Abstract
The incretin effect—the amplification of insulin secretion after oral vs intravenous administration of glucose as a mean to improve glucose tolerance—was suspected even before insulin was discovered, and today we know that the effect is due to the secretion of 2 insulinotropic peptides, glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1). But how important is it? Physiological experiments have shown that, because of the incretin effect, we can ingest increasing amounts of amounts of glucose (carbohydrates) without increasing postprandial glucose excursions, which otherwise might have severe consequences. The mechanism behind this is incretin-stimulated insulin secretion. The availability of antagonists for GLP-1 and most recently also for GIP has made it possible to directly estimate the individual contributions to postprandial insulin secretion of a) glucose itself: 26%; b) GIP: 45%; and c) GLP-1: 29%. Thus, in healthy individuals, GIP is the champion. When the action of both incretins is prevented, glucose tolerance is pathologically impaired. Thus, after 100 years of research, we now know that insulinotropic hormones from the gut are indispensable for normal glucose tolerance. The loss of the incretin effect in type 2 diabetes, therefore, contributes greatly to the impaired postprandial glucose control.
Keywords: glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), hormone antagonists, hormone coagonists, exendin 9-39
Bayliss and Starling, who in 1902 discovered secretin (1), the very first hormone, were convinced that not only the exocrine, but also the endocrine secretion from the pancreas, discovered by von Mering and Minkowski in 1889 (2), was regulated by intestinal factors, and both they and other scientists investigated the hypoglycemic effects of intestinal extracts and secretin preparations. Thus, the incretin concept, the idea that the gut regulates glucose levels via effects on the endocrine pancreas, is older than insulin itself. Although Zunz and La Barre, like Bayliss and Starling, were convinced that secretin was the most important hypoglycemic substance (3), they nevertheless tried to distinguish between “excretins” (stimulating the exocrine secretion from the pancreas) and “incretins” with predominant hypoglycemic effects. In those days, the physiologists made many impressive and daring (and ethically problematic) experiments such as anastomosing a pancreatic vein from a donor dog to the jugular vein of a recipient dog, to demonstrate that blood glucose falls in the latter after injection of a secretin preparation into the donor dog, but it was only after the development of the radioimmunoassay for insulin (4) that it became possible to quantify and determine the importance of the incretin effect for insulin secretion. In 1964, McIntyre and colleagues (5) reported that intestinal administration of glucose elicited a much greater insulin response than intravenous (IV) injections and they correctly deduced that intestinal factors, incretins, were responsible. In 1971, Brown and Dryburgh had sequenced a new peptide, gastric inhibitory polypeptide, GIP, isolated from impurities of cholecystokinin on the basis of its inhibitory effects on acid secretion from canine gastric pouches (6), but in 1973 Dupre and Brown identified the insulinotropic effects of GIP (7) (which, because the inhibitory effects on gastric secretion turned out not to be physiologically relevant, was renamed “glucose-dependent insulinotropic polypeptide,” retaining the nice acronym, GIP). Many subsequent studies confirmed its insulinotropic actions, and in 1989, Nauck et al could show in mimicry experiments that GIP fulfilled rigorous criteria for being a physiologically important incretin hormone (8). Thus, GIP, infused intravenously in amounts that copied its plasma concentrations after oral glucose, had effects on insulin secretion that were similar to those elicited by the oral glucose.
But what about secretin? Secretin had been isolated and sequenced in Stockholm in 1970 by Mutt and colleagues (from extracts of intestines from 10 000 hogs) (9), and it was now possible to synthesize the peptide and develop radioimmunoassays. Infusion experiments clearly confirmed the old observations that secretin would stimulate insulin secretion (actually even in people with type 2 diabetes [10]), but the circulating concentrations of secretin were extremely low (1-2 pmol/L) and even after maximal stimulation with intraduodenal acid, they did not reach levels sufficient to stimulate insulin secretion (11). But there had to be other incretin(s). With the insulin radioimmunoassay, it was possible to quantify accurately the incretin effect by comparing insulin responses to IV and oral administrations of glucose given in amounts that resulted in identical glucose concentrations (isoglycemia), and it could be calculated that up to 70% of the response to oral glucose could be due to the incretin effect (12, 13). However, in animal experiments, immunoneutralization of GIP with potent antiserum samples did not eliminate the incretin effect (14), and in people with resections of different parts of the small intestine, there was no correlation between the incretin effect and the GIP responses (15). Clearly, something was missing. The missing hormone was glucagon-like peptide-1 (16), a product of intestinal expression of proglucagon, the precursor of the pancreatic hormone glucagon. The gene encoding proglucagon is also expressed in the so-called L cells, endocrine cells of the intestinal epithelium (17), where the precursor is processed to release 2 glucagon-containing peptides, glicentin and oxyntomodulin, and 2 glucagon-like peptides, GLP-1 and GLP-2 (18, 19). Of these products, oxyntomodulin and GLP-1 both are insulinotropic, but whereas the concentrations of oxyntomodulin normally are too low to stimulate insulin secretion (20), the 100-fold more potent GLP-1 provides a very powerful stimulus (21) and in mimicry experiments clearly qualified as a new incretin; actually, at first sight, more potent than GIP (22). Today, there is agreement that GLP-1 and GIP are the most important incretin hormones, and it was determined early on that in mice with deletions of the receptors for both GIP and GLP-1, very little incretin effect remained (23). However, it is also clear that these mouse experiments do not tell us much about the importance of the incretin effect. After oral glucose gavage, there are observable differences between the glucose excursions in animals with and without receptor deletions, but they are small and barely significant. In addition, it is possible that rodents, not the least mice, depend less on the incretin effect in their regulation of glucose homeostasis than humans and more on neural regulation (24, 25), and it is therefore questionable how much these experiments can tell us about human conditions.
