Abstract
Chronic drug use is theorized to induce cortico-striatal neuroplasticity, driving an allostatic process marked by increased sensitivity to drug-related cues and decreased sensitivity to natural rewards that results in anhedonia and a dearth of positive affect. As such, positive emotion regulation represents a key mechanistic target for addictions treatment. This paper provides a conceptual model detailing how mindfulness may synergize a range of positive affective mechanisms to reduce addictive behavior, from savoring the hedonic pleasure derived from natural rewards, to self-generating interoceptive reward responses, and ultimately to cultivating self-transcendent meaning. These therapeutic processes may restructure reward processing from over-valuation of drug-related rewards back to valuation of natural rewards, and hypothetically, “reset” the default mode network dysfunction that undergirds addiction.
Keywords: hedonic, Mindfulness-Oriented Recovery Enhancement, opioid, positive affect, reward, savoring
1. Introduction
Throughout history humans have used drugs to alter consciousness, and in particular, to modify negative emotions, blunting the sting of loss, the ache of sadness, the fire of rage, the bite of fear. Yet, individuals often engage in substance use not only to regulate negative emotions, but also to induce positive ones. Though the independence vs. bipolarity of negative and positive emotion has been long debated in the literature, modern theory suggests that negative and positive affect are not mere opposites on a bipolar continuum, but rather are constructed by the brain as phenomenologically distinct action tendencies [1]. Thus, in addition to the well-established connection between addiction and negative affectivity, there may be a unique association between positive affect and addictive behavior. In fact, a robust body of clinical and pre-clinical research indicates that recurrent substance use is associated with deficient positive affectivity and concomitant dysregulation of brain reward systems. Whether deficits in positive affect are the cause, correlate, or consequence of addiction remains uncertain, with rigorous longitudinal studies of this phenomenon just beginning to emerge.
The premise of this paper is that regardless of their point of origin in the natural history of substance use disorder (SUD), positive affective deficits fuel addictive behavior as a means of obtaining hedonic equilibrium, and therefore, positive emotion regulation represents a key mechanistic target in addiction treatment. To underscore this point, here I first provide a brief review of deficient positive affectivity in addiction, using opioid use disorder (OUD) as the paradigmatic example. Next, I present a conceptual model of the positive affective mechanisms by which interventions can reduce addictive behavior. Finally, I present empirical data from my own mindfulness research to support this model.
2. Deficient Positive Affectivity in Addiction: The Example of OUD
2.1. Brain Reward Deficits in Addiction.
Chronic substance use is thought to induce neuroplasticity in cortico-limbic-striatal circuitry, driving an allostatic process marked by increased sensitivity to drug-related cues and aversive stimuli (e.g., stressors and pain), coupled with an increasing insensitivity to natural rewards that results in reduced hedonic tone and mounting dysphoria. By stimulating the same neural systems that evolved to process the value of natural rewards like food and sex [2,3], drugs come to usurp the value of such primary rewards, as well as the secondary rewards (e.g., money) that serve as their proxy. To compensate for this shift in reward threshold and maintain hedonic equilibrium, individuals may escalate substance use, which elicits opponent processes in the brain that propel a downward spiral of emotion dysregulation, blunting positive affective responses to natural rewards while increasing reactivity to aversive states [4].
The allostatic effects of chronic drug use on hedonic neural functions are particularly pernicious in the case of opioid misuse and OUD insofar as exogenous opioids interact with endogenous opioid systems that mediate regulation of emotion and hedonic responses to natural rewards (including social rewards) [5]. Theoretical models suggest that chronic use of exogenous opioids (e.g., heroin, oxycodone) dysregulate positive emotional responding, promoting opioid dose escalation as a means of preserving a dwindling sense of well-being [6]. In that regard, survey research has consistently demonstrated that both opioid misuse and OUD are associated with elevated levels of anhedonia [7] – the self-reported inability to experience pleasure. Similarly, a growing body of psychophysiological studies demonstrate positive affective and reward-related deficits among individuals with opioid misuse and OUD. Compared with healthy controls, patients with current OUD and those in remission exhibited blunted emotional responses to positive stimuli relative to neutral stimuli [8]. Furthermore, patients with OUD evidence reduced neurophysiological responses to representing natural rewards relative to drug cues that significantly predicts future heroin use [9]. Reduced positive affective reactivity is also evident among people who misuse and are addicted to prescription opioids, who evidence reduced attentional, autonomic, and self-reported reward responsiveness, as well as blunted prefrontal cortical response to natural rewards [10–13]. Among opioid misusers, reduced positive affect in response to viewing positive images statistically mediates the association between opioid misuse severity and craving [10], suggesting that opioid misusers may crave opioids when they experience anhedonic responses to naturally-rewarding stimuli.
Such deficient positive affectivity among opioid users may stem from the long-term neuropsychopharmacological effects of opioids on the structure and function of brain reward circuitry. Cross-sectional human positron emission tomography studies indicate that patients with OUD have lower dopamine D2 receptor availability in the striatum than healthy controls [14]. Other cross-sectional studies found that patients with OUD and prescription opioid dependent patients exhibit reduced striatal grey matter [15], decreased amygdala volume and decreased functional connectivity between limbic and striatal regions, with greater years of opioid use associated with weaker nucleus accumbens functional connectivity [16]. Complementing these findings, children who were exposed to opioids in utero exhibit volumetric decreases in reward-related basal ganglia structures including nucleus accumbens, caudate, and putamen [17]. As suggestive as these data are, longitudinal studies are needed to determine the impact of opioid exposure on brain reward function. Few such studies exist. However, a longitudinal neuroimaging study of opioid-naïve chronic pain patients who initiated a one-month course of opioid therapy found volumetric decreases in gray matter of the amygdala and orbitofrontal cortex from pre- to post-treatment via tensor-based morphometry that were maintained approximately 6 months after cessation of opioid administration [18]. Pathophysiological changes in these two brain structures subserving reward processing support the notion that opioid exposure can undermine positive affectivity.
