Abstract
Two specialized functions of cholesterol during fetal development include serving as a precursor to androgen synthesis and supporting hedgehog (HH) signaling activity. Androgens are produced by the testes to facilitate masculinization of the fetus. Recent evidence shows that intricate interactions between the HH and androgen signaling pathways are required for optimal male sex differentiation and defects of either can cause birth anomalies indicative of 46,XY male variations of sex development (VSD). Further, perturbations in cholesterol synthesis can cause developmental defects, including VSD, that phenocopy those caused by disrupted androgen or HH signaling, highlighting the functional role of cholesterol in promoting male sex differentiation. In this review, we focus on the role of cholesterol in systemic androgen and local HH signaling events during fetal masculinization and their collective contributions to pediatric VSD.
Keywords: cholesterol, male sex differentiation, androgen, disorders of cholesterol synthesis, hedgehog signaling, disorders of sex development
Mammalian sex development proceeds from the level of sex chromosomes to gonad specification and finally the expression of male or female phenotype. Genetic sex is determined by the chromosome composition at the time of fertilization that also provides the molecular basis for differentiation of biopotential gonads into testes or ovaries. Once the gonads have differentiated into testes in males, secretion of testicular hormones facilitate masculinization of the reproductive tract and genitalia. Discordance in the development of chromosomal, gonadal, or anatomical sex results in congenital conditions that historically have been labeled variations of hermaphrodite, intersex, or disorders/differences of sex development (DSDs) (1). Although the bulk of literature to date describe atypical reproductive phenotypes as DSDs, variations of sex development (VSDs) is the most recently accepted terminology and thus is used throughout this review (2). VSDs are broadly classified into sex chromosome VSDs such as 46,XX VSD or 46,XY VSD. 46,XY VSD are further classified into errors in sex determination specific to testis development and errors in sex differentiation associated with androgen synthesis or action (3). 46,XY VSD patients exhibit a wide spectrum of phenotypes ranging from mild to severe variations, including cryptorchidism, hypospadias, micropenis, ambiguous genitalia, feminized external genitalia, and complete sex reversal. Although causes are often difficult to trace, a combination of genetics, maternal health, and environmental factors have been implicated in the etiology of these conditions (4, 5).
After chromosomal sex is determined, establishment of primary and secondary sex organ phenotypes require intricate interactions between systemic endocrine and local signaling pathways (6, 7). Specifically, we and others have shown that hedgehog (HH) and androgen pathways collaborate to orchestrate male sex differentiation (8-10). Cholesterol has independent roles in each of these activities: it acts as a substrate for androgen synthesis and it is required for HH ligand processing and receptor activation. Cholesterol is the precursor of all steroid hormones, providing the backbone of the steroid molecule. The availability of cholesterol and intracellular processing are crucial for cholesterol metabolism into steroids, including androgens. In turn, several key participants of the HH pathway are sensitive in different ways to cholesterol (11). First, HH ligands undergo cholesterol modification to enhance their activity. Second, the HH receptor, Patched (PTCH) and the signal transducer, Smoothened (SMO), contain sterol sensing domains and mediate cholesterol transfer. In the absence of HH ligand, PTCH pumps cholesterol across the bilayer to divert substrate from SMO, resulting in suppression of SMO activity. In turn, HH binding to PTCH inhibits the pump activity, thereby restoring access to a cholesterol gradient that promotes SMO activity. Thus, cholesterol is deemed essential for 2 of the most fundamental resources required for male sex differentiation.
Cholesterol is essential for health and is ubiquitous in every cell of the body with strikingly diverse structural, endocrine, and signaling functions. Ever since its discovery, physiological and pathological roles of cholesterol have been studied extensively. Reduced or excessive levels of cholesterol lead to fetal or adult diseases, highlighting the importance of maintaining cholesterol levels within a tight physiological range (12). During development, the fetus grows at a rate unparalleled to any other stage of life and therefore requires a massive amount of cholesterol for normal growth. Consequently, disrupted cholesterol synthesis causes several congenital diseases. Disorders of cholesterol synthesis have emerged as common inborn errors of metabolism because of their strong association with fetal dysmorphogenesis. Common to all of these conditions is a deficiency in cholesterol and the accumulation of precursor sterols determined by the affected enzyme in the biosynthetic pathways (13). Male genital and gonadal anomalies are frequently observed in disorders of cholesterol metabolism with phenotypes ranging from mild to severe VSDs such as ovotestes, cryptorchidism, hypospadias, ambiguous genitalia, and complete sex reversal (14). As such, this review will be used to describe the essential roles of cholesterol in male sex differentiation and how defective cholesterol synthesis may contribute to pediatric VSDs.
Cholesterol Synthesis
Almost all mammalian cells can synthesize cholesterol de novo through the mevalonate pathway. Briefly, cholesterol is synthesized from acetyl-coenzyme A (CoA) in 3 parts, which include synthesis of mevalonate, squalene, and then into the final product, cholesterol. First, acetyl-CoA is transported from mitochondria to cytosol, where it is converted to 3-hydroxy-3-methylglutaryl-CoA. It is then converted to mevalonate by hydroxymethylglutaryl-CoA reductase (HMGCR) in the endoplasmic reticulum (ER). This is the rate-limiting and irreversible step of the pathway, and HMGCR is the pharmacological target of statins, a common class of cholesterol lowering drugs. Notably, HMGCR also functions at a critical juncture between cholesterol synthesis and alternative lipid pathways that are less understood but are also important for posttranslational modifications, including farnesylation and geranylation. In addition, HMGCR is incorporated in feedback pathways to control cholesterol abundance via cholesterol and insulin sensor proteins that regulate transcription factor access to cholesterol conversion enzyme genes.
Mevalonate is converted to squalene following 2 successive phosphorylations, with the last reaction being catalyzed by squalene synthase. Squalene is oxidized and undergoes cyclization to yield lanosterol, which is then converted to cholesterol through a 19-step process. The final reaction to cholesterol from lanosterol occurs via 2 major routes with either 7-dehydrocholesterol or desmosterol as the ultimate precursor of cholesterol (Fig. 1). Cholesterol synthesis is generally divided into 2 major pathways: presqualene and postsqualene cholesterol synthesis. The presqualene pathway contributes to both sterol and isoprenoid synthesis, whereas postsqualene pathway represents a commitment to sterol and vitamin D synthesis (15).
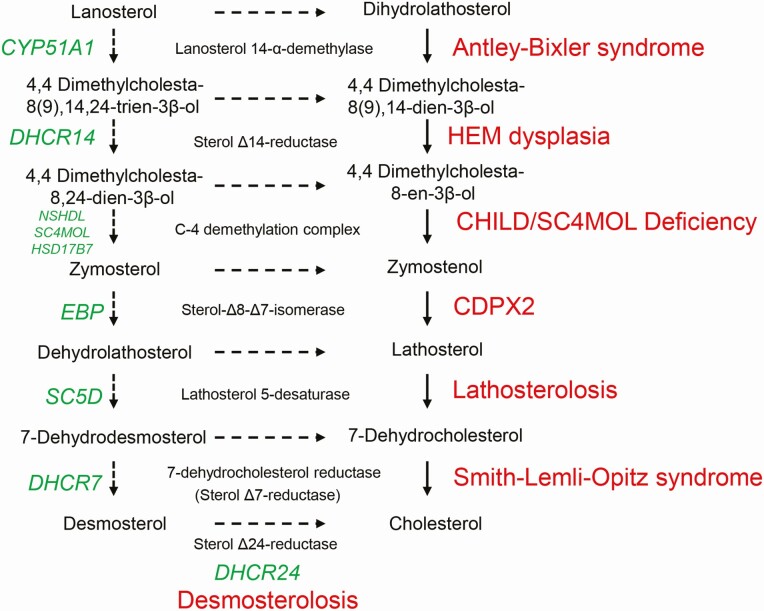
Figure 1.
Cholesterol synthesis disorders. The postsqualene cholesterol synthesis pathway shows conversion of lanosterol to cholesterol in a series of enzymatic reactions. The genes encoding each of the enzymes are shown in green and the associated cholesterol synthesis disorders are shown in red (Reproduced from J Lipid Res 2011;52:6–34. Porter FD and Herman GE. Malformation syndromes caused by disorders of cholesterol synthesis (24)).
Besides de novo synthesis, most mammalian cells also acquire cholesterol via uptake of exogenously derived cholesterol from the low-density lipoprotein (LDL) particles in blood circulation. The balance between endogenous and exogenous pathways depends on cell type and the availability of LDL-derived cholesterol. Free cholesterol can also be absorbed from dietary sources by enterocytes in the intestine and from bile in the biliary ducts by hepatocytes in the liver. Both endogenously synthesized and exogenously acquired cholesterol is converted to cholesteryl ester for storage in lipid droplets or for secretion as lipoproteins while excess cholesterol is exported to the blood by ATP-binding cassette transporters. All of these processes are tightly controlled by multiple transcriptional and posttranslational regulatory circuits to maintain cholesterol homeostasis within each cell. Although it is synthesized in the ER, the cholesterol content of ER is very low because most of the synthesized cholesterol is transported to other organelles and the plasma membrane. The mobilization of cholesterol across aqueous cytoplasmic spaces occurs through vesicular transport mediated by membrane fusion or nonvesicular transport via soluble carrier proteins. Intracellular cholesterol transport varies among different cell types depending on their specialized functions and cellular requirements. The distribution between cellular membranes plays an important role in determining cholesterol synthesis, uptake, export, and storage to ensure cholesterol homeostasis (15, 16).