The Importance of the Incretin Effect
So, how important is the incretin effect in people? This is really an important question, because one of the characteristics of type 2 diabetes is a more or less complete loss of the incretin effect (13). This raises the question whether type 2 diabetes is an incretin disease, although the loss of the incretin effect does not usually top the list of pathogenic factors. The importance of the incretin effect in healthy individuals can be illustrated in experiments in which the so-called gastrointestinally mediated glucose disposal (GIGD [26]) is determined. The approach is simple: One measures the total amount of IV glucose that is required to mimic the plasma excursions after a given oral dose and compares the two. Using this technique, Nauck et al (27) first showed that GIGD strongly depends on the amount of glucose ingested. Thus, with 25-g glucose given orally, an infusion of about 20 g of glucose was required to copy the plasma glucose response. However, with 50 g of glucose, it also took about 20 g to copy the curve, and with 75 g it also took 20 g to copy the curve. It follows that the plasma excursions after the 3 doses of oral glucose were virtually identical. In other words, the body is capable of disposing of orally ingested glucose so rapidly and efficiently that the plasma glucose excursions remain constant and low in spite of widely different amounts taken in. Thus, this gastrointestinal mechanism is capable of removing up to three-quarters of the absorbed glucose from the circulation. This shows how efficiently we normally regulate our postprandial glucose concentrations. But what is the mechanism behind this remarkable glucose disposal? it turns out to be insulin secretion, which increases progressively with increasing glucose doses (27). In other words, the incretin effect secures normal and healthy postprandial glucose excursions almost no matter how large the dosing is (125 g has also been investigated with similar results [28]). Funnily enough, the importance of the incretin effect was nearly missed in some early studies (29); it was shown that a 25-g dose of glucose resulted in almost the same insulin secretion (measured as C-peptide) whether it was given intravenously or orally, and it was concluded that the “incretin effect” was a hoax. But we now know that a) a dose of glucose as small as 25 g elicits a GIGD of only 20% (because it took 20 g of IV glucose to mimic the 25-g oral dose—see earlier), and b) IV administration gives a much larger plasma glucose response than oral dosing, ruining the principle of isoglycemia, and therefore the possibility to gauge the incretin response properly.
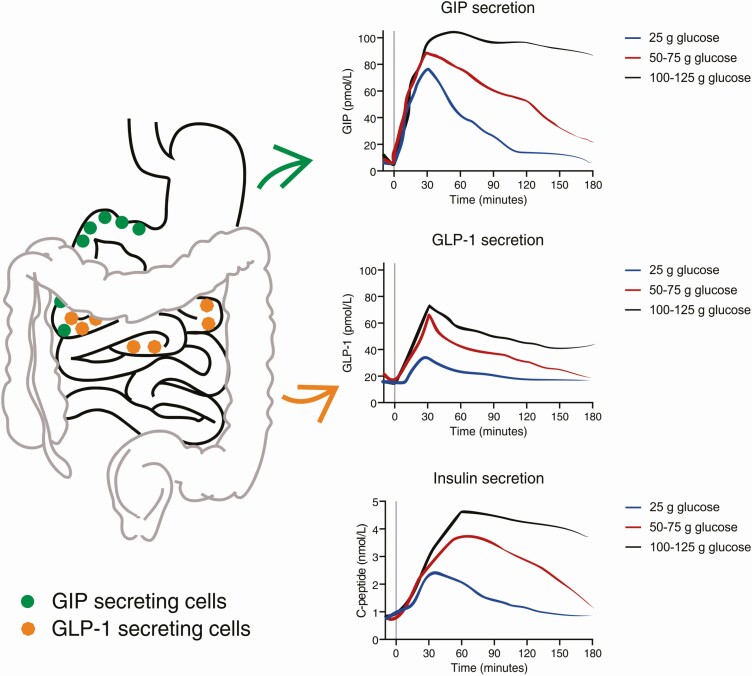
The incretin hormone responses were also determined in the cited dose-response experiments (Fig. 1) and show some interesting features (27, 28). GLP-1 secretion showed a dose response relationship that corresponded more or less with the insulin responses. GIP secretion was a bit different, showing an early rise to a peak value, which was more or less the same with all doses, and a subsequent plateau, the duration of which was proportional to the glucose dose. This profile probably reflects the gastric emptying of glucose, which is regulated so as to ensure a relatively constant emptying rate of nutrients, corresponding, in the case of glucose, to about 4 kcal/min (30, 31). Since this translates into a more or less constant exposure to glucose of the duodenal mucosa, from where most of the GIP is secreted, an enhanced secretion of GIP will be maintained as long as there is glucose left in the stomach. GLP-1 secretion occurs from slightly more distal gut segments, which means that most of the L cells are likely to escape stimulation because of early absorption of glucose. Actually, the grossly exaggerated GLP-1 responses to more distal glucose exposure in patients with gastric bypass operations (32) is another illustration of this. Both the L cells and the GIP-secreting K cells express the sodium-coupled glucose transporter, SGLT1, and their secretory responses can be demonstrated to depend on this transportation. In this way, the secretion of the incretin hormones becomes proportional to the rate of glucose absorption in the gut segment, where this occurs. (33, 34).
Figure 1.
Localization of glucose-dependent insulinotropic polypeptide (GIP)- and glucagon-like peptide-1 (GLP-1)-secreting cells in the gastrointestinal tract (left) and secretion profiles of GIP, GLP-1, and insulin in response to ingestion of low (25 g), medium (50-75 g) or high (100-125 g) amounts of glucose (right). Curves adapted from Nauck et al (27) and Bagger et al (28).
The conclusion is that normal postprandial glucose tolerance to a very large extent is determined by the incretin effect, which in turn is due to the secretion of the incretin hormones. Thus, the stimulatory effect of glucose alone on insulin secretion is far from sufficient. As briefly mentioned earlier, the incretin effect is very much reduced or frankly missing in patients with type 2 diabetes, and one may ask why that is. When it became possible to measure the plasma concentrations of GIP and GLP-1, it was natural to look for secretion of these hormones in diabetes. There are strong indications that the postprandial secretion of GLP-1 is impaired in type 2 diabetes (35); perhaps not the early phase of meal responses (36), but particularly the late phase of the response, and the impairment is probably more related to the negative influence of obesity than it is related to diabetes. However, it is also clear that impaired secretion is not the most important explanation for the decreased incretin effect. Rather, the insulinotropic action of both hormones is impaired (37). The impaired incretin effect is a very early event in type 2 diabetes, but it is the prevailing concept that the defect develops in response to diabetes rather than being a primary deficiency (38). More about this later.