2.2. The Restructuring Reward Hypothesis.
If progression to addiction involves blunted natural reward processing and anhedonia which promotes drug use as a means of compensating for the resultant dysphoria, then it stands to reason that reversing this allostatic process might be an effective means of treating addiction. Thus, my colleagues and I proposed the restructuring reward hypothesis: shifting valuation from drug-related rewards back to valuation of natural rewards by strengthening positive emotion regulation will reduce craving and addictive behavior [19]. Unfortunately, extant pharmacotherapies for OUD (and other SUDs) have not been shown to remediate the aforementioned psychobiological deficits in positive emotion regulation. Blunted reactivity to non-drug rewards has been observed in dorsolateral prefrontal cortex (dlPFC) and ventral striatum among patients receiving medication for OUD (i.e., methadone) [20] – suggesting that reward deficits remain untreated by one of the most effective first-line medications for OUD. However, novel behavioral interventions may have particular promise in modulating positive affective and reward-related mechanisms. In the next section of this review, I provide a conceptual model of these potential therapeutic targets.
3. Conceptual Model
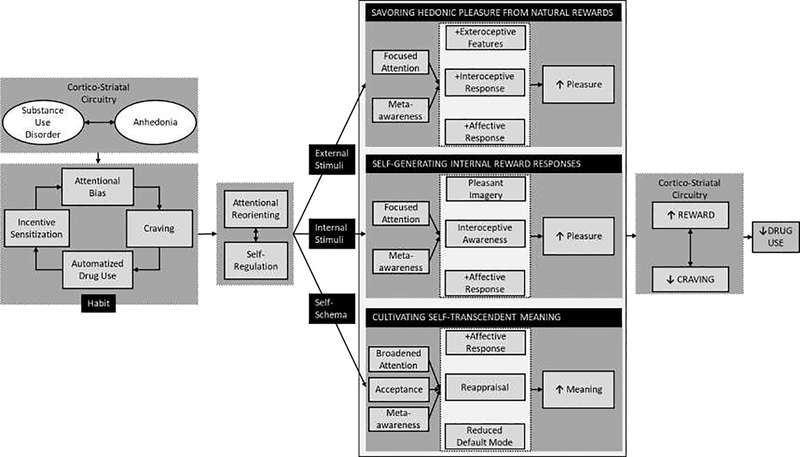
As shown in Figure 1, biobehavioral interventions for OUD and other forms of addiction could be optimized to target three core positive affective/reward mechanisms:
Figure 1. Conceptual model depicting positive affective/reward-related processes to be targeted by substance use disorder interventions.
Substance use disorders (SUD) and anhedonia amplify one another through reward/antireward processes kindled by neuroplastic changes to cortio-striatal brain circuits. Psychosocial interventions may leverage positive affective/reward-related processes to SUDs. Mindfulness and other cognitive-behavioral techniques can be used to disrupt the habit loop of attentional bias, craving, and automatized drug use by enhancing self-regulating of consummatory behaviors and re-orienting attention from drug cues to focus on three therapeutic targets: positive external stimuli, positive internal stimuli, or self-schemas. Through focused attention and meta-awareness, one may savor hedonic pleasure from natural rewards, noticing and appreciating the pleasant exteroceptive (i.e., perceptual) features of a naturally rewarding stimulus while cultivating meta-awareness of the positive emotions and pleasurable interoceptive sensations occasioned by that stimulus. Focused attention and meta-awareness may also enable one to attend to and self-generate internal reward responses in spite of aversive sensations linked with craving or withdrawal, by adopting fine-grained interoceptive awareness of extant pleasurable sensations, or using imagery to evoke the experience of embodied bliss. Finally, meta-awareness and acceptance may facilitate reappraisal of the relative value of drug and natural rewards, and attenuate default model processing of addiction-laden self-schemas, that can, with recurrent practice of contemplative techniques, cultivate self-transcendent meaning. By virtue of their effects on corticostriatal circuitry function, these three processes in turn restructure reward processing by shifting valuation from drug-related rewards back to natural rewards, thereby decreasing craving and addictive behavior.
Savoring hedonic pleasure from natural rewards by focusing on pleasant features of naturally rewarding stimuli and the resulting positive emotions and bodily responses that occur while doing so.
Attending to and self-generating internal reward responses by cultivating interoceptive awareness to delimit aversive somatic experiences (i.e., negative emotionality, pain, and craving), increase awareness of pleasurable sensations, and self-generate internal reward responses.
Reappraising meaning and cultivating self-transcendence by modulating associative learning and “default mode” self-referential processing, thereby tapping into stimulus-independent happiness and eudaimonic well-being.
Given its putative effects as a “domain general” cognitive amplifier [19], I hypothesize that mindfulness may facilitate these three downstream positive psychological mechanisms, which, by virtue of their potential effects on corticostriatal circuitry function, may restructure reward processing from valuation of drug-related rewards back to valuation of natural rewards. As an example of how addiction treatments might integrate these mechanisms of action, here I briefly detail my research on Mindfulness-Oriented Recovery Enhancement (MORE), a biobehavioral intervention that integrates mindfulness with reappraisal and savoring techniques to amplify positive emotion regulation and thereby reduce addictive behavior.
4. Savoring Hedonic Pleasure from Natural Rewards
By virtue of sensitization of the mesocorticolimbic dopamine system, repeated drug use imbues drug-related cues with incentive salience [21], resulting in an attentional bias towards such cues [22] that contributes to craving and addictive behavior [23]. Concomitantly, increased attentional engagement towards drug cues coupled with attentional disengagement from natural rewards predicts relapse [9]. Countering this process by re-orienting attention away from drug-related cues and towards natural rewards might therefore be therapeutic – and indeed, patients who successfully maintain abstinence exhibit this reversed attentional bias profile [24]. To effect such a reversal, cognitive behavioral therapy (CBT) often includes behavioral activation to increase engagement in rewarding life activities. To the extent that behavioral activation increases exposure to natural rewards, it may increase activation in brain reward circuity [25]. However, merely being physically present with a natural reinforcer may not counter cognitive biases that divert attention away from natural reward and toward drug-related stimuli. In that regard, a recent review found no evidence that behavioral activation (or CBT as a whole) can significantly improve anhedonia in OUD [7].
