower decline into dementia, but faster decline after diagnosis. Our finding that low neuroticism was associated with better overall cognition is consistent with prior literature suggesting that having lower anxiety, less intrusive thinking and other characteristics associated with neuroticism, may help individuals be less cognitively vulnerable (Graham et al., 2021). Prior work has shown that neuroticism is associated with higher levels of perceived stress (Jiang et al., 2017). Perceived stress predicts the onset of amnestic mild cognitive impairment and is associated with cognitive decline in the EAS (Katz et al., 2016; Jiang et al., 2017). While the current study did not explicitly test underlying behavioral mechanisms, we recommend that future studies explore whether specific behavioral factors can explain the associations between openness to experience and compression of morbidity.
Limitations and Constraints on Generality.
A number of limitations should be noted in the current study. First, the studies used in this coordinated analysis were not fully representative. The four datasets were from the U.S. and Sweden, and had relatively high racial homogeneity (i.e., mostly white); (Henrich, Heine, & Norenzayan, 2010). This means that our results may not generalize to other cultures, less-industrialized countries, or to under-represented minorities. Much more research is needed to better understand patterns of cognitive aging within these populations, as well as whether personality traits can account for variation in these trajectories (Weuve et al., 2018b). Additionally, two of the four studies had relatively few dementia cases (~3-5% of the total sample), with relatively few measurement occasions of cognition after diagnosis. As such these studies were somewhat low powered to detect changes in cognitive function after diagnosis. We are fairly confident that, given the consistency of our null findings across these datasets, personality is not associated with overall cognitive decline, whether before or after incident dementia. We recommend that this line of inquiry continue, and researchers should interrogate these same questions using higher power samples, as the data come available, and test the robustness of these associations using other analytic or methodological approaches.
All told, we recommend that future studies continue to ask these research questions using more diverse samples and samples from non-Western countries. Investigators should attempt to replicate our findings as studies add measurement occasions (in particular, additional occasions after dementia diagnosis), and more participants are diagnosed with dementia. The current study also focused on global cognition, which was a composite of harmonized common tests across the four studies. Future work should seek to test whether these associations are detectable within specific domains of cognition (e.g. episodic memory, reaction time, reasoning ability, verbal fluency). Additionally, we recommended that investigators conduct new coordinated analyses examining whether traits are associated with risk of and time to dementia. Lastly, to further interrogate the compression of morbidity theory, we recommend that a future study aligns participants at time of death, as well as using change point analysis to test alternative models.
We believe that the format of this paper is of high importance to the cognitive aging literature. To be specific, we coordinated identical complex models across multiple independent datasets, thereby providing evidence for the replicability of these findings (Weston et al., 2019). In a prior era of psychological science, these results may have become victims of the file drawer. However, as a registered report, this paper was accepted in-principle based on the peer-reviewed theoretical framework and detailed analysis plan. This allows null findings to enter into the scientific record more easily, which decreases publication bias, lowers the risk of false positives, and increases the overall credibility of this research area. This paper in particular shows the usefulness adopting the registered reports for existing longitudinal data, and for coordinated data analysis.
Conclusion.
Our results indicated that low neuroticism and high openness were associated with better cognitive function, and this replicates prior work. Most personality traits were not associated with overall cognitive decline, or with decline before or after dementia diagnosis. The exception to this was for openness to experience, which was associated with a steeper decline in cognition post dementia diagnosis. Using coordinated data analysis with random effects meta-analysis, we replicated these findings across four independent longitudinal studies. This study is among the first to rigorously examine whether personality is associated with cognitive aging outcomes using this coordinated analysis approach. This paper joins a growing body of literature (Stephan, Sutin, Luchetti, & Terracciano, 2020; Sutin et al., 2019) seeking to increase the replicability, generalizability, and credibility of research into individual differences in cognitive aging and dementia.
Supplementary Material
Highlights.
Low Neuroticism and High Openness were associated with high cognitive function.