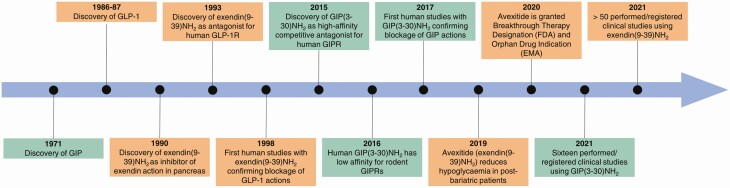
The evidence regarding the importance of the incretin hormones for glucose tolerance presented so far is based on the principles of the mimicry experiments: If we mimic the plasma profiles observed in vivo with infusions of glucose/hormones, do we get the same responses? Although such experiments represent necessary conditions for conclusions on causality, they are not sufficient. Other hormones could easily be involved and neural, renal, hepatic and, in fact, gut mechanisms could be overlooked. The double-knockout experiments referred to previously could be considered sufficient evidence (with the trivial reservation that the receptors were truly knocked out and that knockout did not result in compensatory adaptations, which is more likely than not), but were not carried out in the relevant species. To solve the problem, the application of hormone receptor antagonists would be a useful approach. Such antagonists must be acceptable for human use, which is a major hindrance, but this has recently become possible for both hormones (Fig. 2).
Figure 2.
Central events in the history of the 2 incretin receptor antagonists, exendin(9-39)NH2 and GIP(3-30)NH2.
Glucagon-Like Peptide-1 Receptor Antagonism
In 1992, Eng et al isolated a 39 amino acid peptide, exendin-4, from the saliva of the Gila monster (Heloderma suspectum) (39), which was capable of activating receptors om pancreatic acinar cells, but also found that a truncated version, exendin 9-39 (40), could antagonize the actions of exendin-4 (39). Very soon after its discovery, exendin-4 was demonstrate to be a full and potent agonist for the GLP-1 receptor, which had just been cloned, and the truncated form, exendin 9-39 (Ex-9), was demonstrated to antagonize the actions of exendin-4 as well as GLP-1 on the GLP-1 receptor (41) (see Fig. 2). Interestingly, exendin-4, which has about 50% sequence homology with mammalian GLP-1 (regarding the first 30 amino acids) is not the GLP-1 of the Gila monster—this poisonous lizard has its own GLP-1 (which is much closer to mammalian GLP-1) (42). Back to the antagonist: Two laboratories soon carried out infusion experiments in humans of Ex-9 to elucidate the role of the endogenous GLP-1 by blocking its receptor (43, 44). Regarding safety, these experiments went well, which is ironic considering that the use of synthetic exendin-4 (exenatide) for diabetes therapy was subsequently suspected of causing acute pancreatitis (45)—leading a Food and Drug Administration officer to remark in the New England Journal of Medicine (46) that this was an expected result considering the toxicity of being bitten by a Gila monster (the GLP-1 receptor agonists, including exendin-4, were later completely acquitted with respect to the pancreatitis suspicion [47]). Since then numerous experiments have been carried out with Ex-9. Indeed, it is currently being developed as a therapeutic agent (avexitide) to prevent reactive postprandial hypoglycemia after bariatric surgery, which appears to be due to exaggerated GLP-1 secretion and similarly exaggerated insulin responses, sufficient to cause temporary hypoglycemia (ClinicalTrials.gov identifier: NCT03373435). In fact, avexitide was granted breakthrough therapy designation by the Food and Drug Administration in 2019 for this indication (postbariatric hypoglycemia).
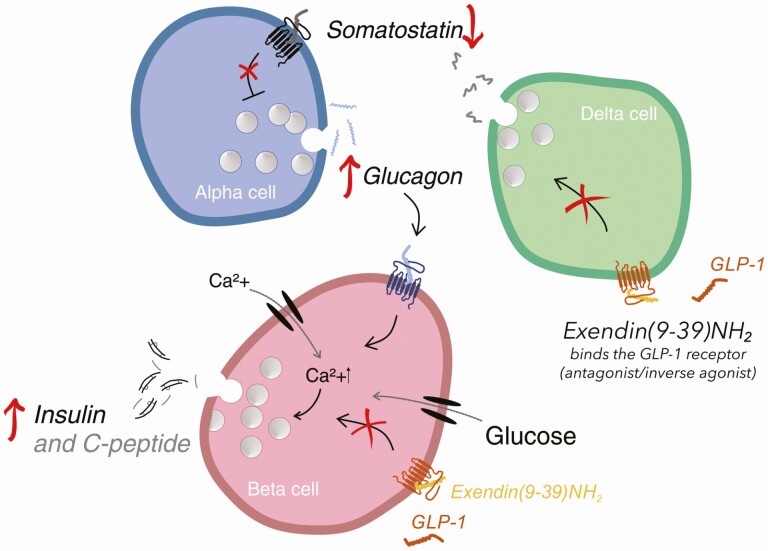
In human experiments involving intraduodenal glucose infusions, Ex-9 increases plasma glucose excursions, decreases insulin responses, and increases glucagon responses, which would be expected based on the actions of exogenous GLP-1 (43, 48). The results of Ex-9 infusion in the fasting state and during simple oral glucose administration are less easy to understand, however. The infusions invariably elevate glucose concentrations and increase glucagon concentrations (44), but insulin responses are more variable, and decreases as well as increases have been found. The increases in glucagon would be assumed to arise because of antagonism of GLP-1 receptors, but where? The α cells do not seem to express GLP-1 receptors (very few at least), and most people agree that the inhibition would be due to a relief of the paracrine inhibition within the islets by somatostatin produced by neighboring δ cells, which express stimulatory GLP-1 receptors (49-51). But in the fasting state there is not much GLP-1 secretion. It has been shown that Ex-9 may act as an inverse agonist (52, 53), inhibiting the activity of the receptor it binds to, and in that case it is conceivable that Ex-9 may stimulate glucagon secretion by inhibiting the tonic activity of the δ cells (because of some constitutive activity of the GLP-1 receptor) to which the α cells are coupled in a paracrine feedback loop (54). Stimulated insulin secretion after Ex-9 may then be due to a paracrine action of glucagon on the β cells via their glucagon receptors, potentiated by the increasing glucose levels (Fig. 3). This is not pure speculation: Glucose levels and glucagon concentration do increase during Ex-9 administration. In previously diabetic individuals who undergo gastric bypass surgery, Ex-9 invariably reduces meal-induced insulin responses and impairs glucose tolerance, while increasing GLP-1 responses further (55); the GLP-1 increase seems to be due to interference with another paracrine interaction, this time in the gut between L cells and somatostatin-producing δ cells or somatostatinergic neurons (56), which are known to be stimulated by the increased GLP-1 secretion. When this effect of GLP-1 is blocked by Ex-9, somatostatin release falls and GLP-1 secretion increases.