Specific attentional training in savoring may be needed to disrupt addiction attentional bias and increase sensory-perceptual contact with the rewarding stimulus. Savoring involves attending to the pleasant features (e.g., visual, auditory, gustatory, olfactory, tactile, or kinesthetic) of a naturally rewarding stimulus while tuning meta-awareness toward the positive emotions and pleasurable sensations that emerge during contact with the stimulus [26]. In that sense, savoring involves an element of mindfulness, and mindfulness-based amplification of sensory-perceptual contact, evident in electrocortical markers of emotional attention [27], may increase reward experience from naturally rewarding stimuli (i.e., social affiliation, food, natural beauty, for example see [28]). As such, we proposed that mindfulness promotes savoring of natural rewards by stabilizing and reorienting attention from distraction onto the pleasant stimulus, and then by deepening meta-awareness of positive emotional and interoceptive responses to the stimulus [29].
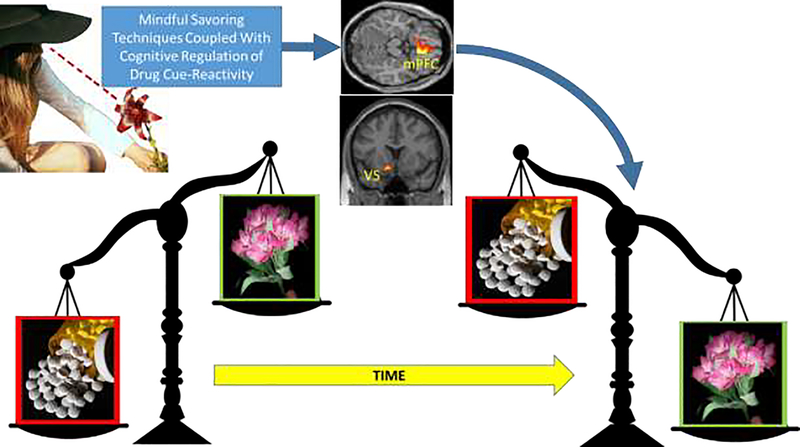
MORE leverages this synergy via a mindful savoring technique in which patients are first taught to attend to the pleasant colors, textures, and scents of a rose, as well as the touch of its petals against the skin, while remaining sensitive to their own emotional response to the flower (Figure 2). When patients become aware of sensations of pleasure or positive emotions, they are then instructed to turn attention inward, and mindfully savor the pleasant internal experience and the arising of any higher-order affective associations until they begin to fade, at which point attention shifts outward again to appreciate the flower once more. Hypothetically, this toggling of exteroceptive and interoceptive attention on pleasant perceptions, sensations, cognitions, and emotions may overcome the “hedonic treadmill effect” [30] to intensify and prolong the pleasant experience. After learning this technique, patients in MORE are instructed to practice mindful savoring with other, more personally meaningful pleasant stimuli that naturally occur their everyday lives. In multiple studies, MORE has been shown to increase subjective, autonomic, and neurophysiological responses to natural reward stimuli among samples exhibiting opioid misuse [31,32], nicotine addiction [33], and obesity [34]. In these studies, enhanced responsiveness to natural reward following treatment with MORE was associated with reductions in craving, substance use/misuse, and attentional bias, providing robust support for the restructuring reward hypothesis.
Figure 2. Restructuring reward processing through savoring.
Savoring in tandem with cognitive regulation of drug cue-reactivity may shift the relative salience of drug and natural rewards, from over-valuing drug-related rewards relative to natural rewards, back to valuing natural rewards more than drug-related rewards. This restructuring reward process is likely instantiated by changing corticostriatal responses during reward anticipation and receipt.
5. Attending to and Self-Generating Internal Reward Responses
Insofar as the allostatic effects of addiction may produce dysphoria, patients with SUD may become insensitive to fleeting, pleasure responses to natural rewards, especially when overwhelmed by aversive body sensations associated with craving –including tension, jitteriness, restlessness, shallow breathing, and stomach cramps. To remedy this biased form of cognitive processing, nuanced and fine-grained use of mindfulness to interoceptively map and delimit the spatial distribution of aversive sensation may facilitate balanced and holistic appraisals of the body’s physiological condition by increasing awareness of pleasant sensations occurring contemporaneously with unpleasant ones. Although mindfulness-based interventions aim to cultivate a non-evaluative, non-reactive awareness of present moment experience regardless of its affective valence, nonetheless the practice of mindfulness may magnify embodied pleasure in response to stimulation, as in sex and eating [28,35], and also induce positive emotions and blissful body sensations in the absence of pleasant external stimulation [36].
MORE provides training in a mindful interoceptive mapping technique to limit aversive sensation and self-generate positive emotion and pleasant body sensations (Figure 3). Specifically, patients are instructed to practice mindfulness to “zoom in” and decompose unpleasant internal experiences (e.g., craving, negative emotions, or pain) into their constituent sensations (e.g., tightness, vibration, heat) and heighten awareness of the center, edges, and permeability (versus solidity) of these sensations while noticing any proximal or distal pleasurable sensations. In accordance with ancient Indo-Tibetan contemplative traditions, this practice may yield the insight that all sensations, regardless of their valence, can be marked by the qualia of bliss upon mindful introspection [37].