Openness was associated with steeper cognitive decline after dementia diagnosis.
Models were coordinated and harmonized across 4 independent longitudinal studies.
Acknowledgments
All co-authors contributed to the development of this manuscript. Co-A’s James, Mroczek, Bennett, Reynolds, Pedersen, Lipton, Bennett, Wilson, Boyle, & Katz all helped with the study conceptualization. Co-A’s James, Jackson, Bennett, and Mroczek assisted with the model development. Co-A’s Beam, Luo, Willroth, Jackson, Bryan, and Graham assisted with data analysis. All authors have contributed to the preparation and revision of this manuscript. Funding support for this project was provided by: P01AG03949, Czap Foundation, Sylvia & Leonard Marx Foundation; 3R01AG018436-18S1; K01AG050823, R01AG017917, P30AG010161.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aartsen MJ, Smits CHM, Tilburg T van, Knipscheer KCPM, & Deeg DJH (2002). Activity in older adults: Cause or consequence of cognitive functioning? A longitudinal study on everyday activities and cognitive performance in older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 57(2), P153–P162. 10.1093/geronb/57.2.P153 [DOI] [PubMed] [Google Scholar]
- Aust F, & Barth M (2018). papaja: Create APA manuscripts with R Markdown. Retrieved from https://github.com/crsh/papaja
- Baltes PB (1987). Theoretical propositions of life-span developmental psychology: On the dynamics between growth and decline. Developmental Psychology, 23(5), 611–626. [Google Scholar]
- Baltes PB, Lindenberger U, & Staudinger U (2006). Life span theory in developmental psychology. In Damon W & Lerner R (Eds.), Handbook of child psychology theoretical models of human development (pp. 569–595). New Jersey. [Google Scholar]
- Bates D, Mächler M, Bolker B, & Walker S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Bäckman L, Jones S, Berger A-K, Laukka EJ, & Small BJ (2005). Cognitive impairment in preclinical alzheimer’s disease: A meta-analysis. Neuropsychology, 19(4), 520–531. 10.1037/0894-4105.19A520 [DOI] [PubMed] [Google Scholar]
- Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, & Schneider JA (2018). Religious Orders Study and Rush Memory and Aging Project. Journal of Alzheimer’s Disease, 64(s1), S161–S189. 10.3233/JAD-179939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, & Wilson RS (2012). Overview and Findings from the Religious Orders Study. Current Alzheimer Research, 628–646. 10.2174/156720512801322573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, & Wilson RS (2012). Overview and findings from the Rush Memory and Aging Project. Current Alzheimer Research, 9(6), 646–663. 10.2174/156720512801322663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JP, & Rothstein HR (2010). A basic introduction to fixed-effect and random-effects models for meta-analysis. Research Synthesis Methods, 1(2), 97–111. 10.1002/jrsm.12 [DOI] [PubMed] [Google Scholar]
- Borenstein M, Higgins JP, Hedges LV, & Rothstein HR (2017). Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Research Synthesis Methods, 8(1), 5–18. [DOI] [PubMed] [Google Scholar]
- Borland E, Stomrud E, Westen D van, Hansson O, & Palmqvist S (2020). The age-related effect on cognitive performance in cognitively healthy elderly is mainly caused by underlying ad pathology or cerebrovascular lesions: Implications for cutoffs regarding cognitive impairment. Alzheimer’s Research & Therapy, 12(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman BP, Duberstein PR, & Lyness JM (2007). Personality traits, education, and health-related quality of life among older adult primary care patients. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 62(6), P343–P352. [DOI] [PubMed] [Google Scholar]