Figure 3.
Exendin(9-39)NH2 actions on pancreatic δ (delta), α (alpha), and β (beta) cells, which can result in increased insulin secretion. Glucagon-like peptide-1 (GLP-1) receptors are red, glucagon receptors are blue, and somatostatin receptors black. The yellow peptide is exendin(9-39)NH2, GLP-1 is red, glucagon is blue, and somatostatin black. Blockage of the GLP-1R on δ cells by exendin(9-39)NH2 prevents somatostatin inhibition of α cells. Increasing glucagon secretion may stimulate insulin secretion via the glucagon receptor in the β cells. Exendin(9-39)NH2 may also inhibit GLP-1 action on beta cells or act as inverse agonist.
What happens to GIP secretion during Ex-9 infusion? The answer is: nothing (57). This tells us that the K cells are not subject to a similar somatostatinergic regulation as the L cells, and that there is no “compensatory” increase in GIP secretion, when GLP-1 action is blocked.
Glucose-Dependent Insulinotropic Polypeptide Receptor Antagonism
Various methods for producing GIP antagonism have been implemented in animal experiments during previous decades, but only recently has it been possible to antagonize GIP action in humans (see Fig. 2). In a systematic search for antagonistic effects of truncations and substitutions of the GIP sequence, a peptide with a high affinity for the GIP receptors, which also turned out to be a potent competitive antagonist, was identified, namely GIP 3-30 NH2 (58, 59). A truncated form of GIP, GIP 1-30 NH2, which is a full and equipotent agonist of the GIP receptor, had been known to exist for some time (60), and because GIP, like GLP-1, is substrate for the proteolytic activity of dipeptidyl peptidase-4 (DPP-4), an N-terminally truncated form of this molecule, GIP 3-30NH2, was predicted to exist. GIP 1-30 NH2 is found in the circulation in very low picomolar concentrations (57), so the concentrations of endogenous GIP 3-30NH2 are likely to be similarly low. However, infused in sufficient quantities, the antagonist dose-dependently blocks all the actions of GIP to a very high degree (> 80%), be it on insulin secretion, stimulation of adipose tissue blood flow and triglyceride uptake, or inhibition of bone resorption (61, 62). Whereas Ex-9 works equally well in experimental animals and humans, the GIP system shows clear differences between species, necessitating the use of species-specific agonists and antagonists in studies of GIP receptor–mediated activities (59).
Combinations of Glucose-Dependent Insulinotropic Polypeptide and Glucagon-Like Peptide-1 Receptor Antagonism
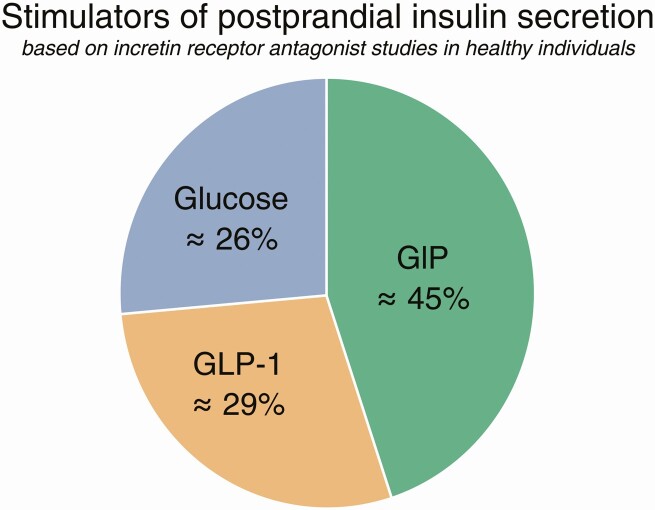
In a recent study (57), antagonists of GIP and GLP-1 were combined in an attempt to finally define the incretin actions of the 2 hormones during an oral glucose tolerance test in healthy volunteers. Indeed, and as expected, both of the antagonists significantly impaired glucose tolerance, and when given together their effects were additive, bringing the glucose responses into the range of pathologically impaired glucose tolerance (peak levels increasing by ~ 3 mmol/L and 2-hour values from 6.4 to 7.5 mmol/L), clearly confirming the importance of the incretin hormones for postprandial glucose tolerance. Their immediate effect on insulin secretion was difficult to gauge because of the increasing blood glucose concentrations (compared to the saline control infusion). However, when C-peptide responses (as a more accurate measure of β-cell secretion) were adjusted for the different glucose responses, a clear impairment of glucose-induced insulin secretion became apparent, more with the GIP antagonist GIP(3-30)NH2 than with Ex9, and again roughly additively with the combination of the 2 antagonists. In an accompanying editorial, Nauck and Meier calculated the individual contributions to the effects (63) and concluded that the incretin effect, deduced from the contributions of each of the components, was distributed with 26% from glucose alone (similar to the value found in traditional estimates of the incretin effect) and with 45% from GIP and 29% from GLP-1. These figures were subsequently confirmed by calculations from the individual data (Fig. 4) (64). Thus, it may be concluded that in healthy individuals, GIP is the most important incretin, perhaps consistent with the proximal location of the K cells (duodenum and proximal jejunum) and their immediate dependence on the outflow of carbohydrate from the stomach, whereas the GLP-1 effect sets in when ingested glucose reaches a bit farther down into the intestine. Indeed, after a gastric bypass operation, as alluded to earlier, the nutritional load is pushed farther down the small intestine, and the GLP-1 responses are huge (typically 10-fold increased), whereas GIP responses are unchanged, and almost all the increased insulin secretion disappears with Ex-9 (55). It should not be forgotten, as discussed previously, that Ex-9 is a difficult tool to work with; therefore, the quantitative data obtained may be somewhat misleading.