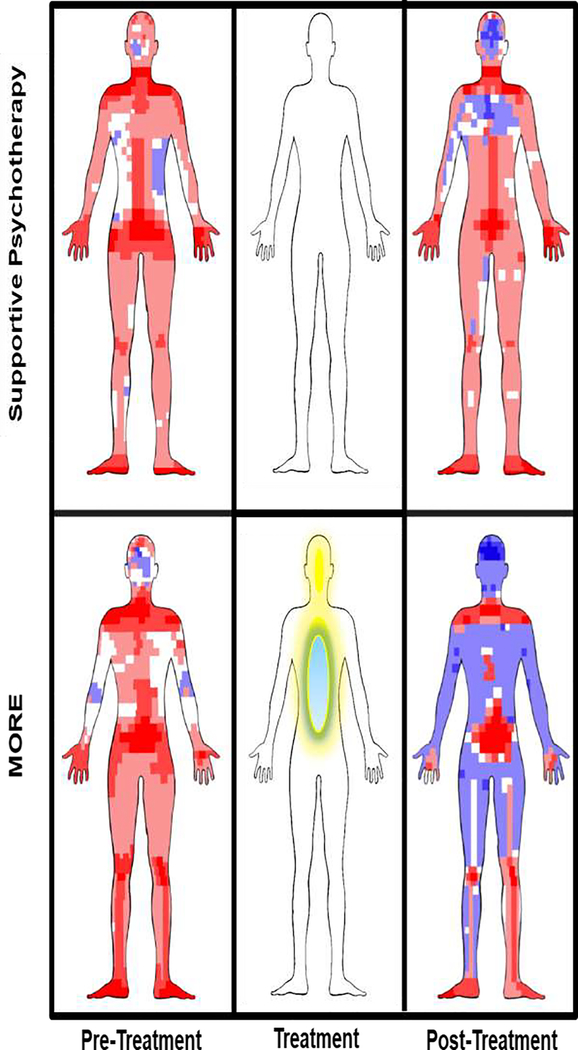
Figure 3. Attending to and self-generating internal reward processes.
Rendering of sensation reports on a computerized sensation manikin for participants treated with Mindfulness-Oriented Recovery Enhancement (MORE) or a social support group control condition, adapted from [64]. Blue represents pleasant sensations and red represents unpleasant sensations. The density of the hue reflects the frequency of pleasant or unpleasant sensation reports at a given sensation pixel. The middle panel illustrates that during treatment with MORE, meditation techniques are used to induce internalized experiences of pleasure.
Using a digital sensation manikin capable of quantifying the location and spatial distribution of pleasant and unpleasant sensations, we found in a randomized experiment that following treatment with MORE, chronic opioid users reported nearly 1.5 pleasant sensations for every 1 unpleasant sensation - representing a 7.5 fold increase in the ratio of pleasant to unpleasant sensations over the course of treatment [38]. Although the neurobiological mechanisms of this therapeutic process remain unknown, meditative self-generation of hedonic pleasure and ecstatic states are likely instantiated by activation in corticostriatal circuits implicated in reward and positive affect and undergirded by increased endogenous dopamine and opioid release, not unlike the neurochemical release occasioned by drug use itself [39]. If so, inducing pleasant sensations through meditative practices might provide a safe, non-addictive, and non-drug means of reward – a “natural high” and endogenous form of intracranial self-stimulation [40] that may replace the craving for drug-induced reward.
6. Reappraising Meaning and Cultivating Self-Transcendence
Addiction is associated with aberrant resting state functional connectivity in the default mode network (DMN), the neural hub of self-referential processing [41]. Indeed both the frequency and intensity of excessive and maladaptive self-referential processing are positively associated with DMN activity [42,43]. On a psychological level, this neural dysfunction may parallel the entrenchment of addiction into the sense of self, where stress- and withdrawal-related dysphoria, craving, and obsessive ruminations on drug use become the primary objects of self-referential thought – leading to a narrowing of one’s identity in which self-other relations (e.g., social, occupational, or recreational) that were once personally meaningful become empty of value. In contrast, according to the Mindfulness-to-Meaning Theory [29], via an array of mechanisms including deautomatization, decentering, attention regulation, and perspective-shifting, adopting a mindful orientation in the context of difficult life experiences may disrupt schematized, self-referential appraisals (e.g., “The only thing that means anything to me is getting high”) and broaden awareness to encompass an expanded set of contextual data from which adaptive reappraisals of self and world can be generated (e.g., “Getting high has hurt me and those I love, but recovery from addiction has brought meaning to my life”).
6.1. Meaning, Default Mode Processing, and Self-Transcendence.
In MORE, patients are taught to cope with craving by engaging in a mindful reappraisal process whereby they contemplate the adverse consequences for engaging in substance misuse. Such reappraisal of the meaning of drug-related stimuli has been shown to decrease craving by modulating corticostriatal circuitry function [44]. Via mindful reappraisal, MORE also instructs patients to find positive meaning in the face of adversity, a process known to activate nodes of the DMN and corticostriatal reward circuit, including mPFC and ventral striatum [45]. This meaning-making process may reach its apex in self-transcendence – the experience of interconnectedness or oneness with something greater than the self – classically held to be the sine qua non of addiction recovery (e.g., surrendering to a higher power in Alcoholics Anonymous) and a source of ultimate meaning. Though self-transcendence can arise spontaneously during awe and other naturally-occurring peak experiences, it may also be engendered by mindfulness meditation and other contemplative practices [46] whose original soteriological purpose was to foster states in which the subject-object dichotomy that structures ordinary human consciousness is transcended by the experience of a nondual awareness in which subject and object are unified [47]. During such meditative self-transcendent experiences, DMN connectivity increases with the dorsal attentional and salience networks – reducing the normative anticorrelation between extrinsic (i.e., “task positive” brain regions devoted to processing sensory information) and intrinsic networks (i.e., “task negative” brain regions involved in self-referential processing) [48] (Figure 4) – similar to functional connectivity patterns observed during the experience of ego dissolution induced by psychedelic drugs [49]. In view of the central role of aberrant DMN function in addiction, modulating functional connectivity patterns by cultivating self-transcendent experiences might “reset” default mode network dysfunction, reducing excessive self-referential processing and thereby transforming the maladaptive cognitive-affective habits driving addictive behavior.