Origin of fetal cholesterol
Cholesterol is absolutely essential for fetal development. Because of its rapid growth, the fetus has elevated cholesterol requirements and therefore synthesizes cholesterol at a higher rate compared with the adult. As a result, cholesterol homeostasis is not at an equilibrium as it is in adults, in which a steady-state mechanism is maintained. Instead, there is a lack of feedback regulation despite cholesterol accumulation and the only net loss of cholesterol is through fetal steroid hormone synthesis (17). The fetus acquires cholesterol mostly from de novo synthesis, with a second source of cholesterol derived from the maternal circulation (18). Studies with plant sterols and labeled lipoprotein complexes have confirmed that cholesterol is actively transported from the mother to the fetus during gestation. This has been supported by studies that detected low cholesterol levels in children with congenital cholesterol synthesis disorders who lack endogenous cholesterol (19). The maternal-fetal cholesterol transfer takes place through lipoprotein receptors and sterol transporters in visceral endoderm in the yolk sac, trophoblasts in the placenta, and fetal endothelial cells (20). Although maternal hypercholesterolemia is associated with fatty streaks in the fetal aorta, low maternal cholesterol level as well as defective fetal de novo cholesterol synthesis results in intrauterine growth restrictions (21). Previous studies have shown a correlation between VSDs and intrauterine growth restrictions, suggesting an association between placental insufficiency and abnormal genital development (22).
Disorders of cholesterol synthesis
Cholesterol biosynthesis disorders affect both presqualene and postsqualene parts of the pathway. Two enzyme defects have been identified in the presqualene segment of the pathway: the classical form of mevalonic aciduria caused by a deficiency of mevalonate kinase and the hyperimmunoglobulinemia D syndrome caused by a partial deficiency of mevalonate kinase. Further, there are 8 inborn errors of cholesterol synthesis that affect each step of the postsqualene pathway, including Antley-Bixler syndrome (ABS), hydrops-ectopic calcification-motheaten (HEM) dysplasia, congenital hemidysplasia with ichthyosiform erythroderma and limb defects syndrome, sterol-C-4 methyloxidase-like (CHILD/SC4MOL) deficiency, X-linked dominant chondrodysplasia punctate (CDPX2), lathosterolosis, Smith-Lemli-Opitz syndrome (SLOS), and desmosterolosis (Fig. 1) (14, 23). Among these rare disorders, SLOS is the most common and most studied syndrome. In each of these cases, pathogenesis arises from both deficiency of cholesterol and accumulation of metabolic precursor sterols. Sterol levels and their biochemistry in each of these disorders are described elsewhere (24). A spectrum of congenital malformations has been observed in these disorders, with common phenotypic features including neurodevelopmental defects, craniofacial defects, skeletal defects especially limb malformations, skin defects, and genital defects. Less frequently observed abnormalities affect major organs such as liver, kidney, bone, heart, lung, gut, and intestine (14). Successful management of cholesterol synthesis disorders by cholesterol supplementation in infants and children has resulted in improved overall growth and behavior (25). Prenatal developmental defects, especially brain functions, however, are often unable to be reversed. Animal models with genetic or pharmacological inhibition of cholesterol synthesis mimic biochemical abnormalities and clinical features of human SLOS patients, many of which overlap those observed with defective HH signaling, suggesting that the SLOS phenotypes are due, in part, to impaired HH signaling (26).
HH Signaling
The HH pathway plays essential roles in embryonic development with functions in cell fate specification, patterning, differentiation, and growth of multiple organs by establishing epithelial-mesenchymal interactions (27). HH signaling encompasses a signal transduction cascade whereby an activated HH ligand is secreted by epithelial cells and then diffuses through the extracellular space to act on nearby mesenchymal cells to modulate the expression of HH target genes in a concentration-dependent manner. Dysregulation of the HH pathway during development causes a variety of congenital malformations in animal models and humans. Cholesterol is absolutely required for HH signaling, and the main components of the HH network show enhanced activity when covalently modified or bound by cholesterol as discussed in detail in the next section. Notably, perturbations in cholesterol homeostasis phenocopy HH signaling defects, indicating the essential role of cholesterol in mediating HH signals (28).
HH ligand processing
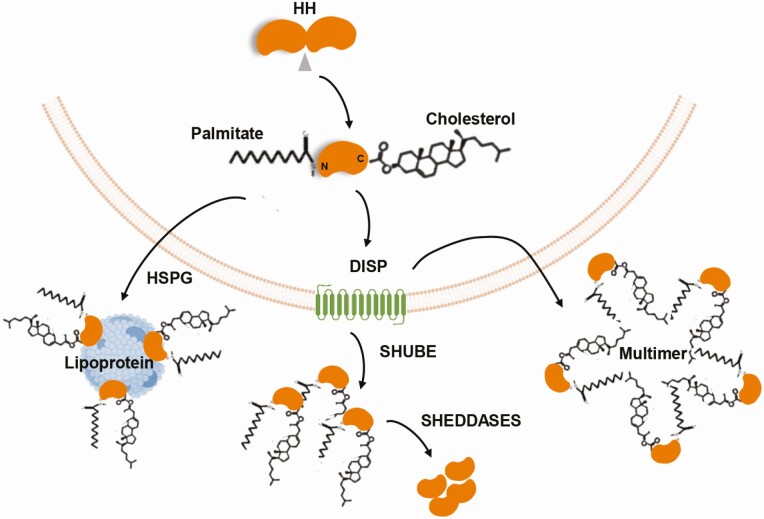
In mammals, there are 3 hedgehog ligands, Sonic hedgehog (SHH), Desert hedgehog (DHH), and Indian hedgehog (IHH), of which SHH is the most studied; the signaling mechanisms are presumed to be the same for all 3 ligands (29). The movement of HH between the donor and acceptor cells is tightly controlled and does not occur by simple diffusion. Instead, lipid modifications are incorporated onto the ligand to facilitate intracellular trafficking, secretion, and signaling activity (30, 31). The association between HH signaling and cholesterol metabolism was discovered more than 2 decades ago, when it was shown that the ligand is modified by cholesterol in an autocatalytic reaction (32). The HH proteins are initially synthesized as inactive 45-kD pro-peptides that subsequently undergo internal cleavage and concomitant modifications to become functional. A cholesterol molecule is covalently attached to the C-terminal end of HH, and a palmitate molecule is added at the N-terminal end by hedgehog acetyl-transferase; this dual lipidation processing increases the efficacy of the ligand as a functional signaling molecule (11, 33). Cholesterol modification renders the ligand highly hydrophobic and increases the affinity of HH for the plasma membrane. Lipid-modified HH can achieve short- and long-range signaling. For example, lipid-modified HH can diffuse short distances without dissociation from the plasma membrane and then be transferred to adjacent cells (34, 35). In contrast, for long-range signals, the ligand is extracted from the plasma membrane by the transmembrane protein, Dispatched (DISP), and then released as freely diffusing aggregates, multimers of HH themselves, or packaged into lipoprotein particles (34, 36). DISP contains a sterol sensing domain (SSD) and binds HH in a cholesterol-dependent manner. Mutations that cause a loss of DISP results in intracellular accumulation of lipid-modified HH within the secreting cells (37). Notably, cholesterol-unmodified HH can be secreted independent of DISP, but only provides short-range activity (35). Ultimately, the secreted ligand moves through the extracellular space, where it interacts with SCUBE proteins (which are also cholesterol dependent (38)) that serve as chaperones to assist ligand interactions with receiving cells (Fig. 2).
Figure 2.
Hedgehog processing and release. Hedgehog (HH) ligands are initially synthesized as inactive pro-peptides, which undergo internal cleavage and covalent modifications to attach cholesterol at the C-terminal end and palmitate at the N-terminal end. These dual-lipidated ligands are released from the cells by Dispatched (DISP) as multimers of HH themselves or as freely diffusing aggregates, with the help of SHUBE and SHEDDASE proteins. The ligands are also packaged into lipoprotein particles through their association with heparan sulfate proteoglycans (HSPGs) (adapted from J Dev Biol 2016; 4:23. Ramsbottom SA and Pownall ME. Regulation of hedgehog signaling inside and outside the cell (32)).
The receptors, PTCH and SMO
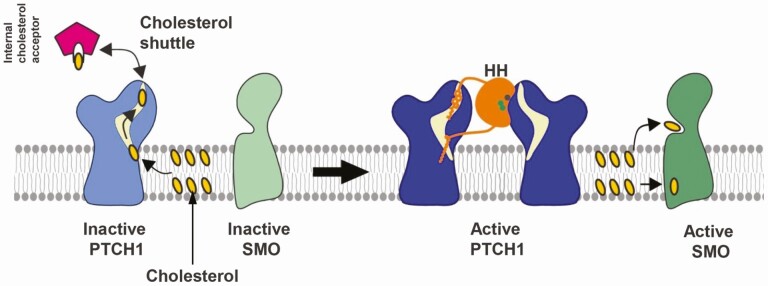
The main receptor for HH ligands is the 12-pass transmembrane protein, PTCH (27). In the absence of ligands, the antagonizing receptor PTCH catalytically suppresses the activity of SMO, thus precluding downstream signaling. HH binding to PTCH subsequently liberates SMO to adopt an active conformation, which then triggers a cascade of events culminating in the activation of GLI transcription factors (Fig. 2) (39, 40). PTCH has several important functional domains: 2 large extracellular loops required for HH binding, a SSD in the transmembrane region (TM2-6) required for inhibition of SMO activity, and a C-terminal domain required for internalization and degradation of the PTCH-HH complex. A second sterol sensing-like domain is also located in another transmembrane region (TM7-12) with similar function as the SSD (41, 42). PTCH shares high homology with other membrane transporter type proteins, such as NPC1, DISP, and SCAP, and various pump proteins including the bacterial resistance-nodulation division family transporters, indicating that PTCH participates in cholesterol trafficking and pumping actions (43).
The exact mechanism by which PTCH inhibits SMO has been under high speculation. Evidence shows that PTCH and SMO do not physically interact with each other (44). Instead, in the absence of HH ligand, PTCH translocates small molecules such as sterols, including cholesterol and oxysterol, across the membrane and away from SMO (45). Structural and biochemical analysis revealed a hydrophobic tunnel within the SSD of the PTCH molecule through which cholesterol and other sterols are removed from the inner leaflet of the plasma membrane. HH binding of PTCH occludes this tunnel effectively blocking sterol transport, which allows it to accumulate in the inner leaflet and activate SMO (Fig. 3) (44-46). Indeed, mutations in the PTCH SSD affects HH affinity in vitro and HH activity in Drosophila (47, 48). SMO is a 7-pass transmembrane protein that belongs to the G protein–coupled receptor superfamily and contains 2 small-molecule-binding sites, 1 in the extracellular cysteine-rich domain and the other in the transmembrane domain, each of which bind cholesterol, oxysterols, or sterol-like SMO agonists (49-51). Although oxysterols have long been proposed to bind SMO, studies show that cholesterol can also act as a ligand and directly activate SMO (50-52). Blocking of sterol binding by small molecule inhibitors impedes SMO activation to transmit HH signals (53).