Figure 4.
Contribution of gut-derived factors to postprandial insulin secretion, expressed in per cent of the total C-peptide secretion (area under curve, 0-240 minutes) following ingestion of 75-g glucose orally with or without incretin receptor antagonist infusions in healthy individuals. Calculations performed by Gasbjerg et al (64). GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1.
The Incretins and Type 2 Diabetes
As mentioned, patients with type 2 diabetes have an impaired or absent incretin effect, and in an experiment in patients with rather long-standing diabetes, infusions of the 2 hormones in amounts recapitulating normal meal responses, there was absolutely no effect on insulin secretion during the conditions of a 15-mmol/L hyperglycemic clamp (65). In agreement with these findings, infusion of the GIP antagonist in patients with type 2 diabetes had no effect on postprandial glucose levels (66), whereas Ex-9 slightly increases postprandial glucose (67). Likewise, whereas even very high rates of GIP infusion had very little effects on insulin secretion and glucose turnover in additional individuals with type 2 diabetes (68), supraphysiological GLP-1 infusions were capable of restoring insulin secretion levels to values similar to those observed in healthy controls during glucose infusion alone (whereas glucose alone was similarly ineffective in the patients). The reason for this difference between the 2 incretin hormones is still unknown. In a careful dose-response study involving increasing infusion rates of GLP-1 on top of a step-wise clamping of plasma glucose at increasing levels, the slopes of the GLP-1 effects on the β-cell responsiveness to glucose (the latter calculated from cross-correlations of insulin secretion rates and glucose clamp levels) could be determined (69). This slope was clearly impaired in patients compared to controls, even if adjusted to the estimated maximal β-cell performance. This clearly illustrated the decreased responsiveness of the diabetic β cells to GLP-1, but it also showed that GLP-1, even at relatively low infusion rates, was capable of restoring β-cell function to the same levels as those observed in the controls given glucose alone (69). In other words, in patients with type 2 diabetes, supraphysiological administration of GLP-1 may improve β-cell secretion to levels similar to those of healthy controls in response to glucose alone (whose responses to combinations of glucose and GLP-1 would, of course, be much larger). This effect of GLP-1 forms the background for its therapeutic use in patients with type 2 diabetes, in whom a GLP-1 infusion may actually restore fasting glucose levels to completely normal levels (70). It must be emphasized, however, that the β-cell responses of the individual patient with type 2 diabetes depend strongly on the residual β-cell function, and in patients with very poor β-cell reserve, the effect of GLP-1 may not be sufficient to normalize glucose levels (71). Thus, in patients with fasting glucose levels between 7 and 10 mmol/L, a “standard” 1.2-pmol/kg × min infusion of GLP-1 was capable of completely normalizing glucose levels (72); in those with fasting glucose levels between 10 and 15 mmol/L, glucose levels were much improved and reached 6 to 7 mmol/L within 4 hours; and in those with fasting values greater than 15 mmol/L there was a decrease in glucose levels of up to 10 mmol/L, but in spite of extended infusion, levels did not fall below 8 mmol/L, indicating that even the slightest meal stimulus would bring glucose levels up again, so it would never be possible to cause a sufficient and lasting improvement in glycemic control in this way (72). In such a patient, a combination of basal insulin and GLP-1 works wonders (73).
As is evident from the process described earlier, the focus has been on the development of GLP-1 analogues for type 2 diabetes, whereas GIP was considered unattractive. Some experiments indicated that improved glycemic control in the patients might lead to recovery of some of the insulinotropic effects of GIP (74). But the truth is that these improvements were very small and far from levels that would be therapeutically relevant. However, more recently monomolecular GIP/GLP-1 coagonists have been developed, and one of these, tirzepatide, showed very strong antidiabetic properties in phase 2 studies, exceeding those of an established, long-acting GlP-1 receptor agonist (dulaglutide), and it was suggested that its effectiveness was due to a combination of the GIP and the GLP-1 effect (75). The coagonist also has strong inhibitory effects on appetite and food intake (and eventually body weight), something that has never been observed in human studies with acute GIP administration (and it has not yet been excluded that the effect could be due solely to GLP-1 receptor agonism). In contrast, in other experimental studies, GIP antagonists lowered body weight, particularly in combination with GLP-1 (76). The surprising effects of the coagonism are currently under intense investigation, and some studies indicate that differential internalization of the GIP and GLP-1 receptors after exposure to the coagonist may explain its unexpected effect (77). At any rate, the phase 3 studies with tirzepatide (78) support its suitability for therapy of type 2 diabetes (according to press releases from Eli Lilly), and future studies will probably show whether GIP agonism or antagonism is preferable for metabolic interventions.
However, the incretin story presented here clearly shows that in healthy individuals the β cells do not work alone—for normal glucose tolerance, the gastrointestinal tract and the incretin hormones are absolutely indispensable. Although not expected originally, the incretin system also plays an important role in the regulation of food intake, and the most recent research has shown that GLP-1 receptor agonists and surprisingly also GIP/GLP-1 receptor coagonists have powerful effects on the treatment of not only diabetes but also obesity. The incretins certainly have come of age.
Glossary
Abbreviations
- Ex-9
exendin 9-39
- GIGD
gastrointestinally mediated glucose disposal
- GIP
glucose-dependent insulinotropic polypeptide
- GLP-1
glucagon-like peptide-1
- IV
intravenous
Additional Information
Disclosures: J.J.H. appears on advisory boards for NovoNordisk and MSD, and J.J.H. and M.M.R. have collaborated with Lilly on coagonist projects. J.J.H. and M.M.R. are founders of Antag Pharmaceuticals. L.S.G. has nothing to disclose.