- Contador I, Bermejo-Pareja F, Pablos DL, Villarejo A, & Benito-León J (2017). High education accelerates cognitive decline in dementia: A brief report from the population-based NEDICES cohort. Dementia & Neuropsychologia, 11(3), 297–300. 10.1590/1980-57642016dn11-030012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, & McCrae R (1989). NEO five-factor inventory (neo-ffi). Odessa, FL: Psychological Assessment Resources, 3. [Google Scholar]
- Costa PT, & McCrae RR (1985). The neo personality inventory. Psychological Assessment Resources; Odessa,FL. [Google Scholar]
- Costa PT, & McCrae RR (1992). Professional manual: Revised neo personality inventory (neo-pi-r) and neo five-factor inventory (neo-ffi). Odessa, FL: Psychological Assessment Resources, 61. [Google Scholar]
- Curtis RG, Windsor TD, & Soubelet A (2015). The relationship between big-5 personality traits and cognitive ability in older adults–a review. Aging, Neuropsychology, and Cognition, 22(1), 42–71. [DOI] [PubMed] [Google Scholar]
- Duberstein PR, Chapman BP, Tindle HA, Sink KM, Bamonti P, Robbins J, … Franks P (2011). Personality and risk for Alzheimer’s disease in adults 72 years of age and older: A 6-year follow-up. Psychology and Aging, 26(2), 351–362. 10.1037/a0021377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck HJ (1975). Manual of the eysenck personality questionnaire (junior and adult). Hodder; Stoughton London. [Google Scholar]
- Friedman HS. Long-term relations of personality and health: Dynamisms, mechanisms, tropisms. Journal of Personality. 2000;68:1089–1107. doi: 10.1111/1467-6494.00127. [DOI] [PubMed] [Google Scholar]
- Fries JF (1980). Aging, Natural Death, and the Compression of Morbidity. New England Journal of Medicine, 303(3), 130–135. [DOI] [PubMed] [Google Scholar]
- Fries JF (2005). Frailty, heart disease, and stroke: The compression of morbidity paradigm. American Journal of Preventive Medicine, 29(5), 164–168. 10.1016/j.amepre.2005.07.004 [DOI] [PubMed] [Google Scholar]
- Fries JF, Bruce B, & Chakravarty E (2011). Compression of morbidity 1980–2011: A focused review of paradigms and progress. Journal of Aging Research, 2011. 10.4061/2011/261702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatz M, Pedersen NL, Berg S, Johansson B, Johansson K, Mortimer JA, … Ahlbom A (1997). Heritability for alzheimer’s disease: The study of dementia in swedish twins. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 52(2), M117–M125. 10.1093/gerona/52A.2.M117 [DOI] [PubMed] [Google Scholar]
- Goldberg LR (1992). The development of markers for the Big-Five factor structure. Psychological Assessment, 4(1), 26–42. [Google Scholar]
- Graham EK, Rutsohn JP, Turiano NA, Bendayan R, Batterham PJ, Gerstorf D, … Mroczek DK (2017). Personality predicts mortality risk: An integrative data analysis of 15 international longitudinal studies. Journal of Research in Personality, 70, 174–186. 10.1016/j.jrp.2017.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham EK, Weston SJ, Turiano NA, Aschwanden D, Booth T, Harrison F, … Mroczek DK. Is Healthy Neuroticism Associated with Health Behaviors? A Coordinated Integrative Data Analysis. Collabra Psychol. 2020;6(1):32. doi: 10.1525/collabra.266. Epub 2020 Jul 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham EK, James BD, Jackson KL, Willroth EC, Boyle P, Wilson R, … & Mroczek DK (2021). Associations between personality traits and cognitive resilience in older adults. The Journals of Gerontology: Series B, 76(1), 6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham EK, Willroth EC, Piccinin A, Weston SJ, Muniz G, Clouston SAP, Hofer SM, Mroczek DK (in press). Coordinated data analysis: An approach to knowledge accumulation in the lifespan developmental psychological sciences. Psychology and Aging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P, MacLeod CJ (2016). “simr: an R package for power analysis of generalised linear mixed models by simulation.” Methods in Ecology and Evolution, 7(4), 493–498. doi: 10.1111/2041-210X.12504, https://CRAN.R-project.org/package=simr. [DOI] [Google Scholar]