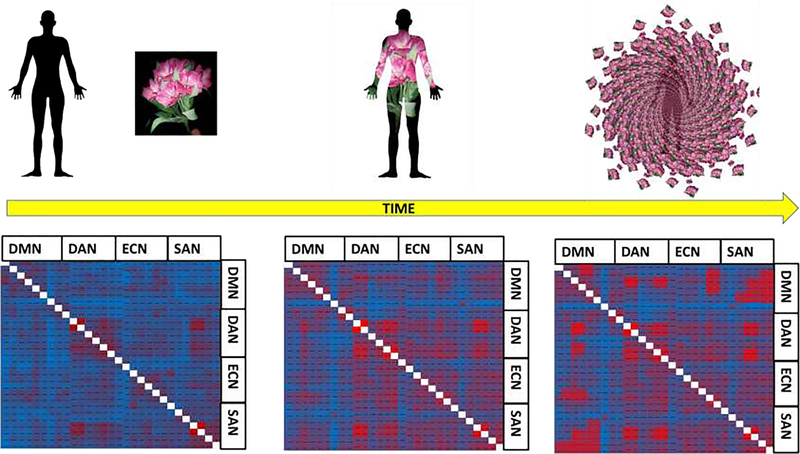
Figure 4. Modifying functional connectivity between intrinsic and extrinsic brain networks during the emergence of self-transcendence.
At baseline, the normative subject-object dichotomy (left panel) is subserved by strong anticorrelations between task-negative brain circuits devoted to self-referential processing (e.g., default mode network-DMN) and task-positive brain circuits devoted to sensory processing (e.g., dorsal attentional network-DAN; executive control network-ECN; salience network-SAN). During meditation, these anticorrelations weaken (middle panel), as attention and awareness become absorbed into the meditative object. Finally, intrinsic and extrinsic brain networks co-activate when meditation produces a nondual state of awareness in which the distinction between subject and object is dissolved – potentially providing a “reset” of default mode network dysfunction (right panel).
To wit, in a Stage 2 RCT of opioid-treated chronic pain patients in primary care (N=95) MORE significantly improved a latent positive psychological functioning variable comprised of positive affect, savoring, meaning in life, and self-transcendence measures [50]. This latent variable predicted decreases in pain severity, and in turn, reduced opioid misuse, with the self-transcendence indicator (operationalized as a sense of oneness with something greater than the self and dissolution of the self-world boundary) exerting the largest effect size.
6.2. Neural Evidence of the Link Between Self-Transcendence and Clinical Target Engagement in Addiction Treatment.
In a second randomized controlled experiment (N=62), chronic opioid users treated with MORE reported increased self-transcendence and reduced self-world boundaries coupled with increased frontal midline theta (FMT) EEG coherence and power during mindfulness meditation, which statistically mediated the effect of MORE on reducing opioid use [51]. New data from a recently completed, full-scale clinical trial of MORE (NCT02602535) replicate this effect in an even larger sample of opioid misusers. Although FMT is well-established as a biomarker of cognitive control [52], FMT also increases during states of flow [53], in which self-referential processing is suspended and transcended during deep cognitive absorption with ongoing experience. Indeed, the link between FMT and self-transcendence has been established in prior studies: theta power has been shown to increase monotonically with depth of meditative absorption towards ego dissolution and self-transcendent states [54]. And, following meditation training, increased frontal midline theta, which is associated with activation in medial prefrontal cortical structures (e.g., ACC), is mechanistically implicated in white matter plasticity surrounding this brain region [55]—providing a potential physiological substrate for the coupling of intrinsic and extrinsic brain networks following repeated instances of self-transcendence achieved during deep meditative states.
Hypothetically, meditative self-transcendence might produce a potent experience of natural reward and stimulus-independent happiness, undergirded by increased functional connectivity among intrinsic and extrinsic brain networks and driven by increased theta oscillations in the mPFC, which may in turn facilitate restructuring of reward processing in addiction. This hypothesis is especially intriguing, given the role of the mPFC (a key node of the DMN) in self-transcendent experiences occasioned by psychedelics [56], as well as the mPFC’s role in reward and addiction.
7. Transdiagnostic Applications of Mindful Positive Emotion Regulation
The same neurobiological reward deficits underpinning OUD have been identified in other forms of psychopathology, including major depressive disorder (MDD) [57] and post-traumatic stress disorder (PTSD) [58]. Thus, deficient reward responsiveness may be construed as a transdiagnostic mechanism [59], and as such, mindful positive emotion regulation interventions might be effective for treating a wide array of disorders. In that regard, MORE has been shown to significantly reduce traumatic stress, anhedonia, and depression symptoms in samples of people with co-occurring SUD and MDD/PTSD [60–62]. Given its effects on reward responses, it is likely that MORE would exhibit robust anti-depressant activity outside of the context of SUD treatment.
8. Personalized Medicine with Mindful Positive Emotion Regulation.
Individual differences may moderate the impact mechanisms outlined in Figure 1. For instance, people who seek positive reinforcement and reward through substance use may experience greater craving reduction from mindful savoring techniques that enhance natural reward responsiveness, whereas those who engage in substance use for negative reinforcement (e.g., to alleviate physical and emotional pain) might find mindful interoceptive mapping of sensation is a more potent means of reducing drug craving. Such speculations require further refinement and testing through individual difference research examining biobehavioral endophenotypes, substance use patterns, and personality characteristics as moderators of addiction treatment outcomes following mindful positive emotion regulation interventions.