Data Availability
No new data have been included in this review.
References
- 1.Mering Jv, Minkowski O. Diabetes mellitus nach Pankreasexstirpation. Archiv f experiment Pathol u Pharmakol. 1890; 26: 371-387. [Google Scholar]
- 2. von Mering J, Minkowski O. Diabetes mellitus and pancreas extirpation. Arch Exp Path Pharmak. 1889;21:371-390. [Google Scholar]
- 3. Zunz E, La Barre J. Contributions a l’étude des variation physiologiques de la sécrétion interne de pancréas: relations entre les secretions externe et interne du pancréas. Archs Int Physiol Biochim. 1929;31(1):20-44. [Google Scholar]
- 4. Berson SA, Yalow RS. Quantitative aspects of the reaction between insulin and insulin-binding antibody. J Clin Invest. 1959;38(11):1996-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McIntyre N, Holdsworth CD, Turner DS. New interpretation of oral glucose tolerance. Lancet. 1964;2(7349):20-21. [DOI] [PubMed] [Google Scholar]
- 6. Brown JC, Dryburgh JR. A gastric inhibitory polypeptide. II. The complete amino acid sequence. Can J Biochem. 1971;49(8):867-872. [DOI] [PubMed] [Google Scholar]
- 7. Dupre J, Ross SA, Watson D, Brown JC. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab. 1973;37(5):826-828. [DOI] [PubMed] [Google Scholar]
- 8. Nauck M, Schmidt WE, Ebert R, et al. Insulinotropic properties of synthetic human gastric inhibitory polypeptide in man: interactions with glucose, phenylalanine, and cholecystokinin-8. J Clin Endocrinol Metab. 1989;69(3):654-662. [DOI] [PubMed] [Google Scholar]
- 9. Mutt V, Jorpes JE, Magnusson S. Structure of porcine secretin. The amino acid sequence. Eur J Biochem. 1970;15(3):513-519. [DOI] [PubMed] [Google Scholar]
- 10. Deckert T. Stimulation of insulin secretion by glucagon and secretin. Acta Endocrinol (Copenh). 1968;57(4):578-584. [DOI] [PubMed] [Google Scholar]
- 11. Fahrenkrug J, Schaffalitzky de Muckadell OB, Kühl C. Effect of secretin on basal- and glucose-stimulated insulin secretion in man. Diabetologia. 1978;14(4):229-234. [DOI] [PubMed] [Google Scholar]
- 12. Perley MJ, Kipnis DM. Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic subjects. J Clin Invest. 1967;46(12):1954-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29(1):46-52. [DOI] [PubMed] [Google Scholar]
- 14. Ebert R, Unger H, Creutzfeldt W. Preservation of incretin activity after removal of gastric inhibitory polypeptide (GIP) from rat gut extracts by immunoadsorption. Diabetologia. 1983;24(6):449-454. [DOI] [PubMed] [Google Scholar]
- 15. Lauritsen KB, Moody AJ, Christensen KC, Lindkaer Jensen S. Gastric inhibitory polypeptide (GIP) and insulin release after small-bowel resection in man. Scand J Gastroenterol. 1980;15(7):833-840. [DOI] [PubMed] [Google Scholar]
- 16. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409-1439. [DOI] [PubMed] [Google Scholar]
- 17. Eissele R, Göke R, Willemer S, et al. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest. 1992;22(4):283-291. [DOI] [PubMed] [Google Scholar]
- 18. Orskov C, Holst JJ, Knuhtsen S, Baldissera FG, Poulsen SS, Nielsen OV. Glucagon-like peptides GLP-1 and GLP-2, predicted products of the glucagon gene, are secreted separately from pig small intestine but not pancreas. Endocrinology. 1986;119(4):1467-1475. [DOI] [PubMed] [Google Scholar]
- 19. Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L, Habener JF. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem. 1986;261(25):11880-11889. [PubMed] [Google Scholar]
- 20. Holst JJ, Albrechtsen NJW, Gabe MBN, Rosenkilde MM. Oxyntomodulin: actions and role in diabetes. Peptides. 2018;100:48-53. [DOI] [PubMed] [Google Scholar]
- 21. Holst JJ, Orskov C, Nielsen OV, Schwartz TW. Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Lett. 1987;211(2):169-174. [DOI] [PubMed] [Google Scholar]
- 22. Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2(8571):1300-1304. [DOI] [PubMed] [Google Scholar]
- 23. Hansotia T, Baggio LL, Delmeire D, et al. Double incretin receptor knockout (DIRKO) mice reveal an essential role for the enteroinsular axis in transducing the glucoregulatory actions of DPP-IV inhibitors. Diabetes. 2004;53(5):1326-1335. [DOI] [PubMed] [Google Scholar]
- 24. Pacini G, Thomaseth K, Ahrén B. Contribution to glucose tolerance of insulin-independent vs. insulin-dependent mechanisms in mice. Am J Physiol Endocrinol Metab. 2001;281(4):E693-E703. [DOI] [PubMed] [Google Scholar]
- 25. Ahrén B, Pacini G. Glucose effectiveness: lessons from studies on insulin-independent glucose clearance in mice. J Diabetes Investig. Published online October 23, 2020. doi:10.1111/jdi.13446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hare KJ, Vilsbøll T, Holst JJ, Knop FK. Inappropriate glucagon response after oral compared with isoglycemic intravenous glucose administration in patients with type 1 diabetes. Am J Physiol Endocrinol Metab. 2010;298(4):E832-E837. [DOI] [PubMed] [Google Scholar]
- 27. Nauck MA, Homberger E, Siegel EG, et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab. 1986;63(2):492-498. [DOI] [PubMed] [Google Scholar]
- 28. Bagger JI, Knop FK, Lund A, Vestergaard H, Holst JJ, Vilsbøll T. Impaired regulation of the incretin effect in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96(3):737-745. [DOI] [PubMed] [Google Scholar]
- 29. Madsbad S, Kehlet H, Hilsted J, Tronier B. Discrepancy between plasma C-peptide and insulin response to oral and intravenous glucose. Diabetes. 1983;32(5):436-438. [DOI] [PubMed] [Google Scholar]