- Grober E, Lipton RB, Hall CB, & Crystal H (2000). Memory impairment on free and cued selective reminding predicts dementia. Neurology, 54(4), 827–832. [DOI] [PubMed] [Google Scholar]
- Hall CB, Derby C, LeValley A, Katz MJ, Verghese J, & Lipton RB (2007). Education delays accelerated decline on a memory test in persons who develop dementia. Neurology, 69(17), 1657–1664. 10.1212/01.wnl.0000278163.82636.30 [DOI] [PubMed] [Google Scholar]
- Hall CB, Lipton R, Sliwinski M, Katz M, Derby C, & Verghese J (2009). Cognitive activities delay onset of memory decline in persons who develop dementia. Neurology, 73(5), 356–361. 10.1212/WNL.0b013e3181b04ae3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson SE, Goldberg LR, Vogt TM, & Dubanoski JP (2007). Mechanisms by which childhood personality traits influence adult health status: Educational attainment and healthy behaviors. Health Psychology, 26(1), 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich J, Heine SJ, & Norenzayan A (2010). The weirdest people in the world? Behavioral and Brain Sciences, 33(2-3), 61–83. 10.1017/S0140525X0999152X [DOI] [PubMed] [Google Scholar]
- Hill PL, & Roberts BW (2011). The role of adherence in the relationship between conscientiousness and perceived health. Health Psychology, 30(6), 797–804. 10.1037/a0023860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer SM, & Piccinin AM (2009). Integrative data analysis through coordination of measurement and analysis protocol across independent longitudinal studies. Psychological Methods, 14(2), 150–164. 10.1037/a0015566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JM, Seng EK, Zimmerman ME, Sliwinski M, Kim M, & Lipton RB (2017). Evaluation of the reliability, validity, and predictive validity of the subscales of the perceived stress scale in older adults. Journal of Alzheimer’s Disease, 59(3), 987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz MJ, Derby CA, Wang C, Sliwinski MJ, Ezzati A, Zimmerman ME, … & Lipton RB (2016). Influence of perceived stress on incident amnestic mild cognitive impairment: Results from the Einstein Aging Study. Alzheimer disease and associated disorders, 30(2), 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz MJ, Lipton RB, Hall CB, Zimmerman ME, Sanders AE, Verghese J, … Derby CA (2012). Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and Alzheimer dementia in blacks and whites: A report from the Einstein Aging Study. Alzheimer Disease & Associated Disorders, 26(4), 335–343. 10.1097/WAD.0b013e31823dbcfc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB (2017). “lmerTest Package: Tests in Linear Mixed Effects Models.” Journal of Statistical Software, 82(13), 1–26. doi: 10.18637/jss.v082.i13. [DOI] [Google Scholar]
- Luchetti M, Terracciano A, Stephan Y, & Sutin AR (2016). Personality and Cognitive Decline in Older Adults: Data From a Longitudinal Sample and Meta-Analysis. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 71(4), 591–601. 10.1093/geronb/gbu184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipnicki DM, Makkar SR, Crawford JD, Thalamuthu A, Kochan NA, Lima-Costa MF, … Sachdev PS; for Cohort Studies of Memory in an International Consortium (COSMIC). Determinants of cognitive performance and decline in 20 diverse ethno-regional groups: A COSMIC collaboration cohort study. PLoS Med 2019. July 23;16(7):e1002853. doi: 10.1371/journal.pmed.1002853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarrey AC, An Y, Kitner-Triolo MH, Ferrucci L, & Resnick SM (2016). Sex differences in cognitive trajectories in clinically normal older adults. Psychology and Aging, 31(2), 166. 10.1037/pag0000070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mella N, Fagot D, Renaud O, Kliegel M, & De Ribaupierre A (2018). Individual differences in developmental change: Quantifying the amplitude and heterogeneity in cognitive change across old age. Journal of Intelligence, 6(1), 1–14. 