Figure 3.
PTCH-SMO activation. PTCH1 prevents access of sterols to SMO or pumps sterols away from SMO, thereby inhibiting SMO activation. HH binding to PTCH1 prevents this inhibition, thus allowing sterols to activate SMO. (Reproduced from Curr Opin Struct Biol. 2019; 57:204–214. Kowatsch et al., Structures of vertebrate Patched and Smoothened reveal intimate links between cholesterol and Hedgehog signaling (43)). Abbreviations: HH, hedgehog; PTCH, Patched; SMO, Smoothened.
Primary cilia
Canonical HH signaling requires a functional primary cilium for its activity. At the cellular level, PTCH and SMO differentially accumulate in the primary cilia. In the basal state, PTCH is enriched in the primary cilia and inhibits SMO activation and entry into cilia. Upon HH binding, PTCH is cleared from the cilia, which allows accumulation of SMO in the ciliary membrane. Importantly, membrane lipids in cilia are distinct from those in the rest of the plasma membrane and are particularly conducive for the cholesterol mobility that is critical to SMO signaling. Much like the adjacent plasma membrane and other cell membranes, cholesterol in cilia is divided into 3 types based on availability: structural cholesterol that is functionally unavailable, cholesterol associated with sphingomyelin that has variable availability, and mobile cholesterol susceptible to cholesterol-binding proteins since cholesterol does not readily move between the bilayers. The high cholesterol requirements for primary cilia are such that restrictions on synthesis or availability prevents formation or, more acutely, changes its activity. Cholesterol-sensitive fluorescent probes show that accessible cholesterol availability is relevant for the activation of SMO because blocking this mobile cholesterol within the cilia affects SMO signaling (54-56).
HH and male sex differentiation
HH signaling has been implicated in the differentiation and development of testes and secondary sex organs with distinct members of the HH pathway expressed in these organs in a temporal and cell-type specific manner (6, 8, 57). First, DHH stimulates testis development and function, including differentiation of cells that produce androgens (58). The circulating androgens will then bind androgen receptors in target organs to stimulate IHH to promote male genital tubercle development and cooperate with SHH in prostate bud formation and masculinization of external genitalia (9, 59, 60). Disruption of HH signaling by targeting each pathway component interferes with male sex differentiation processes, thus causing VSD phenotypes as outlined in Table 1 (61-63).
Table 1.
Effect of disrupting HH pathway components in the development of VSD phenotypes in mice
| HH members | Anogenital development | External genitalia | Prostate | Wolffian duct | Testis descent | FLC differentiation | Testosterone levels |
|---|---|---|---|---|---|---|---|
| SHH | Perineal defects (61) | Complete agenesis, hypospadias (60-62) | Abnormal (59) | Abnormal (58) | N/A | N/A | N/A |
| IHH | N/A | Micropenis (9) | N/A | N/A | N/A | N/A | N/A |
| DHH | N/A | Feminized (130, 131) | N/A | N/A | Cryptorchidism (130, 131) | Impaired (58, 131) | Low (131) |
| SMO | N/A | Complete agenesis, hypospadias (61, 62) | Abnormal budding (59) | N/A | N/A | Impaired (79, 80, 132) | N/A |
| GLI1 | N/A | Normal (10) | Normal (10) | N/A | Normal (132) | Normal (132) | N/A |
| GLI2 | Normal (10) Reduced AGD (133) |
Hypospadias (10) | Abnormal budding (100) | N/A | Normal (132) | Normal (132) | Normal (10) |
| GLI3 | Reduced AGD (8, 63) | Hypospadias (8, 133) | Normal (100) | N/A | Cryptorchidism (8,13) | Impaired (8, 133) | Low (8, 100) |
| GLI inhibitor (GANT61) | N/A | N/A | N/A | N/A | N/A | Impaired (132) | N/A |
Abbreviations: AGD, anogenital distance; DHH, Desert hedgehog; VSD, variations of sex development; HH, hedgehog; IHH, Indian hedgehog; N/A, not available; SHH, Sonic hedgehog; SMO, Smoothened.
Male sex differentiation
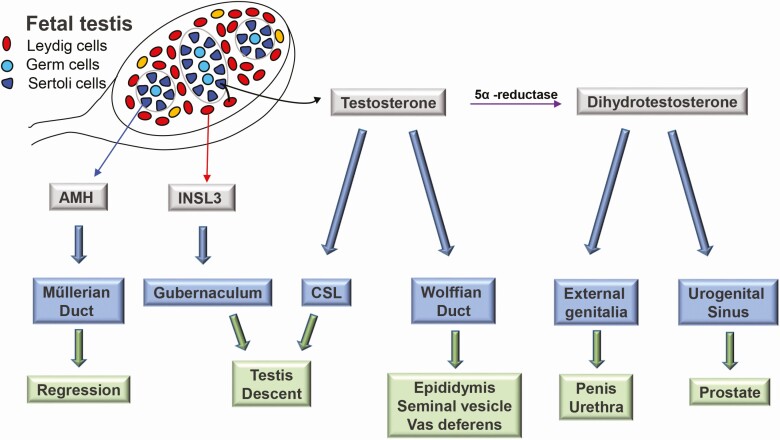
The temporal events in fetal sex development begin with sex determination and followed directly by sex differentiation. Sex determination is the initial event that refers to the commitment of the bipotential gonad to a testis or ovary fate, which then sets the stage for subsequent sex differentiation to ultimately produce a male or female phenotype (64). The male phenotype relies on secretion of 3 testicular hormones: anti-Müllerian hormone, produced by Sertoli cells, which induces regression of the Müllerian ducts; androgens, produced by fetal Leydig cells, which stabilize Wolffian ducts, promote prostate development, and masculinize the external genitalia; and insulin-like 3 peptide, also produced by fetal Leydig cells, which mediates testicular descent together with androgens (65, 66) (Fig. 4). Consequently, testicular dysgenesis and aberrant hormone production or action causes VSD. Further, abnormal early development of the testis can also culminate in adult-onset disorders such as hypogonadism and infertility (67).
Figure 4.
Male sex differentiation. Testicular hormones mediate male sex differentiation: anti-Müllerian hormone (AMH), produced by Sertoli cells, induces regression of the Müllerian ducts; testosterone, produced in partnership between fetal Leydig cells and Sertoli cells, stabilize Wolffian ducts; insulin-like 3 (INSL3), also produced by fetal Leydig cells, mediates testicular descent together with androgens. Testosterone is locally converted to dihydrotestosterone (DHT) in peripheral tissues by 5α reductase to promote prostate development and masculinize external genitalia. (adapted from Front Pharmacol 2019;10:1309. Wang et al. Phthalate-induced fetal Leydig ell dysfunction mediates male reproductive tract anomalies (69)).
Development of fetal Leydig cells
Testis differentiation is initiated with the expression of Sex Determining Region of the Y chromosome, which directs supporting cell precursors to differentiate into Sertoli cells. Sertoli cells function as the organizing center of the testis to orchestrate differentiation of key somatic cell types, including Leydig cells (68). Fetal Leydig cells (FLCs) are the steroidogenic cells in the fetal testis that are distinct from adult Leydig cells, which appear after birth (69). The development of FLCs is a complex process involving differentiation from progenitor precursor cells that mature and then undergo functional involution after birth (70). FLCs appear in the testicular interstitium starting around embryonic day (E) 12.5 in mice and gestational week (GW) 7 to 8 in humans (71, 72). Testosterone synthesis in mouse fetal testes begins at approximately E13.5 and approaches its peak by E16.5, the duration of which is termed the masculinization programming window (MPW) (73). In humans, this corresponds to GW 8 to 14. FLCs undergo a functional regression after the MPW and generally diminish after birth. There is, however, evidence that shows persistence of FLCs in the postnatal testes with unknown biological significance (74).
In the adult testis, testosterone production is regulated by pulsatile secretion of pituitary-derived LH (75). In contrast, FLC activity, at least in the early phase of fetal development, is gonadotropin and pituitary independent and, therefore, presumably relies on paracrine secretions from Sertoli cells for its differentiation and steroidogenic function (70, 76). Although murine androgen synthesis begins around E13.5, FLC do not express LH receptors until later stages, starting at E16; however, they do respond to LH stimulation to produce androgens (70, 72). While the source of FLC stimulation remains unknown in mice, it has been reported that future FLC differentiate in response to coordinated events between active DHH and PDGFA signals and a loss of the NOTCH pathway (77, 78). The essential contributions of DHH-SMO-GLI signaling have been implicated in the onset and maintenance of FLC steroidogenesis (8, 58, 79, 80) and steroidogenic enzymes have been identified as direct transcriptional targets of GLI2 or GLI3 in vitro (81). Genetic and pharmacological studies established that DHH functions through SMO signaling to promote differentiation of interstitial cell fate, including FLC; however, loss of either Gli1 or Gli2 did not affect FLC development or male sex differentiation in mice (82). Instead, loss of Gli3 showed phenotype abnormalities of male secondary sex characteristics as a result of diminished FLC numbers and disrupted androgen and HH actions. In addition, we recently showed that the transactivator form of GLI3 is necessary to maintain FLC identity by promoting the fidelity of both HH and steroidogenic pathways to coordinate and promote development of male sex characteristics (8) (Table 1). This is the first time that GLI3 has been implicated in an active role to stimulate and optimize cell function within the fetal testis.