- 30. Horowitz M, Edelbroek MA, Wishart JM, Straathof JW. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia. 1993;36(9):857-862. [DOI] [PubMed] [Google Scholar]
- 31. Holst JJ, Gribble F, Horowitz M, Rayner CK. Roles of the gut in glucose homeostasis. Diabetes Care. 2016;39(6):884-892. [DOI] [PubMed] [Google Scholar]
- 32. Jørgensen NB, Jacobsen SH, Dirksen C, et al. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with Type 2 diabetes and normal glucose tolerance. Am J Physiol Endocrinol Metab. 2012;303(1):E122-E131. [DOI] [PubMed] [Google Scholar]
- 33. Tolhurst G, Reimann F, Gribble FM. Nutritional regulation of glucagon-like peptide-1 secretion. J Physiol. 2009;587(1):27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kuhre RE, Frost CR, Svendsen B, Holst JJ. Molecular mechanisms of glucose-stimulated GLP-1 secretion from perfused rat small intestine. Diabetes. 2015;64(2):370-382. [DOI] [PubMed] [Google Scholar]
- 35. Færch K, Torekov SS, Vistisen D, et al. GLP-1 response to oral glucose is reduced in prediabetes, screen-detected type 2 diabetes, and obesity and influenced by sex: the ADDITION-PRO study. Diabetes. 2015;64(7):2513-2525. [DOI] [PubMed] [Google Scholar]
- 36. Nauck MA, Vardarli I, Deacon CF, Holst JJ, Meier JJ. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: what is up, what is down? Diabetologia. 2011;54(1):10-18. [DOI] [PubMed] [Google Scholar]
- 37. Holst JJ, Knop FK, Vilsbøll T, Krarup T, Madsbad S. Loss of incretin effect is a specific, important, and early characteristic of type 2 diabetes. Diabetes Care. 2011;34(Suppl 2):S251-S257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Knop FK, Vilsbøll T, Højberg PV, et al. Reduced incretin effect in type 2 diabetes: cause or consequence of the diabetic state? Diabetes. 2007;56(8):1951-1959. [DOI] [PubMed] [Google Scholar]
- 39. Eng J, Kleinman WA, Singh L, Singh G, Raufman JP. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem. 1992;267(11):7402-7405. [PubMed] [Google Scholar]
- 40. Raufman JP, Singh L, Eng J. Exendin-3, a novel peptide from Heloderma horridum venom, interacts with vasoactive intestinal peptide receptors and a newly described receptor on dispersed acini from guinea pig pancreas. Description of exendin-3(9-39) amide, a specific exendin receptor antagonist. J Biol Chem. 1991;266(5):2897-2902. [PubMed] [Google Scholar]
- 41. Thorens B, Porret A, Bühler L, Deng SP, Morel P, Widmann C. Cloning and functional expression of the human islet GLP-1 receptor. Demonstration that exendin-4 is an agonist and exendin-(9-39) an antagonist of the receptor. Diabetes. 1993;42(11):1678-1682. [DOI] [PubMed] [Google Scholar]
- 42. Chen YE, Drucker DJ. Tissue-specific expression of unique mRNAs that encode proglucagon-derived peptides or exendin 4 in the lizard. J Biol Chem. 1997;272(7):4108-4115. [DOI] [PubMed] [Google Scholar]
- 43. Schirra J, Sturm K, Leicht P, Arnold R, Göke B, Katschinski M. Exendin(9-39)amide is an antagonist of glucagon-like peptide-1(7-36)amide in humans. J Clin Invest. 1998;101(7):1421-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Edwards CM, Todd JF, Mahmoudi M, et al. Glucagon-like peptide 1 has a physiological role in the control of postprandial glucose in humans: studies with the antagonist exendin 9-39. Diabetes. 1999;48(1):86-93. [DOI] [PubMed] [Google Scholar]
- 45. Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011;141(1):150-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ahmad SR, Swann J. Exenatide and rare adverse events. N Engl J Med. 2008;358(18):1970-1972. [PubMed] [Google Scholar]
- 47. Nauck MA. A critical analysis of the clinical use of incretin-based therapies: the benefits by far outweigh the potential risks. Diabetes Care. 2013;36(7):2126-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Salehi M, Vahl TP, D’Alessio DA. Regulation of islet hormone release and gastric emptying by endogenous glucagon-like peptide 1 after glucose ingestion. J Clin Endocrinol Metab. 2008;93(12):4909-4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Adriaenssens AE, Svendsen B, Lam BY, et al. Transcriptomic profiling of pancreatic alpha, beta and delta cell populations identifies delta cells as a principal target for ghrelin in mouse islets. Diabetologia. 2016;59(10):2156-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xu SFS, Andersen DB, Izarzugaza JMG, Kuhre RE, Holst JJ. In the rat pancreas, somatostatin tonically inhibits glucagon secretion and is required for glucose-induced inhibition of glucagon secretion. Acta Physiol (Oxf). 2020;229(3):e13464. [DOI] [PubMed] [Google Scholar]
- 51. Kuhre RE, Christiansen CB, Ghiasi SM, et al. Neuromedin U does not act as a decretin in rats. Cell Metab. 2019;29(3):719-726.e5. [DOI] [PubMed] [Google Scholar]
- 52. Svendsen B, Larsen O, Gabe MBN, et al. Insulin secretion depends on intra-islet glucagon signaling. Cell Rep. 2018;25(5):1127-1134.e2. [DOI] [PubMed] [Google Scholar]
- 53. Serre V, Dolci W, Schaerer E, et al. Exendin-(9-39) is an inverse agonist of the murine glucagon-like peptide-1 receptor: implications for basal intracellular cyclic adenosine 3′,5′-monophosphate levels and β-cell glucose competence. Endocrinology. 1998;139(11):4448-4454. [DOI] [PubMed] [Google Scholar]
- 54. Svendsen B, Holst JJ. Paracrine regulation of somatostatin secretion by insulin and glucagon in mouse pancreatic islets. Diabetologia. 2021;64(1):142-151. [DOI] [PubMed] [Google Scholar]