10.3390/jintelligence6010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mroczek DK, & Spiro A (2007). Personality change influences mortality in older men. Psychological Science, 18(5), 371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mroczek DK, Spiro A, & Turiano NA (2009). Do health behaviors explain the effect of neuroticism on mortality? Longitudinal findings from the VA Normative Aging Study. Journal of Research in Personality, 43(4), 653–659. 10.1016/j.jrp.2009.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opdebeeck C, Martyr A, & Clare L (2016). Cognitive reserve and cognitive function in healthy older people: A meta-analysis. Aging, Neuropsychology, and Cognition, 23(1), 40–60. 10.1080/13825585.2015.1041450 [DOI] [PubMed] [Google Scholar]
- Pedersen NL, McClearn GE, Plomin R, Nesselroade JR, Berg S, & DeFaire U (1991). The Swedish Adoption Twin Study of Aging: An update. Acta Geneticae Medicae et Gemellologiae: Twin Research, 40(1), 7–20. 10.1017/S0001566000006681 [DOI] [PubMed] [Google Scholar]
- R Core Team. (2019). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Retrieved from https://www.R-project.org/ [Google Scholar]
- Rönnlund M, Nyberg L, Bäckman L, & Nilsson L-G (2005). Stability, Growth, and Decline in Adult Life Span Development of Declarative Memory: Cross-Sectional and Longitudinal Data From a Population-Based Study. Psychology and Aging, 20(1), 3–18. 10.1037/0882-7974.20.1.3 [DOI] [PubMed] [Google Scholar]
- Salthouse TA (2009). When does age-related cognitive decline begin? Neurobiology of Aging, 30(4), 507–514. 10.1016/j.neurobiolaging.2008.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, & Stern Y (2004). Cognitive reserve: Implications for diagnosis and prevention of Alzheimers disease. Current Neurology and Neuroscience Reports, 4(5), 374–380. 10.1007/s11910-004-0084-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Albert S, Manly J, & Stern Y (2006). Education and rates of cognitive decline in incident alzheimer’s disease. Journal of Neurology, Neurosurgery & Psychiatry, 77(3), 308–316. 10.1136/jnnp.2005.072306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie KW (1989). Individual differences in rate of cognitive change in adulthood. In Bengston VL & Schaie KW (Eds.), The course of later life (pp. 65–85). New York. [Google Scholar]
- Simons DJ, Shoda Y, & Lindsay DS (2017). Constraints on Generality (COG): A Proposed Addition to All Empirical Papers. Perspectives on Psychological Science, 12(6), 1123–1128. 10.1177/1745691617708630 [DOI] [PubMed] [Google Scholar]
- Smith TW. Personality as risk and resilience in physical health. Current Directions in Psychological Science. 2006;15:227–231. doi: 10.1111/j.1467-8721.2006.00441.x. [DOI] [Google Scholar]
- Soubelet A, & Salthouse TA (2011). Personalitycognition relations across adulthood. Developmental Psychology, 47(2), 303–310. 10.1037/a0021816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan Y, Sutin AR, Luchetti M, & Terracciano A (2020). Personality and memory performance over twenty years: Findings from three prospective studies. Journal of Psychosomatic Research, 128, 109885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y (2012). Cognitive reserve in ageing and alzheimer’s disease. The Lancet Neurology, 11(11), 1006–1012. 10.1016/S1474-4422(12)70191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin AR, Stephan Y, Damian RI, Luchetti M, Strickhouser JE, & Terracciano A (2019). Five-factor model personality traits and verbal fluency in 10 cohorts. Psychology and Aging, 34(3), 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, Sutin AR, An Y, O’Brien RJ, Ferrucci L, Zonderman AB, & Resnick SM (2014). Personality and risk of Alzheimer’s disease: New data and meta-analysis. Alzheimer’s & Dementia, 10(2), 179–186. 10.1016/jjalz.2013.