8. Conclusion
In conclusion, addiction may be conceptualized as a hedonic dysregulatory process involving an imbalance in the relative salience of natural rewards and drug rewards, which however may be altered by targeted therapeutic approaches, such as MORE. As addiction worsens, the pleasure derived from natural rewards plummets, resulting in a deficit of positive affectivity and a loss of meaning in life, driving a downward spiral of behavioral escalation toward compulsive drug use. To counter this process, biobehavioral interventions might integrate sequences of mindfulness, reappraisal, and savoring techniques designed to promote positive emotion regulation with those that aim to cultivate self-transcendent meaning independent of stimulus-driven pleasure. Internally-generating reward and meaning in life through such an approach might restore the balance between intrinsic and extrinsic reward, and thereby generate the intrinsic motivation needed for durable changes in addictive behavior [63]. Interdisciplinary efforts uniting behavioral treatment development research with affective science, contemplative science, and neuroscience will drive the next wave of innovation needed to halt the global addiction crisis.
Highlights.
Substance use disorders (SUD) involve blunted positive affective and physiological responses to natural rewards.
Savoring hedonic pleasure from natural rewards may restructure over-valuation of drug-related rewards.
Self-generating internal reward responses through meditation may provide a safe, non-addictive form of pleasure.
Self-transcendence may enhance meaning in life and “reset” aberrant default mode network dysfunction in addiction.
Acknowledgements:
E.L.G. was supported by R01DA042033 (PI: Garland) from the National Institute on Drug Abuse during the preparation of this manuscript. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health. I would like to thank Mathias Sanyer for his illustrations in Figures 2–4.
Footnotes
Conflict of Interest: Eric Garland, PhD, LCSW is the Director of the Center on Mindfulness and Integrative Health Intervention Development. The Center provides Mindfulness-Oriented Recovery Enhancement (MORE), mindfulness-based therapy, and cognitive behavioral therapy in the context of research trials for no cost to research participants; however, Dr. Garland has received honoraria and payment for delivering seminars, lectures, and teaching engagements (related to training clinicians in mindfulness) sponsored by institutions of higher education, government agencies, academic teaching hospitals, and medical centers. Dr. Garland also receives royalties from the sale of books related to MORE.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barrett LF, Satpute AB: Historical pitfalls and new directions in the neuroscience of emotion. Neurosci Lett 2019, 693:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelley AE, Berridge KC: The Neuroscience of Natural Rewards: Relevance to Addictive Drugs. J Neurosci 2002, 22:3306–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfarr S, Schaaf L, Reinert JK, Paul E, Herrmannsdörfer F, Roßmanith M, Kuner T, Hansson AC, Spanagel R, Körber C: Choice for drug or natural reward engages largely overlapping neuronal ensembles in the infralimbic prefrontal cortex. J Neurosci 2018, 38:3507–3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koob GF, Schulkin J: Addiction and stress: an allostatic view. Neurosci Biobehav Rev 2019, 106:245–262. [DOI] [PubMed] [Google Scholar]
- 5.Koob GF: Neurobiology of Opioid Addiction: Opponent Process, Hyperkatifeia, and Negative Reinforcement. Biol Psychiatry 2020, 87:44–53. [DOI] [PubMed] [Google Scholar]
- 6.Garland EL, Froeliger B, Zeidan F, Partin K, Howard MO: The downward spiral of chronic pain, prescription opioid misuse, and addiction: Cognitive, affective, and neuropsychopharmacologic pathways. Neurosci Biobehav Rev 2013, 37:2597–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiluk BD, Yip SW, DeVito EE, Carroll KM, Sofuoglu M: Anhedonia as a Key Clinical Feature in the Maintenance and Treatment of Opioid Use Disorder. Clin Psychol Sci 2019, 7:1190–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aguilar de Arcos F, Verdejo-García A, Ceverino A, Montañez-Pareja M, López-Juárez E, Sánchez-Barrera M, López-Jiménez Á, Pérez-García M, PEPSA team: Dysregulation of emotional response in current and abstinent heroin users: negative heightening and positive blunting. Psychopharmacology (Berl) 2008, 198:159–166. [DOI] [PubMed] [Google Scholar]
- 9.Lubman DI, Yucel M, Kettle JW, Scaffidi A, Mackenzie T, Simmons JG, Allen NB: Responsiveness to drug cues and natural rewards in opiate addiction: associations with later heroin use. Arch Gen Psychiatry 2009, 66:205–12. [DOI] [PubMed] [Google Scholar]
- 10.Garland EL, Bryan CJ, Nakamura Y, Froeliger B, Howard MO: Deficits in autonomic indices of emotion regulation and reward processing associated with prescription opioid use and misuse. Psychopharmacology (Berl) 2017, 234:621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huhn AS, Brooner RK, Sweeney MM, Antoine D, Hammond AS, Ayaz H, Dunn KE: The association of prefrontal cortex response during a natural reward cue-reactivity paradigm, anhedonia, and demoralization in persons maintained on methadone. Addict Behav 2021, 113:106673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garland EL, Froeliger B, Howard MO: Allostatic dysregulation of natural reward processing in prescription opioid misuse: Autonomic and attentional evidence. Biol Psychol 2015, 105:124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garland EL, Trøstheim M, Eikemo M, Ernst G, Leknes S: Anhedonia in chronic pain and prescription opioid misuse. Psychol Med 2019, doi: 10.1017/S0033291719002010. [DOI] [PubMed] [Google Scholar]
- 14.Wang G-J, Volkow ND, Fowler JS, Logan J, Abumrad NN, Hitzemann RJ, Pappas NS, Pascani K: Dopamine D 2 receptor availability in opiate-dependent subjects before and after naloxone-precipitated withdrawal. Neuropsychopharmacology 1997, 16:174–182. [DOI] [PubMed] [Google Scholar]