Fetal steroidogenesis pathway and requirement for cholesterol
Cholesterol is the precursor of all steroid hormones, providing the backbone of the steroid molecule. Steroidogenesis is a multistep process carried out within distinct subcellular organelles, by which cholesterol is converted to biologically active steroid hormones (83, 84). Cholesterol molecules derived from endogenous synthesis or intracellular stores are transported from cytoplasm, through the outer mitochondrial membrane and into the inner membrane by a complex made up of multiple proteins, including the essential component, steroidogenic acute regulatory protein D1. Once delivered to the inner mitochondrial membrane, cholesterol is converted to pregnenolone by cytochrome P450 side chain cleavage enzyme, CYP11A1. Pregnenolone is then transferred to the ER, where it is converted by the dual-purpose enzyme, CYP17A1, first to 17-hydroxypregnenolone and then to dehydroepiandrosterone. Dehydroepiandrosterone is then converted either through the intermediacy of androstenediol or androstenedione to testosterone by the enzymes HSD3B1 and HSD17B3 (83, 84). FLC identity is marked by expression of a complete set of genes for synthesis of androgens, with the exception of the terminal reductase, HSD17B3 (85, 86). Thus, FLCs produce only the androgen, androstenedione, which must make its way back to the Sertoli cells, where HSD17B3 is present for the final conversion to testosterone. As development progresses beyond the fetal stage, Sertoli cells lose, whereas progenitor adult Leydig cells acquire, expression of HSD17B3, which facilitates direct testosterone production in prepubertal life (87). Conversion of testosterone to a more potent dihydrotestosterone by 5α reductase occurs in target organs, including prostate and external genitalia (88).
Cholesterol requirements are different in steroidogenic versus nonsteroidogenic cells. FLCs have an augmented need for cholesterol because of their role in steroidogenesis. The availability of cholesterol and intracellular processing are crucial for cholesterol metabolism into steroids. Studies show that early steroid production in fetal mouse testis is mostly dependent on intracellular cholesterol stores because FLCs do not start expressing genes involved in cholesterol synthesis until E13.5 (89). A similar trend is observed in human fetal testis where de novo cholesterol synthesis is not initiated during early steroidogenesis (90). Both species can support endogenous cholesterol synthesis following the expression of cholesterol biosynthetic genes, after which FLCs rely heavily on de novo cholesterol for androgen production. The additional demands for cholesterol are met by exogenously derived cholesterol that include (1) hydrolysis of cholesterol from cholesterol esters stored in lipid droplets, (2) cholesterol internalized from the plasma membrane, and (3) cholesterol obtained from plasma LDL and high-density lipoprotein (91, 92). The effect of low cholesterol on testosterone synthesis is not clear, possibly because of cholesterol availability through dietary sources and maternal transfer. Severe cholesterol deficiency will lead to fetal demise, precluding further analysis. Studies in the early 1980s showed that statins (pharmacological inhibitor of HMGCR) do not affect testosterone synthesis in MA-10 (mouse Leydig cell tumor) cells or rat testes (93); however, statins have been shown to suppress testosterone levels in human adults and human testicular homogenates (94, 95). Similar results were observed during prenatal exposure to statins in vivo and in cultured fetal rat testes (96). More studies are warranted to study such conflicting results. As such, statins are contraindicated during pregnancy because cholesterol is essential for fetal development; therefore, animal models and ex vivo testicular cultures would be required to give us more information on the effect of low cholesterol on fetal steroidogenesis.
Secondary sex differentiation
Secondary sex differentiation is hormone dependent and requires additional developmental signals, such as HH, fibroblast growth factor, and WNT along with specific transcription factors (97). Androgen-dependent secondary sex characteristics include testicular descent, stabilization of Wolffian ducts, prostate development, and differentiation of external genitalia. Masculinization of these structures does not occur simultaneously; however, hormonal priming or programming occurs during a common fetal programming window followed by masculinization or sexual differentiation in the prenatal stage (71). Additional growth of urogenital organs to their final size occurs after birth and depends on androgen stimulation. As alluded to previously, the critical window of androgen action, termed the MPW, occurs between E13.5 and 16.5 in mice and GW 8 and 14 in humans (73). Prenatal exposure to antiandrogens or endocrine disruptors demonstrated that impaired androgen secretion and action confined to this window affect male sex differentiation and exhibit VSD phenotypes (98, 99). In addition to the timing, certain concentrations of circulating androgens must be achieved to promote optimal male sex differentiation. For example, prostate bud formation is sensitive to even low levels of androgens, whereas the development of Wolffian ducts and testis descent require relatively high concentrations of androgens (8, 100, 101). Morphogenesis of secondary sex organs involves fundamental epithelial-mesenchymal interactions that require the actions of androgens/androgen receptor (AR) activity (102-104). AR expression is observed in the mesenchyme, epithelium, or both, depending upon the organs and the stages of their development (59, 103). Thus, conditional inactivation of AR at different levels determined the severity of VSD phenotype indicating that AR activity is cell-type specific as well as dose dependent (9, 105-107).
A brief description of development of each of the secondary sex characteristics is provided here.
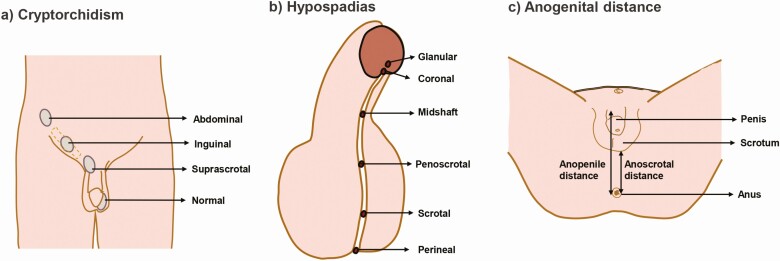
Testis descent: The undifferentiated gonads of both male and female embryos are held near the abdominal wall in a pararenal position by 2 ligamentous structures, the cranial suspensory ligament, and gubernaculum. After sex differentiation, the testes migrate to the bottom of the abdomen and then through the inguinal canal into the scrotum, whereas the ovaries remain positioned lateral to the kidneys. Testis descent requires regression of the cranial suspensory ligament and the outgrowth and subsequent regression and inversion of the gubernaculum, which translocates the testes into the scrotum under the action of androgens and insulin-like 3 peptide. Failure of 1 or both testes to descend from the abdomen into the scrotum results in a condition called cryptorchidism (108) (Fig. 5A). Undescended or maldescended testes are strong risk factors for infertility and testicular cancers.
Figure 5.
Variations of sex development (VSDs). (A) Cryptorchidism: failure of testis to descend with transabdominal or suprascrotal retention. (B) Hypospadias: urethral tube openings that present anywhere along the ventral side of the penis shaft instead of the distal-most tip. (C) Measurement of anogenital distance (AGD): AGD is measured from the center of the anus to the base of the scrotum (anoscrotal) or to the anterior base of the penis (anopenile). A short male AGD is strongly associated with genital malformations at birth and reproductive disorders in adulthood.
Wolffian duct: Before sexual differentiation, both male and female embryos have paired primitive Müllerian and Wolffian duct (WD) systems. Male embryos retain their WD through the action of androgen, whereas female embryos lose their WD as a result of a lack of androgens. The WD is the progenitor of the future male reproductive tract components including the epididymis, vas deferens, and seminal vesicles (109). Morphological development of the WD involves stabilization followed by tubular elongation, coiling, and terminal differentiation into the respective structures (110, 111), and each of these processes are dependent on continuous androgen stimulation. Anti-Müllerian hormone, produced by Sertoli cells, induces regression of the Müllerian ducts in the male embryo. When Müllerian duct derivatives such as uterine and fallopian duct tissues are retained in males with normal masculinization, the condition is called persistent Müllerian duct syndrome. Infertility is the most common feature in these individuals (112).
Prostate: The primitive urogenital sinus gives rise to, in rostral–caudal position, the bladder, urogenital sinus, and urethra. The urogenital sinus develops into the prostate in males and the lower portion of vagina in females (113, 114). Prostate morphogenesis is initiated by androgen signaling and further growth and function are continually dependent on circulating androgens. Development of the prostate is divided into several stages, which begins with prenatal emergence of prostate buds from the epithelium (115, 116). During subsequent ductal morphogenesis, the buds elongate and branch extensively into the surrounding mesenchyme to canalize into solid cords. The final phase involves differentiation of luminal and basal epithelial cells, followed by secretory cytodifferentiation. Later development, including ductal branching and terminal differentiation, occurs after birth with the prostate reaching its mature size during puberty (117, 118).
External genitalia: The genital tubercle (GT) constitutes the anlage of external genitalia that differentiates into a penis in males and a clitoris in females. Development of external genitalia has been separated into pre- and postnatal phases, with distinct sensitivity to both androgens and estrogens (119). The prenatal phase in males includes early patterning and initial outgrowth of the GT and then subsequent androgen regulated sexual differentiation. Masculinization encompasses increased distal outgrowth of the GT along with formation of the urethral tube, both of which are spatiotemporally controlled. Successful urethral tubularization is essential for uresis and ejaculation, and the proximodistally elongated penile structure is necessary for copulation (119). Defects in penile outgrowth result in congenital anomalies such as ambiguous genitalia or micropenis, whereas defects in urethral formation result in tubal opening located ectopically on the ventral side of the penis rather than at its distal-most tip, causing a congenital defect named hypospadias (120) (Fig. 5B).
Similar to genital development, sexual dimorphism extends to the perineum, where a muscular complex develops in males but not in females. Perineal growth including expansion of anogenital distance (AGD) (Fig. 5C) is dihydrotestosterone dependent, and reduced AGD serves as a reliable biomarker for impaired fetal androgen action. AGD is approximately twice as long in males compared with females and short male AGD is strongly associated with genital malformations at birth and reproductive disorders in adulthood (121, 122).
Variations of Sex Development
VSDs are among the most common defects in all live births with an estimated prevalence of 1% to 3%. Current methods of VSD diagnosis requires a multidisciplinary team consisting of a pediatric endocrinologist, pediatric urologist, pediatric radiologist, geneticist, and psychologist that perform clinical phenotype, biochemical, and genetic analyses. VSDs can be identified at any time in life ranging from prenatal or neonatal stages or later, at puberty, or adulthood. Although less common, prenatal ultrasonography can detect abnormal genitalia as early as 14 weeks of gestation and later in pregnancy, 3-dimensional ultrasound and Doppler ultrasound can be useful in identifying malformations of the external genitalia and hypospadias (123). Possible VSDs in neonates include ambiguous genitalia, varying degrees of hypospadias, micropenis, clitoromegaly, unilateral or bilateral undescended testes, or nonpalpable testes. External masculinization is typically scored based on a grading system that may allow a more discriminating and objective assessment of the external genitalia. Recently, an external genitalia score has been introduced that includes AGD in addition to the assessment of the genital difference (124). When a VSD is suspected, chromosome analysis is performed and the karyotype will determine the subgroup of VSD (chromosome VSD, 46,XY VSD, or 46,XX VSD). In parallel, steroid hormones are measured in blood or urine and imaging studies are used to examine internal sex organs. This is followed by diagnostic genetic testing that may include single-gene analysis or targeted gene panels, with results of candidate gene screening confirming a diagnosis meant to help guide treatment and gender assignment decisions. Prenatal genetic testing is typically performed to confirm monogenic familial contributions. Depending on the results of steroid panels and imaging analysis, clinical therapy may be recommended, and genetic counseling is offered for further familial management of the condition (125, 126).