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turiano NA, Chapman BP, Agrigoroaei S, Infurna FJ, & Lachman M (2014). Perceived control reduces mortality risk at low, not high, education levels. Health Psychology, 33(8), 883–890. 10.1037/hea0000022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turiano NA, Whiteman SD, Hampson SE, Roberts BW, & Mroczek DK (2012). Personality and substance use in midlife: Conscientiousness as a moderator and the effects of trait change. Journal of Research in Personality, 46(3), 295–305. 10.1016/jjrp.2012.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viechtbauer W (2010). Conducting meta-analyses in R with the metafor package. Journal of Statistical Software, 36(3), 1–48. Retrieved from http://www.jstatsoft.org/v36/i03/ [Google Scholar]
- Wechsler D (1997). Weschsler adult intelligence scale-iii. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Weston SJ, & Jackson JJ (2015). Identification of the healthy neurotic: Personality traits predict smoking after disease onset. Journal of Research in Personality, 54(C), 61–69. 10.1016/jjrp.2014.04.008 [DOI] [Google Scholar]
- Weston SJ, Graham EK, & Piccinin A (2019). Coordinated data analysis: A new method for the study of personality and health. In Hill P & Allemand M (Eds.), Personality and healthy aging in adulthood. Springer Nature. 10.31234/osf.io/k9up8 [DOI] [Google Scholar]
- Weston SJ, Hill PL, & Jackson JJ (2014). Personality Traits Predict the Onset of Disease. Social Psychological and Personality Science, 6(3), 309–317. 10.1177/1948550614553248 [DOI] [Google Scholar]
- Weuve J, Barnes LL, Leon C. F. M. de, Rajan KB, Beck T, Aggarwal NT, … Evans DA (2018a). Cognitive aging in black and white americans: Cognition, cognitive decline, and incidence of alzheimer disease dementia. Epidemiology (Cambridge, Mass.), 29(1), 151. 10.1097/EDE.0000000000000747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J, Barnes LL, Leon C. F. M. de, Rajan KB, Beck T, Aggarwal NT, … Evans DA (2018b). Cognitive aging in black and white americans: Cognition, cognitive decline, and incidence of alzheimer disease dementia. Epidemiology (Cambridge, Mass.), 29(1), 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H (2016). Ggplot2: Elegant graphics for data analysis. Springer-Verlag; New York. Retrieved from https://ggplot2.tidyverse.org [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, & Bennett DA (2002). Individual differences in rates of change in cognitive abilities of older persons. Psychology and Aging, 17(2), 179–193. 10.1037/0882-7974.17.2T79 [DOI] [PubMed] [Google Scholar]
- Wilson RS, Boyle PA, Yu L, Barnes LL, Sytsma J, Buchman AS, … Schneider JA (2015a). Temporal course and pathologic basis of unawareness of memory loss in dementia. Neurology, 85(11), 984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Boyle PA, Yu L, Segawa E, Sytsma J, & Bennett DA (2015b). Conscientiousness, dementia related pathology, and trajectories of cognitive aging. Psychology and Aging, 30(1), 74–82. 10.1037/pag0000013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Yu L, Lamar M, Schneider JA, Boyle PA, & Bennett DA (2019). Education and cognitive reserve in old age. Neurology, 92(10), e1041–e1050. 10.1212/WNL.0000000000007036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Boyle P, Wilson RS, Segawa E, Leurgans S, De Jager PL, & Bennett DA (2012). A random change point model for cognitive decline in alzheimer’s disease and mild cognitive impairment. Neuroepidemiology, 39(2), 73–83. 10.1159/000339365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit AR, Hall CB, Lipton RB, Katz MJ, Muniz-Terrera G. Identification of Heterogeneous Cognitive Subgroups in Community-Dwelling Older Adults: A Latent Class Analysis of the Einstein Aging Study. J Int Neuropsychol Soc. 2018. May;24(5):511–523. doi: 10.1017/S135561771700128X. Epub 2018 Jan 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