- 15.Bach P, Frischknecht U, Reinhard I, Bekier N, Demirakca T, Ende G, Vollstädt-Klein S, Kiefer F, Hermann D: Impaired working memory performance in opioid-dependent patients is related to reduced insula gray matter volume: a voxel-based morphometric study. Eur Arch Psychiatry Clin Neurosci 2019, doi: 10.1007/s00406-019-01052-7. [DOI] [PubMed] [Google Scholar]
- 16.Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, Wallin D, Pendse G, McDonald L, Griffin M, et al. : Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain 2010, 133:2098–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sirnes E, Oltedal L, Bartsch H, Eide GE, Elgen IB, Aukland SM: Brain morphology in school-aged children with prenatal opioid exposure: A structural MRI study. Early Hum Dev 2017, 106–107:33–39. [DOI] [PubMed] [Google Scholar]
- 18.Younger JW, Chu LF, D’Arcy NT, Trott KE, Jastrzab LE, Mackey SC: Prescription opioid analgesics rapidly change the human brain. PAIN 2011, 152:1803–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garland EL: Restructuring reward processing with Mindfulness-Oriented Recovery Enhancement: novel therapeutic mechanisms to remediate hedonic dysregulation in addiction, stress, and pain. Ann N Y Acad Sci 2016, 1373:25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moningka H, Lichenstein S, Worhunsky PD, DeVito EE, Scheinost D, Yip SW: Can neuroimaging help combat the opioid epidemic? A systematic review of clinical and pharmacological challenge fMRI studies with recommendations for future research. Neuropsychopharmacology 2019, 44:259–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olney JJ, Warlow SM, Naffziger EE, Berridge KC: Current perspectives on incentive salience and applications to clinical disorders. Curr Opin Behav Sci 2018, 22:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacLean RR, Sofuoglu M, Brede E, Robinson C, Waters AJ: Attentional bias in opioid users: A systematic review and meta-analysis. Drug Alcohol Depend 2018, 191:270–278. [DOI] [PubMed] [Google Scholar]
- 23.Field M, Munafò MR, Franken IH: A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychol Bull 2009, 135:589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parvaz MA, Moeller SJ, Malaker P, Sinha R, Alia-Klein N, Goldstein RZ: Abstinence reverses EEG-indexed attention bias between drug-related and pleasant stimuli in cocaine-addicted individuals. J Psychiatry Neurosci JPN 2017, 42:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mori A, Okamoto Y, Okada G, Takagaki K, Takamura M, Jinnin R, Ichikawa N, Yamamura T, Yokoyama S, Shiota S, et al. : Effects of behavioural activation on the neural circuit related to intrinsic motivation. BJPsych Open 2018, 4:317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bryant FB, Veroff J: Savoring: A new model of positive experience. Psychology Press; 2017. [Google Scholar]
- 27.Egan RP, Hill KE, Foti D: Differential effects of state and trait mindfulness on the late positive potential. Emotion 2017, doi: 10.1037/emo0000383. [DOI] [PubMed] [Google Scholar]
- 28.Arch JJ, Brown KW, Goodman RJ, Della Porta MD, Kiken LG, Tillman S: Enjoying food without caloric cost: The impact of brief mindfulness on laboratory eating outcomes. Behav Res Ther 2016, 79:23–34. [DOI] [PubMed] [Google Scholar]
- 29.Garland EL, Farb NA, Goldin PR, Fredrickson BL: Mindfulness Broadens Awareness and Builds Eudaimonic Meaning: A Process Model of Mindful Positive Emotion Regulation. Psychol Inq 2015, 26:293–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brickman P, Campbell DT: Hedonic relativism and planning the good science. In. Appley MH (Ed.), Adaptation level theory: A symposium (pp. 287–302). New York: Academic Press; 1971. [Google Scholar]
- 31.Garland EL, Atchley RM, Hanley AW, Zubieta J-K, Froeliger B: Mindfulness-Oriented Recovery Enhancement remediates hedonic dysregulation in opioid users: Neural and affective evidence of target engagement. Sci Adv 2019, 5:eaax1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garland EL, Froeliger B, Howard MO: Effects of Mindfulness-Oriented Recovery Enhancement on reward responsiveness and opioid cue-reactivity. Psychopharmacology (Berl) 2014, 231:3229–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Froeliger B, Mathew AR, McConnell PA, Eichberg C, Saladin ME, Carpenter MJ, Garland EL : Restructuring reward mechanisms in nicotine addiction: A pilot fMRI study of Mindfulness-Oriented Recovery Enhancement for cigarette smokers. Evid Based Complement Alternat Med 2017, 2017:e7018014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas EA, Mijangos JL, Hansen PA, White S, Walker D, Reimers C, Beck AC, Garland EL: Mindfulness-oriented recovery enhancement restructures reward processing and promotes interoceptive awareness in overweight Cancer survivors: mechanistic results from a stage 1 randomized controlled trial. Integr Cancer Ther 2019, 18:1534735419855138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Velten J, Brotto LA, Chivers ML, Hirschfeld G, Margraf J: The Power of the Present: Effects of Three Mindfulness Tasks on Women’s Sexual Response. Clin Psychol Sci 2020, 8:125–138. [Google Scholar]
- 36.Dambrun M, Berniard A, Didelot T, Chaulet M, Droit-Volet S, Corman M, Juneau C, Martinon LM: Unified consciousness and the effect of body scan meditation on happiness: alteration of inner-body experience and feeling of harmony as central processes. Mindfulness 2019, 10:1530–1544. [Google Scholar]
- 37.Dyczkowski MS: The Doctrine of Vibration: An Analysis of the Doctrines and Practices Associated with Kashmir Shaivism. SUNY Press; 1987. [Google Scholar]
- 38.Hanley AW, Garland EL: Mapping the Affective Dimension of Embodiment With the Sensation Manikin: Validation Among Chronic Pain Patients and Modification by Mindfulness-Oriented Recovery Enhancement. Psychosom Med 2019, 81:612. [DOI] [PubMed] [Google Scholar]