VSD screening for potential candidate genes has revealed an increasing number of genes critical for sex determination and sex differentiation. Animal models recapitulating the human phenotypes have increased our understanding of the mechanism and action. Despite recent advances in genetic screening such as next-generation sequencing, most cases of 46,XY DSD do not have a confirmed genetic diagnosis. VSD gene panels can be categorized into those that are analyzed “first line” or “second line” with more than 80 genes. The first line of genes is directly involved in sex determination and sex differentiation, whereas the second line of genes is not directly involved in these processes and generally exhibits phenotypes other than those involved in VSDs. VSDs may present on their own or as a part of other conditions such as disorders of cholesterol synthesis. Pertaining to the scope of this review, we present here 46,XY VSDs resulting from deficits in androgen, HH pathway mediators, or cholesterol.
46,XY VSDs from androgen deficits: These are broadly classified into the following: (1) impaired Leydig cell differentiation as a result of luteinizing hormone/chorionic gonadotropin receptor defects leading to Leydig cell hypoplasia, (2) defects in adrenal and testicular steroidogenesis from mutations within steroidogenic pathway factors (STAR, CYP11A1, HSD3B, CYP17A1, or HSD17B), (3) altered steroidogenesis because of disrupted electron transfer resulting from cytochrome P450 reductase and cytochrome b5 defects, (4) testosterone metabolism defects as a result of 5α-reductase type 2 deficiency that interferes with the conversion of testosterone to dihydrotestosterone, and (5) androgen receptor defects resulting in complete or partial androgen insensitivity syndrome (127). Several mutations have been identified in each of these associated genes, and the severity of the phenotype depends on the gene dosage and penetrance. Diagnosis of androgen production defects typically shows low testosterone levels and elevated steroids past the enzymatic blockage. While postpubertal diagnosis is made through basal testosterone levels, prepubertal diagnosis requires an hCG-stimulation test because basal testosterone levels are normal in these individuals (127, 128).
46,XY VSD from deficits in the HH pathway: HH signaling operates at every level of male sex differentiation, including testis development, androgen synthesis, and development of secondary sex characteristics, and therefore its disruption affects male sex differentiation processes, ultimately leading to 46,XY VSDs. In humans, mutations in DHH have been identified in patients with gonadal dysgenesis (129). Dhh knockout mouse models exhibit a similar phenotype and present with impaired FLC differentiation, low testosterone levels, defective testis cord formation, and disrupted spermatogenesis (130, 131). Although mutations in SHH, PTCH1, and SMO affect HH signaling in vitro, they have not been linked to VSD, likely because of their essential role in embryonic survival. Studies have shown, however, that pharmacological inhibition of SMO or GLI factors in ex vivo testis culture affect differentiation of FLC (132). Mutations in GLI3, found in Greig cephalopolysyndactyly syndrome (GCPS) and Pallister-Hall syndrome (PHS), as well as their respective mouse models, exhibit VSD phenotypes (133). We showed that VSD phenotypes in Gli3XtJ mutant mice, a model of GCPS, are due to local effects of Gli3 loss in addition to systemic effects of testicular hormone deficiency (8). Further, a recent study reported a HH acetyl-transferase gene (HHAT) mutation in a syndromic form of 46,XY VSD with complete gonadal dysgenesis and other HH phenotypes (134). This mutation disrupted the ability of the HHAT protein to palmitoylate both DHH and SHH, which explains the observed phenotypes. Hhat−/− mice display defective gonad development including impaired FLC differentiation, Sertoli cell polarity, and testis cord formation (134).
46,XY VSD associated with disorders of cholesterol synthesis: These VSDs are classified under 46,XY VSDs due to androgen defects because of the role for cholesterol acting as a substrate in androgen synthesis. In addition, morphological features of disorders of cholesterol synthesis overlap those observed in a defective HH signaling pathway, suggesting a functional connection (26, 28). Although cholesterol levels are normal in genetic disorders of HH signaling, SLOS clearly shows evidence of impaired HH signal transduction and associated phenotypes (135). Overall, 70% of male SLOS patients present with ambiguous genitalia, 25% with female external genitalia, and 50% with cryptorchidism and/or hypospadias (128, 136, 137). Importantly, VSD genetic screening panels include DHCR7 and DHCR24 as candidate genes that are mutated in SLOS and desmosterolosis, respectively (138). Antley-Bixler syndrome (ABS) is caused by mutations in POR or fibroblast growth factor receptor genes. In some patients with ABS, reduced activity of the cholesterogenic CYP51A1 has also been reported. Many cases of ABS exhibit a VSD phenotype (139). Fluconazole, an antifungal medication that inhibits CYP51A1 when exposed in utero causes the same phenotype as ABS, including ambiguous or underdeveloped genitalia (140). Prenatal diagnosis of SLOS is possible by analyzing sterols in amniotic fluid, chorionic villus, and cord blood. Further, prenatal screening is offered to families with history of previous affected children and carriers for SLOS (141-143). In such cases, prenatal diagnosis of SLOS can include fetal androgen levels as well. Activation of the hypothalamic-pituitary-gonadal axis and testosterone synthesis are observed in infants in the first 6 months. This phase, called minipuberty, is linked to the onset of puberty and the adult reproductive capacity. In many cases of VSD, hormone therapy is offered during this period (144). Therefore, including testosterone in endocrine screening of SLOS infants would help in the long-term outcome of DSDs, which is fertility potential.
Summary
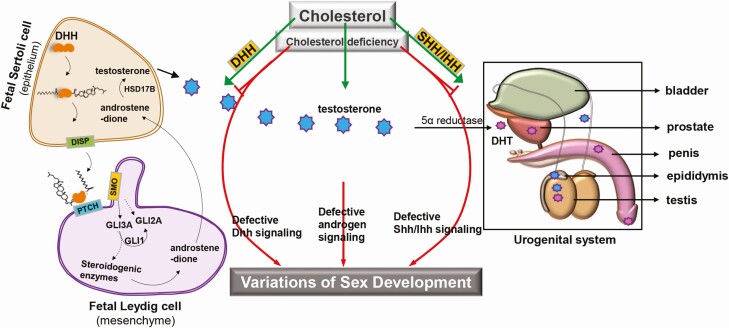
At the molecular level, male sex differentiation requires fetal testis-derived hormones and a number of local signaling pathways. In particular, intricate interactions between androgen activity and HH pathways are necessary for optimal development of primary and secondary sex organs with multiple pathway interactions for each tissue. Interestingly, the interaction between androgen and HH signaling pathways occurs at various levels. Evidence shows that the HH/androgen interface initiates within FLC at the level of the fetal testis and sets the foundation for HH/androgen interactions in mediating male sex differentiation as the embryo develops. At a critical time point in fetal testis development, paracrine DHH signals initiate SMO activity and hence, transactivator form of GLI3, likely in concert with GLI1 and GLI2, to stimulate steroidogenic enzyme pathway genes to provide the foundation for androgen synthesis within the FLC. Circulating androgens must reach critical threshold levels to mediate testis descent, stabilization of Wolffian ducts, stimulate IHH to promote male genital tubercle development, and cooperate with SHH in prostate development and masculinization of external genitalia. Timing of these events is crucial. Cholesterol is an integral part of both androgen synthesis and HH signaling activity, indicating a functional connection to male sex differentiation. Thus, cholesterol deficiency can lead to defective DHH signaling and androgen production within the fetal testis, and defective SHH/IHH signaling in the urogenital organs, ultimately resulting in VSDs (Fig. 6).
Figure 6.
VSD associated with cholesterol deficiency. Cholesterol is essential for 2 of the most important resources required for male sex differentiation: androgen synthesis and HH signaling. Within the fetal testis, Sertoli cells produce DHH, which diffuses to the interstitium where it interacts with PTCH receptors to stimulate fetal Leydig cell differentiation. Bound PTCH releases SMO to stabilize the activated GLI3 transcription factor (GLI3A), which, likely along with GLI2A, stimulates steroidogenic pathway genes to initiate and maintain androgen production. Fetal Leydig cells produce androstenedione, which is converted to testosterone in Sertoli cells. Testosterone is released into fetal circulation and converted to dihydrotestosterone (DHT) in target tissues to promote urogenital development in cooperation with SHH/IHH signaling pathways. Cholesterol deficiency leads to defective androgen production and DHH signaling within the fetal testis, and defective SHH/IHH signaling in the urogenital organs, ultimately resulting in VSD. Abbreviations: DHH, Desert hedgehog; HH, hedgehog; IHH, Indian hedgehog; PTCH, Patched; SHH, Sonic hedgehog; VSD, variations of sex development.
Acknowledgments
Financial Support: This work was supported by the National Institutes of Health (R01HD090660).