- 55. Jørgensen NB, Dirksen C, Bojsen-Møller KN, et al. Exaggerated glucagon-like peptide 1 response is important for improved β-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes. 2013;62(9):3044-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jepsen SL, Grunddal KV, Wewer Albrechtsen NJ, et al. Paracrine crosstalk between intestinal L- and D-cells controls secretion of glucagon-like peptide-1 in mice. Am J Physiol Endocrinol Metab. 2019;317(6):E1081-E1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gasbjerg LS, Helsted MM, Hartmann B, et al. Separate and combined glucometabolic effects of endogenous glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1 in healthy individuals. Diabetes. 2019;68(5):906-917. [DOI] [PubMed] [Google Scholar]
- 58. Hansen LS, Sparre-Ulrich AH, Christensen M, et al. N-terminally and C-terminally truncated forms of glucose-dependent insulinotropic polypeptide are high-affinity competitive antagonists of the human GIP receptor. Br J Pharmacol. 2016;173(5):826-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sparre-Ulrich AH, Gabe MN, Gasbjerg LS, et al. GIP(3-30)NH2 is a potent competitive antagonist of the GIP receptor and effectively inhibits GIP-mediated insulin, glucagon, and somatostatin release. Biochem Pharmacol. 2017;131:78-88. [DOI] [PubMed] [Google Scholar]
- 60. Yanagimachi T, Fujita Y, Takeda Y, et al. Pancreatic glucose-dependent insulinotropic polypeptide (GIP) (1-30) expression is upregulated in diabetes and PEGylated GIP(1-30) can suppress the progression of low-dose-STZ-induced hyperglycaemia in mice. Diabetologia. 2016;59(3):533-541. [DOI] [PubMed] [Google Scholar]
- 61. Asmar M, Asmar A, Simonsen L, et al. The gluco- and liporegulatory and vasodilatory effects of glucose-dependent insulinotropic polypeptide (GIP) are abolished by an antagonist of the human GIP receptor. Diabetes. 2017;66(9):2363-2371. [DOI] [PubMed] [Google Scholar]
- 62. Gasbjerg LS, Christensen MB, Hartmann B, et al. GIP(3-30)NH2 is an efficacious GIP receptor antagonist in humans: a randomised, double-blinded, placebo-controlled, crossover study. Diabetologia. 2018;61(2):413-423. [DOI] [PubMed] [Google Scholar]
- 63. Nauck MA, Meier JJ. GIP and GLP-1: stepsiblings rather than monozygotic twins within the incretin family. Diabetes. 2019;68(5):897-900. [DOI] [PubMed] [Google Scholar]
- 64. Gasbjerg LS, Bergmann NC, Stensen S, et al. Evaluation of the incretin effect in humans using GIP and GLP-1 receptor antagonists. Peptides. 2020;125:170183. [DOI] [PubMed] [Google Scholar]
- 65. Højberg PV, Zander M, Vilsbøll T, et al. Near normalisation of blood glucose improves the potentiating effect of GLP-1 on glucose-induced insulin secretion in patients with type 2 diabetes. Diabetologia. 2008;51(4):632-640. [DOI] [PubMed] [Google Scholar]
- 66. Stensen S, Gasbjerg LS, Krogh LL, et al. Effects of endogenous GIP in patients with type 2 diabetes. Eur J Endocrinol. 2021;EJE-21-0135.R1. [DOI] [PubMed] [Google Scholar]
- 67. Aulinger BA, Bedorf A, Kutscherauer G, et al. Defining the role of GLP-1 in the enteroinsulinar axis in type 2 diabetes using DPP-4 inhibition and GLP-1 receptor blockade. Diabetes. 2014;63(3):1079-1092. [DOI] [PubMed] [Google Scholar]
- 68. Vilsbøll T, Knop FK, Krarup T, et al. The pathophysiology of diabetes involves a defective amplification of the late-phase insulin response to glucose by glucose-dependent insulinotropic polypeptide-regardless of etiology and phenotype. J Clin Endocrinol Metab. 2003;88(10):4897-4903. [DOI] [PubMed] [Google Scholar]
- 69. Kjems LL, Holst JJ, Vølund A, Madsbad S. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes. 2003;52(2):380-386. [DOI] [PubMed] [Google Scholar]
- 70. Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36(8):741-744. [DOI] [PubMed] [Google Scholar]
- 71. Jones AG, McDonald TJ, Shields BM, et al. ; PRIBA Study Group . Markers of β-cell failure predict poor glycemic response to GLP-1 receptor agonist therapy in type 2 diabetes. Diabetes Care. 2016;39(2):250-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Toft-Nielsen MB, Madsbad S, Holst JJ. Determinants of the effectiveness of glucagon-like peptide-1 in type 2 diabetes. J Clin Endocrinol Metab. 2001;86(8):3853-3860. [DOI] [PubMed] [Google Scholar]
- 73. Billings LK, Doshi A, Gouet D, et al. Efficacy and safety of IDegLira versus basal-bolus insulin therapy in patients with type 2 diabetes uncontrolled on metformin and basal insulin: the DUAL VII randomized clinical trial. Diabetes Care. 2018;41(5):1009-1016. [DOI] [PubMed] [Google Scholar]
- 74. Højberg PV, Vilsbøll T, Rabøl R, et al. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia. 2009;52(2):199-207. [DOI] [PubMed] [Google Scholar]
- 75. Frias JP, Nauck MA, Van J, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018;392(10160):2180-2193. [DOI] [PubMed] [Google Scholar]
- 76. Killion EA, Wang J, Yie J, et al. Anti-obesity effects of GIPR antagonists alone and in combination with GLP-1R agonists in preclinical models. Sci Transl Med 2018;10(472):eaat3392. [DOI] [PubMed] [Google Scholar]
- 77. Willard FS, Douros JD, Gabe MB, et al. Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight 2020;5(17):e140532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Min T, Bain SC. The role of tirzepatide, dual GIP and GLP-1 receptor agonist, in the management of type 2 diabetes: the SURPASS clinical trials. Diabetes Ther. 2021;12(1):143-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data have been included in this review.