- 39.Spagnolo PA, Kimes A, Schwandt ML, Shokri-Kojori E, Thada S, Phillips KA, Diazgranados N, Preston KL, Herscovitch P, Tomasi D, et al. : Striatal Dopamine Release in Response to Morphine: A [11C]Raclopride Positron Emission Tomography Study in Healthy Men. Biol Psychiatry 2019, 86:356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olds J, Milner P: Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol 1954, 47:419. [DOI] [PubMed] [Google Scholar]
- 41.Zhang R, Volkow ND: Brain default-mode network dysfunction in addiction. Neuroimage 2019, 200:313–331. [DOI] [PubMed] [Google Scholar]
- 42.Philippi CL, Cornejo MD, Frost CP, Walsh EC, Hoks RM, Birn R, Abercrombie HC: Neural and behavioral correlates of negative self-focused thought associated with depression. Hum Brain Mapp 2018, 39:2246–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Misaki M, Tsuchiyagaito A, Al Zoubi O, Paulus M, Bodurka J: Connectome-wide search for functional connectivity locus associated with pathological rumination as a target for real-time fMRI neurofeedback intervention. NeuroImage Clin 2020, 26:102244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN: Prefrontal–striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci 2010, 107:14811–14816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doré BP, Boccagno C, Burr D, Hubbard A, Long K, Weber J, Stern Y, Ochsner KN: Finding positive meaning in negative experiences engages ventral striatal and ventromedial prefrontal regions associated with reward valuation. J Cogn Neurosci 2017, 29:235–244. [DOI] [PubMed] [Google Scholar]
- 46.Wahbeh H, Sagher A, Back W, Pundhir P, Travis F: A Systematic Review of Transcendent States Across Meditation and Contemplative Traditions. EXPLORE 2018, 14:19–35. [DOI] [PubMed] [Google Scholar]
- 47.Josipovic Z: Neural correlates of nondual awareness in meditation. Ann N Y Acad Sci 2014, 1307:9–18. [DOI] [PubMed] [Google Scholar]
- 48.Froeliger B, Garland EL, Kozink RV, Modlin LA, Chen N-K, McClernon FJ, Greeson JM, Sobin P: Meditation-state functional connectivity (msfc): strengthening of the dorsal attention network and beyond. Evid Based Complement Alternat Med 2012, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tagliazucchi E, Roseman L, Kaelen M, Orban C, Muthukumaraswamy SD, Murphy K, Laufs H, Leech R, McGonigle J, Crossley N, et al. : Increased Global Functional Connectivity Correlates with LSD-Induced Ego Dissolution. Curr Biol 2016, 26:1043–1050. [DOI] [PubMed] [Google Scholar]
- 50.Garland EL, Hanley AW, Riquino MR, Reese SE, Baker AK, Salas K, Yack BP, Bedford CE, Bryan MA, Atchley R, et al. : Mindfulness-oriented recovery enhancement reduces opioid misuse risk via analgesic and positive psychological mechanisms: A randomized controlled trial. J Consult Clin Psychol 2019, 87:927–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hudak J, Hanley AW, Marchand WR, Nakamura Y, Yabko B, Garland EL: Endogenous theta stimulation during meditation predicts reduced opioid dosing following treatment with Mindfulness-Oriented Recovery Enhancement. Neuropsychopharmacology 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cavanagh JF, Frank MJ: Frontal theta as a mechanism for cognitive control. Trends Cogn Sci 2014, 18:414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katahira K, Yamazaki Y, Yamaoka C, Ozaki H, Nakagawa S, Nagata N: EEG Correlates of the Flow State: A Combination of Increased Frontal Theta and Moderate Frontocentral Alpha Rhythm in the Mental Arithmetic Task. Front Psychol 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeLosAngeles D, Williams G, Burston J, Fitzgibbon SP, Lewis TW, Grummett TS, Clark CR, Pope KJ, Willoughby JO: Electroencephalographic correlates of states of concentrative meditation. Int J Psychophysiol 2016, 110:27–39. [DOI] [PubMed] [Google Scholar]
- 55.Tang YY, Tang R, Rothbart MK, Posner MI: Frontal theta activity and white matter plasticity following mindfulness meditation. Curr Opin Psychol 2019, 28:294–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mason NL, Kuypers KPC, Müller F, Reckweg J, Tse DHY, Toennes SW, Hutten NRPW, Jansen JFA, Stiers P, Feilding A, et al. : Me, myself, bye: regional alterations in glutamate and the experience of ego dissolution with psilocybin. Neuropsychopharmacology 2020, doi: 10.1038/s41386-020-0718-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pizzagalli DA: Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol 2014, 10:393–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nawijn L, van Zuiden M, Frijling JL, Koch SB, Veltman DJ, Olff M: Reward functioning in PTSD: a systematic review exploring the mechanisms underlying anhedonia. Neurosci Biobehav Rev 2015, 51:189–204. [DOI] [PubMed] [Google Scholar]
- 59.Nusslock R, Alloy LB: Reward processing and mood-related symptoms: An RDoC and translational neuroscience perspective. J Affect Disord 2017, 216:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garland EL, Roberts-Lewis A, Tronnier CD, Graves R, Kelley K: Mindfulness-Oriented Recovery Enhancement versus CBT for co-occurring substance dependence, traumatic stress, and psychiatric disorders: proximal outcomes from a pragmatic randomized trial. Behav Res Ther 2016, 77:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garland EL, Fix S, Hudak J, Bernat EM, Nakamura Y, Froeliger B, Hanley AW, Donaldson GW, Marchand WR: Mindfulness-Oriented Recovery Enhancement remediates anhedonia in chronic opioid use by enhancing neurophysiological indices of natural reward responsiveness. Rev 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garland EL, Hanley AW, Nakamura Y, Baker AK, Reese SE, Riquino MR, Froeliger B, Donaldson GW, Howard MO: Mindfulness-Oriented Recovery Enhancement for opioid misuse and chronic pain in primary care: A randomized clinical trial. Rev 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sayegh CS, Huey SJ Jr., Zara EJ, Jhaveri K: Follow-up treatment effects of contingency management and motivational interviewing on substance use: A meta-analysis. Psychol Addict Behav 2017, 31:403–414. [DOI] [PubMed] [Google Scholar]