Glossary
Abbreviations
- ABS
Antley-Bixler syndrome
- AGD
anogenital distance
- AR
androgen receptor
- CoA
coenzyme A
- DHH
Desert hedgehog
- DISP
Dispatched
- DSD
disorders/differences of sex development
- E
embryonic day
- ER
endoplasmic reticulum
- FLC
fetal Leydig cell
- GT
genital tubercle
- GW
gestational week
- HH
hedgehog
- HMGCR
hydroxymethylglutaryl-CoA reductase
- IHH
Indian hedgehog
- LDL
low-density lipoprotein
- MPW
masculinization programming window
- PTCH
Patched
- SHH
Sonic hedgehog
- SLOS
Smith-Lemli-Opitz syndrome
- SMO
Smoothened
- SSD
sterol sensing domain
- VSD
variations of sex development
- WD
Wolffian duct
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Lee PA, Houk CP, Ahmed SF, Hughes IA; International Consensus Conference on Intersex organized by the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology . Consensus statement on management of intersex disorders. International Consensus Conference on Intersex. Pediatrics. 2006;118(2):e488-e500. [DOI] [PubMed] [Google Scholar]
- 2. Krege S, Eckoldt F, Richter-Unruh A, et al. Variations of sex development: the first German interdisciplinary consensus paper. J Pediatr Urol. 2019;15(2):114-123. [DOI] [PubMed] [Google Scholar]
- 3. Erdoğan S, Kara C, Uçaktürk A, Aydın M. Etiological classification and clinical assessment of children and adolescents with disorders of sex development. J Clin Res Pediatr Endocrinol. 2011;3(2):77-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kalfa N, Philibert P, Sultan C. Is hypospadias a genetic, endocrine or environmental disease, or still an unexplained malformation? Int J Androl. 2009;32(3):187-197. [DOI] [PubMed] [Google Scholar]
- 5. Foresta C, Zuccarello D, Garolla A, Ferlin A. Role of hormones, genes, and environment in human cryptorchidism. Endocr Rev. 2008;29(5):560-580. [DOI] [PubMed] [Google Scholar]
- 6. Franco HL, Yao HH. Sex and hedgehog: roles of genes in the hedgehog signaling pathway in mammalian sexual differentiation. Chromosome Res. 2012;20(1):247-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morohashi K, Baba T, Tanaka M. Steroid hormones and the development of reproductive organs. Sex Dev. 2013;7(1-3):61-79. [DOI] [PubMed] [Google Scholar]
- 8. Kothandapani A, Lewis SR, Noel JL, et al. GLI3 resides at the intersection of hedgehog and androgen action to promote male sex differentiation. Plos Genet. 2020;16(6):e1008810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zheng Z, Armfield BA, Cohn MJ. Timing of androgen receptor disruption and estrogen exposure underlies a spectrum of congenital penile anomalies. Proc Natl Acad Sci U S A. 2015;112(52):E7194-E7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miyagawa S, Matsumaru D, Murashima A, et al. The role of sonic hedgehog-Gli2 pathway in the masculinization of external genitalia. Endocrinology. 2011;152(7):2894-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jeong J, McMahon AP. Cholesterol modification of Hedgehog family proteins. J Clin Invest. 2002;110(5):591-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cortes VA, Busso D, Maiz A, Arteaga A, Nervi F, Rigotti A. Physiological and pathological implications of cholesterol. Front Biosci (Landmark Ed). 2014;19:416-428. [DOI] [PubMed] [Google Scholar]
- 13. Platt FM, Wassif C, Colaco A, et al. Disorders of cholesterol metabolism and their unanticipated convergent mechanisms of disease. Annu Rev Genomics Hum Genet. 2014;15:173-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kelley RI, Hennekam RC. The Smith-Lemli-Opitz syndrome. J Med Genet. 2000;37(5):321-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luo J, Yang H, Song BL. Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol. 2020;21(4):225-245. [DOI] [PubMed] [Google Scholar]
- 16. Incardona JP, Eaton S. Cholesterol in signal transduction. Curr Opin Cell Biol. 2000;12(2):193-203. [DOI] [PubMed] [Google Scholar]
- 17. Woollett LA. Maternal cholesterol in fetal development: transport of cholesterol from the maternal to the fetal circulation. Am J Clin Nutr. 2005;82(6):1155-1161. [DOI] [PubMed] [Google Scholar]
- 18. Woollett LA. Where does fetal and embryonic cholesterol originate and what does it do? Annu Rev Nutr. 2008;28:97-114. [DOI] [PubMed] [Google Scholar]
- 19. Baardman ME, Kerstjens-Frederikse WS, Berger RM, Bakker MK, Hofstra RM, Plösch T. The role of maternal-fetal cholesterol transport in early fetal life: current insights. Biol Reprod. 2013;88(1):24. [DOI] [PubMed] [Google Scholar]
- 20. Woollett LA. Review: transport of maternal cholesterol to the fetal circulation. Placenta. 2011;32(Suppl 2):S218-S221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pecks U, Bornemann V, Klein A, et al. Estimating fetal cholesterol synthesis rates by cord blood analysis in intrauterine growth restriction and normally grown fetuses. Lipids Health Dis. 2019;18(1):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nemec SF, Nemec U, Brugger PC, et al. Male genital abnormalities in intrauterine growth restriction. Prenat Diagn. 2012;32(5):427-431. [DOI] [PubMed] [Google Scholar]
- 23. Waterham HR. Defects of cholesterol biosynthesis. FEBS Lett. 2006;580(23):5442-5449. [DOI] [PubMed] [Google Scholar]
- 24. Porter FD, Herman GE. Malformation syndromes caused by disorders of cholesterol synthesis. J Lipid Res. 2011;52(1):6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Svoboda MD, Christie JM, Eroglu Y, Freeman KA, Steiner RD. Treatment of Smith-Lemli-Opitz syndrome and other sterol disorders. Am J Med Genet C Semin Med Genet. 2012;160C(4):285-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blassberg R, Macrae JI, Briscoe J, Jacob J. Reduced cholesterol levels impair Smoothened activation in Smith-Lemli-Opitz syndrome. Hum Mol Genet. 2016;25(4):693-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kong JH, Siebold C, Rohatgi R. Biochemical mechanisms of vertebrate hedgehog signaling. Development. 2019;146(10):dev166892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cooper MK, Wassif CA, Krakowiak PA, et al. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat Genet. 2003;33(4):508-513. [DOI] [PubMed] [Google Scholar]
- 29. Pathi S, Pagan-Westphal S, Baker DP, et al. Comparative biological responses to human Sonic, Indian, and Desert hedgehog. Mech Dev. 2001;106(1-2):107-117. [DOI] [PubMed] [Google Scholar]
- 30. Gallet A, Rodriguez R, Ruel L, Therond PP. Cholesterol modification of hedgehog is required for trafficking and movement, revealing an asymmetric cellular response to hedgehog. Dev Cell. 2003;4(2):191-204. [DOI] [PubMed] [Google Scholar]
- 31. Blassberg R, Jacob J. Lipid metabolism fattens up hedgehog signaling. BMC Biol. 2017;15(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274(5285):255-259. [DOI] [PubMed] [Google Scholar]
- 33. Buglino JA, Resh MD. Palmitoylation of Hedgehog proteins. Vitam Horm. 2012;88:229-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ramsbottom SA, Pownall ME. Regulation of Hedgehog signalling inside and outside the cell. J Dev Biol. 2016;4(3):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lewis PM, Dunn MP, McMahon JA, et al. Cholesterol modification of sonic hedgehog is required for long-range signaling activity and effective modulation of signaling by Ptc1. Cell. 2001;105(5):599-612. [DOI] [PubMed] [Google Scholar]
- 36. Ma Y, Erkner A, Gong R, et al. Hedgehog-mediated patterning of the mammalian embryo requires transporter-like function of dispatched. Cell. 2002;111(1):63-75. [DOI] [PubMed] [Google Scholar]
- 37. Kawakami T, Kawcak T, Li YJ, Zhang W, Hu Y, Chuang PT. Mouse dispatched mutants fail to distribute hedgehog proteins and are defective in hedgehog signaling. Development. 2002;129(24):5753-5765. [DOI] [PubMed] [Google Scholar]
- 38. Tukachinsky H, Kuzmickas RP, Jao CY, Liu J, Salic A. Dispatched and scube mediate the efficient secretion of the cholesterol-modified hedgehog ligand. Cell Rep. 2012;2(2):308-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Taipale J, Cooper MK, Maiti T, Beachy PA. Patched acts catalytically to suppress the activity of Smoothened. Nature. 2002;418(6900):892-897. [DOI] [PubMed] [Google Scholar]
- 40. Chen Y, Struhl G. In vivo evidence that Patched and Smoothened constitute distinct binding and transducing components of a Hedgehog receptor complex. Development. 1998;125(24):4943-4948. [DOI] [PubMed] [Google Scholar]
- 41. Zhang Y, Bulkley DP, Xin Y, et al. Structural basis for cholesterol transport-like activity of the Hedgehog receptor Patched. Cell. 2018;175(5):1352-1364.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Qi X, Schmiege P, Coutavas E, Wang J, Li X. Structures of human Patched and its complex with native palmitoylated sonic hedgehog. Nature. 2018;560(7716):128-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hall ET, Cleverdon ER, Ogden SK. Dispatching sonic Hedgehog: molecular mechanisms controlling deployment. Trends Cell Biol. 2019;29(5):385-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Qi X, Li X. Mechanistic Insights into the generation and transduction of Hedgehog signaling. Trends Biochem Sci. 2020;45(5):397-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kowatsch C, Woolley RE, Kinnebrew M, Rohatgi R, Siebold C. Structures of vertebrate Patched and Smoothened reveal intimate links between cholesterol and Hedgehog signalling. Curr Opin Struct Biol. 2019;57:204-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Radhakrishnan A, Rohatgi R, Siebold C. Cholesterol access in cellular membranes controls Hedgehog signaling. Nat Chem Biol. 2020;16(12):1303-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fleet A, Lee JP, Tamachi A, Javeed I, Hamel PA. Activities of the cytoplasmic domains of Patched-1 modulate but are not essential for the regulation of canonical Hedgehog signaling. J Biol Chem. 2016;291(34):17557-17568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Johnson RL, Zhou L, Bailey EC. Distinct consequences of sterol sensor mutations in Drosophila and mouse patched homologs. Dev Biol. 2002;242(2):224-235. [DOI] [PubMed] [Google Scholar]
- 49. Byrne EF, Luchetti G, Rohatgi R, Siebold C. Multiple ligand binding sites regulate the Hedgehog signal transducer Smoothened in vertebrates. Curr Opin Cell Biol. 2018;51:81-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Luchetti G, Sircar R, Kong JH, et al. Cholesterol activates the G-protein coupled receptor Smoothened to promote Hedgehog signaling. Elife. 2016;5:e20304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Huang P, Nedelcu D, Watanabe M, et al. Cellular cholesterol directly activates Smoothened in Hedgehog signaling. Cell. 2016;166(5):1176-1187.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nedelcu D, Liu J, Xu Y, Jao C, Salic A. Oxysterol binding to the extracellular domain of Smoothened in Hedgehog signaling. Nat Chem Biol. 2013;9(9):557-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Qi X, Liu H, Thompson B, McDonald J, Zhang C, Li X. Cryo-EM structure of oxysterol-bound human Smoothened coupled to a heterotrimeric Gi. Nature. 2019;571(7764):279-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kinnebrew M, Iverson EJ, Patel BB, et al. Cholesterol accessibility at the ciliary membrane controls hedgehog signaling. Elife. 2019;8:e50051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Maerz LD, Burkhalter MD, Schilpp C, Wittekindt OH, Frick M, Philipp M. Pharmacological cholesterol depletion disturbs ciliogenesis and ciliary function in developing zebrafish. Commun Biol. 2019;2:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Weiss LE, Milenkovic L, Yoon J, Stearns T, Moerner WE. Motional dynamics of single Patched1 molecules in cilia are controlled by Hedgehog and cholesterol. Proc Natl Acad Sci U S A. 2019;116(12):5550-5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Walterhouse DO, Lamm ML, Villavicencio E, Iannaccone PM. Emerging roles for hedgehog-patched-Gli signal transduction in reproduction. Biol Reprod. 2003;69(1):8-14. [DOI] [PubMed] [Google Scholar]
- 58. Yao HH, Whoriskey W, Capel B. Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev. 2002;16(11):1433-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Berman DM, Desai N, Wang X, et al. Roles for Hedgehog signaling in androgen production and prostate ductal morphogenesis. Dev Biol. 2004;267(2):387-398. [DOI] [PubMed] [Google Scholar]
- 60. Haraguchi R, Mo R, Hui C, et al. Unique functions of Sonic hedgehog signaling during external genitalia development. Development. 2001;128(21):4241-4250. [DOI] [PubMed] [Google Scholar]
- 61. Seifert AW, Bouldin CM, Choi KS, Harfe BD, Cohn MJ. Multiphasic and tissue-specific roles of sonic hedgehog in cloacal septation and external genitalia development. Development. 2009;136(23):3949-3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lin C, Yin Y, Veith GM, Fisher AV, Long F, Ma L. Temporal and spatial dissection of Shh signaling in genital tubercle development. Development. 2009;136(23):3959-3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Murashima A, Akita H, Okazawa M, et al. Midline-derived Shh regulates mesonephric tubule formation through the paraxial mesoderm. Dev Biol. 2014;386(1):216-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. MacLaughlin DT, Donahoe PK. Sex determination and differentiation. N Engl J Med. 2004;350(4):367-378. [DOI] [PubMed] [Google Scholar]
- 65. Nef S, Parada LF. Hormones in male sexual development. Genes Dev. 2000;14(24):3075-3086. [DOI] [PubMed] [Google Scholar]
- 66. Wang Y, Ni C, Li X, et al. Phthalate-induced fetal Leydig cell dysfunction mediates male reproductive tract anomalies. Front Pharmacol. 2019;10:1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Witchel SF. Disorders of sex development. Best Pract Res Clin Obstet Gynaecol. 2018;48:90-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ross AJ, Capel B. Signaling at the crossroads of gonad development. Trends Endocrinol Metab. 2005;16(1):19-25. [DOI] [PubMed] [Google Scholar]
- 69. Shima Y. Development of fetal and adult Leydig cells. Reprod Med Biol. 2019;18(4):323-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. O’Shaughnessy PJ, Fowler PA. Endocrinology of the mammalian fetal testis. Reproduction. 2011;141(1):37-46. [DOI] [PubMed] [Google Scholar]
- 71. Sharpe RM. Androgens and the masculinization programming window: human-rodent differences. Biochem Soc Trans. 2020;48(4):1725-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Habert R, Lejeune H, Saez JM. Origin, differentiation and regulation of fetal and adult Leydig cells. Mol Cell Endocrinol. 2001;179(1-2):47-74. [DOI] [PubMed] [Google Scholar]
- 73. Welsh M, Suzuki H, Yamada G. The masculinization programming window. Endocr Dev. 2014;27:17-27. [DOI] [PubMed] [Google Scholar]
- 74. Shima Y, Miyabayashi K, Sato T, et al. Fetal Leydig cells dedifferentiate and serve as adult Leydig stem cells. Development. 2018;145(23):dev169136. [DOI] [PubMed] [Google Scholar]
- 75. Hayes FJ, DeCruz S, Seminara SB, Boepple PA, Crowley WF Jr. Differential regulation of gonadotropin secretion by testosterone in the human male: absence of a negative feedback effect of testosterone on follicle-stimulating hormone secretion. J Clin Endocrinol Metab. 2001;86(1):53-58. [DOI] [PubMed] [Google Scholar]
- 76. Saez JM, Tabone E, Perrard-Sapori MH, Rivarola MA. Paracrine role of Sertoli cells. Med Biol. 1986;63(5-6):225-236. [PubMed] [Google Scholar]
- 77. Tang H, Brennan J, Karl J, Hamada Y, Raetzman L, Capel B. Notch signaling maintains Leydig progenitor cells in the mouse testis. Development. 2008;135(22):3745-3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Brennan J, Tilmann C, Capel B. Pdgfr-alpha mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev. 2003;17(6):800-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yao HH, Capel B. Disruption of testis cords by cyclopamine or forskolin reveals independent cellular pathways in testis organogenesis. Dev Biol. 2002;246(2):356-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Szczepny A, Hogarth CA, Young J, Loveland KL. Identification of Hedgehog signaling outcomes in mouse testis development using a hanging drop-culture system. Biol Reprod. 2009;80(2):258-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tang C, Pan Y, Luo H, et al. Hedgehog signaling stimulates the conversion of cholesterol to steroids. Cell Signal. 2015;27(3):487-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Barsoum I, Yao HH. Redundant and differential roles of transcription factors Gli1 and Gli2 in the development of mouse fetal Leydig cells. Biol Reprod. 2011;84(5):894-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32(1):81-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Miller WL, Bose HS. Early steps in steroidogenesis: intracellular cholesterol trafficking. J Lipid Res. 2011;52(12):2111-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. McClelland KS, Bell K, Larney C, et al. Purification and transcriptomic analysis of mouse fetal Leydig cells reveals candidate genes for specification of gonadal steroidogenic cells. Biol Reprod. 2015;92(6):145. [DOI] [PubMed] [Google Scholar]
- 86. Miyabayashi K, Shima Y, Inoue M, et al. Alterations in fetal Leydig cell gene expression during fetal and adult development. Sex Dev. 2017;11(2):53-63. [DOI] [PubMed] [Google Scholar]
- 87. Rebourcet D, Mackay R, Darbey A, et al. Ablation of the canonical testosterone production pathway via knockout of the steroidogenic enzyme HSD17B3, reveals a novel mechanism of testicular testosterone production. Faseb J. 2020;34(8):10373-10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Marchetti PM, Barth JH. Clinical biochemistry of dihydrotestosterone. Ann Clin Biochem. 2013;50(Pt 2):95-107. [DOI] [PubMed] [Google Scholar]
- 89. Büdefeld T, Jezek D, Rozman D, Majdic G. Initiation of steroidogenesis precedes expression of cholesterologenic enzymes in the fetal mouse testes. Anat Histol Embryol. 2009;38(6):461-466. [DOI] [PubMed] [Google Scholar]
- 90. Carr BR, Parker CR Jr, Ohashi M, MacDonald PC, Simpson ER. Regulation of human fetal testicular secretion of testosterone: low-density lipoprotein-cholesterol and cholesterol synthesized de novo as steroid precursor. Am J Obstet Gynecol. 1983;146(3):241-247. [DOI] [PubMed] [Google Scholar]
- 91. Eacker SM, Agrawal N, Qian K, et al. Hormonal regulation of testicular steroid and cholesterol homeostasis. Mol Endocrinol. 2008;22(3):623-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hu J, Zhang Z, Shen WJ, Azhar S. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr Metab (Lond). 2010;7:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Freeman DA, Ascoli M. Studies on the source of cholesterol used for steroid biosynthesis in cultured Leydig tumor cells. J Biol Chem. 1982;257(23):14231-14238. [PubMed] [Google Scholar]
- 94. Smals AG, Weusten JJ, Benraad TJ, Kloppenborg PW. The HMG-CoA reductase inhibitor simvastatin suppresses human testicular testosterone synthesis in vitro by a selective inhibitory effect on 17-ketosteroid-oxidoreductase enzyme activity. J Steroid Biochem Mol Biol. 1991;38(4):465-468. [DOI] [PubMed] [Google Scholar]
- 95. Schooling CM, Au Yeung SL, Freeman G, Cowling BJ. The effect of statins on testosterone in men and women, a systematic review and meta-analysis of randomized controlled trials. BMC Med. 2013;11:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Beverly BE, Lambright CS, Furr JR, et al. Simvastatin and dipentyl phthalate lower ex vivo testicular testosterone production and exhibit additive effects on testicular testosterone and gene expression via distinct mechanistic pathways in the fetal rat. Toxicol Sci. 2014;141(2):524-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Windley SP, Wilhelm D. Signaling Pathways Involved in Mammalian Sex Determination and Gonad Development. Sex Dev. 2015;9(6):297-315. [DOI] [PubMed] [Google Scholar]
- 98. Dean A, Smith LB, Macpherson S, Sharpe RM. The effect of dihydrotestosterone exposure during or prior to the masculinization programming window on reproductive development in male and female rats. Int J Androl. 2012;35(3):330-339. [DOI] [PubMed] [Google Scholar]
- 99. Macleod DJ, Sharpe RM, Welsh M, et al. Androgen action in the masculinization programming window and development of male reproductive organs. Int J Androl. 2010;33(2):279-287. [DOI] [PubMed] [Google Scholar]
- 100. Doles J, Cook C, Shi X, Valosky J, Lipinski R, Bushman W. Functional compensation in Hedgehog signaling during mouse prostate development. Dev Biol. 2006;295(1):13-25. [DOI] [PubMed] [Google Scholar]
- 101. Tong SY, Hutson JM, Watts LM. Does testosterone diffuse down the wolffian duct during sexual differentiation? J Urol. 1996;155(6):2057-2059. [PubMed] [Google Scholar]
- 102. Murashima A, Kishigami S, Thomson A, Yamada G. Androgens and mammalian male reproductive tract development. Biochim Biophys Acta. 2015;1849(2):163-170. [DOI] [PubMed] [Google Scholar]
- 103. Archambeault DR, Tomaszewski J, Joseph A, Hinton BT, Yao HH. Epithelial-mesenchymal crosstalk in Wolffian duct and fetal testis cord development. Genesis. 2009;47(1):40-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Perriton CL, Powles N, Chiang C, Maconochie MK, Cohn MJ. Sonic hedgehog signaling from the urethral epithelium controls external genital development. Dev Biol. 2002;247(1):26-46. [DOI] [PubMed] [Google Scholar]
- 105. O’Hara L, Welsh M, Saunders PT, Smith LB. Androgen receptor expression in the caput epididymal epithelium is essential for development of the initial segment and epididymal spermatozoa transit. Endocrinology. 2011;152(2):718-729. [DOI] [PubMed] [Google Scholar]
- 106. Miyamoto J, Asanuma H, Nakai H, Hasegawa T, Nawata H, Hasegawa Y. Mutational analysis of androgen receptor (AR) gene in 46,XY patients with ambiguous genitalia and normal testosterone secretion: endocrinological characteristics of three patients with AR gene mutations. Clin Pediatr Endocrinol. 2006;15(4):151-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Akcay T, Fernandez-Cancio M, Turan S, Güran T, Audi L, Bereket A. AR and SRD5A2 gene mutations in a series of 51 Turkish 46,XY DSD children with a clinical diagnosis of androgen insensitivity. Andrology. 2014;2(4):572-578. [DOI] [PubMed] [Google Scholar]
- 108. Virtanen HE, Cortes D, Rajpert-De Meyts E, et al. Development and descent of the testis in relation to cryptorchidism. Acta Paediatr. 2007;96(5):622-627. [DOI] [PubMed] [Google Scholar]
- 109. Zhao F, Yao HH. A tale of two tracts: history, current advances, and future directions of research on sexual differentiation of reproductive tracts†. Biol Reprod. 2019;101(3):602-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Joseph A, Yao H, Hinton BT. Development and morphogenesis of the Wolffian/epididymal duct, more twists and turns. Dev Biol. 2009;325(1):6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Welsh M, Saunders PT, Sharpe RM. The critical time window for androgen-dependent development of the Wolffian duct in the rat. Endocrinology. 2007;148(7):3185-3195. [DOI] [PubMed] [Google Scholar]
- 112. Farikullah J, Ehtisham S, Nappo S, Patel L, Hennayake S. Persistent Müllerian duct syndrome: lessons learned from managing a series of eight patients over a 10-year period and review of literature regarding malignant risk from the Müllerian remnants. BJU Int. 2012;110(11 Pt C):E1084-E1089. [DOI] [PubMed] [Google Scholar]
- 113. Cunha GR, Vezina CM, Isaacson D, et al. Development of the human prostate. Differentiation. 2018;103:24-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Cunha GR, Baskin L. Development of human male and female urogenital tracts. Differentiation. 2018;103:1-4. [DOI] [PubMed] [Google Scholar]
- 115. Keil KP, Mehta V, Abler LL, Joshi PS, Schmitz CT, Vezina CM. Visualization and quantification of mouse prostate development by in situ hybridization. Differentiation. 2012;84(3):232-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Prins GS, Putz O. Molecular signaling pathways that regulate prostate gland development. Differentiation. 2008;76(6):641-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Georgas KM, Armstrong J, Keast JR, et al. An illustrated anatomical ontology of the developing mouse lower urogenital tract. Development. 2015;142(10):1893-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Keil KP, Abler LL, Laporta J, et al. Androgen receptor DNA methylation regulates the timing and androgen sensitivity of mouse prostate ductal development. Dev Biol. 2014;396(2):237-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Matsushita S, Suzuki K, Murashima A, et al. Regulation of masculinization: androgen signalling for external genitalia development. Nat Rev Urol. 2018;15(6):358-368. [DOI] [PubMed] [Google Scholar]
- 120. Suzuki K, Matsumaru D, Matsushita S, et al. Epispadias and the associated embryopathies: genetic and developmental basis. Clin Genet. 2017;91(2):247-253. [DOI] [PubMed] [Google Scholar]
- 121. Salazar-Martinez E, Romano-Riquer P, Yanez-Marquez E, Longnecker MP, Hernandez-Avila M. Anogenital distance in human male and female newborns: a descriptive, cross-sectional study. Environ Health. 2004;3(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Schwartz CL, Christiansen S, Vinggaard AM, Axelstad M, Hass U, Svingen T. Anogenital distance as a toxicological or clinical marker for fetal androgen action and risk for reproductive disorders. Arch Toxicol. 2019;93(2):253-272. [DOI] [PubMed] [Google Scholar]
- 123. Chitty LS, Chatelain P, Wolffenbuttel KP, Aigrain Y. Prenatal management of disorders of sex development. J Pediatr Urol. 2012;8(6):576-584. [DOI] [PubMed] [Google Scholar]
- 124. van der Straaten S, Springer A, Zecic A, et al. The External Genitalia Score (EGS): a European Multicenter Validation Study. J Clin Endocrinol Metab. 2020;105(3):e222-e230. [DOI] [PubMed] [Google Scholar]
- 125. Vasundhera C, Jyotsna VP, Kandasamy D, Gupta N. Clinical, hormonal and radiological profile of 46XY disorders of sexual development. Indian J Endocrinol Metab. 2016;20(3):300-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. García-Acero M, Moreno O, Suárez F, Rojas A. Disorders of sexual development: current status and progress in the diagnostic approach. Curr Urol. 2020;13(4):169-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Mendonca BB, Costa EM, Belgorosky A, Rivarola MA, Domenice S. 46,XY DSD due to impaired androgen production. Best Pract Res Clin Endocrinol Metab. 2010;24(2):243-262. [DOI] [PubMed] [Google Scholar]
- 128. Mendonca BB, Domenice S, Arnhold IJ, Costa EM. 46,XY disorders of sex development (DSD). Clin Endocrinol (Oxf). 2009;70(2):173-187. [DOI] [PubMed] [Google Scholar]
- 129. Paris F, Flatters D, Caburet S, et al. A novel variant of DHH in a familial case of 46,XY disorder of sex development: insights from molecular dynamics simulations. Clin Endocrinol (Oxf). 2017;87(5):539-544. [DOI] [PubMed] [Google Scholar]
- 130. Clark AM, Garland KK, Russell LD. Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biol Reprod. 2000;63(6):1825-1838. [DOI] [PubMed] [Google Scholar]
- 131. Park SY, Tong M, Jameson JL. Distinct roles for steroidogenic factor 1 and desert hedgehog pathways in fetal and adult Leydig cell development. Endocrinology. 2007;148(8):3704-3710. [DOI] [PubMed] [Google Scholar]
- 132. Barsoum I, Yao HH. Redundant and differential roles of transcription factors Gli1 and Gli2 in the development of mouse fetal Leydig cells. Biol Reprod. 2011;84(5):894-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. He F, Akbari P, Mo R, et al. Adult Gli2+/-;Gli3Δ699/+ male and female mice display a spectrum of genital malformation. Plos One. 2016;11(11):e0165958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Callier P, Calvel P, Matevossian A, et al. Loss of function mutation in the palmitoyl-transferase HHAT leads to syndromic 46,XY disorder of sex development by impeding Hedgehog protein palmitoylation and signaling. Plos Genet. 2014;10(5):e1004340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Biesecker LG, Kang S, Schäffer AA, et al. Exclusion of candidate loci and cholesterol biosynthetic abnormalities in familial Pallister-Hall syndrome. J Med Genet. 1996;33(11):947-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Joseph DB, Uehling DT, Gilbert E, Laxova R. Genitourinary abnormalities associated with the Smith-Lemli-Opitz syndrome. J Urol. 1987;137(4):719-721. [DOI] [PubMed] [Google Scholar]
- 137. Bashamboo A, McElreavey K. Consanguinity and disorders of sex development. Hum Hered. 2014;77(1-4):108-117. [DOI] [PubMed] [Google Scholar]
- 138. Kolesinska Z, Acierno J Jr, Ahmed SF, et al. Integrating clinical and genetic approaches in the diagnosis of 46,XY disorders of sex development. Endocr Connect. 2018;7(12):1480-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kelley RI, Kratz LE, Glaser RL, Netzloff ML, Wolf LM, Jabs EW. Abnormal sterol metabolism in a patient with Antley-Bixler syndrome and ambiguous genitalia. Am J Med Genet. 2002;110(2):95-102. [DOI] [PubMed] [Google Scholar]
- 140. Pursley TJ, Blomquist IK, Abraham J, Andersen HF, Bartley JA. Fluconazole-induced congenital anomalies in three infants. Clin Infect Dis. 1996;22(2):336-340. [DOI] [PubMed] [Google Scholar]
- 141. Abuelo DN, Tint GS, Kelley R, Batta AK, Shefer S, Salen G. Prenatal detection of the cholesterol biosynthetic defect in the Smith-Lemli-Opitz syndrome by the analysis of amniotic fluid sterols. Am J Med Genet. 1995;56(3):281-285. [DOI] [PubMed] [Google Scholar]
- 142. Kratz LE, Kelley RI. Prenatal diagnosis of the RSH/Smith-Lemli-Opitz syndrome. Am J Med Genet. 1999;82(5):376-381. [PubMed] [Google Scholar]
- 143. Tamura M, Isojima T, Kasama T, et al. Novel DHCR7 mutation in a case of Smith-Lemli-Opitz syndrome showing 46,XY disorder of sex development. Hum Genome Var. 2017;4:17015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Johannsen TH, Main KM, Ljubicic ML, et al. Sex differences in reproductive hormones during mini-puberty in infants with normal and disordered sex development. J Clin Endocrinol Metab. 2018;103(8):3028-3037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.